O
pen
A
rchive
T
OULOUSE
A
rchive
O
uverte (
OATAO
)
OATAO is an open access repository that collects the work of Toulouse researchers and
makes it freely available over the web where possible.
This is an author-deposited version published in :
http://oatao.univ-toulouse.fr/
Eprints ID : 14569
To link to this article : DOI:10.1016/j.postharvbio.2015.02.001
URL :
http://dx.doi.org/10.1016/j.postharvbio.2015.02.001
To cite this version : Severo, Joseana and Tiecher, Aline and Pirrello,
Jullien and Regad, Farid and Latché, Alain and Pech, Jean-Claude and
Bouzayen, Mondher and Rombaldi, César Valmor UV-C radiation
modifies the ripening and accumulation of ethylene response factor
(ERF) transcripts in tomato fruit. (2015) Postharvest Biology and
Technology, vol.102. pp.9-16. ISSN 0925-5214
Any correspondance concerning this service should be sent to the repository
administrator:
staff-oatao@listes-diff.inp-toulouse.fr
UV-C
radiation
modifies
the
ripening
and
accumulation
of
ethylene
response
factor
(ERF)
transcripts
in
tomato
fruit
Joseana
Severo
a,*
,
Aline
Tiecher
b,
Jullien
Pirrello
c,
Farid
Regad
c,
Alain
Latché
c,
Jean-Claude
Pech
c,
Mondher
Bouzayen
c,
Cesar
Valmor
Rombaldi
daIFF,InstitutoFederalFarroupilha,EixodeProduçãoAlimenticia,RuaFábioJoãoAndolhe,1100,BairroFloresta,CEP98590-000,CampusSantoAugusto,
SantoAugusto,RS,Brazil
bUNIPAMPA,UniversidadeFederaldoPampa,CampusItaqui,RuaLuizdeJoaquimdeSáBritto,s/n,BairroPromorar,CEP97650-000,Itaqui,RS,Brazil cUMR990INRA/INP-ENSAT,PoledeBiotechnologieVégétale,24ChemindeBordeRouge,P.O.Box107,31326CastanetTolosanCedex,France dUFPel/FAEM,DepartamentodeCiênciaeTecnologia,CampusUniversitário,CaixaPostal354,CEP90010-900,Pelotas,RS,Brazil
Keywords: Abioticstress Ripening Senescence Solanumlycopersicum 1-Methylcyclopropene UltravioletC ABSTRACT
Ultraviolet-C(UV-C) radiationisused asapostharvest treatmenttoprolongthe shelflifeoffruit. However,thisstressfulprocessmayalsoaffectethyleneproductionand,consequently,theexpressionof genesencodingethyleneresponsefactors(ERFs).Totestthishypothesis,MicroTomtomatoesharvested atthebreakerstageweresubjectedto:1–applicationof3.7kJm2UV-Cradiation,2
–applicationof 2mLL11-methylcyclopropene(1-MCP)followedbyUV-Cradiation;and3
–without1-MCPorUV-C (controltreatment). Aftertreatmentall fruitwerestored for12dat 21 2!Cand 80 5% relative
humidity(RH).AlthoughUV-CradiationincreasedACCoxidasetranscripts andstimulatedethylene production,theripeningevolutionwasdelayed.FruittreatedwithUV-Cshowedloweraccumulationof lycopene,b-carotene,lutein+zeaxanthinandd-tocopherol;butretainedhigherlevelsofchlorogenic acid,r-coumaricacidandquercetinafter6d.Additionally,UV-Ctreatedfruithadhighercontentsof polyamines(putrescineandspermidine).Amongthe14ERFsstudied,11(Sl-ERFA.1,Sl-ERFA.3,Sl-ERFB.1, Sl-ERFB.2,Sl-ERFB.3,Sl-ERFC.6,Sl-ERFD.1,Sl-ERFD.3,Sl-ERFE.1,Sl-ERFF.5,Sl-ERFG.2)exhibitedincreased transcriptaccumulation,2ERFs(Sl-ERFE.2andSl-ERFE.4)showeddecreasedtranscriptaccumulationand only1ERF(Sl-ERFE.3)wasnotsignificantlyaffectedbyUV-Ctreatment.Asexpected,thetranscript profilesof1-MCPand/orUV-C-treatedtomatoesdemonstratethatethyleneplaysanimportantroleinthe expressionofERFs.ThedelayinfruitripeningmaybecausedbytheactivationofERFsthatcouldactas regulatorsofmetabolicpathwaysduringripening.However,thishypothesisneedstobebettertested.In conclusion,arelationshiphasbeenestablishedbetweenUV-Ctreatmentandripeningdelay,correlated tochangesin13ERFtranscriptsevaluatedduringpostharvesttreatment.
1.Introduction
UV-Cradiation(100–280nm)isa treatmentwithgermicidal
capabilitiesthathasbeenusedtopreventpostharvestrotinfruits
andvegetables(Stevensetal.,1998;Liuetal.,2011;Syamaladevi
et al., 2014). Because it is a stressor, UV-C can also accelerate
ethylene production and therefore activate the expression of
ethyleneresponsefactor(ERFs)genes.Alteringtheexpressionof
ERF, either through hormonal induction or abiotic stress, can
induce secondary metabolic pathways; these pathways may
activatepathogenesis-related(PR)genesrelatedtothesynthesis
of phytoalexins, phenols and terpenoids (Maharaj et al.,1999;
Charlesetal.,2008a,b;Liuetal.,2011;Pomboetal.,2011).Pombo etal.(2011)reportedthatUV-Ctreatmentofstrawberrieshelps
prevent rotnot onlyby direct inoculum reduction,but also by
activatinggenesencodingenzymesinvolvedinplantdefense.The
beneficial effects of the application of UV-Ccan vary between
species, cultivars and time of application. Bu et al. (2013)
previouslyreportedthatUV-CmaintainedthefirmnessofCherry
tomatoes(SolanumlycopersicumL.cv.Zhenzhu1.),withdecreased
expressionofcellwalldegradingenzymes.Incomparison,Tiecher
et al. (2013) observed delay in fruit maturation without a
commensurateprolongationof tomatofirmness(S.lycopersicum
cv. Flavortop). Obande et al. (2011) reported maintained the
*Correspondingauthor.Tel.:+555332757258;fax:+555332757258. E-mailaddress:joseana.severo@iffarroupilha.edu.br(J.Severo).
firmnessofpreharvestUV-Ctreatmentoftomatoes(S.
lycopersi-cumL. cv.Mill.) withvaryingresults dependingontheapplied
dose.
It iswidelyknownthatthephytohormoneethylenecontrols
manyeventsrelatedtogrowthanddevelopmentinplants,andis
expressed in response toabiotic and biotic stressors(Cara and
Giovannoni,2008;Bapat etal.,2010).1-Methylcyclopropene
(1-MCP)isapotentinhibitorofethyleneperception,whichhasbeen
usedsuccessfullyinstudiestounderstandtheactionofethylenein
ripeningprocessandconsequentlytheexpressionofrelatedgenes
(Hoeberichtsetal.,2002;OpiyoandYing,2005).
Ethylene is formed from the amino acid methionine by
S-adenosyl-L-methionine(AdoMet)and1-carboxylic
acid-1-amino-cyclopropane. The enzymes that catalyze the conversion of
AdoMettoACCandACCtoethyleneareACCsynthase(ACS)and
ACC oxidase (ACO), respectively. During ripening of climacteric
fruit,this biosynthesispathwayis autocatalyticallyregulatedby
ethylene (Barry et al., 1996; Cara and Giovannoni, 2008). In
responsetoethylene,theexpressionprofileofseveraltranscription
factorsmaybealtered,whichresultsintheactivationofpathways
thatinduceordelaysenescence(Ohme-TakagiandShinshi,1995;
Chenetal.,2008;Erkanetal.,2008;Liuetal.,2009,2011).
After synthesis, ethylene is recognized by receptors (ETRs)
locatedinthemembraneoftheendoplasmicreticulum.Asignaling
cascadewhich includespositiveandnegativeregulators,
modu-latestheexpressionofERF,whicharesubsequentlyresponsiblefor
changesinthemetabolicpathwaysinvolvedinripeningandplant
defense (Barry et al., 1996; Bapat et al., 2010). This process
culminates in biochemical and physiological responses suchas
chlorophylldegradation,carotenoidaccumulation,softening,and
changesintomatoaromaandflavor.Inaddition,therearechanges
in the levels of L-ascorbic acid, tocopherols and phenolic
compounds (Stevens et al.,1998; Cara and Giovannoni, 2008).
TheERFsbelongtotheAP2/ERFfamilyoftranscriptionfactorsthat
arecharacterizedbythepresenceofaDNAbindingdomaincalled
AP2/ERF, which is present exclusively in plants. This family of
transcription factorshasa 58-59amino acidconserveddomain
(ERFbindingdomain)thatcanbindtotwocis-elements:(i)
GCC-box, which is present in the promoter regionof PR-genes that
conferaresponsetoethylene,and(ii)C-repeat
(CRT)/dehydration-responsive element(DRE),whichisinvolvedintheexpressionof
genesrelatedtodehydrationand responsetolowtemperatures
(Singhetal.,2002;Xuetal.,2008,2011).Whereassomeofthese
transcription factorsbindtoonlyoneoftheseciselements(Gu
etal.,2002;Singhetal.,2002),othersmaymodulateresponsesto
stresstolerancethroughinteractionswithboth(GCC-boxandDRE)
ciselements(Huangetal.,2004;Zhangetal.,2004;Xuetal.,2007,
2011).
Since the first ERF binding domain was identified in four
tobaccoproteins(Ohme-TakagiandShinshi,1995),newERFgenes
have been identified in other plant tissues (Zhou et al., 1997;
Tournieretal.,2003;Wangetal.,2007;Xuetal.,2007;Zhangetal., 2010;Yinetal.,2012; Girardietal.,2013).Severalstudieshave
soughttorelatetheinfluenceofbioticandabioticstressorstothe
expression of these transcription factors (Singh et al., 2002;
GuttersonandReuber,2004;Xuetal.,2007,2011;Yinetal.,2012).
In general, studiesthat havemodified ERFexpressionin plants
havedemonstratedanincreasedtolerancetosalinity(Huangetal.,
2004; Wang et al.,2004; Zhang et al., 2004;Pan et al., 2010),
drought(Chenetal.,2008;Zhangetal.,2010),temperature(Chen
et al., 2008; Zhang and Huang et al., 2010) and/or pathogen
infection(Heetal.,2001;Panetal.,2010).Yinetal.(2012)showed
that 13 ERFs sequences are differentially expressed during
postharvestabioticstresses(lowtemperature,hightemperature,
highCO2andhighwaterloss)inKiwifuit.Liuetal.(2011),using
microarraytechniques,determinedthatUV-Cirradiationinduced
the expression of defense response genes (such as PR related
proteins,
b
-1,3-glucanase and chitinase), signal transductiongenes(such asethylene relatedgenes,IAA receptorproteinand
calmodulin) and protein metabolism genes. At the same time,
somegenesrelated tocell walldisassembly (such asexpansin,
pectinesterase and endo-
b
-1,4-D-glucanase), photosynthesis(such as chlorophyll a/b binding protein precursor) and lipid
metabolism(suchaslipoxygenase)seemtobesuppressedinthe
tomatofruitafterUV-Cradiation.
The tomato is one model for the studyof the relationships
betweenstress,hormonalresponsesandfruitquality.Tomatoes
are a good model because their structural genomics are
well-known,theirtranscriptomeandproteomedatabasesarerelatively
rich,andbecausetheyareaspeciesofgreateconomicimportance
(CaraandGiovannoni,2008;Bapatetal.,2010;Barsanetal.,2010).
ThegoalofthisresearchwastounderstandhowUV-Caffects
thetranscriptionalprofilesofACO1andERFsaswellaslevelsofthe
majorsecondary metabolitesintomatoes.Theapplication of
1-MCPpriortoUV-Ctreatmentwasusedtodistinguishiftheeffectof
UV-C treatmenton gene expressionwas mainlydependent on
ethylene.
2.Materialandmethods
2.1.Plantmaterial
Tomato plants (S. lycopersicum Mill., “MicroTom”) were
cultivatedinpotswithpeatsubstrate(Klasmann-Deilmann,R.H.
P.15).Growing conditionswere:a14:10hlight/darkcyclewith
temperatures of 25!C during the dayand 20!C overnight, 70%
relativehumidity(RH)andalightintensityof250
m
molm2s1.Tomatofruitwereharvestedatthebreakerstageoftheripening
processandtransportedatroomtemperature(RT)fortreatment.
Theaveragetimebetweenharvestandtreatmentwas30min.
2.2.UV-Ctreatment
ForUV-Ctreatment, theharvestedtomatoes werepackedin
traysandplaced underUV-Clamps(TUVG30T8, 30W,Philips).
Fourlamps were placed at a distance of 30cm from thefruit,
providing a UV-C dose of 3.7kJm2 as measured by a digital
radiometer(ModelMRUR-203,Instrutherm1).Toachievethetotal
dose,4minofexposurewererequiredoneachofthefoursidesof
the fruit, totaling 16min of treatment. To isolate the effect of
ethylene,atreatmentof1-MCPwasappliedtothefruitinthe
1-MCP+UV-C group at a concentration of 2
m
LL1 before UV-Ctreatment.TheseconditionswerepreviouslyoptimizedbyTiecher
et al. (2013). Thus, the experimental design contained the
followingtreatments:1–UV-C:fruitwereharvestedandtreated
withUV-Cat3.7kJm2andstoredatRT(20 3!Cand80 5%RH)
for12d.2–1-MCP+UV-C:fruitwereharvestedandtreatedwith
1-MCPat2
m
LL1for12h,followedbytreatmentwithUV-CasdescribedaboveandstoredatRTfor12d.3–Control(untreated
fruit):fruitwereharvestedandimmediatelyplacedatRTfor12d.
2.3.RNAextraction,cDNAsynthesisandrealtimePCR(qPCR)
Theexocarpsoftheharvestedtomatofruitwereusedtostudy
the transcriptional expression of ACO1 and ERF genes by
quantitative PCR (qPCR).The samples described in Section 2.2
werecollectedafter6hofstorage.TotalRNAwasextractedusing
PureLinkTMreagent(Invitrogen1)accordingtothe
manufacturer’s
instructions.ThequalityandconcentrationofRNAextractswere
evaluatedusinganAgilent2100Bioanalyzer1(Agilent
Technolo-gies,CA),inwhichonlyRNAsamplesthathadRIN(RNAintegrity)
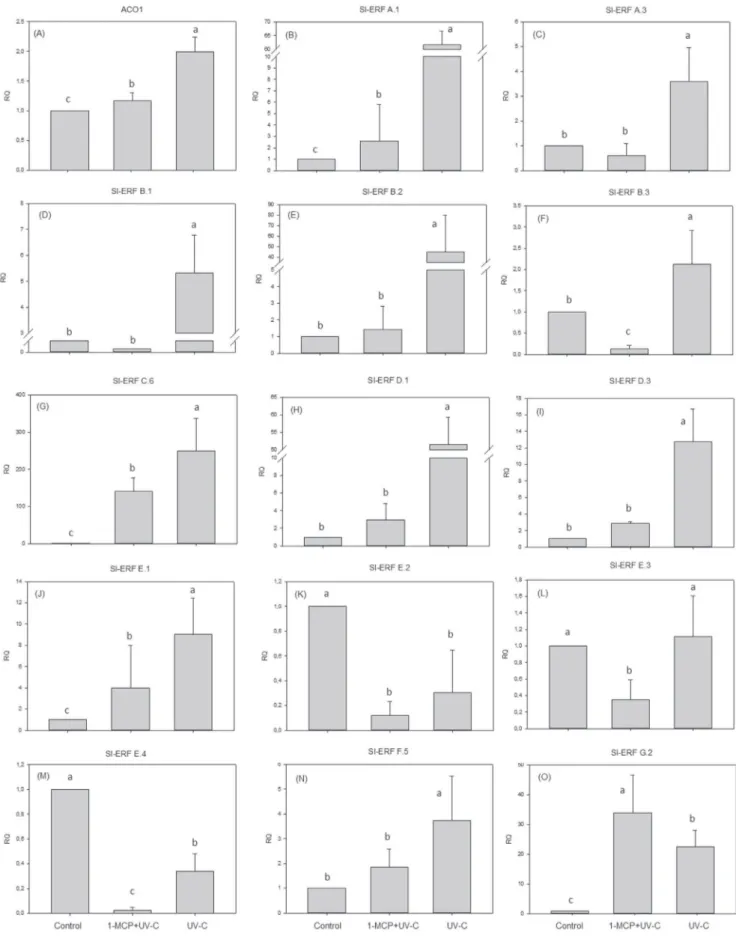
Fig.1.EffectsofUV-CtreatmentonrelativeaccumulationofACO1(A)andERF(B–O)genetranscriptsin“MicroTom”tomatofruitafter6hofstorage.Therelative quantificationoftranscripts(RQ)isrelativetocontrolfruitandnormalizedwithb-actintranscripts.Verticalbarsrepresentthestandarddeviation.
2
m
gofRNAextractwastreatedwithDNase(Qiagen,Valencia,CA,USA). Reverse transcription of mRNAwas completedusing the
OmniscriptReverseTranscriptionkit(Qiagen,Valencia,CA,USA),
resultinginatotalvolumeof20
m
L.ForqPCR,2m
LofcDNAwasaddedto25
m
Lofreactionagent-SYBRGREENPCRMasterMix(PE-AppliedBiosystems,FosterCity,CA,USA),andanABI7900ht
sequence-detection system was used. The Sl-ACO1 gene (Barry
etal.,1996)and14ERFgenes(Sl-ERFA.1–Pirrelloetal.,2012;
Sl-ERFA.3–Zhouetal.,1997;Sl-ERFB.1–Pirrelloetal.,2012;Sl-ERF
B.2–Pirrelloetal.,2012;Sl-ERFB.3–Tournieretal.,2003;Sl-ERF
C.6–Zhouetal.,1997;Sl-ERFD.1–Pirrelloetal.,2012;Sl-ERFD.3–
Pirrelloetal.,2012;Sl-ERFE.1–Tournieretal.,2003Sl-ERFE.2– Zhang etal., 2004; Sl-ERFE.3 –Wang etal., 2004; Sl-ERFE.4 – Pirrelloetal.,2012;Sl-ERFF.5-Tournieretal.,2003;Sl-ERFG.2
-Zhouetal.,1997)wereused.Primerswereusedataconcentration
of50nM,andtheqPCRconditionswereasfollows:50!Cfor2min,
95!Cfor10min,40cyclesat95!Cfor15s,60!Cfor1min, 1cycleof
95!Cfor15sand1cycleof60!Cfor15s.Analyseswereperformed
in triplicate onplates witha capacity of 384reactions. The Ct
(threshold cycle) values were calculated for each sample. The
relative quantification (RQ) was calculated with the method
proposedbyLivakandSchmittgen(2001),using
b
-actin(Pirrelloetal.,2006)asaninternalstandard(nonaffectedby1-MCP+UV-C,
UV-C, fruit growth and development) and control fruit for
calibration.
2.4.Ethyleneproductionandfruitcolor
Fruitethyleneproductionwasquantifiedbygas
chromatogra-phy 1h, 6h, and 12h, and daily (up to 12d) after the UV-C
application.Thefruitineachgroupwasplacedina100mL
screw-capglassvial.After30minofincubation,1mLofheadspacewas
collectedtodeterminetherate ofethyleneproduction, andthe
resultswereexpressedinngkg1s1.
Thecolorofalleachofthesixfruitineachgroupwasmeasured
daily (upto 12d) on 4 sides with a colorimeter (Minolta
CR-300 TM),and the results wereexpressedas thehueangle “H”
[H=tan1(b/a)whena>0andb>0orh=180+tan1(b/a)when
a<0andb>0].
2.5.Levelsoflycopene,
b
-carotene,luteinandzeaxanthinExtraction techniques and chromatographic analysis were
performed following methods described by Rodriguez-Amaya
(2001) followedbysaponificationoftheetherextract.Levelsof
lycopene,
b
-carotene,luteinandzeaxanthinwerequantifiedusinga highperformanceliquid chromatography(HPLC) systemfrom
Shimadzuequippedwithanautomaticinjector,UV–visdetectorat
450nm, a RP-18CLC-ODS (5mm, 4.6mm"150mm, Shimadzu)
reverse-phase column and CLC-GODS (5mm, 2mm"4mm,
Supelco)guardcolumn.Separationwasperformedusingagradient
elutionsystemwithmethanol(solventA),acetonitrile(solventB)
andethylacetate(solventC)asthemobilephaseataflowrateof
16.7
m
Ls1(i.e.1mLmin1).Theinitialphaseconsistedof30%Aand70%B;after10minthecompositionwaschangedto10%A,80%
Band10%C;after35minthecompositionwaschangedagainto5%
A,80%Band15%C;theinitialcompositionwasrepeatedat40min
and maintainedfor 2.5mintorebalance thesystem.The peaks
were identified by comparison with the retention times of
standardsandquantifiedbycomparisonwithexternalcalibration
curves forlycopene,
b
-carotene,lutein andzeaxanthin (Sigma–Aldrich1)standards.TheHPLCresultsareexpressedasmgkg1of
freshmaterial.
2.6.
d
-tocopherollevelsTocopherolextractionwasperformedas describedby
Rodri-guez-Amaya (2001), using a method similar to that used for
carotenoid extraction. The tocopherols were separated and
quantifiedusingHPLCinamanneridenticaltothatdescribedin
item 2.5. The separation was performed by a gradient elution
systemwithamobilephaseof:methanol(solventA),isopropanol
(solventB),andacetonitrile(solventC)ataflowrateof16.7
m
Ls1(i.e. 1mLmin1). The gradient began with a ratio (A/B/C) of
40:50:10 (v/v/v), which was changed linearly to 65:30:5 over
10min,thendecreasedto40:50:10overanother2minandheld
constantfor15min.Thepeakwasidentifiedbycomparisonwith
theretentiontimeofthestandardandquantifiedbycomparison
with an external calibration curve for
d
-tocopherol (Sigma–Aldrich1).Resultsonafreshweightbasisareexpressedasmgkg1.
2.7.Levelsofp-hydroxybenzoicacid,p-coumaricacidandquercetin
The extraction and identification of individual phenolic
compoundswasperformedfollowingthemethodsdescribedby
Häkkinenetal.(1998).Phenoliccompoundswereextractedwith
methanol acidified with6M HCl and separated and quantified
usinganHPLCprocessidenticaltothatdescribedinitem2.5.The
mobilephaseconsistedofanelutiongradientwithaceticacidin
water(99:1)(solventA)andmethanol(solventB)ataflowrateof
15
m
Ls1(i.e.0.9mLmin1).Thestartingpercentageof100%Awasgraduallychangedto60%Aand40%Boveraperiodof25min,held
constantatthisratioforafurther2min,graduallychangedto95%
Aand5%Bat37min,heldconstantforanadditional5minand
thenreturned tothestarting proportionfora totalrun timeof
45min.Thephenoliccompoundswereidentifiedbycomparison
with the retention time of standards and quantified based on
calibrationcurvesofexternalstandardsforp-hydroxybenzoicacid,
p-coumaricacidandquercetin(Sigma–Aldrich1).Theresultsare
expressedonafreshweightbasisasmgkg1.
2.8.Polyaminelevels
Polyamine extraction and quantification was carried out
following Vieira et al. (2007) with minor changes. Polyamines
wereextractedwithtrichloroaceticacid(5%inwater)andanalyzed
byHPLCseparatedinaC18column(30cm"3.9mmi.d."10
m
m,Waters).Polyamineanalysesusedanelutiongradientprogramin
which mobilephase Awas acetate buffer(0.1M) containing
1-octanesulfonicsodiumsalt(10mM),adjustedtopH4.9withacetic
acidandeluentBwasacetonitrile,ataflowrateof11.7
m
Ls1(i.e.0.7mLmin1.Afterseparation,theamineswerederivatizedwith
o-phthalaldehyde(OPA)anddetectedfluorometricallyat340nm
excitationand445nmemission.Resultswereexpressedonafresh
weightbasisasmgkg1.
2.9.Experimentaldesignandstatisticalanalysis
Theexperimentaldesignwascompletelyrandomized,
consist-ingof3UV-Ctreatmentgroups(control,1-MCP+UV-C,UV-C)with
3 analytical replicates. Data was verified for normality using
Shapiro–Wilks’testandforhomoscedasticityusingHartley’stest.
ResultswereanalyzedusingANOVA,withaP#0.05 considered
significant.Post-hoc analysis was performed using Tukey’stest
(p#0.05).SASsoftwarewas usedforallstatisticalanalysis(Sas
3.Results
3.1.TheeffectsofUV-Ctreatmentonthetranscriptionalaccumulation
ofACO1andERFgenes
As revealed by the relative accumulation of ACO1 and ERF
transcripts,UV-Ctreatmentaffectedtheexpressionofmostgenes
investigated(Fig.1).Therewasanincreaseintheaccumulationof
ACO1 gene transcripts when fruits were treated with UV-C
(Fig.1A),andtheapplicationof1-MCPprior toUV-Ctreatment
reducedlevelsofACO1transcriptscompared toUV-Ctreatment
alone; however, levels were still above those observed in the
controlfruit.
Amongthe14ERFgenesstudied(Fig.1B–O),11ERFs(Sl-ERFA.1,
Sl-ERFA.3,Sl-ERFB.1,Sl-ERFB.2,Sl-ERFB.3,Sl-ERFC.6,Sl-ERFD.1,
Sl-ERF D.3, Sl-ERF E.1, Sl-ERF F.5, Sl-ERF G.2) increased withUV-C
treatment, 2 ERFs (Sl-ERF E.2 and Sl-ERF E.4) had decreased
transcriptaccumulationand1ERF(Sl-ERFE.3)wasnotaffectedby
UV-Ctreatment.Whentheethyleneactioninhibitor(1-MCP)was
applied prior to UV-C treatment, there was less transcript
accumulationcompared toUV-C treatment alone for allof the
ERFsstudied,withtheexceptionofSl-ERFG.2.
3.2.TheeffectsofUV-Ctreatmentonethyleneproductionandcolor
ThefruitsubjectedtoUV-Ctreatmentshowedhighethylene
production in the first hour after treatment. The evolution of
ethyleneproductioninalltreatmentsfollowedaclassicclimacteric
pattern,withincreasedethyleneproductioncorrespondingtothe
climactericpeak. However,the maximumclimacteric peak was
delayedby1dwithUV-Ctreatment(Fig.2A),and3to4dwiththe
application of 1-MCP prior to UV-C,as compared withcontrol
tomatoes(Fig.2A).
TheapplicationofUV-Chelpedtomaintainthegreencolorof
the fruit, and 1-MCP+UV-C treatment further inhibited color
change,and retaineda higher !Hue value(Fig.2B),despitethe
increasedethyleneproductionofthisfruit(Fig.2A).Additionally,
bettervisualappearanceinUV-Ctreatedfruitwasobservedafter
12dofstorage(Fig.2C).
3.3.EffectofUV-Consecondarymetabolitelevels
TheUV-Ctreatmentdelayedtheripeningevolution,withlower
levels of lycopene,
b
-carotene, lutein and zeaxanthin andd
-tocopherolobserved,aftersixdaysofstorage.Sloweraccumu-lation was observed when 1-MCP was applied before UV-C
treatment(Table1).Thetreatmentalsoresultedinhigherlevels
ofallmeasuredphenoliccompounds(Table1,chlorogenicacid,
p-coumaricacidandquercetin).
Putrescine and spermidine were predominant among the
polyaminesdetected intreated fruit(Table 1).It wasclearthat
the application of UV-C promoted a greater accumulation of
putrescineandspermidineafter6doftreatment.Infruitthatwas
previously subjected to treatment with 1-MCP before UV-C,
polyaminelevelswerelowerthaninfruittreatedonlywith
UV-Cbuthigherthancontrol.
4.Discussion
Thereisalargebodyofresearchdemonstratingthebeneficial
effectsofUV-Cradiationtreatmentonfruit(Maharajetal.,1999;
González-Aguilaretal.,2007;Charlesetal.,2008a,b;Erkanetal., 2008;Liuetal.,2009;Pomboetal.,2011;Stevensetal.,1998;Liu etal.,2011;Tiecheretal.,2013;Maharajetal.,2014;Syamaladevi
etal.,2014).Theresultsobservedinthisworkhaveconfirmedthat
UV-Ctreatmentstimulatesethyleneproduction,especiallyinthe
first few hours after treatment (Fig. 2A, Maharaj et al., 1999).
Additionally, UV-C treatmentcauses an increase in ACO1 gene
transcripts(Fig. 1A)thatcodefortheenzymeACCoxidase,whichis
activeduringthelaststepofethylene biosynthesis(Barryetal.,
1996;CaraandGiovannoni,2008).Thisphysiologicalresponseis
consistentwiththefactthat UV-Cisa stressorand thatplants
Fig.2.EffectsofUV-Ctreatmentonethyleneproduction(A),!Hue(B)andtomatofruits(C):(i)control,(ii)1-MCP+UV-C,(iii)UV-C,in“MicroTom”tomatofruitsstoredfor
generally increase ethylene production under stress, likely by
actingonsystem2autocatalyticethylene(González-Aguilaretal.,
2007;Liuetal.,2011;VandePoeletal.,2012;Tiecheretal.,2013).
UV-Cdelayedripeningintomatofruit,indespiteoftheincrease
in ethylene production (Fig. 2A) and ACO1 transcriptional
expression(Fig.1A).Infact,infruittreatedwithUV-Cradiation
thedevelopmentofcolorationwasslowerthaninthecontrolfruit,
andthetreatedfruitshowedthefewestsenescencesignals(Fig.2
B,C).ThisfindingisconsistentwithStevensetal.(1998),Maharaj
etal.(1999),Liuetal.(2009)andTiecheretal.(2013)whoalso
foundthat UV-C treatmentled toa reduction in ripening and
delayedtheonsetofredcolorationintomatoes.TheeffectofUV-C
on the development of fruit coloration may be due to its
interferencewithcarotenoids(Table1),whicharethe
predomi-nantpigmentsintomatoes (Stevensetal.,1998;Maharajetal.,
1999;Liuetal.,2009).Liuetal.(2011)reportedachangeinthe
profilecarotenoidsgenesexpressionsintomatoestreatedwith
UV-C.Achangeincolorisoneofthemostobvioustransformationsthat
takesplaceduringtomatofruitripeningandinvolvesthe
ethylene-dependenttransitionofchloroplaststochromoplasts(Opiyo;Ying,
2005;Barsanetal.,2010).Moreover,UV-Ctreatmentmaycause
changesinotherantioxidantpathways,suchastheproductionof
antioxidantenzymes(Erkanetal.,2008)andsynthesisofphenolic
compounds (Charles et al., 2008b) and/or bioactive amines
(Stevens et al., 1998; Maharaj et al., 1999; González-Aguilar etal.,2004;Tiecheretal.,2013).Thesecompoundsmayprevent
thedegradationofchlorophylland/orslowcarotenoiddegradation
(Maharajetal.,1999;Liuetal.,2009;Tiecheretal.,2013).
The effects of UV-C treatment on the levels of compounds
derivedfromplantsecondarymetabolism,previouslyreportedby
several authors (Charles et al., 2008a,b; Erkan et al., 2008;
González-Aguilar et al., 2004; Liu et al., 2009; Pombo et al., 2011;Tiecheretal.,2013)werepartiallyconfirmedbythiswork
(Table 1). The UV-C slows the accumulation of lycopene and
b
-carotene in fruit, which explains the lower intensity of thecharacteristicredcolor(Fig.2B).Whenapplying1-MCPpriorto
UV-C, this physiological response was strengthened (Fig. 2B).
Variations inthe fruitprofile of thesecompounds accordingto
differences in variety, ripening stage, growth, and postharvest
conditionsiswidelyreported(Charlesetal.,2008a,b;Erkanetal.,
2008;Liuetal.,2009;Pomboetal.,2011).UV-Cradiationinduced
the accumulationof at least three of the phenolic compounds
investigated(Table1),which isinagreementwithCharlesetal.
(2008b), who also found higher concentrations of phenolic
compounds,anacceleratedlignificationprocessandtheformation
ofsuberinintomatoestreatedwithUV-C.
Thefactthat UV-Cradiationstimulatedtheaccumulationof
thesecompoundsisinterestingnotonlyforprolongingshelf-life,
but also for increasing plant defenses, and for increasing
potentially bioactive compound levels (Stevens et al., 1998;
González-Aguilaret al.,2004;Charles etal., 2008;Erkanetal., 2008;Tiecheretal.,2013).ItisplausiblethattheERFsinfluenced
by UV-C (Fig.1)control the biosynthesis of these compounds
becausethetomatoisaclimactericfruit,andethyleneisinvolved
inthecontrolof severalof itsbiosyntheticpathways(Cara and
Giovannoni,2008).
Thedelayofsenescencesignals(Fig.2)maybecorrelatetothe
levelsofpolyamines(Table1).Theseresultssupportwhathasbeen
reportedinthepeachbyGonzález-Aguilaretal.,(2004) andby
Maharajetal.(1999)andTiecheretal.(2013)intomatoes.These
authorssuggestedthatbyactingasastressor,UV-Cinitiatesthe
synthesisofpolyaminesthatmaybeinvolvedintheregulationof
ripening.
Becauseethylene can activatedifferenttranscription factors,
including regulators of metabolic pathways involved in fruit
ripening and those related to the stress response, the
T able 1 Levels of caro tenoids, d -to copherol, phenolics, and pol y amines fr om e x ocarps of “ Micr o T om ” tomato fruit 6 d after the UV-C tr eatment. Caro tenoids T ocophero l Phenolics Pol y amines Ly copene (m g k g 1) b -caro tene (m g k g 1) Lut ein + zeaxanthin (mg kg 1) d -to copherol (m g k g 1) Chlor og enic acid (m g k g 1) r -coumaric acid (m g k g 1) q uerce tin (mg kg 1) Putr escine (m g k g 1) Spermidine (mg kg 1) Contr ol 69. 1a * 1 2a 3.6a 1 4.64a 1 92.8c 7 .8a 6.8b 5. 1c 17 .3b 1-MCP + UV-C 20c 6.3b 2.4b 7 .43b 256.9b 9.7b 7 .9b 5 7 .1b 40.2a UV-C 26.6b 8.2b 2.5b 9.67b 36 7 .6a 1 0. 1b 1 9.8a 94.2a 43. 1a * Means with the same lo w er case lett er in the column ar e no t statisticall y differ ent according to the Tuke y’ s test (p # 0.05).
transcriptionalexpressionofERFswasalsoevaluated.UV-Cwas
foundtohavedifferenteffectsontheexpressionofthesegenes
(Fig. 1B–O).Ohme-TakagiandShinshi(1995)characterizedthefirst
fourERFsintobaccodemonstratingthattheyresponddifferently
toethylene.Chenetal.(2008)reportedthatintomatoesERFsmay
be differentially regulated during ripening and in response to
stress.
MostoftheERFsstudiedhere(Sl-ERFA.1,Sl-ERFA.3,Sl-ERFB.1,
Sl-ERFB.2,Sl-ERFB.3,Sl-ERFC.6,Sl-ERFD.1,Sl-ERFD.3,Sl-ERFE.1,
Sl-ERFF.5,Sl-ERFG.2)showedhighertranscriptaccumulationwhen
thetomatoesweretreatedwithUV-Csuggeststhatthesegenesare
strong candidates for explaining the UV-C response, and its
relationshiptoethylene.Thedelayintheripeningprocess,despite
the increase in ethylene production, ACO1 level, and ERFs
transcription level, could be due to activation of metabolic
pathways of antioxidant protection for these ERFs (Liu et al.,
2011;Erkanetal.,2008;Tiecheretal.,2013).Ingeneral,when
1-MCPwas applied prior toUV-C,reduced accumulation of ERFs
transcriptswasobserved,thusconfirmingthattheexpressionof
thesetranscriptionfactorscanberegulated byethylene(Zhang
etal.,2004;Pirrelloetal.,2006;Wangetal.,2007).Moreoverthis
datasuggeststhatregulationofERFtranscriptsbyUV-Cisethylene
dependent.
Inthiswork,theclassificationproposedbyPirrelloetal.(2012),
whoclassifiedtomatoERFsinto8sub-classes(A,B,C,D,E,F,G,H)
wasused;however,membersofsub-classHwerenotevaluated.Of
the14ERFsassessedinthepresentstudy,6(Sl-ERFA.1,Sl-ERFB.1,
Sl-ERFB.2,Sl-ERFD.1, Sl-ERFD.3andSl-ERFE.4) wereisolated and
characterizedbyPirrelloetal.(2012),whowasthefirsttorelate
theseERFstoothertypesofplantstress.
Zhouetal.(1997),whostudiedERFsSl-ERFA.3,Sl-ERFC.6and
Sl-ERFG.2,(describedinhisworkaspti4,pti5andpti6,respectively),
reportedtheabilityoftheseERFstobindspecificregionsofEREBR’s
(ethylene-responsiveelement-bindingproteins),alsoknownasthe
GCC-boxof PR-genes,increasingthetolerance ofplantstobiotic
stress.TheregulationoftheseERFgenesthroughphosphorylation
mayalsoinfluencetheinteractionofthesetranscriptionfactors
withtheGCC-boxregionsofPR-genes(Guetal.,2000;Xuetal.,
2008, 2011). In the present study, these ERFs were strongly
influenced by the abiotic stress generated by UV-C treatment,
showingasignificantincreaseintheaccumulationoftranscripts,
especially Sl-ERFC.6,which showed anapproximately 250-fold
increaseinexpressionrelativetocontrolfruit.Thisindicatesthat
theinductionofERFsmaycontributetotheacquisitionoftolerance
toadverseconditions(Heetal.,2001;Guetal.,2002;Chenetal.,
2008).Liuetal.(2011)alsoreportedasignificantincreaseinthe
expression of these three genes, especially Sl-ERF C.6, which
correspondstopti5.Byover-expressingSl-ERFC.6intomatoes,He
etal.(2001)reportedincreasedlevelsofGluBandcatalasegene
transcripts,whichareassociatedwithresistancetodiseasessuch
asPseudomonassyringae pv. Tomato.Likewise,Guet al. (2002),
observedthatinArabidopsisthalianaplantstheERFsSl-ERFA.3,
Sl-ERFC.6andSl-ERFG.2interact withtheGCC-boxregions of
PR-genes,resultinginpathogendefense.Chenetal.(2008)reported
thatwaterstressandlowtemperaturesreducethelevelsofSl-ERF
A.3transcripts.However,mechanicaldamagealsoincreasedthe
expressionofthisgene,whichsuggeststhattheremaybedifferent
regulatorymechanismsdependingonthestimulus.
The increase of transcript accumulation ofSl-ERF B.3agrees
withtheresultspublishedbyLiuetal.(2011),whichshowedthe
relationshipofthisgenetotheripeningtomatoesprocessand,Liu
etal.(2014)thatalsoreporteddelayoftheonsetofripeningcaused
forover-expression ofSl-ERF B.3-SRDX(a climactericdominant
repressorreversion).
Results from the present study on Sl-ERF E.1, previously
characterized by Tournier et al. (2003) as LeERF2, reveal the
strongimpactofethyleneonERFexpression,inagreementwith
theresultsofPirrelloetal.(2006),Liuetal.(2011),Zhangetal.
(2009) and Zhang and Huang (2010) who also related the
expression of this transcriptionfactor tothe hormoneethylene
intomatoandtobaccoplants.
Sl-ERF E.2 and Sl-ERF E.4 showed significantly reduced
accumulation of transcripts after UV-C treatment. Zhang et al.
(2004), who described Sl-ERF E.2 as JERF1, demonstrated that
expressionofthis ERFintomatoeswasinducedbyanumberof
factors: ethylene,methyl jasmonate(MeJA), abscisic acid(ABA)
and salt treatment.In rice, plants over-expressing JERF1 show
increaseddroughttolerance(Zhangetal.,2010).Incontrast,the
resultspresentedhereinsuggestthatSl-ERFE.3isnotsignificantly
involvedin theresponse toUV-C,although Wang etal. (2004)
reportedthatSl-ERFE.3respondstojasmonicacid,ethylene,cold,
saltstressandabscisicacidbybindingtoGCC-boxandDREregions
oftargetgenes.
Inthisstudy,Sl-ERFF.5alsoshowedincreasedtranscriptionasa
resultofUV-Ctreatment.Chenetal.(2008),studyingSl-ERFF.5
(whichtheyrefertoasLeERF3b),relatedtheexpressionofthisERF
tostressgenerated bydroughtand low temperatures.This ERF
possessesaamphiphilicrepressorbindingdomain(EAR)(Xuetal.,
2008;Panetal.,2010;Pirrelloetal.,2012).Panetal.(2010)deleted
theEARofSl-ERFF.5(referredtoasSl-ERF3in theirstudy)and
observed the induction of PR-gene expression, with increased
tolerancetosaltstressandreducedlipidperoxidationinRalstonia
solanacearum.
Herein, arelationshipbetweenUV-Ctreatment andripening
delay was established, and correlated with changes in 13 ERF
transcriptsevaluatedduringpostharvesttreatment.Theethylene
actioninresponsetoUV-Ctreatmentwasconfirmedwith1-MCP
applicationbeforeUV-C.ItisclearthatalthoughUV-Cpromotesan
increaseinethyleneproduction,theconcomitantincreasesinthe
ACO1expressionprofile, andvirtually allof theERFsevaluated,
resultinextendedfruitpreservation.Thedelayinfruitripening
maybecausedbytheactivationofERFsthatcouldactasregulators
ofmetabolicpathwaysduringripening.However,thishypothesis
needstobebettertested.
Acknowledgements
WethankCapes-Cofebubforthestudyscholarshipandresearch
funding.ResearchfundingwasalsoprovidedbyCNPqandFapergs.
We alsothankDr. RachelKopec forvaluable help withEnglish
revisions. References
Barry,C.S.,Blume,B.,Bouzayen,M.,Cooper,W.,Hamilton,A.J.,Grierson,D.,1996. Differentialexpressionofthe1-aminocyclopropane-1-carboxylateoxidase genefamilyoftomato.PlantJ.9,525–535.
Barsan,C.,Sanchez-Bel,P.,Rombaldi,C.,Egea,I.,Rossignol,M.,Kuntz,M.,etal.,2010. Characteristicsofthetomatochromoplastrevealedbyproteomicanalysis.J. Exp.Bot.61,2413–2431.
Bapat,V.A.,Trivedi,P.K.,Ghosh,A.,Sane,V.A.,Ganapathi,T.R.,Nath,P.,2010. Ripeningoffleshyfruit:molecularinsightandtheroleofethylene.Biotechnol. Adv.28,94–107.
Bu,J.,Yu,Y.,Aisikaer,G.,Ying,T.,2013.PostharvestUV-Cirradiationinhibitsthe productionofethyleneandtheactivityofcellwall-degradingenzymesduring softeningoftomato(LycopersiconesculentumL.)fruit.PostharvestBiol.Technol. 86,337–345.
Cara,B.,Giovannoni,J.J.,2008.Molecularbiologyofethyleneduringtomatofruit developmentandmaturation.PlantSci.175,106–113.
Charles,M.T.,Mercier,J.,Makhlouf,J.,Arul,J.,2008a.Physiologicalbasisof UV-C-inducedresistancetoBotrytiscinereaintomatofruitI:roleofpre-and post-challengeaccumulationofthephytoalexin-rishitin.PostharvestBiol.Technol. 47,10–20.
Charles,M.T.,Goulet,A.,Arul,J.,2008b.PhysiologicalbasisofUV-Cinduced resistancetoBotrytiscinereaintomatofruitIV:biochemicalmodificationof structuralbarriers.PostharvestBiol.Technol.47,41–53.
Chen,G.,Hua,Z.,Grierson,D.,2008.Differentialregulationoftomatoethylene responsivefactorLeERF3b,aputativerepressor,andtheactivatorPti4in ripeningmutantsandinresponsetoenvironmentalstresses.J.PlantPhysiol. 165,662–670.
Erkan,M.,Wang,S.Y.,Wang,C.Y.,2008.EffectofUVtreatmentonantioxidant capacity:antioxidantenzymeanddecayinstrawberriesfruit.PostharvestBiol. Technol.48,163–171.
Girardi,C.L.,Rombaldi,C.V.,DalCero,J.,Nobile,P.M.,Laurens,F.,Bouzayen,M.,etal., 2013.Genome-wideanalysisoftheAP2/ERFsuperfamilyinappleand transcriptionalevidenceofERFinvolvementinscabpathogenesis.Sci.Hortic 151,112–121.
González-Aguilar,G.A.,Wang,C.Y.,Buta,J.G.,2004.UV-Cirradiationreduces breakdownandchillinginjuryofpeachesduringcoldstorage.J.Sci.FoodAgric. 84,415–422.
Gu,Y.Q.,Yang,C.,Thara,V.K.,Zhou,J.,Martin,G.B.,2000.Pti4isinducedbyethylene andsalicylicacidanditsproductisphosphorylatedbythePtokinase.PlantCell 12,771–785.
Gu,Y.Q.,Wildermuth,M.C.,Chakravarthy,S.,Loh,Y.T.,Yang,C.,He,X.,etal.,2002. TomatotranscriptionfactorsPti4,Pti5,andPti6activatedefenseresponseswhen expressedinArabidopsis.PlantCell14,817–831.
Gutterson,N.,Reuber,T.L.,2004.RegulationofdiseaseresistancepathwaysbyAP2/ ERFtranscriptionfactors.Curr.Opin.PlantBiol.7,465–471.
Häkkinen,S.H.,Kärenlampi,S.O.,Heinonen,M.,Mykkänen,H.M.,Törrönen,A.R., 1998.HPLCMethodforscreeningofflavonoidsandphenolicacidsinberries.Sci FoodAgric.77,543–551.
He,P.,Warren,R.F.,Zhao,T.,Shan,L.,Zhu,L.,Tang,X.,etal.,2001.Overexpressionof Pti5intomatopotentiatespathogen-induceddefensegeneexpressionand enhancesdiseaseresistancetoPseudomonassyringaepv.tomato.Mol.Plant MicrobeInteract14,1453–1457.
Hoeberichts,F.A.,VanDerPlas,L.H.W.,Woltering,E.J.,2002.Ethyleneperceptionis requiredfortheexpressionoftomatoripening-relatedgenesandassociated physiologicalchangesevenatadvancedstagesofripening.PostharvestBiol. Technol.26,125–133.
Huang,Z.,Zhang,Z.,Zhang,X.,Zhang,H.,Huang,D.,Huang,R.,2004.TomatoTERF1 modulatesethyleneresponseandenhancesosmoticstresstoleranceby activatingexpressionofdownstreamgenes.FEBSLett.573,110–116.
Liu,L.H.,Zabaras,D.,Bennett,L.E.,Aguas,P.,Woonton,B.W.,2009.EffectsofUV-C, redlightandsunlightonthecarotenoidcontentandphysicalqualitiesof tomatoesduringpost-harveststorage.FoodChem.115,495–500.
Liu,C.,Cai,L.,Han,X.,Ying,T.,2011.TemporaryeffectofpostharvestUV-Cirradiation ongeneexpressionprofileintomatofruit.Gene486,56–64.
Liu,M.,Diretto,G.,Pirrello,J.,Roustan,J.P.,Li,Z.,Giuliano,G.,etal.,2014.The chimericrepressorversionofanethyleneresponsefactor(ERF)familymember, Sl-ERF.B3,showscontrastingeffectsontomatofruitripening.NewPhytol.203 (1),206–218.
Livak,K.J.L.,Schmittgen,T.D.,2001.Analysisofrelativegeneexpressiondatausing real-timequantitativePCRandthe2DDCtmethod.Methods25,402–408.
Maharaj,R.,Arul,J.,Nadeau,P.,1999.Effectofphotochemicaltreatmentinthe preservationoffreshtomato(LycopersiconesculentumcvCapello)bydelaying senescence.PostharvestBiol.Technol.15,13–23.
Maharaj,R.,Arul,J.,Nadeau,P.,2014.UV-Cirradiationeffectsonlevelsofenzymic andnon-enzymicphytochemicalsintomato.Innov.FoodSci.Emerg.21,99–106.
Obande,M.A.,Tucker,G.A.,Shama,G.,2011.EffectofpreharvestUV-Ctreatmentof tomatoes(SolanumlycopersiconMill.)onripeningandpathogenresistance. PostharvestBiol.Technol.62,188–192.
Ohme-Takagi,M.,Shinshi,H.,1995.Ethylene-lnducibleDNAbindingproteinsthat lnteractwithanethylene-responsiveelement.PlantCell7,173–182.
Opiyo,A.M.,Ying,T.J.,2005.Theeffectsof1-methylcyclopropenetreatmentonthe shelflifeandqualityofcherrytomato(Lycopersiconesculentumvarcerasiforme) fruit.J.FoodSci.Technol.40,665–673.
Pan,I.C.,Li,C.W.,Su,R.C.,Cheng,C.P.,Lin,C.S.,Chan,M.T.,2010.Ectopicexpressionof anEARmotifdeletionmutantofSlERF3enhancestolerancetosaltstressand Ralstoniasolanacearumintomato.Planta232,1075–1086.
Pirrello,J.,Jaimes-Miranda,F.,Sanchez-Ballesta,M.T.,Tournier,B.,Khalil-Ahmad,Q., Regad,F.,etal.,2006.Sl-ERF2:atomatoethyleneresponsefactorinvolvedin ethyleneresponseandseedgermination.PlantCellPhysiol.47,1195–1205.
Pirrello,J.,Prasad,B.N.,Zhang,W.,Chen,K.,Mila,I.,Zouine,M.,etal.,2012. Functionalanalysisandbindingaffinityoftomatoethyleneresponsefactors provideinsightonthemolecularbasesofplantdifferentialresponsesto ethylene.BMCPlantBiol.12,190.
Pombo,M.A.,Rosli,H.G.,Martíneza,G.A.,Civello,P.M.,2011.UV-Ctreatmentaffects theexpressionandactivityofdefensegenesinstrawberryfruit
(Fragaria"ananassaDuch.).PostharvestBiol.Technol.59,94–102.
Rodriguez-Amaya,D.,2001.AGuidetoCarotenoidAnalysisinFoods.ILSIPress, Washington,pp.64.
SasInstitute,2002.StatisticalAnalysisSystem9.1forWindows.SASInstituteInc, Cary,NC.
Singh,K.B.,Foley,R.C.,Oñate-Sánchez,L.,2002.Transcriptionfactorsinplant defenseandstressresponses.Curr.Opin.PlantBiol.5,430–436.
Stevens,C.,Liu,J.,Khan,V.A.,Lu,J.Y.,Wilson,C.L.,Igwegbe,E.C.K.,etal.,1998. ApplicationofhormeticUV-CfordelayedripeningandreductionofRhizopus softrotintomatoes:theeffectoftomatineonstoragerotdevelopment.J. Phytopathol.146,211–221.
Syamaladevi,R.M.,Lupien,S.L.,Bhunia,K.,Sablani,S.S.,Dugan,F.,Rasco,B.,etal., 2014.UV-ClightinactivationkineticsofPenicilliumexpansumonpearsurfaces: influenceonphysicochemicalandsensoryqualityduringstorage.Postharvest Biol.Technol.87,27–32.
Tiecher,A.,Paula,L.A.,deChaves,F.C.,Rombaldi,C.V.,2013.UV-Ceffectonethylene, polyaminesandtheregulationoftomatofruitripening.PostharvestBiol Technol.86,230–239.
Tournier,B.,Sanchez-Ballesta,M.T.,Jones,B.,Pesquet,E.,Regad,F.,Latche,A.,etal., 2003.NewmembersofthetomatoERFfamilyshowspecificexpressionpattern anddiverseDNA-bindingcapacitytotheGCCboxelement.FEBSLett.550,149– 154.
VandePoel,B.,Bulens,I.,Markoula,A.,Hertog,M.L.,Dreesen,R.,Wirtz,M.,etal., 2012.Targetedsystemsbiologyprofilingoftomatofruitrevealscoordinationof theYangcycleandadistinctregulationofethylenebiosynthesisduring postclimactericripening.PlantPhysiol.160,1498–1514.
Vieira,S.M.,Theodoro,K.H.,Glória,M.B.,2007.Profileandlevelsofbioactiveamines inorangejuiceandorangesoftdrink.FoodChem.100,895–903.
Wang,H.,Huang,Z.,Chen,Q.,Zhang,Z.,Zhang,H.,Wu,Y.,etal.,2004.Ectopic overexpressionoftomatoJERF3intobaccoactivatesdownstreamgene expressionandenhancessalttolerance.PlantMol.Biol.55,183–192.
Wang,A.,Tan,D.,Takahashi,A.,Li,T.Z.,Harada,T.,2007.MdERFs,twoethylene responsefactorsinvolvedinapplefruitripening.J.Exp.Bot.58,3743–3748.
Yin,X.-R.,Allan,A.C.,Xu,Q.,Burdon,J.,Dejnoprat,S.,Chen,K.-S.,etal.,2012. DifferentialexpressionofkiwifruitERFgenesinresponsetopostharvestabiotic stress.PostharvestBiolTechnol.66,1–7.
Xu,Z.S.,Xia,L.Q.,Chen,M.,Cheng,X.G.,Zhang,R.Y.,Li,L.C.,etal.,2007.Isolationand molecularcharacterizationoftheTriticumaestivumL.ethylene-responsive factor1(TaERF1)thatincreasesmultiplestresstolerance.PlantMol.Biol.65, 719–732.
Xu,Z.S.,Chen,M.,Li,L.C.,Ma,Y.Z.,2008.FunctionsoftheERFtranscriptionfactor familyinplants.Botany86,969–977.
Xu,Z.S.,Chen,M.,Li,L.C.,Ma,Y.Z.,2011.FunctionsandapplicationoftheAP2/ERF transcriptionfactorfamilyincropimprovement.J.Integr.PlantBiol.53,570–585.
Zhang,H.,Huang,Z.,Xie,B.,Chen,Q.,Tian,X.,Zhang,X.,etal.,2004.The ethylene-jasmonate-,abscisicacid-andNaClresponsivetomatotranscriptionfactor JERF1modulatesexpressionofGCCboxcontaininggenesandsalttolerancein tobacco.Planta220,262–270.
Zhang,Z.,Zhang,H.,Quan,R.,Wang,X.-C.,Huang,R.,2009.Transcriptional regulationoftheethyleneresponsefactorLeERF2intheexpressionofethylene biosynthesisgenescontrolsethyleneproductionintomatoandtobacco.Plant Physiol.150,365–377.
Zhang,Z.,Li,F.,Li,D.,Zhang,H.,Huang,R.,2010.Expressionofethyleneresponse factorJERF1inriceimprovestolerancetodrought.Planta232,765–774.
Zhang,Z.,Huang,R.,2010.Enhancedtolerancetofreezingintobaccoandtomato overexpressingtranscriptionfactorTERF2/LeERF2ismodulatedbyethylene biosynthesis.PlantMol.Biol.73,241–249.
Zhou,J.,Tang,X.,Martin,G.B.,1997.ThePtokinaseconferringresistancetotomato bacterialspeckdiseaseinteractswithproteinsthatbindacis-elementof pathogenesisrelatedgenes.EMBOJ.16,3207–3218.