Vot.
45,
No.
4,
July
1998
BIOCHEMISTRYond
MOLECULAR BIOLOGY INTERNATIONALPoges 813-821
EFFECT
OFSEASONAL
ACCLIMATIZATION
ONTHE EXPRESSION OF
THE
CARPTRANSCRIPTION FACTOR
PIT-I
Gudrun
Kausel,Marfa
Inés Vera, Jaimc Figueroa, Jaime Hernândez,Rody
SanMartin,
Alfredo
Molina, Viviana Marcela
Chavezr*Marc Mullerr*
JosephMartial*
and Manuel
Krauskopf
Institute
of Biochemistry,Faculty
of Sciences, UniversidadAustral
deChile' Valdivia'
Chite; Laboratoire
de BiologieMoléculaire
et de Génie Génétique'Université
deLiège'
Institut
deChimie
86'
B-4000Sart-Tilman,
BelgiumReceived October 2, 1997
Rcceivcd after revision, Decembcr 5, 1997
SUMMARY
We
isolateda
clone comprising
four
exonsof
the
carpPit-l
gene.Using
synthetic otigonucleotide probes derived from the carpPit-l
sequencePit-l
expression was assessedby in
st:tu
hybridization
in
pituitary
sectionsfrom
summer- and winter-acclimatizedcarp.
Semi'
quantitative analyses
ofthe
hybridization signals revealed a significant higherPit-l
expressionin
ùe
proximal pars distalis (PPD) and pars intermedia (PI) of the pituitary glandsfrom
summer-acclimatized carp, comparedto
the winter-acclimatized fish. In both adaptive states, relative to the PPD andPI, only
a basatPit-l
expression was detectedin
the rostral parsdistalis.
Thus,during
seasonal acclimatizationof
an
eurythermalfish,
Pit-l
seemsto
be involved
in
the mechanisms that underlie the compensatory response. Key words:Pit-l,
carp, acclimatization, fish, gene expression.INTRODUCTION
Seasonat changes
in
the
environmentalfactors
demand compensatory responsesin
eurythermal
lish
bothat
the celtular
and molecular level.In
the carp(Cyprinus
carpio)
the mechanisms that underliethis
naturalcyclical
adjustment (r.e. acclimatization process)involve
the modulation
of
transcriptionin
distinctive
gene expression processeswhich
take place in
several tissues
[-6].
To
assess whether thepituitary
gland could be a central coordinating node, supplying molecules accordingto
the homeostatic requirements imposedby
the changing environmental signals, we previously studied the expression of prolactin (PRL) during the acclimatization of the carp and observed a high expression of PRL mRNA in the rostral pars distalis (RPD)of
summer-acclimatized carp white a negligible tevelof
transcription was detectedin
thepituitaries
of
theto39-97 r?/gSt t008 I 3{9$05.00/0
Copyigfu A !98 by Acodenic Prcst Austnlia. All rights o! rcpnduction in ony form rescmcd.
Vol.45,
No.4,
1998 BIOCHEMISTRYond MOLECULAR BIOLOGY INTERNATIoNATu'intcr-acclimatized
fish
[7].
Furthermore \À'e establishcdthat
photoperiod
constitutes
aparticutarly retevant modulator in the neuroendocrine àscade that activates PRL transcription in
the carp [8J.
It
iswell
known that the tissue-specific transcription factorPit-l
isimplied
in
the controlof
PRL expression[9,10].
The transcription factorPit-l
plays an important rotein
the
precise tcmporal and spatial pattems of gene expression in the anterior pituitary gland.Pit-l
controls the developmentand
maintenanceof
the
differentiated stateof
Iactotrophs, somatotrophs and thyrotrophs, which are the cell lines producing PRL, growth hormone (GH) and thep
subunitof
the thyroid stimulating hormone (TSH) respectivelyI
l].
In addition, somâtolacrin(SL),
a PRL-related hormone identified in the pars intermedia (PI)in fish
tl2,l3l,
also seems to be controlledby
Pit-l
[14].
Apart
from
the
autoregulationof
Pit-l
expressionfl51
several transactivating factors are involved in the transcriptionofthe
Pit-l
gene [16].To determine
if
Pit-l
plays a rolein
regulating prolactin gene expressionin
carp during acclimatization, we have isolated and characterized partof
the carpPit-l
gene and assessed its expressionin
pituitary cells in summer- and winter-acclimatized carp.ln
this artictewe
report a genomic sequence which codes for carpPit-I,
thefirst
corresponding to a teleost.Also
we show that a strong seasonal differential transcription of Pit-l
occurs in the GH and SL producing cells ofcarp pituitary glands and that only a negligible amountof
Pit-l
transcripts could be detectedin
both acclimatized states in the PRL producing cells.
MATERIALS
AND METHODS
Male carp (Cyprinus carpio) weighing about 1000
-
1500g
were caughtduring winter
and summer and maintained
in
a fixed 3x
4 m cage submerged 2m in
anaffluent
of
the sameriver. The
temperaturesof
the
rvaterin
winter and
in
summerwere 8-l0oC
and l8-20'C,
r€spectively.Pituitary
glands and other tissues,from
winter-
and summer-acclimatized carp, rvere dissected and either frozenin
liquid
nitrogen and stored at-70'C
for RNA
extraction or
fixed immediately
for
rn silrr hybridization studies, as reported elsewhere [8]. Thefixed
sectionsnrre
kept at -70oC and processed together.Carp genomic
DNA
was prepared according to Garbutt ei al.[
7]. To obtain a carpPit-l
probe, oligonucleotide primers rvere derived from thehighly
conserved POUs regionof
salmonPit-l
cDNA
sequenceU8l.
PCR amplification was performed using carp genomicDNA
(80 ng)ar
template
in a
50
pl
mixture containing
25pM
of
each
primer
(oligol:
5'-rc';
oligo2:
5'-ACAACCATCIGTCGCTTTGAJ'),and
2.5
tJ
TaqDNA
polymerase(GibcoBRL),
accordingto
the
manufacturer'sinstructions.
Following
a denaturation step of 3 min at 95'C, 30 cycles of amplilication $'ere performed (30 sec at93'C,
30sec at 55oC,
l0
sec at 72'C), rendering a 93 bp fragment. High specific labelingof this
fragmentVol.45,
No.4,
1998 BI0CHEMISTRY ond MOLECULAR Bl0LOGY INTERNATI0NALwas
obtained
during PCR amplification
rvhen
cr[]2PJ-dCTP(i000
Cilnrmol,
l0
pCi/ml,
Amersham) rvas used as described
by
Mertz
and Rashtchian[9].
To
isolatePit-I
genomic s€quencesa ÀFixll
carp genomiclibrary
(Stratagene) was screenedwith
the
93 bp
fragment accordingto
Sambrook etal. [20].
Subcloning aswell
as sequencingof DNA by
the dideoxy chain-termination method were performed as described [20].Total
RNA
was preparedwith
Trizol
(CibcoBRL) according to the supplier instructions. Northernblotting
was performed using totalRNA
(10-12 pg) from different carp tissues, which were then fractionatedonal.2oÂ
denaturing agarose gel containing 2.2M
formaldehyde, and transfened to a nylon membrane (Amersham Hybond). Hybridization was caniedout
ovemightat,l2"C
using as probethe
1700bp
fragmentof
GP5, labeledwith
[r?P]by
the random primer method (GibcoBRL). Theblot
was washedtwice
for
l5
min
under stringentconditions (0.2 x
SSC, 0.1% SDS at 60"C) and subjected to autoradiography for 48 h at -70"C using a Kodak
X-Omat film.In situ
hybridization was performedin
thefixed
summer- andwinter-
pituitary
gland sections simultaneously[8].
Carp
Pit-l
specific
oligonucleotidehybridization probes
werederived
from the
furthermost
5'
coding
region
of
pGP5't*.
The
antisense
5'-GTAAGCTGTGGGTCAAAG-3'
and the complementary sense oligonucleotidewere
labeled at the 3'-endwith
digoxigenin using terminal deoxynucleotidyl transferase(GibcoBRL)
[7].Quantification
of
the label
in
the
pituitary
sections u'ascarried
out
as
previously d€scribed [8J using an automated imagedigitizing
system ([JN-SCAN-IT,Silk Scientific,
Inc.).In
the PI and
in
the proximal
pars distalis (PPD)
of
eachpituitary
section
a
squareof
approximately 0.2 mm2 was defined.Five
regions were randomly selectedin the
less reactive arcas of the pituitary sections to obtain the background number of pixels. In thePI
and the PPD, 5 areas were marked,their
density measured and conectedfor
the average background value. These analyses were performedon
sectionsof
different individuals,i.c. winter- and
summer-acclimatized carp lish, using the Student r-test. P<0.01 was considered significant.RESULTS
By
screeningabout
I
million
recombinantsfrom
a
l'Fixll
carpgenomic
library
twoclones
(GPt
and GP5)
containing
Pit-l
coding
sequencesnere
isolated based
on
their
hybridization
to
a
PoUs-specific carp
93 bp DNA
fragment obtainedby PCR.
Restriction mapping and Southem blot analyses revealed that both clones contain the carp POUs sequence. GP5 was selected for further analysis. A Xbal-PstI
fragment comprising approximately 1700 bpofGP5
was subcloned inpUCl9
and sequenced.Comparison to the sequence data bank of the primary struciure of the subclone, pGPS'r*, confirmed that
it
conesponds to sequences coding specificallyfor
Pit-1. Moreover, comparison to the gene structure of mousePit-l
reveals that the clone GP5 encompasses exonsIII
toVI,
thussaning in
intronII
(Fig.l ). As the Xbal-Pstl fragmentof
pGP5 corresponds tothe
S'-endof
the lambdarecombinant, GPS misses the S'regionof
the carpPit-l
gene, however, comprises thecoding
sequencefor
the
entire
POU-domain andpart
of
the amino-terminal domain.
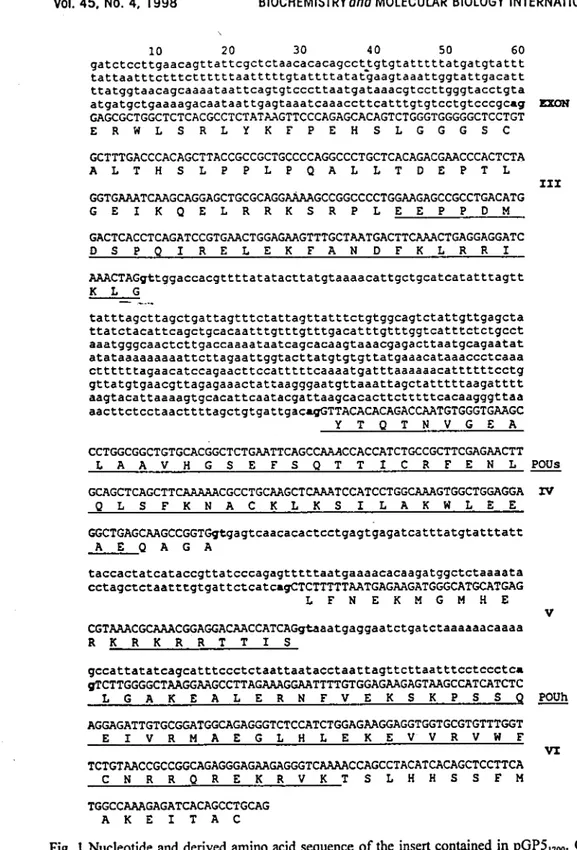
TheVol.45.
No.4,
1998 BI0CHEMISTRY ond MOLECULAR Bl0LOGY INTERNATIONAL10
20
3040
50
60gat
ct,ccttgaacagt
tatt
cactct,aacacacagccttgtgt
attt ttatgatgta
ttt
taÈtaatttctttct
tttttaatttttgtattttat
a Ègaagtaaattggtattqacatt
ttatggtaacagcaâaataatCcagtgtcccttaatgatâaacgtccttgggtacctgta
aÈgatgctgaaaagacaâtaattgagtaaâtcaaaccttcatttgtgtcctgtcccacrg
lEroN GÀæGCTGGCTCTCACGCCTCTATÀÀGTÎCCCAGAGCACAGîCTGGGTGGGGGCTCCTGTE R I{ I, S R L Y K F P E H S L G G G S
C GClTÎGÀCCCACAGCTlÀCCGCCGCTGCCCCAGGCCCTGCTCACAGACGAACCCÀCÎCÎÀÀLlHSLPPLPQALLlDEPl'.
IIT
GGTGÀÀÀÎCÀÀGCÀGGAGCTGCGCÀGGAÀÀÀGCCGGCCCCTGGÀÀGÀGCCGCCTGACATGGEIKQELRRKSRPLEEPPDM
GACTCÀCCTCAGATCCGTGÀÀCTGGAGÀÀGÎTÎGCÎAÀTGACTÎCÀÀACTGÀGGAGGATCDSPOIRELEKFÀNDFKLRRI
MÀCTAGgttggaccacgttttat
atacttatgtaaaacatt
gctgcatcatatttagtt
*
t=nr---tatttagctt,agctgattagtttctattagttatttct
gtggcagtctattgttga gcta
ttatctacattca
gctgcacaatttgtttgtttgacatttgÈttggtcatttctctgcct
aaatgggcaactct
t gà ccaâaâ taatcagcacaagta aacaagactt
aatgcagaatat
atataaaaaaaaattctta gaatÈggtâcttatgtgggt
tatgaaacataaaccctcaaa
cttttttagaacatccagaacÈtccatttttcaaaatgatttaaaâaacattttticctg
gttat
gt gaacattagagaaactatiaagggaatgttaaattagctatttttaa
gatttt
aagtacatt.aa aagtgcacaÈt cà atacgattaagca
cacttct
t tttcacaagggt
t,aaaacttct
ccta act.t t ta gct gt ga t t gaCryGTTÀCACACÀGACCÀÀlGTGGGTGÀÀGCYTOlNVGEÀ
CCTGGCGGCTGTGCACGGCTClGÀÀÎÎCÀGCCÀA/ICCACCATCTGCCGCÎÎCGÀGÀÀCTT
LAAVHGSEFSQTTICRFENT
GCAGCTCAGCÎTCÀÀÂÀÀCGCCTGCAÀGCÎCAÀÀÎCCATCCTGGCÀÀÀGTGGCÎGGAGGA
O L S FKNAC
KL KS I LAKITL
E
EGGCTGÀGCAÀGCCGGTG9t9a
gtcaâcacactcctga
gt gagatcatttatgta
tttatt
ÀEQAGA
taccactatcataccgttatcccâgagttttt.aatgaaaacacaa gatggctctaôaata
cctagctctâattË9t9a
t tctcat
cTgCTCTTTT?AÀTGAGÀÀGATGGGCATGCATGAGLFNEKMGMHE
CGTÂÀACGCÀÀÀCGGAGGACÀACCATCAG9Èaaatgaggaatct ga tctaaaaaacaaaaRKRKRRllIS
POUsw
v
gccattatatcagcatttccctctaattaatacctaattagttcttaatttcctccctcr
9ICÎTGGGGCÎÀÀGGÀÀGCCTTAGÀÀÀGGÀÀTTTÎGTGGÀGÀÀGAGTAAGCCATCATCTCL G A K E A L E R N F V E K S K P S S Q
POUh AGGÀGAT?GÎGCGGATGGCAGÀGGGÎCTCCAÎCTGGAGAAGGAGGIGGIGCGTGI?TGGTE I V R MA E G L H L E K E VV R VT{
Fvt
ÎCÎGTÀÀCCGCCGGCAGAGGGAGAÀGÀGGGTCAAÀACCAGCCTÀCATCACAGCTCCTTCACNRROREKRVKTSLHHSSFM
TGGCCAÀÀGÀGAÎCACAGCCTGCAGAKEITAC
Fig.
I
Nucleotide and derived amino acid sequenceof
the insert containedin
pGP5'106. Coding seguences are indicatedin
upper case letters and exon-intron boundariesin bold
type.From
the several possible AG-sites of the furthermost5'
intron, only one is shown. The POUs and POUh-domain are underlined.Vol.45,
N0.4,
1998 BIOCHEMISTRY ond MOLECULAR BIOLOGY INTERNATIONATsequence of
pGP5,'*
is deposited in the GenBank database. accession No.U92542.Alignment
of
thederived amino acid sequenceof
carpPit-l with.uin'bo*
troutPit-l
reveals6l%
identity in
the amino terminal parl and 94Yo identity in the POU-domain.
When the insert
of
pGP5,roo was used as probeto
hybridizeRNA from
different
carp tissues(Fig. 2), only
pituitary
RNA
reacted renderingtwo
bandsof
2.2
kb
and4.2 kb.
No
expression of
Piçl
was observed in the brain, kidney, heart and liver of the carp.To
determinePit-l
expression during acclimatization,in
siluhybridization
of
pituitary
gtand sections was performed. Signals for
Pit-l
mRNA were localized mainly in the PPD and PI and, to a relatively tow extent, in the RPD (Fig. 3). Molecular hybridizationdid
not occur when the digoxigenin-labeled sense oligonucleotide probe, derived from the carpPit-l
gene, was used. Semi-quantitative analyses of therr
sr'tu hybridization signals confirmed that asignificant
higher reaction was attainedin
the PPD and PI from fish acclimatized to summer as comparedto
the signal attained in the cold-season-acclimatized carp (Fig' 3)DISCUSSION
Alrhough some
Pit-l
cDNAs
from teleosts have been identified[8,
2l-24],
its
genomic organizationis
unknown.To
approach the expressionof
transcriptionfactor
Pit-l
during
the acclimatizationof
the carp,we
isolated and characterized a partial genomic sequenceof
Pit-l
from this fish. The sequence comprises exonslll
toVI,
thus startswithin
intronII.
The deduced partial carpPit-l
amino acid sequence compared to rainbow trout is consislentwith
the expected extentof
homology[25]
in
the POU-domain (94% idenrical). The exon-intron organizationof
the carpPit-l
sequence analyzsd,resembles the genomic structure of the mousePit-l
gene POU-domain [26].The insert
of
the genomic ctone pGP5,ros. comprising abouttwo thirds
of
the
estimated carpPit-l
coding sequence, hybridizeswith two
RNAs (4.2 kb and 2.2kb), depicting
asimilar
pattern to that observed in chum salmon
IS].
As in the case of rat and salmon [18] the carpPiËl
RNA is expressed in the pituitary gland, but not in the Iiver, hean, kidney orbrain.
Becauseonly
a few tissues of the carp were tested by Northern hybridization,
it
is not possibleto
state thatPit-I
is specifically expressed in the pituitary gland.In
situ hybridization
offers,
in
complex
tissues,the
possibility
to
pin
point
gene cxprcssion in specific cell types. This is particularly relevant in the caseof
Pit-l
which
is kndwnVol.45, No.4.
1998 BIOCHEMISTRY ond MOLECULAR BIOLOGY INTERNATIONAL^s
"i'"od*u**"ô
uS
: <.4.2 kb
+21
kbFig.2:
Northernblot
hybridizationof
RNA
from different
carp tissues.RNA
of
various
carp tissues was hybridizedwith
the insertof pGP5,r*
asPit-l
specific probe.The fragment
sizes were estimated by a comparisonwith Hindlll
digests of phageI
DNA.to transactivate at least three important hormones.
Pit-l
appears to be necessary but, onitself,
not suflicient to account for the precise cell type restriction of GH, PRL and SL expressionIl
I].
As
reportedby
Takeoet
al.
1271,Pit-l
protein colocalizeswith
the
hormones whose synthesisis
characteristicin
the RPD, PPD andPI of
rainbowtrout.
In
the presentstudy
the results obtained by in srTa hybridization reveal a remarkablePit-l
expressionin
the PPD and PI and a much lower expression in the RPD in carp.We observed significant differences in the amounts of
Pit-l
transcriptsin
the PPD and PIof
summer- and winter-acclimatized carp. During the warm season carpPit-l
mRNA
was4 to
5folds higher
thanin
winter, implying that
Pit-l
expressionis
involved
in
the
compensatory rcsponse which occurs circannualy, concurrcntlywith
the seasonally environmental changes.It
isof
particular interestto
observe thatPit-l
expressionin
lactotrophs exhibits adifferent
pattern wherea
low
basalPit-l
transcriprion was observedin
the RPD, bothin
summer- andwinter-acclimatized carp. Thus,
the rise
in
PRL mRNA and
protein,
which occurs
in
summer acclimatized mate carp[7],
appeanto
not be associatedwith
a concomitant increaseof
Pit-l
expressionin
lactotrophs. To avoid hormonal changes associated with the reproductive cycle,all
experiments were performed with male carp.
k-r"u
lJ; '
Vol.45,
N0.4,
1998 EIOCHEM ISTRY ONd MOLECUTAR BIOLOGY INTERNATIONAL14@
um
Fig.3.
Insilz
hybridizationof
a sagittal section of a pituitary gland from a summer-âcclimatized."rp.
Mol..utar
hybridization
was
obtainedwith a
digoxigeninlabeled
carp
Pit-l
specific antisense oligonucleotide probe(x30).
The insert(xl5)
shows the results obtainedwhen
the oligonucteotide sense probe was used. RPD, rostral pars distalis; PPD, proximal pars distalis; PI,paÀ
intermedia.The
histogram
depictsthe level
of
Pit-l
expressionof
rvinter
(W)-
and summer(S)-acclimatized carp. Bars represent the meant
standard deviationof
thetotal pixel
values measured
in
five
areasof
carp pituitary sections. (Student's t-test P<0.01; n=5different
fish corresponding to each season). The proximal pars distalis (PPD) and pars intermedia
(PI)
rvere analyzed separately.
In mammals there is evidence that the PRL distal enhancer requires the estrogen receptor
in
additionof
Pit-l
for
extensiveactivity
Il].
Although
in
the male carpthe
steroid-bindingaflinity
to
hepatic
estrogen receptors
is
not
affected
by
seasonalacclimatization'
the concentrationof
receptorsis
increased2.5
fold in
estrogen treated summer-acclimatizedfish
when compared to the cold-season-adapted carp [3]. Thus,it
is possible to conceive ahigh
PRL expressionin
summer-acclimatizedfish [7] in
the absenceof
changesin
Pil'l
transcription. Clearly, the expressionof
Piçl
target genes n'hich are also modulatedby
systemic regulators such as cAMP, steroids and thyroid hormones, isstill
poorly understood [28].In
summary,we
have cloned thefirst
gene sequencesof
Pit-l
from
a
teleostfish
and showed that its expression is seasonally modulatedin
the PI and PPDwhile in
the RPDit
doesnot
show
to
be
significantly
affected. Therefore,it
appearsthat
Pit-l is
involved
in
the compensatory response towards the changes of the habitat of an eurythermal fish. Cloningof
the entire carpPit-l
gene is neededto
gain knowledge on the regulatory sequences whichcould
be involved in this differential expression. We are culrently undertaliing this study'q? 1@ c) J
!m
C).xm
È
!ru
M
s
w
s
w
8t9Vol.45, N0.4,
1998 BIOCHEMISTRYond MOLECUTAR BIOTOGY INTERNATlONATACKNOWLEDGEMENTS
-Our thanks to Professor Oriana Gonzalez and Marco Alvarez Santana
for
critical
reading of the manuscript. This work was supported by grants 2950042 and 1940845 fromFONDECYT,
and GE/G LO /90 /004 LINIDO/l CGEB
Rf,FERENCES
[]
Saez, L., Goicoechea, O., Amthauer, R. and KrauskoplM.
(1982) Comp. Biochem. Physiol. 728, 3l-38.
[2] Gerlach, G.F., Turay,
L.,
Malik,
K.T.A.,
Lidq
J., Scutt,A.
and Goldspink' G.(1990) Am.
J.Physiol. 259, R23 l -R244.
[3] Hernandez, I., Pobtete,
A.,
Amthauer, R., Pessot, R. andKrauskopl M.
(1992) Biochem. Int. 28,559-567.[4] Vera, M.1., Norambuena,
L.,
Alvarez,M.,
Figueroa, J., Molina,A.,
Leôn, G. and Krauskopf, M. (1993) Cell.Mol. Biol.
Res. 39,665-674.[5] Gotdspink, G. (1995) J. Therm.
Biol.
20, 167-174.[6]
Vera,
M.1.,Rios,
H.M.,
de
la
Fuente,E.,
Figuero4 J. andKrauskopl
M.
(1997)
Comp. Biochem. Physiol. In press.[7t Figueroa, J., Molina,
A.,
Alvarez,M.,
Villanueva, J., Reyes,A.,
Leôn, G. and Krauskopf,M.
(1994) Comp. Biochem. Physiol. 1088, 551-560.[8]
Figueroa, J., Reyes,A.,
Rios,M.,
Vera,M.l.
and Krauskopf,M.
(1997)Zoological
science 14, 353-357.[9]
Netson,C., Albert,
v.R.,
Elsholtz,H.P.,
Lu, L.l.-w.
and Rosenfeld,M.G.
(1988)
Science 239, 1400-1405.[t$f
Poncelet,A.-C.,
Levavi-Sivan,B.,
Multer,
M.,
Yaron,Z.,Matlial,
J.A.
andBelayew, A.
(1996)
DNA
and CellBiol.l5,
679-692.il
ll
Andersen, B. and Rosenfeld, M.G. (1994) J.Biol.
Chem. 269, 29335-29338[tZ1 Ono, M.,
Takayama,Y.,
Rand-Weaver,M',
Sakat4
S.,
Yasunaga'T.,
Noso,
T.
and Kawauchi, H.(1990)
Proc. Natl. Acad. Sci. USA 87,4330-4334.[3]
Rand-Weaver,M.,
Noso,
T.,
Muramoto,K.
and Kawauchi,H.
(1991) Biochemistry
30, 1509-l 5 I 5.[4]
Ono,
M.,
Harigai,
T.,
Kaneko,T.,
Sato,Y.,
Ihara, S. andKawauchi,
H.
(199a) Mol'
Endocrinol. 8, 109-l 15.[5]
Chen,R.,
Ingraham,H.A.,
Treacy,M.N., Albert,
V.R,
rililson,
L.
and Rosenfeld,M.G.
(1990) Nature 346, 583-586.
[l6]
Delhase,M.,
Castrillo,J.-L.,
de taHoya'
M',
Rajas, F., and Hooghe-Peters,Biol.
Chem. 27l,
323 49 -32358.E.L.
(1996) J.[7]
Garbutt, G.J., Wilson, J.T., Schuster, G.S., Leary, J.J. and Ward, D.C. (1985)Clin'
Chem. 31,1203-1206.fl81Ono, M.
and Takayama,Y.
(1992) Gene226,275'279.[l9]
Merta L.M.
and Rashtchian,A.
(1994) Focus 16,45-48.iZOi
SumUrook,J.,
Fritsch,
E.F.
and Maniatis,
T.
(1989) MolecularCloning.
A
Laboratory-
Manuat, second edition,
Cold
Spring Harbor Laboratory Press, Cold Spring Harbor,NY.
[21] Yamada" s., Hara, J. and Yamashita, s. (1993) J. Biol. Chem.268,24361-24366.izzjLor"nr,
J.8., Aasland, R., Brunstad, H., Bergh, H. and Male, R. (1996) J.Mol.
Endocrinol.t7,225-236.
Vol.45.
N0.4.
1998 El0CHEMlSTRYond M0LECULAR BlOtOGY INTERNATIONAL[23] Majumdar,
S.,lrwin,
D.M.
and Elsholtz, H.P. (1996) Proc. Natl. Acad. Sci.usA
93,
10256-r 0261.
[24] Martinez-Barberâ, J.P.,
Vila,
V., Valdivia,M.M.
and Castrillo. J.L. (1997) Gene 185, 87-93.[25]Li,
S., CrenshawIII,
E.8.,
Rawson, E.J., Simmons,D.M.,
Srvanson,L.W.
and Rosenfeld, M.G. (1990) Nature 347,528-533.[26]Vila,
V.,
Jimene4
O., Giiell, A.,
Vallejo,
D., de
la
Hoya,
M',
Burgos,A.,
Etxabe, J.,Martinez-Barberâ, J.P. and Castrillo, J.L. (1995) Netherlands Joumal
oîZoology 45,229-234.
[27] Takeo, J., Yamada, S., Hara, J. and Yamashita, S. (1996) Gen. Comp. Endocrin.
102,28-33.
[28] Argenton, F., Ramoz,N.,
Charlet, N., Bernardini, S., Colombo, L. and Bortolussi,M.
(1996)Biochem. Biophys. Res. Comm. 224., 57 -66.