© Jennifer Larouche, 2019
Processing methods for the black soldier fly (
Hermetia
illucens
) larvae
: From feed withdrawal periods to killing
methods
Mémoire
Jennifer Larouche
Maîtrise en sciences animales - avec mémoire
Maître ès sciences (M. Sc.)
Processing methods for the black soldier fly (Hermetia
illucens) larvae
From feed withdrawal periods to killing methods
Mémoire
Sciences animales
Jennifer Larouche
Sous la direction de :
Résumé
Les larves de mouches soldats noires représentent un ingrédient alternatif prometteur pour le bétail, mais les étapes de transformation peuvent affecter leur qualité. Les périodes de vidange gastrique utilisées pour évacuer les excréments du tractus digestif afin de réduire sa charge microbienne, et les méthodes d’abattage sont variables et peu documentées. Ce projet vise à optimiser la vidange gastrique et l’abattage des larves pour en maximiser la qualité. En effet, un jeûne prolongé et une méthode d’abattage inadéquate pourraient altérer la composition et la microbiologie du produit. Le temps d’évacuation du tractus digestif des larves alimentées de Gainesville a été déterminé en suivant l’excrétion des fèces aux douze heures. Puis, l’impact du temps de jeûne sur la composition et la contamination ont été mesurés quotidiennement pendant quatre jours. Également, les effets de dix méthodes d’abattage sur la composition, la microbiologie et la coloration ont été comparés : ébouillantage (40 s), dessiccation (60 °C, 30 min), congélation (-20 °C et -40 °C, 1 h; azote liquide, 40 s),
hautes pressions hydrostatiques (3 min, 600 MPa), broyage (2 min) et asphyxie (CO2 et conditionnement sous
vide, 120 h; N2, 144 h). Bien que le temps d’évacuation du tractus digestif médian fût de 72 h, un jeûne de 96 h
n’a pas permis de réduire la contamination. Certaines méthodes d’abattage ont affecté le pH, la stabilité de la couleur ainsi que la charge microbienne. De plus, ébouillantage, asphyxie et dessiccation ont affecté la composition proximale et l’oxydation des lipides. Malgré l’incapacité de la vidange gastrique à réduire la contamination des larves, l’ébouillantage apparait comme la méthode la plus appropriée en réduisant la charge microbienne et l’humidité tout en minimisant l’oxydation des lipides. Nous proposons donc un protocole pour abattre les larves répondant aux exigences réglementaires canadiennes en matière de transformation des insectes.
Abstract
Black soldier fly (BSF) larvae represents a promising alternative ingredient for animal feed, but post-production processing can affect their quality. Feed withdrawal periods (FWP) used to evacuate fecal matter from the gastrointestinal tract, reducing the microbial load (ML), and killing methods are variable and poorly documented. This project aims to optimize the FWP and killing methods of BSF larvae to maximize product quality. Indeed, a prolonged FWP and an inappropriate killing method could alter larvae composition and ML. The gastrointestinal evacuation time (GET) of BSF larvae fed on coloured Gainesville diet was determined by following frass excretion every 12 h for 108 h. Then, FWP impact on the proximate composition and ML was measured daily over four days. Finally, the effects on the chemical composition, ML and colour of 10 killing methods were compared, i.e., blanching (B = 40 s), desiccation (D = 60 °C, 30 min), freezing (F20 = − 20 °C, 1 h; F40 = − 40 °C, 1 h; N = liquid nitrogen, 40 s), high hydrostatic pressure (HHP = 3 min, 600 MPa), grinding
(G = 2 min) and asphyxiation (CO2 = 120 h; N2 = 144 h ; vacuum conditioning, V = 120 h). Although, the median
GET was 72 h, a 96 h FWP did not reduce larvae ML. Certain killing methods affected the pH (B, asphyxiation), total moisture (B, asphyxiation and D), ash (B), lipid content (asphyxiation) and lipid oxidation (B, asphyxiation and D), as well as the colour stability during freeze-drying. FWP were ineffective in reducing the ML. Blanching appeared as the most appropriate method since it minimizes lipid oxidation, reduces ML and total moisture (78.1 ± 1.0%). Our studies propose a standardize protocol to kill BSF that meet the Canadian regulatory requirements of the insect production and processing industry.
Table of contents
Résumé ... ii
Abstract... iii
Table of contents ... iv
Table of figures ... vi
Table of tables ... vii
List of abbreviations and acronyms ... viii
Remerciements... xi
Avant-propos ... xii
Introduction ... 1
Chapter 1. Literature review ... 2
1.1 Edible insects ... 3
1.2 Black soldier fly (Hermetia illucens) ... 4
1.2.1 Life cycle of the black soldier fly ... 4
1.2.2 Proximate composition of the black soldier fly ... 5
1.2.3 Fatty acid profile ... 5
1.2.4 Microbiota of the black soldier fly ... 5
1.3 Insect processing ... 9
1.3.1 Harvesting ... 9
1.3.2 Post-harvest processing steps ... 9
1.3.3 Killing ... 10
1.3.4 Decontamination... 13
1.3.5 Drying ... 16
1.3.6 Other processing steps ... 17
1.4 Insect quality ... 18
1.4.1 Proximal composition (macronutrient metabolism) ... 19
1.4.2 Lipid quality: peroxidation ... 20
1.4.3 Protein quality: digestibility ... 21
1.4.4 Microbial safety... 22
1.4.5 Colour stability ... 23
1.5 Aim and outline ... 27
References ... 29
Chapter 2. Effect of feed withdrawal periods on the proximate composition and microbial load of black
soldier fly (Hermetia illucens) larvae ... 36
2.1 Résumé ... 37
2.2 Abstract ... 37
2.3 Introduction ... 38
2.4 Materials and methods ... 39
2.4.1 Gastrointestinal evacuation time ... 39
2.4.2 Impact of feed withdrawal period ... 40
2.4.3 Proximate composition and pH ... 40
2.4.4 Microbial analysis ... 41
2.4.5 Statistical analysis ... 41
2.5 Results... 41
2.5.1 Gastrointestinal evacuation time ... 41
2.6 Discussion ... 45
2.6.1 Gastrointestinal evacuation time ... 45
2.6.2 Effect of starvation on the initiation of pupariation. ... 46
2.6.3 Effects of a prolonged feeding period on the larvae ... 46
2.6.4 Effects of a feed withdrawal period on the larvae ... 46
2.7 Conclusion ... 47
References ... 49
Chapter 3. Effects of killing methods on lipid oxidation, colour and microbial load of black soldier fly
(Hermetia illucens) larvae ... 51
3.1 Résumé ... 52
3.2 Abstract ... 52
3.3 Introduction ... 53
3.4 Materials and Methods ... 54
3.4.1 Biological material ... 54
3.4.2 Killing methods ... 55
3.4.3 Analysis ... 56
3.4.4 Statistical analysis ... 58
3.5 Results... 58
3.5.1 Chemical composition and pH ... 58
3.5.2 Lipid oxidation... 59
3.5.3 Larval colour ... 59
3.5.4 Microbial analysis ... 61
3.6 Discussion ... 64
3.6.1 Chemical composition ... 64
3.6.2 Colour ... 65
3.6.3 Microbiology ... 65
3.6.4 Heating ... 66
3.6.5 Freezing ... 67
3.6.6 Asphyxiation ... 68
3.6.7 Mechanical disruption ... 68
3.7 Conclusions ... 69
Appendix A ... 71
References ... 72
General conclusions ... 76
Bibliography ... 78
Table of figures
Figure 1.1 Life cycle of the black soldier fly ... 4
Figure 1.2 High hydrostatic pressure batch system ... 15
Figure 1.3 Atmospheric direct and indirect cold plasma process ... 16
Figure 1.4 Tyrosine related substrates, reactions and enzymes involved in enzymatic browning,
together with subsequent non-enzymatic reactions in the black soldier fly larvae. ... 24
Figure 1.5 Representations of reactions involved in BSF browning at different pH. The colour behind
the structures represents the colour shade induce by the reaction... 25
Figure 2.1 Black soldier fly larvae excreting coloured Gainesville diet on a moist Whatman filter ... 40
Figure 2.2 Gastrointestinal evacuation time and pupariation time of starving and isolated 12-d-old
black soldier fly larvae ... 42
Figure 2.3 Effects of feeding and starving on individual black soldier fly larvae composition: moisture;
lipids; proteins; carbohydrates ... 44
Figure 3.1 Colour analysis of thawed and freeze-dried and granulated BSF larvae after different
killing methods: lightness; colour change between thawed and freeze-dried and granulated larvae;
chroma; hue angle ... 60
Figure 3.2 Freeze-dried and granulated BSF larvae killed by different methods: desiccation;
Table of tables
Table 1.1 Proximal composition, polyunsaturated fatty acids, minerals content and production
characteristics of the black soldier fly larvae and prepupae, mealworms larvae and domestic cricket. 3
Table 1.2 Fatty acid profile of the black soldier fly larvae and prepupae ... 5
Table 1.3 Microbial load of black soldier fly larvae under various rearing conditions ... 8
Table 1.4 Killing methods used in industry according to Erens et al., 2012 ... 11
Table 1.5 Microbial load reduction of decontamination methods applied to insects ... 14
Table 1.6 Impact of processing methods on protein quality of insects and shrimps ... 22
Table 1.7 Microbial safety criteria for ready-to-eat food according to Health Canada ... 23
Table 1.8 Impacts of processing technologies on polyphenol oxidase activity of crustaceans... 26
Table 2.1 Proximal composition of fed and starved black soldier fly larvae at 27 °C ... 43
Table 2.2 Microbial load of fed and starved 10 d-old black soldier fly larvae at 27 °C ... 44
Table 3.1 Chemical composition, primary and secondary lipid oxidation levels, and pH of black
soldier fly larvae killed by different methods ... 59
Table 3.2 Microbial load of thawed black soldier fly larvae killed by different methods ... 62
Table A1. Colour measures, colour intensity, hue angle and colour change while drying of thawed
and freeze-dried and granulated BSF larvae killed by different methods. ... 71
List of abbreviations and acronyms
AKH Adipokinetic hormone
ANOVA Analysis of Variance
AOAC Association of Official Analytical Chemists
AOCS American Oil Chemists’ Society
Aw Water activity
B Blanching
BSF Black soldier fly
CFU Colony forming unit
CHP Cumene hydroperoxide
CO2 Carbon dioxide
CPS Coagulase positive Staphylococci
C-F-C Cetrimide, fucidin and cephalosporin
C* Chroma D Desiccation DM Dry matter E Enterobacteriaceae EE Ether extract F20 Freezing at -20 ºC F40 Freezing at -40 ºC
FAO Food and Agriculture Organization of the United Nations
FDG Freeze-dried and granulated
Fe2+ Ferrous iron
Fe3+ Ferric iron
FOX Ferrous oxidation-xylenol orange assay
FWP Feed withdrawal period
G Grinding
GET Gastrointestinal evacuation time
GRIPHA Groupe de Recherche Intégré en Physiologie et Sciences Animales, Université Laval
h Hue angle
HHP High hydrostatic pressure
LAB Lactic acid bacteria
LH Lipid hemolytic
Log logarithms to the base 10
LOOH Lipid hydroperoxides
LOO· Peroxide radical
LO· Alkoxy radical
LTA Laboratoire de Transformation des Aliments, Université Laval
L· Lipid radical
L* Lightness
MDA Malondialdehyde
ML Microbial load
MRS deMan, Rogosa and Sharp agar
n. d. Not detected
N Liquid nitrogen
N2 Nitrogen
O2 Oxygen
PCA Plate count agar
pH Potential hydrogen
ROS Reactive oxygen species
spp. Species
TBA Thiobarbituric acid
TBARS Thiobarbituric acid reactive substance
TI Total inactivation
TVC Total aerobic viable count
V Vacuum packaging
VRBA Violet Red Bile Agar
VRBG Violet Red Bile Glucose Agar
À Philippe, my Sun and Stars.
À mon grand-père Raynald, dont la curiosité
Remerciements
Au terme de ma maîtrise en sciences animales, j’aimerais souligner les personnes qui m’ont soutenu et appuyé, et qui m’ont encouragé à me dépasser pour pouvoir vous offrir ce mémoire.
Je tiens d’abord à remercier Prof. Grant W. Vandenberg, mon directeur de recherche, qui m’a toujours encouragé à laisser libre place à ma créativité dans la mise en place de mon projet et l’élaboration de mes hypothèses et théories, me permettant même de valider toutes celles qui me semblaient les plus passionnantes. Son écoute, sa disponibilité, ses conseils et surtout sa confiance m’ont permis de progresser à une vitesse que je ne me croyais pas capable. La seconde personne à l’honneur est Dr Marie-Hélène Deschamps, la professionnelle de recherche au laboratoire, mais surtout mon modèle qui a su m’aider à garder le cap sur mes objectifs. Sa franchise, son jugement et son empathie font d’elle une personne qui nous motive à nous améliorer et à attendre la critique avec confiance.
Je me dois également de remercier le programme Innov’Action agroalimentaire, "un programme issu de l’accord cultivons l’avenir 2 conclu entre le ministre de l’Agriculture, des Pêcheries et de l’Alimentation du Québec, et Agriculture et Agroalimentaire Canada", pour le soutien financier du projet. J’aimerais également remercier les organismes subventionnaires m’ayant accordé des bourses d’études me permettant de me concentrer sur mon projet, le Conseil de recherches en sciences naturelles et en génie du Canada et le Fonds de recherche du Québec – Nature et technologie.
Je tiens également à remercier toutes les personnes qui ont contribué de près ou de loin à ce projet, que ce soit par une aide technique ou un simple conseil ou encouragement : Prof. Linda Saucier, Prof. Alain Doyen, Yolaine Lebeuf, Dominic Gagné, l’ensemble des étudiants du laboratoire Vandenberg, le personnel technique du Groupe de recherche en physiologie animale (GRIPHA) et l’équipe du Laboratoire de recherche en sciences aquatique (LARSA).
Merci aux membres de ma famille qui ont su m’encourager à persévérer en célébrant toutes les petites victoires que j’ai connues lors de mon parcours. Un merci particulier à mes parents, Nathalie Verreault et Guy Larouche, mon frère et ma sœur, Eddy et Véronick Larouche, à mes grands-parents, à ma belle-famille, les Clavet, et à mes amis qui ont toujours été présents pour moi.
Finalement, mais non le moindre, un merci particulier à mon amoureux, Philippe Clavet, qui m’a supporté dans ma décision de poursuivre mes études et lors des défis qui ont suivi. Ta présence réconfortante, ta joie de vivre et ton énergie contagieuse m’ont encouragé à me lever chaque matin, les yeux rivés vers mon objectif et prête à affronter tous les obstacles se trouvant sur ma route. Tu es le meilleur.
Avant-propos
Ce mémoire présente les résultats de deux études visant à optimiser le conditionnement préabattage et l’abattage des larves de mouches soldats noires, une source de protéines alternatives pour l’alimentation humaine et animale. Ces travaux ont permis de proposer un protocole optimisé pour la transformation des insectes, de la vidange gastrique jusqu’à l’abattage.
Les travaux réalisés dans le cadre de ce mémoire et portant sur l’optimisation de la vidange gastrique feront prochainement l’objet d’une courte communication scientifique dont je serai l’auteur principal accompagné de M.-H. Deschamps, Y. Lebeuf, L. Saucier, M. Cissé et G.W. Vandenberg.
De plus, les résultats obtenus lors de l’optimisation des méthodes d’abattage ont conduit à la publication d’un article scientifique, avec comité de lecture, dans le journal Animals en avril 2019 intitulé « Effects of Killing
Methods on Lipid Oxidation, Colour and Microbial Load of Black Soldier Fly (Hermetia illucens) Larvae »1.
1 Larouche, J.; Deschamps, M.-H.; Saucier, L.; Lebeuf, Y.; Doyen, A.; Vandenberg, G.W. Effects of killing methods on lipid
Introduction
The Food and Agriculture Organization of the United Nations (FAO) estimates that food production will need to increase by 50% to sustain projected 2050 global population growth [1]. Furthermore, nearly a third of the comestible food currently produced is wasted (~ 1.3 GT/year) and diverted to landfills and other waste management options [2]. In North America, this represents nearly 300 kg of high-value organic matter per capita per year [2]. Since farming conventional livestock has important impacts on the environment [3], several studies have focused on the development of alternative protein sources to support future food and feed demand [4]. Insects are particularly interesting due to their high nutritional quality and bioconversion rate when fed on waste organic residues, their short life cycle as well as low water and space requirements [5-6]. The black soldier fly (BSF) larvae, Hermetia illucens (Diptera: Stratiomyidae), a saprophagous insect, stands out from other edible species by its higher ability to convert residual organic matter into a high-quality protein and lipid source, and as a soil amendment [7]. Since the production of insects on an industrial scale is quite recent, it is necessary to establish standardized production and processing methods to ensure the quality and safety of insect products. The next chapter will focus on the edible insect species, insects processing methods and their impact on the quality of the product. Therefore, it will summarize earlier research on the studied field while emphasing on the quality of the product and recommended threshold. The second chapter describes the effects of a feed withdrawal period on the product quality while the third one compares ten killing methods and their effects on the product quality.
1.1 Edible insects
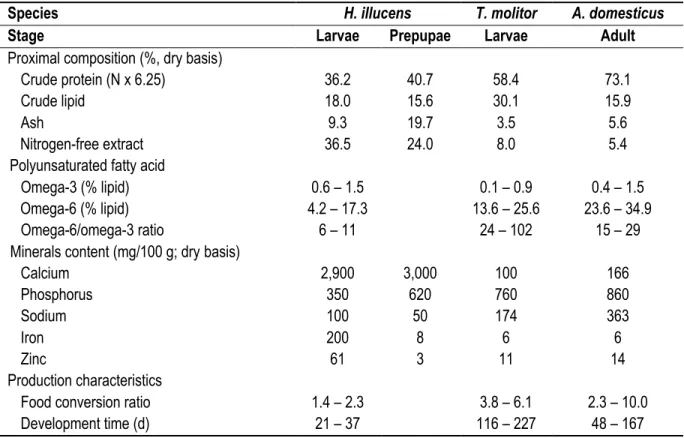
Among the edible insect species, the domestic cricket (Acheta domesticus), the mealworm (Tenebrio molitor) and the BSF are the most often farmed [8]. The BSF appears very promising for large scale rearing systems because it possesses the shortest development time, the lowest food conversion ratio (i.e., kg of feed needed to produce 1 kg of biomass) and offers a great nutritional quality as shown in Table 1. In human nutrition, polyunsaturated fatty acids contribute in the prevention of inflammatory-related disease including, among others, arthritis, asthma and cardiovascular heart disease and thus should be part of the diet [9]. Even if the BSF contains less omega-6 than mealworms and crickets, its n-6/n-3 ratio is closer to the recommended value for human consumption [10]. Furthermore, feeding BSF larvae for only 3 h with 40% fish meal inclusion, allows to reduce this ratio to 2.8 while increasing the total unsaturated fatty acid content by 5% [11]. Furthermore, the BSF possesses two to four times more minerals than mealworms and crickets. Since they are required in all animals feed, it could reduce the need for supplementation [12]. Indeed, on a dry basis, BSF larvae contains 26 times more calcium than crickets and mealworms, at least twice less sodium and fourfold more iron [13]. Finally, the BSF has high-quality protein since their essential amino acid profile corresponds to fish meal requirements [14].
Table 1.1 Proximal composition, polyunsaturated fatty acids, minerals content and production
characteristics of the black soldier fly (Hermetia illucens) larvae and prepupae, mealworms (Tenebrio
molitor) larvae and domestic cricket (Acheta domesticus) [13-17].
Species H. illucens T. molitor A. domesticus
Stage Larvae Prepupae Larvae Adult
Proximal composition (%, dry basis)
Crude protein (N x 6.25) 36.2 40.7 58.4 73.1
Crude lipid 18.0 15.6 30.1 15.9
Ash 9.3 19.7 3.5 5.6
Nitrogen-free extract 36.5 24.0 8.0 5.4
Polyunsaturated fatty acid
Omega-3 (% lipid) 0.6 – 1.5 0.1 – 0.9 0.4 – 1.5
Omega-6 (% lipid) 4.2 – 17.3 13.6 – 25.6 23.6 – 34.9
Omega-6/omega-3 ratio 6 – 11 24 – 102 15 – 29
Minerals content (mg/100 g; dry basis)
Calcium 2,900 3,000 100 166 Phosphorus 350 620 760 860 Sodium 100 50 174 363 Iron 200 8 6 6 Zinc 61 3 11 14 Production characteristics
Food conversion ratio 1.4 – 2.3 3.8 – 6.1 2.3 – 10.0
1.2 Black soldier fly (Hermetia illucens)
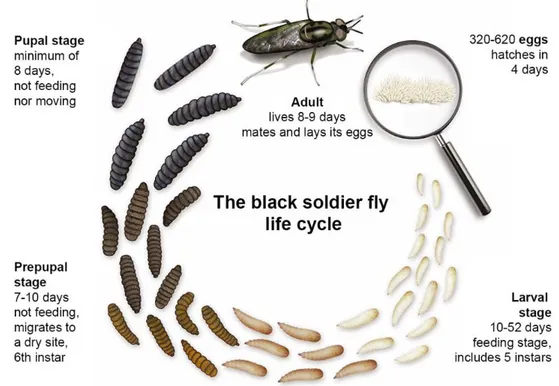
1.2.1 Life cycle of the black soldier fly
The BSF, a tropical and temperate dipteran insect [18], is adapted to large-scale production considering its short life cycle, the great size of its immature stage, the elevated number of eggs and its bioconversion efficiency. Females lay between 320 and 620 eggs [19]. Four days later, the larvae hatch and go through six larval instars, including the prepupae, then pupate [20-21]. The larvae are beige and possess photoreceptors which allow them to flee light [22]. They can eat a wide variety of organic matter such as manure, fruits and vegetables, food waste and fish offal [23-24], and can reduce, up to 50%, their feeding substrate on a dry basis [18]. They also have a high conversion efficiency since they can produce 1 kg of larvae biomass with 1.4 kg of ingested feed compared to cricket and mealworms which require 2.3 and 3.8 kg, respectively [15]. The BSF larvae reach the prepupal stage in 10 to 52 days and weigh 300 mg depending on the feed offered and the rearing temperature [23, 25]. It then stops eating and initiates melanisation, resulting in a darker colouration of the cuticle a few hours before moulting to become a prepupa [26-27]. During this stage of 7 to 10 d, the prepupa migrates to a dry place to metamorphose into a pupa [18, 28]. The pupal stage, during which larvae do not move nor eat for at least 8 days, ends with the adult emergence [29]. Because the imago does not eat, it uses its accumulated reserves to meet its metabolic needs [19]. The fly mates and lays its eggs inside its 8 to 9 living days [30]. The most nutritious stages are the larval ones (larva and prepupa) and are, therefore, the most often harvested by BSF producers [17].
1.2.2 Proximate composition of the black soldier fly
The nutritional quality of the BSF larvae mainly refers as its lipid and protein content [31], oxidation and digestibility as well as its minerals and vitamins content [8]. The composition of the BSF is highly variable and depends on the feeding substrate and the stage of harvest as shown in Table 1.1. There are two stages of interest for the use of BSF as food or feed, the larvae and the prepupae. The pupae are less suitable for use as a feed since they have lost about 20% of their lipid content compared to the larval stage [17]. When reared in the same conditions, the larvae contain more lipids and nitrogen-free extract, but lower ash and protein contents than the prepupae and the pupae [14, 17]. The prepupae also possess 20% more water than larvae [15, 32] and therefore require a longer drying time. From a nutritional point of view, the choice of the harvested stage will depend on the later use of the BSF meal.
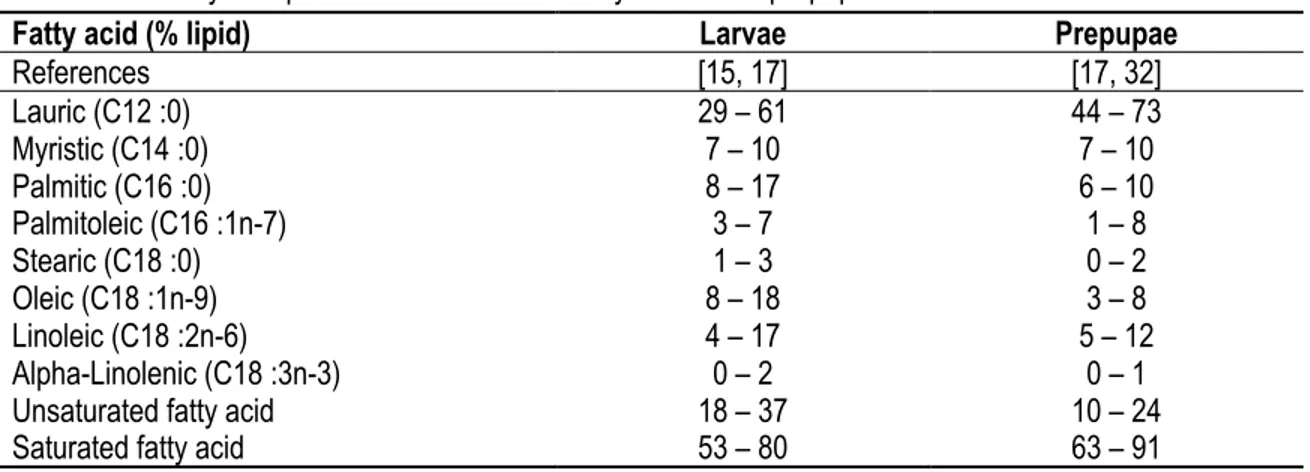
1.2.3 Fatty acid profile
The BSF fatty acid profile is also variable depending on the development stage and contains mostly saturated fatty acid allowing it to tolerate a wide temperature range, as shown in Table 1.2 [33]. Indeed, because the BSF cannot regulate its body temperature and must survive at temperatures up to 40 °C, high concentration of lauric acid allows it to reduce the fluidity of the membrane and its oxidation [33]. Moreover, the composition of the BSF lipid also depends on the feeding substrate [17, 24]. Indeed, it is possible to increase the proportion of valuable unsaturated fatty acid up to 37% of the lipid content by feeding the larvae a diet containing fish meal inclusions a few hours prior killing it [24, 34]. However, unsaturated fatty acids are vulnerable to oxidation which could reduce its nutritional value if not inhibited [35]. It is therefore essential to maintain the quality of the fatty acids by preventing their oxidation.
Table 1.2 Fatty acid profile of the black soldier fly larvae and prepupae.
Fatty acid (% lipid) Larvae Prepupae
References [15, 17] [17, 32] Lauric (C12 :0) 29 – 61 44 – 73 Myristic (C14 :0) 7 – 10 7 – 10 Palmitic (C16 :0) 8 – 17 6 – 10 Palmitoleic (C16 :1n-7) 3 – 7 1 – 8 Stearic (C18 :0) 1 – 3 0 – 2 Oleic (C18 :1n-9) 8 – 18 3 – 8 Linoleic (C18 :2n-6) 4 – 17 5 – 12 Alpha-Linolenic (C18 :3n-3) 0 – 2 0 – 1
Unsaturated fatty acid 18 – 37 10 – 24
Saturated fatty acid 53 – 80 63 – 91
1.2.4 Microbiota of the black soldier fly
The BSF is associated with an elevated microbiological risk related to the diversity of the feeding conditions. To our knowledge, only three studies investigated the microbiota of the BSF and only one its mycobiota. The
the authors, the majority of the yeasts detected on the BSF fed on several substrates are producers of antimicrobial compounds [36]. The impact of the feed on the microbiota has been evaluated on the larvae entire digestive tract [37] and on specific parts of the midgut as well [38]. The microbiota of all BSF stages has also been tracked when fed the Gainesville diet [39], a reference diet for Diptera (50% wheat bran, 30% alfalfa meal and 20% corn meal). The microbiota of the whole larva contained 54% Bacteroidetes, 20% Firmicutes, 28% Proteobacteria and 9% Actinobacteria [39] which is similar to its midgut microbiota when fed on Gainesville diet [38]. It seems that the bacteria taxa of the BSF provided with a balance diet are dominated by Bacteroidetes (i.e., peptidoglycan degraders) and contains 9–20% Firmicutes and 16–28% Proteobacteria [38-39]. Hence, when fed an unbalanced diet such as 100% fish meal and 100% cocked rice, the microbial diversity completely changes. Indeed, the larva digestive tract do not contain Bacteroidetes anymore, but is now colonized by Proteobacteria (54–56%) and Firmicutes (43–47%), which may be problematic since most food-poisoning microorganisms are part of these taxa [37-38]. However, it is not necessarily problematic since lactic acid bacteria also belong to the Firmicutes.
Proteobacteria is a group of gram-negative bacteria, meaning that they possess an external membrane of lipopolysaccharides and a thin layer of peptidoglycan, and which includes some important pathogen bacteria [40]. Indeed, Escherichia coli, which some are known to induce gastroenteritis, Salmonella spp., responsible for salmonellosis, and Shigella spp., which provoke shigellosis in humans, are members of the Enterobacteriaceae family (Gammaproteobacteria) which are Proteobacteria [41]. Another well-known Gammaproteobacteria is
Pseudomonas aeruginosa, which is an opportunist pathogen [42]. Campylobacter spp. are also Proteobacteria
(Epsilonbacteria) which is one of the most often reported etiologic agent of gastroenteritis (i.e., campylobacteriosis) [41].
Firmicutes is a group of gram-positive bacteria which possess a thick layer of peptidoglycan allowing them to increase their resistance to physical disruption, heat and desiccation, but are more vulnerable to antibiotics and other chemical antimicrobial compounds than gram-negative bacteria [43]. Firmicutes also includes major pathogens which are mainly in two classes, Clostridia and Bacilli [42]. Clostridia, including Clostridium
perfringens, a sporulated food-spoilage bacteria, are often found in vacuum-packed food or food poorly
refrigerated [41]. Other important food-poisoning microorganisms are Listeria monocytogenes, Bacillus cereus and coagulase positive Staphylococci (Bacilli) [41].
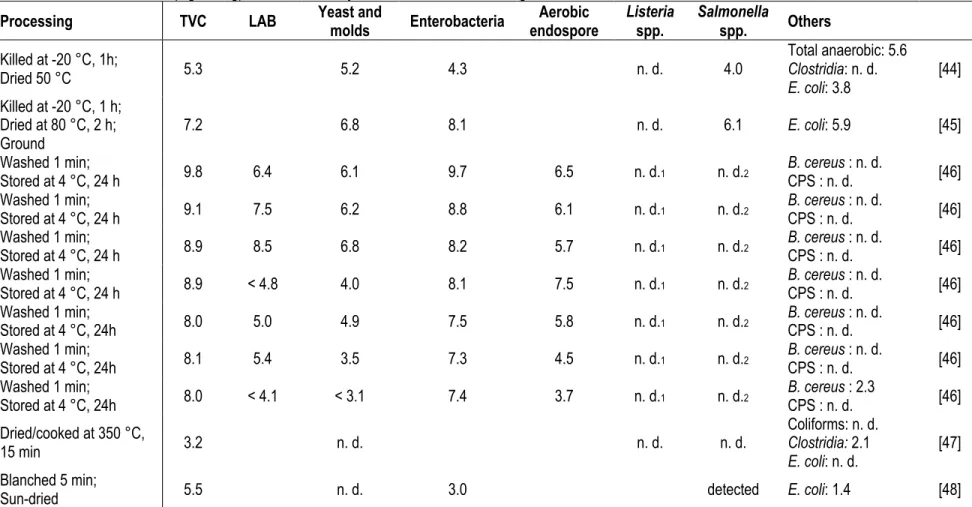
In addition to the feeding substrates that greatly influence the microbial load of the BSF larvae, the stage at harvest is also important to consider. Indeed, when fed the Gainesville diet, it has been shown that microbial community of prepupae contains 20% less Bacteroidetes, 10% less Firmicutes and 25% more Proteobacteria than larvae [39]. As a result, the prepupae may include more pathogens and should be further investigated. For
higher counts [41]. It is therefore critical to quantify the presence of these pathogens per gram to establish the risk they represent. The Table 1.3 reports the only published microbial load data on the BSF larvae or prepupae until now. As far as we know, Listeria spp. and Shigella spp. has not been detected in BSF larvae, but B. cereus,
E. coli, Salmonella spp., Staphylococcus spp., Pseudomonas spp. and Clostridia have been. Further processing
Table 1.3 Microbial load (log CFU/g) of black soldier fly larvae under various rearing conditions.
Processing TVC LAB Yeast and molds Enterobacteria endospore Aerobic Listeria spp. Salmonella spp. Others
Killed at -20 °C, 1h; Dried 50 °C 5.3 5.2 4.3 n. d. 4.0 Total anaerobic: 5.6 Clostridia: n. d. E. coli: 3.8 [44] Killed at -20 °C, 1 h; Dried at 80 °C, 2 h; Ground 7.2 6.8 8.1 n. d. 6.1 E. coli: 5.9 [45] Washed 1 min; Stored at 4 °C, 24 h 9.8 6.4 6.1 9.7 6.5 n. d.1 n. d.2 B. cereus CPS : n. d. : n. d. [46] Washed 1 min; Stored at 4 °C, 24 h 9.1 7.5 6.2 8.8 6.1 n. d.1 n. d.2 B. cereus CPS : n. d. : n. d. [46] Washed 1 min; Stored at 4 °C, 24 h 8.9 8.5 6.8 8.2 5.7 n. d.1 n. d.2 B. cereus CPS : n. d. : n. d. [46] Washed 1 min; Stored at 4 °C, 24 h 8.9 < 4.8 4.0 8.1 7.5 n. d.1 n. d.2 B. cereus CPS : n. d. : n. d. [46] Washed 1 min; Stored at 4 °C, 24h 8.0 5.0 4.9 7.5 5.8 n. d.1 n. d.2 B. cereus CPS : n. d. : n. d. [46] Washed 1 min; Stored at 4 °C, 24h 8.1 5.4 3.5 7.3 4.5 n. d.1 n. d.2 B. cereus CPS : n. d. : n. d. [46] Washed 1 min; Stored at 4 °C, 24h 8.0 < 4.1 < 3.1 7.4 3.7 n. d.1 n. d.2 B. cereus CPS : n. d. : 2.3 [46] Dried/cooked at 350 °C, 15 min 3.2 n. d. n. d. n. d. Coliforms: n. d. Clostridia: 2.1 E. coli: n. d. [47] Blanched 5 min;
Sun-dried 5.5 n. d. 3.0 detected E. coli: 1.4 [48]
1.3 Insect processing
Insect processing starts with harvesting and should end in obtaining a safe and stable product from a microbiological and physico-chemical point of view. Processing has a great influence on the final product quality and can reduce the microbial load while enhancing the colour and nutritional stability of the product. It can also increase or decrease the nutritional quality and change protein functional properties. Depending on the final use of the insect product, processing steps may include post-harvest processing, killing, macronutrient extraction (i.e., proteins, lipids and chitin), decontamination, drying and grinding. In industry, every processing step should aim to reduce microbial load while increasing or maintaining the nutritional quality.
1.3.1 Harvesting
Harvesting represents the separation of the larvae from their feeding substrate and is carried on by sieving or by using their behaviours to let them collect themselves, also called auto-collecting. The choice of the method used will depend on the BSF stage (i.e., larvae and prepupae) at harvest and the feeding substrate humidity and particle size. The first method, and the most used in industry, consists to sieve the substrate while keeping the larvae or prepupae into the sieve. Sieving, using a rotary drum system or a vibrating sieve, can be applied to both stages and allows collecting the substrate which represents a potential soil amendment [49]. Therefore, the substrate must have been completely digested, the particle size must be small enough to be easily sieved and it must contain a maximum of 50% humidity at time of harvest to prevent sieve obstruction [49]. The second harvesting method is that via autocollection, which can only harvest prepupae since it takes advantage of their migration behaviour to a drier place prior to pupate, but tolerates higher humidity substrate [18, 50]. Because they try to flee the substrate, it is possible to orient their movement on a ramp to let them fall into a collecting bucket [30]. It has the advantage to ensure that only the living ones are harvested, but may induce energetic reserve mobilization, reducing the nutritional value of the product, though this still need to be investigated further. The harvesting efficiency is also lower since not all prepupae will migrate according to plan.
1.3.2 Post-harvest processing steps
To obtain a stable insect product and to enhance its quality, large-scale production uses post-harvest processing steps. Although not every producer uses them, they could contribute in enhancing the microbial quality of the product. They include a feed withdrawal period and a washing step.
1.3.2.1 Feed withdrawal period
Because it is difficult to remove the insect gastrointestinal tract, undigested feed, frass and microbial load associated with them usually become a part of the resulting insect meal. To prevent it, insects are sometimes degutted, i.e., applying pressure on it to force its content evacuation, or starved. The FAO recommends including a feed withdrawal period (FWP), also called post-harvest starvation or gut purging, to promote the reduction of
the insect microbial load [6]. The egestion time, which means the time required for a feed bolus to be excreted when feed is continuously ingested, is of 100 min for Musca domestica and between 60 min and 90 min for
Lucilia sericata, two Diptera larvae, respectively [51-52]. The egestion time increase when no more feed is
available and corresponds to the gastrointestinal evacuation time (GET). For instance, the GET of adult Blatella requires 3 days while the egestion time is of 120 min [53]. Because there is only little information on insects GET, it is generally assumed that a FWP of 12 to 48 hours is enough to empty most of the digestive tract [54-56]. To date, only mealworms were investigated for the suitability of applying a FWP to reduce microbial load [57]. The authors concluded that a FWP of up to 48 h with or without faecal contact does not affect the mealworms microbial load [57]. However, since the GET of mealworms is unknown yet, frass may have remained in the digestive tract resulting in a remaining high microbial load. Besides, starvation may induce energetic reserve metabolism and its evaluation appears necessary when estimating the optimal FWP [58]. Since the BSF larvae eat contaminated residual organic matter, research should focus on the effectiveness of a FWP since it could reduce the microbial load at low cost.
1.3.2.2 Washing
As for many crustaceans, some insect species have been reported to be washed with water prior or after killing to remove any feed or frass remaining on their cuticle, including Coleoptera (T. molitor) and Diptera (M.
domestica, Piophila casei, H. illucens) larvae [59-60]. Washing carcasses is an effective process frequently used
in animal production since it allows to physically remove organic matter and the microbes it may contain at low cost and enhance product self-life [61]. Washing can be done by water dipping or by spraying. Washing can be optimized by increasing pressure, duration, temperature, dipping repetition and by adding sanitizers into the water, such as chlorine and organic acids (acetic, lactic and propionic acids) [61]. The effect of dipping in agitated water during 1 min on the microbial load of T. molitor has been investigated and showed no significant microbial reduction [57]. However, as far as we know, this is the only study reporting the efficiency of rinsing edible insects and further research should focus on the optimization of the washing process. Although pre-consumption diet is currently used as feed for BSF larvae, washing the larvae should be investigated since it could allow them to be used to convert highly contaminated residual organic matter.
1.3.3 Killing
Killing is a key step in insect processing since it can impact on the nutritional quality, microbial safety, colour stability and taste of the product [62]. Being necessary in any animal production, killing should be fast and effective and contribute to reducing the microbial load while maintaining the nutritional quality of the product. Invertebrates are killed by several methods such as cold, heat, asphyxiation and mechanical disruption. Therefore, the killing method should be adapted to the specie requirement. Freezing and blanching are the most frequently used methods, but many other methods have potential to be just as effective [5, 8]. The Table 1.4
1.3.3.1 Killing by cold
Freezing is frequently used since it allows product preservation while slowly killing the insect. Because insects are poikilotherms, killing by cold allows reducing metabolism of the insects preventing any potential pain or suffering. It also has the advantages of reducing microbial growth and spoilage-enzyme activity. It can be carried out by freezing, or freeze-drying, and immersion in ice water [63]. A few studies investigated the impact of killing BSF larvae by freezing on the lipid and protein quality and on the colour stability [64-65]. Since enzymes remain active after freezing, spoilage enzyme has been reported to induce lipid degradation and polyphenol oxidation [64-65]. In the BSF larvae, enzyme responsible for lipolysis has been shown to be highly active, even at freezing temperature, inducing lipid deterioration during storage at -20 °C [64]. Consequently, it appears very important to inhibit those enzymes rapidly in order to maintain the nutritional quality of the insect.
Table 1.4 Killing methods used in industry according to Erens et al., 2012 [66].
Individual or company Species Killing method
Tarique Arsiwalla,
Protix Biosystems H. illucens Mechanically crushed by centrifugation
Jagran B. V. Insect Rearing
Concepts M. domestica Grinding and freezing; experimenting asphyxia with high N2/low 02.
Leon Westerd,
Wageningen University
Various insects Small insects: stunned with CO2 and sprayed
with hot water
Large insects: freeze-drying
Kreca Orthoptera, Coleoptera and Diptera Freeze or freeze-drying
Van der Ven T. molitor Boiling or freeze-drying
1.3.3.2 Killing by heat
Killing by heat has been used for many invertebrates including lobster, shrimps and insects. Blanching is one of the most frequent killing methods used in insects production since it allows microbial reduction while being very fast [67]. Some insects start dying at 50 °C but higher temperature is generally used to inactivate microorganisms at the same time [68]. It can be carried on by dry heat, in an oven, or by moist heat such as blanching and steaming. In addition to reducing the microbial load of the insects, heat treatment can induce endogenous spoilage-enzyme denaturation thus limiting product deterioration during storage [69]. Dry heat can allow a high level of non-enzymatic browning by Maillard reaction compared to blanching which minimize it because of the high-water content [70]. Immersive method such as blanching has also been reported to reduce the protein and water content in shrimp, which could reduce the drying time [71]. In insects, blanching has been investigated as a killing method for BSF and mealworms. It was reported to increase the pH by 0.5 unit for at least 48 h, indicating a higher product stability compared to raw product [72]. Blanching also reduces the water-holding capacity of insects, reducing the drying time and inactivating spoilage enzymes while maintaining colour and nutritional quality [64-65, 72].
1.3.3.3 Asphyxia
The use of carbon dioxide (CO2) is one of the most popular methods to anesthetize invertebrates and requires
exposition time between 3 and 60 minutes depending on the insect species [66, 73]. A concentration of CO2
above 40% induces neuron depolarization without affecting its conductance inducing insects immobilization [74]. However, when applied for a longer period of time, it can also be lethal by reducing the hemolymph pH and inducing insect dehydration by promoting spiracle opening [75-76]. In addition, to increase their survival time under anoxic condition, oxygen is spared by reducing their metabolic activity [77]. However, in the presence of
CO2 the metabolism activity is maintained promoting oxygen depletion and thus, reducing their survival [77]. The
time required to effectively kill insects with carbon dioxide is highly variable and depends on the species, the
insect stage, the temperature, the CO2 and O2 concentration, as well as the humidity [78]. Pure carbon dioxide
is lethal for Drosophila melanogaster (Diptera) larvae in only 30 min [79] while it requires 72 h in Cadra cautella (Lepidoptera) and Tribolium castaneum (Coleoptera) larvae [78]. Even if carbon dioxide asphyxiation requires a relatively long time, it can anesthetize the insect prior to killing it, and reduce drying time by initiating dehydration resulting from spiracle opening.
Asphyxiation can also be applied by air saturation with N2 and requires that the oxygen level is below 3% [80].
Because air saturation can be hard to achieve on a large scale, vacuum packaging and drowning can also be used [81]. Insects have different resistance to asphyxia amongst species and stages. Indeed, M. sexta pupae can survive 5 d when immersed in water while the larvae only survive 4 h [82]. Adult grasshopper species can survive between 7.5 to 22 h when immersed in water, while the nymph of the same species can only survive
between 3 and 13 hours [83]. The mortality of larval stages of the Ephestia cautella (Lepidoptera) to 98% N2 is
also highly variable and requires between 96 to 144h which is 1 to 2 days longer than aerobic atmosphere with
60% CO2 [80]. The BSF larvae have not been investigated for its resistance to anoxic condition and could be as
efficient as for D. melanogaster exposed to 100% CO2. However, prolonged anaerobic conditions could allow
anaerobic microbial growth and thus could require a drastic decontamination step.
1.3.3.4 Mechanical disruption
Mechanical disruption of the neuronal system appears to be a promising killing method because of its low cost and rapidity. It can be applied by grinding or centrifugation according to Protix [66]. Even if mechanical disruption appears to be applied in industry (Table 1.4), no study has evaluated the impact of this method on the quality of the resulting product [66]. It was reported that grinding induces browning for several insect species [13, 84]. It could also allow lipid oxidation by exposing insect constituents to oxygen [85]. Besides, grinding could enhance drying capacity by increasing surface evaporation [85]. In addition, Jagran, a housefly rearing company, stated that among others, grinding appeared to be most animal-friendly killing method [66].
1.3.3.5 Other killing methods
Other killing methods have been reported for invertebrates such has electrocution for crustaceans [86]. Electrocution at 120 volts and 20 amps for 10 s has been reported to effectively stun crustaceans if applied for 1 s and to kill lobsters and crabs if applied for 5 and 10 s respectively [87]. However, electrocution has not been investigated yet as a stunning or killing method for insects. In summary, a high diversity of methods is used to kill insects, but only blanching and freezing have been under focus. Therefore, it appears primordial to optimize the killing process for BSF larvae in order to maximize its quality while minimizing potential pain and suffering.
1.3.4 Decontamination
After killing, the resulting product is the whole wet larva, whose microbial load has potentially been reduced, depending on the killing method used, and whose pH is close to neutrality [46] indicating that it is a product of perishable nature and that it is treated as a low-acid food [88]. It is therefore necessary to process the larvae to extend their shelf life. Several decontamination techniques have been developed for the food industry, but only a few have been applied to insects. Amongst them, blanching, desiccation, high hydrostatic pressures, direct and indirect cold plasma and microwave have been investigated for their capacity to reduce the microbial load of insects as shown in Table 1.5.
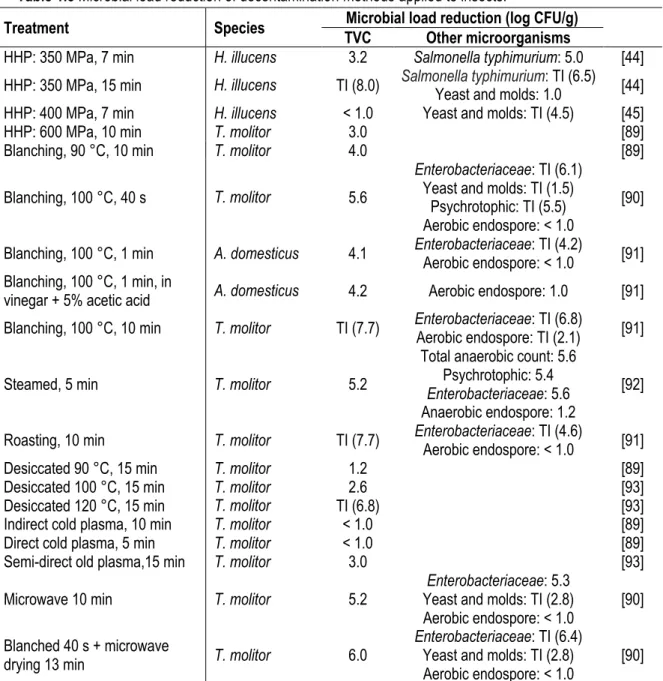
Table 1.5 Microbial load reduction of decontamination methods applied to insects.
Treatment Species TVC Microbial load reduction (log CFU/g) Other microorganisms
HHP: 350 MPa, 7 min H. illucens 3.2 Salmonella typhimurium: 5.0 [44]
HHP: 350 MPa, 15 min H. illucens TI (8.0) Salmonella typhimuriumYeast and molds: 1.0 : TI (6.5) [44]
HHP: 400 MPa, 7 min H. illucens < 1.0 Yeast and molds: TI (4.5) [45]
HHP: 600 MPa, 10 min T. molitor 3.0 [89]
Blanching, 90 °C, 10 min T. molitor 4.0 [89]
Blanching, 100 °C, 40 s T. molitor 5.6
Enterobacteriaceae: TI (6.1)
Yeast and molds: TI (1.5) Psychrotophic: TI (5.5) Aerobic endospore: < 1.0
[90]
Blanching, 100 °C, 1 min A. domesticus 4.1 EnterobacteriaceaeAerobic endospore: < 1.0 : TI (4.2) [91]
Blanching, 100 °C, 1 min, in
vinegar + 5% acetic acid A. domesticus 4.2 Aerobic endospore: 1.0 [91]
Blanching, 100 °C, 10 min T. molitor TI (7.7) EnterobacteriaceaeAerobic endospore: TI (2.1) : TI (6.8) [91]
Steamed, 5 min T. molitor 5.2
Total anaerobic count: 5.6 Psychrotophic: 5.4
Enterobacteriaceae: 5.6
Anaerobic endospore: 1.2
[92]
Roasting, 10 min T. molitor TI (7.7) EnterobacteriaceaeAerobic endospore: < 1.0 : TI (4.6) [91]
Desiccated 90 °C, 15 min T. molitor 1.2 [89]
Desiccated 100 °C, 15 min T. molitor 2.6 [93]
Desiccated 120 °C, 15 min T. molitor TI (6.8) [93]
Indirect cold plasma, 10 min T. molitor < 1.0 [89]
Direct cold plasma, 5 min T. molitor < 1.0 [89]
Semi-direct old plasma,15 min T. molitor 3.0 [93]
Microwave 10 min T. molitor 5.2 Yeast and molds: TI (2.8) Enterobacteriaceae: 5.3
Aerobic endospore: < 1.0 [90]
Blanched 40 s + microwave
drying 13 min T. molitor 6.0
Enterobacteriaceae: TI (6.4)
Yeast and molds: TI (2.8)
Aerobic endospore: < 1.0 [90]
TI = Total inactivation; HHP = High hydrostatic pressure
1.3.4.1 High hydrostatic pressure
High hydrostatic pressure (HHP) technology is a non-heating decontamination technique able to maintain most product quality indices while reducing its microbial load. In a batch system, the product will enter a room that will be filled with a compressible fluid followed by the air removal (Figure 1.2). The fluid will then be compressed directly or indirectly resulting in an instantaneous and uniform pressure on the product allowing it to maintain its structure [94]. Although HHP is considered as a non-thermal treatment, a high temperature might be applied during pressurization to increase effectivity [28]. Three parameters must be optimized to increase the efficiency of the treatment, the pressure applied (MPa), the pressure holding time, and the temperature [28].
Figure 1.2 High hydrostatic pressure batch system (from https://www.hiperbaric.com/en/high-pressure).
Although HHP is still very expensive, it is increasingly used in food processing. This technology offers many advantages over traditional methods depending on the conditions used such as the possibility of pressurizing foods at low temperatures, reducing the load of some bacteria, yeast and moulds, inhibiting enzyme activity and allowing to create new functional foods [94-98]. High pressure is very effective in reducing gram-negative bacteria, yeast and moulds, but less in reducing gram-positive and spore-forming bacteria. When applied to insects, HHP has highly various effects on total viable counts (from < 1.0 to 8.0 log reduction) depending on the research resulting from the presence of spore-forming bacteria able to resist the treatment [44-45]. Therefore, HHP should be optimized according to the microbial community of the product which in turn, varies from insect species, stages and facilities.
Very few studies have attempted to demonstrate the ability of HHP to inhibit enzymatic activity in insects. However, HHP treatment at 400 and 500 MPa for 3 min were able to prevent browning in mealworms suggesting that enzymatic inhibition did occur [72]. It was also reported that HHP reduces water-holding capacity in mealworms which this effect increased with higher pressure [72]. Lower water-holding capacity could also reduce drying time which would be a great advantage for the industry. However, HHP can also have negative effects on the quality of the product since it can promote lipid peroxidation [99] as it was reported for giant tiger shrimp where lipid oxidation levels increased from 300 MPa [100]. Considering the wide variety of effects of HHP, it is necessary to further evaluate the potential utility of this technology for insects, especially BSF larvae.
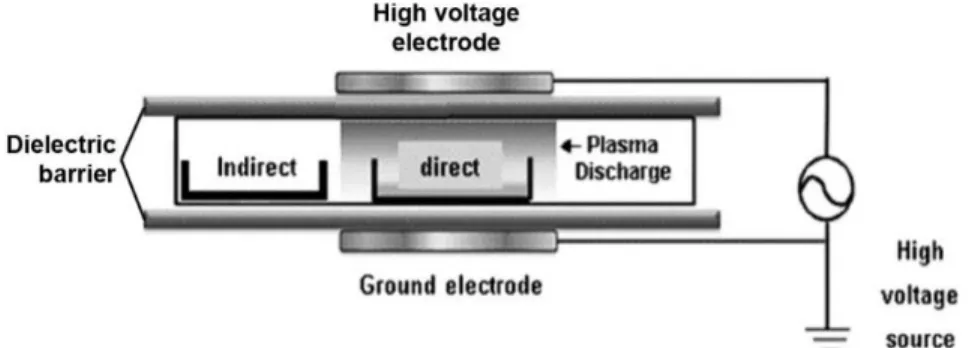
1.3.4.2 Cold atmospheric pressure plasma
Cold atmospheric pressure plasma is a non-thermal technology that allows reducing a wide range of microorganisms including spore-forming bacteria and viruses. Although this technology is gaining interest in food processing, it is an expensive one [93]. Cold plasma is composed of neutral particles and a lower quantity of
Therefore, gram-positive bacteria and spores are more resistant to this treatment [101]. As shown in Figure 1.3, the plasma discharge can be applied directly, semi-directly or indirectly on a product resulting in different antibacterial effects [101]. Applied on fresh mealworms, direct and indirect cold plasma has little effect [89], however, it could reduce by 3 log total viable counts when applied semi-directly on the powder [93]. Cold atmospheric pressure plasma could be useful in controlling microbial loads of insect powders and should be further investigated.
Figure 1.3 Atmospheric direct and indirect cold plasma process (adapted from Almeida et al. 2015) [102].
1.3.4.3 Other decontamination methods
Several other decontamination methods can be used to reduce the microbial load of insects such as pulse electric field, low-energy electron beam, ultrasound, microwave and heat treatment [101]. Among them, only microwave and heat treatment have been used on insects to inactivate microorganisms. Microwave has been highly effective in reducing the microbial load of T. molitor, but was not able to reduce spore-forming bacteria [90]. Heat treatment can be separate in two kinds, moist and dry (desiccation) heat. Blanching during only 40 s in water is very effective in reducing most microorganisms, but spore-forming bacteria remain unaffected [90]. However, blanching in an acidic solution appears to be more effective on aerobic endospores in A. domesticus [91]. Moreover, increasing the blanching time to 10 min allows to totally inactivate aerobic endospores (2.2 log) in T. molitor [91]. At 100 °C, dry heat appears to be less effective than blanching, but higher temperature can be achieved. Desiccation at 120 °C for 15 min was able to totally inactivate total viable counts (6.8 log) [93]. However, no information is available on its effect on insect spore-forming bacteria. Since spore-forming bacteria are the most challenging type of microorganism, decontamination methods should focus on their inactivation.
1.3.5 Drying
Drying insect products has many advantages since it improves preservation by reducing microbial growth and spoilage-enzyme activity. Several drying methods can be applied to insects which have different effects on the final dry product. Indeed, the method can affect the colour, water activity, proximate composition, lipid oxidation and protein solubility. Sun-drying, oven-drying, freeze-drying and microwave-drying are the most frequent
1.3.5.1 Oven drying
Oven drying has been shown to have a great potential as a drying method for insects [103-104]. Many kind of system can be used such as vacuum and rotating oven which may have different effects on the product [105]. Temperatures between 50 °C and 120 °C are generally applied from an hour to a few days, but lower temperature is favourable in order to maintain protein solubility and to reduce Maillard reaction, shrinkage and tissue collapsing [105-107]. Drying at 60 °C appears as the optimal drying temperature to prevent those effects while reducing the drying time [108].
1.3.5.2 Freeze-drying
Freeze-drying is a non-thermal treatment frequently used in insect processing, but is relatively expensive and requires a minimum of 24-53 h to dry mealworms [105, 109]. It is one of the best drying methods to maintain insect colour since it does not induce Maillard reaction resulting in the whitest powder [109]. However, it also induces a slight protein solubility reduction of 10% [105]. Moreover, freeze-drying induces the greatest lipid oxidation resulting in important quality loss [105], but blanching prior freeze-drying was able to reduce by half the oxidation [109]. In addition, it can enhance BSF larvae customer acceptability since the whole freeze-dried larvae appears inflated instead of shrank.
1.3.5.3 Microwave drying
Microwave is also expensive but is the fastest drying method for insects requiring only 10 to 15 min depending on the microwave parameters [109]. It also produces inflated whole dried larvae but allows browning to occur [103]. Microwave drying of mealworms has been reported to reduce protein solubility of 40% [105]. On BSF larvae, microwave drying induces protein polymerization resulting in a lower digestible amino acid score and digestibility, and in a powder of bigger particle sizes compared to oven-drying at 60 °C [103].
1.3.5.4 Other drying methods
Other drying methods may be used on insects such as sun drying and fluidized bed drying. Sun drying is a traditional low-cost drying method, considered as a low temperature and extended-time treatment, which has been applied to many insect species including grasshopper, caterpillar, termite and maggot [60, 107]. Fluidized bed drying is a fast, high-temperature, short-time technology that can also be used since it induces only a small lipid oxidation increase when applied at 130 °C during 110 min, but could induce browning [105]. Most drying studies on edible insects focused on T. molitor and few have investigated the effects of drying on the BSF larvae or prepupae quality. Therefore, a better understanding of the effects of drying on the BSF could contribute in improving the final product quality.
1.3.6 Other processing steps
Once the product has been decontaminated and dried, it is now stable and should be able to be preserved for at least a few months without much nutritional quality change. Further processing steps are optional since the
product is already stable enough to be shipped and store but could give a plus value or enhance the quality of the product.
1.3.6.1 Grinding
Grinding can appear as particularly attractive, since edible insects are frequently used as a powder in a diet or in a meal. For food, insects are more easily accepted by Western customers if they cannot be perceived [110] and thus incorporated as a powder into a recipe. However, grinding fresh insects has been reported to induce browning in many insects resulting from enzymatic and non-enzymatic reaction involved with polyphenols, which may reduce product quality [13, 55, 84]. Therefore, grinding should be applied after product stability is achieved.
1.3.6.2 Extraction of insect components
Extraction of lipids, proteins and chitin may enhance the value of the product by increasing the control of the proportion of their inclusion during diet formulation. However, extraction needs to be optimized for every species in order to maximize extraction yield and purity and to obtain a product with desirable functional protein properties. Indeed, extraction can alter the flavour, the colour and protein functionality, induce protein denaturation and reduce protein extraction yield of the residual defatted meal [54, 111]. Lipid extraction can be done by mechanical pressing, aqueous or solvent extraction and by differential density. Protein extraction is generally applied to defatted insects and can be done by alkali or sonication [112-113]. Finally, chitin extraction consists of product demineralization followed by deproteination and can be done using chemicals or lactic acid
bacteria [8]. New extraction technologies should also be further investigated such as supercritical CO2,
microwave and ultrasound [106, 114]. Some processing steps can enhance component extractability, such as HHP and blanching [65, 115]. Therefore, processing methods should be considered as a whole from the harvest until obtaining the desire final product. Indeed, several processing methods discussed above can contribute in many ways to the final product characteristics allowing the reduction of the number of processing steps or enhancing the final quality. As an example, blanching can be used to kill, decontaminate and to enhance extraction. Further study should focus on the interactions of processing steps in order to provide effective pathways in obtaining different quality products.
1.4 Insect quality
Insect processing can greatly affect the insect product including its nutritional and microbiological quality as well as colour stability. The nutritional quality refers mainly to the proximate composition, the lipid oxidation and the protein digestibility, while the microbial quality refers to the abundance of spoilage and pathogenic microorganisms. Understanding mechanism involved in food spoilage and how processing impacts them is primordial to obtain a product of great quality. Therefore, even if criteria have not been established to evaluate insect product quality, some references in other food systems may be applied to insects.
1.4.1 Proximal composition (macronutrient metabolism)
Processing steps from harvesting to killing can induce a stress on the insects resulting in energy mobilization as for starvation. In addition, most processing techniques can also affect the insect composition depending on the method applied. Understanding the impact of processing on the chemical composition of the insect is primordial in order to increase the control of the resulting product.
1.4.1.1 Stress response
From harvesting to killing, every processing step can induce significant stress on the insect which could strongly affect the composition of the final product. The stress response in insects varies with the development stage, the duration, the nature and the intensity of the stress [116-117]. The response begins with the secretion of octopamine, a stress hormone equivalent to mammalian adrenaline, which promotes gluconeogenesis and glycogenolysis by inducing adipokinetic hormone (AKH) secretion, a neurohormone synthesized and stored in cardiac bodies [118]. In insect larvae, AKHs induce glycogen mobilization, glycogenolysis, whereas in the adult stage they induce the mobilization of triglycerides by lipolysis [117].
The amount of energy stored during the larval stages is even more important in insects in which the imago stage is not feeding such as in BSF. Therefore, the larva must have accumulated enough energy in the form of glycogen and triglycerides to allow reproduction and oviposition [117]. Though the amount of glycogen stored in the fat body is lower than for triglycerides, glycogen is the primary source of energy being mobilized in response to starvation or environmental changes [117]. Glycogen phosphorylase, an enzyme used to mobilize glycogen in the form of trehalose, is active since the larval stage and its activity increases particularly in the prepupal stage [117] in anticipation of a very energy-intensive stage, the pupa. It should be recommended to minimize the stress impose on insects prior and during killing in order to prevent any glycogen or triglycerides loss.
1.4.1.2 Starvation
During the fasting period, triglycerides are normally mobilized after depletion of glycogen [117]. Triglycerides are stored as droplets in association with cholesterol and surrounded by phospholipids in the fat body [117]. Lipase access to triglycerides is regulated by the presence of droplet-associated proteins similar to perilipins in mammals [117]. One of these proteins, Lsd1, is present at the prepupal stage and is activated by AKH which activates lipolysis [117]. The other regulatory protein, Lsd2, is present at all stages of development [117]. It is associated with lipid accumulation and promote droplet formation. The absence of trehalose in the haemolymph under fasting conditions, caused by the depletion of glycogen, induces the mobilization of triglycerides resulting in production of glycerol and fatty acids [117]. Depending on the fasting length, this mechanism could highly reduce the lipids content of the insect. In addition, protein can also be mobilized as a source of energy. The effect of starvation on lipid and protein metabolism is highly variable amongst insect species. Starved adult
Crickets start to oxidize their proteins after 2.5 d, while grasshoppers do not really use them [119]. Their survival under starvation is also quite different since 50% of crickets died after 5 d while it takes 16 d for grasshoppers [119]. Cockroaches initiate lipolysis after 3 d and metabolize their proteins after 36 d. Furthermore, 50% of them die after 52 d which makes them extremely resistant to starvation [119]. Mealworm larvae initiate lipolysis after 2 d but do not rely on their proteins and 50% die in 5.5 d, while M. sexta larvae do not rely on their lipids nor their proteins and 50% die in 3 d [119]. To our knowledge, the effect of starvation on the BSF has not been investigated yet and should be evaluated considering the great diversity of strategy used in insects to survive starvation.
1.4.1.3 Effects of endogenous degradation enzyme post-killing
For BSF prepupae, slow killing methods such as freezing promotes glycogenolysis, lactic acid production from anaerobic metabolism, consumption of citric acid and lipolysis [64-65]. It seems that lipolytic enzymes remain active during cold storage, promoting triglyceride degradation and resulting in lower lipid content [64]. Therefore, it would be useful to inactivate these enzymes as fast as possible to minimize lipid degradation.
1.4.2 Lipid quality: peroxidation
Lipid quality generally refers to their level of oxidation as it reduces fatty acid availability. Because valuable unsaturated fatty acids are highly vulnerable to oxidation, processing methods must focus on minimizing their oxidation in order to increase product quality [35]. Indeed, oxidized polyunsaturated fatty acids are no longer available when ingested and can be potentially harmful via the production of secondary products such as alkenals and malondialdehyde [35]. In addition, the oxidation and hydrolysis of the unsaturated fatty acids lead to the formation of volatile compounds, a sign of lipid degradation, resulting in alteration of sensory properties, notably smell and taste [35]. It is therefore important to minimize oxidation in order to optimize the quality of the product. Considering that in certain circumstances, BSF larvae is enriched in polyunsaturated fatty acid [11], it is important to prevent lipid oxidation during processing.
Lipid oxidation takes place in three stages, namely initiation, propagation and termination. Initiation is generally induced by the presence of reactive oxygen species (ROS) and corresponds to the lipid hemolytic (LH) cleavage liberating water and a radical (L·) [120]. The autocatalytic propagation immediately follows the initiation and corresponds to a reaction between molecular oxygen and L· to form a peroxide radical (LOO·) [120]. The propagation represents the attack of a peroxide radical on an oxygen atom from another lipid resulting in the formation of hydroperoxides (LOOH) and a lipid radical (L·) [120]. The termination phase corresponds either to the combination of two hydroperoxides, to form various products such as volatile compounds, or to the decomposition of the monohydroperoxide by an alkoxy radical (LO·) resulting in the formation of 4-hydroxyalkenals, 2-alkenals and malondialdehyde (MDA) [35, 120]. Lipid oxidation is measured from the propagation and the termination steps, also called primary and secondary lipid oxidation, respectively [121]. The
primary lipid oxidation can be established by measuring the level of hydroperoxides while the secondary one is measured by the MDA content [122]
It is already known that light, heat, the presence of oxygen and iron as well as the presence of reaction producing ROS, such as mitochondrial electron transport and metal-catalyzed oxidation reaction, contribute to the initiation of the peroxidation [120]; ferrous iron can promote initiation by activating oxygen [123]. Therefore, inhibition of
lipid peroxidation initiation may be done by reducing the concentration of ROS and ferrous iron (Fe2+) and by
promoting antioxidant activity [124]. Antioxidant enzymes such as superoxide dismutase, catalase and peroxidase catalyze the conversion of ROS into water while ferrous iron can be inactivated by oxidation into ferric iron by ferroxidase or physically by chelation [85, 125-126].
Several processing methods put the larvae under such conditions which can initiate lipid peroxidation. Low temperature can promote peroxidation since enzymes’ antioxidant activity is reduced resulting in an increase in ROS [127]. Heating and HHP can promote lipid oxidation by protein denaturation, which reduces antioxidant activity and increase iron by releasing them from haem proteins [99]. In fact, heat treatment can reduce the activation energy required to initiate peroxidation while high pressures from 300 MPa promote the reduction of ferric iron into active ferrous iron [99]. However, processing methods can also prevent lipid peroxidation by oxygen removal using modified atmosphere or vacuum packaging and by Maillard reaction products, resulting from heating, which can exhibit antioxidant properties [128]. Moreover, blanching and microwave drying did not promote insect lipid oxidation while freeze-drying did [109]. Because no acceptable threshold for insect lipid oxidation has been established yet, reference quality value for fish oil is being used. Therefore, secondary lipid oxidation values below 1.5 mg MDA/kg on a wet basis are considered as not rancid (excellent quality), between 1.6 and 3.6 as slightly rancid (good quality) and above 3.7 as rancid (unacceptable quality) [129].
1.4.3 Protein quality: digestibility
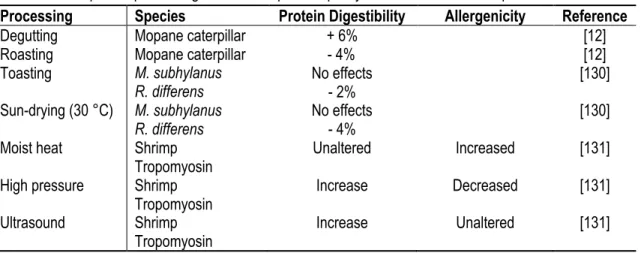
The protein quality refers to the amino acid profile, crude protein content and protein digestibility. Since the amino acid profile and the crude protein content of the BSF was established to be of good quality [14], the main concern is to maintain them as much as possible during processing. The high protein digestibility of the BSF makes it an interesting ingredient for food and feed. Because BSF prepupae has higher chitin content than BSF larvae reaching 82-90% and 78%, respectively, larvae are more digestible and appears as a better choice for use as an ingredient [12]. Processing greatly affects protein digestibility and therefore should be optimized in order to maximize it. The degutting of mopane caterpillar increases crude protein content by 35% and increases protein digestibility by 6%, while roasting decreases protein content and digestibility by 10% and 4%, respectively [12]. As shown in Table 1.6, heat can have different effects on insect protein digestibility depending on the species. While heat treatment affects protein digestibility of grasshoppers, it does not affect that of termites [130].
![Table 1.4 Killing methods used in industry according to Erens et al., 2012 [66].](https://thumb-eu.123doks.com/thumbv2/123doknet/3153971.89858/24.918.130.801.410.632/table-killing-methods-used-industry-according-erens-et.webp)

![Table 1.7 Microbial safety criteria for ready-to-eat food according to Health Canada (log CFU/g) [135]](https://thumb-eu.123doks.com/thumbv2/123doknet/3153971.89858/36.918.130.800.221.573/table-microbial-safety-criteria-ready-according-health-canada.webp)