Fractionation of Peptides from Protein Hydrolysate
by Electrodialysis with Filtration Membrane:
Process optimization, Fouling characterization and Control
mechanisms
Thèse
Shyam
Suwal
Doctorat en sciences et technologie des aliments
Philosophiae Doctor (Ph.D.)
Québec, Canada
© Shyam Suwal, 2015
Résumé
Des peptides bioactifs ont déjà été fractionnés par électrodialyse avec membrane de filtration (ÉDMF) à partir d’hydrolysats de sous-produits de crabe des neiges. L’optimisation des paramètres apparaît maintenant indispensable pour perfectionner le procédé. Ainsi, le taux de migration des peptides, leur sélectivité et l'évolution des paramètres électrodialytiques ont été étudiés pour différents paramètres (configuration, concentration en KCl et types de champ électrique). La configuration (2) de la cellule d’ÉDMF comprenant deux compartiments d'alimentation et un compartiment de récupération a démontré des valeurs de champ électrique local relativement stables par rapport à la configuration (1) constituée d’un compartiment d’alimentation et de deux compartiments de récupération. Des peptides contenant des glutamates, des aspartates, et des glycines ont été séparés avec la configuration 1 et des peptides composés d’arginines et de lysines avec la configuration 2. Un taux de migration peptidique de 13,76 ± 3,64 g/m2h a
été obtenu par le maintien constant de la conductivité des solutions. La sélectivité a été accrue en augmentant la concentration en KCl de 1 à 5 g/L dans le compartiment de récupération. Une augmentation de la force ionique a amplifié la charge de surface, agrandissant ainsi la taille effective des pores et réduisant la couche d'hydratation de la membrane d’ultrafiltration. Toutefois, les membranes échangeuses d’anions et de cations ont été colmatées par des peptides et des acides aminés et détériorées pendant l’ÉDMF. Pour résoudre ces problèmes, l’effet de l’application du champ électrique pulsé (PEF) et de l'inversion de polarité (PR) a été étudié. Le taux de migration des peptides n'a pas été affecté sauf avec PR à 40 V. La sélectivité a été maximale avec PEF à 20 V. La dissociation de l'eau a été réduite tout en conservant les propriétés physico-chimiques des membranes grâce à l’application du PEF et de la PR par rapport au courant continu (DC). En outre, la plus faible quantité d'énergie a été consommée avec le PEF. Par conséquent, il a été possible d’optimiser la technologie d’ÉDMF du point de vue de l’efficacité énergétique, de la sélectivité peptidique et de l’encrassement membranaire grâce à l’application du PEF et tout en maintenant la conductivité électrique des solutions.
Abstract
Bioactive peptides were efficiently separated by using electrodialysis with filtration membrane (EDFM) from snow crab byproduct hydrolysate. Meanwhile, optimization of parameters is indispensable for scaling-up. The peptide migration rate and selectivity as well as evolution of electrodialytic parameters were studied with different parameters such as EDFM cell configuration, KCl concentration and type of electric field. The EDFM stack with two feed and one recovery compartments (configuration 2) has relatively stable electric field strengths (local) than the configuration with one feed and two recovery compartments (configuration 1). Peptides containing anionic amino acids: glutamic and aspartic acid as well as glycine and cationic amino acids: arginine and lysine were fractionated using configuration 1 and 2, respectively. Maintenance of solution conductivity upheld the local electric field and peptide migration throughout the treatment resulting in a higher peptide migration rate of 13.76±3.64 g/m2.h never observed so far. The
selectivity of cationic peptides containing arginine and lysine increased significantly with increase in KCl concentration from 1 to 5 g/L. An increase in ionic strength amplified the surface charge density of filtration membrane subsequently increasing effective pore size and reducing hydration layer. However, both anion- and cation-exchange membranes were fouled by peptides and amino acids and were deteriorated during EDFM treatment. To address these problems, the effect of applying pulsed electric field (PEF) and polarity reversal (PR) was studied. The peptide migration rate was unaffected among PEF, PR and DC modes except with PR at 40 V. The selectivity of cationic peptides was maximum with PEF at 20 V. Fouling and water dissociation were significantly reduced and physicochemical properties of IEMs were better-protected with PEF and PR than DC. Moreover, the least amount of energy was consumed with PEF mode. Therefore, the parameters affecting EDFM process were optimized in terms of energy efficiency, selectivity and lower deterioration of membranes by applying PEF regime with configuration 2 and maintaining the constant electrical conductivity of solutions.
Table of contents
Résumé ... iii
Abstract ... v
Table of contents ... vii
List of tables ... xvii
List of figures ... xix
List of abbreviations ... xix
Acknowledgements ... xxxi
Foreword ... xxxv
Introduction ... 1
Chapter 1: Literature review ... 5
1.1 Functional foods and bioactive peptides ... 5
1.2 Bioactive peptides from marine biomass ... 7
1.3 Production of bioactive peptides from marine biomass ... 13
1.3.1 Chemical hydrolysis ... 13
1.3.2 Enzymatic hydrolysis ... 13
1.4 Separation and purification processes of bioactive peptides ... 16
1.4.1 Chromatographic separation and purification processes ... 16
1.4.1.1 Ion exchange chromatography ... 17
1.4.1.2 Size-exclusion chromatography ... 17
1.4.1.3 Affinity chromatography ... 17
1.4.1.4 High pressure liquid chromatography ... 18
1.4.1.5 Fast protein liquid chromatography ... 18
1.4.2. Isoelectric focusing ... 19
1.4.3 Membrane based separation and purification processes ... 20
1.4.3.1 Pressure driven membrane filtration ... 20
1.4.3.2 Electrically-enhanced membrane filtration ... 25
1.4.3.3 Electrophoretic membrane contactor ... 29
1.4.3.4 Electrodialysis with filtration membrane ... 33
1.4.3.4.2 Ion-exchange membranes ... 34
1.4.3.4.3 Principal phenomena in ED ... 36
1.4.3.4.3.1 Donnan exclusion ... 36
1.4.3.4.3.2 Permselectivity ... 37
1.4.3.4.3.3 Mass transport ... 38
1.4.3.4.3.4 Concentration polarization and limiting current density ... 39
1.4.3.4.4 Electrodialysis with filtration membrane for peptide separation ... 43
1.4.3.4.5 Pilot-scale applications of EDUF technology ... 45
1.4.3.4.6 Advantages of fractionation by using EDUF ... 46
1.4.3.4.7 Bioactive peptides separation from snow crab byproduct hydrolysate by EDUF ... 47
1.4.3.5 Parameters affecting the electromembrane process ... 50
1.4.3.5.1 Electric field ... 50
1.4.3.5.2 Membrane surface area ... 50
1.4.3.5.3 Membrane properties ... 51
1.4.3.5.4 Solution properties and flow rate ... 52
1.4.3.5.5 Fouling of membrane ... 54
1.4.3.6 Characterization of Protein, Peptide and Amino Acid Fouling on Membrane stacked in Electromembrane Processes: Review of Current and Recently Developed Methods ... 55
1.4.3.6.1 Abstract ... 56
1.4.3.6.2 Introduction ... 57
1.4.3.6.3 Qualitative characterization based on membrane morphology (or structure) and physicochemical properties ... 59
1.4.3.6.3.1 Characterization based on membrane morphology ... 59
1.4.3.6.3.1.2 Scanning electron microscopy (SEM) ... 60
1.4.3.6.3.1.3 Atomic force microscopy (AFM) ... 63
1.4.3.6.3.2 Characterization based on physicochemical properties ... 65
1.4.3.6.3.2.1 Ion exchange capacity ... 66
1.4.3.6.3.2.2 Water content or water swelling degree ... 67
1.4.3.6.3.2.3 Streaming potential ... 69
1.4.3.6.3.2.4 Contact angle ... 71
1.4.3.6.3.2.5 Membrane electrical resistance ... 75
1.4.3.6.4 Quantitative characterization (based on composition of fouling) ... 79
1.4.3.6.4.1 Fourier transform infrared (FTIR) spectroscopy ... 79
1.4.3.6.4.2 X-ray photoelectron spectroscopy ... 82
1.4.3.6.4.3 Confocal Laser Scanning Microscopy ... 84
1.4.3.6.4.4 Matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) ... 85
1.4.3.6.4.5 LECO analysis ... 88
1.4.3.6.4.6 Coupling of desorption and analytical methods ... 88
1.4.3.6.5 Conclusion ... 92
1.4.3.7 Control of membrane fouling ... 93
Chapter 2: Problematic, Hypothesis and Objectives ... 97
2.1 Problematic ... 97
2.2 Hypothesis ... 97
2.3 Objectives ... 98
2.3.1 General objective ... 98
2.3.2 Specific objectives ... 98
Chapter 3: Electrodialytic Separation of Peptides from Snow Crab By-product Hydrolysate: Effect of Cell configuration on Peptide Selectivity and Local Electric Field ... 101
Contextual transition ... 102
3.2 Introduction ... 104
3.3 Materials and Method ... 105
3.3.1 Materials and ED cell ... 105
3.3.1.1 Hydrolysate ... 105
3.3.1.2 Chemicals ... 105
3.3.1.3 Membranes ... 106
3.3.1.4 Electrodialysis cell and configurations ... 106
3.3.2 Electroseparation protocol ... 109
3.3.3 Analyses ... 109
3.3.3.1 Solution conductivity ... 109
3.3.3.2 Demineralization rate ... 109
3.3.3.3 Total number of charge transported ... 110
3.3.3.4 In situ membrane resistance ... 110
3.3.3.5 Local electric field calculation ... 111
3.3.3.6 Kinetics of peptide migrations ... 111
3.3.3.7 Total peptide determination in freeze-dried samples ... 111
3.3.3.8 Relative energy consumption of the EDUF process ... 111
3.3.3.9 LC-MS analyses ... 112
3.3.3.10 Amino acid analysis ... 113
3.3.3.11 Statistical analyses ... 113
3.4 Results and Discussion ... 114
3.4.1 Electrodialytic parameters ... 114
3.4.1.1 Conductivity, demineralization rate and charge transported ... 114
3.4.1.2 In situ membrane resistance ... 117
3.4.1.3 Current Intensity and relative energy consumption ... 119
3.4.1.4 Local electric field ... 121
3.4.2 Fraction analysis ... 126
3.4.2.1 LC-MS analysis ... 126
3.4.2.2 Amino acid analysis ... 128
3.5 Conclusion ... 131
3.6 Acknowledgements ... 131
Achievement of objectives and advancement of knowledge ... 132
Chapter 4: Recovery and Utilization of Valuable Compounds from Food Processing By-products by Electrodialysis with Ultrafiltration Membrane: Impact of Ionic strength ... 133
Contextual transition ... 134
4.1 Abstract ... 135
4.2 Introduction ... 136
4.3 Materials and Method ... 137
4.3.1 Materials and EDUF cell configuration ... 137
4.3.1.1 Hydrolysate ... 137
4.3.1.2 Chemicals ... 138
4.3.1.3 Membranes ... 138
4.3.1.4 Electrodialysis cell and configuration ... 138
4.3.2 Electroseparation protocol ... 139
4.3.3 Analyses ... 140
4.3.3.1 Solution conductivity ... 140
4.3.3.2 Total number of charge transported ... 140
4.3.3.3 Global system electrical resistance ... 141
4.3.3.4 Local electric field calculation ... 141
4.3.3.5 Kinetics of peptide migrations ... 141
4.3.3.6 Total peptide determination in freeze-dried samples ... 141
4.3.3.7 Relative energy consumption of the EDUF process ... 142
4.3.3.8 Measurement of zeta potential of ultrafiltration membrane ... 142
4.3.3.9 LC-MS analyses ... 143
4.3.3.11 Statistical analyses ... 144
4.4 Results and Discussion ... 145
4.4.1 Electrodialytic parameters ... 145
4.4.1.1 Initial conductivity, current intensity and local electric field ... 145
4.4.1.2 Number of charge transported and consumption of energy ... 147
4.4.1.3 Peptide concentration and migration rate ... 149
4.4.2 Analysis on freeze dried fractions ... 151
4.4.2.1 Total peptide percentage in freeze-dried samples ... 151
4.4.2.2 LC-MS analysis ... 151
4.4.2.3 Amino acid analysis ... 152
4.5 Conclusion ... 158
4.6 Acknowledgement ... 158
Achievement of objectives and advancement of knowledge ... 159
Chapter 5: Impact of fouling on system performance and membrane properties during consecutive fractionation of peptides of protein hydrolysate by electrodialysis with ultrafiltration membrane ... 161
Contextual transition ... 162
5.1 Abstract ... 163
5.2 Introduction ... 164
5.3 Materials and Method ... 165
5.3.1 Materials and electrodialytic cell ... 165
5.3.1.1 Hydrolysate ... 165
5.3.1.2 Chemicals ... 166
5.3.1.3 Membranes ... 167
5.3.1.4 Electrodialysis cell and configuration ... 167
5.3.2 Electroseparation protocol ... 168
5.3.3 Analyses ... 169
5.3.3.1 Kinetics of peptide migrations ... 169
5.3.3.2 Solution conductivity ... 169
5.3.3.4 In-situ membrane resistance ... 170
5.3.3.5 Membrane electrical conductivity ... 170
5.3.3.6 Membrane thickness ... 170
5.3.3.7 Membrane nitrogen content ... 170
5.3.3.8 Ion exchange capacity ... 170
5.3.3.9 Water content ... 171
5.3.3.10 Infrared spectroscopy ... 172
5.3.3.11 Statistical analyses ... 172
5.4 Results ... 172
5.4.1 Peptide concentration ... 172
5.4.2 Global system resistance ... 174
5.4.3 In-situ membrane resistance ... 175
5.4.4 Membrane conductivity and thickness ... 177
5.4.5 Water content and ion exchange capacity ... 179
5.4.6 Membrane nitrogen content ... 179
5.4.7 Infrared spectroscopy ... 181
5.5 Discussion ... 184
5.6 Conclusion ... 187
5.7 Acknowledgement ... 188
Achievement of objectives and advancement of knowledge ... 189
Chapter 6: Effect of pulsed electric field and polarity reversal on peptide migration, selectivity and fouling mitigation ... 191
Contextual transition ... 192
6.1 Abstract ... 193
6.2 Introduction ... 194
6.3 Materials and Method ... 196
6.3.1 Materials and ED cell ... 196
6.3.1.1 Hydrolysate ... 196
6.3.1.2 Chemicals ... 196
6.3.1.4 Electrodialysis cell and configuration ... 197
6.3.2 Electroseparation protocol ... 198
6.3.3 Analyses ... 199
6.3.3.1 Kinetics of peptide migrations ... 199
6.3.3.2 Amino acid analysis ... 199
6.3.3.3 Total number of charges transported ... 200
6.3.3.4 Global system electrical resistance ... 200
6.3.3.5 Relative energy consumption of the EDUF process ... 200
6.3.3.6 Solution conductivity ... 200
6.3.3.7 In-situ membrane resistance ... 201
6.3.3.8 Membrane electrical conductivity ... 201
6.3.3.9 Membrane thickness ... 201
6.3.3.10 Water content ... 201
6.3.3.11 Membrane nitrogen content ... 202
6.3.3.12 Infrared spectroscopy ... 202
6.3.3.13 Statistical analyses ... 202
6.4 Results ... 203
6.4.1. Effect on electrodialytic parameters ... 203
6.4.1.1 Peptide migration rate ... 203
6.4.1.2 Global system resistance and energy consumption ... 206
6.4.2 Effect on selectivity: total amino acid profile ... 208
6.4.3 Effect on membrane physicochemical properties and fouling ... 209
6.4.3.1 In-situ membrane resistance ... 209
6.4.3.2 Membrane electrical conductivity ... 213
6.4.3.3 Water content ... 214
6.4.3.4 Membrane nitrogen content ... 215
6.5 Discussion ... 222
6.6 Conclusion ... 223
6.7 Acknowledgement ... 224
Achievement of objectives and advancement of knowledge ... 225
Chapter 7: General discussion, conclusion and perspectives ... 227
7.1 General discussion and conclusion ... 227
7.2 Perspectives ... 230
List of tables
Table 1.1: The marine bioactive peptides from biomass hydrolysate, enzyme used and amino acid sequence. N.D: Not determined. ... 9 Table 1.2: Summary of characterization techniques for different types of fouling agents and
applicable membranes. ... 90 Table 3.1: The demineralization rate, relative energy consumption and peptide migration
rate in configurations 1 and 2. na: not applicable. ... 121 Table 4.1: Relative energy consumption, peptide migration rate and peptide concentration
in post treatment KCl and hydrolysate. ... 149 Table 5.1: Composition of total and free amino acids in initial snow crab byproduct
hydrolysate. ... 166 Table 5.2: Evolution of electrical conductivity, water content and ion exchange capacity of
IEMs and UFM used in EDUF process. ... 178 Table 6.1: Volumes of KCl (100 g/L) and HCl (1 M) required to maintain the solution
electrical conductivity and pH respectively in the recovery compartment and relative amount of energy consumed in the different EDUF conditions. ... 206 Table 6.2: Electrical conductivity and water content of membranes before and after EDUF
fractionation. ... 214
List of figures
Fig. 1.1: Main disciplines in the research and development of a functional food and actors involved. Adapted and modified from [55]. ... 6 Fig. 1.2: Schematic diagram of different membrane filtration processes with different
particles size retained by the membrane. Adapted from [134, 136]. ... 22 Fig. 1.3: Schematic diagram of the three-step recycling membrane reactor: TI, temperature
indicator; PI, pressure indicator; FI, flow indicator; P1, recycling pump; P2, feed pump; P3, NaOH pump; PCV, pressure control valve; pHIC, pH indicator controller; FH, first hydrolysates; SH, second hydrolysates; TH, third hydrolysate. Adapted from [24]. ... 24 Fig. 1.4: The schematic diagram of EMF cell (a) when electric field is applied across a flat
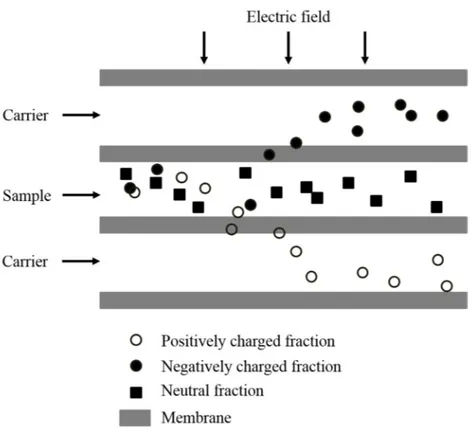
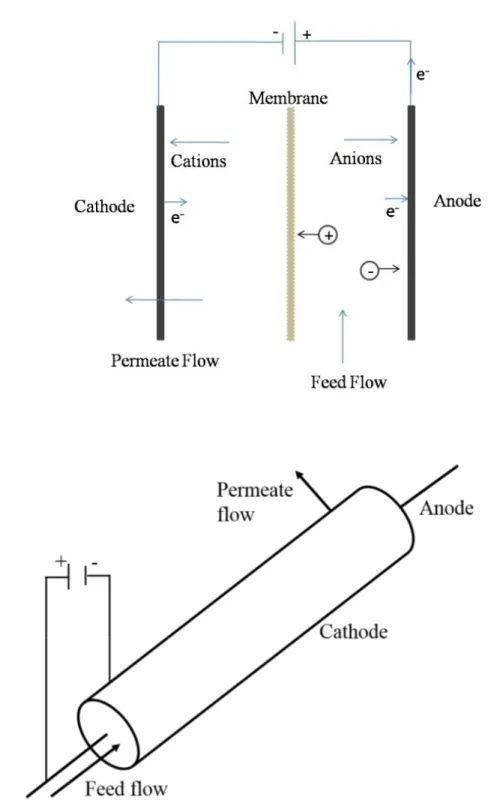
sheet membrane and (b) a tubular membrane is used as an electrode. Adapted from [28]. ... 27 Fig. 1.5: Cell configuration and principle of electromembrane filtration. Adapted and
modified from [29]. ... 28 Fig. 1.6: Schematic diagram of separation process in a multichannel flow electrophoresis.
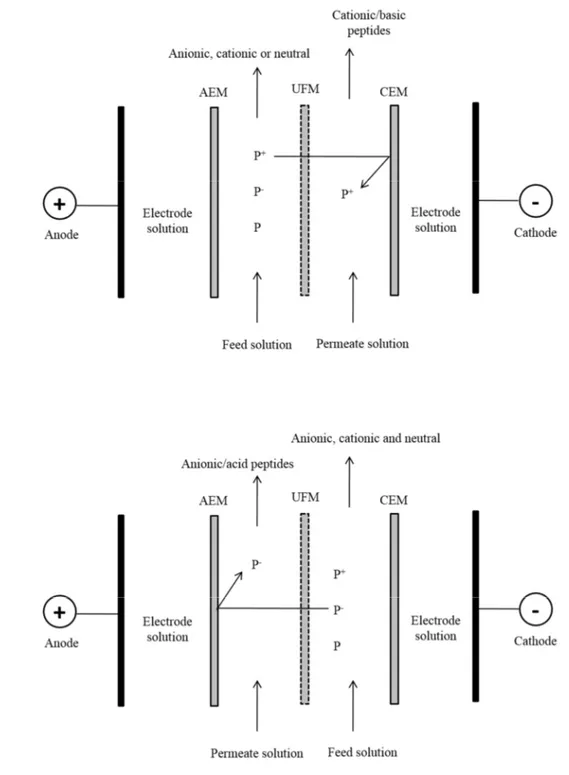
Adapted from [30]. ... 30 Fig. 1.7: Schematic diagram of electrophoretic membrane contactor at (a) separating mode
and (b) eluting mode. CEM: cation-exchange membrane, UFM: ultrafiltration
membrane and AEM: anion-exchange membrane. Adapted from [31]. ... 32 Fig. 1.8: Schematic diagram illustrating the working principle in an electrodialysis process.
Adapted from [49]. ... 34 Fig. 1.9: Schematic diagram of (a) anion-exchange and (b) cation-exchange membrane
preparation. Adapted from [172]. ... 35 Fig. 1.10: Structure, distribution of fixed and mobile (counter) ions and ion transport
mechanism (Donnan exclusion) in a homogenous anion-exchange membrane. ... 37 Fig. 1.11: Formation of concentration gradients in electrodialysis (a) and reaching limiting
current density (b). C+: cations, A-: anions, δ
1: diluate boundary layer, δ2: concentrate
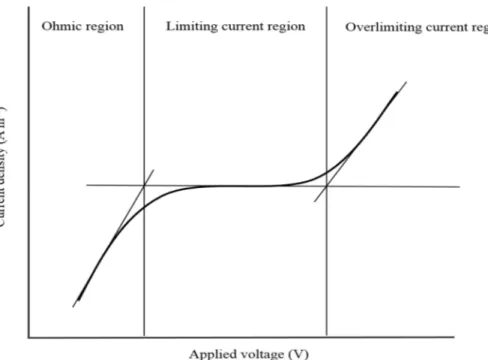
Fig. 1.12: A typical current-voltage curve showing ohmic, limiting and overlimiting
regions. Adapted from [188]. ... 42 Fig. 1.13: Electrodialysis with ultrafiltration membrane (EDUF) configurations for the
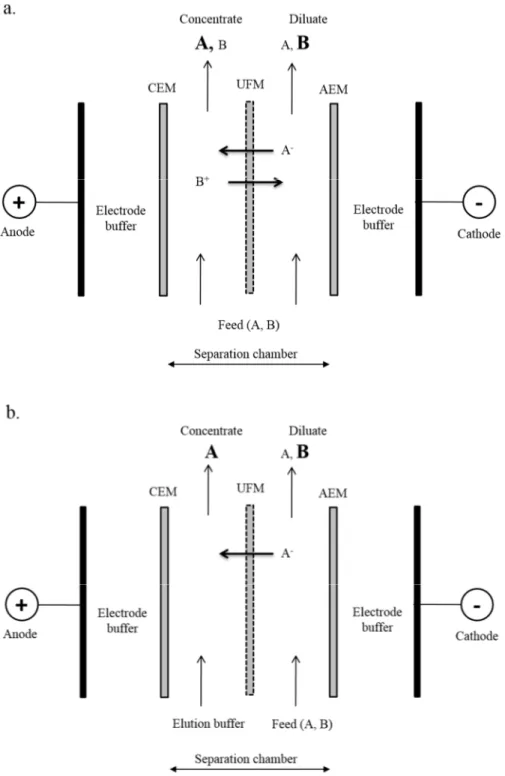
separation of (a) cationic peptides or (b) anionic peptides. AEM: anion-exchange membrane, UFM: ultrafiltration membrane and CEM: cation-exchange membrane. Adapted from [189]. ... 44 Fig. 1.14: Configuration of the electrodialysis module using two ultrafiltration membranes
for the simultaneous separation of cationic and anionic peptides from β-Lg
hydrolysate. UFM: ultrafiltration membrane. AEM: anion-exchange membrane, UFM: ultrafiltration membrane and CEM: cation-exchange membrane. ... 45 Fig. 1.15: Flow scheme of the fractionation process of snow crab by-product. Adapted from [70]. ... 49 Fig. 1.16: Images of PES membrane fouled at lab scale by skim milk protein (a)
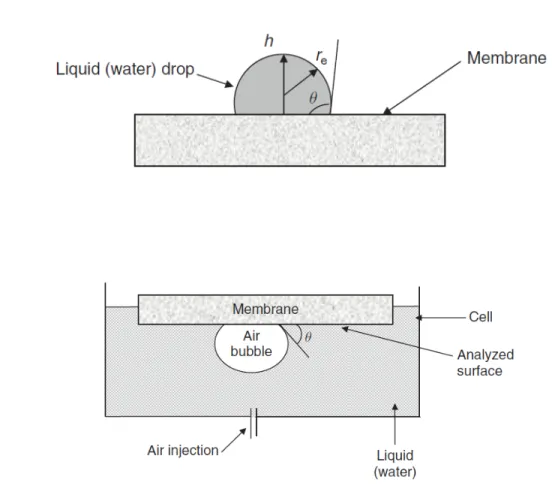
magnification: x 1,000, (b) magnification: x 30,000. Adapted with permission from [249]. ... 62 Fig. 1.17: Measuring of contact angle by sessile drop method (a) and by captive bubble
method (b). Adapted with permission from [233]. ... 73 Fig. 1.18: Illustration of advancing and receding contact angles. Adapted with permission
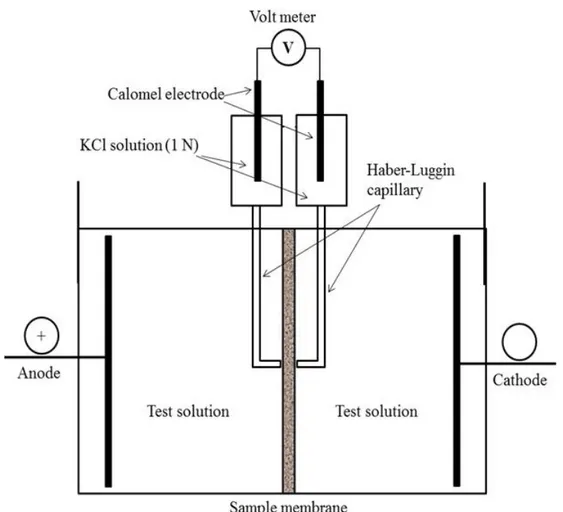
from [313]. ... 74 Fig. 1.19: Schematic representation of conductance measurement of membrane. ... 76 Fig. 1.20: Schematic diagram of the two-compartment electrolytic cell for the
determination of membrane resistance by current-voltage measurements with direct current. Adapted with permission and modified from [187, 292]. ... 77 Fig. 1.21: FTIR absorbance spectra showing the amide I and amide II bands of two soluble
proteins myoglobin, α-helical protein (continuous line) and concanavalin A, β-sheet protein (dashed line) in aqueous solution obtained after subtraction of the background H2O absorption. Adapted with permission from [336]. ... 80
Fig. 1.22: ATR-FTIR spectra of an IEM before and after EDFM process ... 82 Fig. 1.23: Schematic illustration of XPS principle. Adapted with permission and modified
Fig. 1.24: Schematic diagram of a surface-MALDI-MS. Adapted with permission from [365]. ... 86 Fig. 1.25: Mass spectrum obtained from the analysis of membrane fouled by BSA and
β-lactoglobulin (βLG) at pH 4. Conalbumin (CON) and ovalbumin (OVA) were used as internal standards for BSA and βLG respectively. Adapted with permission from [364]. ... 87 Fig. 1.26: Schematic diagram illustrating the removal of deposited negatively charged
colloidal components from the surface of an anion-exchange membrane by reversing the electric field. Adapted from [49]. ... 94 Fig. 3.1: EDUF cell (a) configuration 1 and (b) configuration 2 for the fractionation of
SCBH. AEM: anion-exchange membrane, UFM: ultrafiltration membrane, CEM: cation-exchange membrane, and V: voltmeter. ... 108 Fig. 3.2: Evolution of electrical conductivity with time in (a) configuration 1 and (b)
configuration 2. ... 115 Fig. 3.3: Number of charge transported during EDUF separation in configuration 1 vs. 2.
... 116 Fig. 3.4: Evolution of membrane resistance as a function of time in (a) configuration 1 and
(b) configuration 2. ... 118 Fig. 3.5: Evolution of current intensity as a function of time during EDUF separation
process. ... 120 Fig. 3.6: Evolution of local electric field as a function of time in different compartments of
EDUF cell using (a) configuration 1 and (b) configuration 2. ... 122 Fig. 3.7: Evolution of the peptide concentration as a function of time in (a) configuration 1
and (b) configuration 2 measured by BCA method. ... 125 Fig. 3.8: Distribution of peptide molecular weight present in the initial SCBH, final SCBHs
and KCl fractions for the 2 configurations. ... 127 Fig. 3.9: Abundance (in %) of amino acids in initial and post treatment SCBH and KCl
fractions of EDUF in configuration 1 and 2. ... 130 Fig. 4.1: EDUF cell configuration for the fractionation of SCBH. AEM: anion-exchange
membrane, UFM: ultrafiltration membrane, CEM: cation-exchange membrane, P+:
connected with silver coated platinum electrode placed on the interface of membrane. ... 139 Fig. 4.2: Variation of initial local electric field and conductivity as a function of KCl
concentration in the peptide recovery compartment. ... 145 Fig. 4.3: Evolution of local electric field as a function of time in KCl and hydrolysate
solutions. ... 147 Fig. 4.4: Number of charge transported as a function of time during EDUF treatment at
different KCl concentrations. ... 148 Fig. 4.5: Evolution of peptide concentration in KCl solution as a function of time. ... 150 Fig. 4.6: Distribution of peptide molecular weight in the initial SCBH and different KCl
fractions. ... 152 Fig. 4.7: Abundance of amino acids in initial SCBH and different KCl fractions. ... 154 Fig. 4.8: Evolution of UF membrane zeta potential on filtering side (a) and non-filtering
side (b) as a function of pH at different KCl concentrations. ... 155 Fig. 4.9: Graphical representation of electro-migration of cationic peptides and free Arg and
Lys at ultrafiltration membrane surface at different KCl concentrations during EDUF fractionation. ... 157 Fig. 5.1: EDUF cell configuration for the fractionation of SCBH.AEM: anion-exchange
membrane, UFM: ultrafiltration membrane, CEM: cation-exchange membrane, P+:
cationic peptides, P-: anionic peptides, P±: neutral peptides and V: voltmeter connected
to silver coated platinum electrode placed at the interface of membrane. ... 168 Fig. 5.2: Relative peptide concentration in the KCl fraction as a function of number of
EDUF runs. The value over each bar represents the concentration of KCl (g/L) in recovery compartment. ... 173 Fig. 5.3: Evolution of global system resistance as a function of time at 3 different KCl
concentrations in recovery compartment. ... 174 Fig. 5.4: Evolution of membrane resistance for (a) AEM, (b) UFM, (c) CEM1 and (d)
CEM2 as a function of time in 3 different KCl concentrations in recovery
compartment. ... 177 Fig. 5.5: Variation of total nitrogen content in virgin and used membranes. ... 181
Fig. 5.6: ATR-FTIR spectra of (a) AEM, (b) CEM1 and (c) CEM2 before and after use in EDUF process. ... 183 Fig. 5.7: Fouling mechanism of AEM fouling by peptides/or amino acids and degradation
of quaternary ammonium group by Hofmann elimination. ... 187 Fig. 6.1: Configuration of EDUF cell for the fractionation of SCBH. AEM: anion-exchange
membrane, UFM: ultrafiltration membrane, CEM: cation-exchange membrane, P+:
cationic peptides, P- anionic peptides and P±: neutral peptides and V: voltmeter
connected to silver coated platinum electrode placed at the interface of membrane.. 198 Fig. 6.2: Evolution of peptide concentration as a function of number of charges transported
under three different types of electric field for a constant voltage of (a) 20 V and (b) 40 V. ... 204 Fig. 6.3: Evolution of global system resistance as a function of no. of charge transported
under three different types of electric field for a constant voltage of (a) 20 V and (b) 40 V. ... 207 Fig. 6.4: Abundance of amino acids in initial SCBH and KCl fractions recovered with three different types of electric field at 20 and 40 V. ... 209 Fig. 6.5: Evolution of AEM resistance as a function of no. of charges transported under
three different types of electric field for a constant voltage of (a) 20 V and (b) 40 V. ... 210 Fig. 6.6: Evolution of UFM resistance as a function of number of charges transported under
three different types of electric field for a constant voltage of (a) 20 V and (b) 40 V. ... 211 Fig. 6.7: Evolution of CEM1 resistance as a function of number of charges transported
under three different types of electric field for a constant voltage of (a) 20 V and (b) 40 V. ... 212 Fig. 6.8: Evolution of CEM2 resistance as a function of number of charges transported
under three different types of electric field for a constant voltage of (a) 20 V and (b) 40 V. ... 212 Fig. 6.9: Total nitrogen content in virgin and used membranes recovered after EDUF
List of abbreviations
AA: Amino acidsACE: Angiotensin-converting enzyme AEM: Anion-exchange membrane AFM: Atomic force microscopy Aln: Alanine
Arg: Arginine Asp: Aspartic acid
ATR: Attenuated total reflection BCA: Bicinchoninic acid
BP: Bioactive peptide BPM: Bipolar membrane BSA: Bovine serum albumin BSE: Backscattered electrons CA: Cellulose acetate
CEM: Cation-exchange membrane
CFEMF: Cross flow electromembrane filtration CIP: Cleaning in place
CLSM: Confocal laser scanning microscopy CP: Concentration polarisation
Cys : Cystein
DH: Degree of hydrolysis
DOTM: Direct observation through membranes ED: Electrodialysis
EDBPM: Electrodialysis with bipolar membrane EDFM: Electrodialysis with filtration membrane ÉDMF: Électrodialyse avec membranes de filtration EDR: Electrodialysis reversal
EDUF: Electrodialysis with ultrafiltration membrane EIS: Electrical impedance spectroscopy
EMC: Electrophoretic membrane contactor EMF: Electromembrane filtration
FF: Functional food
FPLC: Fast protein liquid chromatography FTIR: Fourier transform infrared
Glu: Glutamic acid HCl: hydrochloric acid His: Histidine
HPLC: High pressure liquid chromatography IEC: Ion-exchange capacity
IEF: Isoelectric focussing IEM: Ion-exchange membrane Ile: Isoleucine
KCl: Potassium chloride LCD: Limiting current density LEF: Local electric field Leu: Leucine
Lys: Lysine
MALDI-MS: Matrix-assisted laser desorption ionization mass spectrometry
Met: Methionine
MFM: Microfiltration membrane
MICE: Mackerel intestine crude enzyme MW: Molecular weight
MWCO: Molecular weight cut-off Na2SO4: Sodium sulphate
NaOH: Sodium hydroxide NFM: Nanofiltration membrane PEF: Pulsed electric field PES: Polyether sulfone Phe: Phenylalanine
pI: Isoelectric point PR: Polarity reversal Pro: Proline PS: Polysulfone Pt: Platinum PVDF: Polyvinylidene fluoride RO: Reverse osmosis
SCBH: Snow crab by-products hydrolysate SD: Swelling degree
SDBS: Sodium dodecylbenzene sulfonate SDS: Sodium dodecyl sulfate
SEC: Size exclusion chromatography SEM: Scanning electronic microscopy Ser: Serine
Tau: Taurine Trp: Tryptophan Tyr: Tyrosine
UFM: Ultrafiltration membrane Val: Valine
Our greatest weakness lies in giving up.
The most certain way to succeed is
always to try just one more time.
Acknowledgements
It is a great opportunity to express my gratitude to everyone; my family especially my parents, wife and son, friends, supervisors and coworkers who supported me throughout the course of this PhD project. Without your continuous support and love I would not be in the position where I am today. Thank you, thank you so much for everything.
First of all, I would like to thank my parents for bringing me to this wonderful world, teaching me to dream and dare for triumph. Thank you so much letting me go where I wanted to be.
I would like to extend my deep love and gratitude to my son, Sugam who has been the greatest strength and motivation during the entire periods away from home. Your understanding and trust on me has driven me till here.
I am extremely thankful to my life partner, Sakun for changing my life, constant support and love. I am here and in this position because of you. Thanks for bearing the hard times, taking care of our son and having great patience for more than 5 years. I could feel each moment you were going through in my absence. Thank you for everything.
I am deeply thankful to my supervisor Prof. Laurent Bazinet for accepting me for this project in his dynamic team, for bearing me, for his continuous support and aspiring guidance, constructive criticism and friendly advice. Your love for work, time management, care for students and dynamism has always been a key motivation for me to advance and succeed. I would also like to thank you for letting me and encouraging me to participate actively in various national and international conferences and other extracurricular activities such as involving in student association which were very fruitful in developing interdisciplinary competences in me and personal contacts. I have learned a lot from you and I must say that I had a great 3 years of my life working with you. You are a great professor and an excellent supervisor.
I would like to thank my co-director Prof. Jean Amiot for accepting to work in this project, for his valuable remarks and discussions during the project meeting, result analysis and
article writing. His expertise in the research project, endless encouragement and support has provided a good shape to this work.
I am very much thankful to Prof. Lucie Beaulieu, co-director of this PhD project, for her nonstop support, guidance and availability. Thank you so much for providing me an opportunity of an internship and carry-out some microbiological tests that were really great. Rimouski is a beautiful place.
I would like to thank Dr. Alain Doyen for his valuable comments and constructive criticisms in my articles. Moreover, I am grateful to him for accepting to pre-read this thesis.
I am really thankful to Dr. Sylvain Galier (Université Paul Sabatier, Toulouse, France) and Prof. Alain Garnier (Département de Génie Chimique, Université Laval) for accepting to be the external examiners of this thesis and providing valuable and constructive comments. I would like to thank Elodie Rozoy for her continuous support during lab experiments, comments during presentation practices, ordering materials for me and more for being a good friend than just a colleague.
I would also thank Diane Gagnon for her help during experimental works especially with Leco and FTIR analyses and for her availability. Those chocolates had really given me more strength to work. You are an angel of the department.
I am grateful to Jacinthe Thibodeau for her support. It was really fun to work with you though for a very short period of time.
I would like to thank Pascal Lavoie for your technical assistance during my experiments in the pilot lab (cold room) and with sample lyophilisation.
I would like to express my heartfelt thanks to all the Professors who I worked with specially Prof. Muriel Subirade for your encouragement and support during my tenure in the student association.
I would like to thank all other colleagues in the food science and nutrition department specially, Anne-Françoise, Bernard Pouliot, Hélène, France, Marjolaine, Pierre for your collaboration and creating an excellent ambiance.
I would like to express my sincere gratitude to the research team of Québec fisheries and aquaculture innovation center (Merinov, MAPAQ, Gaspé, QC, Canada) especially Mr. Piotr Bryl for providing the snow crab by-products hydrolysate and Marie-Élise Carbonneau for amino acid analyses.
I am really thankful to all the wonderful friends and colleagues who have directly and indirectly helped me realize this dream come true and make my stay in Canada full of memories. My deep appreciation goes to Sergei Mikhaylin (you are a great friend), Valérie Carnovale (Merci d’avoir pris soin de moi ce soir là), Sabrine Naimi (petite forever), Mahder Seifu, Cheslav and Alina (my best Ukrainian couple, sorry for not being able to be your best man), Nassim Naderi, Hasna Hanchi (I miss those coffee breaks), Vanessa Perreault (Merci pour la belle collaboration pendant 2 ans dans l’ACCESTA), Yolande Koumfieg (thanks for helping me with my experiment), Abdel Atia, Luca Lo Verso, Amirouche Djaoudi, Mónica Araya-Farías, Nicolás Cifuentes-Araya, Fatoumata Diarrassouba (my first ever neighbour), Juan Li, Marina Bergoli, Sébastien Goumon, Mathieu Persico, Deepak Kumar Jha, Likun Panda, Mayank Pathak, Élodie Serre, Véronique Perreault, Paridokht Mahallati, Julien Chamberland, Catherine Couturier, Alex and Christiana (sweet couple), Stéphanie Dudonné, Adriana Parades, Laura Chevalier, Sophie Banville (many thanks especially for the memorable snow crab party and your sense of humor, you are just awesome), Ahmed Gomaa (espcially for FTIR analysis), Cyril Roblet (for guiding during the lab experiments), Luis Felipe Gutiérrez, Xixi Fang, Stéphanie Méthot-Hains, Juan and Alejandra (you are a great couple) and all those whom I forgot to mention here. I would like to thank Salona Amatya (Aalu), my best friend, for sharing my journey in France, for taking care of me and supporting me throughout my stay in France and in Québec, you are an amazing friend. Thank you all for those wonderful discussions, tea and coffee breaks, visits, those crazy parties and specially making me forget those difficult moments and nostalgia. Each of you has been very special to me. I would like to thank our small Nepalese community including Narayan dai, Baburam dai, Raj Basnet. I am especially thankful to Shuva Hari Gautam who has been an excellent roommate and a very good friend. Thanks for sharing the ride of PhD in the coffee shop
(Second cup) that helped me enormously to advance in my project, cooking good food while I was busy with my experiments and of course for teaching me how to drive.
Finally, the financial grant from Fonds de Recherche du Québec-Nature et Technologies (FRQNT) is highly appreciated.
Foreword
The present thesis is submitted to the Faculty of Graduate and Postdoctoral Studies of Laval University (Faculté des études supérieures et postdoctorales de l'Université Laval) to meet the requirements for obtaining the Philosophiae Doctor es Sciences (Ph. D) degree in Food Science and Technology at the Faculty of Agriculture and Food Sciences (Faculté des Sciences de l’Agriculture et de l’Alimentation). The research work presented was entirely carried-out at the Department of Food Science and Technology, Faculty of Agriculture and Food Science, Laval University.
Firstly, the introduction will present electro-membrane based techniques used in the separation and purification process of peptides, the parameters and limiting factors affecting the efficiency productivity and selectivity of the separation process.
The first chapter presents the literature review and it deals with the conventional and more recent techniques of production, separation and purification of bioactive peptides from food and marine protein hydrolysates with a special focus on electromembrane processes. The problems associated to membrane fouling used in electromembrane processes and controlling methods are discussed. A part of this chapter entitled “Characterization of protein, peptide and amino acid fouling on membrane stacked in electromembrane processes: Review of current and recently developed methods” is written in the form of a review article and was submitted to Journal of Membrane Science. Authors: Shyam Suwal, Alain Doyen and Laurent Bazinet.
The Chapter 2 presents the problematic, hypothesis and objectives of the present work. Most experimental works and results obtained have been published or submitted for publication in appropriate scientific journals. The manuscripts are summarized in the following chapters from 3 to 6.
The Chapter 3 presents the article entitled “Electrodialytic separation of peptides from snow crab by-product hydrolysate: effect of cell configuration on peptide selectivity and local electric field” published in Separation and Purification technology, 127 (2014)
29–38. Authors: Shyam Suwal, Cyril Roblet, Alain Doyen, Jean Amiot, Lucie Beaulieu, Jean Legault and Laurent Bazinet.
The Chapter 4 presents the article entitled “Recovery of valuable peptides from marine protein hydrolysate by electrodialysis with ultrafiltration membrane: impact of ionic strength” published in Food Research International, 65, Part C (2014) 407-415. Authors: Shyam Suwal, Cyril Roblet, Alain Doyen, Jean Amiot, Lucie Beaulieu, Jean Legault and Laurent Bazinet.
The Chapter 5 presents the article entitled “Impact of fouling on system performance and membrane properties during consecutive fractionation of peptides of protein hydrolysate by electrodialysis with ultrafiltration membrane” submitted to Journal of Membrane Science. Authors: Shyam Suwal, Cyril Roblet, Jean Amiot and Laurent Bazinet.
The Chapter 6 presents the article entitled “Effect of pulsed electric field and polarity reversal on peptide migration, selectivity and fouling mitigation during electrodialysis with ultrafiltration membrane of snow crab byproduct hydrolysate” will be submitted for publication to Journal of Membrane Science. Authors: Shyam Suwal, Jean Amiot, Lucie Beaulieu and Laurent Bazinet.
Finally, the Chapter 7 presents the general conclusions and key findings of the present work and future perspectives which is followed by the references.
In all articles presented above, Shyam Suwal is the first author who was in charge of the conception, experimental design and execution of experimental works, result analysis and article writing. Other authors, Cyril Roblet was involved in experimental design and execution of experiments, Alain Doyen and Jean Legault were involved in correction and revision of manuscripts, Lucie Beaulieu (thesis co-director) was involved in experimental design, correction and revision of manuscripts, Jean Amiot (thesis co-director) was involved in experimental design, correction and revision of manuscripts and Laurent Bazinet (thesis director) was involved in scientific supervision, experimental design, correction, revision and submission of manuscripts.
Introduction
The number of health problems associated with food and dietary habits is increasing day-by-day which has brought the attentions of scientists to link the diet with health. Several chronic diseases such as cardiovascular, diabetes type 2, obesity, cancer etc. are the major causes of death especially in developed countries that are intimidating even more the global health security [1]. Recently, studies have revealed that such diseases are directly associated to human diet which have been a strong motivation for the scientific community to find the ways of prevention and cure by using active or functional ingredients derived from natural food source [2]. Therefore, special focus are being provided in the research and development of functional foods (FFs) and nutraceuticals containing bioactive components suitable for specific type of disease.
Bioactive substances are components of food that can affect biological processes hence have an impact on body functions or conditions and health [3, 4]. Foods of natural origin contain several bioactive molecules. Among them, proteins and their derivatives such as peptides known as bioactive peptides (BP) have demonstrated physiological functions either directly or upon in vivo or in vitro enzymatic hydrolysis [5]. Till date, peptides derived from proteins of various food sources like milk and dairy product, egg, meat, cereals (soya, flaxseed, peas etc.), microalgae and fish are widely investigated for their potential regulatory roles such as anti-microbial, antioxidant, antihypertensive (ACE inhibitor), antithrombotic, antiobesity, hypocholesterolemic, anticarcinogenic, opioid, antiappetizing and immunomodulatory [4, 6-13]. Furthermore, due to a huge biomass and biological diversity available and large quantity of byproducts generated from processing plants, marine biomass has been extensively studied in recent years. However, technological process such as generation of BP, separation and purification process are considered to be the main bottleneck in the product development and their economic feasibility. Moreover, increasing environmental concerns actually demand for green and energetically viable techniques.
Pressure-driven membrane filtration processes, particularly ultrafiltration and nanofiltration, have been used for peptide fractionation [14, 15]. In addition, many works have been carried-out and reviewed to study different parameters such as feed solution,
membrane properties and interaction between them on the migration rate and selectivity of the process [16-22]. Some breakthroughs could be noticed in conventional membrane processes dedicated to increase the selectivity, productivity and decrease production cost. A design for stepwise membrane fractionation [23, 24] and membrane reactor for simultaneous hydrolysis and separation of generated peptides [25] are some good examples. However, main inconvenience of pressure driven membrane techniques are problems associated with membrane fouling and lower selectivity while separating peptides of similar molecular weights [26]. The use of electrical fields in the conventional membrane filtration process have demonstrated to enhance the permeate flux, improve selectivity and reduce membrane fouling [27, 28].
Recently, electrically driven membrane filtration process called electromembrane filtration (EMF) and electrophoretic membrane contactor (EMC) were developed [29, 30]. In an EMF process the migration of solute takes place due to pressure and electrical gradients whereas it takes place only by an electric gradient in an EMC process. Both techniques have been studied for the separation of peptides and amino acids [31-34]. Moreover, electrodialysis with filtration membrane (EDFM) was recently developed and patented by Bazinet et al. [35]. EDFM is a batch process and combines the conventional ED process with one or more filtration e.g. UF membranes which allow the migration of charged molecules such as proteins, peptides or amino acids. EDFM is a very selective process arising from the charge selectivity of conventional ED process and the size exclusion capabilities of filtration membranes [26]. A typical EDFM system consists of an anion-exchange membrane (AEM), a cation-anion-exchange membrane (CEM) and one or more filtration (ultrafiltration, UFM) membranes. The cell configuration can be easily adapted for cationic or anionic peptides fractionation or for simultaneous separation of cationic and anionic peptide. EDFM has been used to fractionate antihypertensive peptides from alfalfa white protein hydrolysate [36], antioxidant peptides from soya protein hydrolysate [37], bovine lactoferrin, an antimicrobial protein from whey [38], antidiabetic and antihypertensive peptides from flaxseed protein hydrolysate [39], antidiabetic peptides from soya protein hydrolysate [40] and anticancer and antibacterial peptides from snow crab byproduct hydrolysate (SCBH) [41, 42].
Different parameters such as pH, applied electric field, membrane molecular weight cut-off (MWCO) and type of material of filtration membrane, have been found to affect EDFM peptide migration rate (productivity) and selectivity [41, 43, 44]. However, the effect of cell configuration and ionic strength of recovery and feed solution have not yet been studied. Moreover, concentration polarization (CP) phenomenon and fouling on membrane especially ion-exchange membrane (IEM) during electrodialytic separation could be the key limiting factors of EDFM process. The CP is an inevitable phenomenon in a membrane separation process. In addition, IEM have been found to be fouled by peptides [45] and amino acids [46, 47] consequently decreasing considerably the process efficiency, selectivity, durability of membranes by degrading their physicochemical and structural properties. Therefore, quantitate and qualitative characterization of membranes during
(in-situ) and after their use (ex-(in-situ) is an important step to understand the fouling mechanism
and to develop fouling control strategy. The conventional method to clean the fouled membrane involves the use of chemical agents [48]. In addition, polarity reversal (PR) of electrode has also been used as tool of fouling control during ED process, known as ED reversal (EDR) [49]. More recently, use of non-stationary current, pulsed electric field (PEF) regime have shown to intensify the ED process and control protein as well as mineral fouling of membrane [50, 51]. Meanwhile, both PR and PEF modes have not been tested in EDUF module during peptide separation, thus their role in peptide migration rate, selectivity and membrane fouling are not well known so far.
The following chapters will present the extensive review of works carried-out previously, problematic, hypothesis and objectives of the present PhD project. The aim of this PhD project is to optimize the EDFM process for the fractionation of peptides from SCBH, to characterize used membranes for potential fouling and deterioration, and to study the effect of a novel method of fractionation by using pulsed electric field and polarity reversal of current on peptide migration rate and selectivity and evolution of membrane properties. The results obtained will be presented and discussed in the form of articles.
Chapter 1: Literature review
1.1 Functional foods and bioactive peptides
The increasing number of health problems associated with food and dietary habits have gained the ever increasing attention of scientists to link the diet with health. Several chronic (non-communicable) diseases such as cardiovascular, diabetes type 2, obesity, cancer etc. are the major causes of death especially in developed countries as their prevention and treatment are extremely difficult and costly. The increasing prevalence and trend of such diseases are intimidating even more the global health security [1]. On the other hand, recent studies have clearly demonstrated a strong relationship between diet and disease [2]. These findings actually lead to an attractive means for the prevention and cure of such diseases by consuming naturally occurring foods that contain functional ingredients. Indeed, large number of biologically active components have been discovered in food sources that have been continuously raising the interest in functional food and/or nutraceuticals and to develop nutritionally rich products designed to suit the specific disease [4]. Bioactive substances are components of food that can affect biological processes or substrates hence have an impact on body function or condition and health [3]. The term “functional foods” (FFs) was first coined in the mid-1980s in Japan and is described as processed foods that contain ingredients such as oligosaccharides, minerals, polyunsaturated fatty acids, and fibers that address chronic diseases such as hypertension, cancer, diabetes, etc. in addition to being nutritious [52]. In addition, FFs have found to be named in various ways such as designed foods, medicinal foods, bioactive foods, therapeutic foods, vita foods, super-foods, foodceuticals, pharmasuper-foods, medical super-foods, etc. Later, Erdmann et al. [4] emphasized that FFs should remain as food or beverages but not in the pharmaceutical forms of tablets or capsules. The notion of “nutraceutical” was derived by combining the terms “nutrition” and “pharmaceutical” in 1989 and can be stated as a food or part of a food which provides medical or human health benefits including prevention and treatment of diseases [53].
The increasing awareness and preference of consumers and their positive effects on health have been the key motivation for researchers and industrials to focus their work and innovation on FFs [54]. The research and development of a FF consist of three interrelated
disciplines: nutritional benefits, health benefits and technological process as shown in Fig. 1.1 [55]. Nutritional values are the first most important points to be considered in a food which refers to the role of nutrients (vitamins, proteins, carbohydrate, lipids and minerals) on growth and maintenance of human body system. As, discussed above, FF should possess health benefits in addition to its nutritional values such as reduction of risk of certain diseases and promote the wellbeing [56]. Finally, the technological processes include processing (preparation, pretreatment, separation and purification) of raw material to final product and characterization of active ingredients. Since this PhD project is about the separation and purification, this chapter will be focused essentially on the technological part. Indeed, the production, accessibility and sustainability of FFs and nutraceuticals depend primarily upon the input cost related to technological processes (extraction and concentration of bioactive molecules) and sources of raw materials [57].
Fig. 1.1: Main disciplines in the research and development of a functional food and actors involved. Adapted and modified from [55].
Bioactive molecules present in FF should be proven to evoke physiological, immunological and behavioral effects [58]. As a consequence, a multitude of bioactive ingredients derived
2. Nutritional benefits: Nutritionists 3. Technological processes: Food technologists 1. Health benefits: Microbiologists Physiologists pathologists etc.
from various food sources has been researched recently for their potentiality to be incorporated in a FF. Among all, proteins and their derivatives such as peptides are most widely known and abundant in nature. In addition, the physiological role of proteins is highly researched and reviewed since the last two decades. Naturally occurring proteins in raw food sources exert physiological function either directly or upon in vivo or in vitro enzymatic hydrolysis [5]. Bioactive peptides (BP) can be defined as the peptides of plant or animal origin which possess regulatory function in human system beyond the normal nutritional value [59]. Generally, peptides are inactive when they are encrypted within precursor protein sequence. Most BPs are composed of 3 to 20 amino acid sequences [60]. The beneficial role of the peptides depends on their amino acid composition and sequences [61]. Till date peptides derived from milk, egg, meat, cereals (soya, flaxseed, peas etc.), macroalgae and fish proteins are widely investigated for their potential regulatory roles [4, 6-13]. A range of BPs having properties like anti-microbial, antioxidant, antihypertensive (ACE inhibitor), antithrombotic, antidiabetic, hypocholesterolemic, anticarcinogenic, opioid, antiappetizing and immunomodulatory are most widely studied [4]. The economically viable and environmentally friendly technology and easily accessible raw materials, e.g industrial byproducts, are the major parameters for the sustainability of FFs and nutraceuticals. Therefore, the industrial byproducts rich in proteins such as marine biomass are gathering more and more attention of industrial, scientific and governmental sectors to valorize them to value added products (FFs).
1.2 Bioactive peptides from marine biomass
Major part of earth (71%) is covered by oceans and is rich in number of biological biodiversity basically microalgae, macroalgae, bacteria, cyanobacteria, fish species and crustacians [53]. These marine organisms represent approximately one half of the total earths biodiversity [62]. Above 50% of world fishing comes from oceans and more than 70% are used in fish processing. As a consequence, marine (seafood) processing plants generate large amount of byproducts and wastes. Byproducts are not ordinarily saleable; however, they can be reused after some treatments [63] while wastes cannot be reused and have to be discarded. In 2012, total of about 160 million tons of fishes worldwide were
captured; thus byproducts generated from processing industries are enormous [64]. The processing byproducts include trimmings, cut-offs, fins, frames, heads, skin, blood and viscera. In addition to fish processing, a large quantity of processing byproducts are produced such as shells of crustaceans and shellfish [8]. Furthermore, crude fish flesh contains 17 to 22% (w/w) of proteins while it varies between 7 to 23% (w/w) in crustaceans and mollusks. However, about 10 to 20% of total fish proteins are found in processing leftovers [62]. Similarly, the marine seaweed contains 3 to 15% (brown seaweed) and 10 to 47% (red seaweed) of proteins of dry weight [65]. Recently, many bioactive molecules have been identified from marine process byproducts such as fish muscle proteins, collagen and gelatin, fish oil, fish bone, internal organs, shellfish and crustacean shells [66-72]. As the marine organisms are rich in proteins, they can be an ideal source for BPs [62]. Naturally, marine invertebrates contain BPs such as antimicrobial peptides as their defense system against infection as they lack acquired immune system [73]. The bioactive compounds and therefore BPs from marine biomass, their functionality and potentiality as a FF have been reviewed by many authors recently [63, 74-76]. It shows that there is a huge prospective in the valorization of these byproducts into value added products such as FFs, nutraceuticals and pharmaceuticals. Unfortunately, at present, the fishery byproducts are discarded back to the sea or end up in landfill sites and only few of them have been recycled but as economically low value products such as fish oil, fishmeal, fertilizer, pet feed and fish silage [8, 77].
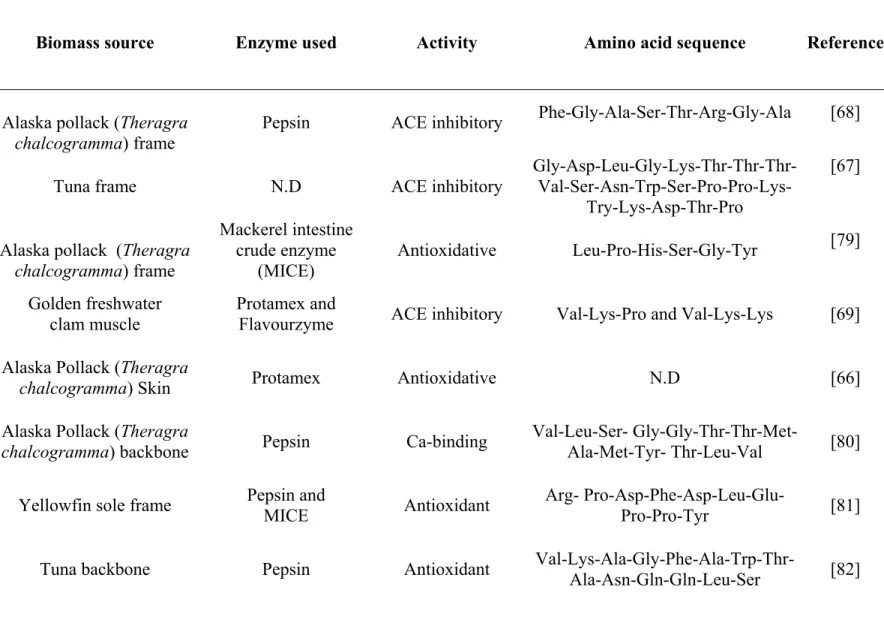
Meanwhile, much attention has been given for the discovery of novel structural, compositional and sequential properties of bioactive peptides [78]. Vidanarachchi et al. [53], have reviewed the applicability of marine based nutraceuticals in dairy industry and pointed out various marine nutraceuticals such as antitumor/anticancer, anti-inflamatory, antioxidant, antidiabetic, antihypertensive, antimicrobial and probiotics. Some of the bioactive peptides discovered in marine biomass, enzymes used for protein hydrolysis, associated bioactivities and amino acid compositions are listed in Table 1.1.
Table 1.1: The marine bioactive peptides from biomass hydrolysate, enzyme used and amino acid sequence. N.D: Not determined.
Biomass source Enzyme used Activity Amino acid sequence Reference
Alaska pollack (Theragra
chalcogramma) frame Pepsin ACE inhibitory
Phe-Gly-Ala-Ser-Thr-Arg-Gly-Ala [68]
Tuna frame N.D ACE inhibitory Gly-Asp-Leu-Gly-Lys-Thr-Thr-Thr- Val-Ser-Asn-Trp-Ser-Pro-Pro-Lys-Try-Lys-Asp-Thr-Pro
[67]
Alaska pollack (Theragra
chalcogramma) frame Mackerel intestine crude enzyme (MICE) Antioxidative Leu-Pro-His-Ser-Gly-Tyr [79] Golden freshwater clam muscle Protamex and
Flavourzyme ACE inhibitory Val-Lys-Pro and Val-Lys-Lys [69]
Alaska Pollack (Theragra
chalcogramma) Skin Protamex Antioxidative N.D [66]
Alaska Pollack (Theragra
chalcogramma) backbone Pepsin Ca-binding
Val-Leu-Ser-
Gly-Gly-Thr-Thr-Met-Ala-Met-Tyr- Thr-Leu-Val [80]
Yellowfin sole frame Pepsin and MICE Antioxidant Arg- Pro-Asp-Phe-Asp-Leu-Glu-Pro-Pro-Tyr [81]
Blood ark shell
(Scapharca brough) N.D Anticoagulant N.D [83]
Hoki (Johnius belengerii)
frame Pepsin Ca-binding N.D [84]
Chum Salmon
(Oncorhynchus keta) Complex protease Immunomodulatory N.D [85]
Cod frame tuna pyloric caeca
crude proteinase Antioxidant (permeate of 10kDa UF) ACE inhibitory (permeate of 3kDa UF) N.D [86]
Blue mussel (Mytilus edulis) Tris-HCl buffer Anticoagulant Gly-Gln-Val- Asn-Tyr-Glu-Glu- Glu-Ala-Asp-Ile-Asp-Gly-Asp-Phe-Val-Ala-Met-Met-Thr-Ser-Lys
[87]
Alaska Pollack (Theragra
chalcogramma) skin gelatin Alcalase, Pronase E,and collagenase Antioxidant
Gly-Glu-Hyp-Gly-Hyp-Gly-Pro- Pro-Hyp-Hyp-Gly, Gly-Pro-Gly-Pro-Hyp- Hyp-Gly-Pro-Hyp-Gly-Pro-Hyp-Gly [88] Snow Crab (Chionoecetes opilio)
byproduct Protamex Antimicrobial N.D [72]
Snow Crab (Chionoecetes opilio)
byproduct
Oyster (Crassostrea gigas) Alcalase and bromelin Antiviral N.D [89] flying fish
(Exocoetus volitans) backbone
Papain, pepsin and trypsin Antioxidant, antibacterial and antiproliferative N.D [90] Oyster (Crassostrea gigas) Alcalase and bromelin Antimicrobial N.D [91]
Spider crab (Hyas araneus)
Haemocyte Solvent extraction Antibacterial N.D [91]
Atlantic rock crab
byproducts Protamex Antibacterial N.D [92]
Blue mussel (Mytilus
edulis) byproducts Protamex Antiproliferative N.D [93]
Red seaweed (P. palmate) Papain Renin inhibitory
Ile-Arg-Leu-Ile-Val-Leu-Met-Pro-Ile-Leu-Met-Ala [94]
Red seaweed (Porphyra
columbina) Trypsin and alcalase Immunosuppressive N.D [95]
Red seaweeds (Solieria
chordalis and Palmaria palmate), green
seaweed (Ulva lactuca) and brown seaweed (Saccharina
longicruris)
Trypsin and chemotrysin
Antioxidant and
Patin (Pangasius sutchi) Alcalase and papain Antioxidant Asp-Pro-Gln-His-Pro-Val-Met-Pro-Arg, Leu-Val-Val-Asp-Ile-Pro-Ala- Ala-Leu-Gln-His-Ala, Gly-Val-Asp-Asn-Pro-Gly-His-Pro [97]
1.3 Production of bioactive peptides from marine biomass
The production of BPs from food proteins is one of the critical steps. The industrial production of BPs from food ingredients and byproducts ought to be economically feasible and should not contain hazardous chemical substances. At the same time the quality, sensorial properties and nutritive values of final products are of equal importance. Two methods such as chemical (solvent extraction and acid/base hydrolysis) and biological (enzymatic hydrolysis and microbial fermentation) hydrolysis are used to produce BPs from marine biomass.
1.3.1 Chemical hydrolysis
Chemical methods such as acid and base hydrolysis were mainly practiced in past due to low processing cost and easy procedure. However, chemical hydrolysis is not considered to be safe for food manufacturing as it leads to variability in composition and functionality of resulting protein hydrolysate [99]. In addition, chemical hydrolysis process is difficult to control in terms of degree of hydrolysis and final products. Traditionally, this technique has been used to produce fish protein concentrates (FPC) during 1960s to add protein intake in developing countries and as animal feed [63]. Solvent extraction method was used to extract Onchidin B, a cyclodepsipeptide from the mollusk Onchidum sp. using methanol and CCl4 [100].
1.3.2 Enzymatic hydrolysis
Enzymatic hydrolysis could be easily controlled and is by far the most important and common method to generate BPs with target functionality [101] as well as to obtain products with high nutritive value [99]. In addition, this process avoid the presence of residual organic solvents or toxic chemicals in the final products [78]. There are several advantages of using proteolytic hydrolysis as compared to organic solvent extraction or chemical hydrolysis [102]. These include: getting the status of a natural product; defined
product stereochemistry due to the high substrate and reaction specificity of biocatalysis; mild reaction conditions; and reduced waste generation.
Proteolytic enzymes also called proteases could be categorized according to their specificity to the peptide bonds and the mechanism of action [99]. Four major classes of proteases were classified according to their specificity to principal functional groups of active sites such as serine, cysteine, aspartic acid and metallo (requiring metal for catalytic activity). According to their mechanisms of hydrolysis, proteases are divided into endoproteinases and exopeptidases. Endoproteinases cleave the peptide bond within protein molecule at specific residue producing larger fragments of peptides while exopeptidases cleave from either side of protein (N or C terminal) to produce amino acids. Therefore, all the four classes of proteases acting on specific sites comply within the group of endoproteinases.
The yield, amino acid composition and functions of resulting peptides from enzymatic hydrolysis depend mainly upon factors: the primary sequence of the protein substrate, the specificity or type of the enzyme(s) and digestive conditions (pH, temperature, time and degree of hydrolysis (DH)) [103, 104]. The DH which is defined as the percentages of peptide bonds cleaved is a crucial parameter to be considered. Indeed, DH is need to be controlled as it directly influences the composition and quality of protein hydrolysates [105]. In addition, some studies were focused to find the relationship between peptide structure, size and composition with its bioactivity. Indeed, the structure, its amino acid sequence and size of peptide play a vital role on its biofunctionality. An example of this structure-activity can be seen in antioxidant peptides. The antioxidative properties arise generally from small size peptides of 2 to 8 amino acids [66, 79, 106]. However, peptides up to molecular weight of 5 kDa and rich in histidine and hydrophobic amino acids are found to possess antioxidative property [101].
Till date several proteolytic enzymes of microbial, plants and animal origin are used for the hydrolysis of fish and macroalgae processing byproducts (Table 1.1). The choice of enzyme is vital for efficient hydrolysis as well as desired end-product. The screening of suitable enzymes is very important. The same enzymes can act differently on different substrates and vice versa. This phenomenon is more apparent in complex substrate like
food and byproducts. Comparison of different enzymes is made in different specific desired biological activity of hydrolysate produced. Jia et al. [66] compared 7 different enzymes or mixes to hydrolyse the Alaska Pollack skin. The hydrolysis with Protamex® (Novozymes, a
complex mixture of proteases) was found to be the most efficient method to produce antioxidant peptides from Alaska Pollack skin as compared to other enzymes such as pepsin, alcalase, papain, flavourzyme, trypsin and neutrase. Indeed, protamex resulted into a maximum peptide yield of 83.44% containing more than 85% of oligopeptides with molecular size ranging from 180 to 1000 Da. Two pancreatic enzymes trypsin and chymotrypsin for the hydrolysis of shrimp meat were used and produced the fractions with different amino acids composition [102].
In addition to the one step hydrolysis process with a single enzyme or enzyme complex (e.g Protamex®), there are also literatures published which used two or more enzymes one after
another. Sequential hydrolysis using two enzyme complexes, primarily by Protamex®
followed by Flavourzyme F®, was used to produce ACE inhibitory peptides from fresh
water clam muscle [69]. Alcalase and neutrase enzymes were successfully employed for Pacific whiting solid waste protein hydrolysis [107]. Serial digestion of gelatin from Alaska Pollack skin using 3 enzymes alcalase, pronase E and collagenase produced antioxidant peptides [24, 88]. The two peptide fractions (13 and 16 amino acid residues) obtained after the second hydrolysis with pronase E exerted antioxidant properties. Two tri-peptides (Gly-Pro-Leu and Gly-Pro-Met) with significant ACE inhibitory activity were identified from Alaska Pollack skin hydrolysate [24].
Another interesting possibility could be the use of crude enzyme isolated from internal organs of marine biomass itself. Je et al. [79] successfully used mackerel intestine crude enzyme (MICE) to hydrolyse Alaska Pollack frame proteins to produce antioxidant peptides. Several BPs have been found in fermented sea foods. The ACE inhibitory BPs were produced from fish proteins by hydrolysis of microbial enzymes during fermentation process [108, 109].
1.4 Separation and purification processes of bioactive peptides
Hydrolysis of native proteins from food or food byproducts leads to the formation of complex polypeptide mixture, non-hydrolyzed protein fraction, lipid and enzyme. In addition, peptides generated differ in terms of molecular mass and amino acid composition. It has been proven that the biological activities of peptides are function of their molecular weight and amino acid composition. Moreover, the functional properties like solubility and foaming of hydrolyzed protein depend on molecular size of peptides produced [110]. Thus, development and selection of appropriate fractionation and further purification methods for recovery and concentration of peptides of interest is crucial for their proficient industrial applications.
Different processes exist for fractionation and purification of molecules of interest. They are mostly based on molecular charge, weight (size) and hydrophobicity. Separation and purification processes of peptides could be divided mainly into two categories. Firstly, membrane based techniques (ultrafiltration and nanofiltration, etc.) are generally used for sample pretreatment and fractionations of bioactive molecules and secondly, chromatographic techniques (HPLC, size exclusion and ion-exchange chromatography, etc.) for further purification, isolation and characterization purposes.
1.4.1 Chromatographic separation and purification processes
Chromatographic techniques such as high pressure liquid chromatography (HPLC), ion exchange chromatography (IEC), size exclusion chromatography (SEC) and fast protein liquid chromatography (FPLC) are used for peptide purification. Generally, a sample pretreatment such as membrane filtration, centrifugation or sieving is required before analytical experiments [84, 87, 111-113]. Moreover, in most cases, a combination of two or more analytical technologies is required to obtain a high degree of purity.
![Fig. 1.5: Cell configuration and principle of electromembrane filtration. Adapted and modified from [29]](https://thumb-eu.123doks.com/thumbv2/123doknet/6486390.173181/64.918.145.713.552.982/fig-cell-configuration-principle-electromembrane-filtration-adapted-modified.webp)