Specificity
of
antibodies
to
heterologous
glomerular
and tubular basement
membranes in
various
strains of
mice
with
different H-2
types
D. MOULONGUET-DOLERIS, D. ERARD, M. T. AUFFREDOU, J. M. FOIDART,* P. MAHIEU* & J. DORMONT INSERM U 131, Clamart, France,
and*Department ofMedicine, State University of Liege, Liege, Belgium (Acceptedfor publication 24April 1981)
SUMMARY
C3H, CBA
(H-2k)
and NZB(H-2d)mice were immunized withdoginsolubleglomerular (GBM)ortubular basement membrane(TBM). The titre ofcirculatingantibodies was sequentially determined and theirspecificitywasanalysedusingvarious solubleantigenic fractions. Glomerular and tubulardeposits were studied on serial biopsies by direct immunofluorescence. After elution from wholekidneys, IgGfixationonnormal mouse kidney sectionswasanalysedbyindirect immunofluorescence. After immunizationwith insoluble GBM, animals from all three strainsdevelop antibodiesmainlydirectedagainst collagenousantigenicdeterminantssharedbyGBM and TBM. Afterimmunization with insoluble TBM, the antibodies are directed in NZB mice against non-collagenous TBM-specificdeterminants,inC3Hmiceagainst collagenousdeterminantsand in CBA mice against both types ofantigenicdeterminants. Thus theability
torespond
to the various antigensof GBM and TBM isgeneticallydetermined and doesnotdepend onlyon themajorhistocompatibilitycomplex.INTRODUCTION
Ithas been shown (Erard et al., 1979) that BALB/c mice (H-2dtype)injected with insoluble dog glomerular basement membranes (GBM) develop circulating and kidney-fixed autoantibodies
mainlydirected against collagenous antigenic determinants shared by GBM and tubular basement membranes (TBM). The same mice injected with insoluble dog TBM also develop circulating and kidney-fixed autoantibodies mainly directed against non-collagenous, TBM-specific, antigenic
determinants(Erardetal., 1979). The production of anti-GBM and/or anti-TBM antibodies, their
specificityandthe magnitude of the antibody response in mice injected with GBM or TBM may be undergenetic control. We have therefore studied the anti-GBM or anti-TBM antibody response in three otherstrains of mice (C3H, CBA, NZB) with two different H-2 types (C3H,H-2k;CBA,H-2k;
NZB, H-2d). Theabove-mentioned characteristics of the immune response were determined by radioimmunoassay and by immunofluorescent microscopy. It has been found that the three strains of mice immunized with insoluble GBM are able to raise specific antibodies against antigenic
determinants shared by GBM and TBM. These antibodies are mainly directed against the collagenous material of basement membranes. Furthermore, the CBA (H-2k type) and NZB (H-2d type)miceimmunized with insoluble TBM also raise antibodies directed against non-collagenous, Correspondence:D.Moulonguet-Doleris, INSERMUnite131,32rue des Carnets, 92140 Clamart, France.
0009-9104/81/1000-0035$02.00 © 1981 Blackwell Scientific Publications
36
D.
Moulonguet-Doleris
et
al.
TBM-specific antigenic determinants while C3H donot. It appearstherefore that theability to respond to the various antigens of GBM and TBM does not depend only on the major
histocompatibility complex.
MATERIALS AND METHODS
Animals. Three different inbred strains of mice were used: C3H(H-2k),CBA
(H-2k)
and NZB(H-2d). Themice (CNRS, Orleans, France) were 4 weeks old at the time of the first injection. Antigens.Glomerular basement membranes (GBM) and tubular basement membranes (TBM) were purified from dog kidneys as previously described (Erard et
al.,
1979). The final GBM preparation was contaminated by less than 5% of TBM and vice versa.Immunization.The lyophilized antigens were suspended in phosphate-buffered saline (PBS) and emulsified volume-to-volume in Freund's complete adjuvant (FCA). C3H and NZB mice were divided into three groups of 16 mice and CBA into three groups of 12 mice. Groups I and II of each strain respectively received eight weekly subcutaneous injections of GBM orTBM(02mg) in FCA (final volume 0-1 ml), whereas groupsIIIreceived PBS in FCA.
Experimental design. After the second injection each mouse was bled by retro-orbital puncture every2weeks. All sera were stored at -20'Cfordetection of circulating antibodies. Following the sameschedule, two or three mice per group were subjected to kidney biopsy. Kidney fragments were immediately frozen in liquid nitrogen and stored at -70'C for immunofluorescent studies. One week after the lastinjection the animals were killed by bleeding and kidneyfragmentsweretaken.In eachexperiment,two tofour miceper groupwerekeptalive,bled 1month later and killed 3 months later; sera and kidney fragments were taken as above.
Beforekilling,urinewastested forproteinuriawith AmesLabstix. Creatininewasevaluatedin serausing a Eurobio kit.
Detection and specificity of circulating anti-DNA, anti-GBM andanti-TBM antibodies. The presenceof anti-DNA antibodies has been searched foraccordingtothe method of Farr(1968).The presence and the specificityof anti-GBM and anti-TBM antibodies has been searched forbya previouslydescribed radioimmunological method (Mahieu, Lambert & Miescher, 1974;
Grain-dorge&Mahieu, 1978). Tostudythespecificityofanti-basement membrane
antibodies,
typeIVprocollagen and a non-collagenous glycoprotein fraction purified from basement membrane material secretedbyamurinetumour(Timpletal., 1978)wereused. These
antigens
werekindly
provided by Dr J. M. Foidart (NIDR, NIH, Bethesda). The
heteropolysaccharide-containing
glycopeptideswerepurifiedfrom GBMorTBM,aspreviouslydescribed(Mahieu
&Winand, 1970).
Immunofluorescent
studies. The kidneybiopsies
and thekidneys
obtained at autopsy werestudiedforthe presenceofmouseIgGdeposits using
fluorescein-conjugated sheep
anti-mouseIgG.
IgMand C3depositsweresearched forusing
respectively
fluorescein-conjugated
goat anti-mouse IgM (Nordic Immunology) andfluorescein-conjugated
rabbit anti-mouse fIC(Nordic
Immu-nology). The direct immunofluorescenttechniqueofLerner,Glassock & Dixon(1967)
wasused.Kidneysections treatedaspreviouslydescribed(Erardetal.,
1979)
wereexamined underaZeissu.v.microscopeequippedwithanHbO 50 mercurylamp.
Studies ofeluates. Kidneys from each
experimental
group werepooled
andhomogenized
(Ultra-Turrax,IkaWerck).
Antibodieswereelutedusing
0-02 M citratebuffer,
pH
3-2,
according
to the techniquedescribed byLerneretal.(1967).
Eluates from each groupwereconcentratedby
negative pressuredialysisto afinal volumeof about 1 ml.The
protein
contentof the eluateswasdeterminedbythetechniqueofLowryetal.
(1951),
using
purified
humanIgG
asstandard. The presence ofmouse IgG was tested forby
immunoelectrophoresis using
agoat
anti-mouseIgG
(Hyland Laboratory,
Travenol)
andarabbit anti-mouseserum(Dr
Pillot,
InstitutPasteur,
Paris).
In each experiment the eluates from the different groups were tested in vitro
by
indirect immunofluorescenceperformedonkidney
sectionsofnormalsyngeneic
mice. Whenspecified,
the eluates wereinjectedintravenously
into normalsyngeneic
mice.Kidney biopsies
wereperformed
RESULTS
In the three strainstested,mice of allexperimentalgroupsappeareduniformlyhealthyat theend of the experiments. The growth of the animals assessed by body-weight measurement was
comparable,serumcreatininewasidenticalincontrol andimmunizedgroupsand below 5mg/l;no
proteinexcretion could be detected. Results obtainedinC3Hmice (H-2k)
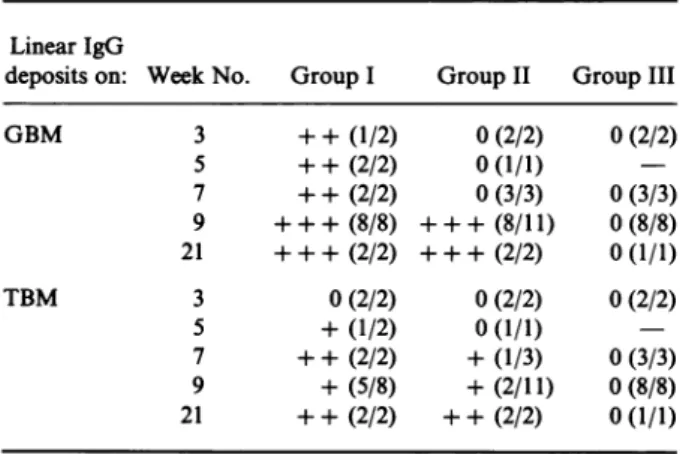
Circulating antibodies. As shown inFig. 1, thepercentage of
1251I-TBM
antigens precipitated remained below 10 in TBM- and GBM-treated mice. Incontrast, antibodiesagainstGBMwere40-(a) 30 I
_0
,° 20 a) 0 40 (b) c, T T 20lo
_
~ T T 3 5 7 9 13 21 Time (weeks)Fig.1.Circulating anti-GBM and anti-TBM antibodies in C3H mice. Tennanogramsof thelabelled GBMor
TBMantigens contained in 0 I ml 0 IMboratebufferwereincubatedwith 0 4 ml of 1/20mouseserumin05 ml
40% polyethylene glycol. Percentage of1251I-GBMantigens (-.*) and1251-TBMantigens(* *)boundby
(a)serafromGBM-treated mice and (b)serafrom TBM-treated mice.
Table 1. Results of directimmunofluorescent studiesonC3Hkidneybiopsies
LinearIgG
depositson: WeekNo. GroupI GroupII GroupIII
GBM 3 ++ (1/2) 0(2/2) 0(2/2) 5 ++ (2/2) 0(1/1) 7 ++ (2/2) 0(3/3) 0(3/3) 9 ++ + (8/8) ++ + (8/11) 0(8/8) 21 ++ + (2/2) ++ + (2/2) 0(1/1) TBM 3 0(2/2) 0(2/2) 0(2/2) 5 +(1/2) 0(1/1) 7 ++ (2/2) +(1/3) 0(3/3) 9 +
(5/8)
+(2/11)
0(8/8)
21 ++(2/2) ++ (2/2) 0(1/1)detected in both groups. The percentage of1251-GBMantigen precipitation reached 40 + 5 at week 9 in group I andremained stable until week 21. The maximum precipitation of1251-GBM antigen in groupII was 30+4 at week 9.
Specificity ofanti-GBM antibodies. To test the specificity of the anti-GBM antibodies elicited in C3H mice of group I, inhibition experiments were performed. One microgram of type IV procollagen,of laminin, and of the disaccharide- or heteropolysaccharide-containing glycopeptides purifiedfrom GBM orTBMwasadded to a solution of anti-GBM antibodies binding specifically
20%
of1251-GBMantigens respectively. The precipitation of labelled GBM antigens was inhibited by 96, 92 and86%
by 1pg
of type IV procollagen and of the disaccharide-containing glycopeptides isolated from GBM and TBM. In contrast, 1 jug of laminin and of theheteropolysaccharide-containingglycopeptides of GBMorTBMdidnotreduce thespecific precipitation of the labelled membrane antigens.
Immunofluorescent studies. The results are summarized in Table 1. Linear IgG deposits were observed on the GBM in groupI assoon asweek 3.Atweek 9astronglinearstaining was observed in all glomeruli of all mice (eight out of eight). Light IgG deposits on the cortical tubules appeared at week 5 and fiveofeightmice exhibitedaweak tubularstainingatweek 9.IngroupII,noglomerular
IgG deposits wereobviousbefore week 9 when allglomeruliofeightof 11 mice exhibitedastrong linear staining. At the sametime light lineardeposits were observedonthecortical tubules intwoof 11 mice. No linear deposits on GBM or TBM were observed in control mice of group III. Immunofluorescent studiesperformedwith FITC anti-mouseIgMexhibitedasimilar pattern of mesangial clusters in immunized and control mice. FITC anti-mouse
/31C
revealed granularmesangial depositsinglomeruliand segmental linearstaining alongTBMand Bowman'scapsulein all groups. Mice withdrawn atweek 9 exhibited asimilar pattern of fluorescent deposits when examinedatweeks 13 and 21.
Studiesofkidneyeluates. Theproteinconcentrationwasabout 1mg/mlin all groups.Theywere
testedfor the presence ofmouse serumproteinswhichprovedtobeIgG onlyin groupsIandII,as
demonstratedbyimmunoelectrophoresis.When testedinvitro
by
indirectimmunofluorescence,
the eluates from groupsI andII fixedlinearly ontheGBM and the TBM. Eluates from group III50 -(o) 17T c 10 40 TJK C
~~
1 o!J
C0 ID0I
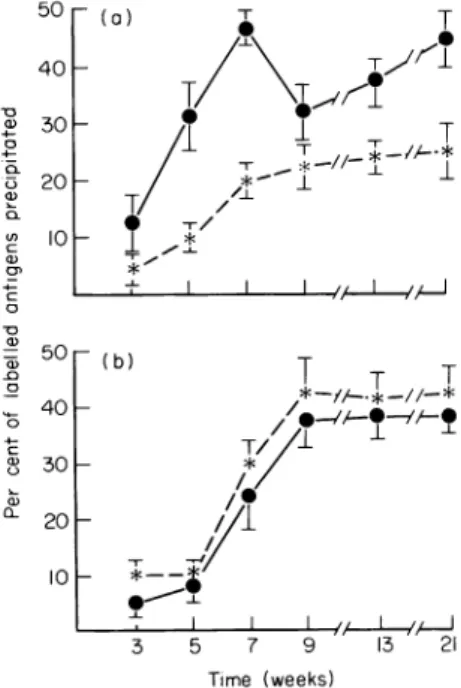
-T // 20 I T I-4 0 3 5 7 9 13 21 Time (weeks)Fig. 2. Circulating anti-GBM and anti-TBM antibodies in CBA mice. Percentage of1251-GBM antigens
( *)and 125I-TBM antigens ( *)bound by (a) sera from GBM-treated mice and (b) sera from TBM-treated mice.
Specificity
of
Table 2.Results of directimmunofluorescent studiesonCBAkidneybiopsies
LinearIgG
depositson: Week No. Group I GroupII GroupIII
GBM 3 0(2/2) 0(2/2) 0 (2/2) 5 ++(2/3) 0(3/3) 0(3/3) 7 ++ (3/3) + ++ (3/3) 0(3/3) 9 + + +(10/10) ++ + (10/10) 0(10/10) 21 +++ (3/3) ++ + (3/3) 0(3/3) TBM 3 0(2/2) 0(2/2) 0(2/2) 5 ++(3/3) + + (3/3) 0(3/3) 7 ++ (3/3) +++ (3/3) 0(3/3) 9 ++ (10/10) ++ + (10/10) 0(10/10) 21 ++ (3/3) ++ + (3/3) 0(3/3)
exhibitednoanti-GBM or TBMreactivity. Three normal C3H micewereinjectedintravenously with 0-5 ml of the eluates from groupsI, IIand III
respectively.
Direct immunofluorescence onkidneybiopsiesperformed 1hr and6daysafterinjectionofeluatesfromgroupsIandII revealed stronglinearIgGdepositsalongGBMonly.After
injection
of the eluates from groupIIInospecificimmunofluorescence could be detected. Resultsobtainedin CBAmice (H-2t)
Circulating antibodies. In groupIthepercentageof
125I-GBM
antigensprecipitated
was31[5+5 atweek 5, reached a maximum of 47+3 atweek 7 anddropped
to 32+±5 atweek 9. In micewithdrawnatthe timeofkilling,the percentage
precipitation
roseslowly
to45+5atweek 21.The percentage of'251-TBMantigensprecipitatedremained between 20+3atweek 7and 25+5atweek 21 (Fig. 2a). In groupII,
thelabelledTBMprecipitatedroserapidlyfrom 10+25%
atweek5to30+4%atweek 7andto42-5+6%atweek9,remainingatthesamelevel until week 21 in themice that hadbeen withdrawn. The precipitation of labelled GBMfollowed thesamecoursewith a
plateauof about 38% between weeks 9 and21(Fig.2b).Ingroup IIIthe percentageprecipitationof bothantigenswas alwaysbelow 6. Thespecificities of the
circulating
antibodies have notbeen determined in thisexperiment.Immunofluorescent studies. As summarized in Table 2, linear IgG fluorescent deposits were obviousonGBM andTBM as soon asweek 5 in group I. At week9,allglomeruliandtubuleswere strongly stained in all mice(10outof10).IngroupII, linear IgGdepositscouldbe observedfirst on tubules(week 5) and then on the glomeruli (week 7).Atweek 9, 10outof10mice hadintense linear IgGdepositsonthe GBM and TBM. No specific staining was obvious in control mice.
Studies
ofkidney
eluates.Theprotein concentration was respectively 1, 0 5 and 0 3 mg/ml in the eluates obtained from groups I,II and III. Only IgG wasdetected byimmunoelectrophoresis in groupsIandII.When incubatedinvitro on normalCBA kidney sections theeluatesfrom groups I andIIfixedonthe GBM and TBM; however, when injected in vivo they fixed on the GBM only. Resultsobtained in NZB mice (H-2d)Circulating antibodies. In groupI,thepercentage of
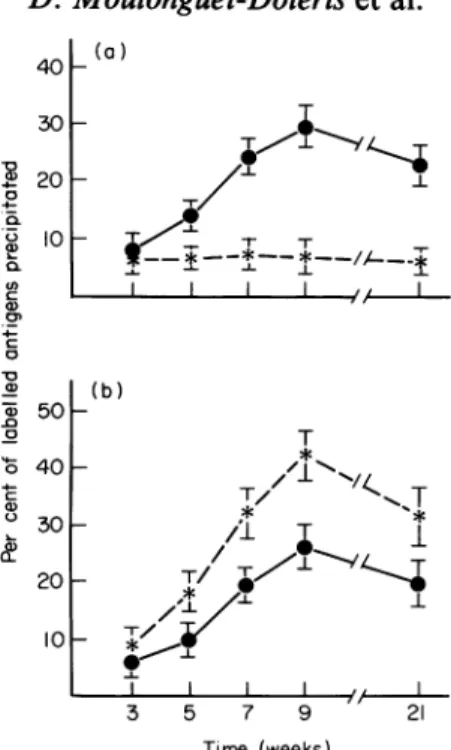
'25I-GBM
precipitated rose progressively to 30+ 3 atweek 9 and decreased slowly to 23 + 3 at week 21; no anti-TBM antibodies could be detected (Fig. 3a). In group II (Fig. 3b), the precipitation of labelled TBM antigens reached 42 5 +4%atweek 9 and dropped to 31-5+5% at week 21. Thepercentage of labelled GBM antigens precipitated was lower (26+3% atweek 9). No anti-GBM and anti-TBM antibodies could be detected inFCA-treated mice.Radioimmunoassaywas used tolook forcirculatinganti-DNA antibodies. The percentages of
labelledDNA precipitated at week 9 wererespectively
I7-5
±2 5, 28±3 and35+4ingroups III, I andII and raised to 38+3% in group I and 45+5% in group II at week 21. The specificity of40
40 30o20
a) (b)D50
-oT 450 T~~T 0 20 T/ 10 3 5 7 9 21 Time (weeks)Fig. 3. Circulating anti-GBM and anti-TBM antibodiesin NZB mice. Percentage of 125I-GBM antigens (. -)and
125I-TBM
antigens (* *) bound by (a) sera from GBM-treated mice and(b) sera from TBM-treatedmice.anti-GBM andanti-TBMantibodies wasstudied byinhibition experiments performedwith the above-mentioned antigens. Whentesting sera from group I the precipitation of labelled GBM antigenswasfoundtobe inhibitedby85% by thedisaccharide-containing glycopeptideobtained from GBM and TBM and by type IV procollagen. In contrast, with sera from group II, the
precipitation of labelled TBM antigens was inhibited by 90% by the
heteropolysaccharide-containing glycopeptidesobtainedfromTBM.The inhibition obtained with type IVprocollagen
wasonly 16%. The precipitation of labelledDNAwasinhibitedby 90%bycold DNAbutnotby GBMorTBM inserafromgroupsIandII.
Immunofluorescent studies. Results of immunofluorescentstudiesaresummarizedinTable 3.In
groupI, linear IgGdepositswereobviousonthe tubulesasearlyasweek 3andontheglomeruliat Table 3. Resultsofdirect immunofluorescent studies in NZB mice
Linear IgG
depositson: WeekNo. GroupI GroupII GroupIII
GBM 3 0(2/2) 0(2/2) 0(2/2) 5 + +(3/3) 0(3/3) 0(3/3) 7 ++ +(3/3) 0(3/3) 0(3/3) 9 ++ +(10/10) ++ (5/11) 0(10/10) 21 ++ +(3/3) ++ +(3/3) 0(4/4) TBM 3 +(2/2) + (2/2) 0(2/2) 5 ++(3/3) +(3/3) 0(3/3) 7 ++ + (3/3) ++ +(3/3) 0(3/3) 9 + + +(10/10) +++(11/11) 0(10/10) 21 ++ + (3/3) ++ +(3/3) 0(4/4)
week 5. After
eight injections
ofGBM,
allglomeruli
and tubules of all mice(10outof10) werehighly positive.
In groupII,
the tubuleswerealready
stainedatweek3,all mice(11outof11)werepositive
atweek9,
while linearIgG
deposits
onthe GBMwereobviousonly
atweek 9 and in four of 11 mice.Allmice of both groups whichwerewithdrawnatweek 9 exhibited strong linear
IgG deposits
onglomeruli
and tubulesonkidney biopsies
performedatweek 21.Studies
of
eluates.Theprotein
concentrationswererespectively
25,
3 and2mg/mlin theeluatesobtained from groupsI, II and III.
Only IgG
wasdetected in eluates from groups I and II byimmunoelectrophoresis.
Forin vitrostudies,
the eluateswereincubatedonnormal NZBkidney sectionsatconcentrationsof1,0 5and 0 25mg/ml
ofprotein.
At these threeconcentrations,
eluates fromgroup Ifixedstrongly
onGBM andTBMwhile eluates from group II fixed withastrongerintensity
onTBMthanonGBMatthe concentrationof 0 25mg/ml.
When the eluateswereinjectedintravenously
into normalNZBmice,
stronglinearIgG deposits
wereobservedonthe GBMonly while TBMappeared lightly
stainedonkidney
biopsies performed
1hrand 6days
afterinjection.DISCUSSION
Regardless
of thedog
renal basement membranepreparations
usedforimmunization,
all the strains of mice testedproduced
antibodies(of IgG
butnotIgM
class)
tobothautologous
TBMandGBM,asdemonstrated
by
indirect immunofluorescenceperformed
invitrowithkidney
eluates.Indeed,
in theseexperiments,
linearIgG
deposits
wereobservedalong
the GBM and TBM. All the strainsthereforepossessthe basement membrane
antigen(s)
thatcross-react(s)
with theimmunizing
dogbasement membranes.
Furthermore,
we have shown that there is no correlation between theantibody
responsetoimmunizationwithTBMorGBMandtwoH-2 types,namely
H-2dandH-2k.Comparable
data have beenobtainedby Hyman,
Colvin &Steinberg (1976)
inguinea-pigs
andbyRudofsky,
Dilwith &Tung (1980)
inmice immunizedby
TBMonly.
Intheirstudies,
nocorrelationexists between the anti-TBM
antibody
responseand thehistocompatibility
typeof the different strains. Ontheotherhand,
weobserved that thelevelofcirculating
antibodieswas notrelatedtotheamountof
antigen used,
assuggested by experiments performed
inC3Hmice with 2 mg ofantigens
per
injection
(Erard
etal.,unpublished data).
In our
work, despite
the presenceof antibodies in the target organ, all animals exhibited a normal renal function and didnotdisplay
histological lesions. No significantC3 depositswere observedalong
the GBMand/or
the TBM of miceinjected
withGBM orTBM antigens. The absence of C3deposits
couldexplain
thelackofdetectablekidneylesions. However, this isnot acompletely satisfactory explanation
asglomerularandtubulointerstitiallesions mayoccurwithout evidence ofcomplement deposition
in certain models of anti-GBM or anti-TBM antibody-mediatednephropathies
(Cochrane, 1969; Couser, Stilmant & Lewis, 1973; Bolton, Benton &Sturgill, 1978).
Since it has beenpreviously
demonstratedin theguinea-pig(Lehman et al., 1974) thatpertussis adjuvant
ismoreeffectivethan FCA ininducing interstitial nephritis, we attempted to inducehistological
lesions by substitutingthe pertussisadjuvant
for FCA in the immunizationprotocol.
Inourhands,
theuseofpertussis adjuvant in mice does not modify the immune response andnorenalhistological
lesionsareobserved (Erardetal., unpublished data). It should also bepointed
outthatdespite
theirparticular abilitytoproduce autoantibodies, NZB mice injected with TBMorGBMantigensdonotdevelopglomerular or tubulointerstitial lesions. Our results suggest,asdo those
published
by Hymanetal.(1976)
inguinea-pigs and by Rudofsky etal.(1980)in mice, that themagnitudeof theantibodyresponse is not the only factor triggering the development of renal lesions orfunctional impairment. Rudofsky et al. (1980) testing mice with seven differenthaplotypes
demonstratedthat the ability to develop tubulointerstitial lesions after one injection of TBM wascontrolledinpartby genes of the major histocompatibility complex. Our contrasting results in BALB/cand NZB micemight be explained by the different antigens used and/or by differentspecificities
of the antibodies developed not defined in Rudofsky's work.Our
previous
studiesonthe specificityof antibodies in BALB/c mice have shown that (a) afterimmunization with insoluble GBM, the antibodies are mainly directed against collagenous
42
D.
Moulonguet-Dokris
et
al.
antigenicdeterminants shared by GBM and TBM; and (b) after immunization with insoluble TBM, the antibodies aremainly directed against non-collagenous TBM-specific antigenic determinants (Erard et al., 1979). In the three other strains tested in the present work, the harvested antibodies are directed against the collagenous material of the membrane, when the immunogen is insoluble GBM. This contention issupported by thein vitrostudies of the eluates and by radioimmunoassay inhibition experiments. Indeed, IgG eluted from kidneys of GBM-treated mice still bind to GBM and TBM. Antibodies raised in GBM-treated mice are mostly directed against the type IV procollagen of the basement membranes. This fraction has been shown (Graindorge & Mahieu, 1978;Lehman et al., 1974) to be shared by the GBM and TBM. These antibodies can therefore be observed by direct immunofluorescence on the GBM and TBM of GBM-injected mice. The specificity of antibodies developed in TBM-treated mice differs according to the strain of mice. In NZBmice(H-2dtype), as in BALB/c mice(H-2dtype), the antibodies are mainly directed against non-collagenous polypeptides present in TBM. This material is not laminin, a non-collagenous glycoproteinfraction purified from basement membrane secreted by a murinetumour (Timpl et al., 1978).The TBM-specific antigenic determinants, however, are well located in the non-collagenous domain of themembrane, since only the heteropolysaccharide-containing glycopeptides purified from TBM are able to inhibit the binding of labelled TBM antigens to anti-TBM antibodies. These
antibodies thereforedeposit specifically on tubules as shown by direct immunofluorescence. The CBA mice (H-2ktype)respond to both the collagenousand non-collagenous material of TBM. Accordingly, linear IgG deposits have been observed along the GBM and the TBM of all the mice of this group.The C3H mice do not respond to the non-collagenous material ofTBM. With both doses
ofTBMantigens, linear depositsareonlyobservedalong the GBM.Theseantibodiesaredirected
against the collagenous material shared by the TBM andthe GBM. The absence of linear IgG
deposits alongthetubulesmaybethe consequence of the relatively weak response of these mice to typeIVprocollagen. Themajorityof theanti-typeIVprocollagen antibodiesare mostprobably
trapped by the GBMduring the ultrafiltration process.
Our resultsthereforedemonstrate that variousmousestrainsareabletodevelopanantibody
response to the collagenous and/or the non-collagenouspolypeptides presentin the GBM and TBM.Thisresponsedoesnotdepend onlyonthemajor histocompatibility complex,since there is nocorrelation between thespecificityof the harvested antibodiesandtheH-2korH-2dtypes.The existence of antibodies with bothcollagenousandnon-collagenous
specificities
further rulesoutthepossibility that the chemical nature of basement membrane antigens triggering the immune responseplaysamajorrole in thedevelopmentof renal
injury
inmice.Other'facilitating
factors' mayplayapathogenicroleasinhuman anti-GBMnephritis,
suchas apersisting impaired
humoraland cellularimmunityor someintercurrentbacterialorviral infections
(Rees,
Lockwood &Peters,
1977;Wilson&Smith, 1972;Perezetal., 1974).
Experiments
are nowbeing developedin orderto testtheresponsibilityof otherenvironmental factorsin thedevelopment
of renal lesions in mice. ThisstudywassupportedbyINSERM CRL 79.5.272.5. Wearegrateful
toDr P.Galanaudfor hisvaluable advice andtoMrs 0.Boudin fortypingthemanuscript.REFERENCES
BOLTON, W.K., BENTON, F.R. & STURGILL, B.C. (1978) Autoimmune glomerulotubular nephro-pathy in mice. Clin. exp. Immunol. 33, 463. COCHRANE, C.G. (1969) Mediation ofimmunologic
glomerular injury. Transplant.Proc. 1,949. COUSER, W.G., STILMANT, M. & LEwis, J. (1973)
Experimental glomerulonephritis in the guinea pig. I. Glomerular lesions associated with anti-glomerular basement membrane antibodydeposits. Lab. Invest. 29, 236.
ERARD,D.,MOULONGUET-DOLERIS,D.,AuFFREDou, M.T.,GRAINDORGE,P.,MAHIEu,P.,GALANAUD, P. & DORMONT, J. (1979) Specificity of antibodiesto
heterologous glomerular and tubular basement
membranes in Balb/c mice. Clin. exp.Immunol.38, 259.
FARR, R.S. (1968) Aquantitative immunochemical measureofthe primary interactionbetween BSA and antibody. J. infect. Dis. 103, 1412.
GRAINDORGE, P.P. & MAHIEU, P.R. (1978) Radio-immunologic methodfordetection ofantitubular basement membrane antibodies. Kidney Int. 14, 594.
HYMAN, L.R., COLVIN, R.B. & STEINBERG, A.D. (1976)Immunopathogenesisofautoimmune tubu-lointerstitialnephritis. I.Demonstrationof differ-ence susceptibility in strain II and strain XIII guinea-pigs. J. Immunol. 116, 327.
Specificity
of anti-basement membrane Ab
43
LEHMAN, D.H., MARQUARDT, H., WILSON, C.B. & DIXON, F.J. (1974)Specificity of autoantibodiesto tubular andglomerularbasement membranes in-duced inguinea-pig.J.Immunol. 112,241. LERNER,R.A.,GLASSOCK,R.J.& DIXON, F.J.(1967)
The role ofantiglomerular basement membrane antibody inthepathogenesis of human glomerulo-nephritis.J.exp.Med. 126,989.
LowRy, O.H., ROSENBROUGH, N.J., FARR, A.L. & RANDALL, R.J. (1951) Protein measurement with theFolinphenolreagent. J.biol.Chem. 193, 265. MAHiEu, P.R., LAMBERT, P.H. & MEscmm, P.A.
(1974) Detection of antiglomerular basement membrane antibodies by a radioimmunological technique. Clinical applicationsinhuman nephro-pathies. J.clin.Invest. 54, 128.
MAHIEU, P. & WINAND, R.J. (1970) Chemical structure of tubular and glomerular basement membraneofhuman kidney: isolationand charac-terization of the carbohydrateunits. Eur. J. Bio-chem. 15, 520.
PEREz,G.O.,BJORNSSON, S., Ross, A.H., AMATO, J.O. & ROTHFIELD,N.(1974)Amini-epidemicof Good-pasture'ssyndrome.Nephron, 13, 161.
REES, A.J., LOCKWOOD, C.M. & PETS, D.K. (1977) Enhanced allergic tissue injuryin Goodpasture's syndrome by intercurrent bacterialinfection. Br. Med. J.fi,723.
RUDOFSKY, V.H., DILWITH, R.L. &TUNG, K.S. (1980) Susceptibility differencesofinbred miceto induc-tionofautoimmune renaltubulointerstitiallesions. Lab. Invest. 43, 463.
TIMPL, R.,ORKYN, R.W., ROBEY, P.G., MARTIN, G.R. &WICK, G.(1978) Chemical and immunological studies on basement membrane collagen from a murine tumor. InBiology andChemistryof Base-mentMembranes (ed. by N. A.Kefalides),Vol. 1,
p. 143.Academic Press, New York.
WILSON, C.B. &SwMTH, R.C. (1972) Goodpasture's syndromeassociated with influenzaA2virus infec-tion.Ann.intern.Med.76, 91.