THÈSE DE DOCTORAT EN COTUTELLE PRÉSENTÉE À L’UNIVERSITÉ PAUL SABATIER TOULOUSE III,
en vue de l’obtention du grade de DOCTEUR EN BIOSCIENCES VÉGÉTALES
DE L’UNIVERSITÉ PAUL SABATIER, TOULOUSE, FRANCE
et de
DOCTEUR EN SCIENCES FORESTIÈRES
DE L’UNIVERSITÉ LAVAL, QUÉBEC, CANADA
par
SYLVAIN LEGAY
C
ARACTÉRISATION
F
ONCTIONNELLE
DE
D
EUX
F
ACTEURS
DE
T
RANSCRIPTION
MYB
R2R3
:
R
ÔLE
DANS
LA
F
ORMATION
DU
B
OIS
CHEZ
LES
A
NGIOSPERMES
Soutenue le 14 Mars 2008, devant la commission d’examen :
A.M. BOUDET, Professeur de l’université Paul Sabatier, Toulouse Président L. LEPINIEC, Directeur de Recherches INRA, Versailles Rapporteur C. MARTIN, Professeure du John Innes Center, Norwich Rapportrice L. BRISSON, Professeure de l’université Laval, Québec Examinatrice J. MACKAY, Professeur de l’université Laval, Québec Co-directeur J. GRIMA-PETTENATI, Directrice de Recherches CNRS, Toulouse Directrice
Le xylème secondaire (appelé bois chez les arbres), est un tissu vasculaire caractérisé par la présence d’un composé phénolique caractéristique, la lignine qui confère hydrophobicité et résistance mécanique aux parois. La différenciation du xylème est un processus complexe qui fait intervenir plusieurs centaines de gènes dont l’expression doit être strictement régulée dans l’espace et dans le temps. Cette coordination spatiotemporelle très fine est assurée au niveau transcriptionnel par des facteurs de transcription. Certains membres de la famille MYB sont par exemple connus pour réguler l’expression des gènes de la voie de biosynthèse des phénylpropanoides, incluant la lignine. Le but de mes travaux était de caractériser fonctionnellement deux facteurs de transcription MYB, EgMYB1 et EgMYB2, isolés à partir d’une banque d’ADNc de xylème d’Eucalyptus gunnii. La majeure partie de mes résultats concerne la caractérisation d’EgMYB1 qui phylogénétiquement, fait partie du sous-groupe 4 des MYB R2R3. Ce sous-groupe comprend plusieurs répresseurs des gènes du métabolisme phénolique. Des expériences de co-expression in vivo dans le tabac appuient l’hypothèse qu’EgMYB1 pourrait agir en tant que régulateur négatif de l’expression des gènes de la voie de biosynthèse de la lignine. Ce rôle potentiel en accord avec l’expression préférentielle d’EgMYB1 dans le xylème de racines et de tiges d’Eucalyptus a été vérifié
in planta chez des peupliers transgéniques. En réponse à la surexpression d’EgMYB1,
le contenu en lignine du xylème secondaire des tiges est réduit. On note également une diminution du nombre de fibres de phloème, et des taux de transcrits des gènes de la biosynthèse de la lignine. De plus, des analyses transcriptomiques réalisées à partir d’ARNs de xylème et d’écorce de tiges de peupliers surexprimant EgMYB1 met en évidence des classes de gènes sous-exprimés ou surexprimés. De nombreuses similitudes sont retrouvées avec les classes de gènes dérégulées chez les mutants affectés dans une des étapes de la voie de biosynthèse de la lignine, renforçant le rôle initialement proposé pour EgMYB1. Les peupliers surexprimant EgMYB1 montrent également d’intéressants phénotypes foliaires qui ont été étudiés. La caractérisation fonctionnelle d’EgMYB2 réalisée chez des tabacs transgéniques surexpresseurs, nous a amenés à proposer un rôle d’activateur transcriptionnel de la voie de biosynthèse des lignines. Les résultats présentés dans cette thèse, apportent des indications claires quant aux rôles opposés des facteurs de transcription EgMYB1 et EgMYB2 dans la lignification lors de la formation du bois.
A
BSTRACT:
Lignin is one of the major characteristics of plant secondary cell walls. In trees, it accumulates mostly during the differentiation of secondary xylem (wood). It provides hydrophobicity to vessels elements and imparts mechanical resistance of the stem. Transcriptional regulation of gene expression during the different stages of xylem cell differentiation is complex; it is thought to involve many molecular actors to enable tight coordination of cellular events. R2R3 MYB transcription factors play a role in this process by regulating the biosynthesis of phenolic compounds including lignin. The goal of my research was to functionally characterize the R2R3-MYB transcription factors, EgMYB2 and EgMYB1, isolated from a Eucalyptus gunnii xylem cDNA library. Most of my results are focused on the characterization of EgMYB1. Phylogenetically, EgMYB1 was classified as part of the R2R3 MYB subgroup 4 containing repressors of phenylpropanoid biosynthesis genes. Together, its expression profile in Eucalyptus and co-infiltration in tobacco results suggested that EgMYB1 could act as a negative regulator of lignin biosynthesis gene expression during wood formation. This putative role was further studied in planta using transgenic poplars over-expressing EgMYB1. Stems of the transgenic plants contained less lignin in the secondary xylem, fewer phloem fibers and lignin biosynthesis genes were down regulated compared to controls. Transcriptomic analysis showed that similar classes of genes were differentially expressed between the transgenic over-expressing EgMYB1 poplars and lignin biosynthesis mutants, consistent with its role as a repressor of the lignin biosynthesis pathway. We also investigated the intriguing foliar phenotypes of poplars over-expressing EgMYB1. Functional characterization of EgMYB2 achieved in transgenic tobacco plants over-expressing this transcription factor, led us to propose a role of activator of the lignin biosynthetic pathway during xylogenesis. The data presented herein provide clear indications as to the antagonist roles of EgMYB2 and EgMYB1 in lignin biosynthesis during wood formation.
P
REFACE:
Au cours de ces cinq dernières années passées à préparer ma thèse, j’ai eu la possibilité de vivre une expérience extrêmement enrichissante au niveau personnel et professionnel. Ceci je le dois notamment aux nombreuses personnes qui m’ont entouré, encouragé et soutenu tout au long de cette épreuve et pour lesquelles je tiens à exprimer toute ma gratitude.
À Jacqueline Grima-Pettenati et John Mackay, mes deux directeurs de thèse qui m’ont permis de prendre part à cette collaboration et qui m’ont guidé tout au long de cette aventure. Merci de votre confiance, patience, soutien, pour tous ces échanges oh combien enrichissants et pour avoir partagé votre passion avec moi.
Aux membres de mon jury de thèse : merci au Professeur Cathie Martin et au Dr Loïc Lepiniec d’avoir accepté d’être les rapporteurs de mon travail de thèse.
Merci au Professeur Louise Brisson et au Professeur Alain Boudet, président du jury, pour avoir évalué ce travail.
Je tiens tout particulièrement à remercier mes parents : pour la confiance que vous m’avez accordé, pour votre soutien tout au long de ma scolarité, pour tous ces sacrifices de votre part pour me permettre de réussir, pour ces nombreuses discussions lors des moments de doutes et pour tout votre amour.
Merci également aux nombreux collaborateurs de ce projet mais plus particulièrement aux Professeurs Catherine Lapierre et Shawn Mansfield pour les analyses chimiques des transgéniques et au Dr Nathalie Pavy pour son aide avec la bioinformatique.
J’adresse mes remerciements aux nombreuses personnes côtoyées dans les différentes équipes et laboratoires au cours de ma thèse. Merci aux membres de l’équipe du groupe « Régulation transcriptionnelle de la formation du bois » : Pierre, Nathalie, Patricia, Colette, etc., aux membres de l’équipe de « Génomique fonctionnelle des Arbres » et plus largement aux membres de l’équipe Arborea: Mario, Fred, Sébastien,
Claude, Pascal, Isabelle, Laurence, Caroline, Armand, Brian, etc. Merci à l’ensemble des membres du laboratoire UMR5546 à Toulouse, du Pavillon Charles-Eugène Marchand et du Centre de foresteries des Laurentides à Québec.
À tous mes amis en France, Daoo, Nono, Simon, Mako, Marie, Frank, Greg, Piquéto et Kesh, Raph, Rom, Nath, Alexi, Tony, les jumelles, Salva, Jordan, Amandine, Bouboune, etc. et au Québec, Dan, Antoine, Joao, Luana et tous les capoeiristes de Sul da Bahia Québec, Fodé, Iris, Jonathan, Eli, Todd, Claude, Monique, etc. Merci d’avoir été là pour me sortir la tête de la thèse, pour votre soutien et votre compréhension notamment sur la fin de la thèse.
Merci à ma chouchou France, pour ta douceur, ta patience et tout ton amour. Promis maintenant je m’occupe de toi!
Enfin à toutes les personnes, qui de près ou de loin, ont contribué à ce travail ou été présentes lors de la thèse, je vous remercie du fond du cœur.
T
ABLE OF CONTENTS:
RÉSUMÉ : ...I ABSTRACT:...III PREFACE : ...V TABLE OF CONTENTS:...VII LIST OF FIGURES AND TABLES: ...XV LIST OF ABBREVIATIONS: ...XIX
CHAPTER I. INTRODUCTION... 1
A. INTRODUCTION... 1
B. LITERATURE REVIEW... 3
B.1. PLANT DEVELOPMENT: PRIMARY AND SECONDARY GROWTH... 3
B.2. VASCULAR TISSUE FORMATION IN THE STEM... 3
B.3. TISSUES AND CELLS OF THE SECONDARY VASCULAR SYSTEM... 5
B.4. THE FORMATION OF WOOD: XYLOGENESIS... 8
B.4.1.MODEL SYSTEMS FOR THE STUDY OF WOOD FORMATION... 8
B.4.1.1. Herbaceous plant systems... 8
B.4.1.2. Woody plant systems: ... 9
B.4.2.CELLULAR ASPECTS OF XYLOGENESIS... 10
B.4.2.1. Dividing cambial initials ... 10
B.4.2.2. Cell elongation ... 10
B.4.2.3. Secondary cell wall biosynthesis... 11
B.4.2.3.a. Cellulose: ... 11
B.4.2.3.b. Hemicellulose: ... 12
B.4.2.3.c. Lignin:... 12
B.4.2.4. Deposition of SCW: ... 12
B.4.2.5. Programmed cell death... 13
B.4.2.6. Hormonal signals controlling vascular tissue differentiation:... 14
B.4.3.MOLECULAR ASPECTS OF XYLOGENESIS... 16
B.4.3.1. First step of xylogenesis: division ... 17
B.4.3.2. Molecular actors of cellular expansion... 18
B.4.3.2.b. Xyloglucan EndoTransglucosylases/hydrolases (XTH): ... 18
B.4.3.2.c. Pectin methylesterases:... 19
B.4.3.2.d. Endo-(1-4)- β-D-glucanases: ... 20
B.4.3.2.e. Others genes involved in cell elongation: ... 20
B.4.3.3. Genes involved in the biosynthesis of the major components of Secondary Cell Wall biosynthesis genes... 20
B.4.3.3.a. Cellulose biosynthesis: SUSY, CESA and KORRIGAN genes ... 21
B.4.3.3.b. Glycosyltransferase direct hemicellulose synthesis: ... 22
B.4.3.3.c. Lignin biosynthesis:... 23
B.4.3.3.c.1. Monolignol biosynthesis:... 25
B.4.3.3.c.2. Incorporation of monolignols into the Secondary Cell Wall: ... 32
B.4.3.3.d. Deposition of the SCW ... 34
B.4.3.4. Programmed Cell Death: ... 36
B.5. TRANSCRIPTIONAL REGULATION AND NETWORKS IN WOOD FORMATION. ... 36
B.5.1.TRANSCRIPTION FACTORS:KEY FUNCTIONAL DOMAINS AND CLASSIFICATION... 37
B.5.2.THE MYB TRANSCRIPTION FACTOR FAMILY... 38
B.5.2.1. Animal MYB proteins ... 38
B.5.2.2. Plant MYB proteins... 39
B.5.2.2.a. Plant R2R3 MYB transcription factors functions... 40
B.5.2.2.b. Regulation of MYB transcription factors activity. ... 42
B.5.2.3. Transcriptional regulators of SCW formation:... 43
B.5.2.4. Regulatory networks of wood formation:... 47
C. HYPOTHESIS AND AIMS OF THESIS... 51
D. PRESENTATION OF THESIS WORK... 55
CHAPTER II. MOLECULAR CHARACTERIZATION OF EGMYB1, A PUTATIVE TRANSCRIPTIONAL REPRESSOR OF THE LIGNIN BIOSYNTHETIC PATHWAY. ... 57
A. PREFACE... 57
B. RÉSUMÉ... 58
D. INTRODUCTION... 58
E. MATERIALS AND METHODS... 59
E.1. RNA ISOLATION... 59
E.2. QUANTITATIVE REVERSE-TRANSCRIPTION POLYMERASE CHAIN REACTION... 60
E.3. BINARY CONSTRUCTS FOR TRANSFORMATION... 60
E.4. TRANSIENT EXPRESSION IN TOBACCO... 61
E.5. EXPRESSION OF GST-EGMYB1 IN E. COLI AND ELECTROPHORETIC MOBILITY SHIFT ASSAY... 61
F. RESULTS... 61
F.1. EGMYB1 SHOWS STRUCTURAL CHARACTERISTICS OF R2R3 MYB REPRESSOR... 61
F.2. EGMYB1 IS LOCALIZED IN THE NUCLEUS... 64
F.3.EGMYB1 TRANSCRIPTS ACCUMULATE PREFERENTIALLY IN EUCALYPTUS SECONDARY XYLEM... 65
F.4. EGMYB1 BINDS SPECIFICALLY EGCCR CIS-REGULATORY ELEMENTS.... 66
F.5. EGMYB1 ACTS AS A TRANSCRIPTIONAL REGULATOR OF EGCCR AND EGCAD2 PROMOTERS IN VIVO... 67
G. DISCUSSION... 67
H. ACKNOWLEDGEMENTS... 70
CHAPTER III. EGMYB1, AN R2R3 MYB TRANSCRIPTION FACTOR FROM EUCALYPTUS, NEGATIVELY REGULATES LIGNIN BIOSYNTHESIS IN TRANSGENIC POPLARS... 71
A. PREFACE... 71
B. RÉSUMÉ... 72
C. ABSTRACT... 73
D. INTRODUCTION... 73
E. MATERIALS AND METHODS... 76
E.1. PLANT MATERIAL... 76
E.2. OVER-EXPRESSION CONSTRUCT... 76
E.3. TRANSFORMATION OF POPLAR AND REGENERATION... 76
E.4. MICROSCOPY TECHNIQUES... 76
E.4.2.PHLOROGLUCINOL STAINING... 77
E.5. CHEMICAL ANALYSIS OF WOOD AND BARK... 77
E.6. DNA/RNA EXTRACTIONS... 77
E.7. EST SEQUENCE PRODUCTION AND ANALYSIS... 78
E.8. SEQUENCE ANALYSIS AND SELECTION OF CDNA CLONES FOR THE MICROARRAY DESIGN... 79
E.9. AMPLICON PRODUCTION AND MICROARRAY PRINTING... 80
E.10. FLUORESCENT ARNA PROBE LABELLING... 81
E.11. MICROARRAY HYBRIDIZATION... 81
E.12. REVERSE-TRANSCRIPTION OF RNA... 82
E.13. REAL TIME QUANTITATIVE POLYMERASE CHAIN REACTIONS... 82
F. RESULTS... 83
F.1. IMPACT OF EGMYB1 OVER-EXPRESSION ON POPLAR GROWTH AND DEVELOPMENT... 83
F.2. HISTOLOGICAL AND CHEMICAL ANALYSIS OF POPLAR TRANSGENIC XYLEM: ... 85
F.3. QUANTITATIVE ANALYSIS OF PHENYLPROPANOID AND MONOLIGNOL GENE EXPRESSION IN TRANSGENIC POPLARS: ... 87
F.4. TRANSCRIPTOMIC ANALYSIS: ... 89
G. DISCUSSION... 93
H. CONCLUDING REMARKS... 97
CHAPTER IV. EGMYB1 OVER-EXPRESSION IN POPLAR INDUCES FOLIAR PHENOTYPES: SUPPLEMENTARY RESULTS. ... 99
A. PREFACE... 99
B. INTRODUCTION... 99
C. MATERIALS AND METHODS... 100
C.1.1.PLANT MATERIAL: ... 100
C.1.2.OPTIC MICROSCOPY TECHNIQUES: ... 100
C.1.3.ELECTRONIC MICROSCOPY TECHNIQUES:... 100
C.1.4.CHEMICAL ANALYSIS OF LEAVES:... 100
C.1.4.1. Anthocyanins:... 100
C.1.4.2. Soluble pehnolics: ... 101
C.1.5.RNA EXTRACTIONS:... 102
C.1.6.MICROARRAY HYBRIDIZATION: ... 102
D. RESULTS... 103
D.1. OVER-EXPRESSION OF EGMYB1 ALTERS THE MORPHOLOGY OF POPLAR LEAVES... 103
D.2. POPLAR LEAVES OVER-EXPRESSING EGMYB1 HAVE INCREASED TRICHOME DENSITY AND CONTAIN LESS ANTHOCYANINS AND SOLUBLE PHENOLICS... 105
D.3.EGMYB1 OVER-EXPRESSION AFFECTS SOLUBLE SUGAR CONTENT IN TRANSGENIC LEAVES... 107
D.4. TRANSCRIPTOME OF LEAVES OVEREXPRESSING EGMYB1 ... 108
E. DISCUSSION... 110
CHAPTER V. EGMYB2, A NEW TRANSCRIPTIONAL ACTIVATOR FROM EUCALYPTUS XYLEM, REGULATES SECONDARY CELL WALL FORMATION AND LIGNIN BIOSYNTHESIS... 113
A. PREFACE... 113
B. RÉSUMÉ... 114
C. ABSTRACT... 114
D. INTRODUCTION... 115
E. MATERIALS AND METHODS... 118
E.1. RECOMBINANT DNA METHODS... 118
E.2. CDNA LIBRARY SCREENING... 118
E.3. RNA ISOLATION... 119
E.4. QUANTITATIVE REAL TIME PCR ... 119
E.5. EXPRESSION OF GST-EGMYB2 IN E. COLI... 120
E.6. ELECTROPHORETIC MOBILITY SHIFT ASSAY (EMSA)... 121
E.7. BINARY CONSTRUCTS FOR TRANSFORMATION... 121
E.8. CO-TRANSFECTION EXPERIMENTS... 122
E.9. TOBACCO PLANT TRANSFORMATION... 122
E.10. MICROSCOPY AND CELL IMAGING... 122
E.11. LIGNIN ANALYSIS... 123
E.12. GENE MAPPING AND QTL ANALYSIS... 123
F.1. EUCALYPTUS EGMYB2 DEFINES A NEW SUBGROUP OF THE R2R3 MYB
FAMILY... 124
F.2.EGMYB2 MAPS TO A UNIQUE LOCUS ON THE E. GRANDIS LINKAGE MAP AND CO-LOCATES WITH A QTL FOR LIGNIN CONTENT... 126
F.3.EGMYB2 IS PREFERENTIALLY EXPRESSED IN DIFFERENTIATING XYLEM TISSUE... 127
F.4. EGMYB2 BINDS SPECIFICALLY THE CIS-REGULATORY REGIONS OF THE EGCCR AND EGCAD2 PROMOTERS... 128
F.5. EGMYB2 ACTS AS A TRANSCRIPTIONAL ACTIVATOR OF EGCCR AND EGCAD2 PROMOTERS IN VIVO... 130
F.6. PHENOTYPIC CHANGES INDUCED BY ECTOPIC EXPRESSION OF EGMYB2 IN TRANSGENIC TOBACCO... 131
F.7. EGMYB2 INCREASES XYLEM SECONDARY CELL WALL THICKNESS... 133
F.8. EGMYB2 CONTROLS THE COORDINATED EXPRESSION OF GENES INVOLVED IN THE LIGNIN BIOSYNTHETIC PATHWAY... 135
F.9. EGMYB2 MAINLY CONTROLS THE MONOMERIC COMPOSITION OF LIGNIN ... 136
G. DISCUSSION... 137
H. ACKNOWLEDGEMENTS... 140
CHAPTER VI. GENERAL DISCUSSION AND CONCLUSIONS... 141
A. EGMYB2 IS AN ACTIVATOR OF LIGNIN BIOSYNTHESIS AND SECONDARY CELL WALL FORMATION... 142
B. EGMYB1 IS A REPRESSOR OF THE LIGNIN BIOSYNTHETIC PATHWAY... 143
C. IS LIGNIN BIOSYNTHESIS CONTROLLED BY THE COMBINATORIAL ACTION OF POSITIVE AND NEGATIVE REGULATORS... 144
D. POSSIBLE MECHANISMS OF REGULATION OF THE ACTIVITY OF EGMYBS.... 145
E. EGMYB1 INTERFERES WITH TTG-DEPENDENT PATHWAYS WHEN OVER-EXPRESSED IN LEAVES... 146
F. CONCLUSIONS... 147
CHAPTER VII. REFERENCES... 149
CHAPTER VIII. APPENDIXES... 187
B. SUPPLEMENTAL FIGURE III-2: ... 188 C. SUPPLEMENTARY TABLE III-1 ... 189 D. SUPPLEMENTAL TABLE IV-2... 213
L
IST OFF
IGURES AND TABLES:
FIGURES:
Figure I-1: Diagram showing the organization of the stem vascular tissues during primary and secondary growth phases in woody species.
Figure I-2: Types of divisions arising from the wood meristem, i.e. the vascular cambium.
Figure I-3: Cells of angiosperm wood vascular tissues. Figure I-4: Cell layers after secondary cell wall formation. Figure I-5: Figure of PCD during TE differentiation.
Figure I-6: General phenylpropanoid biosynthesis pathway and derived pathways including lignin.
Figure I-7: Transcriptional regulation model.
Figure II-1: Sequence analysis of EgMYB1. Figure II-2: Intracellular localization of EgMYB1.
Figure II-3: Transcript accumulation of EgMYB1 in Eucalyptus tissues assessed by RT-qPCR.
Figure II-4: EMSA using a radio labelled fragment (-101/-75) of the EgCCR promoter. Figure II-5: Effects of EgMYB1 on the transcriptional activities of the EgCAD2 and
EgCCR promoters.
Figure III-1: Growth phenotypes of over-expressing EgMYB1 poplar transgenic lines. Figure III-2: Morphology of leaves of the transgenic poplar lines 1 and 6 over-expressing EgMYB1.
Figure III-3: Effect of EgMYB1 over-expression on the vascular system of transgenic poplar lines.
Figure III-4: Chemical composition of the extractive-free cell walls of transgenic poplar plants over-expressing EgMYB1.
Figure III-5: RT-qPCR expression profiles of poplar phenylpropanoid and lignin biosynthesis gene isoforms linked to wood formation.
Figure III-6: Venn diagram of the differentially expressed gene sets in wood and bark of transgenic poplar line 6 over-expressing EgMYB1.
Figure IV-1: Adaxial leaf surface of wild type poplar (A, C) and transgenic line 6 (B, D) observed by scanning electron microscopy.
Figure IV-2: Toluidine blue stained transverse section of wild type poplar and transgenic line 6 leaves observed by optic microscopy.
Figure IV-3: Morphological changes on leaves of the transgenic poplar lines 1 and 6 over-expressing EgMYB1.
Figure IV-4: Chemical analysis of soluble phenolic content in young developing leaves (LPI5-6) of wild type and transgenic line 6 over expressing EgMYB1.
Figure IV-5: Quantification of soluble sugars in young developing leaves of wild type (A, B, C) and transgenic lines 1, 6, 7 over expressing EgMYB1.
Figure V-1: Model of monolignol biosynthesis pathway in angiosperms. Figure V-2: Sequence analysis of the EgMYB2 protein.
Figure V-3: Genetic mapping of EgMYB2 on Eucalyptus RAPD maps. Figure V-4: Expression of EgMYB2 in Eucalyptus tissues.
Figure V-5: Binding of EgMYB2 to the cis-regulatory regions of the EgCAD2 and
EgCCR promoters.
Figure V-6: Effects of EgMYB2 on the transcriptional activities of the EgCAD2 and
EgCCR promoters in vivo.
Figure V-7: Phenotypic changes induced by EgMYB2 ectopic expression in tobacco. Figure V-8: Cytological effects of EgMYB2 over-expression in stem sections.
Figure V-9: Effect of EgMYB2 ectopic expression on the thickness of xylem fibres cell walls.
Figure V-10: Quantitative RT-PCR analysis of transcript accumulation in transgenic tobacco plants over-expressing EgMYB2.
Supplemental Figure III-1: Distribution of differentially expressed sequences from the microarray experiments, into functional annotation categories. A) Representations of wood differentially expressed gene categories. 1: Total number of differentially expressed genes (341). 2: 220 up-regulated genes. 3: 121 down-regulated genes. B) Representations of Bark differentially expressed gene categories. 1: Total number of differentially expressed genes (260). 2: 132 up-regulated genes. 3: 126 down-regulated genes.
Supplemental Figure III-2: Description of different EST libraries generated to produce the Arborea poplar 3,4K custom microarray
TABLES:
Table I-1: Three different plant MYB subgroups, number of isoforms in Arabidopsis and putative functions identified.
Table I-2: Example of R2R3 MYB transcription factor functions in plants.
Table I-3: Summary of transcription factors involved in secondary xylem formation.
Table III-1: JGI accession numbers (http://genome.jgi-psf.org/Poptr1_1/Poptr1_1.home.html) and RT-qPCR primer sequences of the different poplar phenylpropanoid and lignin biosynthesis gene isoforms possibly implicated in wood formation.
Table III-2: Comparison of the values obtained from the microarray and RT-qPCR experiments for the list of clones from the differentially expressed genes on the microarray experiment chosen for validation.
Table VI-1: Lignin analysis in EgMYB2+ plants.
Supplemental Table III-1: List of clones differentially expressed in wood and bark of the transgenic line identified in the microarray experiment.
Supplemental Table IV-1: List of affymetrix poplar genome microarray sequences differentially expressed in leaves of the transgenic poplar line over-expressing EgMYB1.
L
IST OF ABBREVIATIONS:
4CL: 4 hydroxycinnamoyl-CoA Ligase BR: Brassinosteroids
C3H: p-coumarate 3-hydroxylase C4H: Cinnamate 4 Hydroxylase
CAD: Cinnamyl Alcohol Dehydrogenase
CCoAOMT: Caffeoyl-CoA O-methyltransferase CCR: Cinnamoyl CoA Reductase
CHI Chalcone Isomerase CHS: Chalcone Synthase
COMT: Caffeic acid O-methyltransferase CFP: Cyan Fluorescent Protein
DBD: DNA Binding Domain
DFR: DihydroFlavonol-4-Reductase DNA: DesoxyriboNucleic Acid EMSA: Electro Mobility Shift Assay EST: Expressed Sequence Tag F5H: Ferulate 5-Hydroxylase GT: GlycosylTransferases
HCT: hydroxycinnamoyltransferase MBS: MYB Binding Site
NLS: Nuclear Localization Signal PAL: Phenylalanine Ammonia Lyase PCD: Programmed Cell Death PME: Pectin MethylEsterases RNA: RiboNucleic Acid
RT-qPCR : Reverse Transcription quantitative Polymerase Chain Reaction SAM: Shoot Apical Meristem
SCW: Secondary Cell Wall TF: Transcription Factor
CHAPTER I. I
NTRODUCTIONA.
INTRODUCTIONAbout 430 million years ago, during the Silurian period, plants acquired a vascular system enabling them to develop on land (Kenrick and Crane 1997). This event was one of the major steps in the development of what would become the most dominant form of plants on earth: the vascular plants (tracheophytes). Since earlier plants had evolved in water, several problems needed to be solved in order for them to become autonomous from their water dependency. Acquisition of a vascular system, which extends throughout the entire plant thus creating a continuum between all the parts of the plant body, would solve the problems of both water and food transport. The ability to synthesize lignin, which is incorporated into the walls of supporting and water conducting cells of the vascular system, would allow plants to resist to the water pressure accumulated in the stem and provide a rigid structure for the plant body to stand erect. As plants could stand erect and reach great heights, collection of light for photosynthesis was facilitated. Development of leaves would allow for the optimization of photosynthesis. Plants also developed specialized organs for water collection from the soil, allowing them to start evolving outside of water. Because of their success in conquering the terrestrial habitat, vascular plants were free to evolve and diversify. They developed to very large sizes and occupied specialized niches, hence reaching great biodiversity.
Of all the tracheophytes, trees represent the largest biomass in the world. This biomass is largely attributed to the trunk or wood in which plants store enormous amounts of carbon under the form of structural polymers (i.e. ligno-cellulosic polymers). Wood is one of the most impressive examples of the development of plant secondary vascular system. As perennial plants, trees increase the size of their vascular system over hundreds and even thousands of years, reaching sizes, such as in the case of the giant sequoia, of 115 meters of height and 7 meters in diameter (http://en.wikipedia.org/). Mankind has improved its condition through the use of wood and throughout the history of human survival, wood has played an indispensable role (Raven et al. 1999, Table 27-1). Up until today wood has remained an important source of energy or building material in many countries. Today, most of the energy consumed by industries comes from fossilized carbon for which supplies are diminishing, and more importantly their
use no longer satisfies our society’s criteria for a sustainable development, taking into consideration environmental issues such as global warming. Photosynthesis is the only process that enables the production of renewable carbon-based energy but it is also the basis for mankind’s food supplies. Carbon that is captured through photosynthesis is primarily stored in the ligno-cellulosic biomass. Exploiting this renewable carbon resource can be achieved through optimizations of its transformation in the entire plant, through biorefinery approaches and renewable carbon chemistry. In addition improved production must rely on more adequate usage of soils, ecologically adapted agronomy (integrating agroforestry), development of plants possessing better traits through green biotechnologies, etc.
Genetic studies on wood originally focused on identifying the structural genes responsible for the production of the chemical compounds found in its cell walls. Structures, quantities and linkages among the secondary cell wall carbohydrate polymers can be manipulated through genetic engineering, but there is still a lot to discover. Genetic manipulation of structural genes for example leading to the production of lignin, have brought about the idea that controlling key genes, such as transcription factors, would have a greater impact since it counters the adaptive plasticity of the plant genome through the control of not just one but several or even the majority of the structural genes in a biosynthesis pathway. Transcription factors (TFs) are proteins that regulate the transcriptional machinery to ensure the expression of genes and consequently the production of their corresponding protein, therefore they hold a crucial role in the development of an organism. In the case of wood formation, TFs belonging to the MYB family have been shown to impact upon the production of lignin. Therefore dissecting the role of MYB TFs in lignin biosynthesis was set as the central objective of the research presented in this thesis, as it expected to reveal important clues regarding the regulation of this biochemical pathway. Modification of the lignin content and structure, through genetic engineering is one of the strategies that could enable the production of plants with beneficial quantitative and qualitative traits. For example, the production of trees with modified lignin profiles has been widely considered as a potentially beneficial strategy in the pulp and paper industry for which the reduction of lignin content improves pulp yield and reduces the use of environmentally hazardous chemicals. The emerging biofuel industry is also a sector in which modification of wood cell wall structure holds great potential, because of the presence of condensed lignins
which reduce the accessibility of hydrolytic enzymes to the cellulose polymer in the ligno-cellulosic biomass.
B. L
ITERATURE REVIEWB.1. PLANT DEVELOPMENT: PRIMARY AND SECONDARY GROWTH
Unlike animals, plants have the ability to continue growth throughout their life. After the stages of embryogenesis, specialized tissues called meristems generate the different tissues, endlessly adding new cells to the existing body (Raven et al. 1999). In the primary stages of development and throughout the plant’s life, it is from the apical meristems that are derived all the tissues necessary for the formation and elongation of the primary organs (primary stems, leaves, roots). All plants go through this primary growth phase, but some of them also undergo secondary growth that increases the girth of the stems and roots, adding volume to the plant body. This type of growth is the result of the activity of secondary or lateral meristems called the vascular cambium and the cork cambium. The activity of the vascular cambium supports the growth the secondary vascular tissues composing part of the secondary plant body.
B.2. VASCULAR TISSUE FORMATION IN THE STEM
The establishment and growth of the plant vascular system is complex and continues throughout the lifespan of perennial woody plants. As one examines a plant or tree from the tip of the stem to the tip of the roots, he will observe all of the different stages and transitions necessary for setting up the primary and secondary vascular systems (Raven
et al. 1999).
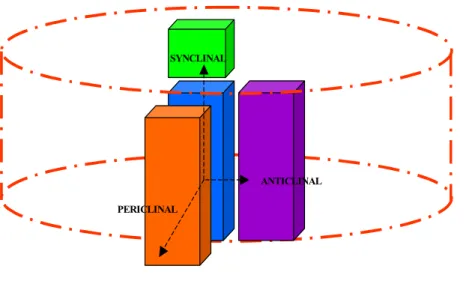
The apical meristems stand at the tip of the stem and are composed of cells called “initials”. A major characteristic of these cells is that upon division, they produce one sister cell that remains an initial of the meristem while the other is committed to differentiation, becoming a specialized cell (Raven et al. 1999; Ye 2002; Scarpella and Meijer 2004). In gymnosperms and woody angiosperms, the primary vascular tissues are organized in vascular bundles (Figure I-1a). Inside of these vascular bundles lie the primary vascular tissue initials, which constitute the procambium. Initials of the procambium are capable of dividing periclinally or synclinally (Figure I-2) and it is the
Figure I-1: Diagram showing the organization of stem vascular tissues during primary and secondary growth phases in woody species. a) Transverse section representing primary vascular tissue organization. b) Transverse section representing secondary vascular tissue organization.
(http://dragon.seowon.ac.kr/~bioedu/bio/ch27.htm)
orientation of the division that enables the formation of three different tissues in the plant body.
Periclinal divisions form the primary xylem and the primary phloem (both make up the primary vascular tissue) where as synclinal divisions perpetuate the procambium tissue along the axis of the plant and later forms part of the initials of the vascular cambium. As the plant body continues to develop, the primary growth undergoes a transition leading to the formation of a secondary meristem called the vascular cambium, which starts to develop in the older parts of the plant (Raven et al. 1999; Ye 2002). At this stage, the procambium is bordered by the primary xylem and primary phloem, together forming a vascular bundle. Vascular bundle organisation is usually circular and separated by interfascicular regions or pith rays (composed of parenchyma). The vascular cambium originates from the procambium and the pith rays; each of these two
will form, respectively, the fascicular cambium at the location of the vascular bundles, and the interfascicular cambium in between the vascular bundles (Figure I-1b). It is the junction of these two that make up the entire circular vascular cambium of mature tree trunks. One of the major characteristics of the secondary vascular system is to support radial growth, increasing the diameter of the plant body. This increase is mainly due to periclinal and anticlinal divisions (Figure I-2) of the vascular cambium. In the case of periclinal divisions, secondary xylem (towards the inner side of the stem) and secondary phloem (towards the outer side of the stem) mother cells are produced, whereas the anticlinal divisions will generate more of the vascular cambium initials, increasing the circumference of the stem.
SYNCLINAL
ANTICLINAL PERICLINAL
Figure I-2: Types of divisions arising from the wood meristem, i.e. the cambium.
Red dashed line represents the axis of the stem. Blue rectangle represents the cambial cell initial.
B.3. TISSUES AND CELLS OF THE SECONDARY VASCULAR SYSTEM
One of the hallmarks of the vascular cambium is that it is a single cell layer (reviewed in Samuels et al. 2006) composed of two different types of initial cells: the fusiform initials and the ray initials (Raven et al. 1999). The main difference between these two initials is in their orientation relative to the main axis of the plant stem or the trunk of the tree. Indeed fusiform initials are oriented vertically whereas ray initials are oriented horizontally; they are thus the axial and radial systems of the vascular system, respectively. These two types of initials also have slight structural differences. The fusiform initials are quite elongated cells that appear flattened in transverse sections whereas the ray initials are only slightly elongated and squarrish (Raven et al. 1999; Mellerowicz et al. 2001 and references therein).
A second particularity of vascular cambium differentiation is that several cell types may be produced from a single initial cell. For example, fusiform initials mainly give birth to xylem and phloem conducting cells as well as fibers whereas the ray initials mainly produce ray parenchyma cells (Raven et al. 1999). As a result, the secondary xylem, and similarly the secondary phloem, is composed of three major cell types: conducting cells, fibers and parenchyma cells (Figure I-3; Aloni 1987; Ye 2002).
¾ CONDUCTING CELLS:
Conducting cells are responsible for the transport of diverse substances throughout the plant body. Generally stacked one on top of the other they will form long tube-like structures by which water as well as dissolved mineral nutrients are taken up from the soil and transported from the roots to the rest of the plant organs through the xylem. Furthermore, organic compounds are transported from the photosynthetic organs to the rest of the plant through the phloem. In the case of angiosperm xylem, there are two different types of functional conducting cells that ensure the transport of water and soil derived nutrients from the roots to the rest of the plant organs (Figure I-3E, F, G). Tracheids, the more primitive form of conducting cells present in gymnosperms, are long, lignified, non-perforated cells with bordered pits. Vessel elements are large, thick, lignified, hollow cells, much more efficient in their transporting function. Both tracheids and vessel elements that have fully matured are dead hollow cells. The conducting cells develop in such a way that they end up stacked one on top of the other generating the vessels of the xylem, which can reach lengths up to 18 m in the case of Fraxinus (Aloni 1987 and references therein).
Conducting cells of the phloem are called sieve elements (Figure I-3A, B, C). At maturity, they lack nuclei and have enlarged end walls covered with pores. These sieve elements are also accompanied by companion cells which are thought to be used for nutrient storage.
¾ FIBERS:
Fibers are present in both xylem and phloem. The major role of fibers is to provide the stem with mechanical strength, helping the plant to stand erect and support the weight of its aerial parts. In consequence, fiber cell structure is adapted to fulfill this role. Indeed they are long and narrow cells possesssing thick secondary walls that are usually highly lignified (Figure I-3D). Due to their impressive capacity for tip growth
(both apical and basal), they are capable of reaching lengths up to 250mm in the case of
Boehmeria nivea (Han 1998); however most trees produce fibers which range from one
to a few millimeters.
Figure I-3: Cells of angiosperm wood vascular tissues. A, B, C: Sieve tubes and companion cells of the phloem. D: Fiber E, F: Xylem tracheid. G: Xylem vessel element. Adapted from http://www.ucmp.berkeley.edu/
¾ PARENCHYMA:
Finally, both xylem and phloem have parenchyma cells. A major role of parenchyma cells is the storage of reserve compounds such as proteins, starch and lipids (Höll 2000). There exist two types of parenchyma cells: axial and ray, depending on whether they are formed from fusiform or ray cambium initials, respectively. The ray parenchyma cells have already been considered as the major radial symplastic transport pathway within trees (Sauter 2000). Chaffey N, (2002b) hypothesized a possible role of parenchyma cells in the formation of a long distance 3-dimensional communication pathway between the phloem, through the cambium into and around the xylem. This super-symplastic continuum would allow transport throughout the whole plant body.
B.4. THE FORMATION OF WOOD: XYLOGENESIS
Through the life of a plant, the vascular cambium generates secondary xylem, which accumulates in trees and constitutes the major part of the trunk, usually referred to as wood. The biological process of secondary xylem formation is termed xylogenesis. Xylogenesis represents the process by which the vascular cambium gives rise to the different cells of the xylem through a series of coordinated events. These events or steps have been reviewed in several papers (Fukuda 1996; Fukuda 1997; Chaffey 1999; Fukuda 2004; Mellerowicz et al. 2001; Ye 2002). In the following section the plant model systems used for studying wood formation as well as the cellular and molecular aspects of xylogenesis, are reviewed.
B.4.1. MODEL SYSTEMS FOR THE STUDY OF WOOD FORMATION
B.4.1.1. Herbaceous plant systems Zinnia: cell culture system.
Zinnia elegans mesophyll cells freshly extracted from developing leaves are
able, in an appropriate hormone inducing medium containing both auxins and cytokinins, to dedifferentiate into procambium initials and undergo tracheary element differentiation (Fukuda 1997). This controlled in vitro transdifferentiation system has the great advantage of enabling single cell visualization of tracheary element differentiation, which is very difficult in planta where tracheary elements are usually embedded into the complex heterogeneous xylem tissue. In addition, fine-tuning of the hormonal balance allowed detailed investigations of the signals leading to xylem differentiation (Pesquet et al. 2005) and the kinetics of the in vitro Zinnia transdifferentiation system has enabled the dissection of the cascade of events taking place one after the other during the course of tracheary element differentiation.
Arabidopsis: model herbaceous species.
Owing to many practical aspects such as its small physical size and high regeneration time as well as the availability of many molecular tools (genome sequence, EST collections, genetically modified or mutagenized collections…), Arabidopsis, has been largely accepted as a model system for studying plant biology. Arabidopsis has been used to study various aspects of wood formation since secondary xylem differentiation can be induced in the hypocotyl region by different treatments (Chaffey
development of Arabidopsis imply that certain aspects of wood formation can not be studied.
B.4.1.2. Woody plant systems: Populus: model tree species.
In the last decade, populus has been used as a model tree species to study woody perennial plant biology. With the recent completion of the first draft genome sequence of populus trichocarpa and the development of many genomic and molecular resources, populus has placed itself as the model tree species (reviewed by Jansson and Douglas 2007). Although its genetic transformation is time and space consuming, poplar offers the possibility of studying many aspects of tree development that can not be encompassed with Arabidopsis. Populus and Arabidopsis are both part of the rosid
clade (Angiosperm phylogeny web site: http://www.mobot.org/MOBOT/Research/APweb/welcome.html), therefore they are
phylogenetically closely related. This suggests that some of the conserved plant developmental processes will probably have common molecular actors between these two species where as more specialized developmental processes such as bud dormancy or secondary xylem formation, very limited and restricted to the hypocotyls region in
Arabidopsis, will probably represent a certain proportion unpreviously characterized
tree specific genes.
Eucalyptus: a future model tree
Owing to its rapid growth rate and high growth adaptability in a wide variety of climates and soils, Eucalyptus has become a key genus on a worldwide scale. Represented by over 700 speciess, it is the most widely planted forestry genus, with over 18 million hectares in 90 countries, and is mainly exploited for the good solid timber wood quality as well as the pulp and paper production (Doughty 2000).
Eucalyptus has also been voted as one of the U.S. Department Of Energy (DOE)
candidate biomass energy crop. In the past years, a vast amount of knowledge and tools have been gained from genomic research, notably in regards to wood formation and stress tolerance (Poke et al. 2005). The proposal for the sequencing of the Eucalyptus genome has been recently approved (IUFRO Tree Biotechnology, 2007) and the first draft sequence is expected to be available in 2 to 3 years to come (http://www.ieugc.up.ac.za). Therefore one can foresee that Eucalyptus, the second
sequenced tree genome, will also become a model species, providing extraordinary opportunities for tree genomic analysis.
B.4.2. CELLULAR ASPECTS OF XYLOGENESIS
For a long time the process of wood formation has been described according to physical observations of the cells undergoing differentiation. This process has been commonly separated in several steps including initiation and division of the cambial cells, xylem mother cell elongation, secondary cell wall (SCW) formation and finally programmed cell death (Fukuda 1997).
B.4.2.1. Dividing cambial initials
As a first step towards the formation of the xylem tissue, cambium initials undergo a series of periclinal divisions giving rise to xylem mother cells. Transmission electron microscopy observations of the high-pressure frozen cambial zone have provided evidence of the evolution of the cellular machinery during the division of the initials, prior to differentiation of the mother cells (reviewed in Samuels et al. 2006). Mitosis in the fusiform initials takes place along the longitudinal axis of the cell and the microtubule spindles drag the separated chromosomes towards the periclinal walls (Rensing et al. 2002). After separation of the chromosomes, the phragmoplast machinery, guided by microtubules, will stretch from the center of the cell moving along the length of the central axis of the initial. Microtubules also drive golgi-derived vesicles, accumulated in the mid-zone of the cell that will be blended into the phragmosome, generating the newly formed cell plate and the complete xylem mother cell.
B.4.2.2. Cell elongation
After the division from the cambial initial, xylem mother cells will elongate longitudinally and radially to reach their final size. The expansion of the xylem mother cells is one of the most impressive in plant cells as it can reach increases up to 30,000-folds in the case of the vessel elements. The turgor pressure inside the cell is one of the driving forces that pushes the walls outwards. Te tensile stress applied will results in stretching of the wall polymers also providing the energy required for extension of the wall (Cosgrove 2005). At one point the elongation of the cell wall must be combined with addition of building materials to the wall to prevent mechanical rupture of the
Anatomically, three different layers, the S1, S2 and S3 layers, are deposited inward of the primary cell wall. These different layers vary in thickness and in the orientation of their cellulose microfibrils (Figure I-4; Plomion et al. 2001). The composition of the SCW varies slightly from layer to layer, but typically it comprises 40-50% of cellulose, 25% of hemicelluloses, 25-35% of lignin, pectins and cell wall proteins (Plomion et al. 2001).
Figure I-4: Cell layers after secondary cell wall formation. S1 layer: 0.1 to 0.35µm thick; S2 layer: 1 to 10 µm thick; S3 layer:
0.5 to 1.1µm thick.
thinning cell wall. During the cell elongation phase, rearrangement of the cortical microtubules is observed, passing from a totally random to a helical organization (Chaffey et al. 2002a), and coincides with the site of SCW deposition.
B.4.2.3. Secondary cell wall biosynthesis
For xylem cell types, differentiation is often finalized by the formation of a SCW on top of the primary cell wall towards the lumen. As the developing cell reaches its final size, the primary cell wall will be cross-linked into its ultimate shape and deposition the SCW occurs in specialized cells. Deposition of this SCW in vascular tissues will serve the cells by providing greater mechanical strength (fibers, and conducting elements) and water resistance (conducting elements).
Components of SCW: B.4.2.3.a. Cellulose:
Cellulose is the most abundant polysaccharide in primary cell walls but is found in higher concentrations in the SCW (Mellerowicz et al. 2001). It is composed of several dozens of linear 1-4-β-D-glucan chains, forming microfibrils throught hydrogen bonds. The current model for cellulose biosynthesis requires at least three steps (Joshi et
al. 2004). First the plasma membrane associated sucrose synthase complex (SUSY)
Cellulose synthesizing complexes, composed of co-ordinately synthesized CESA proteins organized in hexagonal rosettes embedded in the plasma membrane, polymerize glucose monomers into glucan chains. It has been proposed that each subunit of the rosette is composed of six 1-4-β-D-glucan synthase, which joined
together, produce a 36 parallel glucan chain structure called the microfibril (Reiter 2002). Finally the membrane associated KORRIGAN (KOR) protein acts as an editor, monitoring the correct structure of the glucan chains, and repairing them if necessary (Molhoj et al. 2002).
B.4.2.3.b. Hemicellulose:
Hemicelluloses, also called cross-linking glycans, are polysaccharides that coat microfibrils and are thought to span the distance between cellulose microfibrils cross-linking them together. The sugar monomers that compose hemicellulose include glucose, xylose, mannose, galactose, rhamnose, and arabinose, which are attached through linear β-(1-4) linkages. The most abundant hemicellulose chains found in dicot SCWs are xylans and glucomannans (Mellerowicz et al. 2001).
B.4.2.3.c. Lignin:
Lignin, a hydrophobic heteropolyphenolic polymer embedded inside the cellulose and hemicellulose matrix, represents the most distinguishing feature of SCWs. Mechanical and pathogen resistance as well as hydrophobicity characteristics of wood cells have been attributed to its presence in the cell walls. Lignin is composed mainly of three hydroxycinnamyl alcohols (monolignols): the p-coumaryl alcohol, coniferyl alcohol and sinapyl alcohol, which become the p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) units of the polymer. The relative abundance of these three subunits varies greatly amongst different plant speciess and cell types. For example, lignin in angiosperm trees is mainly composed of the S and G units where as gymnosperms lignins are typically composed of G units (Plomion et al. 2001). Furthermore it has been shown that lignin composition of fibers and vessels is different implying that a fine regulation of lignification is necessary during the development of these adjacent cells (Zhong et al., 2000).
B.4.2.4. Deposition of SCW:
SCW deposition is highly organized, especially in the tracheary vessel elements. It does not occur randomly inside the cells but rather follows a certain pattern and also delimits the future perforation plates and pits of the vessel elements. Cortical
microtubules, necessary for the correct deposition of the SCWs (Oda and Hasezawa 2006 and references therein), appear to be responsible for determining the pattern of SCW deposition. It has been hypothesized that microtubule bundles, formed between the primary and secondary walls, will either guide the cellulose synthase complexes or bring vesicles charged with building blocks to the SCW (Oda and Hasezawa 2006). In a similar manner it is thought that sucrose synthase complexes are brought in close distance of the cellulose synthase rosettes by cortical microtubules providing UDP-glucose to the cellulose synthase machinery (Haigler et al. 2001). Lignification occurs during the latter stages of SCW deposition. Once synthesized, monolignols join together to form the lignin polymer through mechanisms that are not entirely understood but referred to as oxidative-polymerization (Boerjan et al. 2003). Whether the polymerization of monolignols occurs at the endoplasmic reticulum or directly at the cell wall is still not clearly understood since enzymes responsible for the synthesis of monolignols are found either associated to the endoplasmic reticulum or in the cytosol (Samuels et al. 2006). In vessel elements lignification (i.e. synthesis and deposition of lignin) is first observed in cell corners, once the SCW S1 layer had already been deposited (Murakami et al. 1999). This process intensifies during the S2 layer formation and deposition is highest in the S3 layer. This patterned deposition is necessary for vessels elements, which once joined together, create a long tube. Fibers, on the other hand, start their lignification latter on in their differentiation process.
B.4.2.5. Programmed cell death
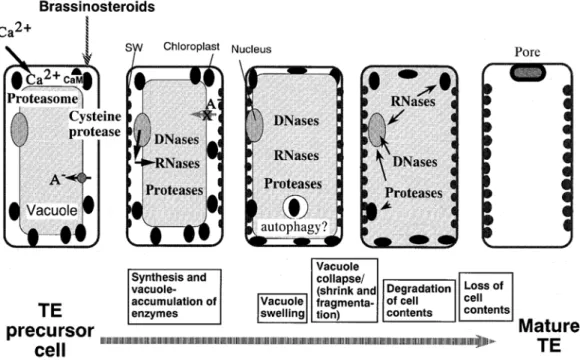
As a final step in the differentiation of the secondary xylem, certain cells of the xylem (vessels, tracheids or fibers) undergo programmed cell death (PCD) generating dead, hollow, tube-like cells. This phenomenon has been best studied in the in vitro tracheary element differentiation of Zinnia elegans (Fukuda 1996; Fukuda 1997; Fukuda 2000, Figure I-5). The principal cellular events which occur in PCD are:
- The increase of vacuole size to the point of rupture of the tonoplast, and consequent collapse of the vacuole.
- As the vacuole collapses, the rapid and progressive degradation proceeds from single to double membrane cellular organelles, and is followed by degradation of parts of the unlignified primary cell wall.
- Rupture of the vacuole induces the release of hydrolytic enzymes, such as DNase, RNases and proteases that are thought to digest the organelles and the cytoplasm content (reviewed in Turner et al. 2007).
Figure I-5: Figure of PCD during TE differentiation. See details in text. CaM: Calmodulin, SW: Secondary Wall. (Taken from Fukuda H. 2000)
Calcium has been shown to be implicated in programmed cell death of the tracheary elements, but since other molecules, such as nitric oxide and cGMP have also been implicated (Lam 2004). PCD in the xylem is different from other types of plant cell death, such as the hypersensitive response or senescence, mainly because the chronology of the degradation events varies (Fukuda 1996).
B.4.2.6. Hormonal signals controlling vascular tissue differentiation:
Hormonal signals have been found to have a great importance in the control and regulation of the differentiation of cambial initials (reviewed by Fukuda 2004).
Auxins are present as a basipetal polar gradient in plants (Sachs 2000). Furthermore it is also present as a gradient all along the transverse axis of the stem with increasing concentrations towards the cambium cells (Uggla et al. 1998). The presence of such
auxin gradients renders possible the establishment of continuous vascular strands along the apical-basal axis of the plant, which is a prerequisite for the proper formation of the vascular system. Molecular evidence obtained by the identification of mutants related to auxin transport or perception, further underlies the importance of auxin in controlling vascular tissue differentiation (Fukuda 2004).
Cytokinins are crucial for the formation and maintenance of procambial cells (Fukuda 2004). They are actively synthesized in vascular bundles of Arabidopsis roots (Miyawaki et al. 2004) and are also a prerequisite, along with auxins, for the differentiation of tracheary element in the in vitro Zinnia elegans system (Fukuda 1996). Histidine kinases (Mahonen et al. 2000), preferentially localized in the procambial cells are thought to be the receptors of cytokinins. Even though the downstream components of this transduction pathway are not clearly known, certain histidine-containing phosphotransfer and response regulators could also be implicated (Fukuda 2004).
Brassinosteroids (BR) are now regarded as hormones that promote the differentiation of procambial cells into xylem mother cells (Fukuda 2004). Abnormal vascular tissue patterning, characterized by fewer xylem cells, was observed in mutants deficient in BR biosynthesis (Clouse and Sasse 1998) and application of brassinolide (an active form of BR) can rescue the effects of the mutation which causes the inhibition of xylem differentiation (Nagata et al. 2001). Clear evidence was obtained with the “Zinnia elegans in vitro tracheary element differentiation system” where drastic increases of BR biosynthesis were observed in the transition phase from procambium cells to xylem precursor cells (Yamamoto et al. 2001). Perception of BR seems to be a vascular cell-specific mechanism involving specific isoforms of BR receptors (Cano-Delgado et al. 2004; Fukuda 2004). BRs promote xylem differentiation by induction of HD-ZIP III class gene expression (Ohashi-Ito et al. 2005).
Finally, other hormones can be linked to xylem differentiation but their role is not clearly understood. Gibberellins, such as gibberellic acid (GA) are know to promote shoot elongation upon application to plants and GA-over-expressing plants have increased number and length of xylem fibers (Eriksson et al. 2000). Some GA-biosynthesis enzymes are preferentially expressed in wood forming tissues and are hypothesized to play a role during the early events of xylem differentiation (Israelsson
et al. 2005). Recently gibberellins (GA) have been linked to lignification in tracheary
due to the presence of several transcripts of the ethylene perception pathway in xylem fibers undergoing cell death (Moreau et al. 2005).
B.4.3. MOLECULAR ASPECTS OF XYLOGENESIS
Up until recently, molecular aspects of wood formation were investigated by approaches such as multi-level characterization of transgenic or mutant plants (Turner et
al. 2001; Anterola and Lewis 2002). With the ongoing technological advances, and the
increasing availability of plant genome resources (Sterky et al. 2004) more global approaches have also been undertaken through the analysis of transcriptome variations associated with SCW formation, either during the course of plant development or in transgenic and mutant plants. Comparative transcriptomic has been very useful in describing sets of genes with precise spatial or temporal regulated expression during development of the secondary xylem. Hertzberg et al., (2001) did pioneering and innovative work in this area. Using the cryodissection technique, they sampled precise zones of the cambium and secondary xylem, representing the different phases of xylogenesis chronology in poplar, and monitored the transcript profiles of approximately 3,000 ESTs, previously isolated from wood-forming tissues (Sterky et al. 1998). Since then, similar approaches have been undertaken covering other aspects of xylem development such as the transdifferentiation of Zinnia mesophyll cells (Demura
et al. 2002), Arabidopsis secondary growth (Oh et al. 2003; Ko and Han 2004; Brown et al. 2005), the 220 µm poplar cambial zone (Schrader et al. 2004), Arabidopsis
primary stems (Ehlting et al. 2005), xylem and phloem of Arabidopsis root-hypocotyl (Zhao et al. 2005), poplar tension wood (Andersson-Gunneras et al. 2006). The availability of the genome sequences of model plants like Arabidopsis and more recently poplar (AGI 2000; Tuskan et al. 2006; Janson and Douglas 2007) provides powerful tools and comprehensive datasets for the identification of candidate genes, as well as their genomic structure and their phylogenetic relationships to other sequences in the genome. Crossing these data with expression profiling analysis allowed the characterization of different classes of genes possibly playing a role in xylogenesis, such as the lignification toolboxes of both Arabidopsis and poplar (Raes et al. 2003; Hamberger et al. in press), the glycosyl transferase families involved in SCW biogenesis (Aspeborg et al. 2005) or the cellulose biosynthesis related families of poplar (Joshi et al. 2004). A further step into the characterization of molecular actors involved in xylogenesis is the analysis of the transcriptomes of lignin mutants or transgenics
(Rohde et al. 2004; Sibout et al. 2005; Dauwe et al. 2007; Leple et al. 2007). These studies enable to observe the consequences of the genetic modifications and their effect on the transcriptomes of plants. Enormous amounts of information documenting the different stages of the xylogenesis process have been generated and are summarized in the following section.
B.4.3.1. First step of xylogenesis: division
Analysis of transcript accumulation profiles across a 220µm window of the cambial zone defined sets of genes specifically expressed in different sections of the cambial zone (Hertzberg et al. 2001; Schrader et al. 2004). These studies indicated that the cambial zone could be subdivided into one region conserving the meristematic activity of the cambial initials and two zones committed to xylem mother cell differentiation. The molecular machinery responsible for maintenance of meristematic initial identity is suggested to have conserved some of the molecular actors implicated in primary meristems (Schrader et al. 2004; Baucher et al. 2007). For instance, in the shoot apical meristem regulation pathway maintenance of meristematic competence is due to the expression of KNOX genes, enabled through the down-regulation of AS1 expression by the STM gene, and is partly conserved in the cambium. Nevertheless it also seems that both processes are not entirely identical, as some key genes of primary meristems are not expressed in cambial initials (Schrader et al. 2004; Baucher et al. 2007). For example transcripts of the Arabidopsis WUS and CLV3 orthologous genes, present in apical meristems, are not expressed in poplar cambium. As daughter cells of the cambial initials move further away towards the xylem they will need to acquire xylem mother cell competence. In Arabidopsis shoot apical meristem this is attributed to the presence of KANADI and HDZipIII genes (Baucher et al. 2007), whose roles are to promote leaf vascular patterning and therefore cell fate. Some of these genes have also been found expressed in the poplar cambium-xylem transition zone suggesting that they help in promoting xylem mother cell differentiation during xylogenesis. Finally, genes of the cell division machinery have also been found preferentially expressed in secondary vascular cambium (Hertzberg et al. 2001; Ko and Han 2004; Schrader et al. 2004).
B.4.3.2. Molecular actors of cellular expansion
As the cells are further pushed away from the cambium following successive divisions of the initials, in xylem mother cells the pool of genes from the transcriptome is modified. Genes, related to cellular elongation, have been identified in secondary xylem mother cells, as they are located farther away from the cambium initials (Hertzberg et al. 2001; Oh et al. 2003; Schrader et al. 2004). These candidate genes are involved in the loosening and expansion of the xylem mother primary cell walls enabling them to reach their final size (Mellerowicz et al. 2001).
B.4.3.2.a. Expansin:
Several lines of evidence first supported the involvement of expansins in cell elongation (Cosgrove 2005). Disruption of acid growth by heating can be almost fully restored by addition of expansins alone and furthermore addition of exogenous expansins to cells stimulates their growth. Although expansins have been largely accepted as actors of cell expansion, their function is still unclear. One hypothesis could be that they disrupt the non-covalent hydrogen bounds between polysaccharide complexes linked to the microfibrils (McQueen-Mason and Cosgrove 1995). In the case of wood formation several expansins have been identified. Two zinnia elegans expansins have been localized in planta predominantly in a single flank of cells adjacent to protoxylem and metaxylem vessels and in cells roughly at the radial position of the fascicular and interfascicular cambium (Im et al. 2000). Recently a poplar expansin,
PttEXP1 was localized preferentially at the tip of xylem fiber, by in situ RT-PCR, and
suggested to be involved in intrusive tip growth (Gray-Mitsumune et al. 2004). Ectopic over-expression of PttEXP1 was recently shown to stimulate stem internode elongation and leaf expansion, producing larger cells in the leaf epidermis, indicating that the PttEXP1 protein is capable of increasing the growth of these organs through enhancement of cell wall expansion in planta (Gray-Mitsumune et al. 2007). Comparative analysis with pine and Arabidopsis suggested that the subfamily of A α– expansins is involved in cell expansion during wood formation.
B.4.3.2.b. Xyloglucan EndoTransglucosylases/hydrolases (XTH):
A side from the expansins, Xyloglucan EndoTransglucosylases/Hydrolases (XTH) belongs to another important class of proteins involved in the process of cell expansion. Whether XTH are involved in the wall loosening is still under debate, nevertheless it seems that they more likely play a role in cell enlargement rather than in reduction of the tensile stress. Xyloglucan EndoTransglucosylases (XET), which are
part of the great family of XTH, catalyze the molecular grafting between xyloglucan molecules by endolytical cleavage of the xyloglucan backbone. This is followed by the formation of a covalent enzyme-substrate intermediate and a deglycosylation step involving a xyloglucan acceptor, which releases the enzyme and leads to the formation of a new β-1,4-glycosidic bond (Rose et al. 2002). XET are involved in many processes requiring modifications of the wall architecture, such as wall degradations needed for fruit ripening, organ abscission, cellular growth and differentiation (reviewed by Carpita and McCann 2000). They have also been reported to be involved in the expansion of the primary cell walls as well as formation of SCWs during wood vascular tissue formation. Indeed several XET have been identified in wood forming tissues of trees. For example,
XET were identified in pine differentiating xylem EST libraries (Allona et al. 1998),
hybrid aspen XET were also found preferentially expressed in cambium and xylem mother cells (Schrader et al. 2004) as well as in the G-layer of tension wood (Nishikubo
et al. 2007). The Arabidopsis xth27 mutant displays a modified xylem development
phenotype, with fewer tertiary veins present (Matsui et al. 2005).
A role in restructuring primary walls at the time of secondary wall layer deposition, probably creating and reinforcing the connections between the primary and secondary wall layers, has been proposed for XET (Bourquin et al. 2002).
B.4.3.2.c. Pectin methylesterases:
Present in the SCW, though in much lower concentrations that in the primary walls (Mellerowicz et al. 2001), as a mixture of heterogeneous, branched, and highly hydrated polysaccharides rich in D-galacturonic acid, pectins appear to play an important role in xylogenesis. Degradation of pectins seems to be important in xylogenesis, since pectins limit wall porosity, the wall-modifying enzymes cited above have more difficulty accessing the cell wall matrix. In addition, when pectins incorporate Ca2+ they become gelatinous rendering the wall unstretchable (McQueen-Mason 1997). It has been suggested that the status of pectin methylation, regulated by Pectin Methylesterases (PME), is an important factor in controlling wall plasticity (Mellerowicz et al. 2001). Nevertheless only molecular proof supports the involvement on PME in xylogenesis. In Zinnia elegans, a Pectate Lyase (ZePEL) gene is associated with vascular tissue formation and its expression is highest in the early stages of induction prior to SCW formation (Domingo et al. 1998). Many ESTs corresponding to this pectinase have also been identified in poplar cambium and xylem EST libraries (Sterky et al. 1998). ESTs corresponding to PMEs were detected in cambium libraries
and recently, highly expressed pectin biosynthesis-related Glycosyl Transferases family 47 genes have been found in developing wood and might function in ray parenchyma cells (Aspeborg et al. 2005). More recently a PME has been found highly induced during formation of tension wood cells in poplar (Andersson-Gunneras et al. 2006).
B.4.3.2.d. Endo-(1-4)- β-D-glucanases:
These proteins catalyze the endohydrolysis of (1-4)-β-D-glucan of xyloglucan chains and are involved in the degradation of non-crystalline cellulose (Ohmiya et al. 2000). As in the case of the PME only molecular evidence support the role of endo-(1-4)-β-D-glucanases in cell elongation during wood formation. Characterization of two poplar genes encoding endoglucanases (PopCEL1 and PopCEL2) provided evidence for a role in relaxation of the cell wall. Over-expression of PopCEL1 in Arabidopsis stimulates growth of internodes and fibers and increases plastic extensibility of cell walls (Shani et al. 2006), whereas antisense suppression reduces leaf growth (Tsabary et
al. 2003).
B.4.3.2.e. Others genes involved in cell elongation:
Aside from the gene families listed above, molecular markers of cell elongation have been identified in the Zinnia elegans in vitro system. ZeTED2,3,4 genes (Tracheary Element Differentiation) were shown expressed during the cell elongation step of tracheary element differentiation (Demura and Fukuda 1994), 12 to 24 hours prior to SCW thickening. But their function remains unknown. Another Arabidopsis gene important for cell expansion corresponds to the det3 mutant (Schumacher et al. 1999) which encodes a V-ATPase. The role of DET3 may be to regulate cell shape, by controlling cell osmosis through solute uptake into the vacuole. Other mutants such as the eli1, lit, rsw1 have created a link between cell expansion and SCW formation (Hauser et al. 1995; Arioli et al. 1998; Cano-Delgado et al. 2000). For example, characterization of the eli1 mutant, encoding a cellulose synthase subunit, showed that it may be responsible for sensing the state of cell expansion, probably monitoring cell wall integrity, and controlling initiation of SCW deposition (Cano-Delgado et al. 2003).
B.4.3.3. Genes involved in the biosynthesis of the major components of Secondary Cell Wall biosynthesis genes
With the help of single and genome wide gene analysis, molecular mechanisms behind the biosynthesis of the SCW of xylem cells are being unravelled for all three of