Contents lists available atScienceDirect
Journal of Microbiological Methods
journal homepage:www.elsevier.com/locate/jmicmethCulture medium for improved production of conidia for identi
fication and
systematic studies of Fusarium pathogens
Raíssa Debacker Moura
a,⁎, Luiza Adami Monteiro de Castro
a, Mark Paul Culik
b,
Antônio Alberto Ribeiro Fernandes
a, Patricia Machado Bueno Fernandes
a, José Aires Ventura
b,⁎aBiotechnology Core, Federal University of Espirito Santo, Av. Marechal Campos, 1468, Maruípe, 29043-910 Vitória, ES, Brazil bCapixaba Institute of Research, Technical Assistance and Rural Extension, INCAPER, Vitória, Espírito Santo, Brazil
A R T I C L E I N F O Keywords: Carboxymethyl cellulose Sporulation Taxonomy A B S T R A C T
Fusarium guttiforme and Fusarium ananatum are the etiological agents of fusariosis and fruitlet core rot in pine-apple, respectively, producing mycotoxins that are harmful to the health of consumers. These two fungi are morphologically similar and difficulty in obtaining macroconidia of the species limits their identification. Different types of media are available for the culture of these pathogens, but not all of them favor F. ananatum and F. guttiforme macroconidia production. Therefore, the objective of this study was to develop a simple culture medium to improve rapid macro- and microconidia formation in both F. guttiforme and F. ananatum to facilitate taxonomic, pathogenicity and mycotoxin studies. In vitro analysis showed that basal medium with carbox-ymethyl cellulose (CMC) was better than other media tested with the highest macroconidia production at 7 days of incubation. The highest production of microconidia was with synthetic nutrient medium (SN) at 7 days. F. ananatum produced a relatively high number of microconidia with one septum in comparison to F. guttiforme when cultured in CMC, which suggests an additional character useful for Fusarium taxonomy. CMC medium may serve as an improved alternative to culture media currently used in Fusarium research and contribute to further knowledge of the taxonomy and mycotoxins of Fusarium species.
1. Introduction
Fusarium fungi are important producers of mycotoxins, which con-taminate food and might affect the health of consumers (Moretti, 2009). The presence of these fungi in plants is also extremely damaging and can cause diseases that lead to total crop loss (Goldschmied-Reouven et al., 1933;Kvas et al., 2009). Therefore, the correct identification of these fungi is extremely important for crop production, to help guar-antee the absence of such pathogens in fruits and the suitability of the final product for consumption and trade based on internationally ac-cepted standards.
The taxonomy of fungi, including their classification, identification and nomenclature, has developed from morphological, metabolic, and genomic information (Chun et al., 2018). However, Fusarium taxonomic knowledge is limited because of the similarity of species and in-sufficiency of distinguishing morphological characters, with identifi-cation based on macro- and microconidia in several species (Burgess et al., 1994;Leslie and Summerell, 2006;Nelson et al., 1983;Ventura, 2000).
Aerial mycelia of Fusarium species produce abundant, unicellular,
ovoid to elliptic microconidia, that are hyaline, nonseptate, and occa-sionally with one or two septa. They are formed in mono- and poly-phialides, and on false heads in short, little-branched hyphae. Macroconidia are also important in Fusarium spp. taxonomy because of their variable size and septation number (Leslie and Summerell, 2006; Nirenberg and O'Donnell, 1998; Ventura, 2000). These reproductive structures are also relevant for the study of pathogenicity and virulence in phytopathogenic Fusarium species. Furthermore, they are important in the production of mycotoxins and are a significant parameter in the evaluation of food quality and safety (Mansour et al., 2012;Seong et al., 2008).
Fusarium mycelial growth and sporulation is influenced by en-vironmental conditions such as culture medium (Souza et al., 2017; Stepień et al., 2013). Some culture media are described as standards for identification of species, and Fusarium may present variable phenotypes when grown with different nutritive conditions (Alfenas and Mafia, 2007). Morphological characters of a species that are common on one medium may be lost or altered when the same isolate is cultivated on a different medium (Leslie and Summerell, 2006). It's impracticable to perform pathogenicity tests and develop a reliable taxonomy of the
https://doi.org/10.1016/j.mimet.2020.105915
Received 14 January 2020; Received in revised form 3 April 2020 ⁎Corresponding author.
E-mail addresses:raissadmoura@hotmail.com,raissadebacker@gmail.com(R.D. Moura).
Available online 04 April 2020
0167-7012/ © 2020 Elsevier B.V. All rights reserved.
species based on these reproductive structures using the currently available culture media (Ventura, 2000).
Studies of the in vitro behavior of Fusarium species are limited, al-though these species have a wide geographical distribution, and great genetic and morphological diversity. Numerous species of Fusarium do not produce a satisfactory amount of macro- or microconidia useful for taxonomic study (Leslie and Summerell, 2006). Therefore, increased knowledge of Fusarium species requires the development of culture methods that enable the production of a sufficient quantity of macro-and microconidia.
Synthetic nutrient-poor agar (SNA), carbohydrate-rich media such as potato dextrose agar (PDA), and carnation leaf agar (CLA) are widely used for culture of Fusarium species (Alfenas and Mafia, 2007;Fisher et al., 1983;Tiedt and Jooste, 1988). However, these media have been shown to induce the formation of irregularly sized and shaped conidia. PDA is potentially degenerative, and a significant difference in the production of conidia in PDA and SNA has been observed (Leslie and Summerell, 2006). Furthermore, the efficiency of these culture media is limited to some species of the genus, and may negatively influence results (Mansour et al., 2012;Seong et al., 2008).
Research has demonstrated that production of macro- and micro-conidia by Fusarium is stimulated by the presence of cellulose com-pounds as in the case of CLA,filter paper, and pieces of banana leaves (Fisher et al., 1983; Leslie and Summerell, 2006; Tiedt and Jooste, 1988). The use of carboxymethyl cellulose in culture media may also be significant in the sporulation process since cellulosic compounds may stimulate sporulation in Fusarium species (Mansour et al., 2012;Ohara et al., 2004).
F. guttiforme Nirenberg & O'Donnell, a member of F. fujikuroi species complex (FFSC), is the etiological agent of pineapple fusariosis. This disease occurs in South and Central America and besides being a lim-iting factor for pineapple production, is a threat to human health, be-cause of the production of the mycotoxin beauvericin. Pineapple fu-sariosis is a quarantine disease for various fruit producing countries where the fungus has not yet been reported (O'Donnell et al., 2015; Ventura and Costa, 2006;Ventura and Zambolim, 2002).
In addition to fusariosis, another significant disease of pineapple is fruitlet core rot, caused by F. ananatum Jacobs, Marasas & van Wyk, another member of the FFSC. The symptoms of this disease are similar to those of fusariosis, but in contrast to F. guttiforme, this pathogen is found in most pineapple production areas of the world (Jacobs et al., 2010).
As fusariosis and the fruitlet core rot are caused by closely related Fusarium species, their accurate identification is difficult and frequently incorrect, leading to confusion among phytosanitary inspection autho-rities of pineapple producing countries (Ventura and Zambolim, 2002). When cultivated in traditional culture media these Fusarium species produce limited numbers of conidia, making taxonomic and biological studies difficult, as for example F. guttiforme, which produces few or no macroconidia (Leslie and Summerell, 2006). Besides that, rapid, accu-rate, and practical methods for identification of species is important for development of management strategies, and for studies of the epide-miology of diseases and resistance of new cultivars developed in breeding programs (Ventura, 2000). Therefore, the development an inexpensive culture medium that promotes rapid macro- and micro-conidia formation in both F. guttiforme and F. ananatum will facilitate taxonomic, pathogenicity, and mycotoxin studies of these important pathogens.
2. Materials and methods 2.1. Fungal isolate collection
Fungal isolates used in this study are from the Fungal Culture Collection of the Phytopathology Laboratory of the Capixaba Institute of Research, Technical Assistance and Rural Extension (INCAPER).
Eight isolates were used: four of F. guttiforme (reference codes: E203/ NRRL25624, E514/CML914, E524/CML921, E724/DMB26), and four of F. ananatum (reference codes: E678/CBS118516, E679/CBS118517, E680/CBS118518, E681/CBS118519).
Isolation, culture, and single-sporing followed the protocol de-scribed for Fusarium species (Leslie and Summerell, 2006). Fusarium isolates were initially identified morphologically and validated by species-specific polymerase chain reaction (PCR) assay (Carnielli et al., 2014).
2.2. Media preparation, macroconidia and microconidia production Four broth culture media were tested to evaluate their effect on F. ananatum and F. guttiforme growth and production of macro- and mi-croconidia: i) sabouraud dextrose (SD; 40 g/L glucose, 10 g/L peptone); ii) 1% sucrose (SUC; 10 g/L sucrose) [2]; iii) synthetic nutrient (SN; 0.5 g/L MgSO4.7H2O, 1 g/L KH2PO4, 1 g/L KNO3, 0.5 g/L KCl, 0.2 g/L glucose, 0.2 g/L sucrose with carnation leaf pieces) (Nelson et al., 1983) and, iv) basal medium with carboxymethyl cellulose (CMC; 0.5 g/L MgSO4.7H2O, 1 g/L NH4NO3, 1 g/L KH2PO4, 1 g/L yeast powder, 1 g/L carboxymethyl cellulose (Sigma-Aldrich #C4021).
Since SNA is a standard medium for the sporulation of Fusarium species, it was used as a control to compare the sporulation between the species (Leslie and Summerell, 2006).
The media were evaluated individually and each replication was inoculated with a disc (60 mm) of 1-week-old actively growing F. ananatum or F. guttiforme PDA culture, 230 rpm and incubated at 25 °C with 12 h photoperiod cycle for 14 days. Sporulation (macro- and mi-croconidia) was measured at 3, 7, 10 and 14 days after inoculation (DAI). On each sampling date a 10μL sample of each replicate was taken and washed in 10 mM phosphate buffer solution (PBS; pH 7.2). After washing, the pellet was resuspended and diluted with PBS to a final volume of 2 mL.
An Attune® Acoustic Focusing Cytometer (Applied Biosystems, Carlsbad, CA, USA) was used with blue laser (488 nm) to quantify the number of macro- and microconidia. Each samples was analized to separate conidia based on size and complexity using, respectively for-ward scatter (FSC) and side scatter (SSC) detectors, both with filter range of 483–493 nm.
2.3. Morphological characterization of macroconidia and microconidia All four isolates of F. ananatum and four of F. guttiforme were grown in CMC for 7 days, 25 °C, 230 rpm, 12 h photoperiod, and 200 macro-and microconidia of each isolate stained with Calcofluor White Stain (Sigma-Aldrich) in the proportion of 1:1, for morphological character-ization of conidia (Leslie and Summerell, 2006).
Macro- and microconidia were analyzed with a NIKON Eclipse TieS Fluorescence Microscope, 100× magnification, and data analyzed with the NIS-Elements software. Morphological characterization of F. ana-natum and F. guttiforme was performed by analyzing the shape and septation of macro- and microconidia and the size (width and length) of these structures (Leslie and Summerell, 2006;Jacobs et al., 2010).
2.4. Statistical analysis
All experiments had four independent replications for each species. Differences in the number of macro- and microconidia produced at each incubation time for each Fusarium species in the four different media were analyzed using a factorial experimental design (4 × 4 × 2) and results were submitted to analysis of variance (ANOVA) with means compared by the Tukey test (p < .05), using the statistical package R (https://www.r-project.org/).
3. Results
3.1. Macroconidia and microconidia production
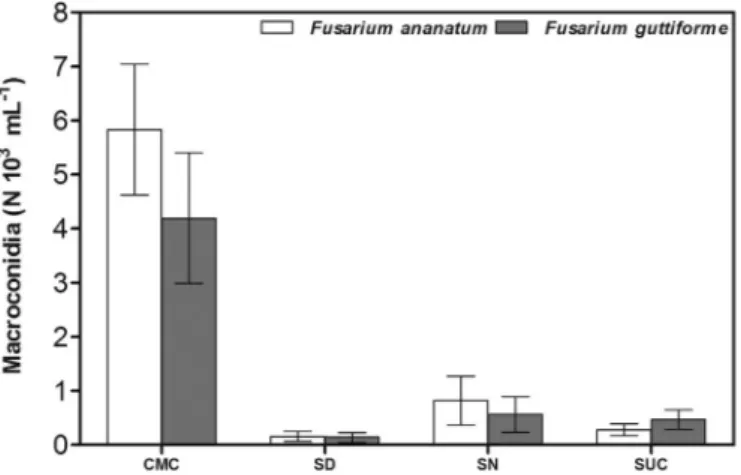
Quantification of macroconidia produced by the isolates of F. ana-natum and F. guttiforme indicated that CMC medium produced macro-conidia at all times tested after inoculation, with peak production on CMC on day 7 (Fig. 1). An advantage of this medium is its capability of inducing macroconidiation in both species tested which in contrast seemed variable on the other media. In comparison to the other culture media, both F. ananatum and F. guttiforme had a high production of macroconidia using CMC at all incubation times.
Fusarium ananatum produced about 6.0 × 103macroconidia/mL in CMC at 7 days. The lowest production of macroconidia by the species (0.1 × 103macroconidia/mL) occurred with SD at 3 days. The other culture media tested had macroconidia production about between 0.2 × 103/mL and 1.0 × 103/mL, which was less than that obtained using CMC medium (Fig. 1).
F. guttiforme produced about 5.0 × 103 macroconidia/mL, at 14 days in CMC. In other culture media tested, production of macro-conidia by F. guttiforme was lower (0.1 × 103/mL to 1.0 × 103 mac-roconidia/mL) with lowest macroconidia production (0.1 × 103/mL) with SD at 3 days.
The highest production of microconidia by both F. guttiforme and F. ananatum was with the SN medium at 7 days, with a production about 2.7 × 104 microconidia/mL. At other incubation times, the highest production occurred in the SD medium for both species (Fig. 1).
The lowest production of microconidia occurred in the SN medium at the 3-day incubation time, with a production about 5.0 × 103 mi-croconidia/mL by F. guttiforme and F. ananatum.
For the 7-day incubation time with CMC medium, F. guttiforme and F. ananatum produced about 1.2 × 104and 1.2 × 104microconidia/ mL, respectively, with no significant difference between the two species (Fig. 1).
3.2. Morphological characterization of macroconidia and microconidia In CMC, F. ananatum produced relatively longer macroconidia (maximum length 85.99μm), with a greater number of septa (Fig. 2) than F. guttiforme. These apparent differences between F. ananatum and F. guttiforme have not previously been noted in other culture media.
In comparison to F. guttiforme, F. ananatum has macro- and micro-conidia that are relatively longer. Macromicro-conidia of F. ananatum have a mean length and width of 34.98μm ( ± 8.83 μm, n = 800) and 4.68 μm ( ± 0.71μm, n = 800), respectively. Macroconidia of F. guttiforme have
a mean length and width of 30.42 μm ( ± 7.92 μm, n = 800) and 3.92μm ( ± 0.75 μm, n = 800), respectively. Fusarium ananatum mi-croconidia, have a mean length and width of 11.04μm ( ± 3.84 μm, n = 800) and 3.26μm ( ± 0.50 μm, n = 800), respectively, and F. guttiforme microconidia, had a mean length and width of 9.27 μm ( ± 2.58μm, n = 800) and 2.81 μm ( ± 0.39 μm, n = 800), respec-tively.
From observation of 800 microconidia of each specie, our results also indicated that F. ananatum produced a relatively high number of microconidia with one septum (78.5%) in comparison to F. guttiforme (8.5%) when cultured in CMC (Fig. 3).
No difference was apparent in other morphological characters ex-amined in F. ananatum and F. guttiforme, such as microconidia form (obovoid) and macroconidia form (straight to slightly curved). 4. Discussion
In this work we tested four culture media for morphological char-acterization and macro- and microconidia production of Fusarium spe-cies. CMC medium was the best medium for macro- and microconidia characterization. To our knowledge, this is thefirst study that provides additional characters useful for taxonomic analysis of the species, since microconidia of both species were previously reported having one septum or none (Leslie and Summerell, 2006;Jacobs et al., 2010).
In comparison to CMC medium, other frequently used culture media that were tested in the present study had a relatively low production of macro- and microconidia. Thus, for F. ananatum and F. guttiforme, the CMC medium proved to be extremely effective for production of mac-roconidia, which should facilitate future studies of the taxonomy, pa-thogenicity, and virulence of these species (Fig. 1).
The evaluation of the production of macroconidia by F. graminearum in different culture media after 14 days indicated that CMC medium positively affected the production of macroconidia, reaching 111 × 105 macroconidia/mL with 14-day incubation time (Mansour et al., 2012). However, some isolates responded differently to the CMC medium by not producing macroconidia indicating that effects of medium on pro-duction of macroconidia depends on the isolate. All isolates of F. gra-minearum exhibited a positive response in macroconidia production using 1% sucrose, confirming that effects of medium on production of macroconidia depends on the species (Mansour et al., 2012).
SNA medium is widely used for sporulation of Fusarium species, but the presence of potassium chloride in this medium has been associated with increased production of microconidia and absence of macro-conidia (Leslie and Summerell, 2006). The present study confirmed that fewer macroconidia were produced with SN compared to CMC. The highest production of macroconidia with SN occurred at 10 days for F. ananatum (1 × 103macroconidia/mL) and 14 days for F. guttiforme (1 × 103macroconidia/mL).
The development of a culture medium that promotes the formation of macro- and microconidia is fundamental for studies of the biology, taxonomy, mycotoxins, and pathogenicity of Fusarium species. F. gutti-forme exhibits low or no production of macroconidia in vitro, presenting challenges in studying this species. In the present study we identified a specific culture medium, CMC, that supported production of macro- and microconidia by F. ananatum and F. guttiforme isolates, enabling us to compare the isolates and, consequently, to identify and differentiate F. ananatum and F. guttiforme, and which may also be useful for studies of other Fusarium species (Leslie and Summerell, 2006).
Differences in total production of macro- and microconidia by F. guttiforme and F. ananatum in different culture media observed in the present study demonstrate differences in the species in response to culture media. However, the total production of conidia cannot be used as a taxonomic characteristic in the genus, because it is inconsistent. The color of mycelia is also not employed for taxonomic purposes be-cause it also differs based on the medium used for culture (Kvas et al., 2009;Leslie and Summerell, 2006).
Fig. 1. Macroconidia production by Fusarium guttiforme and Fusarium ananatum in different culture media (SN, SD, CMC and SUC) at 7 days after incubation (DAI). Bars represent standard error of the mean. (n = four independent re-petition for each specie and media).
The size and septation of conidia are characters that are commonly evaluated in fungal taxonomy (Leslie and Summerell, 2006;Weir et al., 2012). Therefore, the presence of longer macroconidias with a higher number of septa, and more microconidia with one septum in F. ana-narum grown in CMC as observed in the present study, may serve as new morphological markers useful for distinguishing this species from others. Microconidia of both species may have one septum or none (Leslie and Summerell, 2006;Jacobs et al., 2010).
The genus Fusarium does not have enough morphological characters to distinguish most species. For example, morphological marker ana-lysis of members of the F. graminearum species complex associated with
corn has been conducted but could not distinguish F. aethiopicum from F. graminearum, F. vorosii, and F. asiaticum (O'Donnell et al., 2008). Therefore, the discovery of new morphological markers for F. ananatum noted in the present study is of significance for taxonomic analysis and phytosanitary inspection and detection of this important species of agricultural and health concern.
Fusarium circinatum also belongs to the Fusarium fujikuroi species complex and is easily confused with other species of the complex, especially F. subglutinans. For many years, F. circinatum was described as a special form of F. subglutinans, and earlier as a special form of F. la-teritium (Kuhlman et al., 1978). The differentiation between conserved genes is useful for identification of this species (O'Donnell et al., 1998; O'Donnell et al., 2000;Schneider and Seaman, 1977;Steenkamp et al., 1999). However, coiled hyphae are produced only in SNA, and are used as a morphological marker for differentiation of F. circinatum from si-milar species, also indicating the importance of culture media in tax-onomy (Leslie and Summerell, 2000).
5. Conclusions
Results of the present study demonstrate that CMC is an ideal medium to improve macroconidia production for identification of Fusarium pathogens of the Fusarium fujikuroi species complex and can serve as an improved replacement for culture media currently used in Fusarium research. In addition, this medium can facilitate accurate identification of Fusarium species, helping to identify and eliminate fruits mycotoxins producing pathogens, thus benefiting commerce of agricultural products and human health.
Declaration of Competing Interest There is no conflict of interest to declare.
Fig. 2. Production of macro- and microconidia by Fusarium ananatum in CMC. Arrows indicate macroconidia with more than 3 septa, not observed in other media.
Fig. 3. Septation of microconidia of Fusarium guttiforme and Fusarium ananatum grown in CMC medium (n = 200 for each specie).
Acknowledgments
The authors thank the National Council for Scientific Technological Research (PQ- process # 310680/2016-6) and Foundation for Support to Research and Innovation of Espírito Santo (FAPES), for the scholar-ship granted to RDM (#34482.520.19188.27092017), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for granting a post-doctoral fellowship to support this research. References
Alfenas, A.C., Mafia, R.G., 2007. Métodos em Fitopatologia. Ed. UFV, Viçosa.
Burgess, L.W., Summerell, B.A., Bullock, S., Gott, K.P., Backhouse, D., 1994. Laboratory Manual for Fusarium Research. University of Sydney, Sydney.
Carnielli, L., Amorim, W.A., Vaz, A.B., Fernandes, P.M.B., Ventura, J.A., 2014. Molecular diagnosis of Fusarium guttiforme and Pineapple mealybug wilt-associated virus. BMC Proc. 8.https://doi.org/10.1186/1753-6561-8-S4-P121.
Chun, J., Oren, A., Ventosa, A., Christensen, H., Arahal, D.R., Costa, M.S., 2018. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 68.https://doi.org/10.1099/ijsem.0.002516.
Fisher, N.L., Marasas, W.F.O., Toussoun, T.A., 1983. Taxonomic importance of micro-conidia chains in Fusarium section Liseola and effects of water potential on their formation. Mycologia. 75.https://doi.org/10.1080/00275514.1983.12023738.
Goldschmied-Reouven, A., Friedman, J., Block, C.S., 1933. Fusarium spp. isolated from non-ocular sites: a 10 year experience at an Israeli general hospital. J. Mycologie Medicale 3, 1993.
Jacobs, A., Van Wyk, P.S., Marasas, W.F.O., Wingfield, B.D., Wingfield, M.J., Coutinho, T.A., 2010. Fusarium ananatum sp. nov. in the Gibberella fujikuroi species complex from pineapples with fruit rot in South Africa. Fungal Biol. 114.https://doi.org/10. 1016/j.funbio.2010.03.013.
Tiedt, L., Jooste, W., 1988. Aberrant conidiogenesis in a Fusarium subglutinans isolate. Trans. Br. Mycol. Soc. 90.https://doi.org/10.1016/S0007-1536(88)80002-0. Kuhlman, E.G.L.D., Dwinell, P.E., Nelson, C.B., 1978. Characterization of the Fusarium
causing pitch canker of southern pines. Mycologia 70.https://doi.org/10.2307/ 3759311.
Kvas, M., Marasas, W.F.O., Wingfield, B.D., Wingfield, M.J., Steenkamp, E.T., 2009. Diversity and evolution of Fusarium species in the Gibberella fujikuroi complex. Fungal Divers. 34, 1–21.
Leslie, J.F., Summerell, B.A., 2006. The Fusarium Laboratory Manual,first ed. Blackwell Publishing, Ames.
Mansour, M.B., Goh, Y.K., Vujanovic, V., 2012. Rapid macroconidia production in Fusarium graminearum 3-and 15-acetyldeoxynivalenol (ADON) chemotypes using sucrose-water medium. Ann. Microbiol. 62. https://doi.org/10.1007/s13213-011-0335-1.
Moretti, A., 2009. Taxonomy of Fusarium genus, a continuousfight between lumpers and
splitters. Proc. Nat. Sci, Matica Srpska Novi Sad. 117.
Nelson, P.E., Toussoun, T.A., Marasas, W.F.O., 1983. Fusarium Species an Illustrated Manual for Identification. The Pennsylvania State University Press, University Park. Nirenberg, H.I., O’Donnell, K., 1998. New Fusarium species and combinations within the
Gibberella fujikuroi species complex. Mycologia 90.https://doi.org/10.1080/ 00275514.1998.12026929.
O’Donnell, K., Cigelnik, E., Nirenberg, H.I., 1998. Molecular systematics and phylogeo-graphy of the Gibberella fujikuroi species complex. Mycologia. 90.https://doi.org/10. 2307/3761407.
O’Donnell, K., Nirenberg, H.I., Aoki, T., Cigelnik, E., 2000. A multigene phylogeny of the Gibberella fujikuroi species complex: detection of additionally phylogenetically dis-tinct species. Mycoscience. 41.https://doi.org/10.1007/BF02464387.
O’Donnell, K., Ward, T.J., Aberra, D., Kistler, H.C., Aoki, T., Orwig, N., 2008. Multilocus genotyping and molecular phylogenetics resolve a novel head blight pathogen within the Fusarium graminearum species complex from Ethiopia. Fungal Genet. Biol. 45.
https://doi.org/10.1016/j.fgb.2008.09.002.
O’Donnell, K., Ward, T.J., Robert, V.A.R.G., Crous, P.W., Geiser, D.M., Kang, S., 2015. DNA sequence-based identification of Fusarium: current status and future directions. Phytoparasitica 43.https://doi.org/10.1007/s12600-015-0484-z.
Ohara, T., Inoue, L., Namiki, F., Kunoh, H., Tsuge, T., 2004. REN1 is required for de-velopment of microconidia and macroconidia, but not of chlamydospores, in the plant pathogenic fungus Fusarium oxysporum. Genetics 166.https://doi.org/10.1534/ genetics.166.1.113.
Schneider, E.F., Seaman, W.L., 1977. Ontogeny of lipid bodies in the endoplasmic re-ticulum of Fusarium sulphureum. Can. J. Microbiol. 23. https://doi.org/10.1139/m77-027.
Seong, K.Y., Zhao, X., Xu, J.R., Guldener, U., Kistler, H.C., 2008. Conidial germination in thefilamentous fungus Fusarium graminearum. Fungal Genet. Biol. 45.https://doi. org/10.1016/j.fgb.2007.09.002.
Souza, W.C.O., Nascimento, L.C., Oliveira, M.D.M., Porcino, M.M., Silva, H.A.O., 2017. Genetic diversity of Fusarium spp. in pineapple‘Pérola’ cultivar. Eur. J. Plant Pathol. 150.https://doi.org/10.1007/s10658-017-1328-0.
Steenkamp, E.T., Wingfield, B.D., Coutinho, T.A., Wingfield, M.J., Marasas, W.F., 1999. Differentiation of Fusarium subglutinans f. sp. pini by histone gene sequence data. Appl. Environ. Microbiol. 65, 3401–3406.
Stepień, L., Koczyk, G., Waśkiewicz, A., 2013. Diversity of Fusarium species and myco-toxins contaminating pineapple. J. Appl. Genet. 54. https://doi.org/10.1007/s13353-013-0146-0.
Ventura, J.A., 2000. Taxonomia de Fusarium e seus segregados. Parte II: Chaves para identificação. Revisão Anual de Patologia de Plantas. 8, 303–338.
Ventura, J.A., Costa, H., 2006. Controle Cultural. In: De, Oliveira S.M.A., Terao, D., Dantas, S.A.F., SCC, Tavares (Eds.), Patologia pós-colheita: frutas, olerícolas e orna-mentais tropicais. Embrapa Informações Tecnológicas, Brasília, pp. 145–169.
Ventura, J.A., Zambolim, L., 2002. Controle das doenças do abacaxizeiro. In: Zambolim, L., Vale, F.X.R., Do Monteiro, A.J.A., Costa, H. (Eds.), Controle de doenças de plantas: fruteiras. Editora UFV, Viçosa, pp. 445–510.
Weir, B.S., Johnson, P.R., Damm, U., 2012. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 73.https://doi.org/10.3114/sim0011.