Caractérisation de l’implication du cortisol dans la
reprogrammation du comportement en réponse au stress
maternel prénatal chez l’omble de fontaine, Salvelinus
fontinalis
Mémoire
Sergio Cortez Ghio
Maîtrise en biologie
Maître ès sciences (M. Sc.)
RÉSUMÉ
L’environnement maternel peut influencer le développement. Chez les poissons, ce phénomène serait modulé via le dépôt dans les œufs de facteurs maternels, tel le cortisol, une hormone de réponse au stress. L’augmentation de cortisol dans le plasma maternel et dans les œufs a été associée avec des altérations comportementales chez les jeunes. Pour séparer les effets du cortisol de ceux des autres facteurs dans cette reprogrammation comportementale due au stress maternel, nous avons pharmacologiquement et physiquement manipulé des femelles omble de fontaine,
Salvelinus fontinalis, pendant l’oogénèse. Nous les avons soit laissées non dérangées (témoins), (1)
nourries de cortisol additionné à leur nourriture ou (2) manipulées hebdomadairement. Nous avons aussi (3) exposé des œufs des femelles témoins à du cortisol avant la fécondation. Nous avons mesuré les capacités cognitives et l’audace chez les juvéniles. Nous n’avons détecté aucun effet des traitements sur leur comportement.
ABSTRACT
The maternal environment can influence development. In fish, this phenomenon could be modulated by egg deposition of maternal factors, like cortisol, a stress-response hormone. Increased maternal plasma and egg cortisol levels have been associated with behavioral alterations in offsrping. To discriminate between the effects of cortisol and those from other factors in the behavioral reprogramming due to maternal stress, we have pharmacologically and physically manipulated female brook trout, Salvelinus fontinalis, during oogenesis. We either left them undisturbed (controls), (1) fed them cortisol through their food or (2) handled them once a week. We also (3) exposed eggs from the control females to cortisol before fertilization. We measured the juveniles’ cognitive abilities and boldness. We found no effects of treatments on behavior.
TABLE DES MATIÈRES
RÉSUMÉ ... III ABSTRACT ... IV TABLE DES MATIÈRES ... V TABLE DES FIGURES ... VI REMERCIEMENTS ... IX AVANT-PROPOS ... XI
INTRODUCTION GÉNÉRALE ... 1
CHAPITRE 1 : Is cortisol the sole mediator in maternal stress-induced behavioral reprogramming in brook trout, Salvelinus fontinalis? ... 8
Abstract ... 9 Résumé ... 10 Introduction ... 11 Methods ... 13 Results ... 20 Discussion ... 22 Acknowledgements ... 27 Figures ... 28 CONCLUSION ... 34 BIBLIOGRAPHIE ... 38 ANNEXES... 44
TABLE DES FIGURES
Figure 1. Experimental Design ... 28 Figure 2. Cross-sectional view of the maze system ... 29 Figure 3. Plasmatic cortisol levels (ng/ml) of Salvelinus fontinalis females at
stripping and unfertilized egg cortisol levels (ng/ml). ... 30 Figure 4. Latency (s) for Salvelinus fontinalis juveniles to exit the introductory
glass container in the maze system on the first day of training. ... 31 Figure 5. Time (s) for Salvelinus fontinalis juveniles to complete the maze
system over a 5-day training period. ... 32 Figure 6. Time (s) for Salvelinus fontinalis juveniles to complete the maze
Science rules!
REMERCIEMENTS
Je me souviens encore très clairement du jour où Nadia Aubin-Horth, ma directrice de recherche, m’a invité à entrer dans son bureau juste après avoir terminé la majorité des expériences en laboratoire de mon projet de premier cycle d’initiation à la recherche. Elle m’a demandé ce que je comptais faire après mon baccalauréat dans l’optique de m’offrir de faire ma maîtrise dans son laboratoire. La vérité c’est que je comptais la supplier de me donner cette opportunité la semaine suivante. Nadia a été mon mentor pendant presque quatre ans. Son support continuel, sa passion et sa rigueur ont été la catalyse de mon intérêt pour la recherche. Son encadrement et ses conseils m’ont permis de développer toutes les qualités nécessaires pour réussir et me démarquer en tant que scientifique. Non seulement m’a-t-elle encouragé à garder l’esprit ouvert et à m’intéresser à toutes les sphères de la biologie, mais elle était toujours disponible pour me guider dans mon cheminement ou me rassurer quant à la qualité du travail accompli. Nadia, encore une fois, je te suis extrêmement reconnaissant pour la confiance dont tu as fais preuve à mon égard et pour la qualité exceptionnelle de ma formation, à l’aube de ma carrière qui est «la meilleure carrière du monde».
Je tiens également à remercier particulièrement les membres des laboratoires de Nadia Aubin-Horth et de Céline Audet, ma co-directrice. Un merci spécial à Laurence Deneault Tremblay qui a été d’une aide indispensable dans le design de l’étude, les protocoles expérimentaux avec les femelles et une partie de l’échantillonnage et des analyses. Merci à Lucie Grecias, François-Olivier Hébert et Chloé Berger pour leurs commentaires sur mes présentations et sur l’article, ainsi que pour leur support moral tout au long des mes études de deuxième cycle.
Sur une note plus personnelle, je tiens à remercier mes parents, mon frère et ma soeur du fond du cœur pour leur soutien et leurs encouragements. Même s’ils comprenaient rarement ce dont je leur parlais, ils semblaient toujours intéressés par mes travaux et veillaient à ma santé pendant les périodes les plus rudes. Merci de m’avoir poussé à donner le meilleur de moi-même. Je vais avoir besoin de vous dans le futur. Un merci extraordinaire à ma conjointe qui m’a accompagné tout au long de ce périple académique : dans les longues nuits d’analyse et de rédaction comme dans l’écoute répétitive des mes présentations orales. Merci de m’avoir soutenu dans les bons moments et
Finalement, je tiens à remercier les organismes subventionnaires, sans qui ce projet n’aurait pas été réalisable. Merci au RAQ pour m’avoir octroyé une bourse d’étude FONCER-CRSNG et merci au FQRNT pour les fonds de recherche de ma directrice.
AVANT-PROPOS
Le présent mémoire est composé d’une introduction générale et d’une conclusion en français ainsi que d’un chapitre consistant en un article scientifique (manuscrit) inséré en anglais pour soumission au journal Hormones and Behavior. La section discussion de l’article est intentionnellement plus longue qu’elle le sera lors de la soumission au journal pour faciliter la lecture du mémoire et raccourcir la conclusion. Les auteurs de ce manuscrit sont Nadia Aubin-Horth, ma directrice de projet de maîtrise, Céline Audet, ma co-directrice, Antoine Boudreau-Leblanc, un étudiant de premier cycle qui a grandement collaboré à l’analyse des vidéos des tests de comportement dans le cadre de son projet d’initiation à la recherche, et moi-même. Le design de l’étude a été réalisé par Nadia Aubin-Horth, Céline Audet et moi-même. L’échantillonnage, l’analyse des données, l’écriture du manuscrit et les manipulations en laboratoire ont été principalement, mais non-exclusivement, ma responsabilité.
INTRODUCTION GÉNÉRALE
Influence de l’environnement maternel sur le phénotype de la progéniture
Nombre d’études ont décrit une vaste gamme de profondes et permanentes conséquences morphologiques, physiologiques et comportementales attribuables aux conditions environnementales expérimentées par les femelles pendant la période prénatale sur leurs jeunes (Räsänen et Kruuk, 2007). Telles que définies aujourd’hui, ces altérations du phénotype de la progéniture, qui sont la conséquence du phénotype de la mère, sont des effets maternels (Roff, 1998). Ces contributions non-génétiques maternelles au phénotype des rejetons sont ubiquitaires en nature (Mousseau et Fox, 1998). Le lézard de Tasmanie, Pseudemoia pagenstecheri, est un des meilleurs exemples de l’ampleur et de la variété des modifications phénotypiques issues de l’interaction de la mère avec son environnement. Effectivement, lorsque le temps qu’ont les femelles porteuses pour se réchauffer au soleil est réduit, les rejetons naissent plus tard et ont une queue réduite en longueur. Aussi, lorsque la quantité de proies est limitée pour la mère, les jeunes sont significativement plus petits à la naissance. En plus, lorsque les mères sont exposées à l’odeur du serpent prédateur, Drysdalia
coronoides, en période prénatale, les rejetons naissent plus lourds, ont une queue plus longue par
rapport à leur taille et sont deux fois plus sensibles à l’odeur de ces serpents (mesuré par le nombre de fois qu’ils agitent leur langue qui est munie de chémorécepteurs) que les rejetons de mères témoins (Shine et Downes, 1999). On observe également ce genre de phénomène chez les femelles d’une espèce de pucerons, Megoura viciae, qui donnent naissance à des jeunes au phénotype ailé lorsque la densité de conspécifiques est élevée sur la plante natale ou lorsque la nourriture qu’on y retrouve est de qualité moindre (Lees, 1967). Le cas de la mésange charbonnière, Parus major, est un autre bon exemple d’altération du phénotype des jeunes en réponse à l’environnement maternel. Lorsque les femelles sont exposées à l’ectoparasite Ceratophyllus gallinae en période prénatale, le comportement de dispersion des oisillons est affecté; la distance de dispersion par rapport au site de naissance est réduite (Tschirren et al., 2007).
Influence du stress maternel prénatal sur le phénotype de la progéniture
Les exemples énoncés précédemment ont en commun l’induction de stress par la modification de l’environnement des femelles, mettant de l’avant la possibilité que certains effets maternels observés chez les rejetons puissent être dus au stress maternel prénatal (McCormick, 1998). En effet, que ce soit par une diminution dans la quantité ou dans la qualité de l’alimentation, ou encore par un risque de prédation accru en passant par une densité trop élevée de conspécifiques ou même par la présence d’ectoparasites en abondance, les études décrites précédemment supportent fortement l’hypothèse que le stress maternel prénatal joue un rôle important dans le développement de la progéniture.
Le mécanisme proximal le plus probable et le plus étudié actuellement par lequel le stress maternel pourrait influencer le phénotype des rejetons est une forte concentration de glucocorticoïdes (GC, tels le cortisol ou la corticostérone selon l’espèce) dans le plasma maternel (Rhees et Fleming, 1981; Eriksen et al., 2011). Les GC sont des hormones stéroïdiennes sécrétées en réponse à des évènements stressants aigus ou chroniques pour aider les organismes à y faire face et à entraîner un retour à l’homéostasie (Wingfield et al., 1998). Les GC sont également impliqués dans la régulation du métabolisme énergétique et affectent la physiologie d’un organisme de plusieurs façons en se liant à leurs récepteurs exprimés dans divers organes aux fonctions variées tels que le foie, les gonades, le tissu adipeux, le cerveau, la rate et les reins (Becker, 2002; Norris et Carr, 2013). Leur concentration dans le sang est non seulement un excellent indicateur du niveau de stress d’un individu (Barton, 2000), mais se reflète également dans la quantité de GC transférée aux embryons ou aux œufs par les femelles qui les produisent (Hwang et al., 1992; Saino et al., 2005). Par exemple, chez les téléostéens, il a été démontré qu’une femelle stressée a plus de GC en circulation et que ceux-ci sont proportionnellement déposés dans les œufs ou transmis aux embryons (Leatherland et al., 2010), ce qui pourrait altérer le phénotype des rejetons.
Les conséquences d’une concentration élevée en GC dans le sang des femelles en période prénatale sur la morphologie, la physiologie et le comportement des rejetons ont d’ailleurs été démontrées chez diverses espèces vivipares par l’entremise de manipulations pharmacologiques. Chez le lézard commun, Lacerta vivipara, l’augmentation exogène du niveau de GC plasmatique
influence des traits physiologiques et comportementaux des rejetons. Les rejetons provenant d’une mère dont les niveaux de GC ont été augmentés par l’absorption cutanée d’une solution d’huile de sésame et de corticostérone (Meylan et al., 2003), GC principal chez les reptiles, sont moins actifs (Belliure et al., 2004), ont besoin de stimuli plus importants pour commencer à courir, courent moins vite que les rejetons témoins (Meylan et Clobert, 2004), tentent de fuir un environnement inconnu sauf quand l’odeur maternelle est présente et se regroupent davantage lorsqu’ils font face à un stress aigu (De Fraipont et al., 2000). Ce type de relation entre les niveaux de GC de la mère et le phénotype du rejeton a aussi été démontré chez des espèces ovipares. Par exemple, lorsque les femelles hirondelles rustiques, Hirundo rustica, sont stressées par une exposition à un prédateur carnivore pendant la période de ponte (dans ce cas-ci un modèle de chat), leur niveau sanguin de GC augmente et, conséquemment, elles pondent des œufs ayant une concentration en GC plus élevée que les œufs provenant de femelles ayant été exposées à un modèle de lapin. Dans une expérience subséquente, les auteurs ont injecté une solution de corticostérone (le GC en question) directement dans les œufs afin d’obtenir une concentration similaire à celle obtenue lorsque la femelle est naturellement stressée par ce prédateur. Non seulement les œufs survivent moins, mais les jeunes qui en sortent sont significativement plus petits et leur plumage se développe moins vite (Saino et al., 2005). La concentration sanguine maternelle prénatale en GC pourrait donc jouer un rôle important dans le développement de divers traits de la progéniture tant chez des espèces vivipares que chez des espèces ovipares.
Modèle pour l’étude du stress maternel prénatal
Les poissons sont des organismes très étudiés en termes de stress maternel. Ils produisent beaucoup de jeunes, ils affichent une grande variété de formes et de comportements, ils sont faciles à manipuler et leurs œufs aussi. Il est également possible de moduler les niveaux hormonaux du plasma et des œufs, ainsi que d’induire des stress relativement aisément et de façon non-invasive. Chez les poissons, le GC principal, soit le cortisol, est activement accumulé dans les œufs pendant l’oogenèse et il est présent en quantité importante dans les embryons et dans les larves (De Jesus et al., 1991; Auperin et Geslin, 2008; Simontacchi et al., 2009; Pavlidis et al., 2011). De plus, si la concentration de cortisol plasmatique augmente chez la mère en période prénatale, les œufs auront
conditions pour que le stress maternel prénatal puisse avoir un effet sur le phénotype des rejetons et les conditions pratiques pour pouvoir l’étudier sont donc présentes.
Plusieurs équipes de chercheurs se sont penchées, au courant des dernières années, sur la question de l’implication du cortisol dans la reprogrammation du phénotype en réponse au stress maternel prénatal en utilisant les poissons comme modèle. Par exemple, chez le saumon atlantique,
Salmo salar, un stress chronique maternel simulé par un implant de cortisol pendant une semaine
avant l’induction de la ponte cause d’importantes altérations comportementales chez les rejetons. Les jeunes provenant de mères implantées ont plus de difficulté à se nourrir convenablement (plus d’essais infructueux pour attraper les morceaux de nourriture qui coulent vers le fond), sont plus agressifs lorsqu’ils sont dominants et sont moins actifs lorsque confrontés à un stress aigu de confinement (Eriksen et al., 2011). Aussi, les rejetons de mères implantées présentent de nombreuses déficiences morphologiques par rapport aux individus témoins. Ils ont un sac vitellin plus petit et dont l’utilisation est plus lente, ils sont moins longs et moins lourds, ont une tendance accrue aux malformations et sont caractérisés par un taux de mortalité anormal (Eriksen et al., 2006). Chez la truite brune, Salmo trutta, lorsque les œufs baignent dans le fluide ovarien, il est possible d’y ajouter une suspension de cortisol afin d’observer les effets d’une absorption rapide de GC juste avant la fécondation sur le phénotype des rejetons. Les embryons ayant ainsi été exposés à du cortisol avant la fécondation ont non seulement une consommation d’oxygène plus élevée (aussi vrai chez les épinoches à trois épines, Gasterosteus aculeatus;(Giesing et al., 2011), mais excrètent également plus d’ammonium que les individus témoins; différences métaboliques témoignant de possibles modifications dans le développement embryonnaire (Sloman, 2010). De plus, les individus provenant d’œufs ayant baigné dans le cortisol sont significativement plus agressifs envers leur image dans un miroir (Sloman, 2010) ou moins agressifs face à des conspécifiques (Burton et al., 2011). Les rejetons prénatalement exposés à de fortes concentrations de cortisol semblent également être incapables de s’améliorer dans un test de labyrinthe. Tandis que les jeunes témoins prennent moins de temps pour trouver la nourriture cachée entre les essais, les jeunes qui ont baigné dans le cortisol au stade d’œuf prennent autant de temps pour la trouver d’un essai à l’autre (Sloman, 2010). Une autre étude sur les épinoches à trois épines a démontré récemment que les rejetons provenant de mères stressées par la présence d’un prédateur, Esox lucius, ont tendance à tourner le dos à un prédateur de la même espèce lorsqu’ils sont confinés ensemble et, par le fait
même, survivent moins longtemps que les individus provenant des mères témoins (McGhee et al., 2012).
Question
Malgré que plusieurs recherches aient été effectuées en ce qui a trait à la reprogrammation du phénotype en réponse au stress maternel prénatal et au rôle que joue le cortisol dans la reprogrammation du phénotype de la progéniture chez les poissons, il y a lieu de se demander si le cortisol est le seul médiateur des altérations phénotypiques observées chez les rejetons provenant de mères stressées. Afin de pouvoir répondre à cette question, il est essentiel d’isoler les effets directs du cortisol de ceux des autres facteurs maternels sur le phénotype de la progéniture. Pour ce faire, il faut induire du stress prénatal par différentes méthodes (mères stressées physiquement versus augmentation pharmacologique de la concentration de GC dans le sang de la mère ou dans les oocytes) et comparer les possibles modifications sur le phénotype des rejetons issus de ces traitements.
Objectifs et description du projet
Pour tenter de mieux caractériser le rôle du cortisol lorsque le stress maternel résulte en une modification permanente du phénotype de la progéniture, nous avons mené une étude systématique comportant trois méthodes d’élévation des niveaux de cortisol des œufs. Nous avons aussi couplé ces méthodes d’élévation de cortisol à une analyse des effets de ces manipulations sur le comportement des rejetons. Cette approche n’avait jamais été réalisé à ce jour, et ce, chez aucune espèce de vertébré ovipare. Nous avons choisi d’étudier l’omble de fontaine, Salvelinus fontinalis, de par sa facilité d’accès et sa grande importance économique et récréo-touristique au Québec (Ministère des ressources naturelles et de la faune du Québec, 2008).
Notre premier objectif était donc de faire augmenter et de mesurer la concentration de cortisol du plasma et des œufs de femelles ombles de fontaine en employant différentes méthodes décrites dans la littérature au courant de la même période de reproduction. Ces élévations devaient
compromettre la reproduction. Ainsi, pendant plusieurs semaines, nous avons physiquement stressé un premier groupe de femelles par de courtes manipulations hebdomadaires, nous avons enrichi la nourriture d’un second groupe de femelles avec du cortisol en vaporisant une suspension de l’hormone dans de l’éthanol sur leur moulée (ce qui est tout aussi efficace que des implants, mais moins invasif) et nous avons également fait baigner des œufs de femelles non-stressées dans une suspension d’eau saline et de cortisol avant la fécondation. Ces trois protocoles diffèrent de par le type de stress (chronique vs aigu), la vitesse d’absorption de l’hormone par les œufs, la quantité et le temps de circulation du cortisol plasmatique chez les femelles et l’action possible ou non d’autres médiateurs maternels synergiques (outre le cortisol) sur le contenu des œufs.
Notre second objectif était de détecter et de comparer des différences dans le phénotype comportemental des rejetons issus de ces traitements (traitements vs groupe témoin, et possiblement entre les traitements). Nous avons donc mesuré, chez des juvéniles de six mois issus de ces traitements, l’apprentissage et la mémoire spatiale, la témérité (la propension à prendre des risques) ainsi que la néophobie (la peur de la nouveauté). Différetes raisons sous-tendent les choix d’axer cette étude sur le comportement des rejetons et de mettre l’emphase sur le stade juvénile. L’omble de fontaine est une espèce d’aquaculture et a une grande valeur commerciale : la très grande majorité de la production (94%) est destinée à l’ensemencement dans nos plans d’eau pour mettre en valeur la pêche sportive. Ces individus sont généralement ensemencés vers l’âge de six mois (Dumont et Blanchet, 2007). Il est connu que les juvéniles ensemencés ont une moins bonne survie que les individus sauvages, et puisque seuls les poissons ayant survécu jusqu’au stade juvénile, n’ayant aucune malformation et étant d’une taille acceptable sont relâchés en milieu naturel, une divergence au niveau des traits comportementaux pourrait être une des causes les plus probables du faible taux de succès des poissons d’aquaculture en comparaison avec les poissons sauvages. Il a en effet été proposé que le comportement est directement relié à la survie en milieu naturel et que les deux principales sources de mortalité pour un poisson relâché en milieu naturel sont la famine et la prédation (Suboski et Templeton, 1989). En effet, lorsqu’un individu est relâché en milieu naturel, il est, par le fait même, soumis à un environnement non seulement nouveau, mais aussi très variable, contenant de nouvelles sources d’alimentation et dans lequel il existe un risque de prédation (Brown et Laland, 2001). Pour survivre, un individu doit donc pouvoir rapidement acquérir des capacités appropriées pour trouver sa nourriture et gérer les risques associés à
l’alimentation (MacCrimmon et Twongo, 1980) et reconnaître et réagir à ses prédateurs (Brown et al., 2013). La capacité à apprendre, la mémoire, la témérité et la néophobie sont donc d’importants facteurs qui contribuent à la survie d’un juvénile dans un milieu naturel nouveau, instable et possiblement dangereux, en plus d’être aisément quantifiables. Ces comportements sont donc particulièrement intéressants à étudier dans un contexte aquicole.
Hypothèse et prédiction
Notre hypothèse principale était que le cortisol n’est pas le seul médiateur de la reprogrammation du phénotype en réponse au stress maternel prénatal. Pour tester cette hypothèse, nous avons employé trois différentes méthodes d’élévation effective du cortisol dans les œufs de femelles ombles de fontaine, dans le but d’entraîner ou de simuler un stress maternel. Cet aspect novateur de notre projet devait nous permettre de séparer les effets du cortisol sur le comportement de la progéniture de ceux des autres facteurs maternels déposés dans les œufs tout au long de la période prénatale.
La prédiction principale qui découle de cette hypothèse est que si le cortisol n’est pas le seul facteur maternel impliqué dans la reprogrammation du comportement suite au stress maternel, alors il serait possible de mesurer des différences comportementales entre les juvéniles issus des traitements. En contre-partie, si le comportement des jeunes issus des traitements n’était pas significativement différent, alors il serait alors possible que le cortisol soit fortement impliqué ou le seul médiateur de l’altération du phénotype de la progéniture par le stress maternel, infirmant ainsi notre hypothèse.
Pour que cette prédiction soit valable, cependant, il fallait atteindre le premier objectif de cette étude qui était d’induire des différences de niveaux de cortisol des œufs entre les traitements et le groupe témoin, sans quoi une différence de comportement parmi les juvéniles issus des différents traitements (entre eux ou avec les témoins) ne voudrait rien dire.
CHAPITRE 1 : Is cortisol the sole mediator in
maternal stress-induced behavioral reprogramming
in brook trout, Salvelinus fontinalis?
For consideration in Hormones and Behavior
Sergio Cortez Ghio1*, Antoine Boudreau Leblanc1, Céline Audet2 and Nadia Aubin-Horth1
1Département de Biologie and Institut de Biologie Intégrative et des Systèmes, Université Laval,
Québec, Québec, G1V 0A6, Canada
1Institut des sciences de la mer de Rimouski, Université du Québec à Rimouski, Rimouski, Québec,
G5L 2Z9, Canada
*Author for correspondence. Email: sergio.cortez-ghio.1@ulaval.ca. Running title: Prenatal stress and behavioral reprogramming
Keywords: Salvelinus fontinalis, Maternal Stress, Development, Cortisol, Spatial Learning, Spatial Memory, Boldness, Neophobia
Abstract
The environment experienced by females during the prenatal period can have long-lasting effects on offspring phenotype. In fish, these maternal effects are believed to be mainly mediated by the deposition of proteins, RNAs, and hormones in the eggs by the female. One of these maternal factors is cortisol, a steroid hormone involved in the stress response, whose levels in the females’ blood stream are reflected in the eggs. In salmonids, high maternal plasma cortisol levels and elevated egg cortisol content have both been associated with behavioral alterations in offspring. It has thus been hypothesised that a mother’s stress during oogenesis could reprogram the behavior of its progeny through a change in egg-deposited cortisol levels. To understand how maternal stress influences offspring behavior, it is essential to separate the effects of maternally-deposited cortisol from those of other maternal factors. This can be done by using different parallel treatments to increase cortisol levels in female bloodstream or directly in the eggs and by comparing their offspring phenotypes. To achieve this, we used brook trout, Salvelinus fontinalis females; we either left them undisturbed as controls, (1) fed them cortisol through their food or (2) physically stressed them by handling once a week during the whole oogenesis period. Additionally, we (3) exposed half of the eggs from the control females to a cortisol suspension for 3 hours before fertilization. We then measured spatial learning and memory, boldness and neophobia in the 6 month-old resulting juveniles and found no effects of treatment on behavior although egg cortisol concentration was successfully increased in treatment 3.
Résumé
L’environnement maternel pendant la période prénatale peut avoir des effets permanents sur le phénotype de la progéniture. Chez les poissons, ces effets maternels seraient principalement modulés par le dépôt de protéines, d’ARNs et d’hormones dans les œufs des femelles. Un de ces facteurs maternels est le cortisol, une hormone stéroïdienne impliquée dans la réponse au stress et dont la concentration dans le plasma maternel est reflétée dans les œufs. Chez les salmonidés, des niveaux élevés de cortisol dans le plasma maternel et dans les œufs ont tous deux été associés avec des modifications comportementales chez la progéniture. Il a donc été mis de l’avant que le stress maternel pendant l’oogénèse pourrait reprogrammer le comportement des jeunes par l’entremise d’une élévation des niveaux de cortisol déposé dans les œufs. Pour comprendre comment le stress maternel influence le comportement de la progéniture, il est essentiel de séparer les effets du cortisol que les femelles déposent dans les œufs de ceux des autres facteurs maternels. Ceci peut être accompli en utilisant différents traitements en parallèle pour augmenter la concentration de cortisol dans le plasma des femelles ou directement dans les œufs et en comparant le phénotype des jeunes. Dans cette optique, nous avons utilisé des femelles omble de fontaine, Salvelinus fontinalis; nous les avons soit laissées non dérangées en tant que témoins, (1) nourries de cortisol additionné à leur nourriture ou (2) physiquement stressées en les manipulant une fois par semaine pendant toute la période d’oogénèse. Aussi, nous avons (3) exposé la moitié des œufs des femelles témoins à une suspension de cortisol pendant trois heures avant la fécondation. Nous avons ensuite mesuré l’apprentissage et la mémoire spatiale, la témérité et la néophobie chez des juvéniles de six mois issus de ces traitements. Nous n’avons trouvé aucun effet de traitement sur le comportement même suite à une élévation significative de la concentration de cortisol dans les œufs.
Introduction
The environment a female experiences during the prenatal period can have significant and long-lasting effects on the physiology, the morphology and even the behavior of its offspring (Räsänen and Kruuk, 2007). These non-genetic contributions of the maternal phenotype to progeny phenotype are termed maternal effects (Roff, 1998) and are ubiquitous in nature (Mousseau and Fox, 1998). They can cause drastic phenotype changes in offspring in response to maternal environmental pressures and could thus be stress-related (McCormick, 1998). Maternal stress impacts on offspring phenotype have been extensively reported in invertebrates, such as aphids, Megoura viciae who give birth to winged progeny when conspecifics density becomes too elevated on the natal vegetal substrate (Lees, 1967). Similar consequences of maternal stress have also been studied in vertebrates, such as pregnant lizards, Pseudemoia pagenstecheri whose reduced basking time leads to decreased tail length and retarded birth of their offspring (Shine and Downes, 1999). Also, in threespine stickleback,
Gasterosteus aculeatus, progeny of females that are predator-exposed are unable to appropriately
orient themselves towards predators (McGhee et al., 2012).
Elevated maternal plasmatic glucocorticoid (GC) concentration is the proposed main mechanism by which female stress could alter the phenotype of its progeny (Rhees and Fleming, 1981). GCs are steroid hormones involved in the vertebrate stress response (Munck et al., 1984). During oogenesis, GC plasma levels can be transferred in ovo in oviparous species (Hwang et al., 1992; Leatherland et al., 2010; Saino et al., 2005) in addition with a wide variety of other maternal factors such as RNAs, hormones and proteins (Lyman-Gingerich and Pelegri, 2007). Nevertheless, how such mother-offspring proximate communication might function is still poorly understood.
Fish are an excellent model to observe the effects of increased GC levels in ovo on offspring’s phenotype. Several fish species lay considerable amounts of eggs whose hormonal levels can be experimentally manipulated without having to control for the effects of direct molecular communication between the internal maternal milieu and the offspring, something that would be difficult to do in viviparous or ovoviviparous species. In fact, it has been shown in salmonids that pharmacologically elevated maternal plasma cortisol (the primary GC in fish) concentrations are
Salmo salar, are given intraperitoneal implants of cortisol during oogenesis, a procedure that induces
chronic release of the hormone into the blood stream over several weeks, their offspring display feeding capacity disorders, decreased activity when submitted to a confinement stress and increased aggressiveness when dominant (Eriksen et al., 2006; 2011). In brown trout, Salmo trutta, in ovo cortisol concentration manipulation similarly induces considerable behavior modifications and also affects cognitive performance. Juveniles that hatch from eggs that have been directly exposed to cortisol prior to fertilisation (but whose mothers were not stressed during oogenesis) are not only more aggressive towards their mirror image, but are also unable to improve at completing a maze system (Sloman, 2010).
Because previous maternal-stress related phenotype reprogramming studies across taxa mainly focused on comparing offspring phenotype after using only one treatment and one control group, we hypothesised it is possible cortisol might not be the sole factor implicated in this phenomenon, that it could act in synergy with other maternal factors. Our systematic approach and novel design were thus meant to allow separating the direct effects of plasma and egg cortisol from that of other maternal messengers. To effectively isolate the effects of cortisol from that of other maternal factors, multiple maternal stress induction methods were used in parallel in mature farmed brook trout females, Salvelinus fontinalis. Several ecologically relevant behaviors were then measured and compared in the resulting offspring. Three treatments and a control group were used: pharmacological manipulation of cortisol levels in the female bloodstream, physical stress, and pharmacological manipulation of cortisol levels in the eggs. In the first group of females, we aimed to increase plasma cortisol concentration by incorporating cortisol into their food. Weekly physical stress was induced to the second group by short out-of-the-water handling. Finally, a portion of the eggs of untreated control females were exposed to cortisol in an artificial ovarian fluid suspension. Since juveniles are normally released in the wild for stocking purposes when they are 6 month-old, we were particularly interested in behaviors that, when drastically altered, might compromise survival in this extreme environmental-shift scenario (Suboski and Templeton, 1989; Brown and Laland, 2001). We thus examined juvenile spatial learning and memory, boldness (propensity to take risks) and neophobia (fear of novelty). Our main prediction was that if cortisol is not the only factor mediating maternal stress-induced behavior reprogramming, then there would be no significant behavior differences amongst juvenile from all treatments.
Methods
Females and treatments
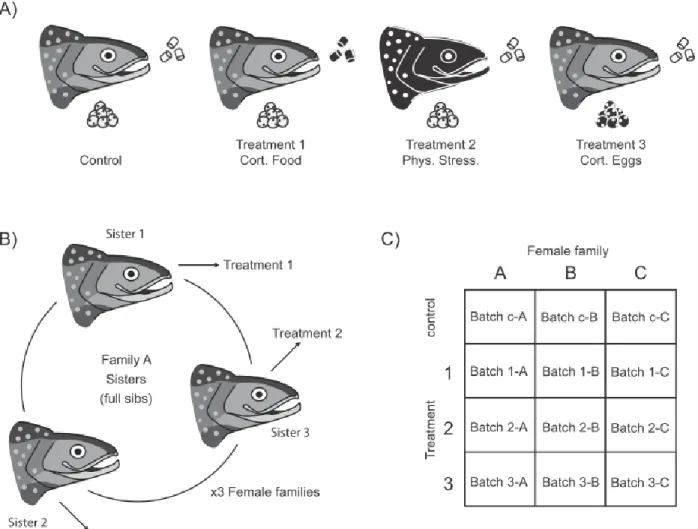
Farmed S. fontinalis broodstock from the Laval strain, which originates from a wild population of anadromous S. fontinalis from the Laval River (48° 44’ N; 69° 05’ W) on the north shore of the St Lawrence River estuary (QC, Canada), were used for this study. In August 2013, individually marked F4 mature females (6 years old) were separated into a control group and 3 different treatment groups (Fig. 1A). They were treated during the total duration of oogenesis, which was from September to December 2013. Females in the control group (n = 3) were left undisturbed (controls). Females in treatment 1 (n = 3) were administered 1.5 mg/kg of cortisol (based on Eriksen et al., 2011) daily through their feed (7.5 mm pellets, Corey Aquafeed). Solid cortisol ( ≥ 98% hydrocortisone, Sigma-Aldrich #H0888-10G) was dissolved in 15 to 20 ml of 100% ethanol (Anhydrous ethyl alcohol, Commercial Alcohols #1019-C) and sprayed over food pellets, which was reported as effective and less invasive than intraperitoneal implants (Gamperl et al., 1994). To ensure females would consume all of the cortisol-incremented food, pellets were given one at a time. Once fish stopped eating, the remaining food was weighed. The next week, cortisol was vaporized on 80% of the total food weight females had consumed the previous week to account for ongoing food consumption reduction during oogenesis. Females in treatment 2 (n = 3) were stressed by being handled (based onBarton et al., 2002) 30 seconds weekly, which consisted in being moved to another tank and then brought back to their original tank using a net. As for treatment 3, when control females were stripped, half of their eggs were immersed, prior to fertilization, in cortisol for 3 hours at 4°C (following Sloman, 2010). To mimic ovarian fluid, cortisol was suspended in NaCl 0.9% (Fisher Scientific #BP358-1) containing 500 ng/ml of solid cortisol (≥ 98% hydrocortisone, Sigma-Aldrich #H0888-10G); only 0.5 ml of this suspension per 100 ml of eggs was added to the eggs to maintain conditions of dry fertilisation. To verify if oogenesis was over and if females were ready to be stripped, females were anaesthetized in buffered MS-222 (0.16 g/l, 3-aminobenzoic acid ethyl ester, Sigma-Aldrich #A5040-250G) and their abdominal cavity was slightly pressed. Females were checked once a week and stripped on the same day if they were ready to release their eggs.
To account for genetic variabilty, females (n = 9; due to limited availability of related reproductive females) used in this experiment originated from 3 families. Three sisters from a given family were distributed across the control group, treatment 1 and 2 (as treatment 3 was a sub-group of the control group; e.g. for family “A”, the first sister was assigned to the control group, its first sister to treatment 1, and its second sister to treatment 2; Fig. 1B). The three groups were scattered over six 500 L indoor tanks (2 tanks per group to avoid tank effects). Tanks contained additional females (n = 11, 18, 13; for the control group, treatment 1 and 2 respectively) and also some males, to preserve normal aquaculture fish density and to stimulate ovulation in focal females. The additional unrelated females were also used to quantify mortality and ovulation disruption effects of treatments on a larger sample size. Fish were maintained at natural temperature (10-12°C) in dechlorinated municipal freshwater and were fed daily (1% mass to body mass ration, 7.5 mm pellets, Corey Aquafeed). Photoperiod and temperature followed the natural seasonal cycles (48° 3’ N).
Tissue collection Maternal plasma
Maternal blood was sampled immediately before females were stripped. Sampling was done between 9.00 AM and 13.00 PM to try and limit bias due to endocrine circadian rhythms. Females were anaesthetized in buffered MS-222 (0.16 g/l, 3-aminobenzoic acid ethyl ester, Sigma-Aldrich #A5040-250G). Blood was then collected by caudal puncture using ammonium-heparinized (porcine heparin ammonium salt, Sigma-Aldrich #H6279-25G) 1 ml syringes (Becton Dickinson #309659; needle size 21G x ½ in. #305167) and was then centrifuged at 7200 g for 3 minutes. The plasma was collected and stored at -80°C until analysis.
Unfertilized eggs
Three hours after stripping and prior to fertilization, roughly 100 eggs per batch (n = 12 egg batches, mother family (n = 3) x control and treatments (n = 4), Fig. 1C) were sampled and placed into weighing scoops, covered with plastic wrap and stored at 4°C for less than 2 hours. Eggs were then wrapped in aluminum foil, snap frozen in liquid nitrogen and stored at -80°C until analysis.
Offspring rearing
Eggs were stripped out of anesthetized females immediately after blood sampling by applying slight pressure on the abdominal cavity. Eggs were divided into 12 batches (n = 12 egg batches, mother family (n = 3) x control and treatments (n = 4), Fig. 1C). The cortisol suspension was added to the treatment 3 egg batches (n = 3). Eggs were stored in cold water baths for 3 hours. Subsequently to egg sampling, all the remaining eggs from a given batch were fertilized using semen from a random male (the use of brothers was avoided and each male was used only once). Three hours post-fertilisation, eggs were disinfected with iodine (100 ppm, West Penetone #8504) and incubated. From egg incubation (December 2013) to exogenous feeding (April 2014), each egg batch was incubated separately in individual trays. Water temperature was first maintained at natural conditions during egg incubation until water temperature dropped to 4.5°C. After hatching, water was heated to 8°C. When natural temperature reached 8°C, heaters were removed and temperature followed natural conditions. The photoperiod was set at 12L:12D (0700-1900 hours). At exogenous feeding, fish were fed daily (5.3% pellet mass to body mass ration, 0.5 mm pellets, Corey Aquafeed). In June, 500 juveniles from each batch were identified using multiple combinations of adipose and pelvic fin clippings. They were then transferred and mixed into three 250 Lindoor tanks (4 randomly selected juvenile batches per tank). They were maintained at natural temperature (10-12°C) in dechlorinated municipal freshwater. Photoperiod followed the natural seasonal cycle (48° 3’ N) and fish were fed daily (7.9% pellet mass to body mass ration, 0.5 mm pellets, Corey Aquafeed). In July, pellet mass to body mass ration was adjusted to 4.9% and pellet size to 0.7 mm. Just prior to behavioral tests, 7 randomly selected juveniles from each batch (n = 84) were transferred to 2 glass aquaria (52 x 26 x 26 cm) with net divisions (15 sections per aquarium; 9 x 9 x 26 cm) and randomly distributed to be able to keep track of individuals for the behavioral trials. This was done over three weeks. We thus transferred 28 juveniles (from 4 randomly selected batches) per week for behavior testing. Water was changed to cleanse feces and tested daily (Nutrafin Minimaster Test Kit #A7865) to ensure normal temperature and pH, and to verify nitrite, nitrate and ammonia levels. Aquaria were placed inside larger raceway tanks with open flowing water to maintain a constant temperature.
Cortisol assays Maternal plasma
Female (n = 9) plasma cortisol levels were measured in duplicates on 25 μl samples using a commercial radioactive immunoassay (125I RIA Cortisol kit #07221102, MP Biomedicals). Coefficients
of variation of cortisol level duplicates did not exceed 5%. One sample from treatment 1 did not yield usable results. Note that this is a data subset analysis from Denault-Tremblay, 2015.
Unfertilized eggs
Cortisol from unfertilized eggs was extracted from pools of 8 eggs per batch (n = 12) in technical duplicates (n = 4 eggs per replicate) using an organic extraction method (Johan Hyllner et al., 1994; Nechaev et al., 2006). Eggs were thawed in 200 μl phosphate buffer (0.1 M) and egg membranes were shredded by sonication for 2 minutes. This was followed by a triple extraction procedure: 2 ml ethyl acetate was added to the mixture, followed by vortexing and centrifugation at 1900 RPM for 2 minutes at 4°C, the supernatant organic phase was then collected and the process was repeated twice by adding 1 ml ethyl acetate to the original solution. The resulting 3 to 4 ml organic phase was dried with compressed air using an Evap-o-rac drying rack (Cole-Parmer #EW-01610-15) submerged in a 60°C water bath. Cortisol was then resuspended in 1 ml PBS (phosphate buffered saline) pH 7.4 (Gibco #10010-023) and samples were stored at -20°C until analysis. Cortisol levels in the eggs were assayed in triplicate using a commercial enzyme immunoassay (Cortisol EIA kit #500360, Cayman Chemicals, Ann Harbor, USA). Three measures were removed from the analyses so the coefficients of variation of cortisol level replicates did not exceed 15%. All samples (n = 12) were diluted 1:100 and assayed on a single plate with randomized positioning. Intra assay coefficient of variation was 9%. Extraction recovery was calculated with cortisol suspension (provided with the EIA kit) spikes added before the sonication step to a pool of eggs from untreated females that was split into a spiked and an unspiked samples. This process was performed on three independent egg pools. Mean recovery using the ethyl acetate phase extraction method was 88%.
Behavioral trials
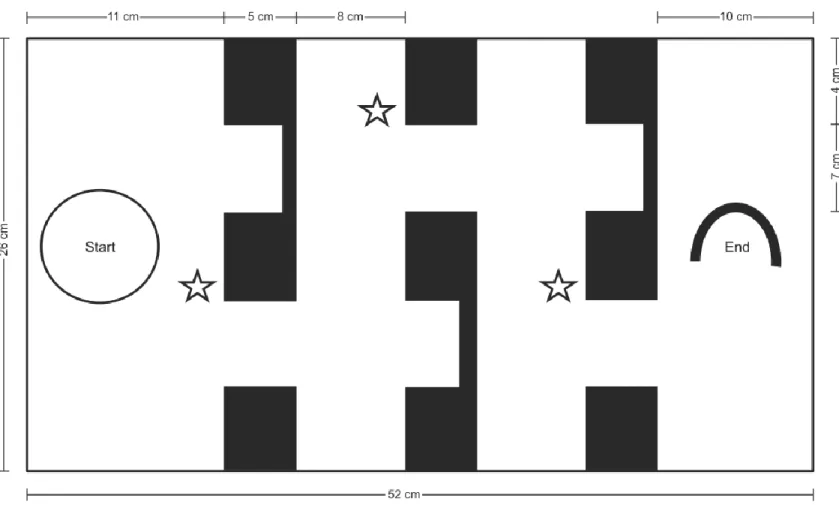
Boldness, learning and memory
In July 2014, we measured boldness, spatial learning and memory in juveniles. Fish were tested in a maze system based on (Girvan and Braithwaite (2000) and Sloman (2010). The maze was 52 cm long by 26 cm high and 26 cm wide. It was divided in four sections: the start section, the end section and 2 middle sections. Sections were separated by walls comprising 2 doors; one was marked with a blue tape outline and a plastic plant and allowed progression into the maze (visual cues help fish learn how to orient themselves in this kind of maze; Girvan and Braithwaite, 2000) and one was a dead end (Fig. 2). The maze walls were made of white opaque Plexiglas and were placed inside a glass aquarium. The sides of the test aquaria (n=4) were covered with white plastic to minimize external disturbance. At the beginning of a maze trial, we introduced a fish in a cylindrical glass container (300 ml) and waited 1800 seconds (30 minutes) for the fish to exit it. We quantified boldness as the latency to exit the glass container on the first day of trials (following Brown and Braithwaite, 2004). If the fish was still inside the glass container at the end of the 30-minute period, we removed the container and gave that fish the maximum latency score for boldness (1800 s.). We used the rest of the trial to assess spatial learning. We allowed the focal individual to explore the maze for 900 seconds (15 minutes) after exiting the glass container. We measured the necessary time for the individual to complete the maze and counted the number of mistakes (entering a dead end or coming back to a previous section of the maze). The motivation at the end of the maze was a 17 cm long PVC pipe cut in half horizontally and an airstone that served as a territory for the fish to hide in and defend (McNicol and Noakes, 1981). If the fish reached the end section within 900 seconds, we rewarded it by letting it stay under or near the PVC pipe for an additional 900 seconds and then returned it to its individual holding tank. If the individual did not complete the maze, we removed it from the maze, gave it the maximum score (900 s.) and immediately returned it to its tank section. Each fish was tested this way once a day for 5 consecutive days to measure learning. We performed the same maze trial once more 3 days after the 5th trial (resulting in a 2-day break) to
measure memory. We conducted trials four at a time and recorded them from above with mounted JVC camcorders (Everio GZ-MS100US). Water was changed at the beginning of each trial to ensure
submitted to the trial in a random order on the first day. The order was then maintained over the next days when trials were repeated.
Neophobia
In July 2014, we performed a neophobia test (following Wilson and McLaughlin, 2007 and Basic et al., 2012) on additional randomly selected juvenile fish (3 from each of the 12 batches; n = 36). We introduced fish to an empty tank (same dimensions and side covers as the maze aquaria) and allowed them to acclimate for 900 seconds (15 minutes). We then dropped a 17 cm long PVC pipe cut in half at the center of the tank and recorded their behavior for 600 seconds (10 minutes). We measured the time to the first approach of the novel object by less than a body length and by contact, and we also counted the number of times the individuals approached the novel object by less than a body length and the number of contacts with the object. Trials were also randomly ordered and video recorded. Water was changed between trials.
Statistical analyses
All statistical analyses were done in R (v3.2.0; RStudio v0.98.1103; R-Core Team, 2015). Differences in female ovulation rate between treatments and mortality during spatial learning and memory trials were analyzed with chi-square contingency tables with the built-in stats package (v.2.15.3; α = 0.05). Correlations between female plasma cortisol concentration and unfertilised egg cortisol concentration were assessed using the stats package. Models were fitted using the nlme package (v3.1-120; Pinheiro et al., 2015). Blood and egg cortisol levels from focal females as well as juvenile boldness data were analyzed with one-way linear mixed models with treatment as a fixed factor and mother family as a random factor. Boldness was also analyzed with egg cortisol concentration as a predictor, with a simple permutation linear model (lmperm package, v1.1-2; Wheeler, 2010). Spatial learning and memory were measured by both the time spent to complete the maze and the number of mistakes comitted during this time. Both learning and memory were analyzed with two-way repeated measures linear mixed models with treatment and day of testing as fixed factors. Learning models were fit with 5 days of testing, whereas memory models were based on only the fifth day of training plus the last trial 3 days later. Mother family and juvenile identity (repeated measures) were used as
random factors. Spatial learning and memory were also analyzed with egg cortisol levels as a fixed factor instead of treatment, with juvenile identity as only random factor. Since we used repeated measures, all individuals who died at any point during the trials were left out of the analyses (the final n, down from n = 28 for each treatment and control group, is displayed in the results section). Normality of residuals (verified by visual assessment of qq-plots) was achieved by data transformation. Homoscedasticity (verified by visual assessment of fitted data ~ residuals) was accounted for by the addition of appropriate weights when necessary. Once assumptions were met, the best fit as described by the Akaike criterion was selected. When models were significant (α = 0.01), Tukey post-hoc tests were performed (multcomp package, version v1.4-0; Hothorn et al., 2008) and significant pair-wise comparisons (α = 0.01) were reported. Statistical power was calculated with the nlmeU package (v0.70-3; Galecki and Burzykowski, 2013). Neophobia data was analyzed with permutation one-way anovas (lmperm package, v1.1-2; Wheeler, 2010) with either treatment or egg cortisol concentration as a fixed factor. The use of permutation models was always due to the impossibility to meet the assumptions of Gaussian parametric linear models (notably normality of the residuals). Permutation models do not provide exact p-values because they are fitted using thousands of iterations, approximated values are thus provided. Weight and length of juveniles did not correlate with behavioral data as revealed by a PCA analysis (FactoMineR package, v1.29; Husson et al., 2015) and were thus excluded from further analysis.
Ethical note
All experiments on brook trout breeders and juveniles were approved by the Université Laval’s (CPAUL, permit #2014-097) and Université du Québec à Rimouski’s (permit #CPA-13-54-29) ethical committees for animal welfare and carried out in accordance with Université Laval’s and Université du Québec à Rimouski’s ethical guidelines. All fish were held under normal and appropriate hatchery settings and water quality conditions.
Results
Treatments
There were no female casualties during treatments. Offspring mortality rates during the spatial learning trials period did not differ amongst treatments (chi-square tests, X2 = 5.94, p = 0.11). Three
females out of 18 in the cortisol-fed group (treatment 1) did not ovulate. This was not observed in other treatments or control females (control group: 0/11, treatment 2: 0/13). However, this difference in ovulation rate was not statistically significant (chi-square test, X2 = 4.31, p = 0.11).
Cortisol assays Maternal plasma
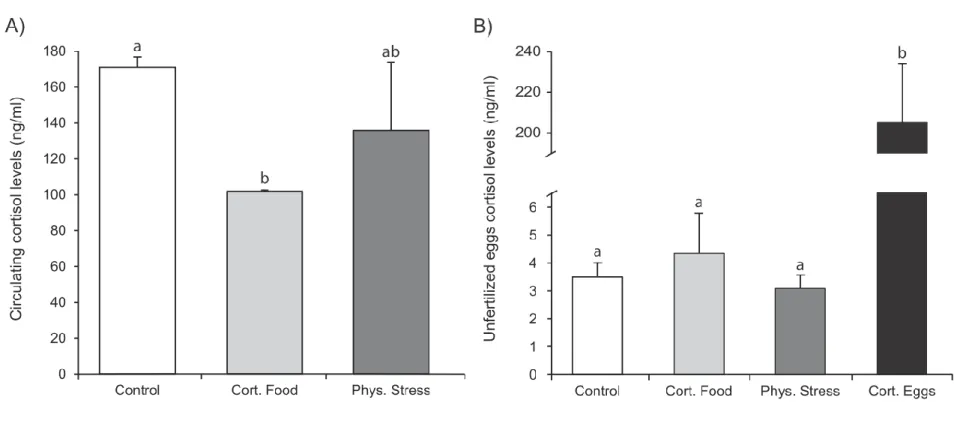
There was a significant effect of treatment on circulating cortisol concentration (one-way linear mixed model, p = 0.003). Control females had significantly higher circulating cortisol levels than females who were fed with cortisol-sprayed food pellets (treatment 1; post-hoc Tukey test, p < 0.001) but not higher than physically manipulated females (treatment 2; Fig. 3A).
Unfertilized eggs
Egg cortisol concentration prior to fertilization differed amongst treatments (one-way linear mixed model, p < 0.0001). Eggs that had been bathed in the cortisol suspension prior to fertilization had an approximately 60-fold increase in cortisol concentration compared with those of other treatments and controls (post-hoc Tukey tests, p < 0.001; Fig. 3B). There was no correlation between female plasma cortisol concentration and their mean egg cortisol concentration (Spearman’s correlation test, ρ = -0.14, p = 0.75, n = 8).
Behavioral trials Boldness
There was no statistically significant effect of treatment on latency to exit the glass container on the first day of training in the maze (one-way linear mixed model, p = 0.74, n = 16, 19, 13 and 18; Fig. 4). There was also no significant effect of egg cortisol concentration on latency to exit the glass container on the first day of training (one-way permutation linear model, p = 0.8).
Spatial learning
A significant effect of day of training on the time for juveniles to complete the maze was detected (two-way repeated measures mixed model, p < 0.001, n = 16, 19, 13 and 18) for all treatments and controls (p = 0.18). There was no significant effect of the interaction between treatment and day of training (p = 0.10; Fig. 5). The significant day of training effect was driven by a difference between days 2 and 5 (post-hoc Tukey test, p < 0.001) and between days 2 and 4 (post-hoc Tukey test, p = 0.004). A significant effect of day of training on the time for juveniles to complete the maze was detected (two-way repeated measures mixed model, p < 0.001) regardless of egg cortisol concentration (p = 0.09). There was no significant effect of the interaction between egg cortisol concentration and day of training (p = 0.09). The significant day of training effect was driven by a difference between days 2 and 5 (post-hoc Tukey test, p < 0.001). There were no significant effect of day of training on the number of errors that fish made while completing the maze (two-way repeated measures mixed model, p = 0.56) or of treatment (p = 0.54) or their interaction (p = 0.05).
Spatial memory
There was no statistically significant effect of day of testing (two-way repeated measures mixed model, p = 0.15, n = 13, 13, 7 and 18; Fig. 6), treatment (p = 0.69) or of the interaction between day of testing and treatment (p = 0.34) on the time for juveniles to complete the maze between the last day of training and the trial that was conducted 3 days later. Results were similar when using egg
cortisol concentration instead of treatment as a fixed continuous factor (two-way repeated measures mixed model, respectively; p = 0.06, p = 0.66, p = 0.15).
Neophobia
There was no significant effect of treatment on time for juveniles to approach the new object for the first time by less than one body-length (one-way permutation linear models, n = 9, 8, 9 and 9, p = 0.5) or to make contact with it (p = 0.7) and there was also no effect of treatment on the number of times juveniles approached the perimeter of the novel object by a fish length radius (p = 0.5) or the number of times they touched it (p = 0.8). There was also no significant effect of egg cortisol concentration for any of these measures of neophobia (one-way permutation linear models, respectively; p = 0.6, p = 0.6, p = 0.8, p = 0.5)
Discussion
The objective of this study was to determine if cortisol was the main mediator of maternal stress-induced behavior reprogramming in offspring by separating the potential effects of cortisol from that of other maternal factors. We used physical and pharmacological methods to modulate cortisol levels in the bloodstream or eggs of S. fontinalis female sisters and we quantified multiple ecologically relevant behaviors in their offspring. We found no effect of maternal treatment or of their egg cortisol levels prior to fertilization on juvenile boldness, spatial learning abilities and memory, or neophobia. However, we cannot conclude that cortisol is not the main maternal factor responsible for offspring behavior reprogramming, because not only did the behaviors and cognitive abilities not differ between controls and all 3 treatments, but our treatments also failed to increase plasma cortisol levels in the females and cortisol concentration in their eggs. Nonetheless, there was one exception : even if no behavior reprogramming was observed, egg cortisol levels from treatment 3 (eggs incubated in a cortisol suspension prior to fertilisation) were significantly higher than that of other treatments and controls. We also found that brook trout juveniles can learn and remember how to complete a maze within a five day training period using a territory-like section and a hide-out at the end of it as sole motivation.
Offspring did not exhibit any behavioral differences when egg cortisol levels were successfully and significantly increased in comparison with control eggs from the same mother and stripping event, suggesting that cortisol did not elicit phenotype reprogramming and might not be the main mediator of this phenomenon in juvenile brook trout of the Laval strain. Our egg cortisol immersion treatment resulted in a roughly 60-fold difference in cortisol concentration in comparison with control eggs but did not produce any alteration in spatial learning and memory, boldness or neophobia in the juveniles. These levels were higher than those reported in previous investigations on brown trout,
Salmo trutta such as Sloman (2010) and Burton et al. (2011) who observed 15-fold and 5-fold
increases in egg cortisol levels respectively, but which were coupled with significant behavior differences between cortisol-exposed eggs and control juveniles. Also in accordance with this possibility, it has also been shown in Coho salmon, Oncorhynchus kisutch, that a physical stressor
applied to females during the last two weeks of oogenesis significantly elevated maternal cortisol circulating levels, as well as egg cortisol levels, but did not modify offspring phenotype (Stratholt et al., 1997). Furthermore, Eaton et al. (2015) demonstrated in a viviparous fish, Poecilia reticulata that cognitive performance and aggressiveness could be reprogrammed in offspring subsequently to a physical maternal stress even when there were no differences in maternal plasma cortisol concentration. Moreover, Sopinka et al. (2014) showed that a repeated maternal chase stressor in the Pacific sockeye salmon, O. nerka, did not significantly elevate egg cortisol levels, but fry reared from stressed mothers swam for shorter periods of time and were better at re-initiating bouts of burst swimming. Part of our results and these studies thus suggest that it is likely that maternal stress could act through egg-deposition of other important maternal factors, rather than cortisol, which would in turn influence offspring phenotype.
The lack of behavioral reprogramming we observed could be partially due to the fact that the particular behaviors and cognitive abilities we tested might not be modified by maternal prenatal stress in brook trout. Within a set of particular phenotypic traits, it is possible that a subset would not be affected by a certain environmental shift, while others would be extensively modified (Sopinka et al., 2014). However, we tested a wide array of behavioral traits that have been previously shown to be affected by maternal stress in offspring or that could be affected by cortisol exposure during development in salmonids (Wilson and McLaughlin, 2007; Sloman, 2010; Eriksen et al., 2011; Frost
target the precise behaviors that would have been modified. It is more probable that we failed to highlight the particular context (Basic et al., 2012) in which behavioral differences would have been observable. For example, Sloman (2010) showed that elevated egg cortisol levels prior to fertilization increased brown trout juveniles’ aggressiveness towards their mirror image, while Burton et al. (2011) demonstrated juveniles of the same species displayed reduced aggressiveness towards a conspecific when eggs were treated similarly. It is thus possible that we did not measure the behaviors that were actually modified by the treatments or that offspring behavior differences in response to maternal stress is context-dependent.
The absence of offspring behavior differences in response to the physical disturbance and pharmacological hormonal manipulation treatments similar to those used in other studies could also be due to several technical issues. First, it is possible that the treatments the females were subjected to were not intense enough to trigger a sustained stress response to deposit cortisol or other maternal factors into the eggs, thus preventing any behavioral phenotype reprogramming in the offspring. Second, it is also possible that females acclimated (retrocontrol) to the long-term stress they were subjected to. While the cortisol circulating levels we measured in females were the opposite of what was expected and suggest these prior statements could be the reasons for the absence of juvenile’s behavioral response (control females had higher or equal circulating cortisol levels with the other treatments), it is possible that maternal plasma cortisol assays were not informative of the levels of stress that were really chronically induced. Since ovulation was not chemically triggered in our study and that females were examined once a week to determine if they were ready to be stripped, the exact day of ovulation is unknown. Salmonid females experience blood cortisol peaks during ovulation (Bry, 1985), therefore the single blood sampling at stripping conducted here did not allow controlling for these sudden circulating cortisol elevations. A solution to this caveat would have been to measure circulating cortisol at several time points during the treatments. However, due to increased circulating stress hormone levels during the reproduction period in salmonids (Robertson et al., 1961; Kubokawa et al., 1999), something that could explain the higher than expected plasmatic cortisol levels (Crespel et al., 2011) we observed, checking females more often could have resulted in an excessive stress response, which could have subsequently led to immunosuppression and other health issues (Pickering, 1989; Huntingford et al., 2006), even in control females. The absence of correlation between maternal circulating cortisol and unfertilized egg
cortisol levels also suggest that the single plasmatic cortisol measurements at spawning were not representative of the levels of stress that were induced by the treatments, as these correlations have been reported in other salmonids (Stratholt et al., 1997; Andersson et al., 2011). Yet using the unfertilized egg cortisol measures as an integrated measure of cortisol levels in females and assuming there should be a correlation between both also suggests that the treatments females were subjected to were not intense enough to result in significant changes in egg cortisol content and thus on downstream reprograming of the offspring phenotype. Of course, this applies only to the two stress treatments targeting females directly and does not explain the lack of response in offspring originating from eggs exposed to cortisol just before fertilisation compared to offspring from the same batch of eggs that were left as controls. Thus, it’s likely that the induced stressors to the females were not intense or sustained enough, or that mothers acclimated to them, and plasma cortisol concentration measures might not be reliable enough to rule out either these possibilities.
Our results show that Salvelinus fontinalis juveniles are able to learn how to complete a maze within 5 days of training with visual cues to aid in progression and with a small territory to hide in and defend as a motivation to complete it. Our results also show that they can remember it as much as three days after the training. By using time to complete the maze across training days as a measure of improvement, we found that, controls and juveniles from treatments had significantly improved in the task on the fourth and fifth day compared with the second. The fact that the first day of training does not seem to follow the general decreasing time trend observed in the other four days of training could possibly be due to novelty. No preliminary introduction of the maze had been made before the learning trials and it is possible a more elevated state of stress could have driven juveniles to speed through the maze on the first day. On the second day, an anticipatory stress response could have resulted in the higher time to complete the maze observed for all treatments and for controls. Also, the lack of significant differences in time to complete the maze between the last day of training and the trial that ensued three days later suggests that juveniles are able to recall a spatial task like a maze system, even after two days without being submitted to the trial. Surprisingly, the number of errors committed by juveniles while completing the maze remained constant throughout the training, which suggests that this measure is not informative of spatial learning in brook trout. Few studies had been conducted on cognitive performance in brook trout and we showed here that this species can
Gasterosteus aculeatus (Girvan and Braithwaite, 2000), zebrafish, Danio rerio (Roberts et al., 2013)
and brown trout, Salmo trutta (Sloman, 2010).
Ultimately, more investigations are needed in order to better understand the role cortisol plays in behavior reprogramming in response to maternal stress in oviparous species. Furthermore, we highly recommend the induction of multiple maternal stressors in parallel when investigating this class of maternal effects. We also suggest the use of different levels and duration of stress. Additionally, our successful cognitive performance trials demonstrated that brook trout juveniles can get better at a spatial learning task and this could open the way to additional investigations. Finally, how behavior reprogramming might differ for genotypes varying in stress responsiveness (between artificially selected lines and wild animals or between wild populations facing different selection pressures) is also of high interest for future studies.
Competing interests
We have no competing interests. Authors’ contributions
SCG, NAH and CA conceived of the study; SCG collected field data; SCG carried out lab work; ABL carried out video analyses; SCG performed the statistical analyses, and drafted the manuscript. All co-authors contributed to manuscript revisions, and gave final approval for submission.
Acknowledgements
The authors wish to thank Laurence Denault-Tremblay for her help in designing the study and for carrying out field work during female treatments and offspring rearing and also for carrying out plasmatic cortisol assays and providing hatchery-related information for this manuscript; Angela Paquet-Walsh, Adeline Piot, Alexe Baillargeon, Isabelle Dumais and Tamara Provencher for their tremendous help at the aquaculture station; Marianne Caouette for optimizing and carrying out most of the egg cortisol assays; Anne Dalziel and Ben Sutherland for their precious comments and contribution to the statistical analyses; Julie Turgeon, Christian Landry, Claude Robert and Conrad Cloutier for their elaborate comments and suggestions from the very beginning of this project; Lene Kleppe for providing a cortisol extraction protocol for salmonid eggs; François-Olivier Gagnon-Hébert, Chloé Berger and Jean-Philippe Rousseau for reading and commenting previous versions of this MS. This project was funded by a Fonds Québécois de la Recherche – Nature & Technologie (FQRNT): Projet de Recherche en Équipe grant to NAH and CA; SCG was supported by a Ressources Aquatiques Québec (RAQ) NSERC CREATE fellowship and a RAQ internship travel fellowship.
Figures
Figure 1. Experimental design. A) Representation of the control and treatment groups Salvelinus fontinalis females were divided into. Images are female heads with a clutch of eggs underneath and
commercial food pellets to the right. Black filling plus white outlines indicate stress induction or cortisol addition. Treatment number and abbreviations for figures are displayed below images. B) Representation of how the three female families were divided amongst treatments. C) Representation of egg and juvenile batches.
Figure 2. Cross-sectional view of the maze system. The maze comprised three walls, each with two doors. One led to a dead end and the other one
allowed passage to the next section. Doors, which allowed progression into the maze, were outlined with blue tape and a plastic plant represented here as open stars. Fish were introduced in a glass container represented as the open circle.