HAL Id: hal-01634034
https://hal.archives-ouvertes.fr/hal-01634034
Submitted on 31 Oct 2019
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Agglomeration of Metals During Pyrolysis of Chromated
Copper Arsenate (CCA) Treated Wood Waste
Mohammed Kemiha, Ange Nzihou, David Mateos
To cite this version:
Mohammed Kemiha, Ange Nzihou, David Mateos. Agglomeration of Metals During Pyrolysis of
Chromated Copper Arsenate (CCA) Treated Wood Waste. High-temperature materials and processes,
de Gruyter, 2008, 27 (5), p.361-368. �10.1515/HTMP.2008.27.5.361�. �hal-01634034�
Copper Arsenate (CCA) Treated Wood Waste
Mohammed Kemiha"*, Ange Nzihou" and David Mateos
ba
Centre RAPSODEE, UMR-CNRS 2392,
Ecole des Mines d'Albi-Carmaux, Campus Jarlard, 81000 Albi, France
bThermya Company,
1 rue Nicolas Appert, 33140 Villenave d'Ornon, France
* corresponding author: mohammed.kemiha(a), mines-albi.fr, Tel: +33563493249, Fax : +33563493243
(Received December 20, 2008)
ABSTRACT
The number of waste disposal sites decreases and redundant poles, piling and lumber, which constitute a large volume of material, m a y not be accepted at the limited number of sites in the future. C C A contaminated wood contains around 1-5% of metal in form of arsenic pentoxide, hexavalent and trivalent chromium compounds and copper sulfate, which act as fungicides and insecticides throughout the life of the wood. While CCA treatment is gradually becoming a banned practice, approximately 4 million tonnes of C C A treated wood is generated in the EU per year, which is set to continue for many decades as the in-service wood is generated in end of its life. Recycling wood waste is important for the effective utilization of natural resources. The charcoal product resulting from C C A treated wood contains Cu, Cr and As. T h e metals appear as both agglomerates and diffused in the solid matrix. Moreover, during pyrolysis an agglomeration process takes place, which induces not only the growth of existing mineral agglomerates but also the formation of new agglomerates. The objective of the present work is the valorisation of C C A metals and charcoal from treated wood waste. T h e research aims at understanding and promoting experimentally, at different pyrolysis heating rates, the generation of charcoal with high specific surface area from pyrolysed treated wood waste.
Keywords:
C C A wood waste, pyrolysis, specific surface area, agglomeration, heavy metals.1. INTRODUCTION
Telephone poles, railway sleepers, timber from landscape and cooling towers, wooden silos, hop-poles, cable drums and wooden play-ground equipment gerterate waste for which environmentally benign technologies need to be developed. The main part of the wood waste is the chromated copper arsenate (CCA-) treated one l \ l . The number of waste disposal sites decreases and redundant poles, piling and lumber, which constitute a large volume of material, may not be accepted at the limited number of sites in the future. So, recycling wood waste is important for the effective utilization of natural resources 121.
It is beneficial to utilize the energy resources of the impregnated wood waste thermal treatment. Meanwhile, thermal treatment must be done with caution to avoid emission of toxic c o m p o u n d s to the surrounding environment, especially when dealing with wood waste containing arsenic 111.
The disposal of CCA-treated waste woods has become a serious environmental problem all over the world. The existing and emerging technologies for managing CCA-treated wood waste include: recycling and recovery, chemical extraction, bioremediation, electrodialytic and thermal destruction /4/.
Vol. 27, No. 5, 2008
Thermal degradation process seems to be a promising technology for material and energy recovery from CCA treated waste wood. Material recovery is based on the formation of clusters or agglomerates, consisting in metals and minerals, during thermal degradation of CCA-treated wood 151. The characteri-zation using a scanning electron microscopy coupled with energy dispersive X-ray analysis (ESEM-EDS) has shown that the metal compounds form agglomerates during pyrolysis, thus suggesting that the metals can be easily recovered from the charcoal in a dry way 16,11.
To dispose of chromated copper arsenate (CCA) treated waste wood, Thermya Company has developed the so-called "Chartherm" process, a novel low temperature thermal process in which "chartherisation" is the thermal part. Practically, this process consists in a staged pyrolytic distillation /8/.
During the chartherisation process, crushed wood is loaded in a reaction column where it is permanently submitted to a flow of neutral gas. The neutral hot gas (low oxygen content, 370°C), coming from a heat generator, is injected at the bottom of this reaction column. An increasing stratified gradient of pressures and temperatures is obtained by controlling the pressure drop between the top and the bottom of the column in order to always maintain the temperature at the top of the column below 65°C. Therefore, the chartherisation process is quite similar to a distillation process: the wood waste, introduced at the upper part of the column, gradually loses all its water and organic matter as it goes down. Finally, its mineral part is the only one remaining at the bottom of the column. In fact, most of the mineral part (>97%) is carbon, which was the wood matrix.
At the bottom of the column, the thermal choc between the hot gas and the wood breaks the hydrogen bonds, which liberates groups of organic molecules and causes their evaporation from the wood. This mixture of gases and vapours of organic molecules is pushed up through the bed, where it is cooled down and condensed by the crushed wood.
The crushed wood particles heat up during their descent in the bed, and the organic molecules, condensed on the wood particles, crack and evaporate, then condense again at a higher level in the column. This phenomenon is repeated until the organics become
Agglomeration of Metals during Pyrolysis of Chromated Copper Arsenate (CCA) Treated Wood Waste
light enough and their condensation point low enough (below 65°C), that they do not condense any more and they make their way to the top of the column. The process is thus characterized by a sequence of evaporation, cooling, condensation, heating, cracking phenomena... where the bed of wood particles acts both as a condenser and a filter.
The charcoal residue extracted from the bottom of the column is cooled, milled to powder and introduced into a pneumatic centrifuge. Centrifugal separation results in a clean carbon product on one side and a powder containing all other minerals (including all metals) on the other side.
The charcoal product resulting from CCA-treated wood contains Cu, Cr and As. The metals appear as both agglomerates and diffused in the solid matrix. So, an agglomeration process takes place during pyrolysis, which induces not only the growth of existing mineral agglomerates but also the formation of new agglomerates 151.
The objective of the present work is the valorisation of CCA metals and charcoal from treated wood waste. The research aims at understanding and promoting experimentally the generation of charcoal with high specific surface area from pyrolysed treated wood waste.
2. MATERIALS AND METHODS 2.1 W o o d s a m p l e s
Two batches of wood waste samples were obtained from Thermya Company: wood waste and CCA-treated wood. Wood waste samples are those obtained from recuperating telephone poles and railway sleepers, etc. CCA-treated wood samples are obtained by impregnation of wood in a preservative CCA solution.
The samples were broken into chips with a size smaller than 2 mm and mixed in order to obtain homogeneous samples.
The metal content of the samples has been determined using ICP-AES after dissolution with aqua regia (HC1/HN03) and HF as proposed in ASTEM's
method (American Society for Testing and Materials) 191. As can be seen in Table 1, the metals'
concentrations in the C C A - t r e a t e d w o o d s a m p l e s are three to fourfold higher than in the w o o d w a s t e samples.
2.2 Thermal characterization
Differential s c a n n i n g c a l o r i m e t r y ( D S C ) and T h e r m o g r a v i m e t r i c a n a l y s i s ( T G A ) are c o m m o n techniques for m e a s u r i n g the heat flux and the weight changes of a material versus t e m p e r a t u r e and time. In this study, T G A and D S C w e r e p e r f o r m e d simultaneously in d y n a m i c m o d e using a S E T A R A M T G A - D S C 111 analyzer. In e a c h e x p e r i m e n t , the w o o d sample, a p p r o x i m a t e l y 25 m g in w e i g h t , w a s introduced into platinum s a m p l e p a n and heated f r o m r o o m temperature up to 7 0 0 ° C at a heating rate of 5 ° C / m i n , under nitrogen inert a t m o s p h e r e .
T h e thermal b e h a v i o u r o f the different s a m p l e s (wood waste and C C A - t r e a t e d w o o d ) obtained by T G A ) are c o m p a r e d in Fig. 1. T h e s e w e i g h t loss versus temperature curves e v o l v e similarly, but at lower decomposition t e m p e r a t u r e for C C A - t r e a t e d w o o d . T h e difference in d e c o m p o s i t i o n t e m p e r a t u r e , c o r r e s p o n d i n g to the temperature peak g a p , is d u e to the catalytic effects of C C A metals / 1 0 , 11/. So, the arsenic, copper, and c h r o m i u m c o m p o u n d s act as accelerators of the degradation of w o o d s a m p l e s c o m p o u n d s . Finally, the T G curves are in a g r e e m e n t with t h o s e in literature /10,
12/.
T h e s e curves point out t h r e e key stages: the volatile loss at low temperature, the main pyrolysis, and the carbonisation. In the e a r l y stage, water begins to volatilize and it is c o m p l e t e l y g o n e at 2 0 0 ° C , this corresponds to the large e n d o t h e r m i c peak in the heat flux curve. Most w e i g h t loss o c c u r s in the main
pyrolysis stage, w h i c h is o b s e r v e d b e t w e e n 2 0 0 ° C and 3 7 0 ° C . T h i s stage c o r r e s p o n d s to the d e c o m p o s i t i o n of h e m i c e l l u l o s e s and cellulose c o m p o u n d s of w o o d . T h e e n d o t h e r m i c p e a k b e t w e e n 3 0 0 ° C and 4 0 0 ° C in the heat flux c u r v e is attributed to t h e cellulose decomposition. T h e carbonisation stage o c c u r s at a t e m p e r a t u r e beyond 3 7 0 ° C , c o r r e s p o n d i n g to t h e lignin degradation and to tHe release o f r e m a i n i n g volatiles f r o m the solid pyrolysis product. Finally, at 7 0 0 ° C the char yield for inert pyrolysis is a b o u t 3 0 % f o r C C A - t r e a t e d wood and 2 4 % f o r w o o d waste.
Temperature (°C)
Flg. 1: M a s s loss and heat f l u x v e r s u s temperature during pyrolysis o f w o o d w a s t e and C C A -treated w o o d , u n d e r nitrogen a t m o s p h e r e and heating rate 5 ° C / m i n .
2.3. Chemical analysis
T h e a m o u n t s of arsenic, c o p p e r and c h r o m i u m in the s a m p l e s o f waste w o o d , of C C A - t r e a t e d w o o d and of pyrolysis residue w e r e m e a s u r e d by inductively coupled p l a s m a ( I C P - A E S ) s p e c t r o m e t r y , after complete dissolution of the s a m p l e s by acid attack in aqua regia solution as p r o p o s e d in A S T E M ' s m e t h o d .
2.4. Physical analysis
T h e specific s u r f a c e a r e a s o f the s a m p l e s and of the pyrolysis solid p r o d u c t s at d i f f e r e n t temperatures ( b e t w e e n 2 0 0 and 4 0 0 ° C ) w e r e d e t e r m i n e d using nitrogen adsorption with the B E T method ( M I C R O M E R I T R I C S G e m i n i V a c P r e p 061). T h e T a b l e 1
Metal (As, Cr and C u ) c o n c e n t r a t i o n s ( m g / k g o f w o o d ) of the C C A - t r e a t e d w o o d and w o o d waste.
Sample A s ( m g / k g ) C r ( m g / k g ) Cu ( m g / k g ) C C A -treated w o o d 25 770 3 2 150 11 520 W o o d waste 5 890 7 6 2 0 4 120
Vol. 27, No. 5, 2008 Agglomeration of Metals during Pyrolysis of Chromated Copper Arsenate (CCA) Treated Wood Waste
samples were examined by environmental scanning electron microscopy ( X L 30 E S E M - F E G , FIE Company).
2.5. Pyrolysis procedure
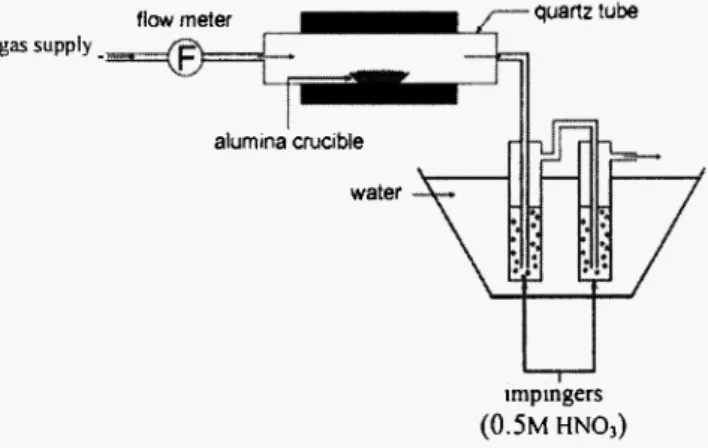
Pyrolysis experiments were carried out in a horizontal tubular furnace C A R B O L I T E 12/65/600 (diameter = 60 mm, length = 600 mm). A few grams of waste sample ( 4 - 5 g) are set in an alumina crucible and introduced into a quartz tube in a preheated furnace, under inert gas (N2), with flow rate set at 100 NL/h (Fig.
2). The gas outlet is connected to a cascade of two impingers filled with a lOOmL solution of 0.5M H N 03
for collecting the vaporized fraction of the heavy metals, thus used as cleaning gas system. Pyrolysis solid residues were analysed at the end of the experiment.
The influence of temperature on the agglomeration and on the metals' release during pyrolysis of wood wastes and CCA-treated wood was studied by carrying out experiments at 200°C, 320°C, 350°C, 370°C, and 400°C. These temperatures were chosen according to the TG curves obtained in the thermogravimetric study. The waste samples were heated from ambient temperature to the preset temperature at 20°C/min for fast heating and at 5°C/min for slow heating, then maintained at the preset temperature during 90 minutes (pyrolysis duration).
gas supply _
flow meter quartz tube
alumina crucible water
impingers
(0.5M HNO,)
Fig. 2: Experimental set-up for pyrolysis study.
3. R E S U L T S
All pyrolysis residues obtained at different temperatures between 200 and 4 0 0 ° C and at two heating rates were analysed to determine their physical and chemical properties. A metals' redistribution within the wood is expected under the pyrolysis thermal effect. Indeed, wood compounds such as cellulose and hemicellulose are decomposed during pyrolysis, and therefore the metals bound to them during the impregnation process have to m o v e to other locations 161.
3.1 ESEM Image analysis
Environmental Scanning Electronic Microscopy ( E S E M ) with X-ray microanalysis has been applied to study the morphology and the chemical components of the pyrolysis residues. In the case of pyrolysis residues of CCA-treated wood, the charcoal keeps a wood structure during pyrolysis (Fig. 3). The CCA precipitated metals (As, Cr and Cu) were detected as white spots (contrast) at the charcoal surface. The EDS analyses of the white spots identified arsenic, chromium and copper as the major metallic components. The pyrolysed CCA-treated wood has a structure similar to that of the wood waste, but with more metallic precipitates and agglomerates at its surface. The C C A metals' concentration increases with the pyrolysis temperature. In contrast, the metal content of residues from CCA-treated wood pyrolysed at 400°C is much higher than that obtained at lower temperature. The high initial metallic concentration in the case of CCA-treated wood explains mainly the abundance of Cr, Cu and As in the pyrolysis products 151.
3.2 Metals' concentrations
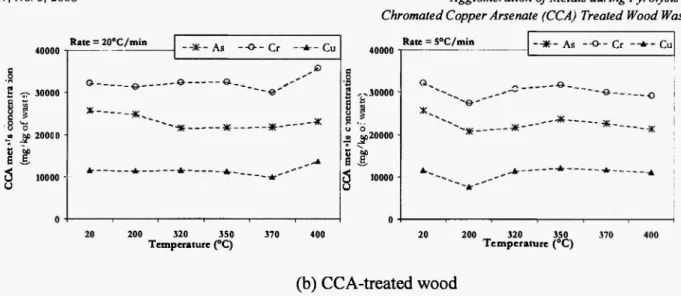
The metallic concentrations in the pyrolysis solid residues were obtained by ICP-AES after acid-attack dissolution. In order to study both temperature and heating rate effects on the C C A metals stability, Fig. 4 summarizes the results by showing the metals' concentrations over the range of experimental
Act V Spül M«)n Out-. WIJ I
»•MM· fix·' use 11 1-AHX OPIwr
Fig. 3: Environmental scanning electronic micrographs ( E S E M ) of C C A treated wood residues at different pyrolysis temperatures (heating rate=20°C/min and pyrolysis duration= 90min).
8000 Rate = 2 0 ° C / m i n 8 ε 1 < 2000 U GL As - - Ο - Cr r - Cu - S · — - - - • Ο - ο —
*
20 200 320 350 370 T e m p e r a t u r e ( ° C ) Β S o υ 0 4000 JASI
j ö 2 υ 400 Rate = 5 ° C / m i n - As --O-Cr - - ΰ τ - Cu - β ^ Ss •& " ""θ — Δ· — —ι Δ 20 200 320 350 T e m p e r a t u r e f°C) 370(a) Wood waste
Vol. 27, No. 5, 2008 Agglomeration of Metals during Pyrolysis of Chromated Copper Arsenate (CCA) Treated Wood Waste 40000 § T> 30000 at jgo 2000 1 1 5 10000 • υ Rate = 20°C/min As - - O - C r Cu » » * - · * * *
-•*:
40000 Rate = 5°C/min ^30000 " υ ο j^) 20000 δ Ifε JL
5 10000 υ - · * - As — O - Cr - - * • - Cu ---•Ο 200 320 350 T e m p e r a t u r e (°C) 370 400 20 200 320 350 T e m p e r a t u r e (°C)(b) CCA-treated w o o d
Fig. 4(b): CCA-treated woodFig. 4: Concentrations of C C A metals in solid residues at different t e m p e r a t u r e s and t w o heating rates.
conditions for both w o o d w a s t e and C C A - t r e a t e d w o o d samples, starting f r o m the initial c o n c e n t r a t i o n s (at 20°C). T h e decreasing o f metal a m o u n t s c o m e s f r o m the volatilisation of C C A c o m p o u n d s during pyrolysis. T h e m e a s u r e m e n t s uncertainties are less then 5 % for C C A -treated wood and about 9 % for w o o d w a s t e . T h e s e uncertainties are very a c c e p t a b l e b e c a u s e the s a m p l e s are wastes of different kinds, particularly, for w o o d waste sample. Fig. 4 s h o w s that for w o o d waste, the a m o u n t of c o p p e r is essentially stable in the system w h a t e v e r the t e m p e r a t u r e and the heating rate. T h e c o p p e r present in the solid residues of pyrolysis is relatively not volatile and its concentration d e c r e a s e is about 10%.
C h r o m i u m is much m o r e volatile than copper; however, its volatility is relatively limited for pyrolysis at 5°C/min heating rate in c o m p a r i s o n to that at 2 0 ° C / m i n . T h e m a x i m u m d e c r e a s e o f c h r o m i u m concentration d e p e n d i n g on the e x p e r i m e n t a l c o n d i t i o n s is about 18%.
Arsenic volatility is higher than both c o p p e r and c h r o m i u m volatilities w h a t e v e r the heating rate. T h e arsenic concentration in the pyrolysis solid p r o d u c t decreases m a r k e d l y with t e m p e r a t u r e . T h i s is particularly observed for t e m p e r a t u r e higher than 3 2 0 ° C . T h e arsenic concentration d e c r e a s e is around
16% at 2 0 0 ° C , up to 3 3 % at 4 0 0 ° C .
In the case of C C A - t r e a t e d w o o d , the c o p p e r
concentration in pyrolysis solid residues is generally very stable, and the concentration d e c r e a s e in residues is lower than 9 % .
T h e c h r o m i u m concentration is relatively stable in pyrolysis residues for the t e m p e r a t u r e s considered. This is especially true f o r pyrolysis at 2 0 ° C / m i n heating rate, w h e r e the concentration d e c r e a s e is less than 6 % . At 5°C/min heating rate, the c h r o m i u m concentration decrease is around 10%.
T h e stability o f arsenic in this case is relatively higher than in the case of w o o d w a s t e pyrolysis. T h e arsenic volatility at 5 ° C / m i n is less than at 2 0 ° C / m i n . In general, the arsenic concentration decrease in residues of pyrolysed C C A - t r e a t e d w o o d is less than 16%.
3.3 Specific surface area evolution
T h e variations of the s p e c i f i c surface area of pyrolysis residues with t e m p e r a t u r e are presented in Fig. 5, at d i f f e r e n t heating rates, and for both C C A -treated w o o d and w a s t e w o o d , the specific surface areas of initial w o o d w a s t e and C C A - t r e a t e d wood samples are 0.7 m2/g and 0.8 m2/g, respectively.
T h e B E T analysis s h o w s that the specific surface area of pyrolysis residues increases with temperature in comparison with that of n o n - p y r o l y s e d waste but with different level, for e v e r y case. So, the specific surface area of the residue is higher than that of the initial w o o d
sample (in both cases), and the m o r e the heating rate the more the increase o f s p e c i f i c s u r f a c e area. At 5 ° C / m i n heating rate,.the specific s u r f a c e area is m o d e r a t e and is
around of 10 m2/ g for both C C A - t r e a t e d w o o d and
w a s t e w o o d residues. At 2 0 ° C / m i n heating rate, the specific s u r f a c e area e x c e e d s 2 0 0 m2/ g for C C A - t r e a t e d
w o o d residue (except at 2 0 0 ° C ) and 3 2 0 m2/g for wood w a s t e residue. 150 200 250 300 350 400 Temperature (°C) 450 150 200 250 300 350 400 Temperature (°C) 450 (a) ( b )
Fig. 5: Specific s u r f a c e area of residues pyrolysis vs. t e m p e r a t u r e at d i f f e r e n t heating rates.(a) W o o d waste; (b) C C A -treated w o o d .
In the case of C C A - t r e a t e d w o o d , the low value of specific area at 2 0 0 ° C can be explained by the lower temperature effect on the d e c o m p o s i t i o n of the C C A -treated w o o d c o m p o u n d s , despite of t h e long pyrolysis duration at this t e m p e r a t u r e . T h i s is d u e to the C C A metals (highly concentrated), w h i c h clog the external pores of the initial s a m p l e and do not f r e e t h e m at this temperature, oppositely to the case of w a s t e w o o d where C C A m e t a l s ' a m o u n t is lower and the pore creation in more important.
Finally, this study points out that the most significant effect of the pyrolysis is to generate a residue with a high specific area, and the higher the heating rate the higher the specific s u r f a c e area, d u e to the creation of pores.
4. D I S C U S S I O N
T h e evolution of the h e a v y metal a m o u n t s at the
pyrolysis residue s u r f a c e indicates that several p h e n o m e n a of metal c o n c e n t r a t i o n s take place in the surface. T h e C C A m e t a l s initially vaporize and concentred at the residue s u r f a c e by c o n d e n s a t i o n and nucleation g r o w t h an a g g l o m e r a t i o n .
T h e higher stability o f C C A metals in the case of the C C A treated w o o d is d u e to the higher initial C C A metal concentrations, w h i c h also p r o m o t e s the a g g l o m e r a t i o n of C C A m e t a l s 151.
T h e increase in the s p e c i f i c s u r f a c e area during pyrolysis can be explained by the higher initial organic content of w a s t e samples. T h e resulting e f f e c t of the d e c o m p o s i t i o n of organic matter c o n t e n t in the waste s a m p l e s is the release of the existing pores and the generation of n e w one. M o r e o v e r , the C C A metal leaves the pores progressively b y v a p o r i z a t i o n . T h e d i f f e r e n c e o b s e r v e d between values of s p e c i f i c s u r f a c e area as f u n c t i o n of heating rate is p r o b a b l y related to the metal concentration at the residue s u r f a c e s .
Vol. 27, No. 5, 2008 Agglomeration of Metals during Pyrolysis of Chromated Copper Arsenate (CCA) Treated Wood Waste
5. C O N C L U S I O N
Experimental results of the pyrolysis of CCA wood waste have been presented. Two different pyrolysis heating rates have been used in order to compare the effect of the heating rate on the characteristics of charcoal during pyrolysis at low temperature (400°C).
The main result pointed out from the environmental scanning electronic microscopy is that the CCA metals are concentred at the charcoal surface. This result can be explained by the release of CCA metals during decomposition of organics and evaporation of water content from wood pores. The amounts of the CCA metals increase with temperatures notably up to 320 °C.
Finally, in this study, the pyrolysis of treated wood waste is used to generate reactive charcoal with higher specific surface area (up to 200 m2/g) by the release of
the existing pores and the generation of new one during the decomposition of organic mater and the progressive vaporization of the CCA metals from the pores.
Since the treated wood waste could be used for the production of materials (charcoal and CCA metal agglomerates) from waste wood, the approach developed in this paper is a promising one for the valorisation of wood waste as useful material in addition to the standard use for the production of energy.
A C K N O W L E D G M E N T S
This research is supported by Thermya Company, and by Region Aquitaine in France.
R E F E R E N C E S
1. J. Zandersons, A. Zhurinsh, G. Dobele, B. Spince, A. Tardenaka and J. Rizhikovs: Thermal Science,
10 (3), 27-38 (2006).
2. L. Helsen, Ε. Van den Bulck and J.S. Hery: Waste
Manag., 185, 7 1 - 578 (1998).
3. L. Helsen, Ε. Van den Bulck, Μ. K. Van Bael and J. Mullens: J. Anal. Appl. Pyrolysis, 68-69, 613-633 (2003).
4. L. Helsen and E. Van den Bulck, Environ. Pollut.,
1 3 4 , 3 0 1 - 3 1 4 (2005).
5. L. Heslen and A. Hacala: J.Hazard. Mater., B137, 1438-1452 (2006).
6. L. Helsen and E. Van den Bulck: Holzforschung,
52, 6 0 7 - 6 1 4 ( 1 9 9 8 ) .
7. Τ. Hata, P.M. Bronsveld, T. Vystavel, B.J. Kooi, J.Th.M. De Hosson, T. Kakitani, A. Otono and Y. Imamura: J. Anal. Appl. Pyrolysis., 68-69, 635— 643 (2003).
8. www.thermya.com. Thermya Company website, France.
9. L. Helsen: Low-temperature pyrolysis of chromated copper arsenate (CCA) treated wood waste, PhD. Thesis, Catholic University of Leuven, Belgium, (2000).
10. L. Helsen, Ε. Van den Bulck, S. Mullens and J. Mullens: J. Anal. Appl. Pyrolysis., 52, 65-86 (1999).
11. T. Hirata, M. Inoue and Y. Fukui: Wood Sei.
Technol, 27(1), 35-47 (1993).
12. A. K. Kercher and D. C. Nagle: Wood Sei.