Regulation of mitotic BubR1 phosphorylation by the BubR1 pseudokinase domain
Mémoire
Michelle Mathieu
Maîtrise en biologie cellulaire et moléculaire
Maître ès sciences (M. Sc.)
Québec, Canada
iii
Résumé
BubR1 est une protéine importante dans le point de contrôle de la mitose pour la stabilisation des interactions entre kinétochores et microtubules (KT-MT). Ces fonctions protègent de la ségrégation anormale des chromosomes et de l’instabilité du génome. BubR1 possède des sites de phosphorylation mitotique hautement conservés dans le domaine régulant l’attachement des kinétochores (KARD), où S676 et S670 sont phosphorylées respectivement par la kinase polo-like 1 (Plk1) et par la kinase cycline-dépendante 1 (Cdk1). Ces sites de phosphorylation sont essentiels pour le recrutement de la phosphatase PP2A-B56, qui stabilise les interactions KT-MT. Nos résultats montrent que la délétion entière ou des mutations qui déstabilisent le domaine pseudokinase de BubR1, causent la perte de phosphorylation des résidus S676 et S670 en mitose. Notre hypothèse est que le domaine pseudokinase de BubR1 peut jouer un rôle essentiel dans la régulation de la phosphorylation du KARD et donc dans la stabilisation des interactions KT-MT.
v
Abstract
The mitotic protein BubR1 functions in the spindle assembly checkpoint (SAC) by stabilizing kinetochore-microtubule (KT-MT) interactions. These functions protect the cell from abnormal chromosome segregation and genome instability. BubR1 has highly conserved mitotic phosphorylation sites in the kinetochore-attachment regulatory domain (KARD); the residue S676 is phosphorylated by polo-like kinase-1 (Plk1) and S670 is phosphorylated by cyclin-dependent kinase-1 (Cdk1). These phosphorylation sites are essential for KARD recruitment of protein phosphatase PP2A-B56, which stabilizes KT-MT interactions. Our results show that mutations that cause pseudokinase domain instability and a highly stable truncation mutant of BubR1 were found to cause loss of mitotic S676 and S670 phosphorylation. We hypothesize that the pseudokinase domain of BubR1 may play an important role in the regulation of KARD phosphorylation and thus the stabilization of KT-MT interactions.
vii
Table of Contents
Résumé ... iii
Abstract ... v
Table of Contents ... vii
List of Tables ... ix
List of Figures ... xi
List of Abbreviations: ... xiii
Dedication ... xv
Acknowledgements ... xvii
I Introduction ... 1
I.1 Cell Cycle ... 1
I.1.1. G1 phase ... 2
I.1.2. Restriction Point ... 2
I.1.3. S phase ... 3
I.1.4. G2 phase ... 3
I.1.5. G2/M Checkpoint ... 3
I.1.6. M phase ... 4
I.1.7. Spindle Assembly Checkpoint ... 6
I.2. Post-translational modifications ... 7
I.2.1. Protein Kinases ... 7
I.2.1.1. Mitotic Kinases ... 9
I.2.1.1.1. Cdk1 ... 9
I.2.1.1.2. Plk1 ... 10
I.2.1.1.3. Mps1 ... 10
I.2.1.1.4. Bub1 ... 11
I.2.1.1.5. Aurora B ... 11
I.2.2. Phosphoprotein Phosphatases ... 12
I.2.2.1. Mitotic Protein Phosphatases ... 12
I.2.2.1.1. PP1 ... 12
I.2.2.2.2. PP2A ... 13
I.3. Pseudokinases ... 13
I.3.1. Mitotic Pseudokinase: BubR1... 14
I.3.1.1. Functions of BubR1 ... 16
I.3.1.1.1. BubR1 in Spindle Assembly Checkpoint ... 16
I.3.1.1.2. KT-MT attachment ... 17
I.3.1.1.3. Timer Function ... 18
I.3.1.2. BubR1 Domains ... 19
I.3.1.2.1. KEN boxes ... 20
I.3.1.2.2. TPR domain ... 20
I.3.1.2.3. GLEBS domain ... 21
I.3.1.2.4. KARD domain ... 22
I.3.1.2.5. Pseudokinase domain ... 23
I.4. BubR1 and Disease ... 24
II. Context of My Study ... 27
III. Materials and Methods... 29
viii
III.2. Mutagenesis ... 29
III.3. Cell Culture ... 30
II.4.Transfections ... 30
III.4.1. siRNA Transfections: INTERFERin ... 30
III.4.2. Plasmid Transfections: JetPrime ... 31
III.4.3. Electroporation: Flip-in System ... 31
III.5. Okadaic Acid Hyperphosphorylation Assay ... 32
III.6. ImmunoPrecipitation and Pull-Down ... 33
III.7. Western blot ... 34
III.8. Immunofluorescence ... 34
III.9.Analysis via ImageJ ... 35
IV. Results ... 41
IV.1. Pseudokinase domain mutations cause a loss of stability and hyperphosphorylation ... 41
IV.2. Pseudokinase BubR1 stability causes loss of BubR1 phosphorylation ... 42
IV.3. Mutation of other conserved kinase domain residues does not alter phosphorylation status ... 43
IV.4. Creation of BubR1 truncated pseudokinase stable cell line ... 45
IV. 5. BubR1 pseudokinase mutations BubR1KD and BubR1731X are never hyperphosphorylated ... 45
IV. 6. Loss of BubR1 phosphorylation S676 and S670 by pseudokinase domain mutations ... 47
IV. 7. BubR1KD and BubR1731X show loss of pS676 and pS670 through immunofluorescence ... 50
IV. 8. Plk1-BubR1 binding remains intact despite loss of Plk1 hyperphosphorylation ... 53
IV. 9. Loss of BubR1-Bub3 interaction causes loss of S676 phosphorylation ... 54
V. Discussion ... 57
VI. Conclusion and Perspectives ... 63
VI. 1. Perspectives ... 63
ix
List of Tables
Table 1. Table of Primers ... 37 Table 2 Table of Plasmids ... 38 Table 3. Table of Antibodies ... 39
xi
List of Figures
Figure 1 The cell cycle. ... 1
Figure 2 Phases of mitosis. ... 4
Figure 3 Spindle assembly checkpoint pathway. ... 6
Figure 4 Critical sequence alignment of conserved motifs. ... 8
Figure 5 Parallel evolution of MADBUB paralogs. ... 14
Figure 6 Model for KT-MT attachment stabilization. ... 18
Figure 7 BubR1 domains. ... 19
Figure 8 GLEBS BubR1 domain. ... 21
Figure 9 BubR1 evolutionary KARD domain conservation. ... 22
Figure 10 MVA Mutants. ... 25
Figure 11 BubR1 conserved pseudokinase residue mutation. ... 27
Figure 12 BubR1 stability and hyperphosphorylation loss due to conserved pseudokinase residue mutation. ... 41
Figure 13 MVA and BubR1KD mutations. ... 42
Figure 14 Pseudokinase motif II, VI and VII mutations and pseudokinase truncation mutation (BubR1731X)... 44
Figure 15 Expression of BubR1. ... 45
Figure 16 No hyperphosphorylation of BubR1KD or BubR1731X. ... 46
Figure 17 The α-pS435 is mitotic and phospho-specific. ... 47
Figure 18 Comparative phosphorylation of equal relative BubR1 levels. ... 48
Figure 19 siBubR1 effectively knock-down endogenous BubR1. ... 50
Figure 20 Loss of pS670 and pS676 in pseudokinase domain mutations were confirmed. . 52
Figure 21 Plk1 binds to all BubR1 cell line mutations. ... 53
Figure 22 Forced localization does not rescue the BubR1 S676 phosphorylation. ... 54
xiii
List of Abbreviations:
A: (Ala) Alanine
APC/C: Anaphase promoting complex or cyclosome ATP: Adenosine-5’-Triphosphate
BUB: Budding uninhibited by benzimidazole BUB1B: Budding uninhibited by benzimidazole 1 beta BUBR1: Budding uninhibited by benzimidazole-related 1
C: (Cys) Cysteine
Cdc20: Cell division cycle 20 Cdk1: Cyclin-dependent kinase 1 CenpE: Centromere-associated protein E
D: (Asp) Aspartate
DNA: Deoxyribonucleic acid
E: (Glu) Glutamate
F: (Phe) Phenylalanine
G: (Gly) Glycine
G1: Gap 1
G2: Gap 2
GLEBS Gle2-binding sequence
H: (His) Histadine I: (Ile) Isoleucine K: (Lys) Lysine KD: Kinase dead KEN-box Lys-Glu-Asn-box KMN: Knl1/Mis12/Ndc80 complex Knl1 Kinetochore null 1 KT: Kinetochore L: (Leu) Leucine M: (Met) Methionine
M phase: Mitotic phase
Mad: Mitotic arrest deficient MCC: Mitotic checkpoint complex. Mps1: Monopolar spindle 1
mRNA Messenger RNA
MT: Microtubule
N: (Asn) Asparagine
NEBD: Nuclear envelope breakdown
OA: Okadaic acid
P: (Pro) Proline
PBD: Polo-box domain
PKA: Protein kinase A Plk1: Polo-like kinase 1 PP1: Protein Phosphatase 1 PP2A: Protein Phosphatase 2A PTC : Premature termination codons
Q: (Gln) Glutamine
R: (Arg) Arginine
Rb: Retinoblastoma
RNA: Ribonucleic acid
S: (Ser) Serine
S phase: Synthesis phase
xiv
siRNA: Small interfering RNA
T: (Thr) Threonine
TPR: Tetratricopeptide repeat UTR: Untranslated region
V: (Val) Valine
W: (Trp) Tryptophan WCE: Whole cell extract
xv
Dedication
I dedicate this work and research to my family, my father Gaetan Mathieu, my mother Karen Marks, my brother Georges Mathieu and the sister of my heart Katherine Rheuark.
xvii
Acknowledgements
My sincere thanks to Dr. Sabine Elowe for her time and the knowledge that she has imparted to me and to Philippe Thebault for training me in the various techniques used in the laboratory. I would like to acknowledge Dr. Elowe for performing the siRNA transfection of the CenpE assay and Philippe Thebault for the creation of the BubR1WT and BubR1KD stable
cell lines, and the testing of the pS435 antibody. Thank you, Danielle Caron and Guillaume Combes for all your help, particularly in French. I would like to thank Adeel Ashgar, Luciano Gama-Braga and Audrey Lajeunesse for all their time and aid during our work together. I would like to thank Dr. Denis Soulet for his aid with ImageJ and a thanks to Dr. Patrick Meraldi for the HHFR5 cells. I would also like to acknowledge the NSERC, FRQNT, and CIHR for providing the funding for my research.
1
I Introduction
The adult human body is comprised of approximately 3.72 ± 0.81 x 1013 cells1. These cells
are derived from a single zygote, which repeatedly divides via the cell division cycle2.
Careful replication of genetic information and equal division of chromosomes is necessary to ensure the integrity and viability of daughter cells. The cell division cycle (cell cycle) is a series of cellular events responsible for cellular growth, replication of genetic information and division of material into two daughter cells3-5. In 1989, Murray and Kirschner suggested
that the cell cycle is controlled by both, cell cycle timers and checkpoints. These timers and checkpoints ensure the careful regulation of the cell cycle, as each successive cell cycle event is dependent on the accurate completion of previous events3.
I.1 Cell Cycle
Figure 1 The cell cycle.
The four major phases of the cell cycle, G1, S, G2 and Mitosis, including Cytokinesis and G0. The cell
depictions indicate relative chromosome content in relation to the cell cycle phases. G1 and G0 are diploid
with two copies of every chromosome. S and G2 are tetraploid with duplicate copies of the original two
chromosomes. The progress through mitosis and cytokinesis begins with tetraploid cells and ends with equal division of chromosome copies resulting in diploid daughter cells. (Figure from website:
http://www2.le.ac.uk/departments/genetics/vgec/schoolscolleges/topics/cellcycle-mitosis-meiosis6)
2
The cell cycle can be divided into two major phases: interphase and the mitotic (M) phase (Figure 1)7. The cell spends most of its life in interphase. Interphase can be further divided
into three phases: the Gap 1 (G1) phase, the Synthesis (S) phase, and the Gap 2 (G2) phase7.
Interspersed between these phases are three major checkpoints that arrest cell cycle progression until essential cell cycle processes can be completed8. These checkpoints are
known as the restriction point, the G2/M checkpoint and the spindle assembly checkpoint
(SAC)7,8.
I.1.1. G1 phase
The first phase of the cell cycle, after the completion of cellular division, is the G1 phase
(Figure 1)7. During the G
1 phase, the cell integrates intercellular, metabolic, stress and
environmental signals9. Then the cell must trigger one of several pathways: 1) To enter a
quiescent state known as G0, 2) to commence the cell division cycle or 3) to activate
programmed cell death. Regulation of cell cycle progression is predominantly controlled by cyclin-dependant kinases (Cdk) and cyclins, regulatory Cdk subunits5. Progression through
G1 requires regulation by Cdk4 or Cdk6, which complexes with cyclin D10. Cyclin D has a
high turnover rate due to its instability and thus, cyclin D levels require continued mitogenic signalling to induce protein expression, synthesis and assembly with their Cdk partners10.
I.1.2. Restriction Point
The first checkpoint, the G1/S checkpoint (restriction point in mammals), is the juncture
at which the cell commits itself to cellular division. This restriction point is controlled by active retinoblastoma (Rb) protein family members that suppress cellular growth through inhibition of gene transcription11. Mitogenic signalling allows for activation of Cdk4 or Cdk6
by cyclin D causing the phosphorylation of Rb protein family members and the subsequent deactivation of Rb protein activity10,11. This reduction of gene transcription inhibition allows
for cyclin E expression10. Cyclin E complexes and activates Cdk2. Cdk2-cyclin E,
irreversibly deactivates Rb proteins through hyperphosphorylation12. Once the cell
overcomes the restriction point the cell is committed to completing the entire cell cycle. The cell activates Cdks, such as Cdk2 via the interaction with cyclins E1, E2 and A promoting entry into the S phase and DNA replication9,13,14.
3
I.1.3. S phase
The second phase of the cell cycle is the S phase, in which DNA synthesis occurs (Figure 1)7. Activated Cdk2-cyclin E is considered essential for facilitating the initiation of DNA
replication; however, this complex is deactivated in early S phase to prevent re-replication of the DNA sequences12. Cdk2-cyclin E autophosphorylates cyclin E, thereby targeting the
cyclin for degradation15. The regulation of Cdk2-cyclin E is tightly controlled, as errors could
cause genomic instability and tumorigenesis. As S phase concludes, cyclin A begins associating with Cdk1. Both complexes Cdk2-cyclin A and Cdk1-cyclin A phosphorylate substrates involved with DNA replication and cell cycle progression12.
I.1.4. G2 phase
The G2 phase follows the S phase (Figure 1)8. During the G2 phase, cyclin A begins to be
degraded and cyclin B is synthesized allowing for Cdk1-cyclin B complex formation12. The
Cdk1-cyclin A functions in the nucleus and Cdk1-cyclin B proteins function in the cytoplasm16. These complexes phosphorylate proteins necessary to ensure that all DNA
synthesis is complete, and errors such as DNA damage are corrected prior to entry into the next phase. This step is controlled by the G2/M Checkpoint.
I.1.5. G2/M Checkpoint
Before the cell can proceed into the M phase, the cell must overcome the G2/M checkpoint.
This checkpoint ensures that the cell’s genetic material is replicated successfully and without DNA damage4. Near the end of the G
2 phase, a large percentage of the Cdk1 is associated
with the cyclin B, and activated Cdk1-cyclin B promotes the entry into mitosis12,17. Cdk1
activity is inhibited by its phosphorylation on T14/Y15, by kinases such as Wee1 and Myt117
A multitude of parallel feedback loops regulates the balance between Cdk1 inhibition and its activation; when the balance shifts in favor of Cdk1 activation progression into M phase occurs.
4
I.1.6. M phase
During the M phase of the cell cycle, the doubled contents of the cell are partitioned into two daughter cells8. The activation of Cdk1-cyclin B complex has been seen to specifically
regulate mitotic entry and targets a variety of mitotic proteins5. This activity triggers a series
of morphological alterations, which have been categorized into five mitotic phases: prophase, prometaphase, metaphase, anaphase, and telophase (Figure 2)8,19.
Prophase is the first stage of cellular division and is marked by the condensation of chromatin into chromosomes, and the separation of the sister chromatids, except for their
Figure 2 Phases of mitosis.
There are five phases of mitosis: prophase, prometaphase, metaphase, anaphase and telophase. The tetraploid cell exits the G2 phase and enters the first mitotic phase, prophase. In prophase, the spindle
fibers appear, and chromosomes condense. The cell then progresses into prometaphase, where the nuclear envelope breaks down (NEBD), and the spindle fibers begin interacting with the chromosomes. In metaphase, the chromosomes become aligned at the metaphase plate. The transition from metaphase to anaphase is irreversible and controlled by the mitotic checkpoint. After checkpoint satisfaction, the cells enter into anaphase, and the chromosomes are separated to the opposing spindle poles. In telophase, the nuclear membrane reforms and chromosome condensation is reversed. In cytokinesis, the cytoplasm is divided between the two daughter cells and the two diploid daughter cells are separated. (Figure from website:
http://www2.le.ac.uk/departments/genetics/vgec/schoolscolleges/topics/cellcycle-mitosis-meiosis18)
5 centromeres (Figure 2)8. The centromere is a region of the eukaryotic chromosome that
becomes visible as a site at which two sister chromatids remain connected2,20. The
centromeres serve as the foundations upon which protein complexes can localize during mitosis2.
The second stage of mitosis is prometaphase during which the nuclear membrane breaks down (NEBD), and the spindle microtubules (MTs) begin interacting with the centromeres of the sister chromatids via their kinetochores (KTs; Figure 2)19. The KT is a large complex
composed of many proteins, localized to the chromosomal centromeres, to which the spindle fibers attach and move the chromosomes in mitosis20.
The third stage of the mitotic phase is the metaphase19. During metaphase, chromosomes
are attached to spindle MTs and the chromosomes are pulled into alignment at the imaginary metaphase plate located between the two opposing spindle poles (Figure 2)20. The SAC is a
mitotic checkpoint that arrests the cell before the onset of anaphase inhibiting mitotic progression until all chromosomes are aligned at the metaphase plate and properly attached to MTs2.
Once the cell progresses through the mitotic checkpoint, anaphase immediately commences by the shortening of MTs, which pulls apart the sister chromatids to opposing spindle poles2. In the final mitotic stage, telophase, the nuclear envelope reforms around the
DNA, and the chromosomes reverse the process of condensation. Cytokinesis occurs dividing the cytoplasm between the two daughter cells by the formation of a plasma membrane between the two daughter nuclei20.
6
I.1.7. Spindle Assembly Checkpoint
The SAC is an important, evolutionarily conserved, surveillance mechanism that delays entry into anaphase until all chromosomes are properly attached to MTs21. This delay
promotes error correction and equal division of genetic material into two daughter cells. Cells failing to achieve proper KT-MT attachments may have either unattached KTs or have non-bioriented chromosomes (Figure 3)22,23. These improper attachments lead to the activation of
SAC signalling and the recruitment of major checkpoint proteins to the affected KTs21,24.
These checkpoint proteins include mitotic arrest deficient (Mad1 and Mad2), budding uninhibited by benzimidazoles (Bub1 and Bub3), Bub1 related-1 (BubR1) and cell division cycle 20 (Cdc20).
The activation of the SAC causes the formation of the mitotic checkpoint complex (MCC) (Figure 3)25. The MCC is composed of Mad2, Bub3, BubR1 and Cdc20. This complex
functions to inhibit the anaphase-promoting complex/cyclosome (APC/C) from degrading key mitotic proteins (Figure 3)25,26. The MCC interferes with APC/C activation by inhibiting
the APC/C’s association with co-activators, Cdh1 or Cdc2025,27. Proteins such as cyclin B1
Figure 3 Spindle assembly checkpoint pathway.
Errors that lead to chromosomal missegregation cause activation of the SAC, and trigger the formation of the mitotic checkpoint complex (MCC). This complex is responsible for the inhibition of the anaphase-promoting complex/cyclosome (APC/C), an E3 ubiquitin ligase. The APC/C ubiquinates key mitotic proteins, such as cyclin B and securin, targeting these proteins for degradation, which then allows for mitotic exit.
7 and securin are targets of ubiquination by APC/C and are subsequently degraded by the 26S proteasome26. Cyclin B1 is necessary for the continued activity of Cdk1 during mitosis.
Securin inhibits separase activity and the degradation of securin frees separase. Separase is then able to cleave the cohesin at centromeres, which hold the sister chromatids together. Inhibition of APC/C, therefore, prevents separase release and prevents premature and potentially unequal separation of the sister chromatids between daughter cells (Figure 3).
I.2. Post-translational modifications
Post-translational modifications (PTMs) of proteins are a major mode of cell cycle activity
regulation28-30. PTMs are enzymatically induced, chemical alterations of proteins after the
protein’s translation from mRNA31. These modifications are highly important and have the
potential to significantly alter the protein’s functional capabilities. PTMs can alter a protein’s physical or chemical properties, conformational status and stability as well as their cellular localization and activity. More than 400 specific PTMs have been discovered and among the most common PTMs are phosphorylation, acetylation, ubiquitination, and sumoylation31,32.
I.2.1. Protein Kinases
The most common PTM discovered is phosphorylation, and kinases (the enzymes that mediate this modification) account for 1.7% of all human genes32,33. Many proteins can
exhibit more than one functional role throughout the cell cycle and PTM by enzymes, such as kinases, allows protein functions to be reversibly fine-tuned towards a specific function34.
A kinase is a protein responsible for the catalytic transfer of a high-energy phosphate, such as the adenosine-triphosphate (ATP) γ phosphate, to another protein substrate20. The
predominant residues that are phosphorylated are serine, threonine and tyrosine and a kinase usually targets residues residing in specific consensus motifs34. A given kinase can vary
greatly in the number of sites they are known to target, from a few key sites to hundreds of sites across many proteins35. In one study on Saccharomyces cerevisiae, kinases were used
against in vitro substrate targets and it was found that 1 to 256 substrates could be recognized by a given kinase, with an average of 47 targets recognized per kinase36.
8
Protein kinases are very similar structurally, however, they still maintain the ability to select specific substrate targets through various mechanisms35,37. Kinase selectivity can be
controlled through structure, charge and hydrophobicity of the catalytic kinase cleft allowing for the binding of only certain substrate motifs35. Proteomic screens have been performed to
discover the ideal substrate, consensus sequence, preferred by a particular kinase38-40. In
addition, other alternative methods of kinase specificity regulation exist. Some kinases make use of docking sites or binding partners with docking sites providing an increased affinity for the substrate to which the kinase interacts35. Phosphorylation specificity may also be
controlled by sequestering the kinase interactions to a specific time or location within a cellular compartment (e.g., protein scaffold or cell cycle time point). Not all methods of controlling kinase specificity are used by any one kinase; yet, these methods along with some other modes of kinase phosphorylation regulation play a major role the control of cell cycle division.
In eukaryotic organisms, protein kinases are one of the largest gene families28,33. The
protein kinase A (PKA) catalytic subunit was the first protein kinase structure to be solved and is still used today as a prototype of the protein kinase superfamily41.
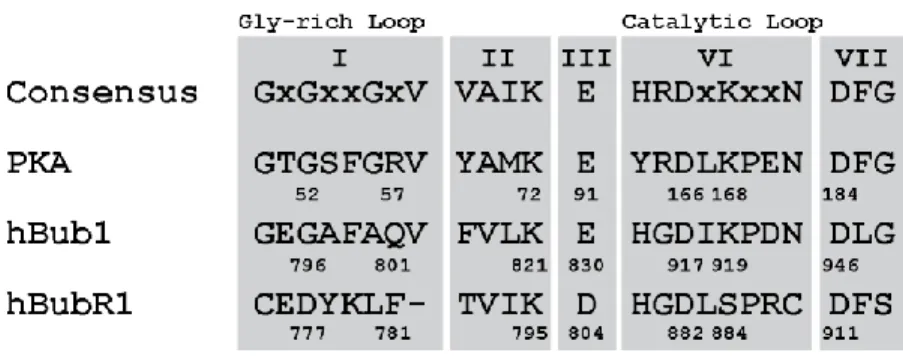
Figure 4 shows several characteristic amino acid, motifs found in functional kinases37.
These motifs are situated to allow for the resulting kinase structure to position both, the ATP, and the protein substrate28. The enzyme then aids in the catalytic transfer of the phosphate
Figure 4 Critical sequence alignment of conserved motifs.
Multiple sequence alignment of important catalytic kinase consensus motifs: protein kinase A (PKA), human Bub1 (hBub1) and human BubR1 (hBubR1) Essential mitotic residues are K72, D166, D184 (PKA numbering) for catalytic activity are conserved in both hBub1 and hBubR1 proteins. (Adapted from Suijkerbuijk, van Dam et al. 2012)
9 between the ATP and the target substrate. Hanks, Quinn et al., using multiple alignment of known protein kinases, were the first to describe the 11 different subdomain motifs conserved in protein kinases37. Subdomains, such as shown in Figure 4, were under intense scrutiny to
ascertain the functions of the each specific conserved region as it pertains to kinase activity37.
The first subdomain (Figure 4), motif I, is a Glycine-rich loop also called the P-loop containing the consensus sequence (GxGxxG) and is found in both proteins that bind to nucleotides as well as kinases42. The Glycine-rich loop acts as a flexible component of ATP
binding, normally lacking a firm structure in the absence of its ATP substrate37. The second
subdomain, motif II (VAIK) in PKA related proteins, carries a charged lysine. This lysine stabilizes the α- and β- phosphates of the ATP to position the γ-phosphate for its catalytic transfer to the substrate, in functional kinases. The Glutamate (E), in the motif III (Figure 4), contains an invariant residue that is highly conserved in active kinases. The biochemical function of the catalytic loop, motif VI (Figure 3), is suggested to aid in substrate proton removal and help position the Mg2+ cofactor. The DFG subdomain, motif VII, is proposed to
be important for chelation of Mg2+ and modification of the ATP-binding site43. Although
these motifs are highly conserved within the PKA family of protein kinases, some kinases within this family may express modified motifs, while still remaining active kinases.
I.2.1.1. Mitotic Kinases
Mitotic regulation relies heavily on PTM of cellular proteins44. A large number of these
proteins are phosphorylated by multiple mitotic kinases45. The regulatory network which
controls activation of the SAC needs to be tightly controlled with reversible PTMs to allow for mitotic progression after SAC signalling has ceased34,46,47. Phosphorylation plays a major
role in SAC regulation, some of the mitotic kinases responsible include Cdk1, Plk1, Bub1, Mps1, and Aurora B5,21.
I.2.1.1.1. Cdk1
Cell-cycle progression is driven by Cdks48. Cdks are a family of serine/threonine kinases,
which bind to and are activated by cyclins49. One mode of Cdk regulation is, in part, driven
10
entry is carefully controlled by Cdk1 in a complex with cyclin B1. Prior to mitosis, Cdk1 is kept in a quiescent state, due to phosphorylation of T14/Y158. This inhibitory
phosphorylation is lost as the cell enters mitosis8. During mitotic progression, various
proteins require phosphorylation by activated Cdk1 to promote downstream protein-protein interactions50,51. Cdk1-cyclin B1 targets substrates with a serine or a threonine that precedes
a proline for phosphorylation44. Active Cdk1 is important for remaining in the M phase, as
the cell progresses to mitotic completion the Cdk1 is rapidly inactivated through degradation of its cyclin co-factor52.
I.2.1.1.2. Plk1
Another family of serine/threonine kinases, which plays an important role in mitosis are the polo-like kinases (Plks)53. These kinases aid in cellular division and proliferation
throughout various eukaryotic species. The Plk family members have a non-catalytic C-terminal domain called the polo-box domain (PBD)53,54. In Plk1, two polo-box motifs appear
in tandem creating a 12-stranded β sandwich domain; this domain is necessary for Plk1 substrate recognition54. Proteomic studies have shown that both polo-boxes are essential for
proper Plk1 PBD-ligand binding to occur and are vital for protein localization and mitotic metaphase to anaphase transition54,55. The prior phosphorylation of a serine or a threonine on
the Plk1 substrate targets is often required to prime the PBD docking site of the target protein54. Both Cdk1 and Plk1 itself may act as priming kinases for generating this
phosphorylated PBD docking site54,56,57. For example, the BubR1 protein is phosphorylated
at T620 by Cdk1 allowing for the Plk1 PBD to target the BubR1 protein51. In the Plk1 PBD
two residues are found to be critical for ligand binding (H538 and K540)54; these residues
when mutated to alanine disrupt PBD ligand recognition, regardless of prior priming phosphorylation of substrate target51,54,58. Plk1 has been seen to play a role in
post-translational regulation of several proteins involved in mitotic control, although only a few Plk1 target substrates have been studied in detail53,59,60.
I.2.1.1.3. Mps1
Monopolar spindle 1 (Mps1) is an essential kinase for proper upstream mitotic surveillance signalling and regulation of segregation61,62; however, only a few regulatory
11 proteins for the dynamic control of its function have been identified61,62. It has been proposed,
that as the Mps1 protein is recruited, increasing levels of Mps1 cause the proteins to homodimerize and autophosphorylate63. This autophosporylation fully activates Mps1 kinase
catalytic activity. The Mps1 kinase protein primes the dividing cell at the end of interphase in preparation for SAC activation64. During mitosis, the active Mps1 kinase is responsible
for the phosphorylation of the kinetochore null 1 (Knl1) MELT motifs allowing the heterodimer Bub1-Bub3 to be recruited to the KT in response to SAC signalling61.
I.2.1.1.4. Bub1
Bub1 is also a serine/threonine kinase that plays an important role in protein recruitment and centromere protein assembly65. Bub1 has been reported to phosphorylate substrates to
inhibit mitotic progression66. The N-terminal, non-catalytic region of the kinase, functions to
recruit proteins critical for mitotic inhibition, such as Mad1, Mad2, Bub3, BubR1, and CenpE (centromere protein E)65,67. Kinase Bub1 and related pseudokinase BubR1 both
heterodimerizes with Bub3, a protein essential for their localization to the kinetochore68-70.
Bub1 is recruited to the kinetochores by prior phosphorylation of the Knl1 protein by Mps171.
Knl1 (Blinkin/Spc105/Spc7 homologue) is an evolutionarily conserved, KT protein that functions as a protein scaffold during mitosis72. The N-terminal region of the Knl1 was found
to play a role in Bub1 phosphorylation of histone H2A, which promotes downstream recruitment of kinase Aurora B73. Bub1 activity has also been cited as a negative regulator
of programed cell death74.
I.2.1.1.5. Aurora B
Aurora B phosphorylation of various KT proteins plays a critical role in mitosis by aiding in the correction of KT-MT attachment error73. For example, the phosphorylation of mitotic
protein Hec1 by Aurora B destabilizes erroneous KT-MT attachments that could lead to chromosome lagging or tearing75,76. Aurora B also performs a very limited role in response
to unattached KTs during the mitotic checkpoint77. During mitosis, Aurora B promotes the
recruitment of mitotic surveillance proteins such as Mad1, BubR1, and CenpE77. Bub1 kinase
12
Bub proteins controls the dynamics of mitotic error correction machinery by regulating Aurora B localization and the phosphorylation status of various Aurora B targets47,79-81.
I.2.2. Phosphoprotein Phosphatases
In contrast to kinases, phosphoprotein phosphatases are enzymes, which dephosphorylate previously phosphorylated protein targets. These protein phosphatases fall into two categories, serine/threonine protein phosphatases and protein tyrosine phosphatases. Proteomic analysis of 6600 human protein phosphorylation sites showed that phosphoserine (pS), phosphothreonine (pT) and phosphotyrosine (pY) accounted for 86.4%, 11.8% and 1.8% of phosphorylated amino acids, respectively82. Interestingly, there are 428
serine/threonine kinases and 90 tyrosine kinases currently reported83, whereas there are 107
tyrosine phosphatases and ~30 serine/threonine phosphatases.
I.2.2.1. Mitotic Protein Phosphatases
The activity of protein phosphatases counterbalances kinase activity allowing for the phosphorylation of target substrates to be reversible84. This reversible signalling permits the
phosphorylation of proteins to be tightly regulated and is used for the integration of cellular signalling and decision-making. Two major serine/threonine protein phosphatases found to be active during mitosis are protein phosphatase 1 (PP1) and protein phosphatase type 2A (PP2A)85.
I.2.2.1.1. PP1
In early mitosis, the protein phosphatase PP1 activity is inhibited by the Cdk1-cyclin B complex86,87, which leads to a state of increased phosphorylation of PP1 target substrates.
Reduced levels of Cdk1-cyclin B allow for PP1 to dephosphorylate itself and return to a state of full activity86,88. The KT protein, Knl1, recruits active PP1 to the SILK/RVSF consensus
motifs found within Knl1 and is regulated by the phosphorylation of the motifs89. This protein
phosphatase recruitment permits the phosphatase mediated dephosphorylation of the Knl1 MELT motif and other KT associated proteins phosphorylated by Mps1, thereby promoting KT-MT stability near the end of metaphase89-91. PP1 is also able to delocalize PP2A-B56
13 mitosis, the recruitment of PP1 to Knl1 can be inhibited by Aurora B phosphorylation of the RVSF motif89, demonstrating tight regulatory control of the phosphorylation status of various
kinase and phosphatase proteins.
I.2.2.2.2. PP2A
Another phosphatase, PP2A, is a heterotrimeric protein consisting of a structural subunit, a catalytic subunit and a variable regulatory subunit92. The protein’s regulatory subunit
provides the essential biochemical factors necessary for fine-tuning substrate specificity and substrate targeting, creating more specialised functioning of the PP2A protein trimer. Currently, 25 different regulatory subunits have been identified for PP2A, among them are B55 and B56, which has been seen to be active in mitosis47.
Both PP1 and PP2A have been shown to counteract the Aurora B and Mps1 activity leading to the silencing of surveillance machinery, which inhibits mitotic progression, to ensure the proper division of genetic material to daughter cells80,89-91,93.
I.3. Pseudokinases
The human genome project (HGP) has uncovered a large amount of data, which has since been closely scrutinized, including information on the human kinase complement of the genome33. From this massive influx of bioinformatics data, it has been found that 10% of
homologous gene sequences to known protein kinases have mutations in gene positions of key catalytic residues33,94-97. The essential residues are K72, D166 and D184, using
traditional nomenclature based on the protein kinase A (PKA) model (Figure 4)41.These
mutations cause a loss of those important catalytic residues and suggest the absence of enzymatic activity98,99. In proteins containing kinase motifs, the lack of catalytic activity
suggested by the loss of these key catalytic residues is why such proteins were given the label of pseudokinase37,94. However, in a few cases proteins have been found lacking these
important catalytic residues while seemingly retaining catalytic activity; therefore, these proteins are not pseudokinases, but functional kinases95,98,99. By contrast, if a protein retains
the key catalytic residues for a kinase but is modified in other critical motifs, this also may result in loss of kinase function and thus is a pseudokinase100.
14
I.3.1. Mitotic Pseudokinase: BubR1
Early genetic screens lead to the discovery of several protein-coding genes that when mutated, caused errors in the SAC of S. cerevisiae101-103. The genes were discovered through
genetic screening and were found to code for proteins Mad1, Mad2, Mad3, Bub1, Bub3, and Mps1101-103. The yeast Mad3 protein (which lacks a kinase domain entirely) is recognized as
an orthologue to human BubR1100. BubR1 has also been noted for its homology to Bub1104.
The BubR1 protein is very unusual. This protein retains the kinase motifs as well as the key residues found in catalytically active kinases (Figure 4). However, in many eukaryotic species the functional role of the BubR1 C-terminal ‘kinase’ domain has been highly controversial. Reports from several laboratories suggest that the C-terminal BubR1 domain is non-essential for the APC inhibition105,106 or the SAC function51,107. In contrast, other
Figure 5 Parallel evolution of MADBUB paralogs.
Of the SAC proteins, BubR1 was the first to show evidence of function outside of the spindle assembly checkpoints and has been seen in other processes and during other cellular phases. The presence of a domain is denoted by colour, KEN box (blue), TPR (green) and kinase/pseudokinase (yellow). *M. pusilla, (orange) has only a partially present domain. The absence of the domains are denoted by (white). (Adapted from Suijkerbuijk, van Dam et al. 2012)
15 research groups suggest that the C-terminal domain activity is important for the full restoration of checkpoint function108-110.
Researchers undertook to study the BubR1 C-terminal domain more closely and resolved to track the evolutionary development of Bub1 and BubR1 from its Last Eukaryotic Common Ancestor (LECA) (Figure 5)100. Their research suggests that the LECA of Bub1 and BubR1,
which they named MADBUB, underwent multiple instances of parallel evolution. This parallel evolution resulted in gene duplication and a subfunctionalization between the two gene facsimiles. Over time, certain domains of each protein were lost, such as the KEN domain in Bub1-like proteins and kinase domain in Mad3/BubR1-like proteins. The N-terminal domains of metazoan BubR1 and Mad3 include an important region for SAC functioning111; loss of this region is detrimental to accurate checkpoint arrest in these cells.
It was assumed that amino acid motifs found from the alignment and sequencing of multiple kinases would reveal key residues, which were essential to those of functional kinases37.
These key residues would thereby be responsible for allowing the phosphate transfer that defines kinase catalytic activity.
The human BubR1 protein contains the key motifs typically required for a catalytically active kinase domain (Figure 4). The retention of these key residues in BubR1 suggests to some that the BubR1 protein might have the ability to interact with ATP37. However, the
human BubR1’s motif I has lost many of the glycine residues, which characterize the glycine-rich loop, a region that normally acts as a flexible component of ATP binding (Figure 4). In addition, in motif VI the conserved lysine, K168 in PKA, is a serine, S884 in BubR1, which becomes phosphorylated completely reversing the charge in the catalytic region of the BubR1 protein (Figure 4)100. This charge reversal would suggest that the interactions, which would
normally take place in this region in an active kinase may not occur.
Finally, the mutation of BubR1D882A, a mutation of one of the three highly conserved
catalytic residues found in BubR1, had no effect on BubR1 function100. In light of this
evidence, the BubR1 C-terminal domain is suggested not to act as a kinase domain, but instead is retained for protein stability and thereby protein the functioning of the other protein domains of BubR1. Thus, despite retention of all the catalytic residues the BubR1 should, actually, be classified as a pseudokinase.
16
I.3.1.1. Functions of BubR1
Three functional roles are suggested for BubR1 during mitosis. These functions are BubR1’s activity in the spindle assembly checkpoint leading to successful mitotic exit21, its
role in stabilizing the KT-MT interactions47,112, and finally its activity in controlling the
duration of mitosis113. To better understand the functional roles of different BubR1 domains,
attempts are being made to separate the functions by domain targeted mutations114. It remains
to be determined if all the various BubR1 functions are independent or interdependent in relation to one another; however, research is currently underway to isolate these functions and perhaps determine this answer.
I.3.1.1.1. BubR1 in Spindle Assembly Checkpoint
The BubR1 protein is an important part of the MCC21. The MCC is composed of BubR1,
Bub3, Mad2, and Cdc20. The activation of the SAC inhibits the degradation of both the cyclin B and securin, preventing the mitotic progression into anaphase25,26. To understand
the role of BubR1 in the SAC, an in-depth look at certain protein pathways that take place during mitosis is required.
The mitotic protein Mad2 exists in two conformations inactive, open (O-) Mad2 and active, closed (C-) Mad2115. Mad1 forms a core complex with C-Mad2. Mad1-C-Mad2
captures Cdc20 and forms C-Mad2-Cdc20. At this time, BubR1 forms a heterodimer with Bub3, through the BubR1 GLE2p-binding sequence (GLEBS) domain70. The BubR1 protein
also contains two Lys-Glu-Asn (KEN) domains, as well as a tetratricopeptide repeat (TPR) domain116. Following preassembly of the C-Mad2-Cdc20, the N-terminal KEN box (KEN1)
and the TPR domains together form the Cdc20 binding region21,117. The two complexes
Mad2-Cdc20 and BubR1-Bub3 then assemble to form the MCC. The MCC functions to prevent the activation of APC/C21.
In recent studies, immunoprecipitation assays have shown that the BubR1 is capable of complexing with a second Cdc20118, this indicates that more Cdc20 is sequestered by the
MCC than originally understood. The C-terminal KEN box (KEN2) has also been found to interact directly with APC/C119, demonstrating that it can directly inhibit the APC/C from
17 binding to Cdc20. Therefore, BubR1 blocks the APC/C activation by preventing the Cdc20 cofactors from interacting with the APC/C in multiple ways. These findings underscore the essential nature of BubR1 function in SAC checkpoint.
Recent work on the BubR1 kinetochore attachment regulatory domain (KARD) has suggested that the BubR1 recruitment of PP2A may play a role in the silencing of the SAC93.
The Mps1 kinase activity activates the SAC120, and the SAC signalling recruits Bub1-Bub3
to the Knl1 at the KT61,64,72,121. This activity further drives SAC signalling, thereby
maintaining Bub1-Bub3 recruitment to the kinetochore61,64. PP2A-B56 recruited to BubR1,
is responsible for KT-MT stabilization and has been implicated in SAC silencing, by dephosphorylating the substrates targeted by Mps1 kinase activity47,79-81,93. Loss of Mps1
phosphorylation causes loss of Bub1 at kinetochore64,93. In brief, BubR1 recruits PP2A-B56,
which dephosphorylates the Mps1 phosphorylated substrates silencing the SAC.
I.3.1.1.2. KT-MT attachment
Creating proper KT-MT attachments are vital for equal cellular segregation of genetic materials. Earlier experiments have suggested that the BubR1 protein plays a role in correcting KT-MT attachment errors and acts in an antagonistic capacity to Aurora B and Mps1 phosphorylation (Figure 6)77,93,112,122. The KT is partly formed by the KMN
(Knl1/Mis12/Ndc80 complex) a protein scaffolding to which other KT proteins interact during prometaphase123,124. The KMN complex binds to the constitutive
centromere-associated network (CCAN) and acts as the linkage site for plus end spindle MT interactions115,123.
When the dynamic MT attachments need to be corrected, the KMN proteins become phosphorylated (Figure 6)61,73; certain kinases, such as Aurora B and Mps1, have been
identified as phosphorylating the KT associated proteins to promote the KT-MT attachment turnover. Aurora B was identified as the principle kinase responsible for the KT-MT destabilization75,76,125. The MT destabilization caused by Aurora B phosphorylation of
incorrect KT-MT attachments allows the opportunity for the MTs to re-connect to a more appropriate attachment site. It was discovered that early KT-MT interactions were protected from destabilization by Aurora B through its dephosphorylation by PP2A-B56 79.
18
Figure 6 Model for KT-MT attachment stabilization.
Kinases phosphorylate Knl1 allowing for the destabilization of improper KT-MT interactions and recruitment of Bub1-Bub3. This recruitment allows for the downstream recruitment of BubR1-Bub3. Kinases such as Cdk1 and Plk1 phosphorylate BubR1 KARD domain at S670 and S676, respectively. This phosphorylation allows for the recruitment of PP2A-B56. PP2A-B56 dephosphorylates Knl1 and other substrates allowing for the disassociation of recruited proteins at the KT and promotes stabilization of KT-MT interactions.
Research has revealed the Mps1 phosphorylation of Knl1 allows docking of Bub3 at the MELT motifs and recruitment of Bub161,126,127. Bub1 activity enables the recruitment of
several downstream proteins, such as Bub3, BubR1, Mad1 and Mad2 (Figure 6)128-134. More
recent studies have shown that phosphorylation of the BubR1 KARD domain by Plk1 and Cdk1 kinases localizes PP2A-B56 directly to BubR147,51,80,135. PP1 and PP2A have been
shown to dephosphorylate the Knl1, opposing both the phosphorylation of Aurora B and Mps1 (Figure 6)61,85,93. PP2A-B56 activity regulates the release of BubR1 and Bub1 from the
KTs, silencing the SAC and stabilizing the MT attachments47,75,76,93,125
I.3.1.1.3. Timer Function
In a population of animal cells, the average duration observed in mitotic cells is fairly consistent113. Roughly 80%, of cells after entering mitosis, will initiate anaphase 20-30
19 cells through mitosis, particularly mutations that disrupt SAC functioning. The average time that a vertebrate cell spends in mitosis, from the point at which the last KT attaches to the start of anaphase, is an average of 23 minutes22. Perturbation of SAC functioning, through
loss of Mad2 or BubR1, shortens the time spent in mitosis136. Other mutations that cause
malorientation of chromosomes have been seen to increase the duration of the mitotic delay113. However, only a certain amount of time in mitosis is possible before the cells will
either enter into apoptosis or the proteins maintaining chromosome cohesion will degrade, and anaphase will commence115.
I.3.1.2. BubR1 Domains
The BubR1 domains can be divided into three predominant regions. The NH2 (N) terminal
region contains the domains important for SAC function, and KT recruitment: KEN1, KEN2, TPR, and GLEBS (Figure 7). This N-terminal region is highly conserved in both the BubR1 and Bub1 homologs of humans as well as those of other organisms100. The middle region
contains the KARD, which is required for stabilizing KT-MT interactions47. Lastly, the
COOH (C) terminal contains the conserved pseudokinase domain. Figure 7 BubR1 domains.
A schematic representation shows several highly conserved domains of the BubR1 protein. The KEN1, TPR, and KEN2 domains are necessary for SAC functioning. The GLEBS domain is essential for BubR1 localization to the KT. Recently, the KARD domain and its phosphorylation sites S670 and S676, have been found to be important for their role in KT-MT stability. However, the C-terminal pseudokinase domain has a conserved pseudokinase domain of undetermined functional significance.
20
I.3.1.2.1. KEN boxes
The two amino acid sequences of Lys-Glu-Asn have been named KEN box domains and are found at in human BubR1 at positions 26-28 (KEN1) and 304-306 (KEN2). The KEN box was originally identified as a motif that is a substrate for poly-ubiquination by APC/C for subsequent degradation by the proteasome137. However, this is not the case for BubR1
causing much interest in their study. These KEN box domains in BubR1 were instead discovered to be important for SAC function and for Cdc20 binding138,139. In one study, the
KEN1 was found to be critical for direct interaction with Cdc20, but not the KEN2 in humans135. These results were also found in yeast111,138. The KEN1 domain exists in a highly
flexible segment of BubR1, which may lead to greater or lesser accessibility of this region to Cdc20 depending on the other, PTMs or protein interactions140,141.
It was recently discovered that the KEN2 box interacts with the APC/C as a pseudosubstrate, further blocking APC/C substrate recruitment119. The KEN2 is suggested to
be targeted by APC/C as if the KEN2 was a true ‘degron’ motif142. However, BubR1 is
acetylated, and this acetylation allows for APC/C targeting, but simultaneously prevents the APC/C from ubiquitinating the BubR1 protein140,142,143. The errors causing loss of BubR1
acetylation would, therefore, allow for BubR1 ubiquitination and subsequent degradation, thereby permitting SAC signalling defects and promoting aberrant chromosome segregation143.
I.3.1.2.2. TPR domain
BubR1 contains TPR region, a consensus of 34 amino acids,triple in tandem repeats. The TPR domain occurs in human BubR1 N-terminal residues 50-204. This amino acid motif creates a structure of a helix-turn-helix, which is then packed into a spiral of alpha-helices144.
This domain aids in the BubR1’s binding to Knl1145. TPR also aids the KEN1 interaction
21
I.3.1.2.3. GLEBS domain
Figure 8 GLEBS BubR1 domain.
The GLEBS domain is vital for BubR1-Bub3 heterodimerization and subsequent localization of BubR1 to the kinetochore. The mutation of the BubR1E413K within the BubR1 GLEBS domain prevents the dimerization
of BubR1 with Bub3.
The GLEBS is a motif that was first identified in budding yeast nuclear pore 116 protein (scNup116p) and acts as a docking site of the GLE2 protein (scGle2p) in the nuclear pore complex68. Human ribonucleic acid export 1 (RAE1), an mRNA export factor, also identified
as GLE2, is found to bind to an NUP98 via this GLEBS motif70. The RAE1 and Bub3 seen
in yeast and higher eukaryotes share sequence homology. The BubR1 protein also contains a GLEBS motif at residues 400-440; however, there has been no evidence that there is protein interaction between BubR1 and NUP98. Studies in mice show that the BubR1 and Bub1 GLEBS motifs were found to be sufficient for Bub3 binding70. Bub3 is an important protein
for BubR1 localization to the KT and is considered a part of the MCC21.
A study was performed using the point mutation BubR1E413K (Figure 8), which prevents
BubR1 heterodimerization with Bub3, to better understand the interaction and functions of the BubR1-Bub3 complex135. The BubR1 protein exhibits a mitotic electrophoretic upshift
caused by Plk1 mediated hyperphosphorylation of the BubR1 protein51. There has been no
evidence that this hyperphosphorylation is necessary for SAC functioning. The mitotic BubR1E413K mutation loses this characteristic hyperphosphorylation, as well as other mitotic
specific phosphorylations on the BubR1 S670 and S676 residues51,135. The phosphorylation
of both S676 and S670 has recently been observed to be important for the regulation of stable KT-MT attachments47. Besides S670 and S676, several other mitotic specific
22
occurs in the GLEBS domain146. However, the kinase phosphorylating this residue has yet to
be discovered.
I.3.1.2.4. KARD domain
Figure 9 BubR1 evolutionary KARD domain conservation.
The KARD domain is highly conserved across multiple species. Three residues have been observed to be important for KT-MT attachment stability. The phosphorylation of S676, S670 and T680 have been noted for importance in the recruitment of PP2A-B56. The residues include phosphorylated residues (red), highly conserved residues (purple), and moderately conserved residues (brown). (Adapted from Suijkerbuijk, Vleugel et al. 2012)
The middle region of the BubR1 protein contains the KARD domain (Figure 9). Phosphorylation of this domain by Plk1 and Cdk1 allows for the recruitment of PP2A-B56 to BubR147,51,80,135. The BubR1 KARD domain includes three well-conserved
serine/threonine residues S670, S676 and T680, which are phosphorylated during mitosis47.
Specific point mutations have suggested that loss of phosphorylation of these three residues in the KARD domain significantly increases the chromosome misalignment. Also, pull-down assays have confirmed that loss of these residues causes a loss of PP2A-B56 recruitment to the BubR1 protein.
BubR1 phosphorylation by Cdk1 at T620 allows for Plk1 binding and phosphorylation to occur at S67651. Cdk1 phosphorylation is also responsible for the phosphorylation of S670
on BubR1135,146. These phosphorylations lead to the recruitment of PP2A-B56 and the
subsequent dephosphorylation by phosphatases permits the stabilization of the KT-MT interactions and the continuation of mitotic progression80.
23
I.3.1.2.5. Pseudokinase domain
The pseudokinase domain of BubR1 has been an area of debate for many years, for both its kinase activity and its functional significance100,106,109,110,147,148. Some recent research has
been fairly convincing of the lack of kinase activity in the human pseudokinase100. This
research suggests that the BubR1 pseudokinase domain has no catalytic kinase function but is, instead, important for structural stability, which in turn supports other BubR1 mitotic functions.
Another avenue of inquiry into the kinase versus pseudokinase debate, suggests that the
BubR1 kinase activity is CenpE-dependent108,109. CenpE is a KT associated MT motor
protein, which may link the SAC to MT capture. Previous research has indicated that CenpE is essential for chromosome alignment at metaphase149-151. This motor protein is associated
with unattached KTs in the rapidly dividing cells of mice149 and is suggested to act as a KT
attachment sensor, which binds both MT and KT recruited checkpoint proteins108. In Xenopus
egg extracts, CenpE was linked to the activation and maintenance of SAC signalling and the loss of CenpE leads to the inhibition of Mad1/Mad2 recruitment152. One protein found to
bind to CenpE is the SAC protein BubR1153, which also happens to be implicated in KT-MT
regulation47. Also, upon immunodepletion of CenpE in Xenopus cells there is no effect on
cellular levels of BubR1108; however, the SAC signalling was eliminated, and recruitment of
BubR1 to the KT was slightly reduced. Therefore, CenpE does have an effect on BubR1 activity via protein localization, although it remains to be fully understood if this effect is confined to KT recruitment or if CenpE aids BubR1 in other functional capacities.
However, not all BubR1 kinase or pseudokinase domains are created equal and in certain species residual kinase function may remain. An example of this is seen in the BubR1 of
Drosophila melanogaster, where the kinase activity is required for proper spindle function
but is unnecessary for the fly SAC114. Thus, each model must be looked at individually as the
BubR1 protein domain functions may be relatively similar, but the exact mechanism of action may be species specific.
24
I.4. BubR1 and Disease
BubR1 is an extremely important cellular protein and loss of this protein is non-viablein eukaryotic cells110. In addition, several disease states have been attributed with lowered levels
of BubR1154,155. Researchers observed that reduced levels of the BubR1 protein in cells
resulted in shortened lifespan, cachectic dwarfism, lordokyphosis, cataracts, impaired healing and loss of subcutaneous fat154. These expressed phenotypes are similar to those
found with advanced age.
Over time, the levels of BubR1 naturally become reduced suggesting that BubR1 plays a regulatory role in aging154. Due to this decrease in BubR1 protein levels, more protein
segregation errors may be found resulting in aneuploidy cells154,155. Aneuploidy is a state in
which a cell does not have the normal chromosome complement. However, it has been noted that artificially increasing BubR1 protein levels has resulted in a reduced levels of aneuploidy cell development156. Mosaic variegated aneuploidy syndrome (MVA; OMIM 257300) is
another disease state caused by mutations in the Bub1-Beta (BUB1B) gene, which codes for the BubR1 protein157,158. MVA is a rare autosomal recessive disorder. This disorder is
characterized by mosaic aneuploidies, trisomies, and monosomies, caused by incorrect chromosome segregation159. Individuals with MVA develop a number of genetic
abnormalities depending on the tissue and the severity of errors involved160-162.
The affected patients with MVA carry either monoallelic or biallelic mutation of the
BUB1B gene157,158. Monoallelic mutations of BUB1B either cause missense or nonsense
mutations, combined with an allelic variant causing a low expression level of the BubR1 protein from the other allele158. Biallelic mutations typically harbor one missense and one
nonsense version of the BUB1B gene mutants157. A missense mutation is caused by a
substitution mutation in the gene sequence, which results in an amino acid change in the final protein product163. Figure 10 shows several missense mutations found in MVA patients158,164.
Nonsense mutations create a premature termination codon (PTC)163,164. In mRNA, when
PTCs are created they cause a failure to remove exon junction complexes and thus mRNA with PTCs are targeted by other proteins recognizing the remaining exon junction complexes163. These proteins degrade the mutant mRNA and thereby prevent the build-up of
25 truncated proteins. The BubR1 mutations that create PTCs cause a low BubR1 mutant protein levels caused by nonsense-mediated decay (NMD) in patients expressing this protein.
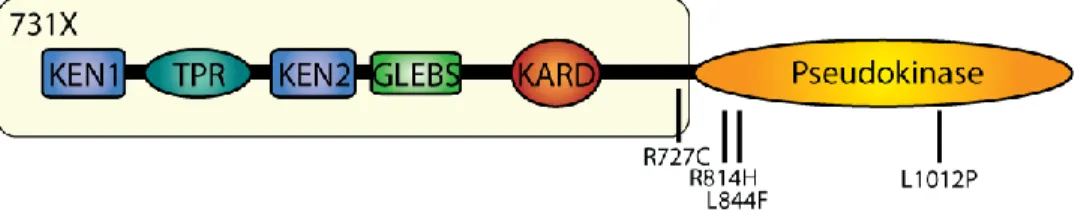
One example of a BubR1 nonsense mutation is BUB1B731X,and this mutation is found in
patients expressing MVA100. The mRNA derived from this mutation causes MVA due to its
production of PTCs and rapid mRNA degradation causing low to non-existent expression of the BubR1731X protein in patient cells. The BubR1731X exhibits a complete truncation of
pseudokinase domain (Figure 10)158,164. Interestingly, BubR1731X when expressed after
plasmid transfection was found to be extremely stable. By contrast, researchers have tested the protein stability of several other BubR1 protein mutants expressed in MVA patients164.
Many of the missense mutations found in MVA patients caused protein instability, and these missense mutations were often discovered to be in or near the BubR1 pseudokinase domain (Figure 10).
Figure 10 MVA Mutants.
The MVA mutations, BubR1R727C, BubR1R814H, BubR1L844F, and BubR1L1012P have been found to cause
BubR1 protein instability. Mutations causing this instability in MVA patients appear to be localized in and around the BubR1 pseudokinase domain.
27
II. Context of My Study
Figure 11 BubR1 conserved pseudokinase residue mutation.
The mitotic HeLaS3 cells were transiently transfected with 3x-Myc-BubR1 mutants. The mutation of the BubR1K795R and BubR1KD within the pseudokinase domain appear to cause a loss of the characteristic mitotic BubR1 upshift causes by Plk1 activity. (Sabine Elowe, unpublished data)
The unusual pseudokinase BubR1 is a SAC protein that is very important for both KT-MT stabilization and SAC signalling21,47,51,135. It is an unusual pseudokinase in that it has
conserved several residues that are important for catalytic kinase activity. However, there has been much debate over the last decade as to whether or not human BubR1 is a functional kinase or a non-functional pseudokinase100,109,165. A recent study by Kops’ team, presented a
convincing article, suggesting that BubR1 is a pseudokinase, and proposed that the retention of this pseudokinase domain in humans was a matter of protein stability100. Based on this
evidence, we proceeded to test not the potential kinase activity of the BubR1 protein, but rather the domain’s function in relation to BubR1’s phosphorylation. One major factor in understanding BubR1 protein functioning is understanding how the PTMs, in particular phosphorylation, affects and controls its functioning47,51,135,146. Certain BubR1
phosphorylations have been found to be important to mitotic regulation47.
Previous results obtained through transient transfections and Western blotting showed that substitution mutations within the pseudokinase domain of human BubR1 exhibiting an altered phosphorylation phenotype during mitosis (Figure 11). Mitotic, BubR1wild-type
(BubR1WT) has a characteristic electrophoretic upshift, caused by a hyperphosphorylation of
28
dead or BubR1KD) are mutations in sites that would inhibit catalytic activity in functional
kinase orthologues of BubR1100, by targeting what would be a region of ATP-binding in the
‘kinase’ subdomains II and VII, K72 and D184 in PKA37. The resultant BubR1 protein mutants
created by these targeted mutations would be unable to perform the catalytic phosphate transfer of a functional kinase, if the kinase was functional. Interestingly, the mutation of these residues caused a loss hyperphosphorylation (Figure 11), and as the BubR1 protein is suggested to be a pseudokinase100, this loss of hyperphosphorylation is not due to loss of
kinase functioning.
It is known that the BubR1WT protein has a highly, unstable, pseudokinase domain, and
several single point mutations, within the pseudokinase domain have the ability to de-stabilize the protein as seen in several patients exhibiting MVA157,158,164. We ventured to
discover why these pseudokinase domain mutations, BubR1K795R and BubR1KD, disrupted
the hyperphosphorylation normally observed in mitotic BubR1 (Figure 11).
We hypothesize that the BubR1 pseudokinase domain may play a role in regulation of BubR1 phosphorylation independent of protein stability. My project set out to study and better understand how the alterations of the BubR1 pseudokinase domain changed the phosphorylation status of several known phosphorylation sites of BubR1. We performed experiments to discover if the phosphorylation of BubR1 was dependent on the stability of the protein or if the BubR1 phosphorylation was necessary for protein stabilization.
29
III. Materials and Methods III.1. Plasmid Preparations
Plasmids containing a gene of interest were transformed into DH5α, Escherichia coli. A measure of 100 ng of plasmid DNA was incubated with DH5α chemically competent bacteria for 30 minutes at 4 ˚C. The mixture was placed at 42˚C for one minute and then again at 4 ˚C for one minute. A volume of 1 ml of Luria-Bertani (LB) media (10 g/L Bacto-Tryptone, 5 g/L yeast extract, and 10 g/L NaCl) was added to the mixture and incubated at 37˚C with agitation for 50 minutes. A sample of the bacteria was plated on selection LB-agar selection media (10 g/L Bacto-Tryptone, 5 g/L yeast extract, 10 g/L NaCl, and 15 g/L Bacto-Agar with Ampicillin (100 µg/ml) and incubated overnight. Bacterial colonies were selected and grown overnight in in LB media, supplemented with antibiotics to maintain selection. The plasmids were then extracted and purified from the bacteria using the manufacturers’ protocol from either the E.Z.N.A. Plasmid Mini Kit I or E.Z.N.A. Plasmid Midi Kit (Omega bio-tek).
III.2. Mutagenesis
The plasmids were derived from 3xMyc BubR1-WT (Dr. Sabine Elowe). All mutagenesis mutations were performed according the protocol of the Phusion Site-Directed Mutagenesis Kit for 50 µl of reaction, using Phusion High-Fidelity DNA Polymerase (New England Biolabs) the appropriate template vector (50 ng) and specific primers were designed (25 μM) (Table 1). After the PCR reactions were performed, the PCR product was incubated at 37˚C for 2 hours with DpnI (20U) (New England Biolabs). A volume of 10 μl of the final product was transformed into DH5α and plated on selection LB-agar media with Ampicillin (100 µg/ml) and incubated overnight. Colonies from the mutagenesis were chosen and mini-preps were performed. All mutagenesis reactions were verified by sequencing (Centre de Recherche du CHUL (CHUQ)). All plasmids, and their selection sensitivity can be found in Table 2.