DEVELOPMENT OF A NEW DAIRY
INGREDIENT FOR THE UTILIZATION OF
BUTTERMILK CONSTITUENTS
Thèse
Maxime Saffon
Doctorat en Sciences et Technologie des Aliments
Philosophiæ Doctor (Ph.D)
Québec, Canada
© Maxime Saffon, 2013
RESUME
L’utilisation du babeurre pour la formulation alimentaire est limitée à cause de la capacité de rétention d’eau importante de ses phospholipides. L’objectif de ce projet était de développer une nouvelle approche de valorisation des constituants du babeurre. Ce sous-produit de l’industrie laitière est riche en composants d’intérêts qui ont des propriétés nutritionnelles, santés, et fonctionnelles prometteuses comme les constituants de la membrane de globule de matière grasse (MFGM) et les phospholipides. Les deux principaux procédés de production d’agrégats protéiques laitiers ont été combinés donnant un traitement thermique intensif à pH acide des protéines du lactosérum en présence des constituants du babeurre. Les résultats ont d’abord montré qu’il était possible de substituer différentes proportions de protéines du lactosérum par des protéines du babeurre et que la présence des constituants du babeurre entrainait la formation d’agrégats protéiques variés avec une capacité de rétention d’eau plus faible. Les résultats ont révélé que des agrégats protéiques étaient préformés lors de la préparation des babeurres incluant les protéines du lactosérum, les caséines et les protéines de la MFGM. Les phospholipides sont intégrés aux agrégats par l’intermédiaire de la MFGM à des températures faibles (65°C) alors qu’ils semblent s’associer directement avec les protéines à des températures plus élevées ( 80°C). Par la suite, les agrégats du babeurre agissent à titre de noyau d’agrégation pour les protéines issues du lactosérum. Le type d’interactions formées entre les protéines a un impact significatif sur les propriétés physiques et fonctionnelles des agrégats. En dernier lieu, il a été possible d’utiliser ces agrégats variés pour la production de yaourt ferme. Les agrégats lactosérum:babeurre ont agi à titre d’agent passif plutôt que d’agent actif mais des interactions entre les agrégats et les protéines du lait écrémé ont été observées. Ces associations seraient initiées par les groupements thiols libres des agrégats présents avant le chauffage. Cependant, la mise en solution de la poudre d’agrégats doit être strictement contrôlée. Ce projet propose une nouvelle approche pour l’utilisation du babeurre ainsi qu’une meilleure compréhension du comportement à la chaleur de ses constituants.
ABSTRACT
The use of buttermilk in food formulation is limited due to the extensive water-holding capacity of its phospholipids. The goal of this project was to develop a new approach for the valorization of buttermilk’s constituents. This by-product is rich in valuable components with promising nutritional, healthy, and functional properties such as the milk fat globule membrane (MFGM) constituents, the phospholipids. The two main processes of production of dairy aggregates have been combined resulting of the intensive heat-denaturation of whey proteins at low pH (4.6) in presence of proteins from buttermilk. First, results showed that it was possible to substitute whey proteins by different levels of buttermilk proteins in the process and that the presence of buttermilk constituents led to the formation of mixed aggregates with new functional properties such as a low water-holding capacity. Results revealed that aggregates are pre-formed during the preparation of the buttermilk concentrates involving whey proteins, casein, and MFGM proteins. Phospholipids are integrated to the aggregates through the MFGM at low temperature (65°C), but seem to directly interact with the proteins at higher temperatures ( 80°C). These pre-formed aggregates from buttermilk can act as aggregation nucleus for the proteins from whey. The types of interactions that occur between the proteins significantly affected the properties of the aggregates such as their water-holding capacity, their size, and the solubility of the powder. Finally, it was possible to use the mixed aggregates in the production of set-type yogurt. Whey:buttermilk aggregates were acting more like a passive than a reactive filler, but some possible interactions with the proteins from the skim milk were observed due to the high concentration of thiol groups of the aggregates before heating. However, the dispersibility of the powder must be strictly controlled. Overall, this project proposed a new approach for the use of buttermilk and allowed a better understanding of the thermal behavior of its constituents.
TABLE OF CONTENTS
RESUME ... III ABSTRACT ... V TABLE OF CONTENTS ... VII TABLE LIST ... XI FIGURE LIST ... XIII EQUATION LIST ...XVII ABREVIATIONS ... XIX ACKNOWLEDGMENTS ... XXI FOREWORD ... XXV
CHAPTER 1 INTRODUCTION... 1
CHAPTER 2 LITERATURE REVIEW ... 5
2.1 BUTTERMILK AND WHEY ... 5
2.1.1 Buttermilk ... 5
2.1.2 Whey ... 7
2.2 CONSTITUENTS FROM BUTTERMILK AND WHEY ... 8
2.2.1 Caseins ... 8
2.2.2 Whey proteins ... 10
2.2.3 Milk fat globule membrane proteins ... 13
2.2.4 Phospholipids from buttermilk ... 17
2.3 PRINCIPLES OF PROTEIN AGGREGATION ... 19
2.3.1 Definitions of native state, denaturation, and reversibility ... 19
2.3.2 Nucleation ... 20
2.3.3 Description of the different pathways ... 20
2.3.4 Bonds, interactions, and exchanges in milk protein aggregation ... 22
2.3.5 Effects of experimental conditions ... 24
2.4 HEAT-INDUCED AGGREGATION OF MILK PROTEINS ... 26
2.4.1 Aggregation mechanisms of
-lactoglobulin and
-lactalbumin ... 262.4.2 Heat-induced interactions with minor whey proteins ... 31
2.4.3 Heat-induced interactions involving casein micelles ... 33
2.4.4 Heat-induced aggregation mechanisms of MFGM constituents ... 36
2.5 APPLICATIONS FOR DAIRY PROTEIN AGGREGATES IN FOOD FORMULATION ... 42
2.5.1 Role of aggregates as fat mimetic ... 42
2.5.2 Role of aggregates as water holder ... 45
CHAPTER 3 HYPOTHESIS, GOAL, AND OBJECTIVES ... 47
CHAPTER 4 THERMAL AGGREGATION OF WHEY PROTEINS IN THE PRESENCE OF BUTTERMILK CONCENTRATE ... 49
4.4 MATERIALS AND METHODS ... 53
4.4.1 Materials ... 53
4.4.2 Preparation of whey and buttermilk concentrates ... 53
4.4.3 Heating experiments ... 54
4.4.4 Treatments with N-ethylmaleimide (NEM) and power ultrasound ... 56
4.4.5 Analytical methods ... 56
4.4.6 Particle size distribution and surface area ... 58
4.4.7 Determination of rheological properties ... 58
4.4.8 Statistical analysis ... 59
4.5 RESULTS ... 59
4.5.1 Aggregation yield ... 59
4.5.2 Water-holding capacity ... 61
4.5.3 Consistency coefficient (k) ... 62
4.5.4 Flow behavior index (n) ... 63
4.5.5 Particle size distribution ... 64
4.6 DISCUSSION ... 66
4.7 CONCLUSIONS ... 68
4.8 ACKNOWLEDGMENTS ... 69
CHAPTER 5 EFFECT OF BUTTERMILK COMPONENTS ON THE HEAT-INDUCED DENATURATION OF WHEY PROTEINS ... 71
5.1 RÉSUMÉ ... 72
5.2 ABSTRACT ... 73
5.3 INTRODUCTION ... 73
5.4 MATERIALS AND METHODS ... 76
5.4.1 Materials ... 76
5.4.2 Preparation of whey protein concentrates ... 76
5.4.3 Preparation of spray dried buttermilk concentrates ... 77
5.4.4 Heat-induced aggregation ... 78
5.4.5 Treatment with N-ethylmaleimide (NEM) ... 78
5.4.6 Analytical method ... 78
5.4.7 Gel electrophoresis ... 79
5.4.8 Confocal laser scanning microscopy ... 80
5.4.9 Statistical analysis ... 80
5.5 RESULTS AND DISCUSSION ... 82
5.5.1 Composition ... 82
5.5.2 Free thiol groups concentration ... 83
5.5.3 Gel electrophoresis ... 85
5.5.4 Confocal laser scanning microscopy images... 89
5.5.5 Proposed mechanism for association of whey proteins with buttermilk constituents 93 5.6 CONCLUSIONS ... 94
5.7 ACKNOWLEDGEMENTS ... 95
CHAPTER 6 EFFECT OF HEATING OF WHEY PROTEINS IN THE PRESENCE OF MILK FAT GLOBULE MEMBRANE EXTRACT OR PHOSPHOLIPIDS FROM BUTTERMILK ... 97
6.1 RÉSUMÉ ... 98
6.2 ABSTRACT ... 99
6.3 INTRODUCTION ... 99
6.4 MATERIALS AND METHODS ... 101
6.4.2 Isolation of the milk fat globule membrane ... 101
6.4.3 Heat treatment ... 102
6.4.4 Analytical method ... 102
6.4.5 Confocal laser-scanning microscopy ... 103
6.4.6 Statistical analysis ... 104
6.5 RESULTS AND DISCUSSION ... 104
6.5.1 Free thiol group concentration ... 104
6.5.2 Gel electrophoresis ... 108
6.5.3 Confocal laser-scanning microscopy images ... 109
6.5.4 Thin layer chromatography ... 113
6.6 CONCLUSIONS ... 115
6.7 ACKNOWLEDGEMENTS ... 116
CHAPTER 7 EFFECT OF FREE THIOL GROUP REACTIVITY DURING THE FORMATION OF HEAT-INDUCED AGGREGATES FROM WHEY AND BUTTERMILK ON THEIR PROPERTIES 117 7.1 RÉSUMÉ ... 118
7.2 ABSTRACT ... 119
7.3 INTRODUCTION ... 119
7.4 MATERIALS AND METHODS ... 121
7.4.1 Materials ... 121
7.4.2 Preparation of the whey and buttermilk concentrates ... 122
7.4.3 Preparation of protein aggregates powders ... 123
7.4.4 Particle size distribution in heated liquid mixtures ... 124
7.4.5 Protein solubility index at pH 6.8 ... 125
7.4.6 Exposition of free thiol groups of the mixed aggregates ... 125
7.4.5 Statistical analysis ... 125
7.5 RESULTS ... 126
7.5.1 Heat-induced aggregation characterization ... 126
7.5.2 Properties of the mixed aggregates ... 128
7.6 DISCUSSION ... 130
7.7 CONCLUSION ... 132
7.8 ACKNOWLEDGMENTS ... 133
CHAPTER 8 EFFECT OF SUBSTITUTION OF SKIM MILK POWDER BY WHEY:BUTTERMILK HEAT-DENATURED PROTEIN AGGREGATES IN MODEL SET-TYPE YOGURT 135 8.1 RÉSUMÉ ... 136
8.2 ABSTRACT ... 137
8.3 INTRODUCTION ... 137
8.4 MATERIALS AND METHODS ... 138
8.4.1 Materials ... 138
8.4.2 Preparation of whey:buttermilk heat-denatured aggregates ... 139
8.4.3 Yogurt production ... 139
8.4.4 Analytical methods ... 140
8.4.5 Confocal laser scanning microscopy ... 142
8.4.6 Statistical analysis ... 143
8.5 RESULTS AND DISCUSSION ... 144
8.5.4 Distribution of particles in enriched skim milk before acidification ... 148
8.5.5 Texture of yogurts ... 148
8.5.6 Water-holding capacity of yogurt gels ... 151
8.5.7 Simulation of the gel formation ... 153
8.7 CONCLUSIONS ... 156
8.8 ACKNOWLEDGEMENTS ... 156
CHAPTER 9 GENERAL CONCLUSIONS... 157
9.1 ACHIEVEMENTS AND ORIGINAL CONTRIBUTIONS ... 157
9.2 SIGNIFICANCE OF THE RESULTS ... 163
9.3 QUESTION YET TO BE ANSWERED AND PERSPECTIVES ... 164
TABLE LIST
Table 1.1: Proposed applications for the use of buttermilk in food formulation (Vanderghem et al., 2010). ... 2 Table 2.1: Comparison between the gross composition of buttermilk and skim milk
(Ramachandra Rao et al., 1995; Walstra et al., 2006). ... 5 Table 2.2: Comparison between the gross composition of milk, acida, and sweetb cheese
whey (Britten et al., 2002; Smithers, 2008). ... 8 Table 2.3: Summary of physical and chemical properties of whey proteins adapted from
Cayot and Lorient (1998), Kinsella and Whitehead (1989a), Morr and Ha (1993), and Rüegg et al. (1977). ... 11 Table 2.4: Summary of physical and chemical properties of bovine milk fat globule
membrane proteins adapted from Cheng et al. (1988), Dewettinck et al. (2008), Heid et al. (1996), Hvarregaard et al. (1996), Pallesen et al. (2001), Singh (2006), and Stammers et al. (2000). ... 15 Table 2.5: Lipid composition of buttermilk (adapted from Keenan and Dylewski (1995) and
Walstra et al. (2006). ... 18 Table 2.6: Possible positive and negative effects of fat replacers (adapted from Senanayake
and Shahidi (2005). ... 43 Table 4.1: Composition (% DM) of the various whey-buttermilk mixtures concentrated at
9.5% protein (w/v) used for the heating experiments ... 57 Table 4.2: Summary of the significance (P values) calculated by the analysis of variance of
data of each contrasts for each variables (n = 3). ... 59 Table 4.3: Average particle size and span of untreated and treated whey-buttermilk
mixtures acidified at pH 4.6, denatured at 90°C and homogenized at 65.50 MPa. ... 65 Table 5.1: Summary of the significance (P value) by the analysis of variance of data of
each contrast for each variable (n = 3). ... 81 Table 5.2: Composition (% DM) of whey protein concentrate (WPC) and the different
buttermilk concentrates (BC) powders used for the heating experiment with rBC: regular buttermilk concentrate, rBCSFE: regular buttermilk concentrate after
supercritical fluid extraction, wBC: whey buttermilk concentrate, wBCSFE: whey
buttermilk concentrate after supercritical fluid extraction. ... 82 Table 6.1: Summary of the significance calculated by the analysis of variance of free thiol
group concentration (n = 3). ... 105 Table 7.1: Summary of the significance (P value) calculated by the analysis of variance of
data of each contrasts for each variables (n = 3). ... 126 Table 8.1: Summary of the significance of the effects of WBAP and time on the texture and
water-holding capacity of yogurt gels, as calculated by analysis of variance (n = 3).143 Table 8.2: Summary of the significance of the effects of WBAP and time on the response of
milk to heating, as calculated by analysis of variance (n = 3). ... 144 Table 8.3: Composition of heat-denatured whey:buttermilk aggregates (WBAP), whey
permeate (WP), and powdered skim milk. ... 145 Table 8.4: Summary of the mean rupture force, adhesiveness and relaxation of yogurt gels,
aggregates as enrichment of the starting milk. A Duncan post-test was applied to compare the means to the control. ... 150
FIGURE LIST
Figure 2.1: Schematic representation of the submicelle model of the casein micelle (represented by Horne (2006). ... 9 Figure 2.2: Schematic representation of bovine milk fat globule membrane (from Jiménez-Flores’ group). ... 14 Figure 2.3: Schematic representation of major protein aggregation pathways ... 20 Figure 2.4: Schematic representation of the currently proposed pathways of formation of
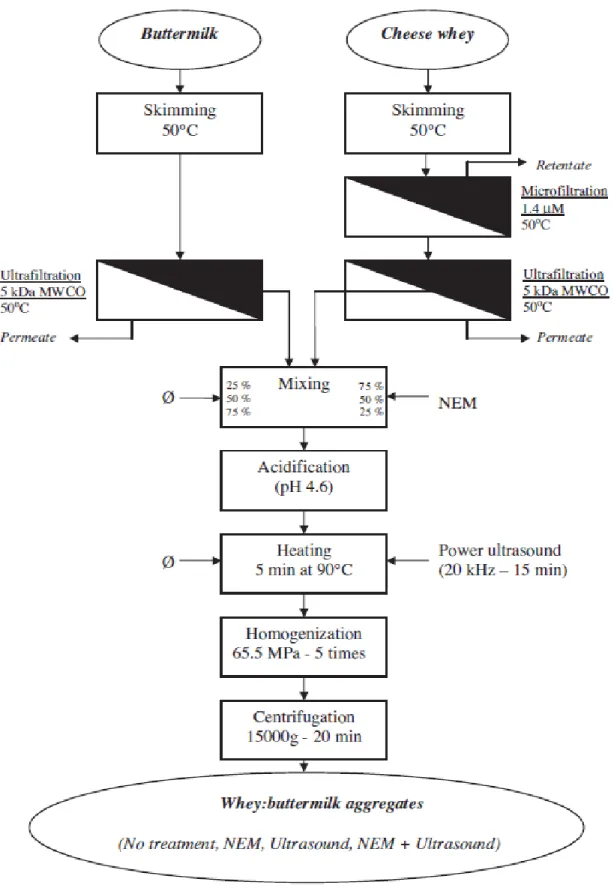
the heat-induced whey proteins/-casein complexes in heated skim milk from Donato et al. (2009). ... 34 Figure 4.1: Experimental procedure used to prepare aggregated buttermilk and whey
proteins mixtures. ... 55 Figure 4.2: Effect of protein composition, NEM and ultrasound on aggregation yield of
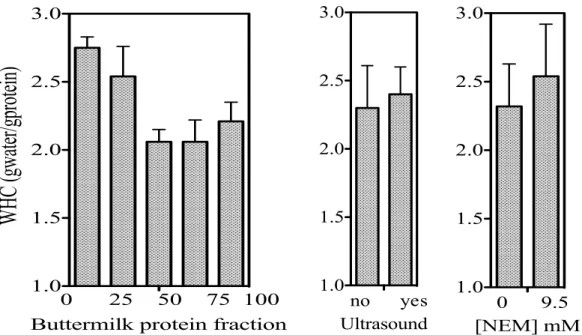
different mixtures of whey and buttermilk concentrates (9.5 % protein), acidified at pH 4.6 and co-denatured at 90°C. ... 60 Figure 4.3: Effect of protein composition, application of ultrasound and addition of NEM
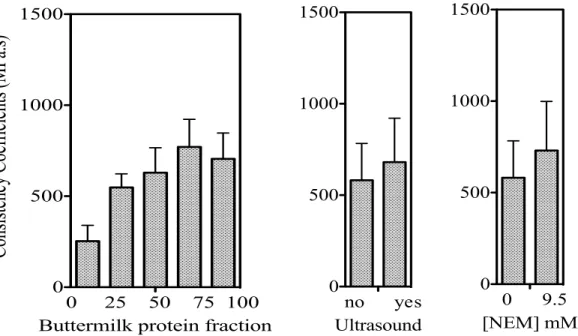
on water-holding capacity (WHC) of mixed buttermilk-whey protein mixture (9.5 % protein) heated at pH 4.6 (90°C). ... 61 Figure 4.4: Effect of protein composition, application of ultrasound and addition of NEM
on consistency index (k) of mixed buttermilk-whey protein mixture (9.5 % protein) heated at pH 4.6 (90°C). ... 62 Figure 4.5: Effect of protein composition, NEM and ultrasound on flow behavior indices of
different mixtures of cheese whey and buttermilk concentrates (9.5 % protein), acidified at pH 4.6 and co-denatured at 90°C. ... 63 Figure 5.1: 15% Non-reducing SDS gels of the mixtures. 1: protein standard, 2: WPC, 3:
WPC + rBC, 4: WPC + rBCSFE, 5: WPC + wBC, 6: WPC + wBCSFE. ... 83
Figure 5.2: Effects of time (a) and composition of the mixture (b) on the accessibility of free thiol groups during heating with constant stirring at temperature up to 90°C at pH 4.6. ... 84 Figure 5.3: Evolution of the protein profile of WPC as a function of heating time (min)
under non-reducing (a) and reducing (b) conditions. ... 86 Figure 5.4: Evolution of the protein profile of the WPC + rBC mixture as a function of
heating time (min) under non-reducing (a) and reducing (b) conditions. ... 86 Figure 5.5: Evolution of the protein profile of the WPC + wBC mixture as a function of
heating time under non-reducing (a) and reducing (b) conditions. ... 86 Figure 5.6: Evolution of the protein profile of the WPC (a), WPC + rBCSFE mixture (b),
and WPC + wBC (c) in presence of NEM as a function of heating time under non-reducing conditions. ... 88 Figure 5.7: Confocal laser scanning miscroscope pictures taken at 100X of WPC at 0, 15,
and 25 minutes of heating before (top) and after (bottom) analysis with ImageJ. ... 89 Figure 5.8: Confocal laser scanning microscope pictures taken at 100X of WPC + rBC
mixture at 0, 15, and 25 minutes of heating before (top) and after (bottom) analysis with ImageJ. ... 90 Figure 5.9: Evolution of the average surface area observed with a confocal laser scanning
time*condition. In a), mixtures were presented as follows: = WPC; = WPC + rBC; = WPC + rBCSFE; = WPC + wBC = WPC + wBCSFE. In b), conditions
were presented as follows: = absence of NEM; = presence of NEM. ... 91 Figure 5.10: Three-dimensional confocal laser scanning microscope pictures of WPC +
rBC mixture before heating. ... 92 Figure 6.1: Effect of interactions composition*temperature (a) and temperature*pH (b) on
the exposition of free thiol groups during heating of WPI (W.), WPI+MFGM extract (W.+M.), and WPI+phospholipid powder (W.+P.) under constant stirring. ... 105 Figure 6.2: Pictures of heated WPI (a), WPI+MFGM (b), and WPI+PL (c) at pH 4.6 at
80°C and after 60 minutes of sedimentation in ice-melted water bath. ... 107 Figure 6.3: Evolution of the protein profiles in the different mixtures at 0 (a), 10 (b), 15 (c),
20 (d) minutes of heating at 80°C using SDS-PAGE under non-reducing conditions. Letters a to d correspond to samples at pH 6.8 and letters a' to d' to pH 4.6. ... 108 Figure 6.4: Three-dimensional confocal laser-scanning microscope pictures of WPI heated
for 25 minutes at 80°C at pH 4.6. ... 110 Figure 6.5: Three-dimensional confocal laser-scanning microscope pictures of the
WPI+MFGM extract heated for 15 minutes at 80°C at pH 4.6. ... 111 Figure 6.6: Three-dimensional confocal laser-scanning microscope pictures of WPI+PL
heated for 15 minutes at 65°C at pH 4.6 ... 112 Figure 6.7: Three-dimensional confocal laser-scanning microscope pictures of WPI heated
for 15 minutes at 80°C at pH 6.8. ... 113 Figure 6.8: Evolution of the phospholipids profiles in WPI+PL at 0 (a), 10 (b), 15 (c), 20
(d), and 25 (e) minutes of heating time at 80°C. Picture was converted in negative mode in order to make the spot easier to see. ... 114 Figure 7.1: Effect of the presence of NEM during formation of mixed aggregates from
heating (90°C – 5min) at low pH (4.6) mixtures containing 50% of the protein from WPC and 50% from buttermilk concentrates on the number-weighted average particle size distribution in the mixtures. ... 127 Figure 7.2: Effect of the presence of NEM during formation of mixed aggregates from
heating (90°C – 5min) at low pH (4.6) mixtures containing 50% of the protein from WPC and 50% from buttermilk concentrates on the aggregation yield. ... 128 Figure 7.3: Effect of the presence of NEM during formation of mixed aggregates from
heating (90°C – 5min) at low pH (4.6) mixtures containing 50% of the protein from WPC and 50% from buttermilk concentrates on the water-holding capacity of the aggregates. ... 128 Figure 7.4: Effect of the composition and the presence of NEM during formation of mixed
aggregates from heating (90°C – 5min) at low pH (4.6) mixtures containing 50% of the protein from WPC and 50% from buttermilk concentrates on nitrogen solubility index of the powders. Aggregates formed in the absence of NEM are represented in black and aggregates formed in the presence of NEM are represented in grey. ... 129 Figure 7.5: Effect of the composition and the presence of NEM during formation of mixed
aggregates from heating (90°C – 5min) at low pH (4.6) mixtures containing 50% of the protein from WPC and 50% from buttermilk concentrates on the accessibility of free thiol groups of the mixed aggregates. ... 130 Figure 8.1: Particle size distribution profile in skim milk after 5 min at 85°C, with added
powdered skim milk substituted 0% () 20% (), 40% (), 60% (), 80% () and 100% () with WBAP. ... 146
Figure 8.2: Effect of milk composition on thiol group exposure following heating at 85°C for 25 minutes (including ramp time). A Duncan post-test was applied to compare the means to the control. ... 147 Figure 8.3: Images of control (enriched with powdered skim milk) skim milk (a) and skim
milk + WBAP aggregates (0.6 g/100 mL; b) at pH 6.8, using the Nikon C1 confocal laser scanning microscope at 60X. Proteins were dye in green and phospholipids in red. ... 148 Figure 8.1: Firmness of control set-type yogurt, and yogurts enriched with whey:buttermilk
protein aggregates. A Duncan post-test was applied to compare the means to the control. ... 149 Figure 8.5: Water-holding capacity of set-type yogurt, and yogurts made from milk
enriched with whey:buttermilk protein aggregates. ... 152 Figure 8.6: Confocal laser scanning microscope (Nikon C1) images taken of gels of control
skim milk (a) and skim milk + WBAP (0.6 g/100 mL; b) at 60X with a zoom of 2.31X; proteins were dyed green and phospholipids are red. Images a’ and b’ were processed with ImageJ to color the cluster in red. ... 153 Figure 8.7: General trends observed in the videos of gel formation as a function of the pH
and time. ... 155 Figure 9.1: Schematic representation of the proposed mechanism of aggregation of proteins
from WPC and the properties of the heat-induced protein aggregates. ... 161 Figure 9.2: Schematic representation of the proposed mechanism of aggregation of
constituents from WPC + BC mixtures and the properties of the heat-induced protein aggregates. ... 162
EQUATION LIST
Equation 4.1: Calculation of the aggregation yield ... 57
Equation 4.2: Calculation of the water-holding capacity of protein aggregates ... 57
Equation 4.3: Formula of the Power Law Model ... 58
Equation 7.1: Calculation of the aggregation yield ... 124
Equation 7.2: Calculation of the water-holding capacity of the aggregates ... 124
Equation 7.3: Calculation of the protein solubility index at pH = 6.8 ... 125
Equation 8.1: Calculation of the relaxation of acid set-type yogurt ... 141
Equation 8.2: Calculation of the water-holding capacity of the gel ... 141
ABREVIATIONS
-LA: alpha-lactalbumin -LG: beta-lactoglobulin -CN: -casein ADPH: adipophilin BC: buttermilk concentrate BSA: bovine serum albumin BTN: butyrophilinCD36: cluster of differentiation 36
CLSM: confocal laser scanning microscope FABP: fatty-acid binding protein
k: Consistency coefficient n: Flow behavior index
MFGM: milk fat globule membrane MUC1: Mucin1
MWP: microparticulated whey proteins NEM: N-ethylmaleimide
PAS 6/7: periodic acid Shiff 6/7 PAS III: periodic acid Schiff III PC: phosphatidyl choline PE: phosphatidyl ethanolamine PI: phosphatidyl inositol
PP: permeate powder PS: phosphatidyl serine
SDS-PAGE: sodium dodecyl sulfate polyacrylamide gel electrophoresis SM: sphingomyelin
SMP: skim milk powder SH/SS: Thiol/Disulfide
TLC: thin layer chromatography WHC: water-holding capacity WPC: whey protein concentrate WPI: whey protein isolate
ACKNOWLEDGMENTS
This work represents an achievement of four years of research during which I crossed the path of many important people that I shall therefore acknowledge.
First of all, I would like to express my sincere gratitude to my advisor Dr. Yves Pouliot. When I met Dr. Pouliot in 2007, I was a kid with a head full of dreams. With his trust, advice, continual support, confidence, and his guidance I grew up quickly in my thinking, the planning of my experiments, and the discussion of my results. Most of all, Dr. Pouliot always had a challenge waiting for me, and at the end the kid was able to realize all of his dreams. I feel very fortunate and I am very thankful for all the opportunities he gave me. As the song goes “Je ne peux pas te dire ce que je ne peux pas écrire. Il faudrait que j’invente
des mots qui n’existent pas dans le dico” but one day I will find a way to express all of my
thankfulness towards you.
I would also like to express my gratitude to my co-advisor Dr. Michel Britten. Dr Britten has always been very generous in his advice, explanations, comments, corrections, and support. His powerful brain has been the cause of few sleepless nights, but I believe that working along Dr. Britten made me stronger in my thinking, the expression of my ideas, and definitely opened my eyes. I hope that our paths will cross again in the future and I will be always ready to discuss with you the different possibilities to explain one result.
I would also like to express my gratitude to my co-advisor Dr. Rafael Jiménez-Flores. We often joke by saying that working along Dr. Jiménez-Flores would be the biggest challenge I faced during this four years of grad school. I found this to be true but it was also the most enjoyable challenge of all. He welcomed me in his arms (no second degree here) in 2010 and later in his scientific team in 2011. Dr. Jiménez-Flores has been a source of inspiration for me and definitely a source of joy. I am thankful for his guidance, his support, and the
advise different candidates in their studies, meet people from industries, and present my results all over California.
I am also thankful toward Dr. Jean-Christophe Vuillemard for accepting to do the pre-reading of this thesis. Dr. Vuillemard has always been very supportive toward this project since day one. He has been generous many times with his comments and his suggestions for the advancement of my experiments. Many thanks to Dr. Fanny Guyomarc’h for joining the committee of my thesis. Dr Guyomarc’h’s work has been a source of inspiration during the planning of many parts of this project.
I am thankful toward Drs. Laurent Bazinet, Sylvie Gauthier, and Sylvie Turgeon. They have been very supportive during the last four years regarding my advancements in my scientific and personal life.
Of course, nothing would have been done without the help of some person. Many many many thanks to the extraordinary Diane Gagnon form the STELA Research Group for teaching me all the lab techniques that I know, for her patience, her availability, her support, and of course her candies … Many thanks to the people from the DPTC to welcome me, for their patience, their understanding, their help, their support, and for never have laughing at my French accent. Many thanks to the interns that have worked at my side: Justine, Eva, Véronique, Sylvain, Camille et Mélanie.
I would like to express all of my gratitude to Frederic Lehance. Frederic was the first person to make me appreciate Quebec City and the Quebec experience in general. He supported from day one (back in 2007) in my studies and in my personal life. He has always been here for me the last six years, listening to my problems, giving me support and advice. He is the belgio-punko big brother that I always wanted.
I also must acknowledge the incredible support I had from my friends at home (particularly Dr. Alexandre). I established very strong relationships since I moved in Canada, and I
would like to express my gratitude to my very best friends Dr. Jeremie and Dr. Romain who have always been here for me. The expression “away from the eyes, away from the heart” is not in their vocabulary, so I never felt alone and lost during my Californian experience. Many thanks to the very good friends that I made during this journey: Benjamin, Laure, Bertrand, Cyril, Ryan, Mason, BJ, Anthony, and David.
I am very grateful to have met my lovely girlfriend Melissa. Her support, comprehensiveness, understanding, encouragement gave me the strength to pursue and complete my degree. Thanks my love for being at my side, even in this scary cold French speaking part of the world. Many thanks to her family for being so supportive and for letting her come in Quebec City.
I am very fortunate to have a wonderful family. I have an enormous respect for them because letting go of their little boy was probably not easy, but they always expressed their understanding and their gigantic support. I am very proud of you and would like to express all of my love to you Danièle & Lucien, Céline & Julien & Justine & Nathan, Marthe & Dominique, Marie-Rose & Hubert.
Last but not least, I am thankful towards Le Fond Québécois de la Recherche sur la Nature et les Technologies (FQRNT) – Novalait Inc. – Ministère de l’Agriculture et de l’Alimentation du Québec (MAPAQ) for their financial support.
FOREWORD
The work carried out in this thesis was aimed to develop a new approach to use whey and buttermilk, and to understand the interactions that occur between components of both liquids. This thesis contains all the results obtained during the course of realization of the project. Results are presented in the form of five published, submitted, or in preparation research articles.
The first article «Thermal aggregation of whey proteins in the presence of buttermilk
concentrate» has been submitted and published in «Journal of Food Engineering N°103» in
2011. Authors are Maxime Saffon, Michel Britten, and Yves Pouliot.
The second article «Effect of buttermilk components on the heat-induced denaturation of
whey proteins» is in preparation for a submission in «Journal of Agriculture and Food
Chemistry». Authors are Maxime Saffon, Rafael Jiménez-Flores, Michel Britten, and Yves Pouliot.
The third article «Effect of heating of whey proteins in the presence of milk fat globule
membrane extract or phospholipids from buttermilk» is pending for submission. Authors
are Maxime Saffon, Rafael Jiménez-Flores, Michel Britten, and Yves Pouliot.
The fourth article «Effect of buttermilk constituents on the properties of heat-induced
protein aggregates» is pending for submission. Authors are Maxime Saffon, Rafael
Jiménez-Flores, Michel Britten, and Yves Pouliot.
The fifth article «Effect of substitution of skim milk powder by whey:buttermilk heat-denatured aggregates in model set-type yogurt» is in preparation for a submission in the
For each article, Maxime Saffona planned and achieved the experiments, presented the results, and wrote the article. In consequence, he is the first author of all of them. Dr. Jiménez-Floresb, co-supervisor of this project, participated to the planning, the discussion of the results, and the revision of the writing from the second to the fifth articles. He is second author on these papers. Dr. Michel Brittenc, co-supervisor of this project, participated to the planning, the discussion of the results, and the revision of the writing of all articles. He is third author on all of them. Dr. Yves Pouliota, supervisor of this project, participated to the planning, the discussion of the results, and the revision of the writing of all articles. He is the last author on all of them.
a STELA Dairy Research Center, Institute of Nutrition and Functional Foods (INAF), Laval University,
Quebec City, QC, Canada, G1V 0A6.
b
Dairy Products Technology Center (DPTC), California Polytechnic State University, San Luis Obispo, CA, USA, 93405.
c
Food Research and Development Center (FRDC), Agriculture and Agri-Food Canada, St-Hyacinthe, QC, Canada, J2S 8E3.
A Danièle, Lucien, Céline Et mes grands-parents
«Les portes de l’avenir s’ouvrent
à ceux qui savent les pousser» -Michel Colucci
CHAPTER 1 INTRODUCTION
The worldwide consumption of milk or dairy products is constantly increasing. It is estimated that the production of milk increases about 1.5% every year in order to satisfy the needs of consumers. The increase of consumption of dairy products such as cheese or butter also increases the production of dairy by-products such as whey or buttermilk. As an example, the production of whey and buttermilk in Canada in 2008 has been calculated around 3,535,000 tons and 82,600 tons, respectively (Statistics Canada, 2009). Over the years, the opinion about these by-products has changed. It is now recognized that whey and buttermilk constituents have interesting biologic, nutritional properties, and health-benefits. Many applications have been developed to use whey in food formulation, such as in cheese, bakery, pastry, and delicatessen productions. Unfortunately, buttermilk has not found as many industrial applications as whey yet. Recently, Vanderghem et al. (2010) summarized ten potential applications for buttermilk or buttermilk constituents that have been tested in research labs over the last 20 years (Table 1.1).
Buttermilk is a unique product due to its concentration of milk fat globule membrane (MFGM) and associated material (proteins, phospholipids, and sphingolipids) that have been associated with very promising health properties ranging from viral to anti-cancer. Buttermilk contains other constituents with potential food applications such as caseins (75% of the total proteins), whey proteins (8 to 15% of the total proteins), and polar lipids. However, it has been demonstrated that the exploitation of the buttermilk constituents is complicated due to some irreversible changes that appear along the process. Previous work of Morin (2006) showed that the pasteurization of the cream is critical because it affects the solubility at pH 4.6 of MFGM proteins, modifies the surface of the fat membrane, and increases the accessibility of phospholipids. Phospholipids have an important capacity to hold water, so the increase of their exposure limits the use of buttermilk in dairy products such as cheese or yogurt. Above all, pasteurization of cream
has been correlated to the poor coagulation properties of buttermilk. Upon the treatment, MFGM proteins initiate heat-induced interactions with other proteins.
Table 1.1: Proposed applications for the use of buttermilk in food formulation (Vanderghem et al., 2010).
Sources Desired Properties Applications Effects Fresh buttermilk Agent for the heat
stability Recombined evaporated milk Stability increased Ultrafiltered sweet buttermilk
Viscosity agent Cheddar cheese Higher apparent viscosity
Condensed sweet cream buttermilk
Agent for the yield, texture, meltability, and coagulation Moisture retention
Pizza cheese Higher cheese yield, moisture content. Decreased chewy, Reduced meltability and gel strength
Buttermilk Moisture retention Agent for the dough and sensory properties
Bread Higher water absorption, Increased resistance to extension, Better sensory score
Whey proteins are also very sensitive to heating. A process has been developed to use whey protein aggregates in food formulation. Some of their industrial applications are, however, limited due to the water-holding capacity of denatured whey proteins. Combination of buttermilk and whey could be the key to better exploit their constituents in food formulation by reducing their individual negative effects such as extensive water retention. It is now clear that caseins, whey proteins, and MFGM proteins interact together under heating. It can be thought that it is possible to control the composition and properties of aggregates by using different ratio and/or experimental conditions.
The goal of this work was to develop a new approach to use buttermilk constituents based on the knowledge of the thermal behavior of its constituents in order to obtain new potential dairy ingredients with texture modification applications for food formulation.
CHAPTER 2 LITERATURE REVIEW
2.1 Buttermilk and whey
2.1.1 Buttermilk
Buttermilk is the aqueous phase expulsed after the formation of large butter grains during churning of cream (Boudreau & St-Amant, 1984). As air is incorporated during the butter-making process, proteins from the cream unfold and form unstable foam with the air bubbles. Upon mechanical stress, the foam is destabilized resulting of fat clumping and phase inversion. Butter becomes solid while the water and soluble particles form the buttermilk. Most of the protein, minerals, lactose, and water from the cream are recovered in the buttermilk (Table 2.1). A high portion of the MFGM is also present in the buttermilk.
Table 2.1: Comparison between the gross composition of buttermilk and skim milk (Ramachandra Rao et al., 1995; Walstra et al., 2006).
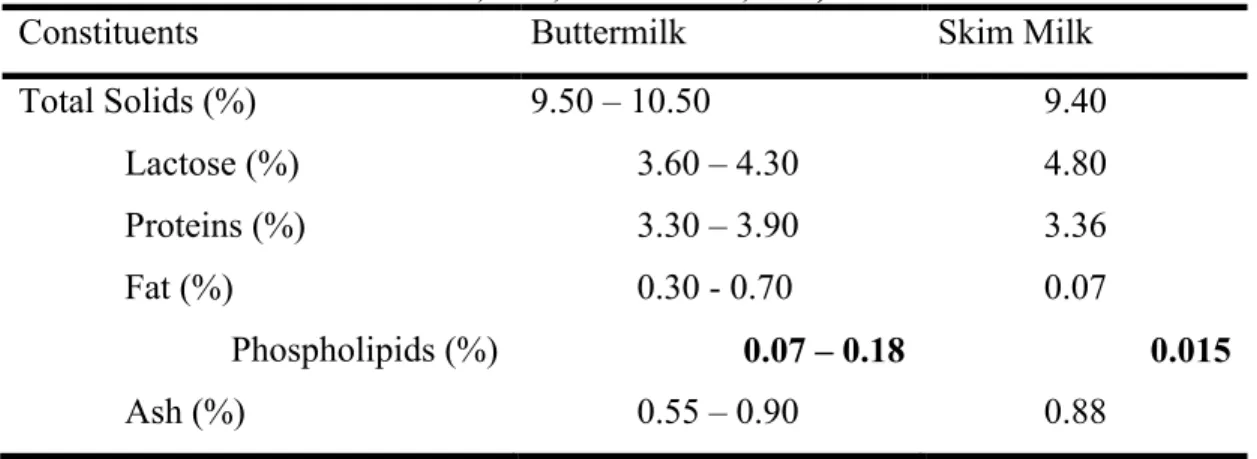
Constituents Buttermilk Skim Milk
Total Solids (%) 9.50 – 10.50 9.40 Lactose (%) 3.60 – 4.30 4.80 Proteins (%) 3.30 – 3.90 3.36 Fat (%) 0.30 - 0.70 0.07 Phospholipids (%) 0.07 – 0.18 0.015 Ash (%) 0.55 – 0.90 0.88
Churning 1 kilogram of cream will typically yield 0.5 kilogram of butter and 0.5 kilogram of buttermilk. Liquid buttermilk is prone to oxidation, so this product is concentrated by evaporation and then sprays dried in order to extend the shelf life. Currently, dried buttermilk is mainly used for animal feeding (swine, bovine), but also used in the food industry for its emulsion properties and its positive impacts on the texture and taste of
Buttermilk is the richest dairy product in phospholipids. In consequence, addition of phospholipids (through substitution of milk by buttermilk) has been tested for the production of low-fat cheese (Turcot et al., 2002; Turcot et al., 2001). Addition of buttermilk increased the cheese yield, but notable changes in the texture and taste of cheese have been recorded. These results have been correlated to the high water-holding capacity of phospholipids. Authors concluded that the amount of phospholipids should be carefully calculated before addition in cheese. They suggested enriching the milk for the cheese-making process (40 gram of proteins per kilogram of milk) with less than one-gram of phospholipids per kilogram of milk to make sure that the texture or taste will not be affected. Earlier, Mistry et al. (1996) have reported that it is possible to substitute milk with liquid buttermilk prior to the cheese-making process without changing the properties of the cheese if the level of substitution is under five percent. Later, Raval and Mistry (1999) observed that cheeses had a lower fat content (lower free oil) and harder bodies if the milk was supplemented with buttermilk (5%). However, cheese had a lower meltability, and a higher apparent viscosity comparatively to the control cheese.
2.1.1.1 Specialized «biorefinery» approach
As described above, buttermilk is rich in minor components associated with the MFGM fragments (proteins, phospholipids). These components have demonstrated promising functional properties and health benefits. Morin (2006) focused on the development of an approach for the separation of MFGM material using microfiltration. Their results showed that the similitarity between the size of the casein micelle and the MFGM fragments made the separation very challenging. Later, they demonstrated that separation can be improved by addition of a cream washing step prior to the microfiltration in order to remove the casein micelles.
However, Bédard ST-Amand (2009) have shown that the industrial HTST pasteurization of the cream is critical for the MFGM fragments. Their results showed that several proteins (probably located at the surface of the bilayer) have broken off the fragments. In
opposition, some of the available Bovine Serum Albumin and β-lactoglobulin proteins are associated with the MFGM fragments.
Regarding the difficulties to separate buttermilk constituents and the modification that occur during the butter-making process (solubilization of some MFGM proteins), a global approach for the utilization of buttermilk seems more appropriate.
2.2.1.2 Global approach
As described in Table 1.1, over ten applications have been proposed for the utilization of buttermilk in food formulation. The proposed applications are ranged from an agent for moisture retention in bread to a viscosity agent for cheddar cheese.
It is also possible to vary the composition of buttermilk for the valorization of a specific constituent. For example, Costa et al. (2010) have proposed an approach to produce a whey buttermilk powder enriched in milk fat globule membrane phospholipids. Whey buttermilk is a very unique product that is richer in MFGM material comparatively to regular buttermilk. By combining ultrafiltration (10X) and CO2-supercritical fluid extraction (350
bars; 50°C) they obtained a powder containing 73% of whey proteins and 21% of lipids of which 61% were phospholipids.
2.1.2 Whey
Whey is the aqueous phase separated from casein network formed through chymosin or mineral/organic acid during cheese-making or casein manufacture process. Depending on the cheese production, whey components represent approximately 50% of the initial milk solids with 20% of the proteins and 100% of the lactose (Table 2.2). Whey has been considered as a waste product during many years and as one of the most polluting food by-products due to its high content in lactose (> 75% of the dry basis).
Table 2.2: Comparison between the gross composition of milk, acida, and sweetb cheese whey (Britten et
al., 2002; Smithers, 2008).
Milk Acid whey Sweet whey
Total solids (%) 12.80 6.60 6.80 Lactose (%) 4.90 4.40 4.90 Total proteins (%) 3.50 0.70 0.70 Caseins (%) 2.80 < 0.1 < 0.1 Whey proteins (%) 0.70 0.70 0.80 Fat (%) 3.70 0.06 0.15 Ash (%) 0.70 0.37 0.31
However, whey represents a rich and heterogeneous content of protein with nutritional, biological and food attributes that give to it potential applications in food processing. Different technologies have been developed to concentrate (35 to 80% of protein) or isolate (> 90% of protein) whey proteins such as ultrafiltration or ultrafiltration/diafiltration. Whey protein concentrates (WPC) or isolates (WPI) have a relatively low value, and are widely produced in dairy industry. These powders have different applications in food processing industries where water binding and texturization are required, such as gelation, thermal stability, foam formation, and emulsification. Their uses vary from manufactured meats to formulated foods or reformed food products (Chatterton et al., 2006; Foegeding et al., 2002).
2.2 Constituents from buttermilk and whey
2.2.1 Caseins
Caseins are the predominant proteins in buttermilk with around 75% of the total protein content (Walstra et al., 2006). Caseins are very different comparatively to other proteins. They have little secondary and tertiary structures, and are hydrophobic with a high charge. The exposed hydrophobic groups give the protein a strong ability for self-interactions or association with other proteins (Walstra et al., 2006).
In a milk system, caseins are present as colloidal particles named casein micelles. Caseins micelles are composed of four major proteins: S1 (33%), (33%), S2 (11%), (11%),
plus calcium phosphate (8 g.100g-1 of casein), a small portion of proteose peptone and some enzymes (Brulé et al., 1997; Walstra et al., 2006). Casein micelles have an average diameter ranged from 40 to 300 nanometers and have a negative charge. The presence of casein micelles in milk is very important because it determines the physical stability of milk products during process and storage and the viscosity of products concentrated in proteins (Walstra et al., 2006).
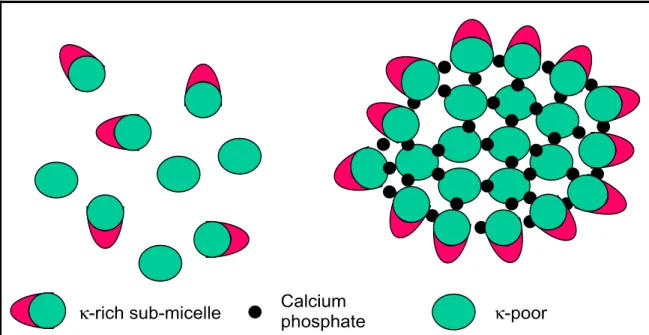
Over the years, various structural models have been proposed for the casein micelles. The most enduring model has been initiated by Schmidt (1980), and consists of submicelles connected together via hydrophobic bonds and calcium phosphate (Figure 2.1).
Figure 2.1: Schematic representation of the submicelle model of the casein micelle (represented by Horne (2006).
of the micelles would contain one or two -caseins. The «hairy layer» of the casein micelles would be formed by the C-terminal end of (~ 75 amino acid residues). The hairy layer would be hydrophilic, negatively charged and would provide colloidal stability to the casein micelle (Walstra et al., 2006).
-casein molecules are mostly present as oligomer of about 120 kDa. Each oligomer contains from 5 to 11 monomers. -casein differs from the other caseins, due to the presence of two cysteine residues that form an intermolecular disulfide bond (Cys11-Cys88),
and because are involved in SH/SS exchanges with whey proteins.
In buttermilk, caseins are also mostly found in micellar form. However, some variation in the size of the casein micelle and its properties due to the processing can be expected. Morin et al. (2008) found that the casein micelles from buttermilk have poor coagulation properties due to the cream pasteurization. As describe later, heat-induced interactions occur between -lactoglobulin and -casein located at the surface of the micelles.
2.2.2 Whey proteins
Whey proteins represent between 8 to 15% of the total proteins in buttermilk (depending of the churning process) (Sodini et al., 2006), while they represent almost 100% of the proteins in whey.
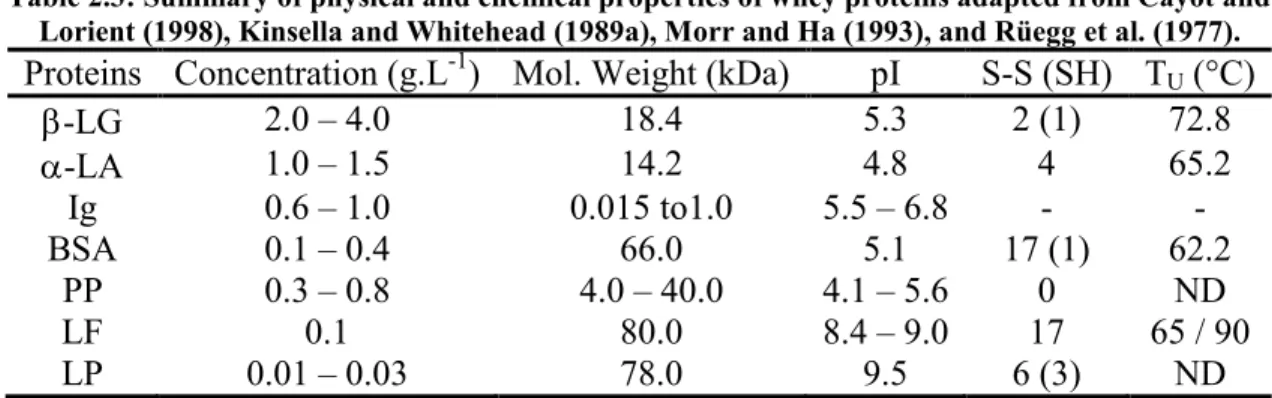
The two major whey proteins are -lactoglobulin (-LG) and -lactalbumin (-LA), and they represent from 70 to 80% of the total protein content. The minor proteins are serum albumin (BSA), immunoglobulin (Ig), proteose peptone (PP), lactoferrin (LF) and lactoperoxydase (LP), and they represent about 20 % of the total proteins (Table 2.3).
Table 2.3: Summary of physical and chemical properties of whey proteins adapted from Cayot and Lorient (1998), Kinsella and Whitehead (1989a), Morr and Ha (1993), and Rüegg et al. (1977). Proteins Concentration (g.L-1) Mol. Weight (kDa) pI S-S (SH) TU (°C)
-LG 2.0 – 4.0 18.4 5.3 2 (1) 72.8 -LA 1.0 – 1.5 14.2 4.8 4 65.2 Ig 0.6 – 1.0 0.015 to1.0 5.5 – 6.8 - - BSA 0.1 – 0.4 66.0 5.1 17 (1) 62.2 PP 0.3 – 0.8 4.0 – 40.0 4.1 – 5.6 0 ND LF 0.1 80.0 8.4 – 9.0 17 65 / 90 LP 0.01 – 0.03 78.0 9.5 6 (3) ND
pI = isoelectric point; TU = unfolding temperature; ND = not determined.
Bovine -lactoglobulin represents about 50 to 55% of total whey proteins with a concentration of 2.0 to 4.0 g.L-1 of milk (Morr et al., 1993). The protein is present as a dimer of 36,400 Da at a neutral pH (Sawyer & Kontopidis, 2000), and starts to dissociate into two identical monomers at a pH above 6.5 or below 3.5 (Hambling et al., 1992; Sawyer et al., 2000; Townend et al., 1969). Monomeric -lactoglobulin is a small acidic globular protein of 162 amino acids with a molecular weight around 18,300 Da (slightly different with genetic variants). The secondary structure of the protein consists of three short helices, an -helix, and nine strands of anti- parallel -sheets (Kinsella et al., 1989a). Hydrophobic, ionic, and hydrogen-bond interactions between the peptide chains stabilize the secondary and tertiary structure of the protein. In the native form, the disulphide bridges Cys66-Cys160 and Cys106-Cys119 also stabilize its structure, and a free thiol group (free –SH)
is available in position Cys121 (Kinsella et al., 1989b). In the native form of the protein, the
free thiol group and the bond Cys106-Cys119 are in the hydrophobic cleft (Sawyer, 2003).
The free thiol group is mainly responsible for the thermal irreversible unfolding and aggregation of the protein. -lactoglobulin is a resistant carrier of retinol, palminate, fatty acids, vitamin D and cholesterol as review by de Wit (1998) and Madureira et al. (2007). Foegeding et al. (2006) have demonstrated that -lactoglobulin has a comparable foam overrun capacity to egg white (albumin) for the formation of meringues, angel food cake or similar products. Their results brought the idea that WPC, WPI or -lactoglobulin isolate could provide a cost-effective alternative to egg albumin in food formulation. The high nutritional value and health benefits (anti-hypertensive, anti-cancer, hypocholesterolemic,
made -lactoglobulin a primary choice for modern food and beverage formulation (Chatterton et al., 2006).
Bovine -lactalbumin represents about 22% of total whey proteins, and it is the second major protein with a concentration of 1.0 to 1.5 g.L-1 of milk (Morr et al., 1993). -lactalbumin is also a small globular protein of 123 amino acids with a 14,200 Da molecular weight. Four disulphide bridges stabilize its structure in position Cys6-Cys120, Cys28-Cys111;
Cys61-Cys77, and Cys73-Cys91 (Morr et al., 1993). -lactalbumin does not have a free thiol
group. The biological function of -lactalbumin is to support the biosynthesis of lactose (de Wit, 1998). Bovine -lactalbumin is an excellent source of the essential amino acids tryptophan and cysteine, and its high homology to human -lactalbumin made it useful for the development of infant formulas.
Bovine serum albumin is present at about 0.1 to 0.4 g.L-1 in cow milk (Morr et al., 1993). Bovine serum albumin is one of the biggest whey protein with 582 amino acid residues, and a molecular weight around 66,000 Da (66,267). This protein possesses seventeen intermolecular disulphide bonds, and a free thiol group at the residue Cys34. The unfolded
protein (rupture of intramolecular disulphide bonds) exposes hydrophobic side groups initially buried to the aqueous phase. Bovine serum albumin binds free fatty acid for transportation in the blood, and is an important source for the production of glutathione in the liver (de Wit, 1998; Morr et al., 1993).
The immunoglobulins (Ig) are an heterogenous family of glycoproteins from 15,000 to 1,000,000 Da, and are present at the concentration of 0.6 to 1.0 g.L-1 in cow milk (Morr et al., 1993). Korhonen et al. (2000) have reported that bovine serums contain three major classes of immunoglobulins that are IgG, IgM, and IgA with a similar structure: two identical light chains (23 kDa) and two identical heavy chains (53 kDa) connected together through disulphide bonds. Approximately 80% of the bovine immunoglobulins are IgG (1 or 2). Immunoglobulins are antibodies that act as a carrier of passive immunity to the newborn, and as an antimicrobial agent (de Wit, 1998; Madureira et al., 2007).
The proteose peptone (PP) fraction of milk is an heterogeneous group of phosphoglycoproteins formed following proteolysis of the N-terminal region in the sequence of -casein by plasmin, and possibly other milk proteins, lipoproteins, and proteolipids. These molecules are amphiphilic because of their charged phosphate groups and sequences of hydrophobic amino acid residues (Kinsella et al., 1989a).
Lactoferrin (LF) is present at 0.02 to 0.35 g.L-1 in bovine milk and 2.0 to 5.0 g.L-1 in human milk. Lactoferrin is a monomeric glycoprotein of 689 amino acids residues and has a molecular weight of around 75,000 Da (76,110). The protein possesses seventeen intermolecular disulphide bonds, but no free thiol group (Kinsella et al., 1989a). Lactoferrin is a transport agent for ferric iron (Fe3+), so it makes iron more available for absorption in the gut. Lactoferrin also has antibacterial activity (de Wit, 1998).
Lactoperoxidase (LP) is present at the concentration of 0.01 to 0.03 g.L-1 of milk. It is one of the most abundant enzyme in whey (0.25 to 0.5 % of the total whey proteins). Lactoperoxidase is a single peptide chain containing 612 amino acid residues. Lactoperoxidase has 4 to 5 potential sites for N-glycosylation. The protein also possesses 15 half cystines (Cals et al., 1991), and a ferric iron (Fe3+). Lactoperoxidase is a main defensive system agent in mammals because of its antimocrobial and antiviral properties. The protein provide a strong protection against invading micro-organisms (Madureira et al., 2007).
2.2.3 Milk fat globule membrane proteins
The bovine milk fat globule membrane (MFGM) represents between 2% and 6% of the total mass of the fat globule and is composed of a complex mix of proteins, glycoproteins, phospholipids, triglycerides, cholesterol, enzymes, and minor constituents. The composition of the MFGM varies as a function of the size of the fat globule, the breed, the
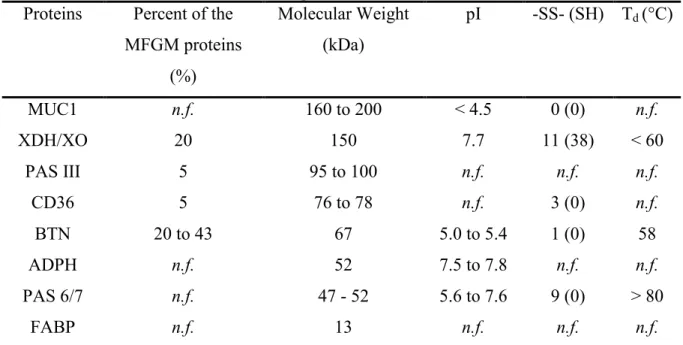
content of the MFGM varies from 25% to 60% depending on the MFGM extraction techniques and the analytical methods. Over 40 proteins have been identified. For a long time, MFGM proteins have been classified according to their relative migration during polyacrylamide gel electrophoresis in presence of SDS. The nomenclature of the MFGM has been clarified by the Milk Protein Nomenclature Committee of the American Dairy Science Association and reported by Mather (2000). The main proteins of bovine MFGM are: Mucin 1 (MUC1), Xanthine dehydrogenase/oxidase (XDH/XO), Periodic acid Schiff III (PAS III), Cluster of Differentiation (CD36), Butyrophilin (BTN), Adipophilin (ADPH), Periodic acid Schiff 6/7 (PAS 6/7), and Fatty-acid binding protein (FABP) (Singh, 2006) (Table 2.4). All these proteins are located through the membrane in specific positions depending on their physical and physiological properties (Figure 2.2). The overall isoelectric point of the MFGM was determined around 4.8 (Kanno & Kim, 1990). At higher pH, the MFGM will be charged negatively.
Figure 2.2: Schematic representation of bovine milk fat globule membrane (from Jiménez-Flores’ group).
Table 2.4: Summary of physical and chemical properties of bovine milk fat globule membrane proteins adapted from Cheng et al. (1988), Dewettinck et al. (2008), Heid et al. (1996), Hvarregaard et al. (1996),
Pallesen et al. (2001), Singh (2006), and Stammers et al. (2000). Proteins Percent of the
MFGM proteins (%) Molecular Weight (kDa) pI -SS- (SH) Td (°C) MUC1 n.f. 160 to 200 < 4.5 0 (0) n.f. XDH/XO 20 150 7.7 11 (38) < 60 PAS III 5 95 to 100 n.f. n.f. n.f. CD36 5 76 to 78 n.f. 3 (0) n.f. BTN 20 to 43 67 5.0 to 5.4 1 (0) 58 ADPH n.f. 52 7.5 to 7.8 n.f. n.f. PAS 6/7 n.f. 47 - 52 5.6 to 7.6 9 (0) > 80 FABP n.f. 13 n.f. n.f. n.f.
pI = isoelectric point; Td= temperature of denaturation; n.f. = not found
Mucin 1 (MUC1) is a mucin-type glycoprotein that is associated with the cream fraction and is found at a concentration of 40 mg.L-1 of milk. It is a polymorphic protein of 160,000 Da to 200,000 Da. Mucin 1 is strongly bound to the serum-exposed side of the MFGM, but can also become soluble after a short heat-shock. In this case, the protein is soluble in the serum. Bovine MUC1 possesses one cysteine residue in the membrane-spanning region and three cysteine residues in the cytoplasmatic tail (Pallesen et al., 2001). Snow et al. (1977) reported that the protein contains 50% of carbohydrate (w/w) including sialic acid (30.5%),
N-acetylglucosamine (22.3%), galactose (15.9%), N-acetylgalactosamine (14.0%), mannose
(11.1%), and fucose (5.8%). The protein is acidic with an isoelectric point under 4.5, due to its high content in sialic acid. The protein also possesses a highly glycosylated filamentous portion located outside the MFGM. The role of Mucin 1 has been uncertain for many years, however Kvistgaard et al. (2004) and Mather (2000) reported that the protein has a protective effect against physical damages and rotavirus. These effects are attributed to the filamentous portion of the protein.
Xanthine dehydrogenase/oxidase (XDH/XO) is the most abundant enzyme of the MFGM. The protein is present in the form of a homodimer of 300,000 Da, where each monomer is estimated to be around 148,000 Da. Twenty-two disulphide bonds reinforce the structure of the protein. XDH/XO also contain thirty eight thiol residues, four of them are available and thirty four are buried in the structure (Cheng et al., 1988). XDH/XO is a peripheral-attached protein that is generally considered as weakly bound to the membrane, because it is possible to remove approximately 60% of the total protein using appropriate buffers. In opposition, the remaining 40% are firmly attached to the membrane (Mather, 2000). The role of XDH/XO has been uncertain for many years, but it is believed that the protein could have an antimicrobial function in the gut due to production of hydrogen peroxide.
Periodic acid Schiff III (PAS III) is a glycoprotein of 95,000 Da to 100,000 Da that has been poorly characterized. However, it is known that PAS III is present in the serum-exposed portion of the membrane (Mather, 2000).
Cluster of Differentiation (CD36) is an integral protein of the MFGM of 76,000 Da to 78,000 Da. The protein possesses ten cysteines residues (Berglund et al., 1996). Six of the ten form the disulfide bonds Cys242-Cys310, Cys271-Cys332, and Cys312-Cys321, and the other
four are acylated near the intracellular side of the membrane (Rasmussen et al., 1998). The protein does not have a free thiol group. CD36 is strongly attached to the membrane; even a centrifugation treatment does not permit to remove the protein from the membrane. CD36 could act as a scavanger receptor by binding to apoptotic cells and cell fragments and precipitating their elimination by phagocytosis (Mather, 2000).
Butyrophilin (BTN) is the most abundant protein in bovine MFGM and is a protein of 67,000 Da. The protein is strongly attached to the membrane and can resist to a centrifugation treatment or extraction with chaotropic agents and detergents (Mather et al., 1977). The structure of the protein is stabilized by a disulphide bond between the two anti-parallel -strands (Stammers et al., 2000). BTN denatures at a temperature of 58°C and
forms aggregates through non-native disulfide cross-bridges at the same temperature (Appel et al., 1982).
Adipophilin (ADPH) is a protein of 52,000 Da strongly attached to the membrane even after extraction with salts and non-ionic detergents (Heid et al., 1996). ADPH has not been widely studied, however it is known that the protein could conserve a hydrophobic binding region that could mediate associations with lipid droplets or eventually proteins. ADPH binds five to six molecules of fatty acids per molecule of proteins (Heid et al., 1996).
Periodic acid Schiff 6/7 (PAS 6/7) are glycoproteins of 52,000 Da and 47,000 Da respectively (Hvarregaard et al., 1996). Nine disulphide bridges stabilized the structure of the proteins (Cys6-Cys17; Cys11-Cys29; Cys31-Cys40; Cys48-Cys59; Cys53-Cys76; Cys78-Cys87;
Cys234-Cys238; Cys252-Cys409; Cys91-Cys247), but the proteins have no free thiol group. PAS
6/7 are poorly attached to the membrane because the proteins can be present as soluble form in skim milk or buttermilk (Mather, 2000). PAS 6/7 is probably the most heat-stable MFGM because the proteins are stable until 80°C (Ye et al., 2002).
Fatty-acid binding protein (FABP) is the smallest protein of the MFGM with a molecular weight of 13,000 Da that is mainly poorly attached to the membrane (Brandt et al., 1988).
2.2.4 Phospholipids from buttermilk
The lipid content of buttermilk usually ranges from 0.3% to 0.7%. Compositions of lipids in buttermilk and in milk fat globule membrane have been well characterized as shown in Table 2.5. Buttermilk is richer in polar lipids such as phospholipids, sphingolipids, and gangliosides with a content ranging from 0.07% to 0.18% of the total lipids comparatively to whole milk (0.035% of the total lipids), skim milk (0.015% of the total lipids) or whey (0.02% of the total lipids). In bovine milk, about 50% to 60% of the phospholipids are integrated to the milk fat globule membrane (fragmented or not), and represent between
2006). The most common classes of phospholipids found in the membrane are Sphingomyelin (SM), Phosphatidyl choline (PC), Phosphatidyl ethanolamine (PE), Phosphatidyl inositol (PI), Phosphatidyl serine (PS), Lysophosphatidyl choline and represent 22%, 36%, 27%, 11%, 4%, and 2% of the total phospholipids from the MFGM, respectively. These classes generally contain high levels of esterified long-chain fatty acid (C16; C18:1), but low levels of short-chain fatty acid (C4 to C10) (McPherson et al., 1983).
The MFGM components and particularly the phospholipids have been extensively investigated over the last twenty years due to their possible roles in nutrition or human health. Agents against colon cancer, gastrointestinal pathogens, Alzheimer’s disease, depression, stress, support for the recovery of the liver from toxic chemical attack or chronic viral are different examples of health-benefits of the phospholipids from the MFGM (Dewettinck et al., 2008; Spitsberg, 2005). It has also been reported that SM and PC are important sources of choline that is important for the synthesis and transmission of important neurotransmitters and even maybe for the brain development.
Table 2.5: Lipid composition of buttermilk (adapted from Keenan and Dylewski (1995) and Walstra et al. (2006).
Constituents In Buttermilk (% of dry basis)
Constituents In MFGM
(% of total lipids) Total lipids 2.9 to 7.4 Triglycerides 62 Phospholipids (PL) 0.6 to 1.9 Diglycerides 9
PE 42.9% of PL Sterols 0.2 to 2.0
PI 8.9% of PL Free fatty acids 0.6 to 6.0
PS 8.6% of PL Phospholipids 26 to 31
PC 19.1% of PL SM 17.9% of PL
2.3 Principles of protein aggregation
Wang et al. (2010) defined protein aggregates as a protein in a non-native state whose size is at least twice as that of the native protein. In chemistry, biochemistry, and pharmacology the term oligomers or critical oligomers is preferred (Modler et al., 2003).
Individual protein molecules have the capacity to fold or (partially) unfold many times. These changes depend on environmental conditions such as intrinsic (structure of protein) or extrinsic factors (pH, temperature, pressure, etc) and lead to an energetically unfavorable unfolded state. This situation is due to the exposition of hydrophobic regions. To regain stability, protein molecules refold again or self-associate to form aggregates.
Aggregation results in the absence of normal functionality of the protein. Protein aggregation has been well-studied because a large number of human disorders, ranging from type II diabetes to Parkinson’s and Alzheimer’s diseases, are associated with protein aggregation (Dobson, 2001; Mattson et al., 1999). Aggregates can also induce a cellular toxicity when they are present in organs such as the liver, heart, and brain (Varadarajan et al., 2000).
2.3.1 Definitions of native state, denaturation, and reversibility
The «native state» of a protein corresponds to its operative or functional form. In their native states, most of the proteins are folded into rigid and well-defined three-dimensional structures. In protein aggregation, the term “native” is used to define the initial state of the protein before denaturation.
“Denaturation” refers to a major change from the original native structure that occurs without severance of any of the primary chemical bonds (Tanford, 1968). However, this definition is subject to controversy, such as the meaning of a major change.
Tanford (1968) simply defined the “reversibility” as the ability of the protein to return (reversible) or to not return (irreversible) to the native conformation. It means that the reaction is reversible only if the native structure can be recovered. In most cases, the process becomes irreversible due to a secondary reaction that follows a major conformational change (i.e unfolding). These reactions can be induced by the use of temperature, acid or basic pH, etc.
2.3.2 Nucleation
Most of the protein aggregation mechanisms are nucleation-dependent and, in consequence, are initiated by the formation of an aggregation nucleus (Wang et al., 2010). There is no definition of an aggregation nucleus in the literature, however, Krishnan (2003) defined a nucleus as small as the size of a dimer. In opposition, Baynes et al. (2005) observed a small multimer as a nucleus. In the case of -lactoglobulin, Schokker et al. (1999) observed the presence of non-native dimers and oligomers in the early stages of the heat-induced denaturation. Later, non-native monomers, denatured proteins, and/or small aggregates are incorporated at the surface of the nucleus to form large aggregates. This second step is named as aggregation, elongation, fibrillation, or polymerization in the literature (Wang et al., 2010).
2.3.3 Description of the different pathways
Wang et al. (2010) summarized the major protein aggregation pathways as presented in Figure 2.3.
Aggregation through unfolding intermediates and unfolded states is the first pathway and named (1) in Figure 2.3. Under normal conditions, a solution of proteins is a balance between proteins in a native state and a small amount of unfolding intermediate proteins (I in Figure 2.3). The latter of the two is divided into native-like intermediate or unfolded-like intermediate depending on the degree of unfolding. These intermediate proteins are considered as precursors of the physical aggregation process because they expose more hydrophobic areas and are more flexible than native proteins. Interactions of these intermediates lead to the formation of aggregates (A in Figure 2.3). Later in the process, aggregates interact with each other. As soon as aggregates reach a certain size or solubility limit, they become insoluble (P in Figure 2.3).
Many proteins do not go through the intermediate state. These proteins can directly form aggregates physically from the native state through self-association (pathways 2a in Figure 2.3). These associations are due to electrostatic, hydrophobic, or van der Waals interactions. Self-association is defined as the formation of reversible aggregates and is considered as the precursor of irreversible aggregates. In fact, the distinction of the formation of intermediates and the self-association can be difficult to demonstrate. So, Wang et al. (2010) reminded that formation of intermediates is related to conformational stability and self-association is related to colloidal stability.
Aggregation can also be due to chemical denaturation and chemical linkage between protein chains (pathways 2b in Figure 2.3). Formaldehyde-mediated cross-linking, dityrosine formation, oxidation, and Maillard reactions have been reported by Wang et al. (2010) as possible cross-linking pathways. Nevertheless, the most common chemical linkage is the intermolecular disulfide bond exchange. Surface-located and internally located cysteines are both involved in the formation of disulfide bonds.