© Hebatoallah Hassan, 2020
Rôle potentiel des cultures bioprotectrices et de leurs
métabolites à activité antimicrobienne pour le contrôle
de Clostridium tyrobutyricum dans les produits laitiers
fermentés
Thèse
Hebatoallah Hassan
Doctorat en sciences et technologie des aliments
Philosophiæ doctor (Ph. D.)
Rôle potentiel des cultures bioprotectrices et de leurs
métabolites à activité antimicrobienne pour le contrôle
de Clostridium tyrobutyricum dans les produits laitiers
fermentés
Thèse
Hebatoallah Hassan
Directeur de recherche
Ismail Fliss
II
Résumé
La présente étude visait à évaluer le potentiel de cultures lactiques bioprotectrices de même que leurs métabolites à activité antimicrobienne (bactériocines) pour le contrôle de Clostridium tyrobutyricum dans du fromage de type Cheddar et la prévention des défauts de texture et de saveur qui lui sont associés.
Trois cent quarante et une souches de bactéries lactiques ont été criblées in vitro pour leur capacité à produire des molécules antimicrobiennes actives contreC. tyrobutyricum ATCC 25755. Trois isolats identifiés comme étant des Lactococcus lactis ssp. lactis ont montré une activité inhibitrice significative contre C. tyrobutyricum. L’opéron codant pour la production de nisine A a été identifié chez ces trois isolats alors que la production de nisine a été confirmée par des tests de diffusion sur gélose contre C. tyrobutyricum. Des essais d’interaction et de biocompatibilité entre ces souches productrices de nisine A et des ferments industriels pour fromage Cheddar ont permis de définir un ferment protecteur mixte comprenant un Lactococcus lactis ssp lactis CUC-H, lactococcus lactis ssp cremoris CUC222 et Lactococcus lactis ssp lactis 32 producteur de nisine A. D’autre part, de la nisine A a été produite et purifiée à partir du surnageant de culture de la souche Lactococcus lactis ssp lactis 32 puis encapsulée dans des billes constituées d'alginate et d'amidon non gélatinisé.
Le ferment mixte protecteur de même que la nisine purifiée encapsulée ont été testés pour leur efficacité à inhiber C. tyrobutyricum dans un caillé modèle de fromage Cheddar en utilisant deux concentrations de sel (1.3 et 2%), pendant deux semaines à 30°C et pendant un mois à 4°C. Les résultats obtenus avec les échantillons de caillé modèle ont été validés lors d’une production à l’échelle pilote de fromage Cheddar. Les résultats ont montré que C. tyrobutyricum n'a pas été détectée dans les échantillons entreposés à 4°C contenant de la nisine encapsulée à partir de la 3e semaine en présence de 1.3% de sel et de la deuxième semaine en présence de 2% de sel. Lorsque le ferment protecteur est utilisé, une diminution d'environ 0.6 log10 de C. tyrobutyricum a été observée à partir de la deuxième semaine dans le caillé modèle entreposé à 4°C. D’autre part, l'analyse de chromatographie en phase liquide à haute performance (CLHP) des caillés a montré que Lactococcus lactis ssp lactis 32 était capable de produire in situ de la nisine à partir de la deuxième semaine. Les résultats de l’analyse métagénomique montrent que l'abondance relative du genre Clostridium a diminuée dans les caillés fromagers en présence aussi bien de la souche protectrice que de la nisine
III
encapsulée, comparativement aux fromages témoins. Ces résultats confirment que l’ajout de Lactococcus lactis ssp. lactis 32 en tant que souche protectrice productrice de nisine permet de contrôler le développement de C. tyrobutyricum dans les caillés modèles de fromage cheddar. Les résultats obtenus dans le fromage Cheddar produit à l’échelle pilote ont montré une réduction de 1 log10 de C. tyrobutyricum dans les groupes traités aussi bien avec la nisine encapsulée qu’avec la souche protectrice. De plus, l’analyse de l’activité protéolytique des fromages a montré une augmentation significative de la fraction d’azote soluble dans l'eau en présence de la souche protectrice ou de la nisine encapsulée. En revanche, les teneurs en azote des fractions TCA et PTA étaient plus élevées dans le groupe contenant de la nisine encapsulée.
En conclusion, les deux stratégies utilisées dans le cadre de cette étude basées sur l’utilisation de la nisine purifiée encapsulée ou de la souche L. lactis ssp lactis 32 productrice de nisine semblent être efficaces à différents niveaux pour le contrôle de C. tyrobutyricum dans du fromage Cheddar. La présence de nisine dans la matrice fromagère semble également avoir des effets significatifs sur l’activité protéolytique globale de la matrice fromagère. Ce résultat suggère des effets potentiels sur la vitesse de maturation des fromages ainsi que sur leurs caractéristiques organoleptiques et sensorielles.
IV
Abstract
The present study aimed to assess the potential of bioprotective lactic acid cultures as well as their metabolites with antimicrobial activity (bacteriocins) for the control of Clostridium tyrobutyricum in Cheddar-type cheese and the prevention of texture and flavour side effects.
Three hundred forty-one strains of Lactococci have been screened in vitro for their ability to produce antimicrobial molecules active against C. tyrobutyricum ATCC 25755. Three isolates identified as Lactococcus lactis ssp. lactis showed significant inhibitory activity against C. tyrobutyricum. The gene coding for the production of nisin-A has been identified in these three isolates while the production of nisin has been confirmed by agar diffusion test against C. tyrobutyricum ATCC 25755. Interaction and biocompatibility assays between these nisin-A producing strains and industrial starter for Cheddar cheese allowed to define a mixed protective starter comprising a Lactococcus lactis ssp lactis CUC-H, a Lactococcus lactis ssp cremoris CUC 222 and Lactococcus lactis ssp lactis 32 as a nisin-A producer. Nisin-A was also purified, then encapsulated in vesicles made of alginate and non-gelatinized starch. The effectiveness of the protective mixed starter as well as the encapsulated nisin were tested for their effectiveness in inhibiting C. tyrobutyricum in a Cheddar cheese slurry using two different salt concentrations (1.3 and 2%), for two weeks at 30 °C or for one month at 4 °C. The results obtained with the cheese slurry samples were validated during a pilot scale production of Cheddar cheese. The results showed that C. tyrobutyricum was not detected in the samples containing encapsulated nisin from third week in the presence of 1.3% salt and from second week in the presence of 2% salt at 4 °C. When the protective starter is used, a progressive decrease in the number of C. tyrobutyricum, of approximately 0.6 log10, was observed from the second week in cheese slurry stored at 4 °C. High-performance liquid chromatography (HPLC) analysis indicated that the protective culture was capable of producing nisin in situ since the second week.
The results of the metagenomic analysis showed that the relative abundance of the genus Clostridium decreased in cheese samples in the presence of both the protective strain and the encapsulated nisin, compared to the control. These results confirm that the addition of Lactococcus lactis ssp. lactis 32 as a nisin-A producing strain helps in controlling C. tyrobutyricum in cheddar cheese slurries. In Cheddar cheese produced at a pilot scale, a reduction of 1.0 log10 in the number of C. tyrobutyricum was obtained in cheeses treated with both the encapsulated nisin and with the protective strain. In addition, analysis of the proteolytic activity of cheeses showed a significant increase in the water-soluble nitrogen (WSN) fraction in the presence of the protective strain or of the encapsulated nisin. On the other hand,
V
the TCA- soluble nitrogen and PTA- soluble nitrogen fractions were higher in the encapsulated nisin group. In conclusion, the two strategies used in this study based on the use of encapsulated nisin or of Nisin-producing Lactococcus lactis appear to be effective at various extend for the control of C. tyrobutyricum in Cheddar cheese. The presence of nisin in the cheese matrix also seems to have significant effects on the overall proteolytic activity of the cheese matrix. This result suggests potential effects on the ripening speed of cheeses as well as on their intrinsic organoleptic and sensory characteristics.
VI
Table des matières
Résumé... II Abstract ... IV Table des matières... VI Liste des tableaux ... XI List des figures ... XIII Liste des abréviations ... XVI Remerciements ... XVIII Avant-propos ... XIX
Introduction générale ... 1
Chapter 1. Literature review ... 3
1.1. Production of Cheddar cheese in Canada ... 4
1.2. Cheddar cheese processing ... 5
1.3. Cheddar cheese quality ... 5
1.4. Cheese microbial ecosystem ... 7
1.5. Cheese defects ... 8
1.6. Cheeses spoilage microorganism ... 9
1.6.1. The genus Clostridium ... 9
1.7. Traditional methods for milk and cheese preservation ... 12
1.8. Bio-preservation ... 13
1.8.1. Protective cultures ... 13
1.8.2. Antimicrobials substances produced by protective cultures ... 14
1.8.2.1. Bacteriocins ... 14 Nisin ... 16 Pediocin ... 17 Enterocin ... 17 Organic acids... 18 Diacetyl ... 18 Reuterin ... 19 Reutericyclin ... 19
VII
1.9. Application of protective cultures and antimicrobial compounds in dairy products .. 20
1.10. Hurdle technology ... 20
1.11. Problems ... 25
1.12. Hypothesis ... 25
1.13. General objective ... 25
1.13.1. Specific objectives ... 26
Chapter 2. Interactions and biocompatibility between nisin-producing Lactococcus lactis strains and lactic acid starter under Cheddar cheese manufacturing conditions ... 27
2.1. Résumé ... 28
2.2. Abstract ... 29
2.3. Introduction ... 30
2.4. Materials and methods ... 31
2.4.1 bacterial strains and culture conditions ... 31
2.4.2. Screening for antimicrobial activity ... 31
2.4.3. Spores production ... 31
2.4.4. Molecular identification ... 32
2.4.5. Molecular identification of subspecies ... 32
2.4.6. Identification of nisin-A encoding gene ... 32
2.4.7. Inhibition of C. tyrobutyricum by nisin and Minimal Inhibitory Concentration (MIC) determination ... 33
2.4.8. Biocompatibility test ... 33
2.4.8.1. Determination of soluble proteins and peptides by OPA ... 34
2.4.8.2. Automated spectrophotometry assays ... 34
2.4.9. Growth Kinetics ... 35
2.4.10. Statistical analysis ... 35
2.5. Results and discussion ... 35
2.5.1. Screening and molecular identification of the bacteriocinogenic strains ... 35
2.5.2. Bio-compatibility test ... 35
2.5.3. Growth kinetics and MIC determination ... 36
2.6. Conclusion ... 37
VIII
2.8. Acknowledgements ... 38
2.8. Conflict of interest statement ... 38
2.9. Authors contributions ... 38
2.10. Tables and figure ... 39
Chapter 3. Novel design for alginate/ resistant starch microcapsules controlling nisin release ... 49
3.1. Résumé ... 50
3.2. Abstract ... 51
3.3. Introduction ... 52
3.4. Material and methods ... 54
3.4.1. Bacterial preparation ... 54
3.4.2. Spores production ... 54
3.4.3. Nisin preparation ... 55
3.4.4. Microencapsulation process ... 55
3.4.4.1. Morphological characterization of microcapsules ... 55
3.4.4.2. Fourier Transform-Infrared (FTIR) Spectroscopy... 56
3.4.4.4. Encapsulation efficiency (EE) ... 56
3.4.4.5. Determination of nisin release ... 56
3.4.4.6. Application of encapsulated Nisin in cheese slurry ... 57
4.4.7. Statistical analysis ... 57
3.5. Results and discussion ... 57
3.5.1. Microscopic examination of microcapsules ... 57
3.5.2. Fourier Transform-Infrared (FTIR) spectroscopy ... 58
3.5.3. Antimicrobial activity of encapsulated nisin ... 58
3.5.4. Encapsulation efficiency (EE) ... 59
3.5.5. Determination of nisin release ... 59
3.5.6. Transmission electron microscope (TEM) ... 60
3.5.7. Application of encapsulated nisin in Cheddar cheese slurry ... 61
3.6. Conclusion ... 61
3.7. Funding... 62
3.8. Acknowledgements ... 62
IX
3.10. Authors contributions ... 62
3.11. Tables and figure ... 62
Chapter 4. Impact of nisin and nisin-producing L. lactis ssp. lactis on Clostridium tyrobutyricum and bacterial ecosystem of cheese matrices... 71
4.1. Résumé ... 72
4.2. Abstract ... 73
4.3. Introduction ... 74
4.4. Material and methods ... 75
4.4.1. Bacterial preparation ... 75
4.4.2. Spore production ... 75
4.4.3. Pearce test ... 76
4.4.4. Nisin preparation ... 76
4.4.5. Cheese slurry preparation and experimental treatments ... 76
4.4.6. Nisin determination by High-performance liquid chromatography (HPLC) ... 77
4.4.7. Metagenomic analysis ... 77
4.4.8. Statistical analysis ... 78
4.5. Results and discussion ... 79
4.5.1. Selection of mixed culture ratio and production of encapsulated nisin ... 79
4.5.2. Chemical and microbiological composition of cheese slurry ... 79
4.5.3. In situ nisin production ... 80
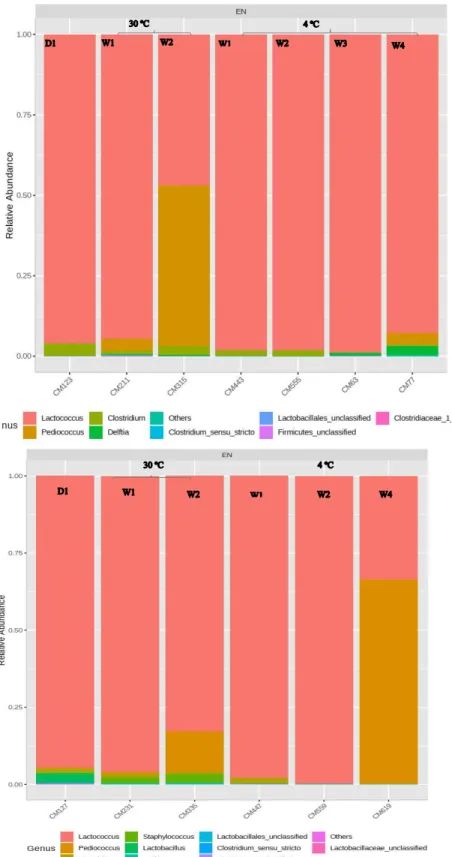
4.5.4. Metagenomics analysis ... 81
4.6. Conclusion ... 82
4.7. Funding... 83
4.8. Acknowledgements ... 83
4.9. Conflict of interest statement ... 83
4.10. Authors contributions ... 83
4.11. Tables and figures ... 84
Chapter 5. Nisin and nisin-producing L. lactis ssp. lactis: a promising natural approach for the control of Clostridium tyrobutyricum in Cheddar cheese .103 5.1. Résumé ... 104
5.2. Abstract ... 105
X
5.4. Materials and methods ... 107
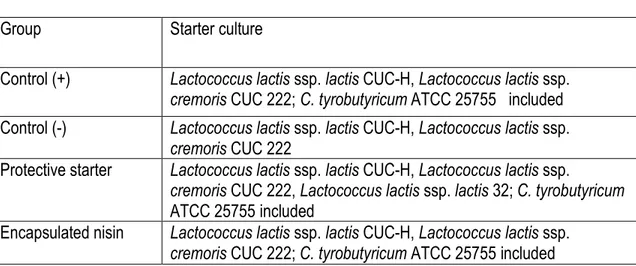
5.4.1. Bacterial culture preparation ... 107
5.4.2. Spore production ... 107
5.4.3. Nisin preparation ... 107
5.4.4. Preparation of encapsulated nisin ... 107
5.4.5. Cheddar cheese production ... 108
5.4.6. Chemical composition ... 109
5.4.7. Microbiological analysis ... 109
5.4.8. Proteolysis during cheese ripening ... 109
5.4.9. Nisin determination ... 109
5.4.10. Metagenomic analysis ... 110
5.4.11. Transmission electron microscopy ... 110
5.4.12. Statistical analysis ... 110
5.5. Results and discussion ... 110
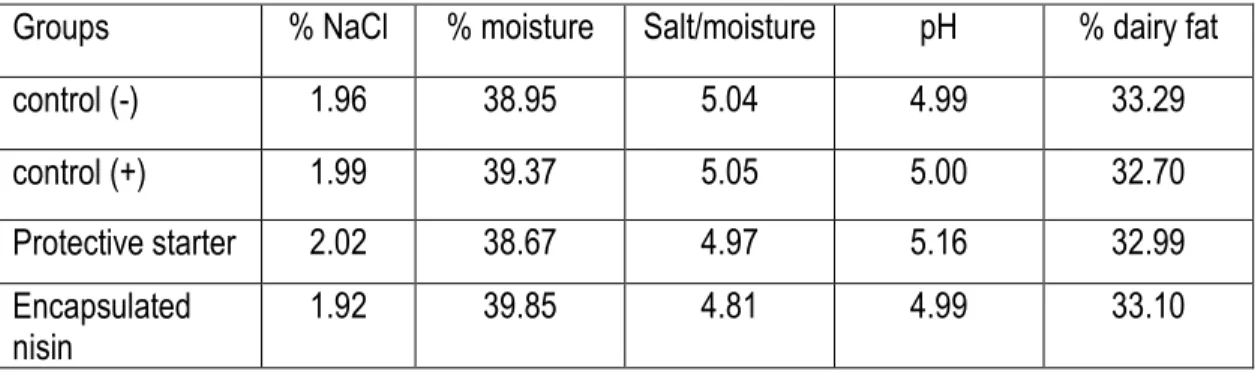
5.5.1. Microcapsule production and chemical composition ... 110
5.5.2. Microbiological analysis ... 111
5.5.3. Microbial diversity based on 16S rRNA MiSeq sequencing ... 112
5.5.4. Proteolysis during cheese ripening ... 114
5.5.5. Nisin determination by High-performance liquid chromatography (HPLC) ... 115
5.5.6. Transmission electron microscopy ... 115
5.6. Conclusion ... 116
5.7. Funding... 116
5.8. Acknowledgements ... 117
5.9. Conflict of interest statement ... 117
5.10. Authors contributions ... 117
5.11. Table and figures ... 118
Conclusion générale ...125
XI
Liste des tableaux
Chapitre 1.
Table 1. Dairy products and typical types of spoilage microorganisms or microbial activity ... 11
Table 2. Applications of bacteriocin-producing LAB in dairy products... … .... ... 22
Table 3. Application of purified and semi-purified bacteriocins in dairy products . ... 24
Chapitre 2.
Table 1. Different specific primers used to determine subspecies ... 39Table 2. Molecular Identification of selected strains ... 40
Table 3. Acidifying capacity and proteolytic activity of Lactococcus strains. ... 41
Table 4. Growth characteristics of selected protective starter inoculated in cell free whey ... 42
Chapitre 3.
Table 1. The polymer mixture composition of different microcapsules. ... 63Table 2. Encapsulation efficiency of different microcapsules (EE%). ... 64
Table 3. The release of nisin (µg/ml) from microcapsules during 26 days in skim milk media... 65
Chapitre 4.
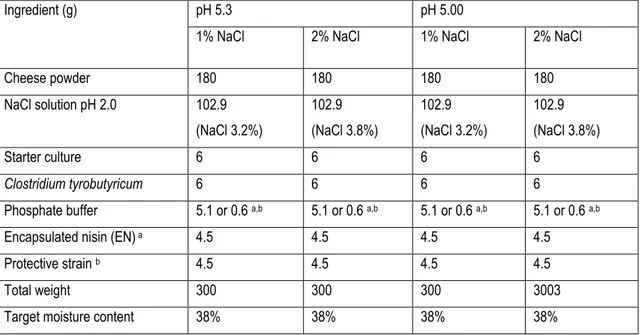
Table 1. The formation of different Cheddar cheese slurry groups .... ………..…84Table 2. Acidification of skim milk by different strains of Lactococcus lactis; pure culture or combination (1% total inoculum volume) under the Cheddar cheese temperature cycle .………..85
Table 3. Viable counts of Lactococcus lactis in skim milk; pure culture or combination (1% total inoculum volume) under the Cheddar cheese temperature cycle. ………...86
Table 4. Nisin-A production by the nisin-producing strain of Lactococcus lactis in skim milk; pure culture or combination (1% total inoculum volume) under the Cheddar cheese temperature cycle ………..………..……..87
Table 5. Chemical analysis of the cheese slurries at time zero ………...……….88
XII
Chapitre 5.
Table 1. Cheddar cheese experimental treatment groups………...…….118 Table 2. Chemical composition of Cheddar cheese in the four experimental treatment groups. …...119
XIII
Liste des figures
Chapitre 1.
Figure 1. The production and shipment of different sectors of food products in Canada, 2019 .... 4 Figure 2. A typical manufacturing process for the production of Cheddar cheese ... ……..6 Figure 3. Late blowing defect in aged chees ... ……..9 Figure 4. Pathway of clostridium spores’ contamination of milk and cheese ….. ... ……..10 Figure 5. Schematic of nisin-A structure. In nisin Z, His- 27is replaced by Asn. Arrows indicate the
amino acid replacements ..……….. …… ... ………16 Figure 6. Schematic of Pediocin PA-1 amino acid sequencing.……….…...17
Chapitre 2.
Figure 1. Agar diffusion assay showing Inhibition zones in M17 culture supernatants of different
Lactococcus lactis strains against C. tyrobutyricum ATCC 25755. Lactococcus lactis ssp. lactis 1145 and Lactococcus lactis biovar diacetylactis 719 were used as a
reference strains………43
Figure 2. Agarose gel electrophoresis of amplicons obtained after PCR amplification using primers specific to nisin-A. Using Lactococcus lactis ssp. lactis ATCC 11454 and Lactococcus lactis biovar diacetylactis 719 as a control. Strains identified as Lactococcus lactis ssp. lactis showed band at 343 bp, for Lactococcus lactis ssp. cremoris showed at 536 bp., and Lactococcus lactis biovar diacetylactis showed positive at 900 bp. ... 44 Figure 3. Electrophoresis gel on amplicons obtained after PCR with primers specific to nisin-A.
Agarose gel 1% migrated for 1 hour at 100 V then stained with Gel-red. Using L. lactis ssp. cremoris SK11 and 190 as negative control (nisin negative), L. lactis biovar diacetylactis 719 as a positive control (nisin-Z producer) and lane C- is control negative (without sample). Strains L. lactis ssp. lactis 32, 33, 209 and the positive control ATTC
11454 (nisin-A producer) showed band at size 900
bp………..………. 45
Figure 4. Values of bacterial strains parameters grown in control whey acidified using
glucono-d-lactone. Where, (A) Tlag, the time when optical density indicates an increase by 0.01 units, (B) Tmax the time when optical density reach the maximum, (C) OD24, the optical density after 24 h, and (D) µmax, the maximum rate at the inflexion point.
………...…..……46
Figure 5. Growth kinetics of Lactococcus lactis ssp. cremoris CUC-H, Lactococcus lactis ssp.
lactis 32 & 222 strains during 30h of incubation in skim milk medium at 30 ºC, a) pH profile and b) growth curve... 47 Figure 6. Growth curve and nisin-A production of Lactococcus lactis ssp. lactis 32 strain during
XIV
Chapitre 3.
Figure1. Macrophotographs of nisin loaded beads prepared with different concentration of
alginate/ gelatinized or non-gelatinized starch: A) alginate 0.5%; A2) 0.5- 1% alginate/ G-starch; A3) 0.5- 1% alginate/ non-starch and B1) alginate 1%; B2) 1- 0.5% alginate/ non-starch; B3) 1- 0.5% alginate/ G-starch; B4) 1- 1% alginate/ G-starch. ... 66 Figure 2. FTIR spectra of Alg, starch, Alg-starch microparticles and nisin-loaded Alg-starch
microparticles. *NG ST = non-gelatinized starch ... 67 Figure 3. Antimicrobial activity of microencapsulated nisin directly using the agar diffusion
assay and Pediococcus acidilactici UL5 as indicator strain. ... 68 Figure 4. Transmission electron micrograph of 1- 0.5%alginate/ non gelatinized-starch
microcapsule. A) microcapsule wall and b) the middle of microcapsule. ... 69 Figure 5. Effect of encapsulated nisin on the starter culture and C. tyrobutyricum ATTC 25755
over the period of 4 weeks at 4 °C in Cheddar cheese slurry. ... 70
Chapitre 4.
Figure 1. Total Lactococcus counts (starter culture) in Cheddar cheese slurries made with 2%
NaCl and stored for four weeks at 4°C, a) pH 5.0; b) pH 5.3 .. .……….….. ... 90 Figure 2. Total Lactococcus counts (starter culture) in Cheddar cheese slurries made with 1.3%
NaCl and stored for four weeks at 4°C, a) pH 5.0; b) pH 5.3 ………91 Figure 3. Total Lactococcus counts (starter culture) in Cheddar cheese slurries made with 1.3%
NaCl and stored for two weeks at 30°C, a) pH 5.0; b) pH 5.3. ... 92 Figure 4. Total Lactococcus counts (starter culture) in Cheddar cheese slurries made with 2%
NaCl and stored for two weeks at 30°C, a) pH 5.0; b) pH 5.3
……… ……….. ……... 93
Figure 5. Clostridium tyrobutyricum counts in Cheddar cheese slurries adjusted to pH 5.3 and
stored for four weeks at 4°C, a) 2% NaCl; b) 1.3% NaCl ... …..94
Figure 6. Nisin production in Cheddar cheese slurry containing bacteriocin-producing Lactococcus lactis ssp. lactis and a) 2% NaCl or b) 1.3% NaCl, during 4 weeks at 4°C
……….………95
Figure 7. Nisin production in Cheddar cheese slurry containing bacteriocin-producing
Lactococcus lactis ssp. lactis and a) 2% NaCl or b) 1.3% NaCl, during 2 weeks at 30°C.
... 96 Figure 8. Nisin released in Cheddar cheese slurry containing the encapsulated bacteriocin and
a) 2% NaCl or b) 1.3% NaCl, during 4 weeks at 4°C. ... 97
Figure 9. Nisin released in Cheddar cheese slurry containing the encapsulated bacteriocin and
XV
Figure 10. Gas swelling of Cheddar cheese slurry (made with in at 1.3% NaCl) by Clostridium
tyrobutricum after one week at 30°C, left: control; right: containing encapsulated nisin-A ... 99 Figure 11. Relative abundance of bacterial genera, based on 16S rRNA, in control Cheddar
cheese slurries made with 1.3% NaCl (top) or 2% NaCl (bottom) and stored for four weeks at 4°C or for two weeks at 30°C (D1 = t0) ……….. ………100 Figure 12. Relative abundance of bacterial genera, based on 16S rRNA, in Cheddar cheese
slurries containing encapsulated nisin-A plus 1.3% NaCl (top) or 2% NaCl (bottom) and stored for four weeks at 4°C or for two weeks at 30°C (D1 = t0) ... 101
Figure 13. Relative abundance of bacterial genera, based on 16S rRNA, in Cheddar cheese
slurries containing a producer of nisin-A plus 1.3% NaCl (top) or 2% NaCl (bottom) and stored for four weeks at 4°C or for two weeks at 30°C (D1 =t0)……….………...102
Chapitre 5.
Figure1. Total viable bacterial counts in the experimental Cheddar cheeses during 6 months of
ripening at 4ºC. ... 120 Figure 2. Effect of encapsulated nisin and nisin-A producing starter culture on C. tyrobutyricum
counts in Cheddar cheese ripened for 6 months at 4ºC ... 121 Figure 3. Different protein fractions in Cheddar cheeses containing encapsulated nisin,
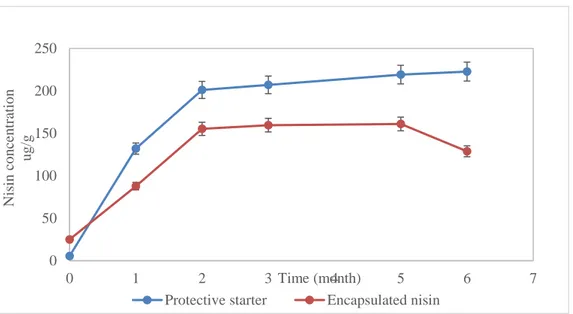
protective starter culture and in the control cheeses (with (+) and without (-) C. tyrobutyricum) ripened for 6 months at 4ºC, and a) Water-soluble nitrogen, b) TCA-soluble nitrogen, C) PTA-TCA-soluble nitrogen... 122 Figure 4. Nisin concentration (HPLC) in Cheddar cheeses ripened for 6 months at 4ºC, produced
by Lactococcus lactis ssp. lactis or released from alginate/starch microcapsules ….123 Figure 5. Transmission electron micrographs showing the inhibitory action of nisin on
Clostridium tyrobutyricum in Cheddar cheese during 6 months of ripening at 4ºC. A) Cells from cheese containing encapsulated nisin, ripened for 3 months; B) Same, after 6 months of ripening, C) In cheese containing the nisin producer,after 6 months of
XVI
Liste des abréviations
Alg-St : Alginate-starch
Alg-St non : Alginate-non gelatinized starch
Alg – St G : Alginate- gelatinized starch
AS: Automated spectroscopy
BL : Bactéries lactiques
CFW: Cell free whey
EE : Encapsulation efficiency
EN-Nisin : Encapsulated nisin
FAO : Food and Agriculture Organization
FTIR : Fourier-transform infrared spectroscopy
HPLC : High Performance Liquid Chromatography
IRTF: Spectroscopie infra-rouge à transformé de Fourrier
KBr : Potassium bromide
LAB : Lactic acid bacteria
MRS: De Man, Rogosa and Sharpe medium
NSLAB: Non-starter lactic acid bacteria
PMA : Propidium monoazide
PTA-SN : Phosphotungstic acid- soluble nitrgen
RCM: Reinforced Clostridial Medium
STELA: Centre de recherche en sciences et technologie du lait
TCA-SN : Trichloroacetic acid-soluble nitrogen
TN: Total nitrogen
XVII
XVIII
Remerciements
Ces travaux de thèse sont l’aboutissement de plusieurs années de travail qui n'auraient pas été possibles sans l'aide et le soutien de nombreuses personnes que je tiens à remercier et à exprimer toute ma gratitude.
Tout d'abord, j’aimerais remercier sincèrement mon directeur de recherche, Dr Ismail Fliss, qui m’a guidé tout au long de cette aventure et m’a offert l’opportunité d’apprendre à ses côtés. Au cours de ces quatre dernières années, j’ai eu la chance de faire partie de son équipe de recherche et surtout de travailler sur un projet aussi passionnant. Je le remercie énormément pour sa présence, ses conseils, et son aide précieuse qu’il m’a accordée durant toute cette période. Je le remercie également pour sa confiance, sa grande patience et ses encouragements qui m’ont toujours aidé à me motiver et à mieux avancer. Enfin, je le remercie pour toutes ces conversations instructives et échanges enrichissants, aussi bien dans le domaine scientifique que dans la vie courante, qui m’ont permis de faire de moi une meilleure personne.
Je remercie énormément mon co-directeur de doctorat, le Dr. Daniel St-Gelais, pour m’avoir transmis son esprit critique, sa passion pour la recherche, ses excellents conseils et pour son dévouement pour mon projet de doctorat. Ses encadrements académiques et professionnels ont été bénéfiques pour la réalisation de ce projet de recherche.
Je remercie l’ensemble de l’équipe du Dr. Daniel St-Gelais, soit Annie Caron, et Gaetan Bélanger pour m’avoir permis d’améliorer mon esprit d’équipe, pour leur aide durant certaines expériences au laboratoire, leur aide technique et leur belle personnalité qui m’ont permis de passer à travers mes objectifs de mon projet.
Un merci très spécial à ma famille qui m’a soutenue dès mon départ d'Egypte. Je voudrais particulièrement remercier mes très chers parents de m’avoir toujours encouragée dans tous mes projets. Je vous aime énormément !!
XIX
Avant-propos
Cette thèse de doctorat comporte une introduction générale suivie de cinq chapitres et d'une conclusion générale.
L’introduction générale présente la problématique, l’hypothèse de recherche, l’objectif général ainsi que les objectifs spécifiques de cette étude.
Le chapitre 1 correspond à une revue de littérature récente qui met en évidence les connaissances actuelles sur le potentiel d’utilisation des bactériocines comme alternative aux additifs chimiques pour la conservation des aliments.
Le chapitre 2 intitulé « Interactions and biocompatibility between nisin-producing Lactococcus lactis strains and lactic acid starter under Cheddar cheese manufacturing conditions » a été réalisé dans le laboratoire du Dr Ismail Fliss et à l’institut sur la nutrition et les aliments fonctionnels (INAF) affilié à l’université Laval.
Le chapitre 3 intitulé « Novel design for alginate/resistant starch microcapsules controlling nisin release » a été réalisé dans le laboratoire du Dr Ismail Fliss. L’ensemble des expériences prévues dans ce cahpitre, le traitement des résultats ainsi que la rédaction de l’article scientifique en lien avec ces travaux ont été réalisés par moi-même. Ce premier article dont je suis le premier auteur a été publié en Octobre 2019 dans International Journal of Biological Macromolecules.
Le chapitre 4 intitulé « Impact of nisin and nisin-producing L. lactis ssp. lactis on Clostridium tyrobutyricum and microbial ecosystem of cheese matrices» a été réalisé à l'usine pilote du centre de recherche et de développement d'Agriculture et Agroalimentaire, Saint-Hyacinthe Canada. L’ensemble des expériences ont été réalisées par moi-même sous la supervision du Dr Ismail Fliss et Dr Daniel st-Gelais. J’ai également effectué l’analyse des résultats ainsi que la plus grande partie de la rédaction. Une partie de l’analyse métagénomique a été réalisée à l'Institut de biologie intégrative et des systèmes (IBIS) de l'Université Laval à l’aide de la technologie Illumina MiSeq.
Le chapitre 5 intitulé « Nisin and nisin-producing L. lactis ssp. lactis: a promising natural approach for the control of Clostridium tyrobutyricum in Cheddar cheese» a été réalisé à l'usine pilote du centre de recherche et de développement d'Agriculture et Agroalimentaire, Saint-Hyacinthe, Canada. L’ensemble des expériences ont été réalisées par moi-même sous la supervision du Dr Daniel
st-XX
Gelais, du Dr Ismail Fliss, et du Dr Ehab Kheadr. J’ai également effectué l’analyse des résultats ainsi que la plus grande partie de la rédaction. Une partie de l’analyse métagénomique incluant le séquençage du gène bactérien ARNr 16S a été réalisée à l'Institut de biologie intégrative et des systèmes (IBIS) de l'Université Laval à l’aide de la technologie Illumina MiSeq.
La dernière section de cette thèse correspond à une conclusion générale résumant les principales réalisations scientifiques de cette thèse ainsi que les perspectives et les travaux de recherche futurs proposés.
1
Introduction générale
La sécurité alimentaire est un des plus grands défis auquel l’humanité devra faire face au cours des prochaines années. Ce défi est d’autant plus important que la population mondiale devrait atteindre 9.2 milliards de personnes en 2050. Selon le FAO, pour atteindre ces objectifs, la production alimentaire devra augmenter d'environ 70% (Gustafsson, Cederberg et al. 2013). Cet objectif peut être atteint grâce au développement de techniques agricoles intensives, massives et industrielles, en adoptant également des méthodes de production plus efficaces et durables (Gustafsson, Cederberg et al. 2013).
La surproduction d'aliments représente des défis supplémentaires en termes de qualité et de sécurité pour l'industrie alimentaire. Tout au long de la chaîne alimentaire, les aliments sont exposés à divers facteurs qui peuvent influencer de manière significative leur qualité nutritionnelle et leur innocuité. On estime qu'un tiers de tous les produits alimentaires destinés à la consommation humaine dans le monde sont perdus. Le déficit économique qui en résulte est considérable (Organization and FAO. 2003). La situation n’est pas différente pour le secteur laitier. La forte incidence d'agents pathogènes et d'altération représente un problème majeur pour ce secteur très vulnérable.
Dans les produits laitiers, en particulier les fromages, la croissance des clostridies pendant la maturation du fromage provoque le gonflement tardif dû à la fermentation de l'acide lactique et à la production d'acide butyrique, de dioxyde de carbone et d'hydrogène (Collins, Lawson et al. 1994; Brown 2000). Un gonflement tardif entraîne une grave perte économique en raison de la déformation défavorable de la saveur et de la texture du fromage, des fissures, des yeux irréguliers et / ou des fentes qui conduisent à un déclassement du fromage (Gómez-Torres, Garde et al. 2015). Il est donc urgent de développer de nouvelles stratégies et de nouveaux composés antimicrobiens pour garantir non seulement la sécurité des produits, mais aussi pour prolonger leur durée de vie (Pichler, Appl et al. 2011).
Au cours des dernières années, un grand intérêt s'est développé autour de l'utilisation des bactéries lactiques (BL) comme cultures protectrices pour préserver la sécurité sanitaire des aliments ou prolonger leur durée de conservation. L'activité bioprotectrice des BL est liée à leur capacité à sécréter différentes substances ayant des activités biologiques à de très faibles concentrations, notamment les acides organiques (acide acétique, lactique et propionique), du peroxyde d'hydrogène, du diacétyle,
2
de l'acétaldéhyde et des peptides antimicrobiens tels que les bactériocines. Certaines souches de bactéries lactiques se sont également révélées capables de produire des substances antifongiques telles que les acides phényllactique et 4-hydroxyphényllactique et plusieurs acides organiques agissant en synergie, notamment les acides acétique, formique, caproïque, propionique, butyrique et valérique (Galvez, Burgos et al. 2014).
Bien que l'activité inhibitrice des BL soit relativement bien documentée dans la littérature, très peu de produits sont autorisés par les organismes de réglementation et sont disponibles dans le commerce pour une utilisation alimentaire. Cette situation peut s'expliquer par le manque de données scientifiques sur la nature et les activités biologiques des composés à activité antimicrobienne produits par des BL. En effet, à part les bactériocines, très peu de molécules antimicrobiennes ont été identifiées et caractérisées. Le spectre d'action de ces composés, les processus pour les produire avec des rendements élevés, leur comportement dans différentes matrices alimentaires complexes telles que les yaourts et les fromages, leur stabilité, leur activité biologique dans des conditions extrêmes de pH, de sels et leur impact sur les caractéristiques intrinsèques des produits sont encore très peu étudiés. Il est évident que des recherches supplémentaires couvrant à la fois les aspects fondamentaux et appliqués demeurent nécessaires et devaient générer des données scientifiques crédibles nécessaires à une utilisation plus efficace et ciblée de ces cultures et de leurs molécules bioactives.
L’objectif général de ce travail de recherche est d’évaluer le potentiel de cultures lactiques bio-protectrices de même que leurs métabolites à activité antimicrobienne (bactériocines) pour le contrôle de Clostridium tyrobutyricum dans du fromage de type Cheddar. Ce travail vise également a étudier l’impact des bactériocines sur la composition et l’évolution de l’écosystème microbien du fromage ainsi que sur les caractéristiques organoleptiques et sensorielles.
3
4
1.1. Production of Cheddar cheese in Canada
The dairy products in Canada represent about 12.6 % of total food products, which is the second sector after meat products (Figure 1). In cheese sector the annual production in 2017 was about 500 kilotons. The Cheddar cheese production during April 2020 was 14.835 kilotons which represent approximately 25% of total cheese production in Canada. Quebec produces approximately the half amount of cheese in Canada (Statistics Canada, 2020).
Figure. 1. The production and shipment of different sectors of food products in Canada, 2019. (Statistics Canada, 2020).
5
1.2. Cheddar cheese processing
The traditional method for Cheddar cheese making consists of pre-treatment of milk, coagulation, cutting, cooking, cheddaring and ripening (Figure 2). Where, the pre-treatment of milk includes pasteurisation and chemical composition standardization. The second step is coagulation of starter acidified milk using rennet, under two major steps. The first step includes the enzymatic hydrolysis of k-casein by rennet, then the second step, the coagulation of casein by calcium ions at temperature above 20 °C. The third step is cutting the coagulum to small cubes for giving more syneresis during cooking. The main purpose of cooking is to reduce the moisture during the acid production by starter culture. After cooking and draining (whey removal), the curd is kept for enough time to develop a proper acidity. The cheddaring is the step which the curd is flipped, and kept to develop acidity followed by salting, then pressing and packaging (Ong, Lawrence et al. 2017).
1.3. Cheddar cheese quality
Several parameters during cheese production contribute to cheese quality, such as the rate of acid production by starter culture, the microbial and chemical composition of milk (fat and protein ratio), the particle size of curd during cooking and cooking temperature.
Milk composition
There is a positive correlation between the protein concentration of milk and the coagulation time, and inverse correlation with the fat content. Thereby, it is important to standardize the milk fat/ protein ratio before cheese production to 0.69 and 0.71 (Ong, Lawrence et al. 2017).
Starter culture
The starter culture used for Cheddar cheese production is usually composed of a mixture of Lactococcus lactis ssp. lactis and Lactococcus lactis ssp. cremoris. For commercial Cheddar production, the optimum starter should provide a continuous acid production throughout cheese processing, to be resistance to phage, minimum flavor defects, such as bitterness and contribute to flavor development during ripening (Bennett and Johnston 2004; Lawrence, Gilles et al. 2004). Thereby most of the Cheddar are produced with a mixture of two or three strains to get all these characteristics. As known, in Cheddar cheese processing each step is dependent on the acid level produced by starter. The rate of acid production during cheese making, especially before the whey removal from the curd,
6
is essential to obtain the proper chemical composition and texture of the cheese (Bennett and Johnston 2004).
Figure.2. A typical manufacturing process for the production of Cheddar cheese (Ong, Lawrence et al. 2017)
7
After cooking and whey drainage, the moisture content is reduced which affects the starter bacteria, thereby, it is important to achieve the desired acidity in the curd at the same time of the required moisture content (Crow, Coolbear et al. 1995). Also, starter culture contributes to development of cheese flavor during the first stage of cheese maturation. The level of starter culture in freshly prepared Cheddar cheese is about 108 cfu /ml. During ripening the number of starter cells and NSLAB reduce, leading to the release of intracellular enzymes into cheese after cell lysis that are responsible for casein hydrolysis, flavor and aroma development (Crow, Coolbear et al. 1995).
1.4. Cheese microbial ecosystem
Cheese is a complex ecosystem which is composed of a wide variety of microorganisms which live together in a nutritionally limited resources environment. Each one has different roles in fermentation (Peláez and Requena 2005) leading to differences in cheeses sensory characteristics (Grappin and Beuvier 1997)
It was observed that the cheese produced under aseptic conditions developed hardly flavor (Visser 1977). Recently, it was known that the non-aseptic cheese develops more flavor which illustrates the role of microbiota (NSLAB) and its interaction in flavor development. Thereby, to manage and control the flavor in a right direction or to develop new flavor, it is necessary to have reliable data on microbial diversity and their dynamics (Fleet 1999). The culture-independent methods, direct analysis of DNA extract, are used to study the microbial diversity in cheese during maturation. Such methods provide full information about the microbial distribution, ecology and biodiversity compare to classical enumeration methods using differential culture media (Garland, Cook et al. 2001; Kelly and Ward 2002).
The progression of microbial communities in cheese is affected by ripening conditions and the type of microbial interaction, which can be antagonistic, neutral or beneficial like the ecosystem present in mould surface cheese or in blue cheeses (van den Tempel and Nielsen 2000; Addis, Fleet et al. 2001). In addition, the proteolytic activity of starter culture in Cheddar cheese is to enhance the growth of non-starter lactic acid bacteria (NSLAB) like the genus Pediococcus and Lactobacillus (Martley and Crow 1993) and Propionibacterium in Swiss cheese (Piveteau, O'Callaghan et al. 2002).
The antagonistic interaction is the most known effect in cheese ecosystem. For example, the inhibitory effect of NSLAB on propionibacteria which control the presence of split defect in Swiss cheese (Jimeno,
8
Lazaro et al. 1995). Another example is bacteriocins which are produced naturally by lactic acid bacteria used as starter culture or as an adjunct culture.
Bacteriocins are used as bioprotective agents to control the growth of cheese spoilage microorganisms to obtain a safe product (Rodrı́guez, González et al. 2000). Using bacteriocins producing strains could significantly reduce the number of NSLAB during cheese ripening. Therefore, the bacteriocin-resistant strain is used as an adjunct culture during cheese production with the bacteriocin-producing strain to avoid this problem (Ryan, Ross et al. 2001). In addition, bacteriocins could be involved in the acceleration of cheese ripening and develop cheese flavor through the induction of cell lysis which release the endogenous enzymes of dead cells (Bierbaum and Sahl 1987).
1.5. Cheese defects
The cheese defect could happen through contamination of milk with spoilage microorganisms, miss controlling of acidification during processing, failure to obtain the optimum cheese composition, and/ or physical damage during storage, or un-well packaging allowing contamination by undesirable molds. In addition, the color defect and surface deposits due to the activity of some NSLAB.
Bitterness
Several factors involved in cheese bitterness including the high retention of chymosin due to cutting the curd at pH lower than optimum or enhance the chymosin activity at low salt concentration of final cheese. In addition, the proteolytic activity of some LAB could have a significant effect in increasing the bitterness, such as the presence of L. casei as adjunct culture in Cheddar cheese (Laridi, Kheadr et al. 2003). Also, using unproper lactococcal starter culture which have high proteinase activity. Thereby, in New Zealand the starter culture is a mixture of lactococcal strains including proteinase- negative one (Habibi‐Najafi, Lee et al. 1996).
Gas production
During Cheddar cheese maturation and due to the starter culture metabolism, a low oxygen environment is generated (Lawrence, Gilles et al. 2004). Such conditions allow enzyme activities, chemical reaction and microbial metabolism to generate the typical Cheddar cheese texture and flavor. On the other hand, other food spoilage bacteria could activate and produce gas such as Clostridium tyrobutyricum due to the fermentation of lactic acid which cause late blowing defect (Collins, Lawson et al. 1994).
9
1.6. Cheeses spoilage microorganism
In dairy products (Table 1) the development of soilage organisms such as Psychrotrophic bacteria is enhanced due to the high moisture content (50- 80%) and pH value (5.0) like soft cheeses (Cottage and Brie cheeses) (Farkye and Vedamuthu 2002). The Pseudomonas, Alcaligenes, Achromobacter or Flavobacterium are examples of gram-negative spoilage bacteria, which cause off-flavour and unpleasant odors due do the release of lipolytic and proteolytic enzymes (Farkye and Vedamuthu 2002). For semi-hard and hard cheese, like Gouda, the coliforms, Clostridium spp, Bacillus spp. and molds are the most spoilage microorganisms due to the low moisture content compared to soft cheeses (Farkye and Vedamuthu 2002). This review is focusing on spores forming bacteria especially Clostridium spp.
1.6.1. The genus Clostridium
Clostridium is a group of gram-positive, rod-shaped, and obligatory anaerobic genus, which form endospores. Clostridium spores are distributed in many environments like soil, water, plants and even human and animal intestinal tract. Several species are commonly found in a wide range of food such as, dairy products, canned or fresh fruits and vegetable, which cause food spoilage and/ or food poisoning (Gómez-Torres, Garde et al. 2015).
In dairy products, especially semi-hard and hard cheese, the outgrowth of clostridia during cheese storage causes the late blowing due to the fermentation of lactic acid and production of butyric acid, carbon dioxide, and hydrogen (Collins, Lawson et al. 1994; Brown 2000). Late blowing lead to a serious economic loss due to the unfavorable flavor and texture deformation of cheese, like the presence of cheese loaf, cracks, irregular eyes and/ or slits which lead to a downgrading of the cheese (Figure 3) (Gómez-Torres, Garde et al. 2015; Bermúdez, González et al. 2016).
Clostridium tyrobutyricum is the most frequent specie responsible for late blowing defect, in addition to other genera are regarded as late-blowing enhancers particularly C. sporogenes, C. beijerinckii, C. butyricum, C. bifermentans, C. perfringens, and C. tertium (Garde, Arias et al. 2011; Cremonesi, Vanoni et al. 2012). The most common way to contaminate the raw milk is through the contaminated silage Figure. 3. Late blowing defect in aged
10
(Figure 4) or other clostridia contaminated feeds (Mathot, Beliard et al. 2003; Gómez-Torres, Ávila et al. 2014). After feeding the spores became concentrated in the gastrointestinal tract and could cross-contaminate the raw milk via the contamination of cow teats during milking process, mainly under improper hygienic condition (Vissers, Driehuis et al. 2007; Vissers, Driehuis et al. 2007; Vissers, Te Giffel et al. 2007).
11
Table. 1. Dairy products and typical types of spoilage microorganisms or microbial activity
Products Organisms Defects References
Raw milk, cottage cheese, buttermilk and sour cream
Pseudomonas species,
Bacillus, Micrococcus, Aerococcus, and Lactococcus
and of the family
Enterobacteriaceae
Reduce the diacetyl content, thereby leading to a “green” or yogurt-like flavor from an imbalance of the diacetyl to acetaldehyde ratio.
(Wang and Frank 1981)
(Cousin 1982)
Buttermilk, sour cream and soft,
mold-ripened cheeses Coliforms
-Reduce the diacetyl content, thereby leading to a “green” or yogurt-like flavor from an imbalance of the diacetyl to acetaldehyde ratio.
- Production of gas
(Wang and Frank 1981)
Butter milk and sour cream
Ripened cheeses
Encapsulated, slime producing Lactococci
Streptococcus thermophilus,
Lactobacillus helveticus and
Leuconostoc
-Excessive viscosity
-Reduce the diacetyl content, thereby leading to a “green” or yogurt-like flavor from an imbalance of the diacetyl to acetaldehyde ratio at 7 ◦C.
-Develop off-flavors and gas which may cause cracks in ripened cheeses at 8 ◦C.
-Metabolism of tyrosine by certain lactobacilli causes a pink to brown discoloration in ripened cheeses (HOGARTY and Frank 1982) (Hutkins 2001) (Shannon, Olson et al. 1977) (Zoon and Allersma 1996)
Buttermilk and sour cream Cottage cheese Geotrichum candidum Candida spp. Kluyveromyces marxianus, Debaryomyces hansenii, and Pichia spp.
- Cause off-flavors (egg odor) -Reduce the diacetyl content, thereby leading to a “green” or yogurt-like flavor from an imbalance of the diacetyl to acetaldehyde ratio
(Wang and Frank 1981)
(Marth and Steele 2001)
Yogurt and fermented milks
Saccharomyces cerevisiae
and Hansenula anomala. -Cause yeasty, fermented off-flavors and gassy appearance
(Giudici 2003)
Cream cheeses Penicillium spp., Cladosporium spp., and Paecilomyces variotii
-Cause off-odor and “kerosene” flavor in vacuum-packaged cheeses
(Hocking and Faedo 1992)
-Raw milk and contaminate milk after processing,
concentrated milks -Aseptically packaged pasteurized milk -Swiss, Emmental, Gouda, and Edam cheeses
- Bacillus licheniformis, B. cereus, B. subtilis, B. mycoides,and B. megaterium, clostidium spp.
- B. circulans and Bacillus stearothermophilus
- Clostridium tyrobutyricum, or C. sporogenes
-Coagulation of the casein of milk -Produces acid but no gas causing the “flat sour” defect in canned milk products
-Production of gas and butyric acid
(Meer, Baker et al. 1991) (Choudhery and Mikolajcik 1970) (Kalogridou-Vassiliadou 1992) (Klijn, Nieuwenhof et al. 1995)
Swiss cheese Enterococcus faecalis ssp. liquefaciens
-Cause white-spot defect and inhibit Propionibacterium and Lactobacillus
fermentum, resulting in poor eye
development and lack of flavor in the cheese
(Nath and Kostak 1986)
UHT milk, butter, some cheeses, and dry whole milk
Pseudomonas fluorescens
and Gram-negative psychrotrophic bacteria.
Pseudomonas fragi
-Produce lipases which cause rancid flavors due to the release of short-chain fatty acids. Also, cause a soapy flavor through long-chain fatty acids. -Cause fruity off-flavor results from lipolysis of short-chain fatty acids
(Adams and Brawley 1981) (Reddy, Bills et al. 1968)
12
1.7. Traditional methods for milk and cheese preservation
In general, foods preservation is still a debated issue not only in low/middle income countries but also in industrialized ones. The major challenges for current food industries are reduction of economic losses due to food spoilage, lowering the food production costs and avoiding the cross contamination through food chain furthermore facing the consumers demands for fresh-tasting, minimally processed and preserved, ready to eat, nutrient and vitamin rich foods (Gálvez, Abriouel et al. 2007).
Conventional heat treatments in the dairy industry (e.g., pasteurization, boiling and sterilization) are
usually used to render cheese milk safe and to allow it to be readily stored and transported. However, these treatments can adversely destroy the texture, flavor, aroma, appearance and sometimes the nutritional quality of milk (Henry 1997).
On the other hand, non-thermal food processing can keep the food safe and maintain its natural characteristics. These methods include high hydrostatic pressure, pulsed electric field, ultrasonic, pulsed high-intensity light, and cool plasma. However, some of these techniques are batch processes and high costly (Henry 1997).
Spores forming bacteria could survive even after heat treatment. Thus, the control of spores’
contamination at dairy farm could be achieved by enhancing cow hygiene, farm environment, in addition to good milking procedure (André, Vallaeys et al. 2017). While, at the dairy factory there are two different strategies, the first is using physical methods to remove the spores from milk, such as bacto-fugation or microfiltration (0.8–1.8 μm) (Tamime, Robinson et al. 2006; Ávila, Gómez-Torres et al. 2014).
Such methods need a specific equipment and large expenses which increase the final cost of product. Also, it could modify milk composition, in addition these methods maybe not enough to avoid the late blowing defect in cheese. Moreover, the microfiltration is suitable to use for skim milk due to the large size of fat globules that’s could block the membrane.
The second strategy is chemical methods using inhibitory substances to inhibit the growth of spores in milk, such as nitrate or lysozyme (Stadhouders 1990; Tamime, Robinson et al. 2006; Ávila, Gómez-Torres et al. 2014). These methods are preferred for large scale cheese production as they are less cost procedure, easy to use without specific equipment. However, there is a carcinogenic risk due to the formation of nitrosamines in the presence of salt or the risk of allergy from hen-egg white lysozyme (Lodi and Stadhouders 1990; Van den Berg, Meijer et al. 2004). Thus, new preservation methods are needed.
13
1.8. Bio-preservation
Bio-preservation or bio-control refers to the use of natural or controlled microbiota, or its antimicrobial products against food spoilage or pathogenic microbes to extend the shelf life and enhance the safety of foods (Stiles 1996). The use of microorganisms or their antimicrobial metabolites for the preservation of foods has been a common practice in the human history (Ross, Morgan et al. 2002).
According to Davidson et al. (2013), the ideal antimicrobial should be effective at low concentrations in its natural form, be economical, cause no sensory changes to the product, also inhibit a wide range of pathogenic and spoilage microorganisms, and non-toxic (Davidson, Critzer et al. 2013).
1.8.1. Protective cultures
Microbes usually live in complex ecosystems where they must interact with the surrounding components of the environment. They compete for space and nutrients or modify the environment conditions by the release of antimicrobial substances as by-products of their normal metabolic activity that inhibit growth or even kill competitors (Gálvez, López et al. 2014).
LAB during fermentation produce different antimicrobial compounds such as organic acids, hydrogen peroxide, diacetyl, bacteriocins or antifungal compounds (Gálvez, López et al. 2014).
Several studies described the significant role of LAB in food bio-preservation such as the bactericidal activity of L. lactis and L casei isolated from Uruguayan artisan cheese against Escherichia coli or Streptococcus bovis (Fraga Cotelo, Perelmuter Schein et al. 2013). In addition, the activity of some LAB isolated from goat milk and artisanal cheese against Listeria monocytogenes ATCC 7644 (Cavicchioli, dos Santos Dornellas et al. 2015). Furthermore, the anti clostridial activity of Lactobacillus rhamnosus LC-705, DSM 7061 in milk media after 48 h of incubation (Mayra-Makinen and Suomalainen 1999).
14
1.8.2. Antimicrobials substances produced by protective cultures
1.8.2.1. Bacteriocins
Bacteriocins are defined as ribosomally synthesized antimicrobial peptides or complex proteins, that can be post-translationally modified or not, which could be produced by many different bacterial species, including numerous members of LAB (Jack, Tagg et al. 1995). Bacteriocins can inhibit microorganisms that are closely related to the producer strain, with the exception of nisin which is inhibitory to a wide range of foodborne pathogens and many other Gram-positive spoilage microorganisms (Tagg, Dajani et al. 1976; Klaenhammer 1993).
Bacteriocins produced from LAB offer several properties which make them suitable for food preservation where they are generally recognized as safe ingredients, have a broad antimicrobial spectrum of food spoilage and pathogenic microorganisms, also they are usually pH and heat-stable, non-toxic for eukaryotic cells, become inactivated by proteases enzyme in digestive tract, so having little influence on the gut microbiota. Moreover, they show a bactericidal mode of action, by acting on the bacterial cytoplasmic membrane which permit no cross-resistance with antibiotics. Finally, they are plasmid-encoded genes which facilities genetic manipulations (Gálvez, Abriouel et al. 2007).
Several studies illustrates that, the application of bacteriocins in food preservation have a several benefits (Naidu 2000), they extended shelf life of foods, provide extra protection during temperature abuse conditions, decrease the risk for transmission of foodborne pathogens through the food chain production.
Furthermore, bacteriocins decrease the economic losses due to food spoilage and reduce the application of chemical preservatives. Which permit the application of less severe heat treatments without compromising food safety leading to better preservation of food nutrients and vitamins, as well as organoleptic properties of foods. Finally bacteriocins permit the marketing of “novel” foods (less acidic, with a lower salt content, and a higher water content), and they may serve to satisfy industrial and consumers demands (Naidu 2000).
Bacteriocins, according to the classification procedure proposed by Klaenhammer ,1993. are divided into four classes according to size, structure and modifications. The majority of those classes are produced by bacteria associated with food belonging to class I or II.
Class I bacteriocins, known as lantibiotics, are small peptides containing the unusual amino acids
15
of the peptides after the protein has been translated through the endoplasmic reticulum (Stoyanova, Ustyugova et al. 2012).
Mode of action: This antimicrobial peptide has a dual mode of action (1) it can form a complex with
the bacterial cell wall precursor lipid II, then inhibit cell wall biosynthesis, after which the complex aggregates, incorporating further peptides, and forms an actual pore through the bacterial membrane, or (2) it can inhibit the synthesis of peptidoglycan (Bierbaum and Sahl 2009) for example; nisin (Mulders, Boerrigter et al. 1991).
Class II (non lantibiotic bacteriocins): It is including small (4–6 kDa), heat-stable peptides with no
modified amino acids (Galvez, Burgos et al. 2014).
Mode of action: (1) Permeabilize the cell membrane of sensitive cells, and research has indicated that
the target of these molecules is a mannose permease protein on the target cell surface (Drider, Fimland et al. 2006), or (2) Create cell membrane channels through a barrel stave mechanism, thus initiating an ionic imbalance in the target cell (Balla, Dicks et al. 2000).
Subclass IIa pediocin-like bacteriocins is the most common subclass are thermostable, consisting
of from 37-48 amino acids and possessing high activity against L. monocytogenes (Drider, Fimland et al. 2006).
Subclass IIb comprises two-peptide bacteriocins and includes α enterocins and lactococcin G
peptides. They inhibit the growth of Enterococcus spp. and a few other Gram-positive bacteria, through the second action (Balla, Dicks et al. 2000).
Subclass IIc: contains circular peptides in which the N- and C-termini are covalently linked, and the
circular molecule is resistant to several proteases and peptidases. These bacteriocins have various modes of action including membrane permeabilization, specific inhibition of septum formation, and pheromone activity (Héchard and Sahl 2002; Kawai, Kemperman et al. 2004).
Class III bacteriocins: consisting of large (greater than 30 kDa) heat-labile proteins, including
helveticin J produced by Lactobacillus helveticus and enterolysin produced by E. faecalis (Joerger and Klaenhammer 1986; Nilsen, Nes et al. 2003).
Class IV bacteriocins: consisting of complex bacteriocins, which require carbohydrate or lipid moieties
16
Nisin
Nisin is lantibiotics (class I) bacteriocins, composed from 34 amino acids (Figure 5), produced by Lactococcus lactis ssp. lactis, was first introduced as a food preservative in 1953 as Nisaplin® (Danisco) and has the EU food additive number E234 (Simha, Sood et al. 2012). Nisaplin® is based on nisin-A which differ from nisin Z in the substitution of His27 for Asn27 (Mulders, Boerrigter et al. 1991). Nisin has an unusually broad spectrum of activity against Gram-positive bacteria. The effect can be either bactericidal or bacteriostatic depending on the physiological status of the target bacteria or the nisin concentration (Mills, Stanton et al. 2011).
However, nisin easily interacts with food ingredients like proteins, lipids and carbohydrates, which lead to a rapid inactivation. In addition, it is affected by the presence of proteolytic enzymes, which reduce
its activity against food pathogenic or spoilage microorganisms (Chi-Zhang, Yam et al. 2004; de Arauz, Jozala et al. 2009). To overcome these limitations, encapsulation is a process that could be implemented in order to protect nisin from food matrix, provide and maintain the gradual release of nisin during food storage. Several matrices have been used for the encapsulation of nisin like liposomes (Laridi, Kheadr et al. 2003), alginate, chitosan or a mixture of polymers such as chitosan/ monolaurin (Lotfi, Tajik et al. 2018); amaranth / pullulan (Soto, Hernández-Iturriaga et al. 2019); zein capsules (Xiao, Davidson et al. 2011); nanoparticles using poly-g-glutamic acid (g-PGA) and poly-L-lysine (PLL) (Cui, Dai et al. 2018); chitosan-coated nisin-silica (Cui, Wu et al. 2016); polyethylene oxide nanofibers containing nisin-loaded poly-g-glutamic acid/chitosan (Cui, Wu et al. 2017) or alginate/ resistant starch (Hassan, Gomaa et al. 2019).
Figure. 5. Schematic of nisin A structure. In nisin Z, His- 27is replaced by Asn. Arrows indicate the amino acid replacements (updated from (Umu, Rudi et al. 2017).
17
Pediocin
Pediocins is non-lantibiotics (class II) sub class pediocin-like bacteriocins, mainly produced from Pediococcus acidilactici AcH, PA-1and JD and P. pentosaceus A, N5p, ST18, and PD1, and composed of 44 amino acids (Figure 6).
Alta™ 2341 is non-purified fermentate commercially available containing pediocins (Simha, Sood et al. 2012), PA1/AcH it is used in some countries to inhibit L. monocytogens, which causes spoilage of meat. Strains of B. cereus, L. monocytogenes, L. innocua, S. aureus, Lc. lactis, C. botulinum, C. tyrobutyricum and C. sporogenes have been reported to be sensitive in vitro (Settanni t al., 2008 & Anastasiadou et al., 2008). Table 4. showed pediocin application in different food products and their inhibition effect against food spoilage and foodborne bacteria.
Enterocin
Enterocin AS-48 is non-lantibiotics (class II) bacteriocins, produced by Enterococcus faecium and E. faecalis. It is composed of 47 amino acids. It is used for the preservation of cider, fruit and vegetable juice against ropy-forming Bacillus licheniformis.
Enterocin CCM4231 is used for the preservation of soya milk to inhibit the growth of Listeria monocytogenes and Staphylococcus aureus (Lauková and Czikková 1999). Furthermore, the enterocin produced from E. faecalis A-48-32 was used to inhibit the growth of Bacillus cereus and Staphylococcus aureus in skimmed milk and low-fat un-ripened soft cheese (Munoz, Maqueda et al. 2004; Muñoz, Ananou et al. 2007). Also, Enterocins A and B inhibit the Listeria monocytogenes growth in Munster and cottage cheeses (Liu, O’conner et al. 2008; Izquierdo, Marchioni et al. 2009).
18
Organic acids
Organic acids such as lactic, acetic and propionic acid are the end products of both homo and hetero LAB fermentations. Lactic acid is the main organic acid produced during fermentation (Galvez, Burgos et al. 2014). It has a broad spectrum activity and inhibits both Gram-positive and Gram-negative bacteria in addition to some yeasts and molds (Galvez, Burgos et al. 2014).
Organic acids decrease the pH of the surrounding environment making it unsuitable for the growth of many non-acidophilic pathogens and spoilage bacteria. Also, they have antibacterial activity through reducing the intracellular pH, which inhibit different metabolic functionsand active transport , and conflict with the maintenance of cell membrane potential (Ross, Morgan et al. 2002; Ricke 2003). For instance, the strains L. brevis, L. acidophilus, P. pentosaceus , L. paracasei, and L. lactis isolated from Baik-kimchi, producing very high levels of lactic acid dramatically decreased the viability of H.pylori ATCC 43504 (Lim 2014).
Also, the yoba mutandabota (dairy product consumed as a major source of proteins and micronutrients in Southern Africa) containing L. rhamnosus inhibit the Campylobacter jejuni, Listeria monocytogenes, Escherichia coli O157:H7, Bacillus cereus, Salmonella Enteritidis, Salmonella Paratyphi B and Salmonella Typhimurium strains (Mpofu, Linnemann et al. 2016).
Diacetyl
Some LAB produces diacetyl, as a by-product of the metabolic activity (Heita 2014). Hetero-fermentative LAB produces active acetaldehyde by decarboxylation of pyruvate. This product condenses with pyruvate, forming α-acetolactate which is then converted by α acetolactate synthesis to diacetyl (Heita 2014).
Therefore, diacetyl (2, 3-butanedione: C4 H6 O2) is an acetonic molecule produced by some strains of LAB such as Streptococcus, Leuconostoc, Lactobacillus, and Pediococcus genera through citrate fermentation (Heita 2014). Also, it is known for the buttery aroma that it imparts to fermented dairy products (Jay 1982). It also inhibits the growth of Gram-negative bacteria by inactivating arginine utilization through the reaction with the arginine-binding protein (Jay 1982).
The antimicrobial activity of diacetyl was reviewed by Jay. (1982) It has been concluded that Gram-positive bacteria were more tolerant to diacetyl than Gram-negative bacteria, yeasts and molds. Generally, diacetyl has a broad antimicrobial activity at a concentration between 200-1000 ppm (Jay 1982).
19
Also, diacetyl has a synergistic effect with other antimicrobial compounds to inhibit the growth of pathogens. The combination with reuterin has been suggested by Langa et al. (2014) to control pathogens in fermented dairy products. Whereby, the antimicrobial activity of reuterin at a concentration of 1 AU/ml was enhanced when diacetyl was added at concentration 50 mg/kg against Escherichia coli O157:H7, Salmonella enteritidis CECT 4300, and Listeria monocytogenes OHIO (Langa, Martín-Cabrejas et al. 2014).
Reuterin
Reuterin (3-hydroxypropionaldehyde (3-HPA) is a potent low molecular weight antimicrobial molecule produced mainly by Lactobacillus reuteri (Talarico, Casas et al. 1988) under anaerobic conditions through the metabolism of glycerol to 1,3-propanediol (Talarico and Dobrogosz 1989). It has the ability to inhibit DNA synthesis and can thus inhibit a wide range of microorganisms, including fungi, protozoa and bacteria including both Gram-positive and Gram-negative (Talarico and Dobrogosz 1989). Reuterin has bacteriostatic activity against Listeria monocytogenes and variable bactericidal activities against Staphylococcus aureus, E. coli O157:H7, Salmonella, Aeromonas hydrophila ssp. hydrophila,Yersinia enterocolitica , and Campylobacter jejuni (Arqués, Fernández et al. 2004), and has a synergistic effect with diacetyl (Langa, Martín-Cabrejas et al. 2014).
Reutericyclin
Reutericyclin is a negatively charged and highly hydrophobic tetramic acid produced by Lactobacillus reuteri when produced at a suitable concentration it influences competitor’s growth during sourdough fermentation. So, the attention turned toward the use of reutericyclin-producing strains in bio-preservation of foods (Gänzle, Höltzel et al. 2000; Gänzle 2004). Its activity has been attributed to act as a proton-ionophore, causing waste of the proton motive force (Gänzle 2004).
Reutericyclin is active against Gram-positive bacteria including Lactobacillus spp., Bacillus subtilis, Bacillus cereus, Enterococcus faecalis, Staphylococcus aureus and Listeria innocua. It inhibits the spore germination of Bacillus species, while the spores remained unaffected (Gänzle 2004). On the other hand, it is not active towards yeasts, fungi and Gram-negative bacteria because of their cell membrane structure. It is only active under conditions that disrupt the outer membrane, including truncated lipopolysaccharides (LPS), low pH and high salt concentrations (Gänzle 2004).