Conception and Validation of Non-Conventional
Mechanical Characterization Protocols Specific to
Soft Tissues for Vascular Application
Mémoire
Audrey Lainé
Maîtrise en génie des matériaux et de la métallurgie
Maître ès sciences (M.Sc.)
Québec, Canada
Conception and Validation of Non-Conventional
Mechanical Characterization Protocols Specific to
Soft Tissues for Vascular Application
Mémoire
Audrey Lainé
Sous la direction de:
Résumé
Les maladies cardiovasculaires représentent une des principales causes de décès dans le monde et différentes actions sont en place depuis des décennies pour comprendre les mé-canismes d’action de ces maladies et leur impact sur le corps humain. Dans l’optique de venir en aide aux personnes souffrant de ces maladies, le génie tissulaire vasculaire émerge comme une technologie prometteuse afin de favoriser la régénération des tissus et des organes endommagés. Cependant, les modèles issus du génie tissulaire, en plus d’être biologique-ment et chimiquebiologique-ment comparables au tissu natif, doivent égalebiologique-ment présenter des propriétés mécaniques permettant aux modèles de répondre aux différentes fonctions physiologiques. Dans cette optique, il est nécessaire de concevoir, optimiser et développer une série d’essais permettant de vérifier les performances mécaniques des constructions regénérées.
De nombreuses approches ont été déployées dans ce domaine contenant chacune ses avan-tages et ses inconvénients, mais il n’existe pas,à l’heure actuelle, aucun standard sur le pro-tocole des essais mécaniques à préconiser avec un échantillon spécifique. Dans ce contexte, le projet de ce mémoire a été de concevoir et de valider des protocoles de caractérisation mécanique non-conventionnels appliqués spécifiquement à des tissus vasculaires.
Tout d’abord, un protocole pour la caractérisation mécanique de gels de collagène en confi-guration plane à été développé. Ensuite, ce protocole à été étendu pour la modélisation numérique afin d’étudier l’applicabilité d’un modèle poro-viscoélastique. Par la suite, des protocoles expérimentaux ont été développés afin de permettre la densification de gel de collagène ainsi que leur caractérisation mécanique. Enfin, une caractérisation expérimen-tale d’aortes de souris calcifiées à été réalisée. Dans son ensemble, l’étude exécutée dans le cadre de cette maîtrise à permis l’approfondissement des connaissances dans le domaine de la caractérisation mécanique des tissus mous viscoélastiques.
Ce mémoire présente également d’autres techniques de caractérisation mécanique pour dif-férents types de tissus vasculaires. D’autres protocoles de tests développés dans le cadre de collaboration avec d’autres groupes de recherche sont décrits dans ce mémoire.
Abstract
Cardiovascular diseases represent one of the principal causes of death worldwide. It is there-fore of high importance to improve our understanding of their mechanisms of action and their impact on health. To help people suffering from these diseases, tissue engineering is emerging as a promising technique for developing regenerated constructs to replace diseased tissues and organs. However, apart from being biologically compatible, the developed con-struct also needs to have mechanical properties like the one of native tissues. It is therefore necessary to perform mechanical characterization on the tissue engineered construct to vali-date its suitability.
When it comes to mechanical characterization, a lot of approaches are used by different research groups as there is absolutely no standard in this field. In this context, the objective of this thesis is to develop and validate non-conventional mechanical characterization protocols specific to soft tissues for vascular application.
First, a testing protocol was developed to characterize disk-shaped collagen gel samples. Sec-ondly, the same collagen gel, but in tubular geometry, were densified and also characterized. Finally, a mechanical testing protocol and device was developed in order to characterize very small caliber blood vessels, such as mouse aortas.
This thesis also presents other techniques for mechanical characterization used for vascu-lar tissue, as along with protocols developed in the process of external collaboration with different research groups.
Contents
Résumé iii
Abstract iv
Contents v
List of Tables viii
List of Figures ix
Ackowledgement xii
Foreword xiv
Introduction 1
0.1 Biomaterials used for cardiovascular repairs . . . 1
0.1.1 Cardiovascular system . . . 1
0.1.2 Cardiovascular pathologies . . . 2
0.1.3 Repair and replacement . . . 3
0.1.4 Vascular tissue engineering . . . 4
0.2 Mechanical characterization of vascular tissues . . . 6
0.2.1 Introduction to mechanical properties of cardiovascular biomaterials 7 0.2.2 Viscoelasticity. . . 10
0.2.3 Poroelasticiy . . . 11
0.2.4 Mechanical characterization technique . . . 12
Testing environment . . . 12
Sample geometry . . . 13
Uniaxial tensile test . . . 13
Compression test . . . 15
Biaxial test . . . 15
Burst pressure test. . . 16
Compliance test . . . 16
Stress relaxation test . . . 17
Creep test . . . 19
Device components . . . 20
1 Poro-viscoelastic models applied to collagen hydrogel scaffolds 24
1.1 Résumé . . . 25
1.2 Abstract . . . 26
1.3 Introduction . . . 27
1.4 Materials and methods . . . 28
1.4.1 Sample preparation . . . 28
1.4.2 Mechanical characterization . . . 29
1.4.3 Modeling and time constant study . . . 29
1.4.4 Structure analysis . . . 30
1.5 Results and discussion . . . 30
1.5.1 Mechanical characterization . . . 30
1.5.2 Modeling and time constant study . . . 32
1.5.3 Structure analysis . . . 32
1.6 Conclusion . . . 34
2 Rotation-based technique for the rapid densification of tubular collagen gel scaffolds 35 2.1 Résumé . . . 36
2.2 Abstract . . . 37
2.3 Graphical abstract . . . 38
2.4 Introduction . . . 39
2.5 Material and methods . . . 41
2.5.1 Preparation of collagen gel-based constructs . . . 41
2.5.2 Stress relaxation testing . . . 41
2.5.3 Immunofluorescence . . . 43
2.5.4 Image analysis . . . 43
2.5.5 Statistical analysis . . . 44
2.6 Results and discussion . . . 44
2.6.1 Effects of densification on collagen fibers . . . 44
2.6.2 Mechanical testing . . . 46
2.7 Concluding remarks . . . 46
3 Mechanical characterization of calcified aortas from Matrix Gla Protein deficient mice 48 3.1 Résumé . . . 49
3.2 Abstract . . . 50
3.3 Introduction . . . 51
3.4 Materials and methods . . . 53
3.4.1 Mice . . . 53
3.4.2 Mechanical characterization . . . 53
3.4.3 Data processing . . . 54
3.4.4 Inductively coupled plasma - Optical emission spectrometry (ICP-OES) . . . 56
3.4.5 Histological analysis . . . 57
3.5 Results and discussion . . . 57
3.5.1 MGP deficient mice . . . 58
3.5.2 Calcium content . . . 58
3.5.3 Circumferential tangent modulus . . . 61
3.5.4 Failure mechanism . . . 63
3.6 Conclusion . . . 65
3.7 Acknowledgement. . . 65
4 General discussion 67 4.1 Mechanical characterization : How to select the appropriate testing protocol 68 4.2 Poro-viscoelastic models applied to collagen hydrogel scaffolds. . . 72
4.3 Rotation-based technique for the rapid densification of tubular collagen gel scaffolds . . . 73
4.4 Mechanical characterization of calcified aortas from Matrix Gla Protein deficient mice . . . 74
4.4.1 Challenges or working with small caliber animal vascular tissue . 74 4.4.2 Choice of component . . . 76
Pressure transducer . . . 76
Syringe pump . . . 76
Camera . . . 77
4.4.3 Calibration of the pressure transducer . . . 77
4.4.4 Development of the LabVIEW interface . . . 78
Conclusion 80 A Development of mechanical characterization device for mouse aortas 81 A.1 Sample preparation protocol for mechanical characterization . . . 81
B Experimental protocols for mechanical characterization of various biolog-ical tissues 85 B.0.1 Mechanical characterization of 3D elastin gels as a model for vas-cular calcification . . . 85
Material and methods . . . 86
Results and discussion . . . 86
B.0.2 Mechanical characterization of calcified septum cartilage from MGP deficient mice . . . 86
Material and methods . . . 87
Results and discussion . . . 88
List of Tables
3.1 Data summary table from calcium content analysis and circumferential tan-gent modulus calculated from the stress-strain curve in the 5-15% strain range for heterozygous (HT), wildtype (WT) and MGP-deficient (MGP) mice at 3
List of Figures
0.1 Physiological biomechanical data on blood circulation throughout the
vascu-lar system . . . 7
0.2 Stress and strain in blood vessels.. . . 9
0.3 Viscoelastic features of soft tissues.. . . 11
0.4 Uniaxial tesile test performed on soft tissues. . . 14
0.5 Compression test on soft tissues. . . 15
0.6 Biaxial testing device . . . 16
0.7 Typical results from compliance test. . . 18
0.8 Schematic of a stress relaxation test . . . 19
0.9 Typical results from cyclic stress relaxation test. . . 20
0.10 Organizational chart showing the scientific structure and approach of this re-search work. . . 23
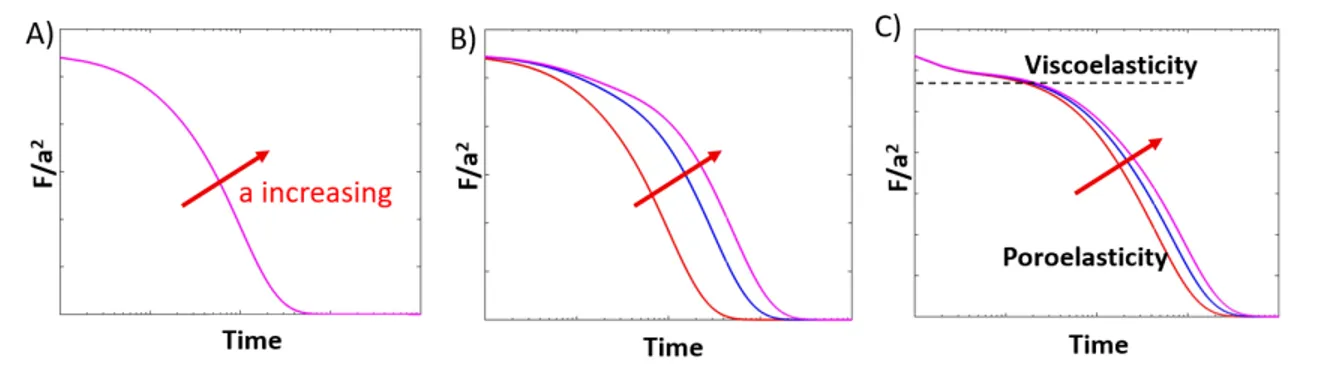
1.1 Schematic curves on separating viscoelasticity and poroelasticity of a gel. . . 30
1.2 Stress relaxation under a 50% strain for disk shape sample of different radius. 31 1.3 Time constants from mechanical behavior modeling. . . 33
1.4 SEM imaging of collagen and gelatin matrix structure. . . 33
2.1 Graphical abstract. This study introduces a novel, rapid, and simple rotation-based technique to produce denser and stronger tubular collagen scaffolds under sterile conditions. . . 38
2.2 Schematic description of the developed technique. . . 42
2.3 Collagen gel scaffolds. . . 45
2.4 Mechanical characterisation of the tubular constructs. . . 47
3.1 Thoracic descending aorta preparation for mechanical testing.. . . 54
3.2 Schematic representation of mechanical testing device. . . 55
3.3 Mouse physiological data. . . 59
3.4 Aortic calcium content and distribution. . . 60
3.5 Stress-strain curve and circumferential tangent modulus. . . 63
3.6 Aortic failure mechanism.. . . 64
4.1 Schematic summary of how the different mechanical characterization tech-nique to chose according to the sample geometry and desired mechanical properties. . . 71
A.1 Mouse aorta upon reception prior to mechanical testing. . . 82
A.2 Aorta preparation for mechanical characterization. . . 84
B.1 Mechanical characterization on elastin gels. . . 87
B.2 Mechanical characterization of nasal septum cartilage from mice. . . 88
I have not failed,
I’ve just found 10 000 ways that won’t work.
Acknowledgement
I first want to acknowledge my supervisor, Diego Mantovani, who allowed me to make my entrance in the broad world of biomaterials. It all started from the class I took during my undergrad in which you succeeded in passing on your passion for this field to me. Diego, you taught me something very important. You showed me that graduate studies are about more than what you learn, it is also what you become. It shows you how to develop great working skills, but also personal skills and scientific curiosity. Being part of the Laboratory of Biomaterial and Bioingineering (LBB) also allowed me to meet some amazing people from everywhere around the world and that was such an experience in itself. I also want to thank you, Diego, for allowing me to participate in a Canadian congress and even a world biomaterial congress where I got to meet with a lot of passionate scientists and students. I want to specially acknowledge my co-supervisor J. Michael Lee from Dalhousie University for giving me the opportunity of coming to his lab for almost a year. It has been such a great learning and personal experience. Thank you very much for having faith in me throughout this entire project and making me feel like I was part of the lab’s family. I really appreciated all the great scientific discussion we had during our meetings. Thank you very much for being almost as keen as I was about my project, and thank you for listening and handing tissue over when times were rougher. I also want to thank him particularly for this one time he drove from home all the way to the lab on the Sunday of Mother’s day to help me fix the camera so I could continue with my testing. Thank you very much, I am extremely grateful for everything you have done for me.
I would like to thank my committee who took the time to review this thesis. I want to thank them for their relevant comments that contributed to greatly improving the quality of this manuscript. A special thank you to Geatan Laroche for his listening and for his remarkable laughter, which you can hear from anywhere in the lab and that always gives you a little smile.
Engineering Research Council for partially founding this research through a MSc scolarship in the frame of the NSERC CREATE program for regenerative medicine (NCPRM).
I want to thank Lucie Lévesque from the LBB for her kind help and guidance throughout this entire thesis. Thank you for answering my questions at any time of the day and always taking the time to help me in any situation. A special thanks to you for reviewing and pressing send on important emails. I also want to acknowledge my dear colleague and friend Caroline Loy for her support during these last years. It was such a pleasure to work on this paper we published together. As the first paper for both of us, it was a great learning experience. Working with you has made work much more than just work but also even fun. I am very lucky I got to meet both of you girls and I know all the best is yet to come for you.
Thank you very much to all the research professional from the LBB : Pascale Chevalier, Stéphane Turgeon for always being all ears when I had issues and for the time they devoted to helping me. Thank you very much Pascale for reviewing my papers, like I always said, you are the best chemist in the entire world ;). I want to acknowledge Daniele Pezzoli for reviewing my papers. You have this magic touch that makes them always so much better.
Thanks to all my colleagues from the LBB and especially the Collagen Team for their help and advices in our meetings.
Thank you to my friends from the Tissue Mechanics Lab at Dalhousie University. Thank you to Jasmin Astle for being an awesome friend and making my stay in Halifax an amazing time. Thank you so much for being my personal interpreter and for being the only one who could understand my Quebec French-English dialect. Thank you for taking the time and having the patience to bring me everywhere with you to show me the city and to make me meet all your people. Thank you very much to Kelina Murdymootoo for being such an amazing and supportive friend during my stay in Halifax. See you both soon in Quebec and I love you so much! A special thanks to Adam Brown for his extreme and almost keener-like devotion when I asked for help. It was awesome to know that there will always be someone to count on when help was needed. Thank you Brendan Grue for all the scientific discussions and help with AAS. I am highly grateful to both of you guys for your kind accommodation toward a very tired girl driving all the way from Quebec to Halifax. Meeting all of the students from the Biomedical Engineering faculty was a blessing for me as all of you were so kind to me. This experience will stay engraved in my memory forever.
Foreword
The mechanical properties of biological soft tissues are a very important aspect of their phys-iological behavior. However, with aging or progressive pathologies, the mechanical integrity of these tissues can become altered, leading to further complications. Sometimes, the un-healthy tissue needs to be replaced with a biomaterial, in which case the mechanical prop-erties are again extremely important to consider. To be suitable for any application, a bio-material must mimic the mechanical properties of the native tissue. This is the reason why mechanical testing is an important part of assessing the morphological and functional per-formance of any biomaterial. All materials have a composition and structure specific to their application and function. These differences also need to be considered in choosing the right testing protocol to validate the required mechanical properties. The purpose of this research project is the development and validation of non-conventional mechanical characterization test protocols, specific to soft tissues for vascular application.
This project was conducted primarily in the Laboratory for Biomaterials and Bioengineer-ing (LBB) at Laval University, located in the research center of the Saint François d’Assise Hospital in Quebec, and under the supervision of Professor Diego Mantovani. This project was funded by the NSERC CREATE program for regenerative medicine (NCPRM) through a Master’s level scholarship. This project was also in collaboration with the Tissue Mechanics Lab of the School of Biomedical Engineering at Dalhousie University in Halifax and under the supervision of Professor J. Michael Lee. This collaboration took the form of a close to one year internship, where a mechanical testing device for mouse aortas was developed and tests were performed. This stay in Halifax also allowed me to tremendously improve my English.
CHAPTER 1 : Poro-viscoelastic models applied to collagen hydrogel scaffolds. Author : Audrey Lainé
in mechanical testing from the LBB. With Bernard, there were many discussions about vis-coelastic and poroelastic mechanical behavior, and the experimental protocol for the mechan-ical testing was developed based on the literature. I performed the experiments and wrote the chapter.
CHAPTER 2 : Rotation-based technique for the rapid densification of tubular collagen gel scaffolds.
Authors : Caroline Loy, Audrey Lainé and Diego Mantovani
Journal : This paper was published in Biotechnology Journal, section Biotech Method, 2016, 11(12), 1673-1679. doi: 10.1002/biot.201600268
This work started when I assisted Caroline with her experiments on creating tubular collagen scaffolds and we developed this densification technique. We made the gels together and I performed the mechanical characterization. Caroline conducted the immunofluorescence and cell viability assay and analyzed the data. The manuscript was mostly written by Caroline as first author; I wrote all parts regarding mechanical characterization. Corrections were made by all the authors. This chapter is an adapted version of the published paper, highlighting the parts on mechanical characterization.
CHAPTER 3 : Mechanical characterization of calcified aortas from Matrix Gla Protein deficient mice.
Authors : Audrey Lainé, Abhinav Parashar, J. Micheal Lee, Monzur Murshed and Diego Mantovani
This paper is now in preparation and its submission is planned for summer 2017.
This work was performed in the Tissue Mechanics Lab at Dalhousie University. Mouse aortas were harvested and shipped from McGill University in Montreal by Abhinav. Abhinav bred the mice and performed the genotyping and histological staining under the supervision of Monzur Murshed. I developed the mechanical testing device and performed all the mechani-cal characterization and data analysis. Abhinav parashar performed the histologimechani-cal analysis. The draft was written by me; all authors provided input to strongly improve the manuscipt to its current status.
Introduction
0.1
Biomaterials used for cardiovascular repairs
0.1.1
Cardiovascular system
The function of the cardiovascular system is to distribute oxygenated blood and required nutrients to organs throughout the body. It is composed of two principal components which are the heart and the blood vessels, which allow for blood circulation.
The heart is a muscle, the most important muscle the body contains. It is totally independent, meaning it contracts by itself, and its rhythmed contraction allows for blood to be in motion in the entire vascular system [1]. It can be compared to a pump, in a mechanical sense, which distributes fluid inside a system. It pumps into three distinct divisions of the circulatory system: the coronary (vessels that serve the heart), pulmonary (heart and lungs), and the systemic (systems of the body). The heart is a four-chambered organ which is composed of right and left atria and ventricles.
The vascular system is composed of a network of blood vessels distributing blood to the entire body. It is divided into three main categories: the arteries, the veins and the capillar-ies. Arteries and veins have their layered structure in common as they are composed of 3 layers: the intima, media and adventitia layer. Each layer has its own composition and this composition will allow for their specific functions.
The intima layer is the internal layer of the blood vessel. This layer is composed of endothe-lial cells which will directly interface with blood [2]. These cells form a very smooth film to minimize friction with blood. They are also responsible for mechanotransduction of the blood vessel. Based on the shear stress they experience from the rate of blood flow, they convert this into a physiological, intracellular signal to allow for vasodilation. Vasodilation allows for blood pressure to be kept constant if there is any change in blood flow rate.
cells, elastin and collagen type I and II. The elastin rich composition of this layer gives it the role of providing elasticity to the blood vessel. This layer is thicker than the two others and the elastic properties it provides to the blood vessel allow for diameter increase and decrease under pulsatile blood flow [2].
The adventitia is the external layer of the blood vessel. It is composed of a strong collagen network and fibroblasts. This collagen network plays a major role in the strength of the blood vessel wall. Under an applied stress, this layer will take on the load as the stress is too great for the medial layer to control the final radial dilation. This layer also anchors the vessel to the surrounding connective tissue.
0.1.2
Cardiovascular pathologies
Cardiovascular diseases are the second greatest cause of mortality worldwide behind cancer. Many factors can increase the risk of cardiovascular diseases such as diabetes, hypertension and high cholesterol level. It is also possible to take actions to limit those risk factors such as increasing activity level, avoiding smoking and having a healthy diet.
The most common cardiovascular disease is atherosclerosis, which mostly takes place within the arteries. Atherosclerosis consists of the creation of atheroma plaque within the arterial intimal layer. This plaque is composed of lipid deposition, cellular debris, macrophages and calcium [3]. The creation of this plaque leads to a thickening of the vessel wall and obstruction of blood circulation. With the alteration of normal blood flow, it is a struggle to bring oxygenated blood and nutrients to the different organs. The atherosclerotic plaque can grow until complete lumen occlusion or even rupture, both scenarios leading to further complications such as thrombosis, cerebral vascular accidents and myocardium infarctions [4,5].
Another cardiovascular disease that is unfortunately widely spread but less understood is vascular calcification. It consists of the mineral deposition within the vascular tissues due to aging, or degenerative diseases such as advanced atherosclerosis, chronic kidney disease and type 2 diabetes[6,7]. The mineral deposits formed within the arteries consists of hydroxya-patite (HA) crystals [8] that are typically only present in mineralized tissues such as bone, suggesting that cardiovascular calcification is due to a process of biomineralization similar to osteogenesis [7]. There are two types of vascular calcification: intimal and medial calcifica-tion. The former occurs when mineral deposition takes place in the intimal layer of the artery and is often due to a degeneration of atherosclerosis plaque ruptures . The latter is totally independent from the intimal calcification and occurs when calcium deposition takes place in
the medial layer of the artery. Medial calcification is characterized by the thickening of the medial layer in parallel with the disruption of the elastic laminae, the concentric elastin-rich ECM layers responsible for arterial elasticity [6]. Due to the bonding of HA to the elastin fibers present in the elastic layers of the arteries, their elastic properties are therefore highly diminished. Elastin being responsible for the elasticity of blood vessel [9], a significant in-crease in the vessel stiffness is expected to be seen upon calcium deposition [10]. Arterial stiffness is one of the most important factor causing increased systolic and pulse pressure and therefore, leading to cardiovascular complications and events, including left ventricu-lar hypertrophy and failure, as well as aneurysm formation and rupture [11, 12, 10]. In the case of the thoracic aorta, composed of up to 40% of elastic fibers [10], decreased elasticity leads to loss of its Windkessel function and severe health issues. Different factors such as the extracellular level of calcium ions and of inorganic phosphate, the presence of a suitable extracellular matrix for mineral deposition, and the absence of mineralization inhibitors such as inorganic pyrophosphate, matrix Gla protein and fetuin-A have been shown to regulate the initiation and progression of Ca deposition within the arterial wall [6].
Cardiovascular diseases affect the physical and chemical structure of the vascular wall. These pathological situations cause the vessels to be thicker and the lumen to be narrowed. These factors alter the mechanical integrity of the blood vessel by increasing wall stiffness and thus preventing the blood vessel from being able to perform its physiological and necessary function of carrying blood throughout the entire body.
0.1.3
Repair and replacement
For those suffering from obstructive diseases, the following are the possible solutions avail-able.
The first and most widely used clinical treatment is the stent implantation [13]. Through an incision into the femoral artery, a catheter surrounded by a stent is inserted inside the body by the surgeon. When the pathologic site is reached, a balloon is deployed, and the stent expanded, to reopen the vessel lumen and bring mechanical support to the vessel. Blood circulation is then re-established. However, stents, which are often made of metal, are ex-panded to a fixed diameter and this can cause a problem for rapidly growing children with congenital disorders. The stented vessel can not grow with the child leading to a relative re-occlusion of the vessel. Furthermore, their permanent presence can lead to complications such as thrombosis, late restenosis and even stent fracture.
total resection and replacement is necessary. To do so, different types of grafts can be used depending on the diameter of the blood vessel that needs to be replaced.
For small caliber blood vessels (Ø<6mm), autologous grafts are commonly performed with a high success rate [14]. The donor site for these replacements is often the saphenous vein, a vein located in the leg, whose diameter and length are well suited for this application. However, when an artery needs to be replace due to cardiovascular diseases, it is likely that the alteration to vascular system takes place at multiple locations. In this case, it might be impossible to harvest enough heathy autologous tissue from the patient to perform all the required grafts.
For superior caliber blood vessels (Ø>6mm), synthetic grafts, made from one of two dif-ferent polymers, can be used to replace the diseased arteries. For diameters between 6 and 10mm, tubular grafts made of Teflon® (polytetrafluoroethylene PTFE) are prescribed [15]. For diameters larger than 10mm, synthetic grafts are created using polyethylene terephtha-late, commonly called Dacron® [16]. Despite these types of grafts being widely used in our hospitals today, these two polymers showed poor success rates in longterm studies [17,18]. Synthetic grafts have higher mechanical strength than the native tissue but also have a sig-nificant compliance mismatch. The difference in radial compliance between the graft and the native vessel is accentuated by the inelasticity of sutures. This has been shown to cause luminal narrowing due to intimal hyperplasia [16]. For the young people suffering from premature cardiovascular diseases, the implantation of synthetic grafts will only solve their problem for a short period of time. As those young people grow, their grafts do not; they will therefore eventually need to have their graft replaced, meaning another surgical procedure for the patient.
0.1.4
Vascular tissue engineering
Tissue engineering is thus emerging as a promising technique for replacement of small cal-iber blood vessels. Tissue engineering combines knowledge from engineering, biology and medicine in the aim of creating a blood vessel, in vitro, to be implanted or to be used as a model for characterization of pathological vascular disease and the effect of various drugs for treatment. With tissue engineering being part of the regenerative medicine field, the produced scaffold should adapt to its physiological environment and have the ability to regenerate and repair itself. To be suitable for vascular application, the engineered construct needs to mimic the biological and mechanical properties of the native tissue. It needs to show great biocom-patibility, meaning that it is not toxic or causing inflammatory response, nor is it rejected by
the body. It also needs to mimic the mechanical properties of the native blood vessel, such as mechanical resistance to blood flow and compliance.
Several approaches have been reported to solving the challenge of vascular tissue engineer-ing. Some of these approaches will briefly be described here.
The first technique relies on using what nature already created by by means of decellularized tissues from another human or even other species. The tissue goes through some physical and chemical treatment to extract cells and genetic material from the tissue. It may then act as a decellularized matrix, ready for seeding of autologous cells, from the patient and eventual implantation. Tissues created by this technique have already been implanted with a great success rate[19, 20]. However, the treatment that tissue must go thought could potentially alter the biochemical and mechanical properties of the native tissue [21].
Vascular substitutes can also be created using an auto-assembly method [? ]. First step consists of culture of the three different types of cells located in the three different blood vessel layers. Cells grow until they form a leaflet, and the three leaflets are then rolled around a mandrel to mimic the vascular structure. The obtained tubular construct is then put in a bioreactor for a certain period of time to allow for the good cohesion of the different layers. After a total of three months, the vascular substitute is ready for implantation. However, even if this technique seems very promising, the long period of time needed to obtain a suitable construct is a major drawback.
A scaffold can also be created using a synthetic polymer base that allows for great mechani-cal support. Most of the polymers will degrade with time, allowing for the regenerated tissue to take over. Polymers such as poly(glycolic acid) (PGA) [22] are used for this applica-tion. However, the degradation rate is sometimes faster than the tissue regeneration rate, thus leading to a severe weakening of the construct [23].
Tissue engineered graft can also be made out of natural polymers. Using a natural material that is already located in body represents major advantages, especially in terms of biocom-patibility. Collagen type I is often used to create scaffolds as it can easily be extracted, sol-ubilized and reconstituted [24]. Cells can be seeded into the scaffold [25] to mimic vascular tissue. However, as the obtained mechanical properties are too weak to represent a suitable graft, many research groups work on enhancing those mechanical properties.
0.2
Mechanical characterization of vascular tissues
Mechanical properties are intrinsic to every material; they allow for a tissue to perform its physiological function. Unfortunately, when diseased, the physical and chemical properties of the biological material can be affected, leading to a change in the mechanical properties. It is therefore important to understand how changes in the mechanical behavior of a material could lead to further health complications. Mechanical properties are a key feature to con-sider in the design of a potential biomaterial. It is therefore important to understand what a biomaterial is made of to be able to perform the adequate mechanical characterization and to analyze the results. Mechanical characterization technique will also depend on the sample geometry.
Before going any further, some concepts and definitions need to be explained to help with understanding the content of this thesis. First, if the strength of a material needs to be deter-mined, it is possible to test a small or a large sample. The larger specimen is more likely to sustain a large force and a smaller specimen is more likely to fail under a smaller force [26]. However, it is the force relative to the size that is considered in mechanical characterization. This brings us to the concept of stress, which represents the force per unit of cross-sectional area. The stress can be calculated as follow:
σ = F
A (1)
where σ is the stress, F is the load and A is the cross-sectional area perpendicular to the load. Another concept mentioned in this thesis is strain, which is the deformation of the sample, which can be related to applied stresses. It is expressed as follows:
ε = L− L0
L0 (2)
where ε is the strain, L0 represents the original length or dimension of the sample and L is
the length at a given time point during the experiment.
Just as skin’s elasticity allows us to move freely, the high strength resistance of our bones allows us to stand straight and the elasticity of our blood vessels allow for blood to circulate through our entire body, allowing us to live. The following chapter aims to show the impor-tant contribution of mechanical properties to the functionality of a tissue and the different techniques to evaluate its mechanical properties. This chapter will also particularly focus on mechanical properties and characterization of vascular tissue.
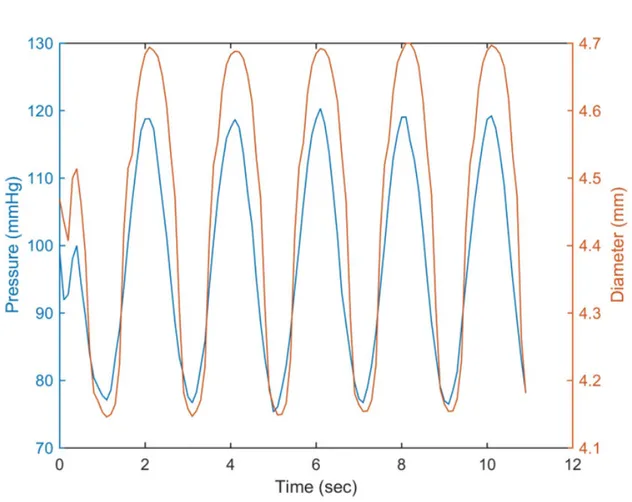
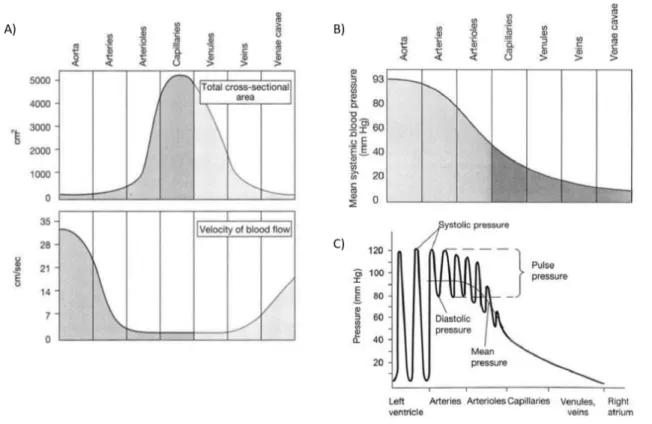
Figure 0.1: Physiological biomechanical data on blood circulation throughout the vascular system.(A) Vessel diameter, total cross-sectional area and blood flow velocity.(B) Pressure throughout the systemic circulation. (C) Pressure gradient in the bloos vessels. Adapted from [27]
0.2.1
Introduction to mechanical properties of cardiovascular
biomaterials
It is important to understand that the closer to the heart a blood vessel is, the more pulsatile the flow and higher the mean systemic blood pressure is, as shown in Figure0.1. As the blood travels through the entire vascular system, the total cross sectional area of vessels exposed to blood flow will increase, with the cross-sectional area of the aorta being significantly lower than the overall area of the capillaries. It also shows that the rate of blood flow is fastest in the arterial system and slowest in the capillaries and venules [27]. These parameters need to be considered when designing a mechanical testing protocol for characterizing vascular substitutes.
The flow pulsatility previously demonstrated shows the high importance of a specific me-chanical property: The elasticity. The elasticity, is described as the ability of a blood vessel to store and return energy via deformation imposed by the blood circulation. In mechanical
terms, the elasticity is characterized by the elastic modulus of a material, which is measured as the relation between the stress applied and the strain achieved. As the pulsation amplitude increases closer to the heart, the more elastic must be the arteries. The aortic elastic modulus is approximately 1 MPa [28]. Under this significant variation in pressure, the arterial wall needs to expand and come back to its initial diameter without any permanent damage. The elasticity is therefore most important for the aorta as it needs to undergo exceptional pressure variation (80-120 mmHg), receiving pulsatile blood flow directly from the heart. The aorta plays a very important role called the Windkessel function. During systole, the stroke volume injected from the left ventricle to the aorta is about 60-100 ml. About half of this volume is sent directly into peripheral circulation. The other half is stored inside the aorta thanks to its elastic expansion. During diastole, the aortic valve is closed and there is no blood injected into the system. With the decrease in aortic pressure, the aorta retracts slowly and the elas-tic forces from the aorelas-tic wall press the stored volume of blood into the circulation. This is how the aorta transforms the rhythmic blood output from the heart into a laminar flow, which is distributed into the peripheral circulatory system [10]. An increase in aortic wall stiffness will therefore affect the Windkessel function and will lead to a pronounced increase in systolic blood pressure and a decrease in diastolic blood pressure. Arterial stiffening is a determinant of cardiovascular complications and events such as left ventricular hypertrophy and failure, as well as aneurysm formation and rupture [12].
Another important property is the compliance. The compliance quantifies the ability of a blood vessel to expand under a change in blood pressure. However, compliance is often cal-culated for the physiological pressure range, 80-120 mmHg, as compared to elastic modulus, which can be measured for different strain ranges.
The blood vessel strength can also be measured to characterize the mechanical resistance of the vessel wall under high pressure. It will often give information about the collagen component since, under higher pressures, the collagen fibres contained in the wall straighten and become engaged, to bear most of the load. This can be characterized by a burst pressure test or by calculating the stress at mean arterial pressure.
Blood vessels, as part of the soft tissue family, demonstrate a nonlinearity of their stress-strain behavior [26] and exhibit a upward concave stress-strain curve. Their mechanical properties are due to their composition: the intimal layer rich in collagen gives to the vessel its mechanical resistance and the elastin rich medial layer allows for the vessel elasticity under blood pressure. In vascular tissue, elastin is responsible for deformation at low pressure as its stiffness is lower than that of collagen. At higher pressure, collagen contained in the wall
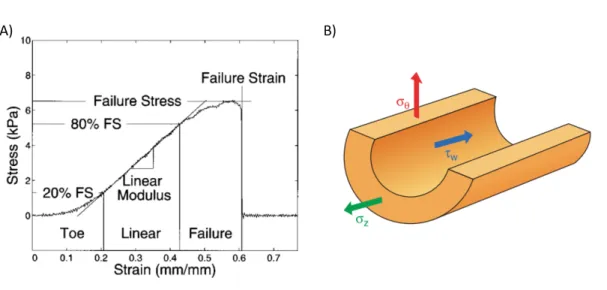
Figure 0.2: Stress and strain in blood vessels. (A) Representative stress-strain curve of a biological soft tissue showing the three distincts parts of the mechanical behavior. Adapted from [31]. (B) Schematic representation of a blood vessel under physiological stresses: cir-cumferential (red), longitudinal (green) and shear stress (blue). Adapted from [32]
straightens and becomes engaged to bear most of the load. Engaged collagen fibers are much stiffer than elastin fibers, causing a rapid increase of the blood vessel stress-strain curve [29]. The stress-strain curve for biological soft tissue is characterized by three distinct parts as shown in Figure 0.2. First, the low strain, non-linear ”toe region”, then a linear region, and finally the failure region. The low strain toe region involves the collapse of the micrometer-scale collagen crimp pattern, and the initiation of stretching of the collagen triple-helix. In the linear region, molecular stretching is responsible for the elastic behaviour. Molecular and fibrillar slippage represent the main mechanism of plastic deformation, which eventually lead to damage to the collagen fibrillar structure in the failure region [30].
Stresses on the vessel wall include shear stress from blood flow through the vessel lumen (represented as τW in Figure0.2B)), longitudinal stress from surrounding tissue (σZ in Figure
0.2B) and circumferential stresses from blood pressure (σθ in Figure0.2B)).
For calculation of mechanical properties, blood vessels can be modelled as thick or thin walled vessels depending on the radius to wall thickness ratio. When the thickness is less than one-tenth of the radius, the thin-walled assumption can be made, and subsequently, the wall can be treated as a surface. Previous studies showed that circumferential stress is very non-uniform, with higher stress at the inner wall and lower at the outer wall [33]. In an unloaded state, arteries are considered as thick-walled tubes, as their radius to thickness
ra-tio is in the range of 2-4, meaning their radius is 2-4 times larger than their wall thickness. During inflation, with blood pressure around 110-130 mmHg, the tube radius increases and the thickness decreases, leading to an increasing radius to thickness ratio. The resulting cir-cumferential inner strain will be greater than the circir-cumferential outer strain, and stress at the inner wall will be more concentrated because of the non-linear stress-strain relationship. However, in most studies, several assumptions are made, including incompressibility and uniform strain across the vessel wall [32]. Assuming incompressibility allows for the mea-surement of the deformation of the inner and outer diameters. This is quite useful as it is often difficult to detect the inner diameter during testing. Therefore, thin-walled equations are usually used to evaluate circumferential σθ and longitudinal stresses σZ.
σθ =Pri
t (3)
σZ =
Pri
2t (4)
Where P is the intraluminal pressure, rithe inner vessel radius and t the wall thickness [32].
0.2.2
Viscoelasticity
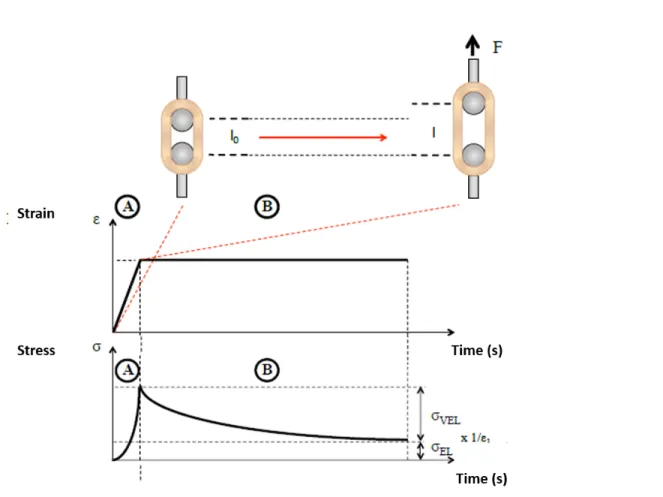
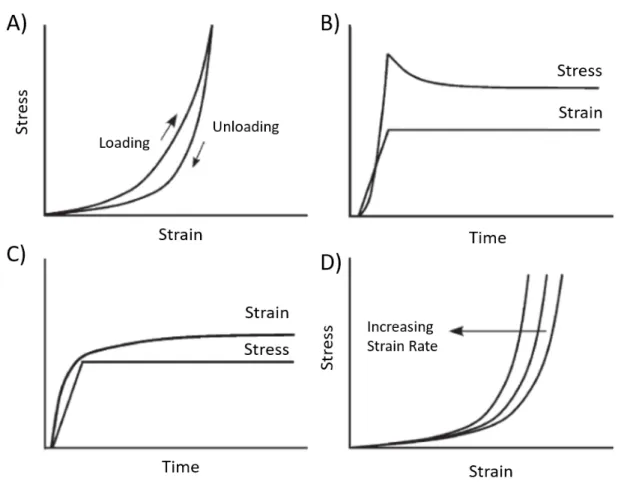
The mechanical properties of biological tissues are the direct result of their highly-hydrated structure. Due to this specific structure, tissues will display viscoelastic behavior, which is especially true for soft tissue such as vascular tissues. When a viscoelastic material is loaded, it stores energy which part of it is returned upon unloading and part of it is dissi-pated. Viscoelastic material exhibits specific mechanical behavior governed by two distinct contributions, elasticity (time-independent) and viscosity (time-dependent). The restored en-ergy represents the elastic component of the behavior; when some of the stored enen-ergy is dissipated because of fluid shear or other processes, this represents the viscous component. Viscoelasticity is also differing from traditional elastic material in its tidependent me-chanical behavior. Figure0.3shows some of its mechanical features [34]. First, Figure0.3A shows the hysteresis phenomena, where mechanical responses are not the same when a sam-ple is loaded and unloaded. The two curves need to be analyzed separately to evaluate the mechanical properties. However, the loading curve is often solely considered for properties measurement. Figure0.3B and0.3C show the stress relaxation behavior and creep behavior, respectively; these are probably the most well-known viscoelastic features. Viscoelastic ma-terials are also very sensitive to strain rate; their stiffness increases with strain rate as shown
Figure 0.3: Viscoelastic features of soft tissues. (A) Hysteresis is seen with different stress-strain curve during loading and unloading of the tissue. (B) Stress relaxation is the decrease in stress when the sample is maintained under constant strain. (C) Creep is the increase in strain when the sample is maintained under constant stress. (D) The stress curves shift toward lower strain with increasing strain rate. Adapted from [34].
in Figure0.3D. To characterize viscoelastic material, creep and stress relaxation tests are the most common techniques used and will be discussed in more detail in the following section of this thesis.
0.2.3
Poroelasticiy
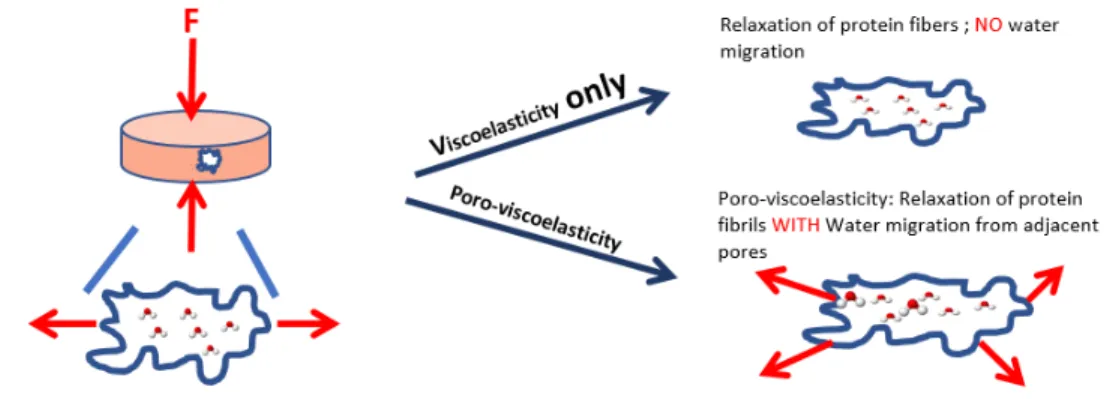
Viscoelasticity manifests itself through various phenomena, as descried previously, with the most studied being stress relaxation. However, the stress relaxation behavior for hydrated constructs, such as collagen gels or other hydrogels, is actually composed of two concurrent molecular processes [35]. First, there is a conformational change of the network, which is the
result of fibrils stretching, buckling, bending, and slipping at interaction points, thus leading to a matrix reorganisation corresponding to viscoelasticity. Secondly, the presence of inter-stitial fluid movement may slow relative motion or pressurize the system [36], resulting in poroelasticity. Even if poroelasticity is rarely taken into consideration, it can be observed in every multiphase medium, including collagen, which is typically composed of less than 0.5% collagen by weight with the rest of the composition being mostly water. It is, however, difficult to differentiate viscoelastic and poroelastic behaviour in mechanical test data. But, there are some techniques, developed by research groups, in order to study the two phenom-ena separately, such as indentation and compression techniques [37]. These two behaviors can be observed mostly by compression tests as interstitial flow resistance is negligible in extension tests, which allow only for the investigation of the tensile behavior of the network phase [36]. Poroelastic behaviour can be observed in samples of different sizes [37]. Indeed, viscoelasticity is not affected by sample length-scale as the sample size is several order of magnitude larger than the mesh size of pores within the material, leading to an independency of the time-constant of viscoelastic relaxation from the geometry of the test [37]. However, various studies have shown that poroelasticity is a scale dependant behavior. When mean load-relaxation curves for gels of different sizes are normalized by the individual sample size and plotted against time, the trends of each curve are offset in time [38]. This is due to the fact that it takes more time for all the fluid to migrate into and out of a larger gel than a smaller one, it therefore takes more time for a larger gel to reach the equilibrium stress value during relaxation. Poroelasticity can be summarized as the fluid movement relative to the solid and it is important to understand its contribution to mechanical behavior. This topic and techniques will be discussed in further detail in the first chapter of this thesis.
0.2.4
Mechanical characterization technique
Testing environment
The environment and conditions in which the sample is tested can also have a notable impact on the obtained results. Unfortunately, there is no standard regulating the mechanical charac-terization of hydrogels and soft tissues in general. In addition to the large number of parame-ters that need to be taken into consideration with the mechanical testing protocol itself, there are also other aspects that need to be given thought [39]. First, mechanical preconditioning is often performed in order to release any residual stresses within the material, prior to the actual experiments. Removing tissue stress and strain history improves reproducibility of the mechanical behavior of living tissue [26]. The hydration state is also important to consider. Testing the sample in a dry or wet environment is likely to greatly impact the results. In an
aqueous saline environment, the viscous part of the material is favored because of the plasti-cizing effect of water [40]. Moreover, as some of the mechanical characterization techniques require a long testing time to account for the viscoelastic behavior, the sample will simply loose some of its water due to evaporation into the surrounding environment during the ex-periment. The measured mechanical properties could therefore change over time within the same sample from a change in the level of hydration. Finally, temperature will also play an important role in the mechanical behavior, as the temperature will impact the molecular mobility of polymer chains and can damage weaker bonds, such as hydrogen bonds. From a study driven by Meghezi et al. in the LBB, mechanical preconditioning does not signifi-cantly improve the reproducibility of results, as the variances were not much different with or without precycles prior to the experiment. However, temperature and hydration were shown to have a major impact on the mechanical behavior. In the perspective of testing biological tissue or a tissue engineered construct that are respectively either coming from, or will be implanted in, a wet environment, it is recommended to test the tissues in wet environment at 37C (body temperature) [39]. For a pseudo-physiological solution, phosphate buffered saline (PBS) is often used for vascular applications. However, the solution to be used always depends on the environment in which the native tissue usually experiences within the body.
Sample geometry
The sample geometry will have a major impact in the decision of the right mechanical testing protocol. Depending on how easy it is to manipulate the sample and to grasp it, different types of testing techniques should be used. The tissue also needs to be characterized in order to understand the relevant mechanical properties for its application and it needs to be tested in conditions as close as possible to its physiological function. The objective is to obtain the accurate in vivo behavior, but under in vitro conditions. So depending on the mechanical properties that need to be measured, along with the geometry and size of the samples, several mechanical characterization techniques are presented below that could be used for soft tissues and vascular applications.
Uniaxial tensile test
Considering the wall as a membrane, the simplest experiment that can be done is uniaxial testing. A rectangular or dog-bone [31] (as shown in Figure0.4A) shaped strip is cut from the wall and is pulled longitudinally at a constant rate, with the force-elongation relationship recorded until failure [26]. In the case of vascular samples, the blood vessel is often cut into rings that are each opened up and cut into a rectangular strip, with the long axis aligned with
Figure 0.4: Uniaxial tesile test performed on soft tissues. (A) Uniaxial tensile test can be performed on dog-shaped samples (adapted from [31]). (B) Vascular tissue can be cut into rings that are opened up and cut into rectangular strip, with the long axis aligned either in the circumferential or longitudinal direction (adapted from [28]). (C) Samples can also be tested into ring shape (from [39]).
either the circumferential or longitudinal axes of the sleeve, as demonstrated in Figure0.4B [28]. The specimens are then usually outfitted with custom made grips and loaded.
When the samples are difficult to manipulate and cannot easily be held by grips, the speci-mens are kept in the ring shape [41,39]. It is often the case with collagen hydrogels as they are often very weak and slippery. Just trying to grip them would induce damage automati-cally. An ”L” shaped fixture can be use to pull on the ring as shown in Figure0.4C.
Uniaxial tensile testing allows for the measurement of the elastic modulus, ultimate tensile strength and elongation at rupture. Elastic modulus is calculated as the slope of the linear region [42]. The slope of the ”toe” region is also sometimes characterized when the tissues needs to be tested for very small deformations.
A pressurized vessel is loaded multidirectionally, but strips and rings are only loaded uniaxi-ally, which does not accurately reflect the actual mechanical behavior. Unidirectional loading may artificially orient the tissue structure in the loading direction to give an inaccurate mea-surement of the wall mechanical properties [43]. Cutting the vessel into ring or strip shape is
Figure 0.5: Compression test performed on collagen disk-shaped smaples
also likely to induce damage into the tissue prior to experiment. It is therefore difficult to cor-relate the obtained properties from strips and rings to the transmural pressures experienced by intact vessels.
Compression test
Compression tests are often used when the sample geometry doesn’t allow for it to be grasped or attached in any way in order for load to be applied. Compression is commonly used for all types of hydrogels as they are often hard to handle and very slippery due to their hydrated state. It is therefore simpler to make disk-shaped samples out of gels for compression testing. The test is performed by compressing the gel at a constant rate and it allows for the calculation of a compression modulus [44].
Biaxial test
A biaxial testing device allows for the characterization of the mechanical properties and anisotropy of a material. An anisotropic material does not have the same mechanical proper-ties in every direction, which is the case for vascular tissues [45]. For blood vessels, it means that the mechanical properties are not the same in the longitudinal and circumferential direc-tions. Biaxial testing can be done on flat strip shaped samples. Usually, the sample is cut into a square and attached on each side to actuators and load cells as shown in Figure 0.6A[46]. Samples are then pulled with different ratios of load applied in the longitudinal and circum-ferential directions. Strain is measured using a camera device by tracking particles glued to the samples surface [34]. Some biaxial testing devices are even adapted to test tubular constructs as shown in Figure0.6B. This is useful for characterizing very small caliber blood vessels, such as mouse aortas, as it would be difficult to cut the vessel open, in order to test
Figure 0.6: Biaxial testing device in (A) planar and (B) tubular configuration. Adapted from [47].
it in flat shape, without causing damage. The vessel is cannulated at both extremities, and a pump is used to push fluid into the sample, in order to increase the intraluminal pressure. Load cells used at the extremities of the vessels can measure the longitudinal load induced from the increase in pressure. Samples can also be stretched longitudinally at different ratio compared to in vivo or ex vivo length and then inflated.
Burst pressure test
Burst pressure test is the equivalent of a tensile rupture test for tubular samples. The pressure is increased and the tubular construct inflated until bursting. The bursting pressure can be compared to ultimate tensile strength obtained from tensile test and diameter-pressure mea-surement allows for the calculation of elastic modulus through Laplace’s Law [41]. Burst pressure test has the advantage of applying a load in a physiological manner to measure the desired properties, but its major drawback is that, for the test to succeed, a long vessel seg-ment is needed, which can be costly and time consuming for tissue engineered scaffolds [41]. To impose pressure, a syringe pump is sometimes used [28] or a pump. Burst pressure val-ues are in the range of 2031-4225 mmHg for a human artery, and 1680-2273 mmHg for a saphenous vein [48].
Compliance test
A compliance test is a measure of the vessel’s ability to vary its diameter under changing blood pressure. This type of testing is performed by imposing a pressure by cyclically inflat-ing a tubular construct [49, 50]. The experiment set-up is therefore very similar to that for
the burst pressure test; however, the compliance test is different in that it is a non-destructive test. Compliance testing is often performed by varying pressure from 80 to 120 mmHg at a frequency of 0.2 Hz[48]. A typical figure for compliance test results is presented in Figure
0.7, where the varying pressure is shown and the diameter changes accordingly. Using the measurement taken from the experiment, a compliance modulus is calculated as follows :
C=
d120− d80
d80
∆P (5)
where the diameters are measured at the specific pressure points and the diameter deformation is divided by the change in pressure. The cyclic way of performing this type of characteriza-tion allows for a testing setup that is closer to physiological condicharacteriza-tions; however it requires a complex testing device to vary the pressure and control the pump. This type of characteriza-tion is sometimes performed in a device called a bioreactor, which is used for the maturacharacteriza-tion of tissue engineered vessels and for the measurement of their mechanical properties [51]. Stress relaxation test
A stress relaxation test gives a lot of information on the mechanical properties of a material at the macroscopic scale, as well as the molecular scale. In the body, tissues tend to be under tension, and the resulting strain, maintained over an extended period, is the reason why a stress relaxation test is one technique that allows for mechanical characterization at conditions closer to what is experienced physiologically. The technique entails applying strain to a material at a constant rate, and then maintaining this strain afterward, as shown in Figure0.8.
The induced stress increases rapidly under imposed strain to reach a maximum value. When the desired strain value is reached, it is then held constant and the induced stress decreases over time to an equilibrium value. At this equilibrium state, the viscous component of the behavior is dissipated, and this value gives information on the elastic responses. As soft tissues are viscoelastic and do not exhibit a linear stress-strain response, this technique is the best way to evaluate their elastic mechanical properties as it allows for it to be isolated from the viscous component and to be independent from strain rate. Using this knowledge, research groups often use multiple stress relaxation tests, with the same sample being held at different values of maximum strain, to calculate the elastic properties of a material [30,
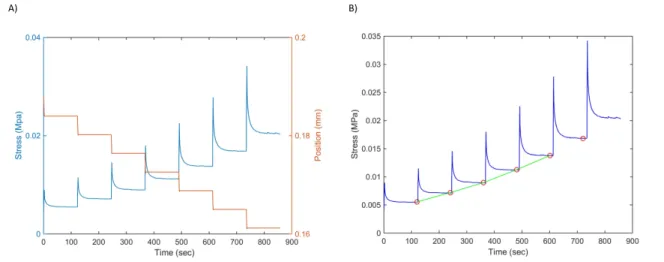
53]. This test consists of applying continual strain ramp at a constant speed, with a constant holding time between each ramp step. Figure 0.9 is showing the imposed strain and the
Figure 0.7: Vessel diameter under compliance test at physiological pressure.
corresponding induced stress. The elastic component of the stress is defined as the stress at equilibrium, while the viscous component represents the difference between the total stress before relaxation and the equilibrium value. The elastic fraction of the behavior can be calculated as the ratio of the elastic stress divided by the total stress at a particular strain [30]. The equilibrium stress value for each ramp, and the corresponding strain, are used to obtain a slope which corresponds to the elastic modulus.
Cyclic loading can also be used to characterize viscoelastic properties, by observing hystere-sis in the induced stress. Achilli et al. performed this type of testing on collagen gel ring samples [54]. They performed cyclic loading for multiple strain ramps and noticed that the hysteresis generally decreases along each set of cycles, while it increases when the load in increased. They also tested crosslinked collagen samples, which exhibited lower hysteresis, showing more elastic behavior than the uncrosslinked material. Indeed when elastic materials are strained without any permanent deformation, the remaining stress when unloaded returns
Figure 0.8: Schematic of a stress relaxation test performed on ring shaped sample. At step (A), the sample is strained and this strain in maintained constant in (B), where stress decreases with time. Adapted from [52].
to the initial stress value, meaning the hysteresis is very small.
Creep test
Compression creep/recovery tests are used to characterize disk-shaped gels [55]. A creep/recovery test is the opposite of a stress relaxation test, as the stress is imposed and the strain is mea-sured. This is performed by putting the gel between two parallel plates and imposing a known stress on the sample. A parallel plate measuring system is then used to measure the induced strain on the sample. This type of test can also be performed in multiple compression ramp by increasing the imposed stress at specific time intervals [56]. The imposed stress and mea-sured strain can then be used to obtain the stress-strain curve and calculate elastic properties. The only downside about creep test is that it can take a significant about of time. This is why frequency-phase lag test is sometimes preferred.
Figure 0.9: Typical results from cyclic stress relaxation test. (A) In orange, the position of the plate in decreasing step wise imposing a more important strain. The blue curve represents the corresponding stress induced into the sample. (B) The elastic modulus represents the slope of the green curve obtained from the equilibrium stress value from each ramp.
Device components
Any mechanical testing device needs to allow for stress and strain calculation. To measure stress, a force transducer needs to be used, in the case of uniaxial testing of ring or strip shaped samples. For compliance and burst pressure tests, a pressure transducer needs to be placed in line with the samples. The ideal type of pressure transducer for vascular application is one that will allow for the measurement of pressure directly inside the tubular construct. However, in the case of small caliber samples, the pressure transducer could affect the flow or cause obstruction of the vessel. To measure strain, the deformation of the sample needs to be tracked during the experiment. This can be done by using video capture system with markers placed on the samples. This method requires post-experiment image analysis as the markers placed onto the samples need to be tracked through the movie or images that were captured. Another way is to use a laser as described by Laterreur et al. to measure the sample’s dimension, in real time, during the experiment [41]. This method allows for certain types of mechanical testing where, for example, pressure needs to change according to a change in diameter.
0.3
Thesis: Structure and Strategy
Mechanical characterization of biological tissues represents a major challenge as the testing protocols and devices need to be adapted to the specific tissue that is being tested. Testing
vascular soft tissues, with their tubular geometry, adds more complexity to the design of the experiments. As it was discussed in the previous section, hydrated biological tissues are dif-ficult to manipulate and their viscoelastic behavior needs to be considered. Finding the right method to perform the mechanical characterization is a real challenge as the chosen tech-nique is highly dependent on the tissue geometry, the desired application, and the mechanical properties that need to be measured. As there is absolutely no standard in mechanical char-acterization techniques, and since every tissue needs a specific testing protocol, comparing results between different studies is complicated.
The objective of this research work is to develop and validate non-conventional mechanical characterization protocols specific to soft tissue for vascular application. Different tissues were tested in order to better understand the impact of their nature, geometry and application on the choice of mechanical testing protocol. This research work will explore testing of different soft tissues for vascular application going from simple geometry to a more complex ones.
In this context, this master’s thesis is constituted of three chapters:
• Chapter 1 presents the mechanical characterization of disk-shaped collagen gels using a poro-viscoelastic model.
• Chapter 2 presents a mechanical testing technique for tubular shaped collagen gels made by the use of a rotational technique to improve the mechanical properties of the gels by collagen densification.
• Chapter 3 presents a mechanical characterization technique of extremely small caliber blood vessels using mouse aortas. It also shows the impact of vascular calcification, as a result of genetically induced disease, on aorta mechanical behavior.
Chapter 1 presents a mechanical characterization technique developed to study viscoelastic and poroelastic properties of collagen gels in disk shape. When the collagen gels are under strain, the induced stress is time dependent. This behavior in due to two molecular process which consist of matrix deformation and migration of solvent trapped in the pores [57]. The former results from viscoelasticity and the latter from poroelasticity. The techniques used here allows for the study of both behaviors as they manifest themselves at different time points of the experiment. The study has allowed the characterization of disk-shaped colla-gen gel samples to be performed, and was good practice for handling such hydrated tissue in further experiments of increased complexity, involving more intricate sample geometries.
To mimic vascular tissue, mechanical properties need to be characterized on tubular-shaped samples and Chapter 2 presents a mechanical characterization technique for a tubular colla-gen construct. In the course of this study, a new manner to produce collacolla-gen tubular scaffolds using a rotation technique to increase collagen wall density was developed. A significant in-crease in elastic modulus was measured for the denser gel compared to the normal gel. This study was published in Biotechnology Journal (2016, 11(12), 1673-1679). Working with col-lagen tubular constructs allowed me to be comfortable handling and characterizing this type of tissue geometry. The construct’s large diameter made it easy to work with and to practice for the work presented in Chapter 3. In the last chapter of this thesis, a mechanical charac-terization technique and the corresponding device were developed in order to perform testing on mouse aortas. These mice suffered from severe vascular calcification due to Matrix-Gla protein deficiency. Working on aortas having a diameter of about 0.5 mm represents quite a challenge in and of itself. The objective of this study was to correlate the impact of calcium deposition within the aortic wall with the mechanical properties of the aorta. Then, a general discussion will present the limitations, challenges and perspective of this research work. The appendix of this thesis will present mechanical characterization protocols developed in the course of collaboration with other research groups as well as the protocol for preparation of mouse aorta for mechanical testing.
Figure 0.10: Organizational char showing the scientific approach taken in the course of this research work.
Chapter 1
Poro-viscoelastic models applied to
collagen hydrogel scaffolds
1.1
Résumé
Le collagène est largement utilisé dans le domaine du génie tissulaire en tant qu’échafaudage dû à sa bonne biocompatibilité. Cependant, son comportement mécanique particulier et son faible module élastique rendent sa manipulation ardue. Tout comme d’autres matériaux in-homogènes composés d’une matrice et de liquide interstitiel, le collagène devrait démontrer des signes de comportement poroélastique, ce qui nécessite donc d’être étudié. Des tests mécaniques et de la modélisation mathématique ont permis d’étudier les propriétés poro-viscoélastiques du collagène et de les comparer à celles de la gélatine, qui est reconnue comme un matériau poroélastique. La technique utilisée permet d’analyser et de distinguer les comportements poroélastiques et viscoélastiques puisqu’ils se manifestent à des moments différents durant la caractérisation mécanique. Les résultats obtenus démontrent que la con-formation de la matrice de collagène est telle que la poroélasticité est négligeable lorsque comparée à la viscoélasticité.
1.2
Abstract
Collagen hydrogels are widely used scaffolds in vascular tissue engineering mainly due to their desirable biological properties, in that they can support and guide the growth of vascular cells. However, their handling is known to be difficult because of their particular mechan-ical response to strain or stress, and their low elastic modulus. Collagen, similar to other inhomogeneous materials containing interstitial fluid, should exhibit poroelastic behavior. Poroelasticity therefore needs to be studied. Mechanical testing and mathematical model-ing allowed the poroviscoelastic properties of collagen gels to be studied and compared to gelatin gels, a known poroelastic material. The technique used allow for both behaviors to be studied, as they manifest themselves at different time points during the experiment. Current results show that collagen does not display poroelastic behavior mainly due to the conforma-tion of its porous structure. The absence of poroelasticity, in addiconforma-tion to viscoelasticity, could give an explanation for the lack in mechanical properties of the collagen gels.
1.3
Introduction
Collagen type I, as the most abundant protein in human body, is an excellent candidate for use as a scaffold. In its native state, collagen has amazing mechanical properties and is re-sponsible for the strength of the vascular walls [34]. However, during the extraction process, the supramolecular organization that provides collagen its strength is lost leading to loss of its mechanical properties [58]. Its weak mechanical strength makes it hard to handle during the processing of the scaffold.
Mechanical characterization is essential to validate the suitability of a scaffold for vascular tissue engineering. A lot of research groups have the same goal: to enhance and better un-derstand the mechanical properties of collagen scaffolds [54,44,39]. As collagen is the most abundant protein in tissues, its viscoelastic mechanical behavior is of high interest. Collagen gel shows high viscoelasticity due to its highly hydrated structure. When under strain, colla-gen gels exhibit strong stress relaxation, revealing the importance of the viscous contribution to the mechanical behavior. Research groups have also investigated the viscoelasticity of collagen at its fibrillar level and results showed that isolated collagen-fibrils are intrinsically viscoelastic [59,60].
In order to better understand the mechanical properties of collagen gels, it is important to know what parameters influence their mechanical behavior. When the collagen gels are under strain, the induced stress is time dependent. This behavior is due to two molecular processes: matrix deformation and the migration of solvent trapped in the pores [57]. The former results from viscoelasticity and the latter from poroelasticity. Material mechanical properties are highly dependent on their structure at the microscopic level, such as pore size and distribution. The study of their mechanical behavior at the microscopic scale is therefore of high importance in understanding their behavior at the macroscopic scale.
Regarding poroelastic materials, they are composed of a solid matrix and a fluid component moving through the pores [36]. This definition fits well with collagen gel, as it is a porous structure containing a lot of water. However, the poroelastic behavior of collagen gel is not often taken into consideration. Having fluid moving through the gel during mechanical testing is very likely to influence the mechanical response of the material. There are therefore two distinct possible conditions for poroelastic materials. First, there is the drained condition, when the fluid is free to escape from the gel and no pressure is built inside the pores during mechanical stimulation. Pores are often interconnected, allowing the fluid to move freely through the entire gel. The second condition is non-drained, when pores are closed and not connected. In this situation, the pressure increases under an applied load as fluid is trapped
![Figure 0.4: Uniaxial tesile test performed on soft tissues. (A) Uniaxial tensile test can be performed on dog-shaped samples (adapted from [31])](https://thumb-eu.123doks.com/thumbv2/123doknet/5532783.132263/29.918.123.789.98.463/figure-uniaxial-performed-tissues-uniaxial-tensile-performed-samples.webp)
![Figure 0.6: Biaxial testing device in (A) planar and (B) tubular configuration. Adapted from [47].](https://thumb-eu.123doks.com/thumbv2/123doknet/5532783.132263/31.918.136.793.114.331/figure-biaxial-testing-device-planar-tubular-configuration-adapted.webp)