HAL Id: hal-01611093
https://hal.archives-ouvertes.fr/hal-01611093
Submitted on 9 Jan 2018
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Capillary rheometry of a binary mixture polymer/CO2
in a single screw extruder
Audrey Common, Martial Sauceau, Élisabeth Rodier, Jean-jacques
Letourneau, Jacques Fages
To cite this version:
Audrey Common, Martial Sauceau, Élisabeth Rodier, Jean-jacques Letourneau, Jacques Fages.
Cap-illary rheometry of a binary mixture polymer/CO2 in a single screw extruder. Chemical Engineering
and Processing: Process Intensification, Elsevier, 2015, 93, pp.21-26. �10.1016/j.cep.2015.04.004�.
�hal-01611093�
Capillary
rheometry
of
a
binary
mixture
polymer/CO
2in
a
single
screw
extruder
Audrey
Common
1,
Martial
Sauceau,
Elisabeth
Rodier,
Jean-Jacques
Letourneau,
Jacques
Fages
*
UniversitédeToulouse,EcoledesMinesd'Albi,CNRS,CentreRAPSODEE,81013Albi,France
Keywords: Hot-meltextrusion Supercriticalcarbondioxide Capillaryrheometry Polymerviscosity
ABSTRACT
Processingbio sourcedpolymerswithsupercriticalfluidsisapromising routetowardsnew green engineeringprocesses.Supercriticalcarbondioxide(sc CO2),issolubleinlargequantitiesinmolten
polymers,whereitactsasplasticizerandswellingagent.Itisused,inblendingorfoamingofpolymers, particleformationandpolymerisationprocess.
Theprocessofhot meltextrusionassistedbysc CO2allowedthedevelopmentofanon lineviscosity
measurementbasedoncapillaryrheometry.Appliedtoabio sourcedpolyamide,itwasvalidatedby comparisonwithaclassiccapillaryrheometer.Bothdatasetswereingoodagreement.
Apseudoplasticfluidbehaviourwasobservedwitha30%viscositydecreasefrom46to32Pasat 5000s 1and220"C,uponadditionofCO
2.However,viscositydecreasedtoaplateaubeforereachingthe
thermodynamicsolubility.Thecomparisonwithamodelcouplingsolubilityandflowallowedtoidentify themethodlimitations,whichwereattributedtothekineticsofdissolutionandmixing.Thehigherthe shearstress,thehighertheamountofCO2atwhichtheviscosityplateauisreached.Thesemeasurements
mayquantifytheimpactoftheCO2ontherheologyofthesystembutalsooftheefficiencyofthemixing
processinourexperimentalsetup.
1.Introduction
Most of the polymers used in industry nowadays have a petrochemicalorigin.However,duetothepredictedexhaustionof theworldpetroleumreserves,itisnecessarytoreplacethemwith bio sourced polymers with equivalent properties. It is also importanttofindnewwaysofprocessing them,whichcomply withtherequirementsofgreenchemistry.Theuseofsupercritical fluidsinpolymerprocessinghasconsiderablygrownupinthelast decades[1 3].Themostusedissupercriticalcarbondioxide(sc CO2).Itissolubleinlargequantitiesinmanymoltenpolymersand
canbeaddedinextrusionprocesseswhereitactsasaplasticizer andswellingagent[4].Itisknowntobeagreenprocessingagentof greatinterestinthepolymerfield,suchasblendingofpolymers, polymerfoaming,particleformationorpolymerizationprocess.
Extrusionisaprocessconvertingarawmaterialintoaproduct ofuniformshapeanddensitybyforcingitthroughadieunder
controlledconditions[5].Ithasextensivelybeenappliedinthe plastic and rubber industries, where it is the most important manufacturingprocess.
Coupling sc CO2 and extrusion modifies the rheological
properties ofthe polymer whileflowing through thebarrel of the extruder [5]. The reduction of viscosity decreases the mechanical constraints and the operating temperature within theextruder.Thus,thismayallowtheuseoffragileorthermolabile molecules, like active pharmaceuticalingredients. Furthermore, theabsenceofresiduesinthefinalmaterialisalsoanadvantagefor pharmaceuticalapplications[6,7].
Using anewsc CO2assistedextrusionprocess,microcellular
foamsofabiocompatibleamorphouspolymerwereelaborated[8]. However,understandingandimprovingsuchaprocess requires theknowledgeofphysicalproperties,likethesolubilityofCO2into
the polymer, the diffusion coefficient andthe viscosityof the mixture.Anextruderprovidesahighshearrate,particularlyinthe die.However,theviscosityofthe binarysystemunderprocess conditionsisverydifficulttoreachinconventionalrheometers. Viscosityunderpressurecanbemeasuredindifferentways[9]. Onegroupofmeasurementsisbasedonvibratingsurfaceorfalling ballrheometers[10].ThesetechniquesrequireNewtonianorlow viscosity polymers. The other main group of measurement
*Correspondingauthorat:EcoledesMinesd'Albi,CampusJarlard,F-81013Albi, France.Tel.:+33563493141;fax:+33563493025
E-mailaddress:Jacques.Fages@mines-albi.fr(J.Fages).
techniques is based on capillary rheometry. This technique is widely usedforviscouspolymersandimplementationsexistto measuretherheologyofmixturesofpolymerandsc CO2[11].
Inthiswork,wehaveimplementedthison linetechniqueona single screwextruderinordertoestimatetheviscosityofabio sourced semi crystalline polymer. Firstly, the validity of the methodwillbecheckedbycomparingtheobtainedresultswith measurementsmadeonaclassiccapillaryrheometer.Secondly,we will applythistechnique tothe binary systempolymer/carbon dioxide. Finally, the comparison with a flow model will be implementedtoidentifysomelimitationsofthetechnique. 2.Theoryofcapillaryrheometry
Theprincipleofcapillaryrheometryistoforcemeltpolymer throughadieoflengthLanddiameterD.Knowingthevolumeflow rateQandthepressureloss
D
P(P Patm)createdbythedie,theshearrate _
g
app,thestresst
pandsubsequentlytheviscosityh
canbe calculated (Fig. 1). To determine this viscosity, several assumptions need to be made. A Poiseuille flow in a tube is assumedwiththefollowingadditionalhypotheses:
# incompressiblefluidwithalaminar,isothermalandestablished flow,
# noendeffects, # nowallslip.
Ifthesehypothesesareverified,thenthefollowingformulas apply:
t
p¼D4LD
P (1) _g
app¼ 32Qp
D3 (2)h
¼g
_t
p app (3)Fornon newtonianfluids,theRabinowitchcorrectionshould apply:
_
g
w¼ _g
app 3mþ1 4m ! "withm¼dIndInQ
t
p (4)Another arguable hypothesis is the absence of end effects, whichmayrequirecorrections.Onewayistousealongdieinorder tomaketheeffectsnegligible.Theothermethodistoimplement theBagleycorrection[12].Itconsistsincarrying out measure ments with dies of different lengths and to use the slope of
D
P f(L/D)tocalculatet
p.Theproblemofthismethodisthehighnumberofexperimentsrequired. 3.Materialsandmethods
Polyamide PA 11, commercial name Rilsan1 (BMFO grade,
Arkema,France),isabio sourcedpolymeramino 11 undecanoic acidobtainedfromcastoroil.Itisalinearpolymerwithanumber averagemolarmassof8700gmol 1andaweightaveragemolar
massof20,010gmol 1.
Melting onset temperature of the PA 11 is in the range 180 200"Candtheglasstransitiontemperaturewithin40 60"C.
Thesoliddensity
r
P,determinedbyheliumpycnometry(Micromeretics,AccuPYC1330)was1027&5kgm 3.Meltdensitywas
found to be 979kgm 3 at 220"C with a magnetic suspension
balance(Rubotherm,Germany).
SolubilitymeasurementsofCO2inthepolymerwerecarried
outonthe samemagneticsuspensionbalance at202,220and 231"Catdifferentpressures.
Theexperimentalsetup(Fig.2)haspreviouslybeenusedwith several polymers including PA 11 [13 16]. The single screw extruderhasa30mm screwdiameterandalengthtodiameter ratio(L/D)of35(Rheoscam,SCAMEX,France).AgreatL/Dratio generallyindicatesagoodcapacityofmixingandmeltingbutan importantenergyconsumption.Thescrewisdividedintothree parts.Thefirstonehasalengthtodiameterratioof20whilethe othershavealengthtodiameterratioof7.5.Betweeneachpart,a restrictionringhasbeenfittedoutinordertoobtainadynamic gastight, which prevents sc CO2 from back flowing. The first
conicalpartallowsthetransportofsolidpolymersandthen,their melting and plasticizing. Then, the screw has a cylindrical geometryfromthefirstgastightringtothedie.Thetemperature insidethebarrelisregulatedatfivelocations:TaandTbbeforethe
CO2injection,TcandTdaftertheinjectionandTeinthedie.All
temperaturesweresetat220"C.
Therearethreepressureandtwotemperaturesensors:P1after
theCO2injector,P2andT1beforethesecondgastightringandP3
andT2bythedie.Thisallowsmeasuringthetemperatureandthe
pressureofthepolymerinsidetheextruder.Errorsassociatedto pressureandtemperaturemeasurementswereabout0.2MPaand 1"C,respectively.
Threedieshavebeenused.Theyallhaveadiameterof1mm andtheirlengthsare7,17and22mm.Thosedieswillbecalledhere L7,L17andL22,respectively.
CO2(N45,AirLiquide,France)ispumpedfromacylinderbya
syringepump(260D,ISCO,USA)andthenintroducedatconstant volumetricflowrateatalengthtodiameterratioof20fromthe feedhopper.ThepressureintheCO2pumpiskeptslightlyhigher
than the pressure P1. The pressure, the temperature and the
volumetricCO2flowratearemeasuredwithinthesyringepump.
CO2 density, obtained on NIST website by Span and Wagner
equationofstate[17,18],isusedtocalculatemassflowrateand thustheCO2massfractionwCO2.CO2flowratewasvariedandits influenceontheviscosityofthemixturewasstudied.Oncesteady stateconditions(testedonP andTmeasurements)arereached, massflowrateandpressure(P3)aremeasured.
Fortheimplementationofthetheoryofcapillaryrheometry, the melt density has been considered as independent of the pressure,andtakenequaltothevaluemeasuredbyamagnetic suspensionbalanceat220"Candatmosphericpressure.Moreover,
inthecaseofCO2injection,wehavestatedthatthehypothesisof
incompressiblefluidandconstantdensityisacceptablesincethe solubilisationoflessthan5%ofdenseCO2(densityofwhichis
400kgm 3) with an increase involume of the same order of
magnitudewillresultinadensityvariationofabout2 3%. Parallelmeasurementsonacapillaryrheometer(CR)(Instron model3211,USA)were madeattheLaboratoire desMatériaux Polymères etdes Biomatériaux(ISTIL EPUL, Lyon, France). This rheometerisequippedwithapistonof0.9525cm2andwithaforce
sensor of 20kN with a precision of0.2%. One die of L/D 140 (L 70mmandD 0.5mm)isused.Measurementswerecarried outat220"C.
4.Results
4.1.Comparisonofbothmethods
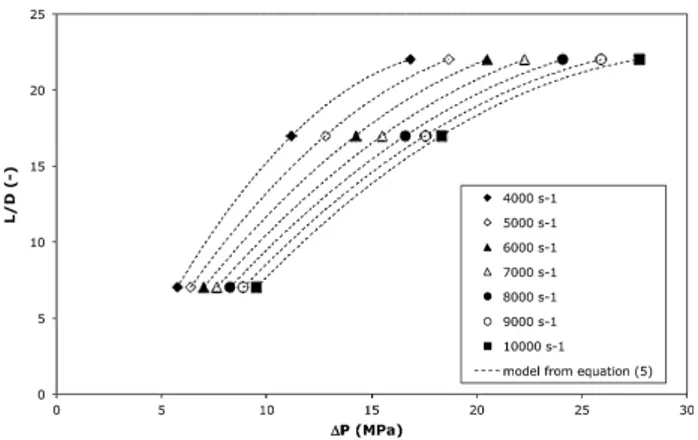
Measurementsontheextruderwerecarriedoutwiththethree dies(Table1).Experimentswerecomparedwithresultsobtainedon the Instron capillary rheometer. At first, end effects could be postulated as negligible with thelongest dieL22 and only the Rabinowitchcorrectionwasapplied(m 0.56).Nevertheless,Bagley correctionwasalsoimplementedontheextruderexperimentsin ordertoevaluateendeffects.Experimentalresultsobtainedwith thethreedies wereinterpolatedatthesameshearrate,which allows plotting the Bagley curves (Fig. 3). According to the procedure ofanalysis describedhereafter, thepressure dropis reportedonthexaxis,whereasthecapillaryaspectratioisreported ontheyaxis.Thenon linearshapeofthesecurvesiscommonly attributedtotheeffectofthepressureontheviscosity[19].
To estimatethe effectof pressureon viscosity, Pantani and SorrentinohaveproposedthefollowingexpressionfortheBagley curves[20]: L D ¼
a
D
P2þbD
P c (5) witht
op¼4b1 e D ¼cb
¼2ab 8 > > > > > < > > > > > : (6)t
opisthestressatzeroentrancepressure,eanadditionallengthwhichtakesintoaccounttheentranceeffectonpressuredropand
b
aparameterdescribingthepressureeffectonviscosityinthe Baruslaw.AscanbeseeninFig.3,thedatawerewellrepresented byEq.(5).Theb
valuesobtaineddecreasedfrom55'10 9Pa 1at4000s 1to35'10 9Pa 1at10000s 1(Fig.4).Thesevaluesare
coherentwith theonesreportedat an averagetemperature of 210"Cforpolypropylene(PP)andpolystyrene(PS),buthigherthan
those obtained for PA66 found to be around 7'10 9Pa 1 at temperatures below 300"C [21]. Finally, theentrance pressure
drops were extrapolated fromFig. 3, allowingto calculatethe corrected stresses. After the application of the Rabinowitch correction(m 0.54),thecorrectedviscositycanbecalculated.
Fig.5showsthecomparisonofresultsobtainedontheextruder andonthecapillaryrheometer.Bothseriesofresultsareingood agreement. However, results on the extruder treated with the Bagley correction give significantly lower viscosity values. Therefore,thecorrectionseemstobeusefulforthisdie.Theset ofviscosityvaluesobtainedwiththecommercialrheometerare higher.
Table1
Dataobtainedontheextruderwiththethreedifferentdies.
L7 L17 L22
DP(MPa) Q(cm3s 1) DP(MPa) Q(cm3s1) DP(MPa) Q(cm3s 1)
5.8 0.50 8.3 0.22 14.5 0.31 7.6 0.65 10.3 0.35 18.0 0.45 9 0.82 12.5 0.50 20.0 0.58 10 1.01 14.9 0.65 23.0 0.71 11 1.20 17.7 0.82 25.5 0.86 11.6 1.38 18.2 0.97 28.0 1.00 19 1.14
Fig.3.Bagleycurvescalculatedatdifferentshearrates.
Thisdiscrepancymightbeascribedto:
# temperaturecontrolduringtheexperiment.Bothmethodsused temperaturesetat220"Cbuttheheatingtakesplacethrougha
staticpolymer inthe CRduring around 1hwhereas there is mixing in the extruder with short residence times (around 3min).
# pressurelevelisquitedifferent.PressureintheCRisintherange 6 200 MPa and particularly within 60 200 MPa, which correspondstotherangeofshearratesencounteredinthedie of the extruder. This high level of pressure may induce an increaseinviscosity.Pressurelevelintheextruderisbetween7 and25MPa.
# samplestorage leading to a possiblechange in themoisture content.PA11isquitesensitivetohumidity.
Inordertofurtherinvestigatethesediscrepanciesandtouse thiscomparisonasavalidationofthemethod,wecouldusealess sensitive polymer andevaluate pressure correctionsto the CR results.Nevertheless,untreatedresultsstillgiveagoodorderof magnitudeoftheviscosity.
4.2.Evolutionofviscosityofthemixture
Experimentson the binary systemwere carried out on the extruderonlywiththeL22die,whichensuresagoodpressurefor solubilisationofCO2intheextruderandminimizesthenumberof
experiments.However,ithastobekeptinmindthateventhough thequantitativevalueisnotexact,duetothelackofcorrection,the qualitativeevolutioniscorrect.
SolubilityexperimentaldatawererepresentedbyaSanchez Lacombeequationofstate[22,23]byusingtheexpressionofthe fugacitycoefficientsofacomponentinthemixtureproposedby Neau[24].Thepurecharacteristicparametersofeachcomponent weretakenasindicatedinTable2.Moreover,thefollowingmixing ruleswereused,endowedwithonefittedbinaryparameterforthe characteristicenergy
e
*:e
(¼X i X jf
if
je
( ijwithe
(ij¼ð1 kijÞðe
(ie
(jÞ0:5 (7)Thismodelwasthenusedtoevaluatethesolubility(weq)ateach
pointofthedie.Solubilityatthedieentranceisnotedweqi.
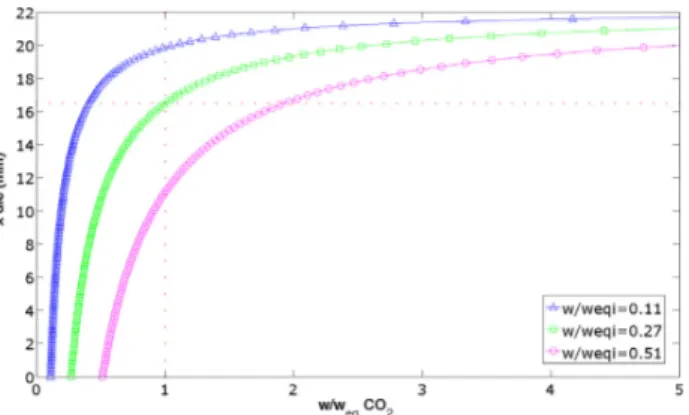
Viscosityofthebinarysystemdecreaseswiththeincreasein CO2contentandshearrate(Fig.6).Thebehaviourintermsofshear
rateisnotsurprisingsincepolymersareoftenshearthinning.The decreaseinviscosityrelatedtoCO2additionisalsoconsistentwith
literatureobservations.Thisdecreaseusuallyfollowsapowerlaw
[26].However,ourmeasurementssuggestaplateau,whichisnot consistentwithsuchalaw.Thismaybeduetothedesorptionofthe CO2inthedieandtheoccurrenceofatwo phasesystemthatcould
disturbmeasurements.
Infact,animportantissuewithbinarymixtureistoknowifthe systemisinasingle phase.Multiplepressuresensorsalongthedie wouldbenecessarytocheckthisassumption[27].However,our systemwassetwithonlyonesensorattheentranceofthedie. Thus,anevaluationoftheexactlocationofdesorptioninthedie was made. A linear depressurisation was assumed from the entrance to the exit [28]. This linear depressurisation was confirmedbymodellingthedieflowwithComsolMultiphysics1
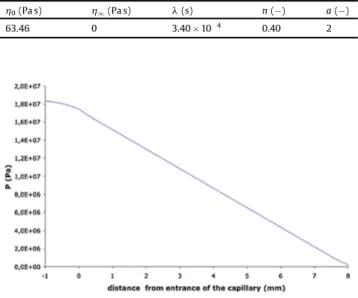
byusingNavier Stokesequationsforanincompressiblefluid.A Carreaulaw(Fig.5)wasusedtorepresenttheviscosity
h
ofthe polymer(Table3):Fig.5.Comparisonofdifferentrheologicalresults.
Table2
PurecharacteristicparametersforSanchez–Lacombeequationofstate. Compounds r*(g/cm3) T*(K) P*(MPa)
PA11[25] 1.035 765.0 465.4 CO2a 1.2518 354.1 329.3 aCalculatedbyusingdataobtainedonNISTwebsitebySpanandWagnerequationof
state[17,18].
Fig.6.EvolutionofthebinarysystemviscosityasafunctionofCO2contentand
shearrate.
Table3
ParametersfortheCarreaulaw.
h0(Pas) h1(Pas) l(s) n(!) a(!)
63.46 0 3.40'10 4 0.40 2
h
¼h
1þðh
0h
1Þ 1þðl
g
_Þa+ n 1=a ð Þh
(8)
h
0istheviscosityatzeroshearrate,h
1theviscosityatinfiniteshear rate,
l
the relaxation time, n the power index and a a dimensionlessparameterdescribing thetransition betweenthe firstNewtonianplateauandthepowerlawregion.An example of the resultsfound by the flow simulationis presentedonFig.7.Figs.8 10showthelocationofthepointof desorptionforeachexperimentalmeasurementatthethreeshear ratesstudiedandfordifferentCO2contents.Desorptionisassumed
tooccurwhenw weq.
AstheCO2contentincreases,desorptionoccursearlierinthe
die.Whendesorptionoccurswithinthefirstthreequartersofthe die length (16.5mm), viscosity measurements differ from the powerlaw,asshownbythelogarithmicchartinFig.11.Theindex
ofthepowerlaw,n,forthethreeshearratesarelistedinTable4. ViscosityresultscanthusbeassumedaccurateaslongastheCO2
contentisnottoohighanddesorptiondoesnotoccurtooearlyin thedie.Inthisstudy,thelimitappearstobew/weqi<0.27.
Finally,adecreaseoftheviscosityaround25%isobservedinthe single phasecriterionregiondefinedabove(w/weqi<0.27,corre
spondingtoamasspercentageupto2%ofCO2).Theappearanceof
theplateauathigherCO2contentisduetothepredominanceofthe
two phaseoccurrencealongthedie.Theviscositymeasuredinthis zonecanbeseenasanapparentviscosityofthemixtureinthe process.Itreflectscouplingofflowingwithphysicalphenomenaof two phase occurrence. Then, it can be used to apprehend phenomenological behaviour of the flowing mixture in the process.
5.Conclusion
Capillaryrheometryisanefficientmethodtomeasureviscosity ofpolymerathighshearrate.Weimplementedthistechniqueon line on an extruder in order to carry out measurement in processingconditions.Validityofthemeasurementswaschecked with a commercialcapillary rheometer. Measurements on the binary mixtureCO2/polymer were thencarriedout. Desorption
pointalongthediewaslocatedbymeansofasolubility model (Sanchez Lacombe) coupled with a flow modeland correlated withexperimentaldata.AminimaldistancebeforeCO2desorption
mustbeattainedtoensureaccuracyofthedata.Thoseexperiments andcalculationdemonstratetheefficiencyofcapillaryrheometry to measure viscosity of polymer and binary system polymer/ sc CO2.Theset upwithonlyonepressuresensorattheentranceof
the dieworksatlowratio ofCO2.Abovea givenCO2 content,
depending on the solubility, pressure and length of the die, depressurisation occurstooearlyinthedieandmeasurements giveaccesstoapparentviscosityinprocessconditions.Toaccess absoluteviscosityofthemixture,asystemwithmultiplepressure sensorswouldbenecessary.
Acknowledgements
This paper is dedicated to the memory of our esteemed colleagueElisabethRodierwholeftusmuchtooearly.
Fig.8.Desorptionpointat3640s 1at3differentinitialratios.
Fig.9.Desorptionpointat5025s 1at3differentinitialratios.
Fig.10.Desorptionpointat7707s 1at2differentinitialratios.
Fig.11.Logarithmicchartoftheevolutionofviscosityofthebinaryasafunctionof CO2contentandshearrate.
Table4
Indexofthepowerlaw. _
gðs1Þ 3640 5025 7707
Thisworkwasmadepossiblethankstofinancialsupportofthe ANR (French National Agency for Research) and to Arkema companythatsuppliedthepolymer.
AuthorswouldliketothankPr.Jean PierrePuauxfromLMPB (Lyon,France)laboratorywhokindlyaccepttocarryoutexperi mentsonhiscapillaryrheometer.ThetechnicalsupportofOlivier Ezequelisalsogratefullyacknowledged.
References
[1]R.B.Yoganathan,R.Mammucari,N.R.Foster,Densegasprocessingofpolymers, Polym.Rev.50(2)(2010)144–177.
[2]H.P.Zhang,M.C.Chen,Polymerizationinsurpercriticalcarbondioxide,Prog. Chem.21(9)(2009)1869–1879.
[3]E.Reverchon,R.Adami,S.Cardea,G.DellaPorta,Supercriticalfluidsprocessing ofpolymersforpharmaceuticalandmedicalapplications,J.Supercrit.Fluids 47(3)(2009)484–492.
[4]M.Sauceau,J.Fages,A.Common,C.Nikitine,E.Rodier,Newchallengesin polymerfoaming:areviewofextrusionprocessesassistedbysupercritical carbondioxide,Prog.Polym.Sci.36(6)(2011)749–766.
[5]C.Rauwendaal,PolymerExtrusion,HanserPublishers,München,2001. [6]Z.K.Nagy,M.Sauceau,K.Nyúl,E.Rodier,B.Vajna,G.Marosi,J.Fages,Useof
supercriticalCO2aidedandconventionalmeltextrusionforenhancingthe dissolutionrateofanactivepharmaceuticalingredient,Polym.Adv.Technol. 23(2012)909–918.
[7]T.Vigh,M.Sauceau,J.Fages,E.Rodier,I.Wagner,P.L.Sóti,G.Marosi,Z.Nagy, EffectofsupercriticalCO2plasticizationonthedegradationand residual crystallinity of melt-extruded spironolactone – non-invasive testing of immediate-releasesoliddispersions,Polym.Adv.Technol.25(2014) 1135–1144.
[8]C.Nikitine,E.Rodier,M.Sauceau,J.-J.Letourneau,J.Fages,Controllingthe structureofaporouspolymerbycouplingsupercriticalCO2andsinglescrew extrusionprocess,J.Appl.Polym.Sci.115(2)(2010)981–990.
[9]A.Shine,Polymersandsupercriticalfluids,Phys.Prop.Polym.Handb.18(2007) 319.
[10]B. Calvignac,E.Rodier, J.-J. Letourneau,P. Vitoux,C. Aymonier,J. Fages, Development of an improved falling ball viscometer for high-pressure measurementswithsupercriticalCO2,J.Supercrit.Fluids55(1)(2010)96–106.
[11]M.Nobelen,S.Hoppe,C.Fonteix,F.Pla,M.Dupire,B.Jacques,Modelingofthe rheologicalbehaviorofpolyethylene/supercriticalCO2solutions,Chem.Eng. Sci.61(2006)5334–5345.
[12]E.Bagley,Endcorrectionsinthecapillaryflowofpolyethylene,J.Appl.Phys.28 (5)(1957)624–627.
[13]M.Sauceau,C.Nikitine,E.Rodier,J.Fages,Effectofsupercriticalcarbondioxide onpolystyreneextrusion,J.Supercrit.Fluids43(2007)367–373.
[14]C.Nikitine,E.Rodier,M.Sauceau,J.Fages,Residencetimedistributionofa pharmaceuticalgradepolymermeltinasinglescrewextrusionprocess,Chem. Eng.Res.Des.87(6)(2009)809–816.
[15]C.Nikitine,E.Rodier,M.Sauceau,J.-J.Letourneau,J.Fages,Controllingthe structureofaporouspolymerbycouplingsupercriticalCO2andsinglescrew extrusionprocess,J.Appl.Polym.Sci.115(2010)981–990.
[16]A.Common, E.Rodier,M. Sauceau, J.Fages,Flow and mixingefficiency characterisationinaCO2-assistedsingle-screwextrusionprocessbyresidence timedistributionusingRamanspectroscopy,Chem.Eng.Res.Des.92(2014) 1210–1218.
[17]Carbondioxide,NISTChemistryWebBook,http://webbook.nist.gov/cgi/cbook. cgi?ID=124-38-9.
[18]R.Span,W.Wagner,Anewequationofstateforcarbondioxidecoveringthe fluidregionfromthetriple-pointtemperatureto1100Katpressuresupto 800MPa,J.Phys.Chem.Ref.Data25(6)(1996)1509–1596.
[19]H.Laun,Polymermeltrheologywithaslitdie,Rheol.Acta22(2)(1983) 1435–1528.
[20]R. Pantani, A. Sorrentino, Pressure effect on viscosity for atactic and syndiotacticpolystyrene,Polym.Bull.5(4–5)(2005)365–376.
[21]S.Ceccia,C.Cocquet,L.Trouillet-Fonti,D.R.Long,Influenceofpressureon polyamide66shearviscosity:acasestudytowardspolarpolymersbehavior, Rheol.Acta53(2014)181–190.
[22]I.C.Sanchez,R.H.Lacombe,Anelementarymoleculartheoryofclassicalfluids. Purefluids,J.Phys.Chem.80(21)(1976)2352–2362.
[23]I.C.Sanchez,R.H.Lacombe,Statisticalthermodynamicsofpolymersolutions, Macromolecules11(6)(1978)1145–1156.
[24]E.Neau,Aconsistentmethodforphaseequilibriumcalculationusingthe Sanchez–Lacombelattice-fluidequation-of-state,FluidPhaseEquilib.2(2002) 133–140.
[25]P.A.Rodgers,Pressure–volume–temperaturerelationshipsforpoly(vinylidene fluoride)andpolyamide-11,J.Appl.Polym.Sci.50(12)(1993)2075–2083. [26]J.R. Royer, Y. Gay,J.M. Desimone,S.A. Khan, Highpressure rheologyof
polystyrene meltsplasticizedwith CO2: experimentalmeasurementand predictivescalingrelationships,J.Polym.Sci.PartB:Polym.Phys.38(2000) 3168–3180.
[27]A.Xue,C.Tzoganakis,Rheologicalpropertiesofpolystyrene/supercriticalCO2 solutionfromanextrusionslitdie,J.Polym.Eng.23(1)(2003)1–22. [28]X.Han,K.W.Koelling,D.L.Tomasko,L.J.Lee,Effectofdietemperatureonthe
morphologyofmicrocellularfoams,Polym.Eng.Sci.43(6)(2003) 1206–1220.