Comportement du gastéropode du médiolittoral
Littorina saxatilis face aux fluctuations météorologiques
journalières
Mémoire
Guadalupe Daniela Fernandez Nieto
Maîtrise en biologie - avec mémoire
Maître ès sciences (M. Sc.)
Comportement du gastéropode du médiolittoral
Littorina saxatilis face aux fluctuations météorologiques
journalières
Mémoire
Guadalupe Daniela Fernández Nieto
Sous la direction de :
Ladd E. Johnson, directeur de recherche
Christopher W. McKindsey, codirecteur de recherche
Résumé
La vie sur la Terre est apparue et a évolué dans un milieu physique qui change dans le temps et l’espace. Les êtres vivants se trouvent dans les environnements qui englobent l’ensemble des conditions physiques, chimiques et biologiques qui les permettent de compléter leur cycle de vie. Certains facteurs environnementaux comme la température et la présence d’eau affectent particulièrement l’abondance et la distribution des espèces autour de la Terre. Il y a aussi des variations du milieu physique dans le temps. La vie sur Terre subit des cycles annuels, saisonniers et journaliers et certains cycles peuvent se compléter en quelques heures. Tous les changements spatiaux et temporaux façonnent le milieu physique et les écosystèmes s’y développant. Par exemple, dans le médiolittoral, les organismes subissent des changements dus à l’action des marées. Le mouvement de l’eau causé par les marées expose, pendant une période de temps, les organismes y habitant à des conditions extrêmes. Ces communautés, majoritairement composées d’algues et d’invertébrés, sont dotées d’adaptations physiologiques ou comportementales qui leur permettent de survivre à ces conditions. Les invertébrés mobiles présentent souvent des changements comportementaux qui leur permettent de trouver des endroits moins stressants servant de refuges pendant la marée basse. Cette étude se consacre à étudier le comportement d’un gastéropode du médiolittoral très abondant dans les milieux tempérés, Littorina saxatilis. Ce gastéropode se retrouve dans le littoral de l’estuaire maritime du Saint-Laurent à l’intérieur des fissures dans les roches et se déplace vers l’extérieur pour brouter des algues vertes éphémères. Nous avons observé son comportement d’utilisation de refuges lors de différentes conditions météorologiques et nous avons fait une expérience de manipulation sur le terrain pour mieux comprendre l’effet de différents facteurs environnementaux qui peuvent affecter son comportement. Les gastéropodes herbivores du médiolittoral est une composante importante de ces communautés et affectent la biodiversité locale. En comprenant mieux leurs réponses comportementales aux changements environnementaux, nous pouvons mieux comprendre l’ensemble de cet écosystème.
Abstract
Life on Earth has appeared and evolved in a physical environment that changes through time and space. Living beings are found in places that bring together all the environmental conditions that allow them to complete their life cycle. Environmental factors such as temperature and the presence of water affect the abundance and distribution of species around the Earth. There are also variations in the physical environment over time. Life on Earth experiences annual, seasonal and daily cycles and can experience environmental changes on an hourly scale. All spatial and temporal changes shape the physical environment and the ecosystems that develop there. In the intertidal environment, organisms undergo changes due to tidal action. The movement of water caused by tides exposes the organisms living in it for a period of time. These organisms, mainly algae and invertebrates, have physiological or behavioral adaptations that allow them to survive aerial conditions. Mobile invertebrates often exhibit behavioral changes that allow them to find less stressful places to take refuge during the low tide. This study investigated the behavior of an intertidal gastropod, Littorina saxatilis, which is widely distributed in temperate environments. In the littoral zone of the St. Lawrence Maritime Estuary, this gastropod is often found inside cracks in the rock surface and forages outward from these refuges to graze on ephemeral algae. We observed the use of refuges under different weather conditions and conducted a field manipulation experiment to better understand the effect of different environmental factors affecting this behavior. The herbivorous gastropods of the intertidal are an important component of these ecosystems that affect local biodiversity. By better understanding their behavior responses to environmental changes, we can better understand this ecosystem and the changes that it is experiencing.
Table des matières
Résumé ... ii
Abstract ... iii
Table des matières ... iv
Liste des tableaux ... v
Liste des figures ... vi
Remerciements ... ix
Introduction ... 1
Chapitre 1 Meteorological conditions and substrate desiccation affect the use of shelters by Littorina saxatilis during low tide. ... 7
Résumé ... 7
Abstract ... 8
Introduction ... 9
Methods and Materials ... 12
Results ... 16
Discussion ... 30
Perspectives ... 35
Conclusion ... 36
Liste des tableaux
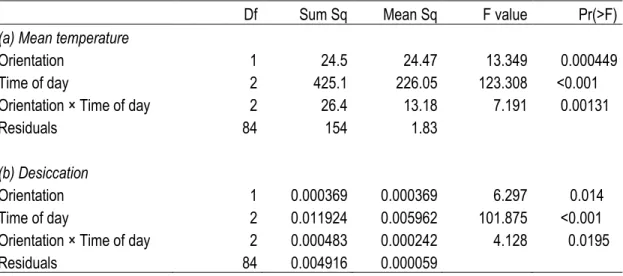
Table 1. Series of ANOVA testing the effect of Orientation and Time of day on (a) Mean temperature and (b) Desiccation
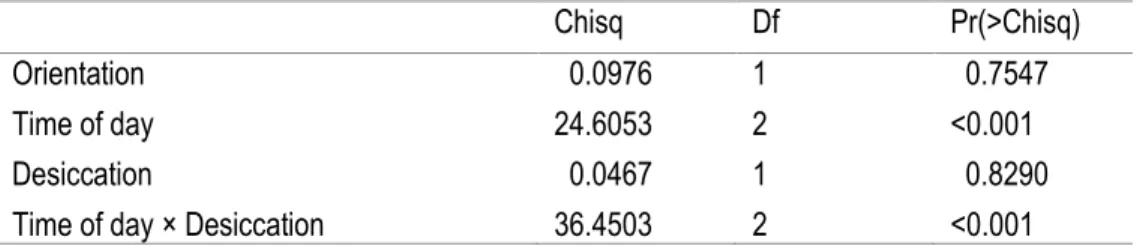
Table 2. Binomial general mix model with repeated measures of the proportion of snails grazing outside crevices
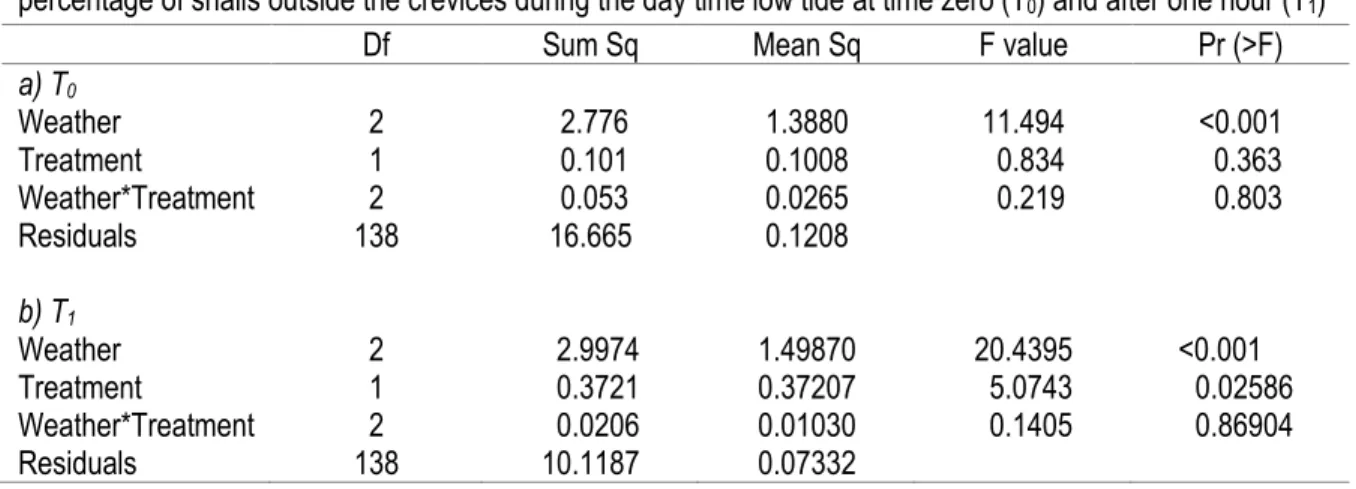
Table 3. Series of ANOVA testing the effect of the addition of water and different categories of weather on the percentage of snails outside the crevices during the day-time low tide at time zero (T0) and after one hour (T1)
Table 4. Result of the Mix Linear Model with Repetitive Measures of the percentage of snails outside crevices Table 5. ANOVA table for testing the effect of day or night and the on the percentage of snails outside the crevices during low tide
Table 6. Result of the Mix Linear Model with Repetitive Measures of the percentage of snails outside crevices for day and night analysis
Liste des figures
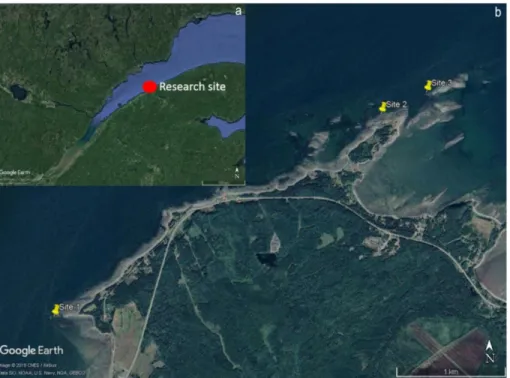
Figure 1. a) Map of the Saint Lawrence Maritime Estuary in Quebec, Canada. b) Location of the three research site in the Mitis region
Figure 2. Mean desiccation rate at different times of the day and orientations (mean - SE)
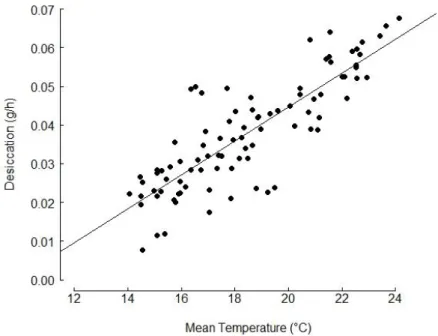
Figure 3. Linear model of the desiccation rate measure over two hours as a function of mean estimated substratum temperature during low tide (R2= 0.7; n = 90)
Figure 4. Mean percentage of snails outside of crevices at different times of the day and different orientations (mean - SE)
Figure 5. Percentage of snails outside of the crevices (mean - SE) at two substrate moisture treatments during different types of Weather: Calm, Breeze, and Windy at day-time low tide at the beginning of the experiment (T0) and after one hour (T1)
Figure 6. Mean of the percentage of snails outside of crevices per day (N=12) as a function of wind speed. The different shapes are associated with different values of relative humidity (mean - SE). R2= 0.44
Figure 7. Percentage of snails outside of the crevices for two substrate moisture treatments during night-time or day-time low tides (mean - SE)
À mes parents, José et Lupita. Car ils m’ont
toujours donné plus que ce dont j’ai besoin.
À mes sœurs, Mari y Ceci, qui me font
toujours rire. À mon mari, Eliud, pour toute
la motivation à la fin de ma maîtrise.
« Quel que soit votre travail, faite-le de tout
votre cœur. » Col 3, 23
Remerciements
Je veux remercier énormément à mon directeur de rechercher Ladd Johnson, car il a été un mentor pour moi du début de ma carrière en science avec mon initiation à la rechercher et jusqu’à maintenant. Merci pour toutes les expériences différentes que j’ai pu vivre tout au long de mes études, j’ai appris énormément dans son laboratoire et je lui serais toujours reconnaissante. Je veux remercier aussi tous mes collègues de laboratoire pour les merveilleuses années que j’ai passé avec vous. Merci pour tous les échanges, les apprentissages et le bel environnement de travail toujours si motivant. Merci aussi pour votre amitié inconditionnelle. Merci à Charlotte, à Marie-Hélène et à Heather pour votre aide sur le terrain dans des conditions si glissantes. Merci à Chris McKindsey pour les commentaires au début de ma maîtrise et les corrections de mon mémoire ainsi que pour l’opportunité d’utiliser les installations de l’Institut Maurice Lamontagne. Merci à Julie Turgeon de m’avoir évalué à chaque étape de ma maîtrise. Merci à mes amis de la paroisse St-Thomas-d’Aquin qui m’ont donné une famille à Québec. Finalement, merci à ma famille et son support et amour si inconditionnel. Tout, du début, est grâce à vous. Merci d’avoir semé en moi l’amour pour la science et la nature. Merci pour l’opportunité que vous m’avez donnée d’aller étudier à l’étranger qui m’a permis de grandir autant. Merci d’avoir cru en moi du départ ¡Gracias!
Introduction
La vie sur la Terre est structurée dans un milieu physique, un environnement composé de tous les facteurs inertes qui l’entourent. Commençant par les phénomènes de rotation et translation de la Terre dans l’espace jusqu’aux éléments se trouvant dans le sol, le milieu physique encadre la vie que nous trouvons sur la Terre et a permis son évolution tout au long du temps géologique.
La vie a colonisé toutes les sortes de milieux existants sur la Terre. Dans chaque milieu, un ensemble d’organismes se sont adaptés aux conditions environnementales présentes. La notion de niche écologique (Grinnell, 1904) nous illustre sur l’ensemble des conditions présentes dans un milieu physique qui vont permettre l’existence d’une certaine espèce dans un espace spécifique. Chaque espèce a une combinaison de facteurs environnementaux qui lui permet de compléter son cycle de vie. Cette combinaison est différente d’une espèce à une autre.
Il y a beaucoup de facteurs environnementaux qui affectent les organismes, mais l’irradiance, la température et la présence d’eau sont eux qui vont façonner le plus la vie sur la terre, car les réactions biochimiques de base chez les êtres vivants en dépendent (Vasseur et al., 2014). L’irradiance et la température vont souvent co-varier dans les milieux parce qu’ils sont tous les deux produits par le Soleil (Raven et al., 2007). Quant à la disponibilité de l’eau, on sait que l’état de l’eau change avec la température. Au tour de la Terre, l’irradiance et la température ne sont pas égales, elles changent en suivant la latitude sur la Terre formant des gradients.
Il existe une variation spatiale dans le milieu physique et un des changements le plus évidents est la différence de température entre l’équateur et les pôles. Ce gradient de température distribue les espèces dans des zones différentes, soit plus dans l’équateur, aux tropiques, aux zones plus tempérées ou encore boréales et polaires, suivant le niveau de tolérance de chaque espèce aux différents écarts de température (e.g. Chen et al., 2011; Bozinovic et al., 2011). À une échelle plus petite, la température change aussi dépendamment de l’altitude sur la terre, étant plus élevée près du niveau de la mer et descendant avec l’altitude. Des espèces différentes se distribuent aussi le long de ce gradient vertical créant des zones de haute altitude avec un paysage semblable à celui qu’on observerait dans la toundra Arctique (Küchler, 1984).
La variation spatiale du milieu physique permet de rassembler des espèces avec des traits semblables qui leur permettront de vivre dans un milieu donné et pas dans un autre. Le milieu impose des conditions qui vont permettre l’accumulation des traits favorisant la survie dans ce milieu. Par des interactions écologiques entre les espèces présentes, comme la compétition, les symbioses, la prédation, etc., les différents écosystèmes se forment et évoluent ainsi dans le temps (Raven et al., 2007).
Le milieu physique n’est pas uniquement variable dans l’espace, mais aussi dans le temps. Il a changé dans l’histoire géologique de la Terre à grande échelle, mais il subit aussi des changements cycliques, routiniers suivant les mouvements de la Terre autour du Soleil (e.g. Küchler, 1984). L’irradiance, la température et la disponibilité de l’eau varient d’année en année avec les saisons. Dans le temps, ces trois facteurs affectent notamment les organismes photosynthétiques, car ils transforment l’énergie solaire et la matière inorganique en matière organique (Raven et al., 2007). Lorsque les journées deviennent plus longues et qu’il y a plus de lumière, les phototrophes réalisent plus de photosynthèse et entrent dans leur saison de croissance et reproduction, changeant la dynamique de tout le reste de l’écosystème.
Les phototrophes rendent disponible cette énergie pour le reste du système et, en se faisant consommer par les herbivores, cette énergie passe vers les animaux et s’y distribue. Alors, les animaux à leur tour s’ajustent aussi à ces cycles globaux profitant les moments où la photosynthèse a été abondante pour se nourrir et combler leurs propres besoins de croissance et de reproduction. On observe dans la nature par exemple des herbivores qui vont se nourrir après le pic de photosynthèse de la journée pour avoir accès à un aliment plus nourrissant (e.g. Gregorini, 2012) ou des animaux qui coordonnent leur période de reproduction avec les saisons suivantes des signaux environnementaux (e.g. Wingfield et al., 1992) ou encore les baleines qui migrent vers le nord en suivant les floraisons phytoplanctoniques au printemps (Visser et al, 2011). Toutes ces interactions biologiques sont encadrées par des changements temporels cycliques de l’environnement physique.
À une échelle temporelle beaucoup plus courte, le milieu physique peut changer en suivant les conditions météorologiques. C’est-à-dire, l’ensemble des conditions atmosphériques de température, humidité relative, vitesse des vents, etc., affectant un lieu spécifique (e.g. Küchler, 1984). Ces changements peuvent être subtiles, mais aussi très rapides et de grande amplitude, créant des conditions parfois stressantes pour les organismes. Il y a des endroits avec des conditions généralement plus stables et des milieux où les changements brusques sont à l’ordre du jour. Dépendamment d’où les espèces ont évoluées, elles peuvent avoir des traits physiologiques où comportementales pour faire face à des conditions difficiles. Les changements de température brusques hors des conditions moyennes peuvent entrainer des niveaux de stress importants aux organismes et même causer une augmentation de la mortalité (Vasseur, 2014).
Dans le monde animal, il y a deux façons de gérer la température corporelle, il y a des animaux endothermes et des animaux ectothermes. Les animaux endothermes sont ceux qui gardent leur température corporelle constante malgré les changements de la température de l’environnement. Ce sont uniquement les mammifères et les oiseaux. L’endotherme les a permis d’être abondants dans toute la superficie de la terre et même dans les milieux terrestres de zones polaires très froides (e.g. Swanson et Garland, 2009). La
température corporelle des ectothermes, par contre, change avec les conditions environnementales. Pour faire face aux changements brusques de l’écosystème elles présentent des traits comportementaux qui leur permettent de trouver des zones d’ombrage ou de s’exposer au soleil pour garder leur température moyennement constante (e.g. Kearney et al., 2009).
Les ectothermes possèdent une courbe de performance qui est en fonction de la température. Cette courbe nous montre dans un premier temps, l’augmentation de la performance écologique (taux de croissance et de reproduction) avec l’augmentation de la température. Par la suite il y a un écart de température où l’espèce réalise sa meilleure performance et finalement le seuil à partir duquel l’augmentation de la température devient stressante et mortelle qui se traduit par une chute de la performance (Vasseur et al., 2014). Les espèces ectothermes sont malgré très abondantes au tour de la terre et pour garder leur température à des valeurs optimales, ils vont déployer tout un grand inventaire de traits comportementaux, métaboliques et physiologiques.
Dans les déserts par exemple, un milieu connu pour les changements drastiques de température entre le jour et la nuit nous observons le changement d’habitat et de comportement entre jour et nuit d’une sauterelle pour éviter la prédation lorsque chaque nuit, la température froide l’empêche de fuir le prédateur (Maeno et al., 2019). Un autre milieu connu et classiquement étudié par ces changements drastiques c’est le milieu médiolittoral. En se trouvant entre la mer et la terre, ce milieu montre de grandes variations temporelles et spatiales à des échelles très petites. Ce milieu est délimité par le mouvement de l’eau lors des marées.
Les marées sont les montées et les descentes du niveau de la mer causées par les forces d’attraction gravitationnelles de la Lune et du Soleil ainsi que par les forces centrifuges du mouvement de la Terre sur l’ensemble des océans. En plus des corps célestes, beaucoup de facteurs locaux vont affecter le cycle des marées comme la distribution des continents, la topographie de la côte et les courants marins. Trois types de cycles de marées ont été décrits, les diurnes, les semi-diurnes et les semi-diurnes mixtes. Les diurnes, il y a juste une marée haute et une marée base chaque vingt-quatre heures. Dans les semi-diurnes, il y a deux marées hautes aux niveaux semblables et deux marées basses aux niveaux semblables par jour. La période de ces marées est de douze heures et vingt-cinq minutes, alors les heures des marées hautes et basses avancent d’à peu près quarante-cinq minutes d’une journée à l’autre. Finalement, les marées semi-diurnes mixtes sont deux marées hautes et deux marées bases aussi, mais à des niveaux différents.
Ces mouvements de l’eau vont créer des variations temporelles très importantes du milieu médiolittoral en passant du milieu aquatique vers un milieu aérien dans une échelle très courte. Ces mouvements d’eau vont créer aussi des gradients spatiaux perpendiculaires à la côte (Raffaelli et Hawkins, 1999). Lors de la marée base, par exemple, les conditions près du niveau de l’eau le plus bas vont être toujours plus humides et
fraîches qu’un niveau plus haut, car cet espace est exposé plus de temps à l’air que plus bas. Sur une échelle due centimètre au mètres, l’humidité ainsi que la température et la salinité changent beaucoup du niveau le plus bas au niveau le plus élevé.
Le médiolittoral, même s’il est entre la terre et la mer, il est majoritairement colonisé par des organismes marins. Ces organismes se sont adaptés au fil du temps pour être capables de vivre à l’extérieur de l’eau lors des marées bases. Étant donné les gradients spatiaux présents, les organismes qui habitent se sont distribué le long des différents niveaux du médiolittoral en suivant leur tolérance aux températures plus élevées ou au manque d’eau dans les niveaux plus élevés (Raffaelli et Hawkins, 1999). Vers les niveaux les plus bas du médiolittoral, où les conditions environnementales sont moins stressantes, c’est la compétition pour une place dans le substrat et la prédation qui vont délimiter les zones de répartition (e.g. Rochette et Dill, 2000). Les animaux présents, qui sont généralement des invertébrés ectothermes, vont se fixer à des microclimats favorables qui tamponnent les variations environnementales et vont réguler leur température corporelle par des changements de comportement (Miller et al., 2014).
Un autre facteur qui covarie avec la température, mais qui a des effets différents est la dessiccation du milieu et des organismes. Après le départ de l’eau au début de la marée base, l’air et l’augmentation de la température évaporent l’eau, le milieu s’assèche et les organismes se déshydratent. Les organismes vont alors préférer des microclimats plus humides qui retiennent l’eau et vont adopter des comportements qui réduisent la déshydratation. Les organismes sessiles comme les algues peuvent produire des substances chimiques qui les protègent contre les effets du Soleil ainsi que contre la déshydratation (e.g. Sampath-Wiley et al., 2008). Les animaux plus mobiles, comme les gastéropodes, vont arrêter leur quête de nourriture, s’enfermer dans leur coquillage ou chercher des refuges ou microclimats comme les crevasses dans les roches (Chapperon et Seuront, 2013).
L’environnement physique affecte énormément la biologie de chaque espèce et par conséquent il affecte aussi les interactions biologiques, comme la prédation et l’herbivore. Les organismes ont besoin de se nourrir, mais dans le médiolittoral il faut que les conditions environnementales soient propices, car, pour les organismes mobiles, sortir d’un refuge signifie s’exposer aux conditions physiques difficiles. Aux conditions environnementales s’ajoute généralement, le risque de prédation. Les animaux vont préférer aller se nourrir lorsque le milieu physique ne présente pas des risques à leur survie et lorsque les prédateurs ne sont pas très abondants ou efficaces.
Les organismes qui vivent dans le médiolittoral doivent trouver l’espace et le moment approprié pour se nourrir en évitant la prédation, mais aussi les stress associés au milieu aérien lors de la marée base, comme la
hausse de température ou la dessiccation, et ceux associés à la marée haute comme le délogement par la force des vagues.
Les conditions météorologiques jouent un rôle très important dans ce milieu en rajoutant une couche de variation temporelle très imprédictible pour les organismes. Les conditions météorologiques à un moment précis peuvent accentuer ou diminuer les conditions environnementales et leurs effets sur les êtres vivants et leurs activités. Les organismes du médiolittoral sont très bien adaptés aux changements physiques, mais peuvent être fragiles lorsque les conditions météorologiques poussent les conditions physiques hors des limites de tolérance de ces organismes. (e.g. Helmuth et al. 2002).
Comme c’est le milieu physique à la base qui façonne la majorité des caractéristiques du milieu médiolittoral, étudier comment tous les organismes répondent aux changements périodiques, mais aussi ponctuels peut nous indiquer beaucoup sur comment les interactions entre les espèces changent et sur les conséquences sur le reste de la communauté.
Un groupe important des rivages tempérés sont les gastéropodes du genre Littorina. Ces escargots sont des organismes herbivores se trouvant tout autour du monde. Par leur comportement d’herbivores, ils modifient la biodiversité d’algues du médiolittoral en augmentant le substrat disponible à la colonisation (Lubchenco, 1978).
Dans le médiolittoral de l’estuaire du Saint-Laurent nous trouvons trois espèces de littorines, Littorina obstusata, L. littorea et L. saxatilis. La répartition dans le médiolittoral dépende de leur tolérance à l’augmentation de température et dessiccation, la présence des prédateurs et leur source et abondance de nourriture. L. littorea c’est la plus grande de taille d’entre les trois et tend à se trouver dans le niveau plus bas du littoral où elle se nourrit préférentiellement de l’algue verte du genre Ulva. Cette espèce est d’origine européen, introduite en Amérique il y a longtemps, cependant, nous avons observé une augmentation de sa population dans l’estuaire du Saint-Laurent pendant les dernières années. L. obstusata se localise habituellement où il y a des macroalgues du genre Fucus car c’est leur source de nourriture. Souvent, ces macroalgues créent un microclimat assez humide et frais où ces littorines peuvent se réfugier des conditions environnementales difficiles. L. saxatilis se trouve traditionnellement dans le niveau plus élevé du médiolittoral cependant, dans l’estuaire du Saint Laurent, nous observons cette espèce distribuée dans tous les niveaux à cause de la manque de prédation (Pardo et Johnson, 2005). C’est l’espèce L. saxatilis que nous avons utilisé pour réaliser la présente étude.
L. saxatilis est très abondante dans les côtes boréales et tempérés de l’hémisphère Nord. Avec sa petite taille, elle est une bonne proie pour les prédateurs marins, comme les crabes alors, elle tende à se retrouver dans
les zones les plus hautes du médiolittoral. Cette zone diminue la pression de prédation cependant, L. saxatilis reste exposée plus de temps aux conditions aériennes et alors à des températures plus élevées et aux niveaux de dessiccation plus importants. Alors, cette espèce a été très étudiée par ces multiples adaptations physiologiques qui lui permettent de vivre dans ces hauts niveaux du médiolittoral (Sokolova, 2003). Ce gastéropode a un écart de tolérance de température est très large, pouvant tolérer de -16 jusqu’à 40°C (Davenport et Davenport, 2005). Il a été décrit comme un herbivore, car il a une préférence par les tapis d’algues vertes et des diatomées, mais il peut manger aussi des détritus (Marzouk, 2015).
Pour faire face au milieu physique, cet escargot a aussi plusieurs réponses comportementales qui lui permettent de rester dans les milieux moins stressants. Il a été observé chez L. saxatilis beaucoup de comportements différents, comme l’agrégation, l’empilement, le choix des microclimats ou le comportement d’utilisation de refuge qui varie dépendamment des conditions du milieu physique (Ng et al., 2017).
Dans les médiolittoral du Saint Laurent, nous observons une grande utilisation de refuge particulièrement par cette espèce. Les littorines se retrouvent en grande abondance à l’intérieur de petites fissures ou cracks dans les roches. Le long de toutes les rives du Québec, les perturbations causées par l'action des glaces créent une abondance de surfaces rocheuses dénudées dans la zone médiolittorale. Ces surfaces sont colonisées par des algues éphémères, principalement des algues vertes filamenteuses et des diatomées, qui fournissent des ressources alimentaires abondantes à L. saxatilis. Lorsque ce petit herbivore abandonne son refuge, il broute le tapis d’algues qui l’entoure (Pardo et Johnson, 2004).
Par tous ces différents traits physiologiques et comportementales, L. saxatilis est un organisme modèle pour étudier les effets du milieu environnementaux sur les organismes vivants et l’écosystème (e.g. Johannesson, 2003). Ce gastéropode est une composante importante dans les écosystèmes du médiolittoral et comprendre son comportement est essentiel pour la compréhension du système. Par ce projet de maîtrise, nous avons étudié le comportement d’utilisation du refuge ou de broutage de L. saxatilis face aux différentes conditions météorologiques qui affectent son milieu physique. Nous avons regardé plus précisément, les effets sur la température et la dessiccation sur le comportement de L. saxatilis en utilisant la variation spatiale et temporelle présentent dans le milieu.
Chapitre 1 Meteorological conditions and substrate
desiccation affect the use of shelters by Littorina
saxatilis during low tide.
Résumé
Le broutage des gastéropodes façonne les communautés du médiolittoral rocheux. Dans ce milieu, leur comportement est affecté par des facteurs biologiques et physiques. Pour étudier les réponses comportementales du gastéropode Littorina saxatilis face aux fluctuations météorologiques, nous avons premièrement fait un échantillonnage pour calculer le pourcentage d’utilisation du refuge à marée basse selon différents moments de la journée et différentes orientations des refuges aux vagues. Deuxièmement, nous avons fait une expérience sur le terrain et calculé le pourcentage des gastéropodes à l’extérieur des crevasses (i.e. hors refuge) à marée basse pour deux conditions différentes d’humidité du substrat (humide et sec). La température du substrat et la dessiccation ont aussi été mesurées lors de l’échantillonnage et lors de l’expérience sur le terrain. Globalement, nous avons vu une augmentation de l’utilisation du refuge lorsque la marée basse était à midi, probablement dû à une dessiccation habituellement plus forte à cette heure. Cependant, nous avons observé plus d’escargots à l’extérieur des crevasses lorsqu’elles sont exposées au sud, en comparaison au nord, malgré que la température soit plus élevée au sud. Lors de l’expérience, nous avons constaté que le pourcentage de gastéropodes à l’extérieur des crevasses diminue avec la vitesse du vent et la force des vagues. Contrairement à nos prédictions, quand le substrat est maintenu humide moins d’escargots se trouvent à l’extérieur. Donc, dans le traitement humide et lorsque la roche fait face au nord, dans l’échantillonnage, les littorines se sont probablement plus déplacées et ont trouvé un refuge. Cela a donné comme résultat une diminution du pourcentage de littorines hors des refuges lorsque le substrat est humide. Nous pouvons donc croire que, dans le médiolittoral sous-boréal, l’action des vagues a un impact plus important sur le comportement d’utilisation de refuge chez L. saxatilis que la température. L’augmentation des tempêtes prévue par les changements climatiques pourra limiter le broutage de cet herbivore et affecter la communauté.
Abstract
Gastropod grazing shapes rocky intertidal communities, but in this environment their foraging behavior can be affected by various physical factors. We studied how variation in meteorological conditions affected the behavior of the periwinkle snail Littorina saxatilis with respect to its use of rock crevices as shelters. First, we sampled the frequency of shelter use during low tide at different times of the day and on different rock orientations. Secondly, we conducted a field experiment to calculate the percentage of snails outside shelters under two conditions of substrate wetness (dry or humid). Temperature and substrate desiccation were simultaneously measured in both cases. Globally, we measured an increase in the frequency of shelter use when the low tide was around noon, probably because of high desiccation at that time. However, we observed more snails outside shelters on the south-facing rock surfaces, compared to the north-facing ones, even though the temperature and desiccation were higher on the former. During the field experiment, the percentage of snails outside the crevices decreased with increases in wind speed, presumably due to increased wave strength. Contrary to our expectations, when the substrate was humid, there were fewer snails outside shelters. Thus, under humid conditions (e.g. north-facing rock surfaces and wetted treatments), the snails probably moved more and were able to find shelter, decreasing of the percentage of snails outside of the shelter. In this sub-boreal intertidal environment it appears that wave action has a greater impact than temperature on the use of shelter in L. saxatilis. Increases of storm frequency with climate change might then limit the grazing of this intertidal herbivore and affect intertidal community structure.
Introduction
In the animal world, the search for food – foraging – is one of the most important activities and challenges that organisms have to accomplish for obtaining the energy needed for other biological functions, such as growth and reproduction. However, the search for food, handling, and eating requires an investment of energy. Organisms should consume the food that optimally provides them the maximum amount of energy for the least amount of time invested into the foraging process (MacArthur and Pianka, 1966; Brown, 1988).
Foraging also implies movements to find prey or a new patch of food. While moving, the risk of mortality is often higher and so strategies that reduce this risk, like moving in herds, are favoured (Krause and Ruxton, 2002; Scott-Samuel et al., 2015; Yoder, 2004). Other organisms opt for the use of a shelter, always staying close to a certain area and foraging nearby (Bakker et al., 2005; Holmes, 1991). This strategy, called central place foraging, creates a gradient of foraging pressure, being stronger close to the shelter and decreasing with distance. The quality and abundance of the food should then increase with distance from the shelter. With time, organisms have to increase the distance they move away from the shelter to find better quality food, increasing the risk of being exposed (Schoener, 1979; Orians and Pearson, 1978). For example, when food is abundant near the refuge, the Whinchat (Saxicola rubetra, an old-world passerine bird) reduces foraging distances and increases time searching near the nest (Anderson, 1981). The gradient created by the difference in foraging pressure is particularly evident in herbivore and plant interactions, where the abundance of the plant eaten by the herbivore is typically lower closer to the herbivore’s shelter (Bakker et al., 2005, Rozen-Rechels et al., 2015). Shelters are not the only thing that increase localized foraging pressure. Areas with a resource essential for survival, like water, can also concentrate foraging pressure around them and become a central place. The foraging pressure then becomes more intense near the source than farther away. This is particularly common in stressful environments, where survival might be more difficult (Lewison and Carter 2004; Kramer, 1988; Rosen-Rechels et al., 2015).
Central place foraging has also been observed in marine systems, with the particularity that the refuges and the feeding areas can be distributed in a three-dimensional space (e.g. within the water column; Friedlaender et al., 2016). Classic examples include marine birds and some marine mammals, such as pinnipeds that live on islands, which have been described as central place foragers because they travel far from their resting place to eat (Massardier-Galatà et al., 2017). As in terrestrial habitats, such foraging implies being away from shelters and exposed to predation risk. However, in marine systems, predation is not the only factor that motivates the use of shelters. Animals living in ecosystems with stressful environmental conditions tend to
aggregate in shelters that will protect them against the physical environment and increase their survival. For example, in the intertidal zone, exposure to aerial conditions during low tide (i.e. emersion) may be stressful for marine organisms living there, and organisms have thus evolved strategies to survive outside of the water, especially to avoid extreme temperatures and desiccation (e.g. Somero, 2002). The stressful environmental conditions in the intertidal zone cause widespread use of shelter (e.g. Chapperon and Seuront, 2013) and central place foraging behavior among several invertebrates living there.
In spite of their tolerance to emersion, most organisms living in the intertidal zone require water for normal activities (e.g. filter-feeders), and thus most animals are more active during high tide and suspend feeding activities during low tide (Raffaelli and Hawkins, 1999). Some organisms are, however, capable of continuing their foraging during the low tide. This group includes gastropods (i.e. snails), which are important consumers in intertidal habitats and can be important components of the community. Indeed, grazing by herbivorous snails has shown to shape the community structure and to increase local biodiversity (Lubchenco, 1978). The behavioral response of intertidal snails to the physical environment experienced during low tide has been extensively studied, and they generally aggregate in microclimates where the substrate is humid and cool (e.g. Chapman and Underwood, 1996; Rickards and Boulding, 2015). On rocky shores, they use shelters, such as crevices in the rock surface, to avoid extreme temperatures or desiccation during low tide or dislodgment by water motion during high tide (Addy and Johnson, 2001; Ng et al., 2017; Pardo and Johnson, 2006).
Gastropod foraging behavior in this environment in combination with their use of shelters can thus be used to better understand central place foraging theory. Some limpets, for example, exhibit homing behaviour, rapidly leaving their shelter on a specific environmental cue to graze intensively and then returning to the same shelter by using the mucus trail left by their earlier movements (e.g. Santini et al., 2015). However, the unique locomotory mechanisms of gastropods, a combination of the contractions of their muscular foot on a mucus-covered surface, requires a wet surface. Thus, they can continue to forage during low tide only as long as there is enough water on the substrate to hydrate their mucus and continue crawling (Norton et al. 1990; Raffaelli and Hawkins, 1999). Knowing this, we hypotheses that when the tide goes down, snails that were foraging outside of refuges can continue crawling until they find a refuge or until the substrate dries. This latter condition will, however, depend on the rate of evaporation of any remaining water, which in turn depends on a complex suite of environmental conditions. Meteorological factors including solar irradiation, relative humidity, air and substrate temperatures, and wind speed (which affects the boundary layer) will all affect the evaporation rate of the water remaining on the substrate. The probability of the snails to find refuge at low tide will thus depend on how fast the meteorological conditions dry the rock.
The periwinkle snail Littorina saxatilis is a very common intertidal grazer in temperate-boreal ecosystems and is distributed at higher latitudes up into the Arctic. It feeds primarily on microalgae growing on rock surfaces (e.g. benthic diatoms) as well as on mats of ephemeral green algae (Marzouk, 2015). This species has been intensively studied for its wide tolerance to low and high temperatures (Davenport and Davenport, 2005), physiological and metabolic adaptations to the dramatic changes in temperature experienced in the intertidal (McMahon, Russell-Hunter, and Aldridge, 1995; Sokolova, 2003) and local adaptation to environmental conditions (Kyle and Boulding, 2000). Littorinid snails also display a vast diversity of behavioral responses to environmental challenges in different places around the world (Addy and Johnson, 2001; Iacarella and Helmuth, 2012; Miller et al., 2014; Muñoz et al., 2005; Ng et al., 2017).
In the intertidal habitats of the St. Lawrence Maritime Estuary (Quebec, Canada), densities of L. saxatilis are very high, and previous studies suggest that is probably due to a lack of predator (Pardo and Johnson, 2005). On rocky shores exposed to waves, snails are usually found inside or close to crevices on the rock surfaces, presumably to avoid dislodgment by hydrodynamic forces (Addy and Johnson 2001). The use of shelters by L. saxatilis increases the grazing pressure around the crevice, creating a pattern of a bare rock area surrounding most of the crevices in the mid and low intertidal zones, where the algal mats grow. The use of shelter and foraging process of this species are similar to central place foraging because of the high foraging pressure around the crevices even though the snails do not necessarily remain associated with a single crevice (L. M. Pardo, unpubl. data).
In spite of the extensive knowledge of the ecology of this species, the details of its foraging strategy remain poorly unknown. Grazing activity in the laboratory follows a circadian rhythm synchronized by the tides, with most activity occurring during the period of the high tide (Konan et al., 1992). In the field, movements were observed to occur during both high and low tides, but net displacements were larger during high tide than during low tide (Pardo and Johnson 2004). It appears then that the snails prefer to forage during high tide but can opportunistically forage during low tide as well. The decision to leave a shelter during high tide depends, however, on the environmental conditions. For example, fewer snails are found outside of shelters (i.e. crevices in the rock surface) during low tide when wave-generated hydrodynamics forces were high during the preceding high tide with the degree of this behaviour changing daily (Addy and Johnson 2001). Likewise, the decision to leave a shelter during low tide should depend on environmental conditions, but in this case, the wetness of the rock surface should affect this behaviour. However, as mentioned above, the wetness of the rock surface will depend on the meteorological conditions at that time. Adding complexity to this situation is the fact that the timing of low tides varies in a predictable way, advancing approximately 40 minutes forward every day. Thus, the time of the day at which the snails and the rock surface across which they are moving are exposed will vary from day to day. Indeed, because tides are semidiurnal in the St. Lawrence Maritime
Estuary, intertidal zones are exposed to air twice daily, usually once during the day and once at night. Evaporation would thus be expected to be highest when emersion by the low tide occurs during the day due to solar irradiance and concomitant increases in air and rock temperatures, but even within the day evaporation rates will vary, being highest during the midday when solar irradiance is greatest.
In this study, we addressed the question of how meteorological conditions affect the foraging and use of shelters by L. saxatilis during low tide. To address this question, we first measured local abiotic conditions (rock temperatures and desiccation rates) and snail behaviour at different moments of the day throughout the summer. We predicted that snails would remain inside shelters when the conditions were warmer and dryer (e.g. at midday) and at locations that were warmer and drier (e.g. south-facing rock surfaces). We then experimentally manipulated environmental conditions during low tide to reduce desiccation and predicted that snail foraging would increase under these conditions.
Methods and Materials
The study took place during the summer of 2016 and 2017 on the rocky intertidal shores of the Grand Métis region, located on the south shore of the St. Lawrence Maritime Estuary (Québec, Canada). This area is colonized by fucoid algae in the mid intertidal and by green algae mats on the low intertidal shore (Pardo and Johnson, 2005). Littorina saxatilis is found on the rocky reefs at all levels of the intertidal zone, although there are demographic differences between the populations at different levels (see Leroux, 2011; Pardo and Johnson, 2005). Close to the rock reefs, there are also mussel beds, boulders fields and tide pools with other invertebrates and algae. The tidal cycle at this location is semi-diurnal with a 4.3 meter amplitude spring tide.
Field sampling
Sampling took place during the summer of 2016 at three wave-exposed sites (Site 1: 48°39’51.68”N 68° 4’38.16”O; Site 2: 48°40’52.65”N 68°2’9.43”O; Site 3: 48°40’59.24”N 68°1’48.37”O) distributed within 4 km of shoreline near Pointe-Mitis, Québec (Figure 1). At each site, we selected 10 crevices in the mid-intertidal zone (between 1 and 1.5 m height): 5 facing south (more exposed to the sun and thus expect to be hotter and drier) and 5 facing north (cooler and moister). A crevice was defined as being a crack across a smooth rock surface of dimensions approximately 50 cm long, 1 cm deep and 1 cm wide and inhabited by L. saxatilis. A fixed quadrat of 25x25 cm was centered on each crevice to define the sample area. To test the effect of temperature on the desiccation rate and snail behavior, we deployed a temperature logger (iButton DS1921G) next to each crevice that recorded substrate temperature every 30 minutes. Loggers were embedded in a green marine epoxy putty to better mimic the thermal properties (e.g. absorption) of the rock surface. To best represent
recorded during the low tide. The behavioral response was defined as the proportion of snails that were observed outside of a crevice during a given low tide, typically measured 1 to 2 hours after emersion by the low tide. Inside of the delimited area, we counted the number of snails inside and outside the crevice, which gave us the percentage of snails outside of the crevice. We used the percentage to be able to compare the data because of the variability of the number of snails present in the quadrat (minimum = 15, maximum = 220, average = 97 snails/quadrat). This sampling was conducted on different days when the low tide occurred around 07:00, 14:00 or 19:00 to have the different meteorological conditions of sun irradiation and temperature typical of each “time of day” (i.e. morning, midday and evening, respectively). As the crevices were usually exposed two hours before the low tide, thus exposed at sunrise (approximately 05:00 at this latitude in summer), noon and the beginning of the sunset, respectively. Care was taken to avoid disturbing the snails since the same crevices were followed for the entire summer. We sampled each crevice a total of five times for each time of day (i.e. 15 days in total).
Each time we sampled, desiccation was measured by deploying two "desiccaps" per crevice for two hours. Desiccaps are circular plastic caps (1-cm diameter by 1-cm depth) filled with water-saturated plaster of Paris. Before deployment, they were immersed in water for 30 minutes, weighed (to a milligram precision) and covered with a plastic lid. After exposure, they were again covered with the lid and weighed again to estimate the rate of water loss (grams per hour, g/h).
Figure 1. a) Map of the Saint Lawrence Maritime Estuary in Quebec, Canada. b) Location of the three research site in the Mitis region Statistical analysis
All statistical analyses were conducted with R software with a critical threshold of p < 0.05 to determine significance. Because of the high variability between all the days sampled, from the original dataset that we obtained, we used the mean of the five times we sampled each crevice for each time of day, reducing the data set from 450 data points to 90 data points. This mean helped us to group the data per time of day before continuing the analysis. With this new data frame, we did two analysis: first, we did two-way ANOVAs to test the effect of orientation and time of day as predictors of the mean temperature and desiccation rate. To explore the relationship between temperature and desiccation rate we used a linear regression. Secondly, to address the question of the effect of orientation, the different time of day and desiccation on the proportion of snails grazing outside of the crevices, we used a generalized (binomial) linear mixed model with repeated measures where each crevice was specified as a random factor. Normality and heterogeneity of variances was checked and for the linear mixed model we also checked leverage with the use of hat-values and the influence data points with Cook’s distance.
Field experiment
To observe the use of refuges under two different desiccation regimes, we randomly selected six pairs of crevices in July 2017 at the same level in the intertidal zone (mid-high, approximately 2 m) at Site 1. The distance between paired crevices was 30-100 cm and distances between pairs were between 2 to 10 m. The two crevices of each pair had similar characteristics of slope, water drainage, orientation and exposure to wave action. In contrast, these characteristics varied among the six pairs of crevices.
For each pair, we manipulated the wetness of one of the crevice and the rock surface surrounding it by adding seawater with a hand mister over a fixed area of 15x15 cm, centered on the crevice, keeping the substrate wet for one hour after emersion by low tide. The other crevice of each pair was allowed to dry naturally. We repeated the experiment twelve times, but each time we randomly selected the crevice that was kept wet and the one that was allowed to dry. We counted the number of snails that were outside of the crevice both at the beginning and the end of each trial but only counted the number of snails inside the crevices at the end of the experiment because crevices were full of water at the beginning of the experiment, making it difficult to sample without disturbing the snails. Assuming that there was no “immigration” of snails into or out of these areas, we were able to use these numbers to calculate the percentage of snails outside the crevices at the beginning and the end of the experimental period. The water we used for keeping the rocky substrate moist was collected directly from the sea at the study site. Since the experiment only ran for one hour and the seawater at this site has a lower salinity (28 psu) than the open sea, we assumed that any salt accumulation on the substrate due to evaporation did not affect snail behavior. Previous trials also show that snails tolerate high salinity better than low salinity conditions (G. Fernandez, pers. obs.). This experiment was repeated 12 times during
day-time low tides. We also did 5 trials during the night-time low tides to compare the behavior observed during the day with the completely different conditions of temperature and desiccation at night. However, during the night-time trials, we only used 4 spatial replicates.
To measure substrate temperature, we again deployed temperature loggers (iButtons) adjacent to each pair of crevices, taking measurements every 15 minutes. Likewise, desiccation rates during the experiment were estimated by measuring the change of weight of water-saturated desiccaps. Wind speed, air temperature, and relative humidity data were provided by an Environment Canada weather station located by the shore at Point au Père approximately 50 km from our site. We chose this location for our meteorological data rather than a closer one located at the airport in Mont-Joli because the substrate temperature measured in situ was better correlated with the air temperature provided by the weather station at Point au Père. This is probably due to the fact that the station at Point au Père is located near the shore and not inland.
Statistical Analysis
We used two statistical analysis: one for the time trials and another for the night-time trials. For the day-time trials the weather conditions changed markedly from one day to another. Temperature, wind speed and relative humidity were very different between days and so we classified the days using a Cluster Analysis to regroup the days that had similar weather conditions. The Cluster Analysis and personal notes taken during the experiment revealed three kinds of weather conditions that we designated Calm, Breezy, and Windy. First, we did two unbalanced two-way ANOVAs for testing the effect of the treatments, Dry and Wet, the three weather conditions and their interaction on the percentage of snails outside the crevices. One was at time zero (T0) and the other after one hour (T1). Post hoc Tukey tests were used to detect where specific differences
occurred. Normality and the homogeneity of the data were verified with graphics tools and the Fligner-Killeen test of homogeneity. We used the asin(x)*square root (x) transformation of the response variable to achieve normality of the data when needed. We continued using the transformed data for the rest of the following analysis.
To find which environmental factor was driving more the use of refuge of the snails between days, we used a mixed linear model with repeated measures (lme). In this model, all the environmental variables (substrate temperature, wind speed, air temperature, relative humidity and desiccation) and treatments were considered as fixed factors for explaining the response variable (percentage of snails outside). Since we did repeated measures on the same crevices for the different time of days, we had to include a random term that will take the intrinsic difference between the crevices into account. We also created a variable that tested for any correlation through time (variable named: Time). To complement this analysis we did a linear regression between the wind speed and the percentage of snails outside.
Finally, we compared day and night trials. Since the size sample was too different between day and night, we randomly choose 5 days to compare with the 5 nights we sampled. We used a two-way ANOVA with the factors day or night and the two treatments, Dry and Wet, to explain the percentage of snails inside crevices. We concluded with another very similar mixed linear model with Repeated Measures with all the environmental variables, the treatments, and the day or night factor as well as the random term used previously to account for the difference between crevices.
Results Field sampling
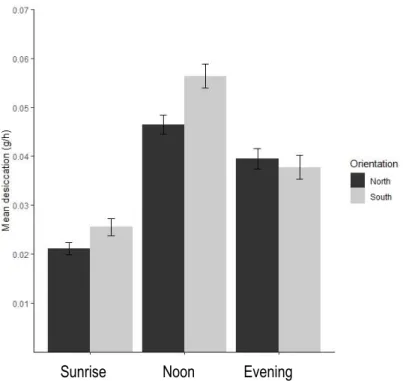
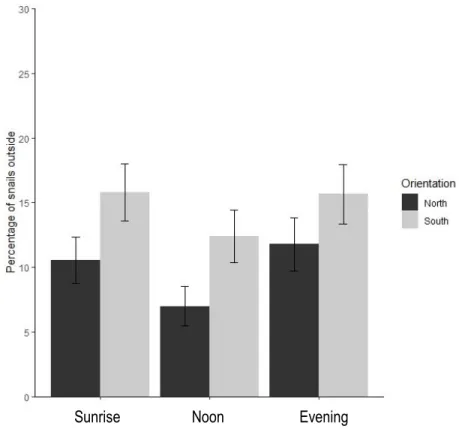
Orientation, time of day and the interaction between these factors all had significant effects on the mean temperature and desiccation rate during the low tide (Table 1a). The mean temperature at noon was around 5°C higher than the mean temperature at sunset and sunrise. This difference was significant, but there was no significant difference between the mean temperatures at sunrise and sunset. The orientation of the rock also had a significant effect on the mean temperature, being around 2°C lower on northern faces compare to southern faces but only at sunrise and noon and not at sunset. The results of the two-way ANOVA of desiccation rate were similar, except the desiccation rate was significantly different at each time of day, being highest at midday (Table 1b; Figure 2). Orientation was significantly different only at low tides around noon. Overall, the desiccation rate and mean temperature were significantly correlated for all data combined (Figure 3, R2 = 0.7, N = 90).
The interaction of time of day and desiccation rate had a significant effect on the proportion of snails outside the crevice (Table 2). Orientation alone was not significant despite the consistent trend of 30-80% more snails being outside the crevices with a southern orientation (Figure 4).
Field experiments
For the experiment conducted during the day-time low tides, at time zero (T0) only the weather had a
significant effect on the percentage of snails outside the crevices (Table 3 (a); there was no wet/dry treatment effect because, of course, it had not yet been applied). Windy weather had fewer snails outside of the crevices at the beginning of emersion by the low tide. At time one (T1) both weather conditions and the wet/dry
treatment had a significant effect on the percentage of snails outside the crevices (Table 3). The percentage of snails outside was significantly lower during windy days than on calm and breezy days (Figure 5). However,
dry treatment (Figure 5). This difference was consistent between the three kinds of weather conditions (i.e. no interaction between these factors).
In the mixed linear model, the only variables that explain a significant portion of the variation in the percentage of snails outside of crevices were wind speed and relative humidity (RH) (Table 4). Wind speed alone predicts 44% of the response variable. In general, the percentage of snails outside crevices decreased with the increase of wind speed (Figure 6). Again, contrary to expectations, the percentage of snails outside of the crevices decreased with increasing relative humidity, and the percentage of snails outside during the most humid days was lower than during the driest days (Figure 6, filled and empty circles, respectively). The days with intermediate relative humidity values largely fell along the regression line, suggesting that extremely high or low values of humidity can alter the effect of wind.
There was a significantly larger percentage of snails outside of crevices during night-time than during day-time low tides, but the treatment of wetting the rock surface did not have an effect on snail behavior (Table 5; Figure 7). However, the mixed linear model did not find an effect of Day-Night on the percentage of snails outside crevices but did find significant effects of relative humidity and desiccation rate (Table 6).
Table 1. Series of ANOVA testing the effect of Orientation and Time of day on (a) Mean temperature and (b) Desiccation
Df Sum Sq Mean Sq F value Pr(>F)
(a) Mean temperature
Orientation 1 24.5 24.47 13.349 0.000449
Time of day 2 425.1 226.05 123.308 <0.001
Orientation × Time of day 2 26.4 13.18 7.191 0.00131
Residuals 84 154 1.83
(b) Desiccation
Orientation 1 0.000369 0.000369 6.297 0.014
Time of day 2 0.011924 0.005962 101.875 <0.001
Orientation × Time of day 2 0.000483 0.000242 4.128 0.0195
Table 2. Binomial general mix model with repeated measures of the proportion of snails grazing outside crevices Chisq Df Pr(>Chisq) Orientation 0.0976 1 0.7547 Time of day 24.6053 2 <0.001 Desiccation 0.0467 1 0.8290
Table 3. Series of ANOVAs testing the effect of the addition of water and different categories of weather on the percentage of snails outside the crevices during the day time low tide at time zero (T0) and after one hour (T1)
Df Sum Sq Mean Sq F value Pr (>F)
a) T0 Weather Treatment Weather*Treatment Residuals b) T1 2 1 2 138 2.776 0.101 0.053 16.665 1.3880 0.1008 0.0265 0.1208 11.494 0.834 0.219 <0.001 0.363 0.803 Weather 2 2.9974 1.49870 20.4395 <0.001 Treatment 1 0.3721 0.37207 5.0743 0.02586 Weather*Treatment 2 0.0206 0.01030 0.1405 0.86904 Residuals 138 10.1187 0.07332
Table 4. Result of the Mixed Linear Model with Repeated Measures of the percentage of snails outside crevices
Value Std. Error DF t-value p-value
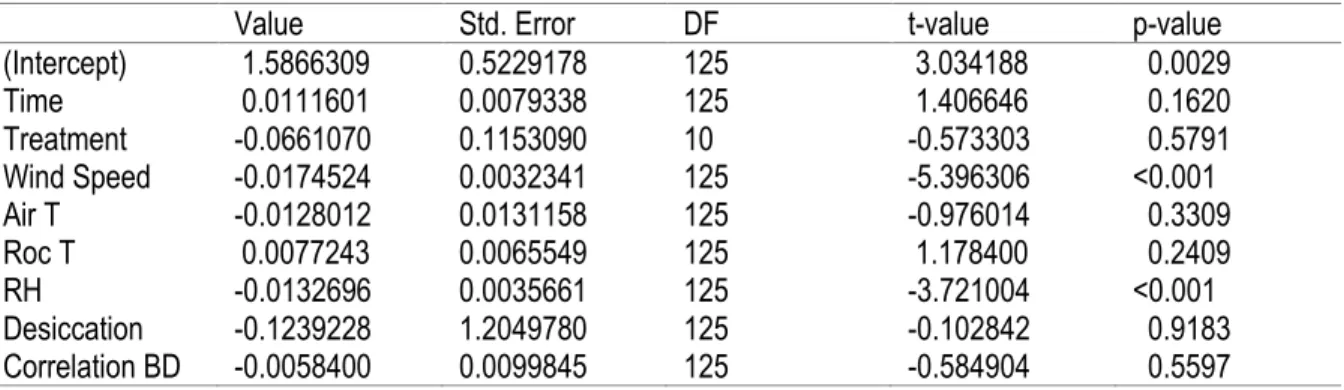
(Intercept) 1.5866309 0.5229178 125 3.034188 0.0029 Time 0.0111601 0.0079338 125 1.406646 0.1620 Treatment -0.0661070 0.1153090 10 -0.573303 0.5791 Wind Speed -0.0174524 0.0032341 125 -5.396306 <0.001 Air T -0.0128012 0.0131158 125 -0.976014 0.3309 Roc T 0.0077243 0.0065549 125 1.178400 0.2409 RH -0.0132696 0.0035661 125 -3.721004 <0.001 Desiccation -0.1239228 1.2049780 125 -0.102842 0.9183 Correlation BD -0.0058400 0.0099845 125 -0.584904 0.5597
Table 5. ANOVA table for testing the effect of day or night and the on the percentage of snails outside of the crevices during low tide
Df Sum Sq Mean Sq F value Pr (>F)
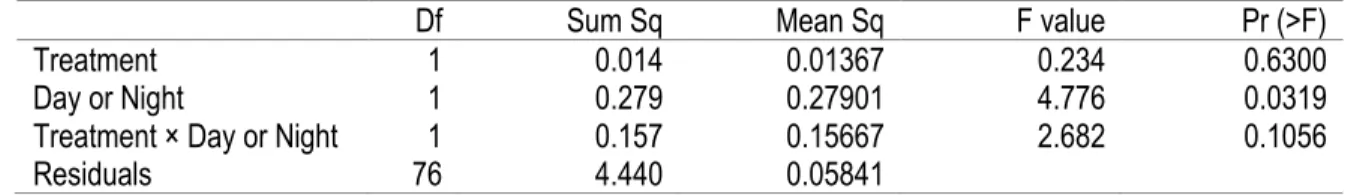
Treatment 1 0.014 0.01367 0.234 0.6300
Day or Night 1 0.279 0.27901 4.776 0.0319
Treatment × Day or Night 1 0.157 0.15667 2.682 0.1056
Table 6. Result of the Mix Linear Model with Repeated Measures of the percentage of snails outside of crevices for day and night analysis
Value Std. Error DF t-value p-value
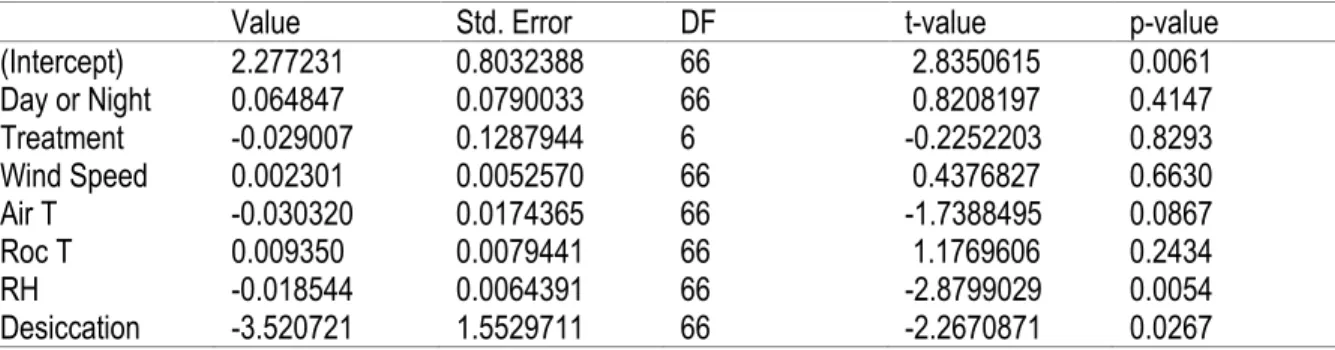
(Intercept) 2.277231 0.8032388 66 2.8350615 0.0061 Day or Night 0.064847 0.0790033 66 0.8208197 0.4147 Treatment -0.029007 0.1287944 6 -0.2252203 0.8293 Wind Speed 0.002301 0.0052570 66 0.4376827 0.6630 Air T -0.030320 0.0174365 66 -1.7388495 0.0867 Roc T 0.009350 0.0079441 66 1.1769606 0.2434 RH -0.018544 0.0064391 66 -2.8799029 0.0054 Desiccation -3.520721 1.5529711 66 -2.2670871 0.0267
Figure 2. Mean desiccation rate at different times of day and orientations (mean SE) Sunrise Noon Evening
Figure 3. Linear model of the desiccation rate measured over two hours as a function of mean estimated substratum temperature during low tide (R2= 0.7; n = 90)
Figure 4. Mean percentage of snails outside of crevices at different times of the day and different orientations (mean SE)
Figure 5. Percentage of snails outside of crevices (mean SE) at two substrate moisture treatments during different types of Weather: Calm, Breezy, and Windy at day time low tide at the beginning of the experiment (T0) and after one hour (T1).
Figure 6. Mean of the percentage of snails outside of crevices per day (N=12) as a function of wind speed. The different shape colours are associated with different values of relative humidity (RH). Mean SE. R2= 0.44
Figure 7. Percentage of snails outside of the crevices for two substrate moisture treatments during night-time or day-time low tides (mean SE).
Discussion
Mobile invertebrates can adjust their behavior in response to biological or abiotic stimuli in their environment. Gastropods have been used as a model system to study behavior in the intertidal zone under different conditions, displaying many kinds of behavioral responses (e.g. Johannesson, 2003; Chapperon and Seuront, 2013). In this study, the use of shelters by the periwinkle snail Littorina saxatilis has been used to explore their behavioral response to different kinds of physical stimuli from the environment.
Our results showed that timing of emersion during the day has an impact on substrate temperature, which in turn affects the desiccation of the rock and ultimately shelter use by snails. This result is consistent with observations elsewhere as intertidal gastropods tend to avoid stressful temperatures and desiccation by aggregating in more humid microclimates (Chapperon and Seuront, 2013). The result that was more surprising was the tendency of snails to be found in greater numbers outside the crevices on southern rock faces (relative to northern faces) where we measured higher mean temperatures and higher levels of desiccation. Because of the sampling and the statistical binomial model, we were not, however, able to detect a significant difference based on orientation, likely due to the very high variability among crevices. This aspect was considered with a random factor in the model, but since each crevice had only one orientation (north or south), the variability associated with the identity of the crevice was probably higher than the variability associated with the orientation, masking the effect of the orientation alone. Nevertheless, our results suggest that there are differences in the behavioral response between snails living on rock surfaces with different orientations. Moreover, it would appear that the snails are unable to return or find new shelters and thus must stop on the open rock surface where they are presumably subject to greater stress (e.g. higher temperatures, desiccation), greater risk of predation (minimal in this system) and greater dislodgment by waves when the tide returns.
We learned that contrary to other places in the world (e.g. Marshall et al., 2015), L. saxatilis is unlikely to be at risk of stress due to high temperatures in this boreal environment. This species has a very high tolerance to temperature (Davenport and Davenport, 2005), and even though we did record substratum temperatures of 40°C, these extreme conditions were rather rare. Thus, L. saxatilis in this environment does not appear to be using crevices as a refuge from high temperatures. Indeed, it has been observed that some snails in high latitudes actually aggregate in crevices to maintain higher temperatures than those found outside of the crevices (Ng et al., 2017). If temperature is having an important effect on snail behavior in this region, it is more probable that this would happen during the winter when extremely low temperatures occur in this system, but this remains unknown in this system.
Substrate temperature does, however, directly affect desiccation rates. Typically, the term “desiccation” refers to the loss of corporal water due to evaporative cooling (McMahon, 1990). In contrast, in the circumstances of our study, desiccation is more relevant to the small amount of water remaining on the rock surface, which in turn affects immediately the snail's mobility and ultimately its use of shelters. Intertidal snails need water on the substrate to continue moving and grazing (Raffaelli and Hawkins, 1999), so snails grazing outside crevices when the rock surface dried completely could not continue moving. Indeed, these snails usually had their foot retracted with the operculum closed. Our observation that there were more snails outside of crevice on south-facing rock surfaces, especially at noon and morning tides, (where desiccation is also generally higher) suggests that these snails were unable to return to their refuges. The capacity of these snails to determine the proximity of refuges is not known although homing behaviours have been observed in other species (e.g. Santini et al., 2015). Regardless, the stress of corporal desiccation incurred by moving over a dry substratum is probably more problematic than the benefit gains by possibly locating a humid crevice. Thus, the better strategy is probably foot withdrawal and shell closure. We conclude then that the difference in the percentage of snails outside of crevices between north and south orientations is probably due to a difference of the desiccation rate between these two microhabitats. During the morning and noon, the north face was protected from the sun, the mean temperature was lower and, in consequence, the desiccation rate was smaller compared to the south face. These conditions allowed the snails to move more, increasing their ability to find a crevice. As a result, we observed fewer snails outside of the crevice compared to the southern face. This explanation seems quite reasonable for the times when initial emersion occurred at sunrise or at noon, and there were clear differences in desiccation rates. However, we observed the same pattern at sunset even though there was no difference in the desiccation rate between north and south faces. Other factors may thus be affecting snail behaviour, including wave action, which has already been documented an important driver of the use of shelter by snails at this location (Addy and Johnson 2001). Since the northern crevices faced the open water and the south crevices the shore, increased wave action associated with end-of-the-day increases in wind speed may have disproportionately reduced the number of snails leaving crevices (and thus the potential number that could be found later outside the crevices) on the north-facing rock surfaces (G. Fernandez, pers. obs.). Unfortunately, we do not have information on wave forces to compare north and south orientations at different times of the day.
To properly address and understand the question of how the environment affects snail behavior, we need to observe separately two times – when snails are still submerged and when they emerge with the falling tide, keeping in mind that past conditions will probably influence the present observations. Our experimental results show that increased wind speed decreased the number of snails outside of crevices. This is probably due to the concomitant increase in wave action when wind speed increases. Wave action thus has an effect on snails while they are still submerged or in the splash-zone of the waves, and two mechanisms can explain why we