© Claudia Mendez Espinoza, 2018
White spruce resistance against the spruce budworm:
Genetic control and insect-host interaction
Thèse
Claudia Mendez Espinoza
Doctorat en sciences forestières
Philosophiæ doctor (Ph. D.)
White spruce resistance against the spruce budworm:
Genetic control and insect-host interaction
Thèse
Claudia Méndez Espinoza
Sous la direction de :
Éric Bauce, directeur de recherche John MacKay, codirecteur de recherche
iii
RÉSUMÉ
Picea glauca (Moench) Voss (l’épinette blanche) est l’un des principaux hôtes de
la tordeuse des bourgeons de l’épinette (TBE), le défoliateur épidémique le plus dommageable de l’est du Canada qui est à l’origine de la mortalité d’arbres et de pertes économiques d’envergure considérables. Un mécanisme constitutif de résistance contre la TBE a récemment été découvert. Dans la présente thèse, nous avons étudié ce mécanisme basé sur l’accumulation foliaire du picéol et du pungénol, deux acétophénones découlant de la surexpression du gène Pgglu-1. Ces trois facteurs sont désignés comme étant des « biomarqueurs de résistance ». Nous avons aussi étudié la picéine, un acétophénone glycosilé qui est le précurseur du picéol, et l’ensemble des quatre facteurs sont désignés « biomarqueurs de défense ». La première partie de la thèse présente une approche de génétique quantitative s’appuyant sur l’analyse de 874 arbres représentant 33 familles et 71 lignées clonales répartis dans sept emplacements de l’est du Canada. Nos objectifs étaient : i) de déterminer le contrôle génétique des biomarqueurs de défense, ii) d’estimer les corrélations génétiques et phénotypiques entre les quatre traits de défense, iii) d’évaluer la présence de compromis entre les biomarqueurs de défense et la croissance primaire. Nous avons conclu que l’héritabilité au sens strict du picéol, du pungénol et de l’expression du gène Pgglu-1 était modérée (0,55, 0,50 et 0,58 respectivement), et obtenu des estimés un peu plus élevées pour l’héritabilité au sens large du picéol et du pungénol (0,66 et 0,60 respectivement), ce qui indique que ces traits de résistance sont soumis à un contrôle génétique additif. Les traits de résistance et la croissance montrent des corrélations génétiques positives (de 0,14 à 0,30), ce qui suggère que le mécanisme de résistance n’entraine pas un effet négatif sur la croissance de l’épinette blanche. Dans la deuxième partie de la thèse, nous avons étudié l’interaction insecte-hôte en menant des essais d’élevage d’insectes sur différents clones d’épinettes blanches. Nos objectifs étaient iv) de caractériser la variation développementale des acétophénones de défense, v) d’évaluer l’influence du stade phénologique de l’hôte sur le niveau de résistance indiqué par
iv
la performance biologique de la TBE et vi) de déterminer si les traits de résistance sont inductibles. Nous concluons que la variation des acétophénones dépend du phénotype de résistance de l’arbre, et que l’efficacité des traits de résistance dépend du synchronisme entre le Picea glauca et l’alimentation des insectes. Finalement, nous avons démontré que ce mécanisme de résistance peut être inductible.
v
ABSTRACT
Picea glauca (Moench) Voss (white spruce) is one of the main hosts of the spruce
budworm (SBW), an epidemic defoliator that is the most damaging in forests of eastern North America causing tree mortality and large economic losses. A constitutive resistance mechanism against the SBW was recently discovered. In this thesis, we studied this mechanism based on the foliar accumulation of aglycon acetophenones ̶ piceol and pungenol ̶ resulting from the expression of the
Pgglu-1 gene; and we refer to them as resistance biomarkers. Picein, the
glycoside precursor of piceol was also investigated and we refer to all four traits together as defense biomarkers. The first part of this thesis presents a quantitative genetic study, which analysed 874 trees representing 33 full-sib families and 71 clonal lines from seven field locations in Eastern Canada. The goals were to i) determine the genetic control of the defense biomarkers, ii) estimate the genetic and phenotypic correlations among the four defensive traits and growth, and iii) evaluate the occurrence of trade-offs between the defense biomarkers and primary growth. Narrow sense heritability of piceol, pungenol and Pgglu-1 gene expression was moderate (0.55, 0.50 and 0.58, respectively). Slightly higher broad sense heritability estimates were obtained for acetophenones (0.66 and 0.60 respectively), indicating that additive genetic effects play a major role in these resistance biomarkers. Positive genetic correlations were found between the resistance traits and growth (from 0.14 to 0.30), suggesting that the resistance mechanism does not compromise growth in white spruce.In the second part of the thesis, we studied the insect-host interaction by use of insect rearing trials in several white spruce clones. Our objectives were to iv) characterize the developmental and phenological variation of the defense acetophenones, v) evaluate the impact of the matched and delayed host phenology windows on the biological performance of the SBW, and vi) assess the inducibility potential of the resistance traits. We show that there are considerable variations in the acetophenone accumulation profiles between individual trees supporting their classification as Resistant (R) and Non-Resistant (NR); that the efficiency of the resistance traits is influenced by the synchronization between the P. glauca
vi
phenology and the insect feeding. Finally, we show that the resistance mechanism can be inducible.
vii
TABLE OF CONTENT
RÉSUMÉ ... iii
ABSTRACT ... v
LIST OF FIGURES ... xi
LIST OF ABBREVIATIONS ... xii
ACKNOWLEDGEMENTS ... xiv
FOREWORD... xv
Chapter 1: General introduction ... 1
1.1 The economic relevance of Picea glauca ... 2
1.2 The spruce budworm and its impact on the forest industry in Canada ... 4
1.3 Conifer defensive strategies against insect pests ... 8
1.3.1 Plant chemical defenses: naturally-occurring constitutive resistance of white spruce against the SBW ... 10
1.4 Genetic control of the natural resistance against the SBW ... 15
1.5 Context of the project ... 18
1.6 Objectives and hypothesis ... 19
1.6.1 Genetic heritability (Chapter 2) ... 19
1.6.2 Role of the acetophenone resistance biomarkers in insect-host interactions (Chapter 3) ... 20
1.7. References ... 22
Chapter 2: Genetic control and evolutionary potential of a constitutive resistance mechanism against the spruce budworm (Choristoneura fumiferana) in white spruce (Picea glauca) ... 31
2.1 Résumé ... 31
2.2 Abstract ... 32
2.3 Introduction ... 33
2.4 Material and methods ... 36
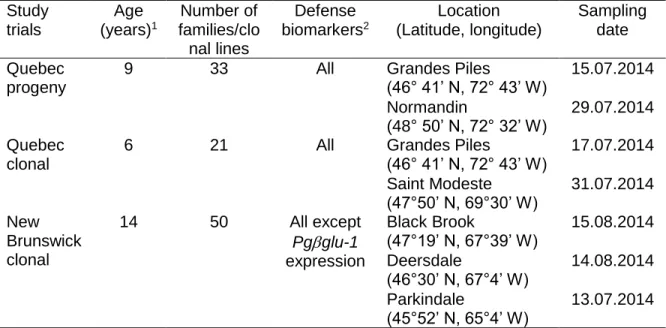
2.4.1 Plant material ... 36
2.4.2 Tissue sampling and preparation ... 37
2.4.3 RNA extraction ... 38
2.4.4 RT Quantitative PCR ... 38
2.4.5 Extraction and quantification of phenolic compounds ... 39
viii
2.5.1 Heritability estimates ... 39
2.5.2 Genetic and phenotypic correlations between defense traits ... 40
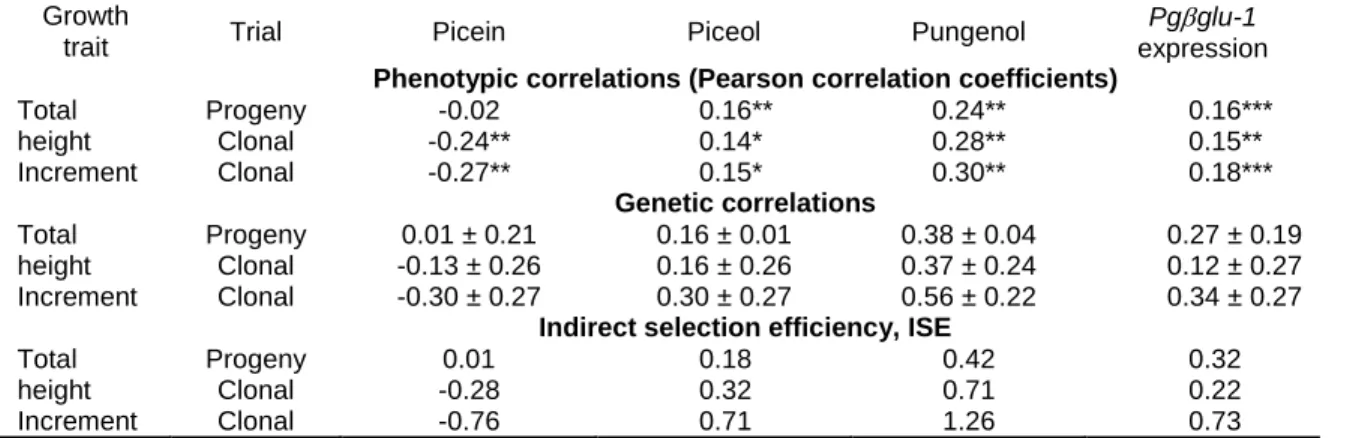
2.5.3 Trade-offs between growth and resistance against the SBW ... 41
2.6 Results ... 42
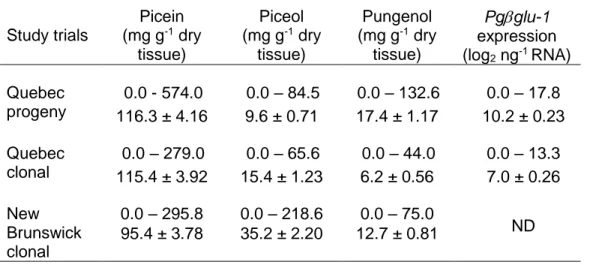
2.6.1 Large phenotypic variation in defense biomarkers ... 42
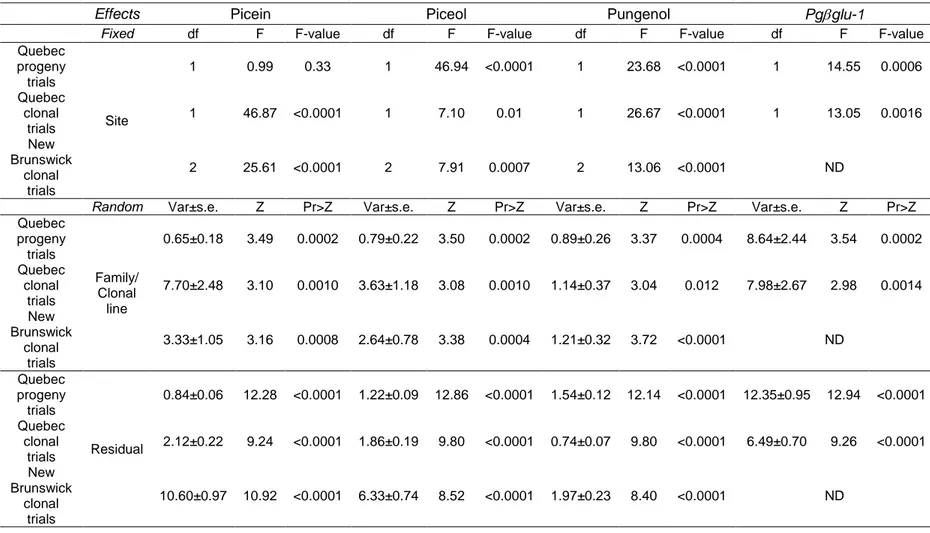
2.6.2 Phenotypic variation in defense traits is under strong genetic control ... 46
2.6.3 The SBW resistance biomarkers are highly correlated genetically and phenotypically ... 47
2.6.4 A trade-off is detected for picein but not between resistance biomarkers and growth ... 48
2.7 Discussion ... 49
2.7.1 Genetic control of SBW defense biomarkers ... 50
2.8 Acknowledgements ... 56
2.9 References ... 57
2.10 Supplementary material ... 61
Chapter 3: Interaction between a Picea glauca defensive mechanism and a major coniferous insect defoliator Choristoneura fumiferana... 63
3.1 Résumé ... 63
3.2 Abstract ... 64
3.3 Introduction ... 65
3.4 Material and methods ... 67
3.4.1 Plant material ... 67
3.4.2 Extraction and quantification of acetophenone compounds ... 68
3.4.3 Insect rearing trials ... 69
3.5 Data analysis ... 70
3.5.1 Developmental variation of the foliar acetophenones ... 70
3.5.2 Inter-annual variation of the acetophenones ... 70
3.5.3 Phenological windows of resistance ... 70
3.5.4 Inducibility of the defense biomarkers ... 70
3.6 Results ... 71
3.6.1 Developmental and intra-annual variation of acetophenones within and between tree phenotypic classes ... 71
ix
3.6.3 Impact of tree phenology on SBW survival, growth and development ... 76
3.6.4 Inducibility of the resistance acetophenones ... 79
3.7 Discussion ... 80
3.7.1 Developmental and intra-annual variation of acetophenone compounds in R and NR trees ... 80
3.7.2 Inter-annual variation of the acetophenones accumulation ... 81
3.7.3 Phenological stages affect host resistance... 82
3.7.3.1 Matched phenological window ... 82
3.7.3.2 Delayed phenological window ... 83
3.7.4 Inducibility of the phenolic compounds piceol and pungenol ... 84
3.8 Conclusions and perspectives ... 86
3.9 References... 86
3.10 Supplementary material ... 92
Chapter 4: General conclusions ... 94
4.1 Major findings and critical assessment of the present work ... 95
4.1.1 Narrow and broad sense heritability of the defense biomarkers ... 95
4.1.2 Genetic and phenotypic correlations between defense biomarkers ... 96
4.1.3 Absence of trade-offs between defense biomarkers and primary growth ... 97
4.1.4 White spruce-spruce budworm interaction ... 99
4.1.5 Intra-annual developmental variation... 100
4.1.6 Inter-annual variation of acetophenones in current year foliage ... 100
4.1.7 Host-tree phenology and its impact on resistance ... 101
4.1.8 Inducibility potential of the resistance biomarkers ... 102
4.2 Perspectives ... 104
4.2.1 Genetic control of the resistance biomarkers ... 104
4.2.2 Trade-offs between resistance biomarkers and growth ... 105
4.2.3 Host-insect interaction ... 105
x LIST OF TABLES
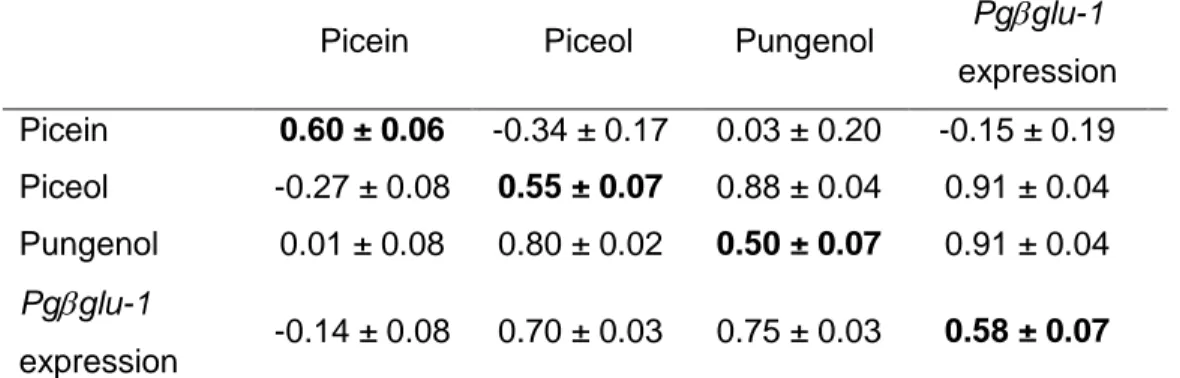
Table 1.1. Estimates of narrow and broad sense heritability of defense traits in different plan taxa. ... 17 Table 2.1. Characteristics and sampling details of study trials. ... 37 Table 2.2. Phenotypic variation of picein and resistance biomarkers. ... 43 Table 2.3. Summary of the mixed model analyses for defense biomarkers from the study trials of Picea glauca ... 45 Table 2.4. Narrow sense heritability estimates (h2 ± standard error, s.e.), genetic
correlations (rG ± s.e.) and phenotypic correlations (rP ± s.e.) for picein and resistance
biomarkers in Quebec progeny trials of Picea glauca. ... 46 Table 2.5. Broad sense heritability heritability estimates (H2 ± standard error, s.e.),
genetic correlations (rG ± s.e.) and phenotypic correlations (rP ± s.e.) for picein and
resistance biomarkers in Quebec and New Brunswick clonal trials of Picea glauca... 47 Table 2.6. Trade-offs between growth traits and picein and defense biomarkers for Quebec progeny and clonal trials of Picea glauca.. ... 49 Supplementary table S1. 1Provenances of Quebec progeny trials ... 61 Supplementary table S2. 1Provenances of Quebec clonal trials ... 62 Table 3.1. Summary of the analysis of variance for main effects and their interactions for the foliar acetophenones picein, piceol and pungenol ... 72 Table 3.2. Summary of the analysis of variance for main effects and their interactions for the foliar acetophenones picein, piceol and pungenol and defoliation in white spruce; larval survival and developmental traits of the SBW evaluated in the two resistance windows.. ... 77 Table 3.3. Mean ± standard error for the matched phenological window and the delayed phenological window.. ... 78 Table S4. Compilation of piceol and pungenol mean foliar content in Picea glauca from diverse locations and age. Means ± SEM are presented when available. ... 93
xi LIST OF FIGURES
Figure 1.1: Estimated ranges of some spruce budworm biotypes in North America (after Harvey, 1985). Image from Volney and Fleming (2007). ... 5 Figure 1.2: Metabolic pathways of phenolic compounds biosynthesis. ... 11 Figure 1.3: a) Theoretical schematic of the biosynthesis of piceol (Negrel and Javelle, 2010; Mageroy et al., 2015) and b) pungenol, the dotted arrows represent reactions not confirmed yet (Mageroy et al., 2017). ... 14 Figure 2.1: Density plots for (a), (b) piceol, (c) pungenol and (d) Pgglu-1 gene
expression of full-sib progeny and clonal trials.. ... 44 Figure 3.1: Intra-annual variation of the acetophenone compounds in two types of foliage of white spruce trees.. ... 74 Figure 3.2: Inter-annual variation of acetophenones in current year foliage from 2014 to 2016.. ... 75 Figure 3.3: Inducibility of resistance markers in white spruce. ... 79 Figure S1: Inter-annual variation of acetophenones in current year foliage for 2015 and 2016.. ... 92
xii
LIST OF ABBREVIATIONS
C4H; cinnamate-4-hydroxylasecDNA; Complementary DNA 4CL; 4-coumaroyl CoA-ligase DIR, Delayed induced response DNA; Deoxyribonucleic acid
E/M ratio; ratio of all eggs laid to the number of locally emerged moths JDI; J.D. Irving Limited
h2; Narrow sense heritability
H2; Broad sense heritability
HPLC-MS; High Performance Liquid Chromatography-Mass Spectrometry IR; Induced response
MFFP; Ministère des Forêts, de la Faune et des Parcs of Quebec NR; Non-Resistant
PAL; phenylalanine ammonia-lyase PCR; Polymerase chain reaction
Pgbglu-1; Picea glauca b-glucosidase-1
PgUGT5b; Picea glauca Family 5b glycosyltransferase R; Resistant
REML; Restricted Maximum Likelihood rG; Genotypic correlation
rp; Phenotypic correlation RIR, Rapid induced response RNA; Ribonucleic acid
RT; Reverse transcription SBW; spruce budworm VA; Additive genetic variance VP; Phenotypic variance
xiii
A la memoria de mi abuelo Roberto Méndez Hernández Te extraño
xiv
ACKNOWLEDGEMENTS
This has been the most challenging period of my life but also the most gratifying, thanks to the people involved.
First, I would like to express all my gratitude and admiration to Dr. John MacKay for giving me the opportunity to be part of his team, and through his unrelenting support, showing me that his patience and commitment have no known boundaries.
I also want to address my special thanks to Dr. Éric Bauce for giving me his support and patience in the most difficult moments of this journey.
I extend my sincere gratitude to Drs. Ilga Porth, Nathalie Isabel and Louis Bernier for evaluating this thesis.
I need to address a special thank you to Geneviève Parent for always raising the bar too high! But at the same time, for being there to help me make the best of the unexpected outcomes of science.
Thank you to Sebastien Caron and Isabelle Giguère for their invaluable guidance in the transcriptomics laboratory. My recognition to Martin Charest for assistance in the field and the Entomology laboratory. I extend my gratitude to my field and laboratory colleagues, principally Patrick Lenz.
I thank the ones who make possible every project I start, my parents Adela and César, my sister Angélica, my brother Roberto and especially my Rafael, as I always say, for filling my life with hope, love and strength. Without you, I would be nothing.
My Jean-François, I had to cross two countries to finally meet you. I started this project thanks to my family but it is because of your support, comprehension and love that I can finish it.
This thesis and PhD studies were possible thanks to the funding and scholarships from Fonds de Recherche du Québec – Nature et Technologie, NSERC of Canada and INIFAP, Mexico.
xv
FOREWORD
This thesis contains a general introduction (chapter 1), two main chapters presenting results of experimental work (2 and 3) written and presented in the format of scientific publications, and general conclusions and perspectives (chapter 4).
Chapter 2 has been submitted on July 19th, 2017 and accepted on December 11th
as: Méndez-Espinoza C, ParentG, Lenz P, Rainville A, TremblayL, AdamsG, McCartneyA, BauceÉ and J MacKay. Genetic control and evolutionary potential of a constitutive resistance mechanism against the spruce budworm (Choristoneura fumiferana) in white spruce (Picea glauca).
Chapter 3 will be submitted after modifications as: Méndez-Espinoza C, Parent G, MacKay J and Bauce É. Interaction between a Picea glauca defensive mechanism and a major coniferous insect defoliator Choristoneura fumiferana.
Field and laboratory work, data analysis and redaction of chapters 2 and 3 have been prepared by Claudia Méndez-Espinoza under the supervision of John MacKay as thesis co-director and formerly research director, and Éric Bauce as the present director and formerly co-director.
1 Chapter 1: General introduction
Vertebrate and invertebrate organisms consume between 5% and 20% of the vascular plant biomass each year (Turcotte et al., 2014) as their primary source of carbon, nitrogen, and of many macro and microelements (Walters, 2011). Consequently, plants have evolved diverse and effective defensive strategies against a wide range of herbivores. In conifers, coevolutionary plant-insect relationships started 400 million years ago (Fürstenberg-Hägg et al., 2013) and defense mechanisms are considered a main factor of their success and vast colonization capacity (Phillips and Croteau, 1999; Franceschi et al., 2005). The genetic variation and genetic control of the defensive characters are essential for evolution and both determine the capacity of populations to respond to natural and artificial selection. Heritability of defensive traits against insect pests is known in diverse plant taxa from the angiosperms Zea mays and Populus tremuloides to the gymnosperms Abies balsamea and Pinus radiata, among others (Rea et al., 2000; Stevens et al., 2007; Edwards et al., 2016; Moreira et al., 2013).
This thesis investigates defense focusing on the spruce budworm (Choristoneura
fumiferana (Clemens)), the most destructive insect defoliator of Picea and Abies
species in North America (Hajek and Frankenhuyzen, 2017) and white spruce (Picea glauca (Moench) Voss), one of the most important species for the forest industry in Canada (NRC, 2016). The search for pest control approaches to reduce the impact of the spruce budworm (SBW) outbreaks is a long-standing problem and is pillar of integrated forest protection. Currently, the main strategy to control SBW damage is the aerial spray with Bacillus thuringensis, and although there have been continuous improvements to the formulation and application methods, the efficacy is still variable, which has been associated with tannin concentrations in foliage of white spruce and balsam fir (Carisey et al., 2004; Bauce et al., 2006).
The use of elevated Btk concentrations has been suggested to improve the efficacy; however, it is expensive and the environmental consequences are
2
unknown (Fuentealba et al., 2012). In addition, Bt spraying is used in only about 5% of areas threatened by SBW (Arsenault, 2015). This thesis contributes to the study of a complementary approach, which consists in exploiting naturally occurring host resistance against the SBW. I specifically investigated a constitutive resistance mechanism against the spruce budworm (SBW), which has been linked to foliar accumulation levels of the acetophenone compounds piceol and pungenol, and to the expression of the Pgglu-1 gene in white spruce. These traits are referred to hereafter as SBW resistance traits or resistance biomarkers and when the glycoside picein is also included, they are named defense traits or defense biomarkers.
The first chapter presents a review of the background literature on the economic importance of white spruce, the main plant defensive strategies against insects, genetic control of resistance in different plant species and the context of the project including the research goals and hypotheses of the thesis. In the second chapter, we investigate the quantitative genetic control of the resistance mechanism and evaluate the potential trade-off with primary growth to gain insights into the evolutionary potential, defined by Harrison et al. (2014) as “the capacity to evolve in response to changing environments”. The third chapter examines parameters that may influence white spruce-SBW interactions including the developmental variation of the resistance acetophenones (piceol and pungenol) in the host, the impact of host tree phenology on the performance of the SBW and the inducibility of the acetophenones following SBW attack. Last, I include the general conclusions and perspectives.
1.1 The economic relevance of Picea glauca
Conifers are woody plants present in six of the seven continents (Gernandt et al., 2011). They make up over 39% of the world’s forests (Armenise et al., 2012) and 70% of the total diversity of the taxa is found on the Northern Hemisphere (Farjon, 2001). Extant conifers are grouped in six families, 71 genera and nearly 670
3
species. The family Pinaceae is the largest, comprising 11 genera and 238 species, (Gernandt et al., 2011). Conifer trees and forests provide habitats for vertebrates, invertebrates and microorganisms (Harmon et al., 1987), are essential for global carbon fixation and include tree species of major economic importance such as spruce (Picea spp.), pine (Pinus spp.), true firs (Abies spp.) and Douglas fir (Pseudotsuga menziesii) (Ralph et al., 2006).
In Canada, from late 1800s to 1950, the strong demand of wood as fuel, railway, for building ships and bridges, for the production of pulp and paper reduced the wood supply available in primary forests; raising the need of a sustainable management of forest resources (NRC, 2017). At the beginning of the timber industry, natural forests were the only source of wood and pulp, but now, commercial plantations based on tree breeding represent a growing proportion of commercial softwood and may serve to reduce the pressure on primary forests.
The genus Picea belongs to the family Pinaceae and is represented by more than 35 species (Pavy et al., 2013). Picea glauca (Moench) Voss (white spruce) is a species native to North America, its natural distribution spans from Newfoundland and Labrador to Alaska, covering all of the Canadian provinces and northeastern United States (Nienstaedt and Zasada, 1990). Due to its high survival and growth rates, it is one of the most widely used species in Canada, the world’s largest producer of softwood pulp and for newsprint (NRC, 2016). It accounts for 55% of the Canadian annual wood harvest, and has provided employment for up to 5% of the country’s population (Attree et al., 1991).
In general, the main breeding goals for white spruce are the improvement of stem growth and straightness, volume, crown form and branch size and wood physical properties while keeping a wide genetic pool for pest resistance (Gernandt et al., 2011). In Quebec, the white spruce genetic improvement program began at the end of 1950’s, when several provenance and half-sib tests were established with the aim of quantifying the genetics of growth, phenology and wood density traits
4
(Beaulieu, 1996). This was followed by the development of a 1st generation of
breeding based on controlled pollinations and the establishment of seed orchards. Around the year 2000, more controlled crosses using 100 plus-trees selected from the populations stablished in the 50’s for the second generation, which is now providing the germplasm for reforestation (Gernandt et al., 2011). White spruce breeding is also used in New Brunswick and Nova Scotia, where it was started between 1978 and 1982 (Carter and Simpson, 1985) and in Newfoundland and Labrador where it was started in the early 1990s (Gernandt et al., 2011).
1.2 The spruce budworm and its impact on the forest industry in Canada Insect outbreaks, diseases and fire are the three main natural disturbances in Canadian forests (MacLean, 2016). At the end of the 80’s, forest losses due to insect attacks in Canada were estimated at 51.0 million m3 of wood per year,
representing 1.5 fold those caused by wildfire and up to 1/3 of the annual harvest volume (Hall and Moody, 1994). Insects are one of the leading biotic agents influencing forest productivity (Volney and Fleming, 2000) and spruce budworm (SBW) outbreaks are the main threat for P. glauca, due to the insect’s epidemic behavior and the tight interaction between both species associated with their transcontinental and sympatric distribution. SBW (Choristoneura fumiferana Clem), is a lepidopteran native to North America known to exhibit epidemic periods at intervals of 25-38 years in eastern Canada, according to the records of the last 300 years (Jardon et al., 2003).
From 1975 to 2000, the SBW caused moderate to severe defoliation (30-100%) of 450 668 000 ha, representing 64% of the total area damaged by insects in such period (MacLean, 2016). More recently, since 2006, a new outbreak has developed, and the affected land area has doubled every year since then. In 2013, the epidemic had extended over more than 3.2 million hectares (Morneau, 2014) and in 2015, nearly 7 million hectares were damaged by SBW (NRC, 2015).
5
SBW is part of a group of budworms that includes a complex of eight species and 15 biotypes that feed on coniferous trees across the Nearctic (Volney and Fleming, 2007). The taxa can be differentiated according to the larval host plant preference, larval and pupal morphology and adult morphology (Freeman, 1967; Stehr, 1967; MacKay, 1953; Harvey & Stehr, 1967 In Lumley and Sperling, 2010) and it is found across all of Canada’s provinces (Figure 1.1).
Figure 1.1. Estimated ranges of some spruce budworm biotypes in North America (after Harvey, 1985). Image from Volney and Fleming (2007).
There are three major host species for C. fumiferana: balsam fir (Abies balsamea (L.) Mill.), white spruce (Picea glauca (Moench) Voss) and black spruce (Picea
mariana (Mill.) BSP) (Nealis and Régnière, 2004). Red spruce (Picea rubens) and
Norway spruce (Picea abies) are minor hosts. SBW moths emerge from mid-July to early August, eggs are laid on the conifer needles and hatch within 10 days
6
(Royama, 1984). Neonate larvae establish hibernacula on the branches, moult to the second instar and overwinter until early May (Nealis & Régnière, 2004). After emergence, they feed on 1-2 year old needles, or in seed and pollen cones. During early June to the beginning of July (third to sixth instars) they consume current-year shoots. In early July, pupation occurs on the foliage and adults eclose 8-12 days later (Royama, 1984).
After almost 70 years of research, there is still no general agreement about the ecological factors governing the SBW epidemic behavior (Sturtevant et al., 2015). Even though some authors consider that there are three main hypotheses to explain the SBW population dynamics (Sylviculture Hypothesis, Epicenter Hypothesis and Multiple Equilibria Hypothesis), we only consider two of them. We exclude the Sylviculture Hypothesis because there is evidence of SBW epidemic behavior much earlier in history than the beginning of the forest management (records of SBW outbreaks from more than 300 years ago (Jardon et al., 2003). The Epicenter Hypothesis and the Multiple Equilibria Hypothesis are briefly described below.
The Epicenter Hypothesis. The idea of epicenters or hot spots appeared from
studies of outbreaks in New Brunswick. These epicenters governed the spread of outbreaks (Greenbank, 1957). This was later supported by the results of analysis of defoliation records in 1967-1978 at seven infested sites in Quebec (Hardy et al., 1983). At the beginning, host species were not the most abundant in epicenters, but ecological (Hardy et al., 1983) and nowadays anthropogenic (Miller and Rusnock, 1993) perturbations promote the proliferation of fir and white spruce. Observations from infestations in Ontario over 1947-1983 showed that SBW densities diminished with distance from known epicenters, the new infestations seemed to originate from previous outbreak sites spreading with the wind and even after outbreak collapse, small remnants persisted (Mattson et al., 1988).
The Multiple Equilibria Hypothesis, also known as the Double Equilibria
Hypothesis, based on a report by Morris (1963) explains how outbreaks start and collapse, but not their cyclic behavior. The hypothesis postulates that predators
7
and parasitoids control the SBW populations, but climatic conditions over several years such as warm summer temperatures favor the insect population growth rates, facilitating escape from the regulation by natural enemies (Wellington et al. 1950). After several years under defoliation, trees are no longer capable of compensating and replacing damaged foliage in quality and quantity (Bauce and Hardy, 1988) so the SBW population decreases and natural enemies regain control. Last, the oscillation is principally determined by intrinsic factors, e.g. parasitoids, diseases and overall unknown causes. The apparent rate of oviposition or E/M ratio (ratio of all eggs laid to the number of locally emerged moths) is the major density-independent determinant of budworm population dynamics (Royama, 1984).
Current altered climate regimes could change historical patterns (Volney and Fleming, 2000). In fact, a new SBW outbreak started in Quebec in 2006 and according to the location of the epicenters it is believed that climate change is already having an effect on the species’ distribution (Régnière et al., 2012). However, higher temperatures should increase the rates of foliage development and hence reduce the 'window of opportunity' when the herbivore has access to immature foliage (Fleming and Volney, 1995).
To reduce the risks of economic losses from insect epidemics, there is a constant search for pest control approaches to reduce the impact of the spruce budworm (SBW) outbreaks. Many of them are still in the research and development phase,for example, the use of fungal anti-insect compounds like rugulosin from the endophyte Phialocephala scopiformis (Frasz et al., 2014), spinosad from
Saccharopolyspora spinosa (Thompson et al., 2002) or the disruption of diapause
and induction of precocious and incomplete moulting by a commercial insecticide which composition is based on tebufenozide (Doucet et al., 2007).
8
1.3 Conifer defensive strategies against insect pests
In general, plant defense against insect herbivory is represented by two main strategies: tolerance and defense. Tolerance consists of recovery by regrowth, minimizing the negative effects on plant productivity or fitness caused by tissue loss. Destruction of apical meristems releases the previously suppressed modules from apical control, and allows the other meristems to continue the plant growth (Haukioja and Koricheva, 2000). There are two main classes of defense: mechanical and chemical. Mechanical defenses prevent or restrict damage through specialized morphological and anatomical structures, while the chemical defenses are manifested through secondary metabolites that interfere with herbivore feeding, settling or reproduction on the host, due to their toxic, repellent and/or antinutritional effects (War et al, 2012; Mitchell et al., 2016).
The mechanical and chemical defense mechanisms can be constitutive or inducible. Constitutive defenses develop independently of insect attack. They represent the first step to prevent an initial attack (Franceschi et al., 2005). For example, constitutive resistance was shown in interior spruce (Picea
glauca-engelmannii), where trees putatively resistant and susceptible to attacks by white
pine weevil (Pissodes strobi) showed differences in traits such as resin duct size and number, bark thickness, and terpene content. Comparison of 18 725 genes for Resistant (R) and Non-Resistant (NR) trees revealed 54 upregulated and 137 downregulated genes in the R genotypes (Verne et al., 2011).
The inducible defenses are triggered upon tissue damage and are an evidence of plant adaptive plasticity (Agrawal, 1998). In Sitka spruce (Picea sitchensis) following attack by the white pine weevil, enhanced –pinene synthase gene expression and terpene synthases activity induced terpenoid defenses associated with the production of traumatic resin ducts in phloem and xylem tissue (McKay et
al., 2003).
Along with the previous cases, we can cite some general types of defenses deployed by conifers:
9
- Bark. Its thickness represents a first barrier against invaders. Contains concentric layers of mechanical and chemical defenses including dead cells with lignified or suberized walls that also contain phenolic compounds, calcium oxalate crystals and resin ducts. One of the most important components of the bark is the polyphenolic parenchyma cells due to the inducible defense responses they produce (Franceschi et al., 2005) and because they have been implicated in defense signaling through their abundant plasmodesmata (Krekling et al., 2000).
- Cortical stone cells (sclereids). They are a type of sclerenchyma cells with very thick and lignified secondary cell walls localized in the cortex of apical shoots (Evert 2006; Franceschi et al. 2005). The stone cells of resistant (R) Sitka spruce (Picea sitchensis) genotypes contain more lignin (G-lignin), cellulose, xylan and mannan than those of susceptible (S) trees. This has been associated with the differential expression of a monolignol biosynthesis gene: coumarate 3-hydroxylase. The stone cells provide a reinforcement considered as an effective defense mechanism against the pine weevil (Pissodes strobi) (Whitehill et al., 2016).
- Oleoresin. It is the best known chemical defense in conifers (Lippert et al., 2007) and a complex mixture of terpenoids (turpentine and rosin). Turpentine evaporates when exposed to the atmosphere, so the resin acids polymerize forming a rigid and chrystalline barrier that seals the damaged site trapping the attackers. The Pinus species produce and store large amounts of oleoresin, while the genus Abies accumulates just small quantities. A majority of conifers have levels that are intermediate to these high and low extremes (Phillips and Croteau, 1999).
- Cuticle and epicuticular wax layer of needles. The plant cuticle is impregnated by cutin and covered by waxes (Dragota and Riederer, 2007). Insects evaluate the chemistry of the plant surface via probing behavior to determine whether or not they will proceed to feeding (Daoust et al., 2010) or oviposition (Steinbauer et al., 2004) as response to the monoterpenes in the epicuticular wax (Despland et al., 2016). The epicuticular wax crystals
10
are ultra-hydrophobic and reduce adhesion of external particles (Barthlott and Neinhuis, 1997).
- Insect-host phenological asynchrony. This is an important defense system against defoliators, since early or late bud development affect the quantity and quality of foliar tissue (Clancy, 2002). Such is the case of white spruce leaf age effects on Zeiraphera canadensis. Young needles in expansion during the 4-5 days after bud burst are the best food source for the first larval stages and survival is significantly reduced after that period (Quiring, 1992). So, the existence of genotypes with a faster or earlier foliar development is advantageous for the tree species. In Douglas fir (Pseudotsuga menziesii var. glauca) R et NR trees were identified based on the level of budworm defoliation. The R individuals had later bud burst than the NR individuals, and thus budworm defoliation depended on the degree of synchrony between bud burst phenology, with less defoliation on the R genotypes (Chen et al., 2001).
1.3.1 Plant chemical defenses: naturally-occurring constitutive resistance of white spruce against the SBW
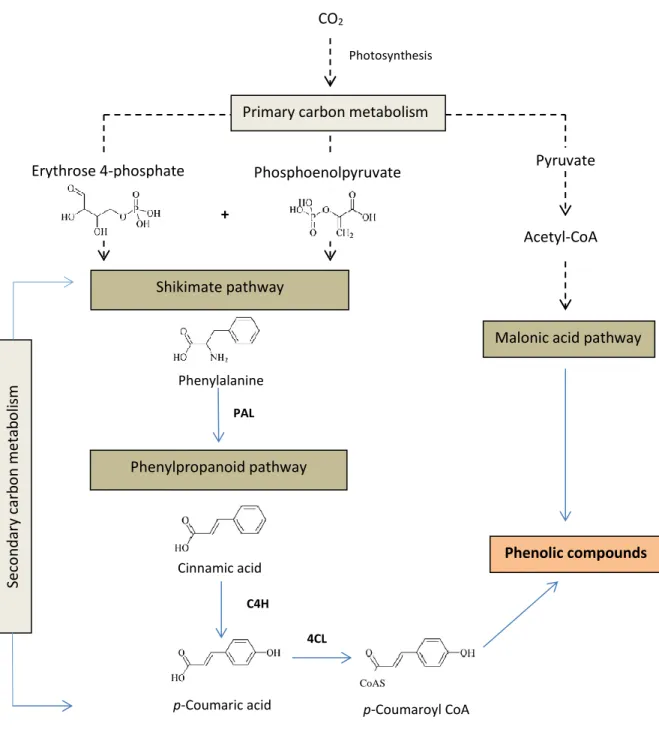
Secondary metabolites have no apparent or direct function in primary physiological processes (Taiz and Zeiger, 2010) and represent from 1 to 10% of the dry mass in plants (Ramawat et al., 2009). Plant secondary metabolites are classified into three chemically different groups: 1) terpenes (pyrethroids, essential oils, limonoids, phytoecdysones, cardenolides and saponins), 2) phenolics (phenylpropanoids, furanocoumarins, flavonoids, lignin, tannins) and 3) nitrogen-containing compounds (alkaloids, cyanogenic glucosides, glucosinolates, non-protein amino acids) (Taiz and Zeiger, 2010). Phenolic compounds are the most widely distributed of the secondary metabolites, accounting for up to 40% of organic carbon in the biosphere (Lattanzio, 2013). Plant phenolics are synthesized from the shikimate/phenylpropanoid or the acetate/malonate/polyketide pathways (Cheynier et al., 2013; Figure 1.2) and are absent in bacteria, fungi and algae.
11
Figure 1.2 Metabolic pathways of phenolic compounds biosynthesis.
Abbreviations: PAL, phenylalanine ammonia-lyase; C4H, cinnamate-4-hydroxylase; 4CL, 4-coumaroyl CoA-ligase. Adapted from Taiz and Zeiger, (2010); Cheynier et al. (2013).
Phenolic compounds are classified in 13 groups according to their skeletons: C6
(simple phenols, benzoquinones), C6-C1 (phenolic acids and aldehydes), C6-C2
4CL H C4H H p-Coumaroyl CoA CoAS Phenolic compounds Cinnamic acid p-Coumaric acid + PAL Phenylpropanoid pathway
Malonic acid pathway Shikimate pathway
Phenylalanine
Photosynthesis CO2
Primary carbon metabolism
Erythrose 4-phosphate Phosphoenolpyruvate
Acetyl-CoA Pyruvate Seco n d ary c arb o n m et ab o lism
12
(acetophenones, phenylacetic acids), C6-C3 (hydroxycinnamic acids, coumarins,
phenylpropanes, chromones), C6-C4 (naphtoquinones), C6-C1-C6 (xanthones), C6
-C2-C6 (stilbenes, anthraquinones), C6-C3-C6 (flavonoids, isoflavonoids,
neoflavonoids), (C6-C3-C6)2,3 (bi-, tri- flavonoids, proanthocyanidin dimers, trimers),
(C6-C3)2 (lignans, neolignans), (C6-C3)n (lignins), (C6)n (catechol melanins,
phlorotannins), (C6-C3-C6)n (condensed tannins) (Cheynier et al., 2013).
Although conifers can defend themselves against many generalist herbivorous insects, some specialized insect species such as bark beetles (Scolytidae), shoot and root weevils (Curculionidae), sawflies (Hymenopteare) and budworms (Lepidopterae) may cause large damages in conifer forests (Ralph et al., 2006). Our special interest on phenolic compounds is derived from the discovery of a constitutive resistance mechanism against Choristoneura fumiferana in the needles of Picea glauca trees (Delvas et al. 2011; Mageroy et al., 2015). The role of phenolic compounds in plant defense has been constantly studied in angiosperms, but the information concerning gymnosperms is limited (Porth et al., 2011).
Two classes of tree phenotypes were identified during a survey from 1998-2007 in a white spruce plantation (Quebec, Canada 45° 53’0’’N, 72° 29’0’’W) severely infested by the SBW. They were named resistant (R) and non-resistant (NR) according to the average defoliation by SBW: 0-20% and 30-70%, respectively (Daoust et al., 2010). Recent work has aimed to explain the occurrence of these contrasted phenotypes (Delvas et al. 2011; Mageroy et al. 2015; Parent et al. 2017).
The foliar chemistry of R and NR trees and the behaviour of sixth instar SBW larvae when fed on needles from both types of white spruce were evaluated before and after the removal of the epicuticular wax layer. Some of the largest differences were represented by 307% more -pinene, 476% more myrcene and 20% more for total phenolics in the R trees. The authors also found that 80% of budworm larvae probed and fed on NR foliage, but only 35% of those on R needles transitioned from probing to feeding (Daoust et al., 2010).
13
Later, Despland et al. (2011) aimed to discriminate between deterrence or toxicity of condensed tannins and simple phenolic compounds extracted from NR and R trees from the same plantation studied by Daoust et al. (2010). There was no difference in the quantity and duration of meals when comparing both types of needles. However, longer pauses between meals were detected when feeding on R foliage, which explained a difference in food consumption of approximately 50% between NR and R foliage. The results suggested a toxic or anti-digestive effect associated with a slower digestion of the R foliage or alternatively, that a toxin in the R foliage slows down the feeding behavior.
Two phenolic compounds were identified: piceol (4-hydroxy-acetophenone) and pungenol (3, 4-hydroxy-acetophenone) which accumulate at high levels in the R trees but are undetected or present at negligible concentrations in the NR trees (Delvas et al., 2011). The addition of both of the aglycon phenolics to artificial diet decreased larval survival in SBW, caused a delayed development and reduced pupal mass. It was also observed that the precursors of piceol and pungenol, the phenolic glucosides picein and pungenin, accumulate in the two white spruce chemotypes. So, the authors hypothesized that R trees have a major enzymatic expression in the phenolic pathway that may be lacking in the NR trees (Delvas et
al., 2011).
A comparative transcriptome profiling of almost 24000 genes from both white spruce phenotypes led to the identification of a gene overexpressed only in the R trees: Pgglu-1 (Mageroy et al., 2015). The R and NR trees were monitored 3 and 6 years after the outbreak, revealing that piceol and pungenol were significantly higher in R than in NR trees. Biochemical tests showed that piceol and pungenol were formed from the glucosides picein and pungenin, respectively, as the result of the enzymatic activity of the glycosyl hydrolase enzyme coded in the Pgglu-1 gene. The study also showed that differential expression of the beta-glucosidase gene was a main factor responsible for the variations in constitutive resistance against C. fumiferana in white spruce (Mageroy et al., 2015). The theoretical metabolic pathway of acetophenones is illustrated in Figure 1.3, although the
14
enzymes involved are not known or not confirmed yet (Negrel and Javelle, 2010; Mageroy et al., 2015, Mageroy et al., 2017). The synthesis of piceol implicates coumaroyl-CoA while pungenol originates from caffeic acid, followed by glucosylation to produce picein and pungenin, respectively. Addition of a monosaccharide group to secondary compounds enhances the bioavailability and solubility, so as reduced toxicity (Tiwari et al., 2016). After, the activity of the
Pgglu-1 enzyme allows the synthesis of the aglycons in the R trees.
Figure 1.3 a) Theoretical schematic of the biosynthesis of piceol (Negrel and Javelle, 2010; Mageroy et al., 2015) and b) pungenol, the dotted arrows represent reactions not confirmed yet (Mageroy et al., 2017).
More recently, the synchrony between the highest accumulation levels of the aglycon compounds and the most damaging phase of the SBW was revealed by a survey in a common garden during a growing season. Moreover, the effect of the
Coumaroyl-CoA Caffeic acid Picein OH Pungenin Piceol Pungenol Pgglu-1 Pgglu-1 4-coumarate: CoA ligase
Caffeoyl-CoA hydratase 3, 4-dihydroxiphenyl- -hydroxypropionyl-CoA 3,4-dihydroxyphenyl--ketopropionyl-CoA dehydrogenase thioesterase 3,4-dihydroxyphenyl--hydroxypropionyl acid decarboxylase Pungenol PgUGT5b a) b)
15
SBW herbivory on the white spruce population structure was elucidated and was partially explained (26%) by historical damage levels from C. fumiferana, temperature and forest type. The authors concluded that the resistance mechanism studied was effective (Parent et al., 2017).
1.4 Genetic control of the natural resistance against the SBW
A gene is a segment of DNA -deoxyribonucleic acid- containing the coding information to synthesize proteins (Conner and Hartl, 2004) and the physical entity carrying the hereditary characters (Hartl, 2000). The totality of DNA in the nucleus, chloroplast or mitochondria of a cell is the genome (Hartl, 2000), the genotype is formed by the genes of an organism and the physical or biochemical expression of the genotype is known as the phenotype (Conner and Hartl, 2004). The traits characterizing a tree are the result of its genotype (species, provenance, within tree), the environment (climate, biotic agents, tree density) where it has grown and their interaction as expressed in the equation P = G + E + G x E (phenotype = genotype + environment + genotype x environment) (White et al., 2007).
Genetic differences may be associated with phenotypic variations between individuals and populations are the basis for enabling both evolution (through natural processes) and genetic improvement (based on artificial selection). Phenotypic variation may result from additive and non-additive genetic effects. The additive genetic variance refers to the contribution of a specific allele to the mean phenotype of the population and the non-additive sources –dominance and epistasis – imply allelic interactions at a particular locus or in different loci, respectively (Bryers, 2008).
On this basis, we can quantitatively define heritability as the ratio of the total variation due to additive or non-additive genetic factors at a specific moment or age (Visscher et al., 2008). The estimation of heritability is widely used to evaluate whether traits or phenotypes are the consequence of biological or environmental causes, to predict gain from artificial selection (Holland et al., 2003) and to know
16
how rapidly the mean phenotype could evolve in response to artificial or natural selection (Conner and Hartl, 2004).
Quantitative genetics lets us estimate two types of heritability, narrow-sense heritability (h2) and broad-sense heritability (H2). Narrow-sense heritability (equation 1) is generally the most useful and relevant in genetic improvement programs because the response to artificial selection depends on additive genetic variance (Wray and Visscher, 2008) and in outbreeding species it has larger effects on evolutionary rates than non-additive components (Conner and Hartl, 2004). Broad-sense heritability (equation 2) includes dominance and epistasis and is indicated for clonal or highly self-fertilizing species (Conner and Hartl, 2004).
Equation 1: h2 = VA/VP,
where h2 is the ratio of additive genetic variance to the total phenotypic variance.
Equation 2: H2 = VG/VP,
where H2 is the ratio of total genetic variance (dominance and epistasis) to total phenotypic variance.
Due to their relevance in evolution and potential application in genetic improvement, narrow and broad sense heritability of resistance against insect pests have been the objectives of many studies in angiosperms and gymnosperms species (Table 1.1). A fundamental property of both types of heritability estimates is that they are trait-specific, population-specific and also specific to the environmental conditions (White et al., 2007), since the individuals within and between populations are different, and every trait has its own evolutionary history.
17
Table 1.1.Estimates of narrow and broad sense heritability of defense traits in different plan taxa.
Plant species Trait/Localization in the plant
Heritability Insect species pest Reference
Zea mays Damage (%) caused by the insect. Not a specific resistance mechanism explained. h2=0.47 ± 0.03 Spodoptera frugiperda, fall armyworm Rea et al., 2000 Gossypium hirsutum Gossypol, terpenoid aldehydes (not directly tested)/Most tissues and organs H2= 0.36 to 0.79 Frankliniella occidentalis, thrips Zhang et al., 2014 Eucalyptus tricarpa Sideroxylonal/leav es H 2=0.60 Anoplognathus montanus, christmas beetle Andrew et al., 2007 Populus tremuloides Condensed tannins, phenolic glycosides Condensed tannins H2 = 0.58 Phenolic glycosides H2 = 0.71 Malacosoma disstria, forest tent
caterpillar; Choristoneura conflictana, large aspen tortrix Stevens et al., 2007 Abies balsamea Damage (%) caused by the insect. Not a specific resistance mechanism explained. H2= 0.50 Mindarus abietinus,
balsam fir twig aphid
Edwards et al., 2016 Pinus radiata Total phenolics/needles , non-volatile resin/stem Total phenolics h2 = 0.18 ± 0.11 Non-volatile resin h2 = 0.91 ± 0.20 Thaumetopoea pityocampa, defoliator; Hylobius abietis, phloem feeder Moreira et al., 2013
18
Besides the estimation of heritability, other genetic parameters can be useful for understanding the genetic basis of phenotypic variation including phenotypic correlations (rp) and genetic correlations (rG) between traits. The rp measures the strength of the phenotypic association between two traits, which may be caused by genetic or environmental factors. Similarly, the rG measures the genetic association between two traits, where the main cause is pleiotropy or linkage. Pleiotropy means that a single gene can affect multiple, apparently unrelated, phenotypes (Lobo, 2008). For linkage, two or more loci affect different traits, but are inherited together since they are in linkagedisequilibrium due to their physical proximity on the same chromosome (Saltz et al., 2017).
Correlation between traits is significant because selection on a given trait may cause indirect effects on the correlated characters and their distribution in the population (Lande and Arnold, 1983). This is known as correlated response to selection or indirect selection (White et al., 2007) and is of major concern since natural and artificial selection act on many traits simultaneously (Lande and Arnold, 1983). There are three types of genetic correlations: 1) trait-trait correlations, the genetic correlation between two different traits; 2) age-age correlations or juvenile-mature correlations, the genetic correlation between the same character expressed at two different ontogenic moments; and 3) the genetic correlation of the same trait in two different environments (White et al., 2007).
1.5 Context of the project
Unlike agricultural practices, most forest insect epidemics cannot be managed with crop rotation or intensive chemical pesticide applications. Forest protection strategies may use natural host defensive mechanisms against insect herbivory, and thus reduce the use of pollutant and persistent pesticides (War et al., 2012). If there is a correlation between plant secondary metabolites and a reduction in the negative impact of a particular pest, then genotypes could be selected according to the composition of specific tissues as biomarkers of resistance (Henery, 2011).
19
In the present thesis, I investigated three resistance biomarkers which I considered as surrogate phenotypes for predicting SBW resistance. The resistance biomarkers included the accumulation of piceol and pungenol and the expression of the Pgglu1 gene (Parent et al., 2017). Picein, the glucoside acetophenone and precursor of piceol, was also evaluated, and when considering the four traits I refer to them as defense biomarkers. Although the use of insect-resistant trees is a promising option for silvicultural techniques with potential for large-scale use, until now, genetic selection of white spruce trees that are resistant against the SBW has not been directly carried out due to lack of knowledge about the genetics of the defensive mechanisms against this lepidopteran.
The central goal of my project was to evaluate whether the traits implicated in white spruce defense against the SBW are genetically controlled and to know the impact that the environment exerts on them. The first signs of the inheritance of the defense biomarkers were obtained from 1-year-old progeny of R and NR trees grown in a greenhouse. Only the progeny of R trees expressed the Beta-glucosidase gene after an induction treatment with methyl jasmonate (Mageroy et
al., 2015). Later, the genetic control of the defensive traits was estimated (Parent et al., 2017), but the environmental effect was not evaluated and the nature of the
samples used could have resulted in an overestimation of the genetic parameters.
1.6 Objectives and hypothesis
My thesis focused on the analysis of SBW resistance biomarkers and aimed to understand their genetic heritability and their role in insect-host interactions. The specific objectives are related to each of these aims.
1.6.1 Genetic heritability (Chapter 2)
Heritability and genetic correlations are considered the two principal genetic parameters that shape evolutionary changes in quantitative characters (Roff, 1996). I studied both types of genetic parameters in full-sib progeny and clonal trials established in different bioclimatic regions in Quebec, Canada. I also
20
assessed the potential trade-offs between the defense biomarkers and primary growth (height and height increment). This work aimed to provide basic knowledge to evaluate the usefulness of the resistance biomarkers in tree breeding and to know the evolutionary potential of the defense traits. Access to progeny tests and clonal trials was facilitated via collaborators at the Ministère des forêts, de la faune et des parcs du Québec and JD Irving, New Brunswick.
Specific objectives were to:
Objective 1. Estimate the narrow and broad sense heritability of the defense
biomarkers.
Objective 2. Evaluate the environmental effects on the defense biomarkers
expression.
Objective 3. Test the potential trade-offs between resistance and primary growth.
Hypotheses:
Hypothesis 1. The additive genetic control of the defense biomarkers is stronger
than the non-additive control in white spruce.
Hypothesis 2. The SBW resistance biomarkers will be expressed at the same order
of magnitude regardless of the environmental differences and variations.
Hypothesis 3. The resource and energy investment to synthetize the SBW
resistance biomarkers imply a metabolic cost that negatively impacts on white spruce growth.
1.6.2 Role of the acetophenone resistance biomarkers in insect-host interactions (Chapter 3)
The study of genetic parameters is a key step for developing strategies to minimize the risks that the SBW outbreaks represent for forest plantations. However, it is also essential to understand the role of resistance biomarkers and the parameters that may influence their impact in the insect-host relationship.
21
Therefore, for the third chapter of this thesis, I investigate the relationship between the acetophenones linked to resistance and insects. I present the first combined analysis of the impact of tree development and phenology on the expression of resistance. The impact of tree phenology on SBW performance was analyzed by comparing the efficacy of host resistance in two phenological windows and last, I evaluated the potential inducibility of the defense mechanism. I used white spruce trees from a clonal bank for insect rearing experiments on the field. This material is advantageous because the trees are 16 years old, represent a broad genetic background, and show extensive variation in the biomarkers of interest to this study.
To perform the insect rearing trials in the field, access to the Valcartier Experimental Forest Station of Natural Resources Canada was facilitated via collaborators of the federal government of Canada.
The specific objectives were to:
Objective 4. Characterize the developmental variation by monitoring seasonal
fluctuation of acetophenones in previous and current year foliage.
Objective 5. Characterize the inter-annual variation of the acetophenones in the
current year foliage of white spruce.
Objective 6. Assess the impact of tree phenology on resistance against SBW by
comparing the effects of two different host tree phenological windows on SBW biological performance.
Objective 7. Evaluate the potential of inducibility of the defense acetophenones
following SBW attack.
Hypothesis
Hypothesis 4. The acetophenones variation depends on the phenological stage of white spruce.
Hypothesis 5. The SBW resistance mechanism is expressed in white spruce at a
consistent level from year to year despite the inter-annual variations of environmental factors.
22
Hypothesis 6. The phenological stage of white spruce trees determines the
efficiency of resistance.
Hypothesis 7. The constitutive resistance mechanism is efficient; therefore the
SBW feeding will not induct the accumulation of the resistance biomarkers.
1.7. References
Agrawal A. 1998. Induced Responses to Herbivory and Increased Plant Performance. Science. 279: 1201-1202.
Andrew RL, Wallis IR, Hardwood CE, Henson M, Foley WJ. 2007. Heritable variation in the foliar secondary metabolite sideroxylonal in Eucalyptus confers cross-resistance to herbivores. Plant animal interactions. Oecologia. 153: 891-901. Armenise L, Simeone M, Piredda R, Schirone B. 2012. Validation of DNA barcoding as an efficient tool for taxon identification and detection of species diversity in Italian conifers. European Journal of Forest Research. 131: 1337– 1353.
Arsenault Y. 2015. Les interventions de lutte directe de la SOPFIM contre la tordeuse des bourgeons de l’épinette. Les colloques du SCF-CFL. Service canadien des forêts. Centre de foresterie des Laurentides.
Attree SM, Dunstan DJ, Fowke LC. 1991. White Spruce [Picea glauca (Moench) Voss] and Black Spruce [Picea mariana Mill) B.S.P.]. In: Biotechnology in Agriculture and Forestry 16. Trees III. Y.P.S. Bajaj (Ed.). Springer-Verlag. Berlin Heidelberg, Germany. 491 pp.
Barthlott W, Neinhuis C. 1997. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta. 202: 1–8.
Bauce E and Hardy Y. 1988. Effects of Drainage and Severe Defoliation on the Raw-Fiber Content of Balsam Fir Needles and Growth of the Spruce Budworm (Lepidoptera, Tortricidae). Environmental Entomology. 17(4):671-674.
Bauce É, Crépin M, Carisey N. 1994. Spruce budworm growth, development and food utilization on young and old balsam fir trees. Oecologia. 97: 499-507.
Bauce É, Kumbaslı M, van Frankenhuyzen K, Carisey N. 2006. Interactions among white spruce tannins, Bacillus thuringiensis subsp. kurstaki, and spruce budworm (Lepidoptera: Tortricidae), on larval survival, growth, and development. Journal of Economic Entomology. 99: 2038–2047.
23
Beaulieu J. 1996. Programme et stratégie d’amélioration génétique de l’épinette blanche au Québec. Catalogue Fo46-18/117, SCF-Québec. Rapport d’information LAU-X-117. Service canadien des forêts.
Broekgaarden C., Snoeren T.A.L., Dicke M. and B. Vosman. 2011. Exploiting natural variation to identify insect-resistance genes. Plant Biotechnology Journal. 9: 819-825.
Byers D. 2008. Components of phenotypic variance. Nature Education. 1 (1): 161. Carisey N, Bauce É, Dupont A, Miron S. 2004. Effects of bud phenology and foliage chemistry of balsam fir and white spruce trees on the efficacy of Bacillus
thuringiensis against the spruce budworm, Choristoneura fumiferana. Agricultural
and Forest Entomology. 6: 55-69.
Carter KK, Simpson JD. 1985. Status and outlook for tree improvement programs in the northeast. Northern Journal of Applied Forestry. 2: 127-131.
Chen Z, Kolb TE, Clancy KM. 2001. Mechanisms of Douglas-fir resistance to western spruce budworm defoliation: bud burst phenology, photosynthetic compensation and growth rate. Tree physiology. 21: 1159-1169.
Cheynier V, Comte G, Davies KM, Lattanzio V, Martens S. 2013. Plant phenolics: recent advances on their biosynthesis, genetics and ecophysiology. Plant Physiology and Biochemistry. 72: 1-20.
Clancy KM. 2002. Mechanisms of resistance in trees to defoliators. In: Mechanisms and deployment of resistance in trees to insects. Wagner MR, Clancy KM, Lieutier F, Paine TD (eds.) Kluwer Academic Publishers. Netherlands. Conner JK, Hartl DL. 2004. A primer of ecological genetics. Sinauer Assciates. Massachussets. 304 pp.
Daoust SP, Mader BJ, Bauce E, Despland E, Dussutour A, Albert PJ. 2010. Influence of epicutilar-wax composition on the feeding pattern of a phytophagous insect: implications for host resistance. Canadian Entomology. 142: 261-270. Delvas N, Bauce É, Labbé C, Ollevier T, Bélanger R. 2011. Phenolic compounds that confer resistance to spruce budworm. Entomologia Experimentalis et Applicata. 141(1): 35 – 44.
Despland E, Gundersen M, Daoust SP, Mader BJ, Delvas N, Albert PJ, Bauce É. 2011. Taste receptor activity and feeding behavior reveal mechanisms of white spruce natural resistance to Eastern spruce budworm Choristoneura fumiferana. Physiological entomology. 36: 39-46.
24
Despland E, Bourdier T, Dion E, Bauce É. 2016. Do white spruce epicuticular wax monoterpenes follow foliar patterns? Canadian Journal of Forest Research. 46: 1051-1058.
Doucet D, Frisco C, Cusson M, Bauce É, Reddy PS, Tomkins B, Arif B, Retnakaran A. 2007. Diapause disruption with the tebufenozide for early-instar control of the spruce budworm, Choristoneura fumiferana. Pest Management Science. 63: 730-736.
Dragota S, Riederer M. 2007. Epicuticular wax crystals of Wollemia nobilis: Morphology and chemical composition. Annals of Botany. 100: 225-231.
Edwards S, Jesson LK, Quiting D, Weng Y, Johns R, Park YS. 2016. Genetically-based resistance of balsam fir (Pinaceae) to damage from the balsam twig aphid (Hemiptera: Aphididae). Canadian Entomology. 00: 1-8.
Evert RF. 2006. Sclerenchyma. In Esau’s Plant Anatomy, pp. 191–209. John Wiley & Sons, Inc.
Farjon A. 2001. World checklist and bibliography of conifers. Royal Botanic Gardens, Kew, UK.
Fleming RA, Volney JA. 1995. Effects of climate change on insect defoliator population processes in Canada’s boreal forest: some plausible scenarios. Water, Air and Soil Pollution. 82: 445-454.
Franceschi VR, Krokene P, Christiansen E, Krekling T. 2005. Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytologist. 167: 353-376.
Frasz SI, Walker AK, Nsiama TK, Adams GW, Miller D. 2014. Distribution of the foliar fungal endophyte Phialocephala scopiformis and its toxin in the crown of a mature white spruce tree as revealed by chemical and qPCR analyses. Canadian Journal of Forest Research. 44: 1138-1143.
Fuentealba A, Bauce É, Dupont A. 2012. Bacillus thuringiensis efficacy in reducing spruce budworm damage as affected by host tree species. Journal of Pest Science. 88: 593-603.
Fürstenberg-Hägg J, Zagrobelny M, Bak S. 2013. Plant defense against insect
herbivores. International journal of molecular sciences.
doi:10.3390/ijms140510242
Gernandt DS, Willyard A, Syring J, Liston A. 2011. The conifers (Pinophyta) In: Genetics, Genomics and Breeding of Conifers. Plomion C, Bousquet J, Kole C (eds.) CRC Press. Florida. 449 pp.
25
Greenbank DO. 1957. The role of climate and dispersal in the initiation of outbreaks of the spruce budworm in New Brunswick. II. The role of dispersal. Canadian Journal of Zoology. 35: 385-403.
Hajek AE, Frankenhuyzen KV. 2017. Chapter 21: Use of Entomopathogens Against Forest Pests. In: Microbial Control of Insect and Mite Pests. Lacey AL (editor).
Hall JP, Moody B H. 1994. Forest Depletions Caused by Insects and Diseases in Canada, 1982-1987, Information Report ST-X-8, Canadian Forest Service, Ottawa, Canada 14 pp
Hardy YJ, Lafond A, hamel L. 1983. The epidemiology of the current spruce budworm outbreak in Quebec. Forest Science. 29: 715-725.
Harmon ME, Cromack K, Smith BG. 1987. Coarse woody debris in mixed-conifer forests, Sequoia National Park, California. Canadian Journal of Forest Research. 17: 1265-1272.
Hartl DL. 2000. A primer of population Genetics. Sinauer Associates. Massachussets. 221 pp.
Harrison KA, Pavlova A, Telonis-Scott M, Sunnucks P. 2014. Using genomics to characterize evolutionary potential for conservation of wild populations. Evolutionary Applications. Doi: 10.1111/eva.12149.
Haukioja E, Koricheva J. 2000. Tolerance to herbivory in woody vs. herbaceous plants. Evolutionary Ecology. 14: 551-562.
Henery M. L. 2011. The constraints of selecting for insect resistance in plantation trees. Agricultural and Forest Entomology. 13: 111–120.
Holland JB, Nyquist WE, Cervantes-Martínez CT. 2003. Estimating and interpreting Heritability for Plant Breeding: An Update. Plant Breeding Reviews. 22: 9-112.
Jardon Y, Morin H, Dutilleul P. 2003. Périodicité et synchronisme des épidémies de la tordeuse des bourgeons de l’épinette au Québec. Canadian Journal of Forest Research 33: 1947-1961.
Krekling T, Franceschi VR, Berryman AA, Christiansen E. 2000. The structure and development of polyphenolic parenchyma cells in Norway spruce (Picea abies) bark. Flora. 195: 354-369.
Lande R, Arnold SJ. 1983. The measurement of selection on correlated characters. Evolution. 37 (6): 1210-1226.