HAL Id: dumas-01377587
https://dumas.ccsd.cnrs.fr/dumas-01377587
Submitted on 7 Oct 2016HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
ECG-gated computed tomography to detect patients
with post-capillary pulmonary hypertension
Luc Moncharmont
To cite this version:
Luc Moncharmont. ECG-gated computed tomography to detect patients with post-capillary pul-monary hypertension. Human health and pathology. 2015. �dumas-01377587�
AVERTISSEMENT
Ce document est le fruit d'un long travail approuvé par le
jury de soutenance et mis à disposition de l'ensemble de la
communauté universitaire élargie.
Il n’a pas été réévalué depuis la date de soutenance.
Il est soumis à la propriété intellectuelle de l'auteur. Ceci
implique une obligation de citation et de référencement
lors de l’utilisation de ce document.
D’autre part, toute contrefaçon, plagiat, reproduction illicite
encourt une poursuite pénale.
Contact au SID de Grenoble :
thesebum@ujf-grenoble.fr
LIENS
LIENS
Code de la Propriété Intellectuelle. articles L 122. 4
Code de la Propriété Intellectuelle. articles L 335.2- L 335.10
http://www.cfcopies.com/juridique/droit-auteur
1
UNIVERSITE JOSEPH FOURIER
FACULTE DE MEDECINE DE GRENOBLE
Année : 2015
N°
ECG-gated computed tomography to detect
patients with post-capillary
pulmonary hypertension.
THESE
PRESENTEE POUR L’OBTENTION DU DOCTORAT EN MEDECINE
DIPLÔME D’ETAT
Luc MONCHARMONT
THESE SOUTENUE PUBLIQUEMENT
A LA FACULTE DE MEDECINE DE GRENOBLE*
Le mardi 6 octobre 2015
DEVANT LE JURY COMPOSE DE
Président du jury : M. le Professeur Gilbert FERRETTI
Membres :
M. le Docteur Adrien JANKOWSKI, directeur de thèse
M. le Professeur Christophe PISON
M. le Professeur Gilles PERNOD
Mme. le Docteur Hélène BOUVAIST
*La Faculté de Médecine de Grenoble n’entend donner aucune approbation ni improbation aux opinions émises dans les thèses ; ces opinions sont considérées comme propres à leurs auteurs.
6 REMERCIEMENTS
AUX MEMBRES DU JURY
Au Président du jury, Monsieur le Professeur Gilbert FERRETTI
Vous me faites l’honneur de présider cette thèse. Votre enseignement m’a donné envie
d’approfondir mes connaissances en imagerie thoracique. J’ai pour vous et votre travail un profond respect.
A mon directeur de thèse, Monsieur le Docteur Adrien JANKOWSKI
Merci de m’avoir confié ce travail. Merci de ton enseignement, ta disponibilité, tes conseils et de tes encouragements pour la préparation de cette thèse. Au plaisir de te croiser un jour en randonnée à pied ou à ski.
Monsieur le Professeur Gilles PERNOD
Merci de m’avoir accueilli durant un semestre au sein de l’équipe de médecine vasculaire. Vos qualités de pédagogue et votre humanité m’ont beaucoup apporté. Merci encore d’avoir accepté d’être membre de mon jury de thèse.
Monsieur Le Professeur Christophe PISON
C’est un grand honneur dont vous me témoignez en acceptant de participer à mon jury de thèse. Je vous en remercie chaleureusement.
Madame le Docteur Hélène BOUVAIST
Vos conseils avisés m’ont beaucoup aidé dans la préparation de cette thèse. J’éprouve un profond respect pour votre travail. Merci de votre participation à mon jury de thèse.
7 A MES PROCHES
A ma Lili
Tu combles mes jours et mes nuits depuis presque 6 ans maintenant. Tu m’as montré ton courage et la force de ton amour au cours de ces trop longues années à distance. Nous voilà enfin réunis et pour de bon! Dans un an, quasi jour pour jour au moment où j’écris ces lignes, tu seras mienne pour le reste de notre vie et cette pensée me comble de joie. Je t’aime.
A mon petit Rémi
Depuis que tu es né je n’imagine plus la vie sans toi. Un regard sur ton visage paisible lorsque tu dors me souffle l’inspiration et me donne envie d’aller toujours plus loin. Je serai toujours là pour toi, te guider, te conseiller et te protéger. J’attends avec impatience de t’emmener crapahuter et grimper en montagne.
A mes parents
Ma petite maman, tu m’as tellement donné que je ne sais par où commencer. Tu m’as toujours soutenu, encouragé et poussé à donner le meilleur de moi-même, surtout dans les moments difficiles. Merci d’être toujours là pour nous tous. Papa, ta « culture G » et tes connaissances médicales m’impressionneront toujours. Tu es pour moi une référence en tellement de domaines que je ne les compte plus! Merci de m’avoir transmis ta passion de la lecture, du cinéma, ta curiosité et ta soif d’apprendre. La vie m’a fait prendre conscience de la place que vous occupez dans mon quotidien et à quel point je tenais à vous.
A ma grande sœur
Tu m’as toujours soutenu, écouté, conseillé, consolé et tu continueras encore de nombreuses
années. Quelle chance j’ai d’avoir une sœur aussi attentionnée que toi. A tous ces moments, ces joies et ces peines que nous avons partagés. Ils nous ont rendus plus forts et plus complices, c’est
probablement là le secret de l’amour fraternel. J’ai beaucoup de respect pour Florent avec qui tu partages ta vie, qui m’a d’emblé été sympathique et que j’ai accueilli dans ma famille comme une évidence. Vous nous avez donné une magnifique Clara et un adorable Sébastien que j’ai hâte de voir grandir et s’épanouir. Sébastien, je connais ton amour pour les motos, heureusement j’ai
l’impression que tu aimes tout autant les casques! Petite Clara, tes yeux vont en faire chavirer plus d’un!
A Catherine et à Laurent
Tantine, j’aime ta générosité, ton amour sans faille et ta simplicité. Je me rappelle avec plaisir tous les bons moments que nous avons partagés quand j’étais enfant. J’adorais nos soirées vidéo avec Flo « chez Tantine et Laurent », j’avais l’impression de partir en colo! Avec Laurent, en animateur et ma tantine de choc, la soirée ne pouvait qu’être drôle et chaleureuse.
A Jean Max
Tonton Jeanno, tu m’as donné l’envie de gravir les plus hauts sommets, de dormir à la belle et de profiter de la vie. Je suis ravi que Cathy soit entrée dans ta vie et dans la nôtre par la même occasion. A mon grand père André
J’aurai aimé que tu sois là aujourd’hui et encore plus que tu ais pu connaitre tes arrières petits enfants. Ils auraient fait ta fierté. Merci pour tout ce que tu m’as transmis : la valeur de la vie humaine, l’amour de la nature, de la montagne, tes connaissances en horticulture et apiculture. Je tâcherai de ne pas oublier tout ce que tu m’as appris.
8 A ma belle famille, tellement nombreuse, mais si chaleureuse
A mes beaux parents qui m’ont donné ma Lili. Vous l’avez plus que réussie, une chance pour moi! Merci Christine d’avoir soutenu Aurélie quand je n’étais pas là. C’était toujours un soulagement de te savoir près d’elle. Merci Patrick pour tous les conseils de bricolage. Continue de courir, je vais enfin pouvoir m’entrainer pour faire un petit marathon avec toi!
Aux Juju, Aurélie a de la chance d’avoir des frères aussi prévenants. J’ai hâte de partager encore de bons moments avec vous et vos petites familles! En tout cas, nous retournerons aux champignons quand vous voulez. On finira par ce petit accrobranche que vous avez réussi à esquiver en
Chartreuse.... Rassurez-vous, après renseignement, il est encore là pour quelques années! En échange, vous aurez le droit de me trainer (une fois!) au stade pour voir un match de foot.
A Aurélie et Sarah, Avec vous deux, ma Lili a tiré le gros lot et moi aussi. Vous l’avez soutenue dans les moments de déprime, quand la distance se faisait trop pesante et je ne vous en remercierai jamais assez.
A Aymeric, Laurine, Anaïs et Margaux. Rémi a de la chance de vous avoir comme cousins, je sais que vous prendrez soin de lui!
A TOUS MES AMISDE LYON, DE GRENOBLE ET D’AILLEURS.
A Sylvain, Mister incroyable! Que de chemin parcouru depuis le collège ! Que d’autres choses à vivre ensemble! Ton amitié et celle de Raths me sont très chères. A bientôt sur Panam, je t’enverrai mes dates!
A Nico, parce que t’es un mec cool et que j’adore ta zen attitude! Et ta petite Céline est au top! A Paris, tu as toujours une solution aux problèmes, surtout en montagne, et tes talents culinaires ont toujours ravi mes papilles de grand gourmand! J’ai hâte de me remettre à la grimpe avec toi.
A Bouchon, en souvenir de cette fichue P1 où tu m’as souvent remonté le moral et surtout pour ta précieuse amitié. Notre Lili a de la chance de t’avoir pour amie.
Au « professeur » Lehingue, à Dine, Mike, pour tous ses bons moments partagés ensembles. A Francky, Zaza, Marc et Aurélien.
Aux collocs de « Jean Perrot », aux apéros sur le balcon, aux séances de blocs en coup de vent, la porte du four et j’en passe!
Jo, je me rappelle encore la première fois que tu as débarqué à la colloc, tu m’as tout de suite fait bonne impression. Tu essaies de me convaincre que l’espèce humaine est foncièrement bonne…défit à moitié relevé. En tout cas, j’envie ton optimisme et ta bonne humeur était un plaisir au quotidien. Caro, je crois que je ne connais pas de personne plus dynamique et investie que toi. Ton amitié compte beaucoup pour moi.
Nad, comme Caro, respect! Je crois que je n’arriverai jamais à te suivre, en course ou en grimpe d’ailleurs. Je t’ai vu t’épanouir (à commencer par ton sourire), depuis que Simon est entré dans ta vie et j’en suis ravi!
9 Merci à tous mes co-internes avec qui j’ai passé ces 5 belles années à Grenoble : Auré, Nico, Lio, Pierre, Béné, Olivier, Moumoune, Finas, Rouchy, Romain, Lison, Tristan, Ju G et Ju C. Forcez quand même pas trop sur le bip 152!!
Un grand merci à mes chefs qui m’ont tellement appris : Julien, Isa, Marie, Anny, Emilie, Ivan, Mathieu, Frédéric, Caro, Jean No, Arnaud.
Merci à tous les manips de radio, de médecine nucléaire, de Chambéry et aux infirmières de médecine vasculaire qui ont partagés ces 5 années au CHU, de jour comme de nuit. J’y ai passé de très bons moments en votre compagnie.
10 TABLE DES MATIERES :
RESUME ABSTRACT ARTICLE
INTRODUCTION
MATERIAL & METHODS RESULTS DISCUSSION CONCLUSION ANNEXES CONCLUSION SIGNEE REFERENCES SERMENT D’HIPPOCRATE
11 RESUME:
Objectif:
L’objectif de cette étude est de détecter en TDM synchronisée à l’ECG les patients souffrant d’hypertension pulmonaire (HP) post-capillaire.
Matériels et méthodes :
Quatre vingt neuf patients suspects ou souffrants d’HP ont bénéficié d’une TDM synchronisée à l’ECG et d’un cathétérisme cardiaque. Dans le plan axial nous avons évalué visuellement la dilatation de l’oreillette gauche (OG) puis son diamètre antéropostérieur (DAP). Dans le plan quatre cavités nous avons calculé la vitesse de relaxation longitudinale maximale du ventricule gauche (VG) (onde Ea-like). Un logiciel de post-traitement cardiaque a modélisé les courbes de remplissage du VG et de l’OG, nous permettant de calculer des critères de dysfonction diastolique (DD) : le ratio fraction d’éjection de l’OG(FEOG)/volume télé-diastolique de l’OG (VTD-OG), le temps de récupération de 70% du volume d’éjection du VG, le pourcentage de récupération du Volume télé-diastolique du VG (VTD-VG) pendant la première moitié de la diastole, l’index volumique de fonction diastolique (DVFi). Résultats :
Les patients avec une HP post-capillaire présentent significativement une élévation du DAP de l’OG et une diminution du ratio FEOG/ VTD-OG (p<0,001) par rapport aux patients sans HP post-capillaire. Le pourcentage de récupération du VTD-VG pendant la première moitié de la diastole est significativement augmenté (p=0,045) alors que le temps de récupération de 70% du volume d’éjection du VG est diminué (p=0,02). Les autres critères ne sont pas significativement différents entre les patients.
Conclusion :
Le scanner synchronisé à l’ECG peut fournir des critères de DD utiles en routine pour détecter les patients souffrant d’HP post-capillaire.
12 ABSTRACT:
Objective: To detect patients suffering from pulmonary hypertension (PH) caused by left heart disease (LHD-PH) using ECG-gated computed tomography (CT).
Methods: Eighty-nine patients suspected or suffering from PH underwent ECG-gated CT and heart catheterization. In axial images we visually evaluated left atrial (LA) enlargement then the LA anteroposterior diameter (LAd). In 4-cavity-axis images we calculated the left ventricular (LV) maximal longitudinal relaxation velocity (Ea-like). Cardiac software generated left ventricle and left atrial filling curves for calculating heart failure preserved ejection fraction (HFpEF) criteria: LA ejection fraction (LAEF)/LA end-diastolic volume (LAVd) ratio, time for left ventricle to recover 70% of stroke volume (SV), percentage of end-diastolic volume recovered during the first 50% of diastole, diastolic volume function index (DVFi).
Results: In patients with LHD-PH the LAd and LAEF/LAVd ratio were respectively increased and reduced significantly compared to patients without LHD-PH (p<0.001). Also, the percentage of diastolic volume recovered during the first 50% of diastole was significantly increased (p=0.045) whereas the time required to recover 70% of SV was significantly reduced in LHD-PH patients (p=0.02). DVFi and Ea-like were not significantly different.
Conclusion: ECG-gated CT can provide HFpEF criteria, useful in routine practice to detect patients with PH-LHD.
Key points:
ECG-gated CT is useful in routine practice to assess PH.
LAd > 49.5mm and LAEF/LAVd ratio < 0.45 usually indicates LHD-PH.
Some LV HFpEF criteria should be useful to diagnose LHD-PH.
Key words: Heart failure with preserved ejection fraction, Pulmonary hypertension, Computed Tomography, heart catheterization, comparative study.
13 CT: Computed Tomography; CMRI: cardiac magnetic resonance imaging; LA: Left Atrial; LAVd: Left Atrial end-diastolic Volume; LAd: LA anteroposterior diameter; LAEF: LA ejection fraction; PFR: Peak Filling Rate; LV: Left ventricule; LVEDP: Left Ventricule End-Diastolic Pressure; HFpEF: Heart Failure with preserved Ejection Fraction; HFrEF: heart failure with reduced ejection fraction; PCWP: pulmonary capillary wedge pressure; DPG: diastolic pressure gradient; RHC: Right heart catheterization; LHC: Left heart catheterization; DVFi: Diastolic Volume Function index.
14 INTRODUCTION
In recent years, our understanding of the epidemiology of Pulmonary Hypertension (PH), defined as an increase of resting mean pulmonary arterial pressure (mPAP) > 25mmHg [1], has been largely revised. The updated classification identifies 5 groups of disorders that cause PH [1]. Left heart diseases (LHD) cause post-capillary PH (group 2), which is diagnosed by a pulmonary capillary wedge pressure (PCWP)>15mmHg [2] or a left ventricular end diastolic pressure (LVEDP)> 15mmHg on heart catheterization [3]. Other groups (ie, 1, 3, 4, 5) have pre-capillary PH (PCWP≤15mmHg).
Among group 2, heart failure with preserved ejection fraction (HFpEF) is more prevalent than heart failure with reduced ejection fraction (HFrEF), but the prognosis of both conditions is similar [4–7]. Fifty to 83% of patients with HFpEF have PH [6,8,9].
There are 2 types of post-capillary PH according to the diastolic pressure gradient (DPG = diastolic PAP-PCWP) value: “isolated post-capillary PH” (PCWP>15mmHg and DPG<7mmHg) and “combined pre and post-capillary PH” (PCWP>15mmHg and DPG≥7mmHg) [2].
In HFpEF left ventricular-arterial uncoupling generates the upstream elevation in left atrial (LA) pressure then an increase in pulmonary venous circulation. These phenomena cause pulmonary hypertensive-HFpEF (PH-HFpEF) syndrome which is initially responsible for functional and reversible PH but in the end leads to structural remodeling (permanent) of the pulmonary circulation [6,10]. It is recommended [11] that the diagnosis algorithm for PH should include clinical examination, chest X-ray, high resolution computed-tomography and trans-thoracic echography (TTE). However, only right heart catheterization (RHC) can confirm the diagnosis [11]. When PH is suspected, a chest CT is acquired to clarify the clinical group of PH [11]. New advances in ECG-gated CT allow accurate assessment of hemodynamic cardiac function with acceptable levels of irradiation [12]. A previous study in our center showed good correlations between morphological criteria of the left atrium (LA) and PWCP [13]. Studies have shown that ECG-gated CT [14], as well as CMRI [15,16], was comparable to 2D echography for HFpEF detection. Nevertheless, despite this progress in ECG-gated
15 CT, it has never been used to evaluate PH-HFpEF syndrome due to left heart disease although all patients with PH undergo a chest CT.
Therefore the aim of this retrospective study was to evaluate the performance of ECG-gated CT versus heart catheterization to detect patients suffering from PH-LHD syndrome among patients suspected or suffering from PH.
MATERIALS AND METHODS: Patients:
Our PH reference center prospectively stores ECG-gated CT data for all suspected or followed pulmonary hypertensive patients. Consecutive patients who underwent ECG-gated CT and RHC with or without left heart catheterization (LHC) within 15 days between September 2008 and March 2014 were included in our study population.
Heart catheterization:
Trained operators performed RHC with or without LHC following current guidelines.
They placed a swan-ganz catheter (Edwards Lifesciences, Irvine, CA, USA) using a femoral or right internal jugular approach. The patient was resting in a supine position both during RHC and CT. Haemodynamic variables including systolic, diastolic and mPAP, PCWP, right ventricular pressures and cardiac output by the thermos-dilution method were recorded. The usual definition was used to diagnose pre and post-capillary PH.
If there was a suspicion that PCWP underestimated Left Ventricular End Diastolic Pressure (LVEDP) a fluid challenge was performed. If necessary the cardiologist improved the diagnosis of HFpEF by Left heart catheterization. A right or left radial artery approach was used to catheterize the left cavities. The LV, LVEDP and LV end-systolic pressures were measured.
16 All procedures were performed with a 64-slice CT scan (Philips Briliance 64, Philips medical systems, Eindhoven, The Netherlands). CT acquisitions were synchronized to the ECG. For each CT, the parameters were as follows: collimation: 64x0.625mm, rotation time: 400ms, pitch: 0.203, tube voltage: 120 kV, tube current 500 mAs. 110 ml of non-ionic low osmolar contrast agent (Iohexol 350, GE Healthcare, Princeton, NJ, USA or Iobitriol, Laboratoire Guerbet, Aulnay-sous-Bois, France) were administered by intravenous catheter at an injection rate of 4ml/s. Bolus tracking in the main pulmonary artery with a trigger at 150 HU allowed contrast arrival monitoring. No beta-blockers were used. Images were retrospectively reconstructed with a slice thickness of 1mm every 10% of R-R’ interval, using a standard reconstruction filter.
Image interpretation:
Three experienced investigators used dedicated cardiac function analysis software (Comprehensive Cardiac, version 4.5.2.40007, Philips Medical systems) to analyze anonymized data on a workstation (EBW, Philips Medical Systems).
Investigators assessed an equal numbers of scans. Ten CT examinations were reviewed to evaluate inter-observer reliability. Investigators were unaware of the patients’ clinical condition or RHC/LHC results.
Reconstructions of short-axis, 2-chamber and 4-chamber views, at all phases of R-R’ interval, were performed using the usual steps.
We used axial views to estimate if the LA was visually enlarged. Secondly, we measured the maximum anteroposterior diameter of the LA on end-diastolic and end-systolic phases.
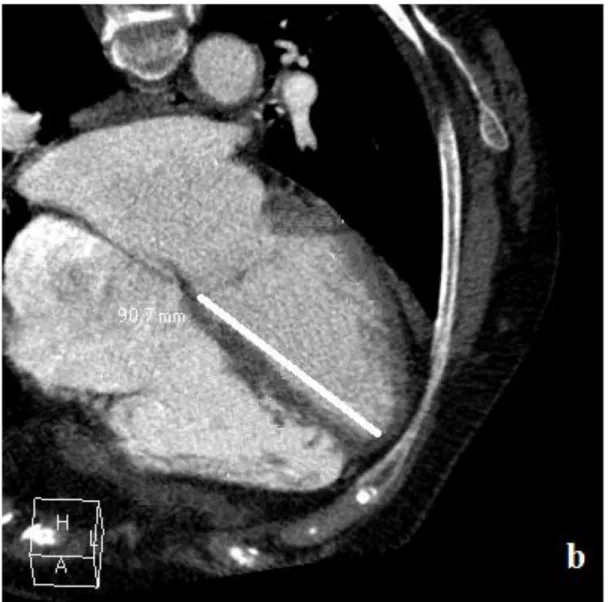
LV length (mm), the distance between the septal side of the mitral annulus and the cardiac apex, was measured on 4-chamber views (figure 1), at every phase from the end-systolic phase to mid-diastole (70 % of R-R interval). Based on these measurements the LV longitudinal relaxation rate was
17 estimated and the highest figure (called “Ea-like”) was considered as an estimation of the maximal mitral septal tissue velocity (Ea).
An automatic segmentation algorithm (Comprehensive Cardiac, version 4.5.2.40007, Philips Medical Systems,) was used to calculate LV volumes in ten phases of the R-R’ interval and LA volumes in end-systolic (LAVs) and end-diastolic (LAVd) phases. If necessary, manual corrections of the automatic segmentation could be made. Each investigator estimated the efficiency of automatic segmentation and when they remained in spite of manual corrections, the automatic data were not used.
Functional measurements:
We calculated the LA ejection fraction (LAEF) [(LAVs-LAVd /LAVs) (= Vmax-vmin/Vmax)] and the ratio LAEF/LAVd.
Post-processing software was used to model LV volumes (LVV) versus RR’ phase curves (figure 2) allowing the calculation of the peak filling rate (PFR) (= maximal filling slope (LV dV/dphase) during diastole phase). Then, we calculated the PFR/Ea-like ratio to estimate LV filling pressures.
Finally, thanks to the volume versus RR’ phase curves, we obtained the stroke volume (SV), the slope a of LV filling between two consecutive RR’ phases including the value at 70% of the SV and the b constant of the linear function F(x) =a(x)+b; where (x) is the percentage of RR’ interval and F(x) the value of LVV at (x) percentage of RR’. Thus, the time required for the recovery of 70% of the SV is expressed as a percentage of the interval RR’. Moreover, the percentage of end-diastolic volume recovered during the first 50% of diastole (this is equivalent to 70% of the RR’ interval) could be calculated (LVV at the phase at 50% of diastole x100/end-diastolic LVV).
Using the same software, semi-automatic Simpson segmentation was performed on short axis views from the mitral valvular annulus to the LV apex at the end-diastolic phase, end-systolic phase and phase at 50% of diastole. Our dedicated cardiac function analysis software calculated corresponding volumes. These data were used to calculate the volume change during the first 50% of diastole
18 (called the diastolic volume function index, DVFi) (= (LVV at 50% of diastole-end-systolic LVV) / (Ejection volume)).
Statistical analysis:
Variables are described by their mean and standard deviation or by their median, first and third quartile if normality was not confirmed (with the Shapiro-Wilk test). Qualitative variables are expressed as numbers and percentages. Quantitative variables for pre- and post-capillary patients were compared using the two-sample t-test or, if inapplicable, the Wilcoxon rank sum test. A Chi-square test or Fisher test (if the sample size was insufficient) was used to compare qualitative variables. A p-value < 0.05 was considered to denote a significant difference between pre- and post-capillary patients.
A PLS-DA (Partial Least Squares – Discriminant Analysis) was implemented for all parameters and ROC curves plotted for variables selected by the VIP (Variable Importance in the Projection) of PLS-DA.
Kappa and intraclass coefficients were calculated to assess inter-observer agreement for qualitative and quantitative variables respectively.
RESULTS:
Of 102 eligible patients 12 with incomplete CT data and 1 with unparsed RHC data linked to intercurrent diseases were not included. Table 1 summarizes the population data. The median time between RHC and ECG-gated CT was 2 [0; 5] days. Among all our patients, 14 (16%) were free of PH, 53 had pre-capillary-PH (59%), 13 post-capillary-PH (15%) and 9 mixed-PH (10%). Our study population had several causes of PH. According to the most recent classification (Nice, 2013) [1], 13 patients had pulmonary arterial hypertension (group 1), 2 had group 1’ PH, 22 had left heart disease (group 2), 11 had PH due to lung disease and/or hypoxia (group 3), 21 had chronic thromboembolic pulmonary hypertension (group 4) and 6 had PH with unclear multifactorial mechanisms (group 5).
19 Patients with post-capillary-PH (n=22) were significantly older than patients with pre-capillary PH (n=53) (p=0.01). Apart from “Ea wave” (ICC= 0.28), all variables measured showed high interobserver reproducibility (ICC=0.6 to 1), (table 2). Table 3 compares heart catheterization parameters between patients with PCWP> and those with PCWP≤15mmHg.
1. Role of LA morphological and functional values in distinguishing patients with PCWP>15 mmHg from patients with PCWP ≤15mmHg:
There was significant increase in the maximal end-systolic anteroposterior diameter (p<0.001) in patients with post-capillary PH. LAEF (p<0.001) and LAEF/LAVd ratio decreased significantly in patients with post-capillary PH (p<0.001). Furthermore, more post-capillary patients had a visually enlarged LA (p<0.001).
2. Role of LV functional criteria in detecting patients with post-capillary PH:
Patients with post-capillary PH had significantly lower time to recovery of 70% of SV (p=0.02) and a higher percentage of end-diastolic volume recovered during the first 50% of diastole (p=0.045). Nevertheless, the diastolic volume function index as well “Ea-like” wave and PFR were not significantly different between post-capillary and pre-capillary patients (p=0.15; p=0.59 and p=0.40 respectively).
3. Multivariate analysis:
The Variable Importance in the Projection (VIP) of the PLS-DA selected LA diameter, LAEF/LAVd, time to recovery of 70% of stroke volume, DVFi and percentage of end-diastolic volume recovered during the first 50% of diastole (VIP>1). From the charts of PLS-DA (figure 3), although pre- and post-capillary groups are not totally separated, we see that the parameters LA diameter and LAEF/LAVd are the most discriminating: post-capillary patients tend to have a higher value for LA diameter and a lower value for LAEF/LAVd.
20 4. Diagnostic performance of variables selected by the multivariate analysis:
According to the results of ROC curves (figure 4), table 4 summarizes the accuracy of individual variables in identifying post-capillary patients. LA diameter and LAEF/LAVd show good diagnostic performance (AUC>0.8 and Se>85%).
DISCUSSION:
To our knowledge, this is the first study that investigates the ability of ECG-gated CT, compared to the gold standard (HC), to assess LV diastolic dysfunction in a specific and complex clinical situation such as PH.We showed that the percentage of end-diastolic volume recovered during the first 50% of diastole can be useful to diagnose post-capillary PH. Nonetheless, LA diameter and LAEF/LAVd seem to be the optimal criteria to distinguish patients with and without PCWP ≥15mmHg.
In substantial agreement with previous studies [13,17–19], our results confirm that LA morphological and functional parameters allow a noninvasive diagnosis of post-capillary PH.
The LAEF/LAVd ratio, LAd, and visual estimation of dilatation of the LA are easily measured with automatic segmentation and without loss of time. Our multivariate analysis and the ROC curves gave LAd and LAEF/LAVd as suitable criterions, LA diameter being the easiest to measure in routine practice. In line with previous findings LAEF was significantly lower in post-capillary patients (13,19].
Dilatation of the LA shows good sensitivity to detect an elevation of left ventricular filling pressure. However, LA remodeling can occur in conditions other than left heart failure, especially in elderly patients and those with mitral valve disease, atrial myopathy, and atrial fibrillation [19–22]. These findings provide evidence that LA morphological and functional assessment is not sufficient to establish a definite diagnosis of post-capillary PH. Epidemiological studies report that HFpEF is more prevalent than HFrEF in PH [4,9]. By studying LV filling, especially HFpEF criteria, we tried to improve the diagnostic accuracy of ECG-gated CT for detecting post-capillary PH.
21 Two-dimensional echocardiography using tissue Doppler Imaging is commonly used to evaluate LV-filling pressure [23–26]. Studies have confirmed that automated segmentation of CMRI can provide accurate LV filling curves which allow diastolic function assessment [15,27,28]. Evaluation of peak filling rate, time to peak filling rate, diastolic volume recovery (mean proportion of diastole required for recovery of a given percentage of stroke volume) have shown good correlation with echocardiographic-evidence of diastolic dysfunction.
Nevertheless, both techniques suffer from several limitations. Echocardiography, notably tissue Doppler echocardiography (TDE) has technical and clinical limitations leading to the dependence of TDE techniques on angle, practitioners’ training, patient’s morphology, acoustic windows, and heart axis [29], whereas CMRI currently suffers from a lack of availability, with long delays [30], and is not yet a recommended standard examination for the initial evaluation of PH.
A study by Boogers et al. [14] [15], showed that ECG-gated CT as compared to TDE, can be used to assess left ventricular diastolic dysfunction on selected patients. The question is therefore “can HFpEF be detected by ECG-gated CT performed during the initial evaluation of PH?”
The majority of PH-HFpEF syndromes occur with increasing severity of HFpEF. Time (percentage of diastole) for LV to fill with 70% of stroke volume (equivalent to diastolic volume recovery) has been shown to be an independent predictor of HFpEF [27]. Our study shows that this time decreases in post-capillary PH patients; in substantial agreement with the CMRI study of Mendoza et al. which found that in patients with echocardiographic-evidence of grade 1 or 2 diastolic dysfunction, diastolic volume recovery increased whereas it decreased in grade 3 patients [15].
As most people recover 80% of stroke volume at 70% +/-15 of R-R’ interval [27] we chose to evaluate the percentage of end-diastolic volume recovered at 70% of R-R’ interval, which is easy to perform in routine practice. Our results using ECG-gated CT suggest a significant increase in diastolic volume recovery at 70% of R-R’ interval in post-capillary patients.
22 Our diastolic volume function index (DVFi) calculated using volume measurements differs from the study of Okayama et al. [31] that used fractal area changes, and has several advantages. It takes into account longitudinal, circumferential and radial relaxation, ie, overall and not only local cardiac function. As suggested in their study, volumetric changes are not the same as area changes, probably due to complex three-dimensional LV deformation [32]. In fact, DVFi tends to increase in post-capillary patients, but not significantly (p>0.05). These findings lend support to the assumption that circumferential deformation is an important predictor of HFpEF. Moreover, our findings agree with the shortened diastolic volume recovery seen in severe HFpEF [15].
With our automated segmentation software we obtained the peak filling rate and Ea-like wave. We expected the ratio of PFR/Ea-like to provide the LV pressure estimation as E/Ea. However both the Ea-like wave and PFR estimations, and thus PFR/Ea-like wave, were not significantly correlated with PCWP. Two facts might have caused these inconclusive results. First it appears difficult in current practice to reconstruct data for 5% R-R’ intervals due to high computing time, so we reconstructed images at 10% intervals (0 to 90% of the R-R’ interval). Consequently our volume-versus-time curves suffer from poor time resolution. Moreover, with 64-slice CT scans, we were unable to obtain the whole cardiac volume in a single rotation as with a large collimation CT-scan. In any case, using images every 5% of the R-R’ interval wouldn’t have been useful due to our temporal image resolution (little more than 200ms). Second, the Ea-like wave assessment by CT shows low interobserver reproducibility. Accordingly, ECG-gated CT couldn’t assess LV filling pressure in patients with PH.
Today, echocardiography is used as the gold standard when assessing HFpEF in many CT or CMRI studies [14,15,27]. Nevertheless, echocardiography often fails to diagnose diastolic dysfunction and heart catheterization remains the gold standard for the diagnosis of elevated LV filling pressure [33]. The use of the recommended gold standard (HC) might partly explain the negative results of our study for DVFi, PFR and PFR/Ea-like.
23 Moreover, due to the studied pathology, our sample population and patient’s clinical presentations were heterogeneous. Patients may have had systolic or diastolic dysfunction but with no left heart disease.
This heterogeneity is an important difference compared to other studies in which patients were either free of or suffered from diastolic dysfunction [14,15,27,31]. The clinical application of LV evaluation criteria to ECG-gated CT, in a medium sized and non-selected population sample, may complicate the positive diagnosis of post-capillary PH. Indeed, our study probably suffers from a lack of statistical power for left ventricular assessment.
A previous study suggests that PCWP frequently underestimates LVEDP [3]. The study by Robbins et al [34] shows that a fluid challenge performed during RHC avoids misclassification of post-capillary PH in pulmonary arterial hypertension. In our study, to limit this bias, cardiologists could perform a fluid challenge and if necessary LHC.
Moreover, this precaution minimized the impact of the delay between cardiac catheterization and ECG-gated CT. Indeed, left heart filling pressures may be normalized by diuresis or vasodilator therapy which could mask the post-capillary component of PH when we performed both ECG-gated CT and HC (35]. Nonetheless, the main issues concerning HC are its invasiveness with potential complications and radiation exposure.
The main limitations of this study are its retrospective design, and the delay between CT and HC which led to misclassification of pre and post-capillary patients. It seems difficult to increase the number of cardiac phases in the reconstruction due to the high computing time needed.
24 CONCLUSION
ECG-gated CT can provide HFpEF criteria to distinguish PH patients with or without PCWP≥15 mmHg. The LAd and LAEF/LAVd ratio are the best parameters to use but some LV criteria may also be useful to diagnose post-capillary-PH in routine practice.
ACKNOWLEDGEMENT:
Study ethics approval was obtained on 11 February 2015 (CECIC Rhône-Alpes-Auvergne, Clermont-Ferrand, IRB 5891). Patient consent was not required.
We thank Dr Alison Foote (Grenoble University Hospital Clinical Research Center) for editing the manuscript and Ms Maud MEDICI, biostatistician, for her statistical expertise.
The authors have no conflicts of interest to disclose. Professor Gilbert FERRETTI is the Guarantor of this study.
25 ANNEXES:
Figure 1: LV length measurement on 4 chamber-view:
Footnotes:
26 Figure 2: LV volume versus RR’ phase curves:
Footnotes:
LV volume versus RR’ phases curve of patient with post capillary (a) and pre-capillary (b) PH. Note the rapid LV filling in the first part of diastolic phase in the patient with post-capillary PH compared to that with pre-capillary PH.
27 Table 1: Population Characteristics
Clinical characteristics N=89 P
Age, median[Q1-Q3] (years)
Age pre-capillary / post-capillary, median [Q1-Q3]
66 [58;75] 62 [56;72.5] / 69 [65.25;77.75] p=0.01 Men/women n(%) 44 (49)/45 (51) NYHA classes: ≤II n (%) ≥III n (%) 40 (45) 49 (55) PH etiology
1. Pulmonary arterial hypertension (PAH) 13 (15%)
1.1 Idiopathic PAH 6 (7%)
1.3 Drug and toxin induced 1.4 Associated with:
1.4.1 Connectivite tissue disease 1.4.4 Congenital cardiopathy
1’. Pulmonary veno-occlusive disease and/or
pulmonary capillary haemangiomatosis
1 (1%)
5 (6%) 1 (1%) 2 (2%)
2. Pulmonary hypertension due to left heart disease 3. Pulmonary hypertension due to lung disease
and/or hypoxemia
4. Chronic thromboembolic pulmonary
hypertension (CTEPH)
5. PH with unclear multifactorial mechanism
No PH 22 (25%) 11 (12%) 21 (23%) 6 (7%) 14 (16%)
Pre-capillary / post-capillary / mixed / normal (%) 53 (59) / 13(15) / 9 (10) / 14 (16) Mean PAP, median [Q1-Q3] (mmHg) 40 [28;50]
PCWP, median [Q1-Q3] (mmHg) 11 [8;16]
Footnotes:
NYHA: New York Heart Association; PH: Pulmonary hypertension; PCWP: Pulmonary Wedge Capillary Pressure; PAP: Pulmonary Artery Pressure. PH: Pulmonary Hypertension, HC: Heart catheterization, RHC: Right HC, LHC: Left HC.
28 Table 2: Interobserver reproducibility, all variables
variables Kappa[IC 95%]
LAVd 1[1 ;1]
LA diameter 0.97[0.91 ;0.99]
Visual estimation of enlargement of LA 0.69[0.21 ;1]
LAEF 1[0.96 ;1]
time of recovery of 70% of stroke volume 0.96[0.7 ;0.99]
percentage of end-diastolic volume recovered
during the first 50% of diastole
0.86[0.04 ;0.96]
Ea-like wave 0.28[0.04 ;0.43]
PFR 0.96[0.88 ;0.97]
Diastolic volume function index 0.87[0.3 ;0.97]
Footnotes:
Below 0.4, interobserver reproducibility is low, middling between 0.4 and 0.6, strong between 0.6 and 0.8 and almost perfect when above 0.8.
LAVd: Left Atrial end-diastolic Volume, LA: Left Atrial, LAEF: LA ejection fraction, PFR: Peak Filling Rate.
29 Table 3: Results of CT evaluation according to heart catherization results
criteria PCWP>15mmHg n=22 PCWP≤15mmHg n=67 P values LA diameter mean (sd) 52.27 (10.07) 40.34 (7.75) <0.001 LAEF/LAVd median[Q1;Q3] 0.2 [0.09;0.41] 0.82 [0.56;0.97] <0.001 LAEF median[Q1;Q3] 19.7 [9.53;29.36] 38.18 [32.83;43.91] <0.001 Time to recovery of 70% of stroke volume (% of RR’) median[Q1;Q3] 28.33 [23.13;34.37] 34.52 [30;42.2] 0.02 Percentage of end-diastolic volume recovered during the first 50% of diastole median[Q1;Q3]
81.2 [75.7 ;89.05] 76.58 [69.76 ;80.19] 0.045
Diastolic volume function index median[Q1;Q3]
63.25 [57.41;78.43] 61.54 [50;69.49] 0.16
Ea wave median[Q1;Q3] 59.16 [45.58;79.29] 60 [45.08;90.5] 0.6
PFR mean (sd) 331.8 (118.34) 306.4 (110.53) 0.40
Footnotes:
LA: Left Atrial, LAVd: Left Atrial end-diastolic Volume, LAEF: LA ejection fraction, PFR: Peak Filling Rate.
30 Figure 3: Multivariate analysis (PLS-DA plot)
Footnotes:
a) On this PLS-DA chart, both pre (blue) and post-capillary (red) groups are not totally dissociated. A majority of the post-capillary patients are located in the upper right of the plot.
31
b) LA diameter and LAEF/LAVd are the most discriminating parameters: the post-capillary patients tend to have a higher value for LA diameter and a lower value for LAEF/LAVd.
32 Figure 4: ROC curves of variables selected by PLS-DA:
35 Footnotes:
LA antero-posterior diameter and LAEF/LAVd seem to be the best diagnostic criteria with AUC ≥0.8. LA: Left atrial; LAEF: Left atrial ejection fraction; LAVd: Left atrial diastolic volume.
36 Table 4: Diagnostic performance of variables selected in multivariate analysis in discerning post-capillary PH: Criteria Cutoff value Se (%) Sp (%) AUC (95% CI) LA diameter (mm) 49.5 90 64 0.82 [0.71;0.93] LAEF (%) 31.2 78 79 0.78 [0.63;0.93] LAEF/LAVd ratio 0.45 85 78 0.80 [0.65;0.94]
time of recovery of 70% of stroke volume (% of RR’) 76.7 44 88 0.68 [0.53;0.82]
percentage of end-diastolic volume recovered during the
first 50% of diastole (it is equivalent to 70% of RR’
interval) (%)
87.5 94 38 0.65 [0.50;0.80]
Footnotes:
LA: Left Atrial, LAVd: Left Atrial end-diastolic Volume, LAEF: LA ejection fraction, PFR: Peak Filling Rate.
39 REFERENCES
1. Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. (2013) Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 62:D34–41 2. Vachiéry J-L, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, et al. (2013) Pulmonary
hypertension due to left heart diseases. J Am Coll Cardiol 62:D100–108.
3. Halpern SD, Taichman DB (2009) Misclassification of pulmonary hypertension due to reliance on pulmonary capillary wedge pressure rather than left ventricular end-diastolic pressure. Chest 136:37–43.
4. Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, et al. (2012) ASPIRE registry: assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 39:945–955.
5. Sharma K, Kass DA (2014) Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res 115:79–96.
6. Guazzi M (2014) Pulmonary hypertension in heart failure preserved ejection fraction: prevalence, pathophysiology, and clinical perspectives. Circ Heart Fail 7:367–377. 7. Leung CC, Moondra V, Catherwood E, Andrus BW (2010) Prevalence and risk factors of
pulmonary hypertension in patients with elevated pulmonary venous pressure and preserved ejection fraction. Am J Cardiol 106:284–286.
8. Guazzi M, Gomberg-Maitland M, Arena R (2015) Pulmonary hypertension in heart failure with preserved ejection fraction. J Heart Lung Transplant 34:273–281.
9. Lam CSP, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. (2009) Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol 53:1119–1126.
10. Rosenkranz S, Bonderman D, Buerke M, Felgendreher R, Freyhaus H ten, Grünig E, et al. (2011) Pulmonary hypertension due to left heart disease: updated Recommendations of the Cologne Consensus Conference 2011. Int J Cardiol 154 Suppl 1:S34–44.
11. Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery J-L, Barbera JA, et al. (2009) Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 30:2493–2537.
12. Huda W, Rowlett WT, Schoepf UJ (2010) Radiation dose at cardiac computed tomography: facts and fiction. J Thorac Imaging 25:204–212.
13. Sauvage N, Reymond E, Jankowski A, Prieur M, Pison C, Bouvaist H, et al. (2013) ECG-gated computed tomography to assess pulmonary capillary wedge pressure in pulmonary hypertension. Eur Radiol 23:2658–2665.
14. Boogers MJ, van Werkhoven JM, Schuijf JD, Delgado V, El-Naggar HM, Boersma E, et al. (2011) Feasibility of diastolic function assessment with cardiac CT: feasibility study in comparison with tissue Doppler imaging. JACC Cardiovasc Imaging 4:246–256.
40 15. Mendoza DD, Codella NCF, Wang Y, Prince MR, Sethi S, Manoushagian SJ, et al. (2010) Impact
of diastolic dysfunction severity on global left ventricular volumetric filling - assessment by automated segmentation of routine cine cardiovascular magnetic resonance. J Cardiovasc Magn Reson 12:46.
16. Caudron J, Fares J, Bauer F, Dacher J-N (2011) Evaluation of left ventricular diastolic function with cardiac MR imaging. Radiographics 31:239–259.
17. Kawasaki M, Tanaka R, Ono K, Minatoguchi S, Watanabe T, Iwama M, et al. (2014) A novel ultrasound predictor of pulmonary capillary wedge pressure assessed by the combination of left atrial volume and function: A speckle tracking echocardiography study. J Cardiol. 18. Safdar Z, Katz M, Frost A (2010) Computed axial tomography evidence of left atrial
enlargement: a predictor of elevated pulmonary capillary wedge pressure in pulmonary hypertension. Int J Gen Med 3:23–29.
19. Posina K, McLaughlin J, Rhee P, Li L, Cheng J, Schapiro W, et al. (2013) Relationship of phasic left atrial volume and emptying function to left ventricular filling pressure: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 15:99.
20. Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. (2005) Diastolic dysfunction and left atrial volume: a population-based study. J Am Coll Cardiol 45:87–92.
21. Aurigemma GP, Gottdiener JS, Arnold AM, Chinali M, Hill JC, Kitzman D. (2009) Left atrial volume and geometry in healthy aging: the Cardiovascular Health Study. Circ Cardiovasc Imaging 2:282–289.
22. Sohrabi S, Hope M, Saloner D, Keedy A, Naeger D, Lorca MCN, et al. (2015) Left atrial transverse diameter on computed tomography angiography can accurately diagnose left atrial enlargement in patients with atrial fibrillation. J Thorac Imaging 30:214–217. 23. Nishimura RA, Appleton CP, Redfield MM, Ilstrup DM, Holmes DR, Tajik AJ. (1996)
Noninvasive doppler echocardiographic evaluation of left ventricular filling pressures in patients with cardiomyopathies: a simultaneous Doppler echocardiographic and cardiac catheterization study. J Am Coll Cardiol 28:1226–1233.
24. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation 102:1788–1794.
25. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and
Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28:2539– 2550.
26. Oh JK, Hatle L, Tajik AJ, Little WC (2006) Diastolic heart failure can be diagnosed by
comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol 47:500– 506.
41 segmentation of routine clinical cardiac magnetic resonance imaging for assessment of left ventricular diastolic dysfunction. Circ Cardiovasc Imaging 2:476–484.
28. Codella NCF, Weinsaft JW, Cham MD, Janik M, Prince MR, Wang Y. (2008) Left ventricle: automated segmentation by using myocardial effusion threshold reduction and intravoxel computation at MR imaging. Radiology 248:1004–1012.
29. Borges AC, Knebel F, Eddicks S, Panda A, Schattke S, Witt C, et al. (2006) Right ventricular function assessed by two-dimensional strain and tissue Doppler echocardiography in patients with pulmonary arterial hypertension and effect of vasodilator therapy. Am J Cardiol 98:530– 534.
30. Pommier M (2013) Groupe d’experts AFIB 2012 : état de l’art en imagerie médicale. IRBM News 34:41–53.
31. Okayama S, Nakano T, Uemura S, Fujimoto S, Somekawa S, Watanabe M, et al. (2013) Evaluation of left ventricular diastolic function by fractional area change using cine cardiovascular magnetic resonance: a feasibility study. J Cardiovasc Magn Reson 15:87. 32. Pedrizzetti G, Sengupta S, Caracciolo G, Park CS, Amaki M, Goliasch G, et al. (2014)
Three-dimensional principal strain analysis for characterizing subclinical changes in left ventricular function. J Am Soc Echocardiogr 27:1041–1050.e1.
33. Zoreka F, Bouvaist H, Vautrin E, Marlière S, Guerbaai R, Labarere et al. (2015) Pulmonary Capillary Wedge Pressure Measurement: A Challenge for Diagnosis of Pulmonary Arterial Hypertension. Int J Respir Pulm Med 2:010.
34. Robbins Robbins IM, Hemnes AR, Pugh ME, Brittain EL, Zhao DX, Piana RN, et al. (2014) High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in
pulmonary hypertension. Circ Heart Fail 7:116–122.
35. Fang JC, DeMarco T, Givertz MM, Borlaug BA, Lewis GD, Rame JE, et al. (2012) World Health Organization Pulmonary Hypertension group 2: pulmonary hypertension due to left heart disease in the adult--a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 31:913– 933.
42
SERMENT D’HIPPOCRATE
En présence des Maîtr es de cette Faculté, de mes chers condisc iples et devant l’effigie d’HIPPOCRATE,
Je promets et je jure d’être fidèle aux lois de l’honneur et de la probité dans l’exercice de la Médecine.
Je donnerai mes soins gratuitement à l’indigent et n’exigerai jamais un salaire au dessus de mon travail. Je ne participerai à aucun partage clandestin d’honor aires. Admis dans l’intimité des maisons, mes yeux n’y verront pas ce qui s’y passe ; ma langue tair a les secrets qui me seront confiés et mon état ne servir a pas à
corrompre les mœurs, ni à favoriser le crime.
Je ne permettrai pas que des considérations de religion, de nation, de race, de parti ou de classe sociale viennent s’interposer entre mon devoir et mon patient. Je garderai le respect absolu de la vie humaine.
Même sous la menace, je n’admettrai pas de faire usage de mes connaissances médicales contre les lois de l’humanité.
Respectueux et reconnaissant envers mes Maî tres, je rendrai à leur s enfants l’instr uction que j’ai r eçue de leurs pères.
Que les hommes m’accordent leur estime si je suis fidèle à mes promesses. Que je sois couvert d’opprobre et mépr isé de mes confrères si j’y manque.