Études des anti-oxydants-antimicrobiens
provenant de fruits et légumes
Thèse
Cuong Ho
Doctorat en sciences et technologie des aliments
Philosophiae Doctor (Ph. D.)
Québec, Canada
© Cuong Ho, 2017
Études des anti-oxydants-antimicrobiens
provenant de fruits et légumes
Thèse
Cuong Ho
Sous la direction de :
Joseph Arul, directeur de recherche
Paul Angers, codirecteur de recherche
iv Résumé
Plusieurs études ont rapporté l’activité antimicrobienne et anti-oxydante d'extraits de sous-produits végétaux. Le recours à l’utilisation de ces extraits dans les sous-produits alimentaires pourrait constituer une stratégie innovante et prometteuse. Les résidus de fruits et légumes peuvent être une source potentielle de composés bioactifs. L’enjeu de cette étude était d’initier des recherches qui pourront aboutir au développement d’agents anti-oxydants-antimicrobiens à partir des sous-produits végétaux destinés à la conservation des aliments, comme une alternative aux produits chimiques synthétiques. L'étude initiale a été d'identifier des extraits de fruits et légumes qui possèdent à la fois de grandes propriétés antimicrobiennes et anti-oxydantes. Enfin, des moyens simples pour augmenter l'activité antimicrobienne de ces extraits ont été étudiés.
Environ 160 extraits aqueux de sous-produits de fruits et légumes ont été criblés pour évaluer leur potentiel antimicrobien et anti-oxydant. L’activité antimicrobienne a été déterminée par l’inhibition de la croissance d’Escherichi coli et de Bacillus subtilis. L’activité anti-oxydante a été mise évidence par le test au DPPH (2,2-diphenyl -1-picrylhydrazyl). L'étude a conduit à l'identification des extraits présentant à la fois un potentiel antimicrobien et des propriétés anti-oxydantes. Les propriétés bioactives des extraits sont influencées par le pH de l'extrait, le type de tissu (fruits, feuilles et racines) et le type physiologique des fruits (climactérique). Les résultats ont montré l’existence d’une relation entre les propriétés anti-oxydantes et antimicrobiennes des extraits de plantes. De ce fait, l'indice anti-oxydant-antimicrobien développé pourrait être utile dans le choix des sources végétales bioactives. Les extraits de plantes aux propriétés antimicrobiennes et anti-oxydantes présentent un potentiel comme agent de conservation sécuritaire, bénéfique pour la santé et économique.
La considération de la vitesse de piégeage des radicaux (facteur du temps) pour définir l'efficacité réelle (capacité) du système anti-oxydant a été considérée comme la meilleure manière d’exprimer avec plus de fiabilité le pouvoir anti-oxydant réel. La nouvelle expression, le pouvoir antiradicalaire - ARP, produite à partir du test DPPH, peut être plus utile pour identifier l'activité anti-oxydante des échantillons biologiques. Certains échantillons présentaient une valeur ARP élevée tels que les extraits de rambutan, feuille de
v
canneberge, feuille de bleuet, feuille de vigne (sauvage), feuille de framboise, feuille de bétel, avocat, grenade et cherimoya. Les extraits de feuilles possèdent, en général, une valeur d’ARP supérieure à celle des fruits tandis que, les extraits de racines présentent les plus faibles ARP. De plus, cette étude montre également que le nombre moyen d’oxydation du carbone de mélanges complexes tels que des extraits de plantes peut prédire leur pouvoir anti-oxydant, en dépit de la disparité entre les tissus de feuilles et de fruits.
Le spectre d'activité radicalaire d'extraits sélectionnés a été exploré. L'activité anti-oxydante a été évaluée par différentes méthodes incluant, capacité anti-anti-oxydante équivalente de Trolox (TEAC), essai de radicaux libres (DPPH), test du pouvoir réducteur d’ion ferrique (FRAP), mesure du potentiel d'oxydoréduction, réduction du peroxyde d'hydrogène, du radical hydroxyle, d’anion superoxyde, d’oxyde nitrique et de l’activité de chélation du fer. Les teneurs totales en composés phénoliques (TPC) et en flavonoïdes (TFC) des extraits ont également été déterminées. Les extraits de feuille de bétel, fruit de bleuet, feuille de cassis, feuille de canneberge ont montré une bonne activité de piégeage des radicaux (essai TEAC); ceux de pomme, oseille, vigne rouge et racine de pissenlit étaient efficaces contre SOA; ceux de feuilles de canneberge, feuille de bleuet, cassis et romarin contre le radical hydroxyle; ceux de bette à carde, panais, brocoli et orange contre H2O2; et ceux de pomme de terre, banane, oseille, feuille d'argousier contre l'oxyde
nitrique. Les extraits de feuille et fruit de bleuet, grenade, cassis et feuille de bétel ont montré un bon pouvoir réducteur d’ion ferrique. Les extraits possédant la capacité élevée de chélation du fer étaient: feuille d’argousier, radis, panais, feuille de bétel et mangoustan. L'extrait de feuille de bétel présentait des activités élevées, y compris de fixation du fer et de piégeage de divers radicaux, à l'exception de l'oxyde nitrique, où l'activité était néanmoins modérée. Les essais de TEAC, de DPPH et de FRAP fournissent essentiellement la même réponse concernant l'activité anti-oxydante des extraits de plantes, ce qui suggère que l'un de ces essais serait suffisant pour évaluer leur capacité anti-radicalaire. Cette étude suggère également que l'activité anti-oxydante d'une substance, déterminée par un ou plusieurs essais, ne donne pas une image complète de son efficacité contre les diverses espèces de radicaux oxygénés; et elle montre que la détermination du spectre de l'activité anti-radicalaire serait nécessaire.
vi
L’activité antimicrobienne des extraits de plantes sélectionnés a été évaluée en détail par turbidimétrie en milieu liquide et par diffusion en milieu solide (gel). L’activité antimicrobienne a été exprimée dans la première méthode par l’inhibition de la croissance (GI), par la concentration minimale inhibitrice (MIC) et par une nouvelle expression, dénommée index antimicrobien (AMI). Dans la seconde méthode, l’activité a été exprimée en zone d’inhibition. L'AMI prend en compte les trois phases de croissance des bactéries. Bien que les méthodes turbidimétriques soient fiables dans l’évaluation de l’activité antimicrobienne des substances, la nouvelle expression (AMI) semble être plus significative car, elle comprend l’information sur l’interaction entre la substance et le micro-organisme et sa vitesse de croissance en présence de cette substance. De plus, l'AMI démarque l’activité des échantillons, même ceux qui sont très actifs. Le spectre d’activité antimicrobienne des extraits de plantes sélectionnés a été également étudié. Seulement quelques extraits ont montré un spectre d’activité antimicrobienne assez large. En effet, seul l’extrait de feuille de bétel a un large spectre d’activité contre les bactéries, les levures et les champignons, suivis des extraits de grenade et de fruits d’argousier qui semblent être efficaces contre les bactéries et les levures. L’extrait de cassis est effectivement une substance antibactérienne.
Finalement, différentes stratégies pour améliorer l’activité antimicrobienne d'extraits anti-oxydants-antimicrobiens sélectionnés ont été explorées. Trois approches, incluant l’extraction par solvants sélectifs, le mélange binaire des extraits et l'addition de composés végétaux, ont été étudiées. L’activité anti-oxydante-antimicrobienne et le profil phytochimique ont été analysés. Le résultat montre que l’eau chaude peut être utilisée comme solvant pour l’extraction d’agents anti-oxydants-antimicrobiens d’origine végétale, bien qu’un solvant polaire extrait en préférence les substances phénoliques et modestement les substances non polaires comme les terpénoïdes. Toutefois, compte tenu du rendement et de l’activité des extraits, l’eau semble être appropriée, et elle peut être considérée comme un solvant efficace pour les fruits qui sont riches en substances phénoliques. Néanmoins, d'autres solvants sélectifs doivent être considérés pour l’extraction des substances actives non-polaires issues de matière première végétale comme les feuilles. Certaines augmentations de l’activité antimicrobienne sont possibles par le mélange de deux extraits de plantes ou par l’addition de composés végétaux. On a aussi observé que la composition
vii
des mélanges peut être importante, car les interactions synergiques ou antagonistes se retrouvent dans certaines proportions. Le mélange des extraits de grenade et cassis d’une part et le mélange des extraits de thé vert et pomme de cajou d’autre part ont montré une amélioration d’activité. L’ajout de glyoxal de méthyle et mono-caprine a également produit une augmentation de l’activité des extraits de plantes. L'addition de glyoxal de méthyle à 25 % (p/p) a amélioré l’activité des extraits de grenade et de cassis.
Dans l'ensemble, ce travail a exploré le potentiel des extraits des sous-produits de fruits et de légumes comme agent anti-oxydant-antimicrobien. Les extraits de plantes montrant des potentiels anti-oxydants et antimicrobiens regroupent: olive, canneberge, noni, feuille de bétel, cassis, grenade, citronnelle, épinard, raisin vert (vin), cassis (résidu), aubergine, ramboutan, prune indienne, feuille de canneberge, feuille de romarin, feuille de vigne (sauvage), thé vert, mangoustan et feuille de framboise. Cependant, de cette liste, seuls quelques extraits (feuille de bétel, grenade, résidus de cassis) présentent un large spectre d’activité anti-oxydante et antimicrobienne. En outre, cette étude a introduit de nouvelles expressions pour l’activité anti-oxydante (ARP) et antimicrobienne (AMI) des mélanges complexes tels que les extraits de plantes. Ces expressions peuvent être utiles pour le criblage de matériels d’origine végétale dans la recherche de ces activités. Un autre enseignement de cette étude est que l'amélioration de l'activité antimicrobienne des extraits de plantes ne peut pas être possible simplement par le mélange d'extraits, en raison des interactions potentielles entre les composants des extraits. La connaissance de la composition phytochimique est essentielle pour comprendre de telles interactions, dans la sélection des mélanges et pour déterminer les approches possibles pour améliorer l'activité antimicrobienne. Les sous-produits de végétaux peuvent être une source potentielle d'antioxydant-antimicrobiens pour une application dans la conservation des aliments, comme alternative aux agents synthétiques, mais beaucoup de travaux sont encore nécessaires pour atteindre ce but, ce qui nécessiterait une approche systémique.
viii Abstract
There is a growing interest in the development of strategies to use agricultural and industrial residues as a source of high value-added, including bioactive products. The residues of fruits and vegetables may be a potential source of bioactive compounds. The major objective of this work is to conduct studies to pave the way for the development of antioxidant-antimicrobials from plant by-products for use in food preservation, as alternative to synthetic chemicals. The starting point was the identification of extracts of fruit and vegetable extracts exhibiting high antimicrobial and antioxidant properties. The selected extracts were then characterized for their spectrum of antioxidant and antimicrobial activities. Finally, simple ways of enhancing the antimicrobial activity of the selected extracts were examined.
Aqueous extracts of about 160 fruit and vegetable by-products were evaluated for their potential as antimicrobial and antioxidant agents. The growth inhibiting activity of all the 160 extracts were tested against Escherichia coli, and Bacillus Subtilis; and the antioxidant activity was determined by DPPH radical scavenging assay. The pH of the extract, type of tissue (fruit, leaf and root) and physiological type of fruits (climacteric) had impact on the bioactive properties. There was some relationship between antioxidant and antimicrobial properties of the plant extracts. The proposed antioxidant-antimicrobial index may be useful in the selection of plant sources of bio-actives.
Consideration of rate of radical quenching (time factor) to define actual efficacy (capacity) of antioxidant system was found to be a better and reliable way of expressing the real antioxidant power. The new expression, antiradical power - ARP, generated from DPPH assay may be more useful in identifying the antioxidant activity of biological samples. Some samples exhibited high ARP value such as rambutan, cranberry leaf, blueberry leaf, grape leaf (wild), raspberry leaf, betel leaf, avocado, pomegranate and custard apple. Leaf extracts possess, in general, higher ARP than fruits, and root extracts possess low ARP. In addition, this study also suggests that average carbon oxidation number of complex mixtures such as plant extracts may portend their antioxidant power, in spite of the disparity between leaf and fruit materials.
ix
In chapter 4, plant extracts selected from the original 160 were investigated for their spectrum of anti-radical activity. The antioxidant activity was evaluated by Trolox equivalent antioxidant capacity (TEAC), DPPH free radical assay (DPPH), ferric reducing power assay (FRAP), redox potential measurement, hydrogen peroxide, hydroxyl radical, superoxide anion, nitric oxide and iron chelating activities. Their total phenolic content (TPC) and total flavonoid content (TFC) were also determined. Betel leaf, blueberry fruit and black currant and cranberry leaf showed high radical scavenging activity (TEAC assay); apple, sorrel, red grape and dandelion root were effective against SOA; cranberry leaf, blueberry leaf, black currant and Rosemary against hydroxyl radical; rainbow chard, parsnip, broccoli and orange against H2O2; and potato, banana, sorrel, sea buckthorn leaf
against nitric oxide. Blueberry leaf and fruit, pomegranate, black currant and betel leaf showed high ferric ion reducing power. The extracts showing high iron binding capacity were: sea buckthorn leaf, radish, parsnip, betel leaf and mangosteen. Betel leaf extract exhibited high activities, including iron binding and scavenging of various radicals except nitric oxide, where the activity was moderate. TEAC, DPPH and FRAP assays provide essentially the same response with respect to the antioxidant activity of plant extracts, suggesting that any one of them would be adequate to evaluate their anti-radical capacity. This study also suggests that antioxidant activity of a substance, determined by one or more related assays, does not give the complete picture of its effectiveness against various species of oxygen radicals; and emphasizes that determination of the spectrum of the anti-radical activity would be necessary.
In chapter 5, the antimicrobial activity of selected plant extracts was evaluated in detail by turbidimetric methods in liquid medium, and by well-diffusion method in gel medium. The antimicrobial activity was expressed in the former by growth inhibition, minimum inhibitory concentration - MIC and by a new expression, the antimicrobial index – AMI; and in the latter, expressed by zone of inhibition. AMI takes into account all the three growth phases of the bacteria. Although the turbidimetric methods were in good agreement in the assessment of the activity of the substances, the new expression, AMI, appears to be more meaningful since it carries the information regarding interaction between the substance and the microorganism and the growth rate in the presence of that substance. In addition, AMI demarcates the activity of samples, even those found to be highly active.
x
Furthermore, the spectrum of antimicrobial activity of selected plant extracts was also examined. Only a few extracts showed some broad spectrum in their activities. In effect, only betel leaf extract showed a broad spectrum of antimicrobial activity against bacteria, yeasts and fungi; whereas pomegranate and sea buckthorn fruit extracts appear to be effective against both bacteria and yeasts. Black currant extract is effectively an antibacterial substance.
In chapter 6, various ways of enhancing the antimicrobial activity of selected antioxidant-antimicrobial extracts were explored. Three approaches, including selective solvent extraction, binary blending of extracts and addition of plant compounds were investigated. Antioxidant, antimicrobial activity and the profile of phytochemical classes were analyzed. The results showed that hot water could be used for solvent extraction of antioxidant-antimicrobials from plant materials, albeit a polar solvent that extracts phenolic substances preferably and only modestly non-polar substances such as terpenoids. However, considering the yield of the extracts and the activity, water appeared to be an effective solvent solvent for fruit sources that are rich in phenolic substances. Other selective solvents must be considered for extraction of active non-polar substances for plant sources such as leaves. Some enhancement in antimicrobial activity was possible by either mixing plant extracts or by the addition of plant compounds. It was also observed that the composition of blends or mixtures might be important, since synergistic or antagonistic interactions occurred at certain proportions. Pomegranate and black currant extract blends and green tea and cashew apple extract blends showed enhancement in activity. The addition of methyl glyoxal and mono-caprin also showed enhancement in the activity of plant extracts. Methyl glyoxal at 25 % (w/w) addition improved the activity of pomegranate and black currant extracts.
Overall, this work explored the potential of extracts of fruit and vegetable by-products as anti-oxidant-antimicrobials. Some plant extracts having potential as antioxidant-antimicrobial agents. They include: olive, cranberry, noni, betel leaf, black currant, pomegranate, lemon grass, spinach, green grape (wine), black currant (residue), egg plant, rambutan, Indian plum, cranberry leaf, rosemary leaf, grape leaf (wild), green tea, mangosteen and raspberry leaf. However, the list is reduced to a few (betel leaf,
xi
pomegranate, black currant residue), should broad antioxidant and antimicrobial activities are taken into account.
In addition, this study introduces new expressions for antioxidant (ARP) and antimicrobial (AMI) activities of complex mixtures such as plant extracts, and they can be useful in the screening of plant materials for these activities. The knowledge of the phytochemical composition is essential to understand such interactions, in the selection of mixtures and to determine possible approaches to enhance the antimicrobial activity. Plant by-product can be a potential source of antioxidant-antimicrobial for use in food preservation, as alternative to synthetic agents, but much work is needed to realize this goal, and that would require a systemic approach.
xii Table des matières
Résumé ... iv
Abstract ... viii
Table des matières ... xii
Liste des tableaux ... xvii
Liste des figures ... xix
Liste des abréviations et des sigles ... xxii
GENERAL INTRODUCTION ... 1
Chapter 1 REVUE DE LITERATURE ... 5
1.1 Plant by-products: eco-friendly source of novel bioactive compounds ... 6
1.1.1 Why plant by-products? ... 7
1.1.2 Source, provider and type of by-products ... 8
1.1.3 Methods of extraction of bioactive compounds from plant ... 9
1.1.4 Trend and challenges in using plant by-products ... 11
1.2 Plant secondary metabolites ... 13
1.2.1 Primary and secondary metabolites ... 13
1.2.2 Plant secondary metabolism ... 14
1.2.3 Biosynthesis of secondary metabolites ... 16
1.2.3.1 Terpenes... 18 1.2.3.2 Phenolic compounds ... 19 1.2.3.3 Nitrogen-containing compounds ... 23 1.2.3.4 Polyketides... 23 1.3 Antioxidants ... 25 1.3.1 Concepts ... 25
1.3.2 Why to examine antioxidants from plants? ... 26
1.3.3 Production of free radical in biology ... 28
1.3.4 Natural sources of antioxidant compounds ... 29
1.3.5 Modulation of free radical by antioxidants ... 30
1.3.5.1 Mechanisms of antioxidant action ... 31
1.3.5.2 Defense system mechanism in vivo against oxidative damage ... 31
1.3.5.3 Plant antioxidant systems ... 33
1.3.6 The structure–activity relationships of antioxidant ... 35
1.3.7 Methods of in vitro antioxidant activity determination ... 37
1.3.7.1 In vitro antioxidant capacity assays ... 37
1.3.7.2 Mode of action of in vitro antioxidant activity assays ... 38
1.3.8 Trends and challenges in application of antioxidants from plant sources. ... 46
1.4 Plants as a source of antimicrobials ... 49
1.4.1 Why antimicrobials from plant sources? ... 49
1.4.2 Major groups of phytochemicals with antimicrobial properties ... 50
1.4.3 Modes of action of antimicrobials ... 53
xiii
1.4.5 In vitro methods to evaluate plant extracts for antimicrobial activity... 57
1.4.5.1 Diffusion method ... 58
1.4.5.2 Dilution method ... 58
1.4.5.3 Broth dilution ... 58
1.4.5.4 Agar dilution ... 59
1.4.5.5 Time kill assay ... 59
1.4.6 Research trends and challenges of antimicrobials from plant products ... 59
1.4.7 Recent literature on bioactivities of some specific fruits and vegetables - A random walk ... 61
1.5 Hypotheses ... 66
1.6 Objectives ... 67
1.6.1 General Objective ... 67
1.6.2 Specific Objectives ... 67
Chapter 2 NATURAL ANTIOXIDANT-ANTIMICROBIALS: SCREENING OF FRUITS, VEGETABLES AND THEIR BY-PRODUCTS ... 68
2.1 Abstract ... 69
2.2 Introduction ... 70
2.3 Materials and Methods ... 72
2.3.1 Plant materials ... 72
2.3.2 Chemicals and reagents ... 72
2.3.3 Preparation of extracts ... 72
2.3.4 Bacterial strains and inoculum preparation ... 72
2.3.5 Antimicrobial activity assay ... 73
2.3.6 Antioxidant assay - DPPH radical-scavenging capacity ... 73
2.3.7 Statistical analyses ... 74
2.4 Results ... 75
2.4.1 Antimicrobial activity ... 75
2.4.2 Antioxidant activity of fruit and vegetable extracts (DPPH assay) ... 84
2.4.3 Correlation between the antimicrobial and antioxidant activity of plant extracts 84 2.5 Discussion ... 86
2.5.1 Antioxidant and antibacterial activities of plant by-products ... 86
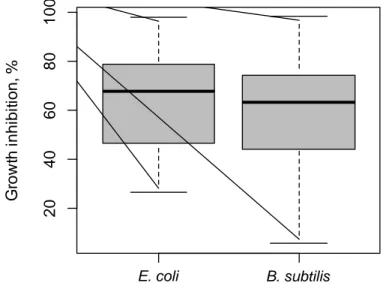
2.5.2 E. coli and B. subtilis sensitivity. ... 86
2.5.3 Effect of pH of extracts on AM and AO properties ... 88
2.5.4 Effect of physiological type of fruit: climacteric vs. non-climacteric... 89
2.5.5 Effect of plant tissue type ... 90
2.5.6 Antioxidant activity of Brassica and Allium extracts ... 90
2.5.7 Correlation between antioxidant and antimicrobial activities ... 92
2.5.8 Antioxidant-antimicrobials index (AO-AM Index) ... 93
2.6 Conclusions ... 95
Chapter 3 ANTI-RADICAL POWER (ARP) OF PLANT EXTRACTS: A NEW MEASURE OF ANTIOXIDANT PROPERTY FROM DPPH ASSAY ... 96
3.1 Abstract ... 97
3.2 Introduction ... 98
3.3 Materials and methods ... 100
xiv
3.3.2 Chemicals and reagents ... 100
3.3.3 Plant extract preparation ... 100
3.3.4 DPPH free radical scavenging assay ... 100
3.3.5 Elemental and metal analyses ... 101
3.3.6 Reaction kinetics and calculation of reaction rate constants ... 102
3.3.7 Anti-Radical Power (ARP): The overall antioxidant activity ... 104
3.3.8 Average carbon oxidation number ... 105
3.3.9 Data analysis ... 105
3.4 Results and discussion ... 106
3.4.1 Estimation of antioxidant activity by DPPH• assay ... 106
3.4.1.1 Antioxidant capacity ... 107
3.4.1.2 Rate of radical scavenging ... 108
3.4.2 Relation between scavenging rate and capacity of antioxidant activity ... 113
3.4.3 Anti-Radical Power ... 116
3.4.4 Average carbon oxidation number (ACON) ... 119
3.5 Conclusions ... 124
Chapter 4 ANTI-RADICAL ACTIVITY SPECTRUM OF SECLETED FRUIT AND VEGETABLE EXTRACTS AGAINST SEVERAL REACTIVE OXYGEN SPECIES ... 125
4.1 Abstract ... 126
4.2 Introduction ... 127
4.3 Materials and methods ... 129
4.3.1 Plant materials ... 129
4.3.2 Chemicals and reagents ... 130
4.3.3 Plant extract preparation ... 130
4.3.4 Total phenolic content ... 130
4.3.5 Total flavonoid assay ... 131
4.3.6 Antioxidant Assays ... 131
4.3.6.1 TEAC (Trolox equivalent antioxidant capacity) ... 131
4.3.6.2 DPPH free radical scavenging assay ... 132
4.3.6.3 Reducing power property ... 132
4.3.6.4 Hydrogen peroxide scavenging activity ... 133
4.3.6.5 Hydroxyl radical scavenging activity ... 133
4.3.6.6 Superoxide anion scavenging capacity ... 133
4.3.6.7 Nitric oxide scavenging activity ... 134
4.3.6.8 Metal chelating assay (Spectrometric assay) ... 134
4.3.7 Data analysis ... 134
4.4. Results and Discussion ... 136
4.4.1 Total phenolic and flavonoid contents ... 136
4.4.2 Antioxidant activity ... 138
4.4.2.1 Test Radicals (ABTS tand DPPH)... 138
4.4.2.2 Relation between anti-radical activities (TEAC, DPPH) and total phenolic content (TPC) and total flavonoid content (TFC) ... 141
4.4.2.3 Reducing power property (FRAP) ... 143
4.4.2.4 Hydroxyl radical scavenging activity ... 146
xv
4.4.2.6 Superoxide anion scavenging activity ... 147
4.4.2.6 Nitric oxide scavenging activity ... 147
4.4.2.7 Metal chelating activity ... 148
4.4.3 Inter-relationships between anti-radical activities ... 150
4.4.4 Anti-radical activity spectrum ... 153
4.5 Conclusions ... 155
Chapter 5 CHARACTERIZATION OF ANTIMICROBIAL ACTIVITY OF SELECTED PLANT EXTRACTS: ANTIMICROBIAL ACTIVITY INDEX AND SPECTRUM OF ACTIVITY ... 156
5.1 Abstract ... 157
5.2 Introduction ... 158
5.3 Materials and methods ... 161
5.3.1 Plant materials ... 161
5.3.2 Extraction and stock solution preparation ... 162
5.3.4 Bacterial strains and inoculum preparation ... 162
5.3.5 Antimicrobial activity assays ... 162
5.3.5.1 Zone inhibition agar gel diffusion assay... 162
5.3.5.2 Micro-titer broth dilution (growth inhibition or inhibition capacity) and time kill curve assay ... 163
5.3.5.3 Broth micro-dilution (MIC) assay ... 163
5.3.5.4 Agar dilution (yeasts) ... 164
5.3.5.5 Anti-fungal assay (agar dilution) ... 164
5.3.6 Empirical expression for antimicrobial activity ... 164
5.3.7 Data analysis ... 167
5.4 Results and discussion ... 168
5.4.1 Antimicrobial activity of plant extracts ... 168
5.4.1.1 Antimicrobial Index (AMI) ... 168
5.4.1.2 Growth inhibition or antimicrobial capacity ... 173
5.4.1.3 Minimum inhibitory concentration (MIC) ... 173
5.4.1.4 Zone inhibition (ZI) ... 173
5.4.1.5 Comparison of methods in the characterization plant extracts ... 174
5.4.2 Spectrum of Antimicrobial activity ... 182
5.4.2.1 Antimicrobial activity of plant extracts against bacteria ... 182
5.4.2.2 Antimicrobial activity of plant extract against yeasts ... 183
5.4.2.3 Antimicrobial activity of plant extract against fungi ... 184
5.5 Conclusions ... 186
Chapter 6 ENHANCEMENT OF THE ANTIMICROBIAL ACTIVITY OF SELECTED PLANT EXTRACTS BY FRACTIONATION AND MIXING ... 187
6.1 Abstract ... 188
6.2 Introduction ... 189
6.3 Materials and methods ... 190
6.3.1 Plant materials ... 190
6.3.3 Plant extracts and stock solution preparation ... 190
6.3.4 Bacterial strains and inoculum preparation ... 191
xvi
6.3.5.1 Growth Inhibition using micro-titer broth dilution assay (GI) ... 191
6.3.5.2 MIC - Broth microdilution assay ... 192
6.3.6 Antioxidant assay - DPPH radical-scavenging capacity ... 192
6.3.7 Phyto-chemical analysis ... 193
6.3.7.1 Total phenolics ... 193
6.3.7.2 Total flavonoid assay ... 193
6.3.7.3 Estimation of total tannin content ... 194
6.3.7.4 Determination of proanthocyanidin content ... 194
6.3.7.5 Determination of alkaloid content ... 194
6.3.7.6 Terpenoids ... 195
6.3.8 Enhancement of antimicrobial activity of plant extracts ... 196
6.3.8.1 Selective solvent extraction ... 196
6.3.8.2 Binary blends of selected extracts ... 197
6.3.8.3 Addition of plant compounds ... 197
6.3.9 Statistical analysis ... 197
6.4 Results and discussion ... 198
6.4.1 Solvent fractionation of extracts ... 198
6.4.1.1 Extract yields ... 198
6.4.1.2 Composition of solvent fractions and their antioxidant-antimicrobial activities ... 199
6.4.2 Binary blends of selected extracts ... 203
6.4.3 Addition of plant compounds ... 206
6.5 Conclusions ... 212
GENERAL CONCLUSIONS AND PERSPECTIVES ... 213
BIBLIOGRAPHIE ... 218
ANNEXES ... 233
Annexe 1. Yields of extracts from plant materials (Chapter 1) ... 234
xvii Liste des tableaux
Table 1.1 Solvents used for active component extraction ... 10
Table 1.2 Natural plant sources of some antioxidants ... 29
Table 1.3 Natural sources of polyphenol ... 30
Table 1.4 In vitro antioxidant capacity assays ... 38
Table 1.5 Major classes of antimicrobial compounds from plants ... 52
Table 1.6 Phyto-chemical composition and bioactivities of fruit and vegetable extracts ... 63
Table 2.1 Antimicrobial and Antioxidant activity of Climacteric fruits ... 78
Table 2.2 Antimicrobial and Antioxidant activity of Non-climacteric fruits ... 79
Table 2.3 Antimicrobial and Antioxidant activity of leaf extracts ... 81
Table 2.4 Antimicrobial and antioxidant activity of root peel extracts ... 83
Table 3.1 Antioxidant activity of fruits and vegetables by products extracts ... 109
Table 3.2 Elemental composition, average carbon oxidation number value of fruits and vegetables by-products extracts ... 120
Table 3.3 Comparison of ACON, Capacity, rate and ARP values of plant by product (fruit and leaf) extracts (1.0 mg/mL) ... 120
Table 4.1 Total phenolic and flavonoid contents of aqueous extracts of 36 selected plant materials: Total phenolic content was expressed as gallic acid (GAE) and ascorbic acid equivalent (AAE) and total flavonoid content was expressed as rutin equivalent (RE). (±) SD ... 137
Table 4.2 Free radical scavenging activity and reducing property of the aqueous extracts obtained from selected plant materials. (±) denotes standard deviation ... 140
Table 4.3 Free radical scavenging activity and reducing property of the aqueous extracts obtained from selected plant materials. (±) denotes standard deviation: Ferric reducing power assay (FRAP) and Nitric Oxide Radical Scavenging (NO) ... 144
Table 4.4 Ferrous ion chelating activity of aqueous extract obtained from selected plant material. This activity was expressed as Na2EDTA equivalent and (±) means the standard deviation ... 149
Table 5.1 Antimicrobial growth kinetic parameters against E.coli for plant extracts at the concentration of 10 mg/mL ... 170
Table 5.2 Comparison of antimicrobial activity (AMI) against E.coli of plant extracts (10 mg/mL) determined by agar gel diffusion assay (Zone of inhibition, ZI), Micro-titer broth dilution (antimicrobial capacity), Broth microdilution assay (MIC) ... 176
Table 5.3 Growth inhibition of bacteria by plant extracts at 10.0 mg/mL (Growth inhibition ± SD) ... 182
xviii
Table 5.4 Zone of inhibition (mm) of bacterial growth by well-diffusion assay at 5.0
mg/mL ... 183 Table 5.5 Inhibitory activity of selected plant extracts on the growth inhibition of
yeasts at 10.0 mg/mL ... 184 Table 5.6 Inhibitory activity of selected plant extracts on the growth inhibition of
fungi (%) at 10.0 mg/mL ... 184 Table 6.1 Solubility parameter of the solvent systems ... 196 Table 6.2 The MIC against E.coli of 12 selected plant extracts and 10 plant products ... 206 Table 6.3 The GI (%) against E.coli of mixture of 12 plant extracts with added plant
products at total concentration of 5.0 mg/mL ... 208 Table 6.4 The GI (%) against E. coli of mixtures of plant extracts and 5 plant
xix Liste des figures
Figure 1.1 Overview of plant primary and secondary metabolism ... 15
Figure 1.2 Biosynthetic relationships of major groups of secondary metabolites ... 17
Figure 1.3 Biosynthetic of terpenes: Mevalonic acid pathway ... 18
Figure 1.4 Biosynthetic phenolic compounds: Shikimate + Malonate pathway ... 19
Figure 1.5 Balance between antioxidant (AO) and reactive oxygen species (ROS) in plants ... 34
Figure 1.6 ROS and antioxidant defense mechanisms ... 34
Figure 1.7 (a) catechol moiety of the B-ring, (b) 2,3-double bond in conjugation with a 4-oxofunction of a carbonyl group in the C-ring and (c) presence of hydroxyl groups at the 3 and 5 positions ... 35
Figure 1.8 Intra-molecular hydrogen bonding of ortho substituted phenols ... 36
Figure 1.9 Radical trapping mechanism of phenolic antioxidants ... 36
Figure 1.10 Radical trapping mechanism of carotenoids ... 36
Figure 1.11 Reduction of ABTS+ radical by Trolox (H-donor)... 39
Figure 1.12 Protonation of DPPH (violet) to most stable DPPH-H form (light yellow color) ... 40
Figure 1.13 Mechanism of Ferric Reduction ... 40
Figure 1.14 Reduction of Titanium compound by H2O2 ... 41
Figure 1.15 Superoxide anion radical generation by PMS-NADH system ... 42
Figure 1.16 Formation of azo dye ... 43
Figure 1.17 Mechanism of metal chelation by ferrozine ... 45
Figure 1.18 Mechanisms of action of antimicrobial agents. PABA, paraminobenzoic acid; DHFA, dihydrofolic acid; THFA, tetrahydrofolic acid ... 54
Figure 1.19 Mechanisms of bacterial resistance to antimicrobials ... 57
Figure 2.1 A comparison of sensitivity of E.coli and B.subtilis against 164 plant extracts ... 75
Figure 2.2 Effect of physiological type of fruits on the growth inhibition of B.subtilis ... 77
Figure 2.3 Effect of physiological type of fruits on the growth inhibition of E.coli ... 77
Figure 2.4 Effect of the plant tissue type on the growth inhibition of E. coli ... 77
Figure 2.5 Effect of the plant tissue type on the growth inhibition of B. subtilis ... 77
Figure 2.6 Effect of ripening process of fruit on DPPH radical scavenging capacity ... 85
xx
Figure 2.8 Correlation between, the pH, growth inhibitory activity (GI) against E.coli
and B.subtilis and DPPH radical scavenging capacity of the extracts. ... 85
Figure 3.1 Time course of DPPH• scavenging by selected extracts ... 107
Figure 3.2 Relation between rate of radical scavenging and capacity of the radical scavenging capacity of plant extracts ... 115
Figure 3.3 Relation between ARP and radical scavenging capacity of plant extracts ... 118
Figure 3.4 Correlations between pH, rate, capacity and ARP ... 119
Figure 3.5 Relationship between ACON and capacity of selected plant extracts ... 121
Figure 3.6 Relationship between ACON and rate of selected plant extracts ... 121
Figure 3.7 Relationship between ARP and ACON of selected plant extracts ... 121
Figure 4.1 Correlation between Trolox equivalent antioxidant capacity and DPPH free radical scavenging activity ... 141
Figure 4.2 Correlation between Trolox equivalent antioxidant capacity (TEAC), DPPH and total phenolic content (TPC)... 142
Figure 4.3 Relationship between redox (potentiometric) and FRAP analysis ... 145
Figure 4.4 Relationship between TEAC and FRAP values of plant extracts ... 146
Figure 4.5 Correlation between Total polyphenol content (TPC), Total flavonoid content (TFC), DPPH, TEAC, Hydroxyl radical (HR), Superoxide anion (SOA), Hydrogen peroxide (HP) and Nitric oxide (NO) ... 151
Figure 4.6 Multi correlation between Total polyphenol content (TPC), Total flavonoid content (TFC), DPPH, TEAC, Hydroxyl radical (HR), Superoxide anion (SOA), Hydrogen peroxide (HP) and Nitric oxide (NO) ... 152
Figure 5.1 Growth curve of E. coli in Nutrient broth at 37oC temperature during 24 h period ... 168
Figure 5.2 Linear representation of growth curves of E. coli according to equation (9). A: Control; B: Nutrient broth containing pomegranate extract at 10 mg/mL concentration ... 169
Figure 5.3 Relationship between interaction constants log Ka and log K of plant extracts ... 171
Figure 5.4 Antimicrobial activity of plant extracts: correlation loading of antimicrobial evaluation methods by PCA ... 179
Figure 5.5 Antimicrobial activity of plant extracts: scatter plot of antimicrobial activity of plant extracts by PCA. The number corresponds to the product as listed in Table 5.1 and 5.2. ... 179
Figure 5.6 Multi-correlation between different methods for assay of antimicrobial activity ... 181
Figure 6.1 Yield of solvent extracts of pomegranate, black currant residue and betel leaf ... 198
xxi
Figure 6.2 Composition of solvent fractions of pomegranate and their antimicrobial
activity against E. coli and anti-radical activity by DPPH assay... 201 Figure 6.3 Composition of solvent fractions of black currant residue and their
antimicrobial activity against E. coli and anti-radical capacity by DPPH assay ... 201 Figure 6.4 Composition of solvent fractions of betel leaf and their antimicrobial
activity against E. coli and anti-radical activity by DPPH assay... 202 Figure 6.5 Antibacterial activity of blends of plant extracts in varying proportions... 203 Figure 6.6 Antibacterial activity of blends of plant extracts in varying proportions
against B.subtilis at total concentration of 5.0 mg/mL ... 205 Figure 6.7 Antibacterial activity (MIC) against E.coli at varying compositions of
xxii Liste des abréviations et des sigles
AAE: Ascorbic Acid Equivalent
ABTS: 2,2-azinobis 3-ethylbenzothiazoline-6-sulphonic acid ACAC: acetone: acetonitrile (solvent)
ACON: Average Carbon Oxidation Number ACS: acridone synthase
AM: antimicrobial
AMI: Antimicrobial Index ANOVA: Analysis of Variance AOA: Antioxidant Activity APX: ascorbate peroxidase ARP: anti-radical power ASH: ascorbic acid
ATCC: American Type Culture Collection ATP: adenosine triphosphate
BHA: butylated hydroxyanisole BHT: butylated hydroxytoluene BPS: benzophenone synthase BS: Bacillus subtilis
CAT: catalase
CE: catechol equivalent CFU: colony-forming unit CHS chalcone synthase
CLSI: Clinical and Laboratory Standards Institute CTAS: coumaroyl triacetic acid synthase
DHAR: dehydroascorbate reductase DHFA: dihydrofolic acid
DMAPP: dimethylallyl pyrophosphate DNA: deoxyribonucleic acid
DPPH: 2,2-diphenyl-1-picrylhydrazyl DR: Dragendorff’s Reagent
EC: Escherichia coli
EC50: effective concentration of a drug that gives half-maximal response EO: Essential oils
ET: electron transfer
EUCAST: European Committee on Antimicrobial Susceptibility Testing FCR: Folin Ciocalteu reagent
FRAP: Ferric ion Reducing Power FW: Fresh weight
GAE: Gallic Acid Equivalent GI: Growth Inhibitory Activity GOPX: guaicol peroxidase GPX: glutathione peroxidase GR: glutathione reductase GSH: glutathione
xxiii GSSG: glutathione disulfide
GST: glutathione-S- transferase H2O: water
HAT: hydrogen atom transfer
HEEA: hexane: ethyl acetate (solvent) HEX: Hexane (solvent)
HFA: tetrahydrofolic acid HH2O hot water
HIV: human immunodeficiency virus
HMG-CoA: 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase HPLC: High Performance Liquid Chromatography
HSD: honest significant difference iNOS: inducible nitric oxide synthase IOU: inhibits oxygen uptake
IPP: isopentenyl diphosphate LDL: Low-density lipoprotein
MBC: minimum bactericidal concentration MDHAR: monodehydroascorbate reductase MDR: multidrug-resistance
MEET: methanol: ethanol (solvent)
MEH2O: methanol 80 (v): water 20 (v) (solvent) MEP: 2-Methyl-D-erythritol-4-phosphat pathway MHB: Mueller-Hinton Broth
MIC: Minimum inhibitory concentration MPO myeloperoxidase
Na2EDTA: sodium ethylene diamine tetra acetate NADH: nicotinamide adenine dinucleotide
NADPH: Nicotinamide adenine dinucleotide phosphate NAO: natural antioxidant
NBT: nitroblue tetrazolium NBT: nitro-blue tetrazolium
NCCLS: National Committee for Clinical Laboratory Standards ND: not detected
NO: nitric oxide OD: optical density OH: hydroxyl
ONOO−: peroxynitrite
ORAC: oxygen radical absorbance capacity PA: proanthocyanidin
PABA: paraminobenzoic acid PAL: phenylalanine ammonia lyase PBS: phosphate buffered saline PCA: Principal Component Analysis PG: propyl gallate
PKS: Polyketide synthases PMS: phenazine methosulfate RE: rutin equivalent
xxiv RNS: reactive nitrogen species
ROS: Reactive Oxygen Species SD: standard deviation
SOA: superoxide anion SOD: superoxide dismutase STS: stilbene synthase
TBHQ: tert-butyl hydroquinone TBHQ: tertiary-butyl-hydroquinone
TEAC: Trolox equivalent antioxidant capacity TFC: total flavonoid contents
TPC: total phenolic content
TRAP: total radical trapping antioxidant parameter UV: Ultraviolet
xxv Dévouement
Je tiens à exprimer mes plus profonds regrets au décès du Professeur Khaled Belkacemi. Il a été une des victimes de l’attentat du 29 janvier 2017 au Centre culturel islamique de Québec, une semaine après avoir siégé au comité de jury de la soutenance de cette thèse. Nous nous souviendrons toujours de Dr. Belkacemi comme d’un grand professeur très passionné, cultivé, dévoué, compétent et aimé de ses collègues et de ses étudiants.
xxvi Remerciements
Je tiens tout d’abord à remercier mon directeur de recherche professeur Joseph Arul qui m’a accueilli au sein de son laboratoire et m’a offert un projet si intéressant ainsi qu’un inestimable appui scientifique et moral. La première leçon que tu m’as donnée est inoubliable, "Attitude et Aptitude font Altitude". Cela m’a guidé tout au long de mes études. Merci Joseph pour ta confiance en moi, tes encouragements, et tes conseils à toutes les rencontres, merci aussi pour ton aide financière. Je voudrais remercie aussi professeur Paul Angers, mon co-directeur de recherche, de m’avoir accepté de travailler dans son laboratoire et utiliser ses équipements, merci pour sa gentillesse et ses encouragements. Je remercie également professeur Khaled Belkacemi, du département des sols et de génie agroalimentaire, d’avoir fait la pré-lecture de cette thèse. Je tiens à merci aussi professeur Jean- Christophe Vuillemard, Dr. Wilhelmina Kalt (cherceure, Agricuture et agroalimentaire Canada, Kentville, Nouvelle-Écosse) d’avoir accepté d’être membre de commité de jury. Vos
commentaires ont vraiment contribué à la qualité de cette thèse. Un remerciement particulier
est également adressé au PCBF-ACDI de m'avoir accordé une bourse d'études de 5 ans. J'aimerais également remercier Ronan Corcuff, pour son support technique dont j’ai pu bénéficier tout au long de mes études. Merci pour sa grande disponibilité et particulièrement pour sa rigueur au labo qui ont contribué aussi à la qualité de tous mes résultats. Un gros merci aussi à Diane Gagnon, Pascal Dubé, Anne-Françoise Allain, Catherine Viel, Marie Michèlle Gagnon, Benoit Fernandez, pour leur supporte technique, leur gentillesse, leurs conseils.
J’ai eu la chance de travailler dans une excellente équipe. Merci pour mes collègues Arturo, Fayaz, Navina, Likun, Maria, Mialy, Tom Richard, Marine Dubois, et mention spéciale pour Mayank Pathak, Deepak Kumar Jha et Denis Dallie pour leurs participations dans mes travaux de thèse et pour leurs connaissances et encouragements. Un merci particulier à Thang et Vinh pour leur amitié.
Un remerciement spécial à mes parents, ma sœur Huyen, ma femme Khuong et mes enfants Han et Phong, pour leurs soutiens lors des moments difficiles et leur amour, leur compréhension de même que pour n'avoir jamais cessé de croire en moi.
1
2
Agricultural industry produces billions of tons of residues in non-edible portions derived from the cultivation and processing of a particular crop. Waste and by-products cause pollution, management and economic problems worldwide (Santana-Méridas et al., 2012). These residues of fruits and vegetables may be an abundant source of bioactive compounds. There is a growing realization and interest in the development of different strategies to use agricultural and industrial residues as a source of high value-added products (Santana-Méridas et al., 2012). Vegetables and some fruits yield between 25% and 75% of non-edible by-products as wastes (e.g., apple, 30 %; pineapple, 45 %; and citrus fruits, 70 %) (Van Dyk et al., 2013), after processing of fruits and vegetables in the food processing industry; and they contain a large amount of bioactive compounds such as polyphenols. The most abundant by-products of minimally processed fresh fruit and vegetable products are fruit peels and seeds which have been reported to contain high amounts of phenolic compounds with antioxidant and antimicrobial properties (Balasundram et al., 2006). The antimicrobial activities of a variety of naturally occurring phenolic compounds from different plant sources have been studied in detail (Cueva et al., 2010b; Daglia, 2012b; Quideau et al., 2011). Phenolic compounds play an important role in the protection of crops against pathogenic agents by damaging microbial cell membrane and causing lysis of the cells. Phenolic compounds from spices such as gingerone, zingerone, and capsaicin have been found to inhibit the germination of bacterial spores (Burt, 2004). Polyphenols from green tea have also been explored for their broad spectrum activity against pathogens (Taylor et al., 2005). In addition, flavonoids, when used in conjunction, have been reported to enhance the antibacterial, antiviral, and anticancer activities of compounds such as naringenin, acycloguanosine, and tamoxifen ( Ayala-Zavala et al., 2010).
Antioxidants play a significant role in protecting the body from damage caused by free radical- induced oxidative stress (Ozsoy et al., 2008). Natural anti-oxidants, like polyphenols, found in medicinal and edible plants, are very effective in preventing oxidative damages (Silva et al., 2005). It is desirable to establish and standardize antioxidant assay methods that can measure the real antioxidant power directly for all classes of anti-oxidants, irrespective of their origin, either plant extracts or biological fluids. Free radicals play a very important role in the oxidative damage of biological
3
systems (McCord, 2000; Scalbert et al., 2005). Plant extracts are complex mixture of compounds, where phytochemicals may function in synergism or antagonism with each other, and they may exhibit ‘Broad spectrum antioxidant activity’ against various radicals of importance in biology.
Plants have been a focus of attention for a longtime as source of antimicrobial compounds and it has been reported that two-thirds of the world’s plant species have medicinal value (Craig, 1999; Krishnaiah et al., 2011). In recent years, there has been an increasing interest in antimicrobial properties of medicinal plants, and enormous attention is being paid in the discovery of new antimicrobial from natural sources. (Brown et al., 2014; Khanam et al., 2015), as multi-drug resistances of microbes are becoming a concern that can have considerable impact on public health with potential treatment failures (Balouiri et al., 2016). However, the research on antimicrobial compounds from plant sources has yet to see a systematic approach with respect to screening for possible candidates active against microbial pathogens, yeasts and fungi (Aqil and Ahmad, 2003). Furthermore, the screening processes, as of now, do not necessarily take into account of the morphological (Gram-positive or negative) or growth requirement (aerobe or anaerobe) characteristics of the bacteria. It is expected that testing of selected plant extracts against wide variety of test microorganisms will be helpful in developing plant extract formulations exhibiting broad spectrum antimicrobial activity as well as new antimicrobial substances.
Several bioassays are used to determine the activity of antimicrobial substances, such as the well-known and commonly used disk diffusion or well diffusion and broth or agar dilution (Balouiri et al., 2016). These methods can be qualitative and quantitative in nature. A qualitative test such as the Kirby-Bauer disk diffusion method helps screening candidate antimicrobial compounds, whereas quantitative methods (such as MIC, growth inhibition)s, also known as end point methods, help quantify the potency of the compound. Absorbance readings can help monitor lag phase, growth rate, and maximum population in real time and ascertain the effectiveness of an antimicrobial agent. Although, only the maximum growth is typically noted to determine the effect of environmental conditions from these assays, other parameters such as the lag time and growth rate reflecting bacterial growth through its life cycle can help to understand bacteria-environment interactions and build bacterial
4
growth prediction models for use in food and fermentation microbiology. Mathematical modeling can be important to incorporate a few parameters, or very useful to help advance the understanding a wide variety of intrinsic and extrinsic factors governing microbial growth.
Many studies have shown that naturally derived compounds and natural products may have applications in controlling pathogens in foods (Lucera et al., 2012; Negi, 2012). However, the use of natural antimicrobials is limited due to the fact that they are of low potency, and it requires high concentrations, which can affect the organoleptic properties of foods (Davidson et al., 2013; Lucera et al., 2012). However, enhancing the antimicrobial activity of plant extracts to increase their potency could enable their use as antimicrobial agents in foods at low levels without affecting the sensory properties of foods. Three approachs for enhancement used in this study were extraction using selective solvents (Miyasaki et al., 2013), binary blending of extracts and addition of plant products that may potentiate the activity of the extracts (Worthington and Melander, 2013).
The objective of this work was to conduct studies to evaluate the potential of extracts obtained from various fruit and vegetable by-products and to develop antioxidant-antimicrobials for use as food preservation agents, as alternatives to synthetic agents. The thesis is organized in six chapters starting with a review on plant by-products, plant secondary metabolites, and antioxidants and antimicrobials from plant sources (chapter 1). The identification of promising antioxidant-antimicrobial extracts from fruits and vegetable by-products, generated during harvesting or processing is described in chapter 2. The chapter 3 introduces a new measure to characterize the antioxidant property of plant extracts from DPPH assay, the anti-radical power (ARP). The spectrum of anti-radical activity of selected fruit and vegetable extracts is reported in chapter 4. The chapter 5 depicts the characterization of antimicrobial activity of selected plant extracts by a new measure, the antimicrobial activity index (AMI) and the evaluation of the spectrum of their antimicrobial activity. Finally, chapter 6 describes the work that explored ways to enhance the antimicrobial activity of selected antioxidant-antimicrobial extracts.
5
Chapter 1 REVUE DE LITERATURE
Cette partie de revue bibliographique porte sur des sous-produits de plantes, les méthodes d’extraction des composés bioactifs, les métabolites secondaires, les antioxydants et les antimicrobiens. Ce chapitre se termine par la présentation de la problématique, de l’hypothèse de recherche, du but et des objectifs spécifiques de cette étude.
6
1.1 Plant by-products: eco-friendly source of novel bioactive compounds Avant propos
The following is a description of some of the terminologies often used in both business and scientific literature to describe product sources. They include: by-products, co-products, secondary products, intermediate products and sub-products We will use the definitions of the Waste Framework Directive (EuropeanUnion, 2006) as follows:
Waste: A material that the holder discards, and indended or is required to discard.
Production residue: A material that is not deliberately produced in a production process
that may or may not be a waste.
By-product: A production residue that can be used directly without any additional
processing, other than normal industrial practice.
There are two main categories of agricultural residues: crop residues (generated in the farm) and agro-industrial residues (generated during post-harvest process).
Crop residues (primary biomass residues) are non-edible plant parts that are left in the field
or orchard after the main crop part has been harvested. These residues, which mainly include straw, stove, stubble, stalks, sticks, leaves, haulms, roots, branches, twigs, brushes, trimmings and pruning, are produced from sources including seeds, fruits, nuts, vegetables and energy crops.
Agro-industrial residues (secondary biomass residues) are those materials from the
processing of the crop into a main resource product, including residues from wood and food processing industries in the form of husks, hulls, peels, dust, straws, bagasse, sawdust, corncobs, pomace, etc. In addition, the remaining residues after the use of processed materials may be considered as a tertiary biomass (UNIDO, 2007).
7 1.1.1 Why plant by-products?
The generation of food waste occurs all through the food life cycle; from agriculture, industrial food processing and manufacturing, retail and up to household consumption. In developed countries, 42% of food waste is produced by households, while 39% losses occur in the food processing industry, 14% in food service sector, and the remaining 5% at the retail and distribution level (Mirabella et al., 2014).
Agricultural industry produces billions of tons of residues in non-edible portions derived from cultivation and processing of a particular crop. Waste and by-products can cause pollution, management and economic problems worldwide (Santana-Méridas et al., 2012). These residues of fruits and vegetables may be an abundant source of bioactive compounds. This is the reason for the development of different strategies to use agricultural and industrial residues as a source of high value-added products (Santana-Méridas et al., 2012). Because the consumers are more and more discouraged by the presence synthetic additives in foods they consume, food industry is increasingly paying attention to obtain functional ingredients from natural sources. This is particularly true for phenolic compounds, which in contrast to certain ingredients such as carotenoids and vitamins, are not chemically synthesized and need to be extracted from plant materials. The exploitation of by-products of fruit and vegetable processing as a source of functional compounds and their application in food is a promising field which requires interdisciplinary research of food technologists, food chemists, nutritionists and toxicologists (A. Schieber, 2001).
Amongst the fruits, vegetables and herbals, agricultural and industrial residues are also sources of natural antioxidants (Moure et al., 2001). Special attention is being paid to their extraction from inexpensive or residual sources from agricultural industries. By-products, remaining after processing of fruits and vegetables in the food processing industry, still contain a considerable quantity of phenolic compounds (Cowan, 1999). Some studies have already been done on by-products, which could be potential sources of antioxidants. Recycling of the by-products has been supported by the fact that polyphenols have been located specifically in the peels (Moure et al., 2001). One of the richest sources of
8
polyphenols are grape berry skins, which during wine and juice making remain as residue are usually converted into compost (Lapornik et al., 2005). The olive mill wastes are also a major potential source of phenolic compounds. The phenolic content of the olive mill waste water is reported to fluctuate between 1.0 % and 1.8 % (Visioli and Galli, 2001), depending on varietal factors and processing effects. Besides olive mill waste water, olive leaves are another by-product of the olive industry that has been explored as a source of phenolics (Lee and Lee, 2009). Besides having a strong antioxidant activity, polyphenols often also exhibit antimicrobial activity (Agourram et al., 2013).
1.1.2 Source, provider and type of by-products
The food processing industry would be the ideal source of such wastes. Residue from juice production, waste from canning factory, harvest, minor crops and other agricultural and processed food by-products would be ideal candidates (Peschel et al., 2006).
The number of studies devoted to exploit residual sources for bioactive compounds has increased considerably, which is driven not only to add value to the by-products, but also to improve recycling of the wastes for sustainable development of the agro- and food industry. Such studies have contributed to the knowledge base regarding specific locations of active compounds and their modifications during processing. Although numerous studies have been carried out on this subject, only a few by-product derived antioxidants have been developed successfully from the vast quantities of plant residues produced by the food processing industry. Various examples of such successes include the work done in Europe, primarily on grape seed and olive waste extracts (Alonso et al., 2002; Amro et al., 2002). Potential crop candidates with a high annual production and already confirmed high antioxidant potential include apple (DuPont et al., 2002), tomato (Lavelli et al., 2000), and artichoke (Jimenez-Escrig et al., 2003).
The peels of several other fruits also contain higher amounts of phenolic compounds than the edible fleshy parts. Apple peels contain up 3.3 % in comparison to apple pomace, which yields 11.8 % of phenolic compounds (Schieber et al., 2003). The peels and seeds of tomatoes have also been reported to be richer sources of phenolic compounds in comparison to their fleshy pulp (George et al., 2004). Louli et al (2004) investigated the
9
effect of various process parameters such as solvent type and feed pre-treatment (crushing, removal of stems) on the efficiency of the extraction of phenolic antioxidants from grape residue; whereas Negro et al (2003) investigated the content of total polyphenols and antioxidant activity of grape residue extracts (Louli et al., 2004; Negro et al., 2003).
The citrus industry produces large quantities of peel and seed residues, which may account for up to 50% of the total fruit weight (Bocco et al., 1998). Citrus industry by-products, if utilized optimally, could be a major source of terpenes and phenolic compounds, particularly flavonoids, as the peels are known to contain higher amounts of total phenolics compared to the edible portions (Balasundram et al., 2006). Likewise, by-products obtained after the processing of artichoke, cauliflower, carrot, celery and onion have also been reported (Larrosa et al., 2002).
Although the antioxidant potential of less small volume crops such as strawberry (Kahkonen et al., 2001), pear (Imeh and Khokhar, 2002), red beet root (Kujala et al., 2001), or broccoli (Kurilich et al., 2002) is known, little information is available in the literature with regards to practical means of obtaining bio-active substances from by-products as well as their utilization in foods. This might be caused by three limiting factors often overlooked in scientific studies: the effectiveness of recovery and extraction, the marketability of resulting extracts and the practical suitability for the food, cosmetic or pharmaceutical products.
1.1.3 Methods of extraction of bioactive compounds from plant
Extraction of bioactive compound from plant is the separation of active portions of plant tissues using selective solvents. During extraction, solvents diffuse into the solid plant material and solubilize compounds with similar polarity. The products so obtained from plants are relatively complex mixtures of metabolites such as alkaloids, glycosides, terpenoids, flavonoids and lignans (Joana Gil-Chávez et al., 2013).
The commonly used methods of extraction of plant are the conventional liquid–liquid or solid–liquid extraction and the advanced include pressurized-liquid extraction, subcritical and supercritical extractions, and microwave- and ultrasound-assisted extractions. In
10
addition, these extraction techniques have been improved with preparative treatment steps (enzyme-and instant controlled pressure drop-assisted extractions), which help to release the compounds from the matrix. These technologies could provide in the next few years an innovative approach to increase the production of specific compounds, particularly flavonoids, for use as nutraceuticals as ingredients in the design of functional foods (Joana Gil-Chávez et al., 2013).
Table 1.1 Solvents used for active component extraction
Water Ethanol Methanol Ether Acetone Chloroform
Anthocyanins Tannins Anthocyanins Alkaloids Phenol Terpenoids Starches Polyphenols Terpenoids Terpenoids Flavonols Flavonoids Tannins Polyacetylenes Saponins Coumarins
Saponins Flavonol Tannins Fatty acids
Terpenoids Terpenoids Xanthoxyllines Polypeptides Sterols Totarol
Lectins Alkaloids Quassinoids
Lactones Flavones Phenones Polyphenols
(Cowan, 1999) Table 1.1 summarizes the use of different solvents for extraction of various bioactive compounds. Solvent is selected for its capacity for the species being separated into it, i.e., the solubility of the targeted compounds in that solvent. The solvent should also be selective, extracting primarily one or more of the related class of compounds from the solid matrix. Thus, polar solvents are more appropriate for polar components, and non-polar solvents for non-polar components. Some times, a solvent mixture may be used to obtain properties that cannot be achieved with a single solvent. For laboratory preparations, hot water, hexane, ethyl acetate, chloroform, dichloromethane, ether, acetone and alcohols (ethanol and methanol) are frequently used solvent mixtures offering a range of polarity. Hot water is a universal solvent, and it is used frequently to extract phyto-compounds. Acetone dissolves many hydrophilic and lipophilic components, and is volatile and miscible with water, and has a low toxicity for use in the bioassay of extracts. Acetone is used for extraction phenolic compounds from plant extracts. Alcohols are effective in the
11
degradation of cell walls, and seed coats that have non-polar character, and facilitate the release of substances from the cells. The frequently used alcohols are ethanol and methanol. Ethanol can permeate through the cellular membrane and facilitates the extraction of intra-cellular components from plant materials. Higher recoveries of bioactive flavonoids can be achieved with aqueous ethanol (70%, v/v) due to the higher polarity of the mixture than pure ethanol (Khoddami et al., 2013). While methanol is more polar than ethanol, it is often a suitable solvent for extraction of components of higher polarity such as polyphenols. Since most of the antimicrobial compounds isolated from plant sources possess relatively more non-polar character (aromatic or unsaturated organic compounds), they are initially extracted using either ethanol or methanol and purified subsequently. Although tannins can be modestly extracted by aqueous medium, they are efficiently extracted by less polar solvents. Ether is commonly used selectively for the extraction of coumarins and non-polar lipids. Terpenoids are generally extracted with more non-polar solvents such as dichloromethane and ethyl acetate, when the former is more selective towards terpenoids. Terpenoid lactones have been obtained by successive extractions of dried barks with hexane, followed by solvent mixture (chloroform-methanol) with higher bioactivity concentrating in chloroform-methanol fraction (Cowan, 1999).
1.1.4 Trend and challenges in using plant by-products
Recently, polyphenolic content was also examined in some plant by-products, which are available in large quantities and at low cost, but are currently used only as animal feed or fertilizer. Their use as food additives could help industries to solve the environmental problems related to the disposal of these materials, and provide new sources of natural antioxidants.
The future of food processing in a sustainable way should warrant not only minimize wastes from processing, but also would require value-addition to the waste streams. An integrated system should also incorporate nutrional, health-functional and food safety characteristics of the foods and by-products. The food processes need optimization to minimize the amounts of waste generation, and focus on complete utilization of by-products resulting from large-scale processing at affordable costs. There is a need for specific analytical methods for the characterization and quantification of organic
12
micronutrients and other functional compounds. The bioactivity, bioavailability and of phytochemicals need to be carefully assessed by in vitro and in vivo studies. In addition, there is also a need to eliminate natural and anthropogenic toxins such as solanin, patulin, ochratoxin, dioxins and polycyclic aromatic hydrocarbons need by efficient quality control systems.
The ‘activity’ of many phytochemicals has only been tested in in vitro models, and this may bear no relationship to the situation in vivo. Undoubtedly, functional foods represent an important, innovative and rapidly growing part of the overall food market. However, their design, i.e. their complex matrix and their composition of bioactive principles, requires careful assessment of potential risks which might arise from isolated compounds recovered from by-products. Furthermore, investigations on stability and interactions of phytochemicals with other food ingredients during processing and storage need to be initiated. Since functional foods are on the boundary between foods and drugs, their regulation still proves to be difficult. In any case, consumer protection must take priority over economic interests, and health claims need to be substantiated by standardized, scientifically sound and reliable studies (A. Schieber, 2001).