HIGH SURFACE AREA MESOPOROUS
PEROVSKITES FOR CATALYTIC APPLICATIONS
Thèse
Mahesh Muraleedharan Nair
Doctorat en Chimie
Philosophiae Doctor (PhD)
Québec, Canada
III
RÉSUMÉ
Les pérovskites sont des oxydes métalliques mixtes qui peuvent être représentés par la formule générale ABO3. Depuis la première revue mettant en
évidence leur activité catalytique, ces matériaux ont attiré l‟attention des chercheurs dans le monde entier. Il a été confirmé que les pérovskites peuvent être considérées comme des alternatives rentables et efficaces aux métaux nobles pour plusieurs applications (les réactions de synthèse à titre d‟exemple). En outre, ces oxydes métalliques mixtes sont bien connus pour leur stabilité à haute température, leur grande mobilité d'oxygène ainsi que la stabilisation des inhabituels états d'oxydation des cations. Pour ces raisons, plusieurs stratégies ont été développées pour la synthèse de ces matériaux. Cependant, les méthodes conventionnelles de synthèse des pérovskites permettent d‟obtenir seulement des matériaux ayant une faible surface spécifique, ce qui constitue un inconvénient majeur du fait que des applications catalytiques sont mis en jeux. La faible surface spécifique est due à un traitement thermique de haute température appliqué au cours de la synthèse de ces matériaux. Le premier objectif de ce présent travail est donc l‟obtention d‟oxydes métalliques mixtes structurés de type pérovskite avec une grande surface spécifique. Le “Nanocasting”, une méthode de gabarits solides récemment développée, a montré son efficacité pour la synthèse de diverses compositions chimiques ayant des valeurs extrêmement élevées de surface spécifique. En se basant sur cette méthode, plusieurs pérovskites LaBO3 (B = Mn ,
Ni , Co, Fe) ont été synthétisées. Ces matériaux se caractérisent par leur grande surface spécifique qui peut atteindre 150 m2 g-1.
Les premiers essais de l'oxydation totale du méthanol, une molécule sonde, out confirmé que ces nouveaux matériaux sont des catalyseurs très actifs, en particulier les LaMnO3. De plus, d'autres études ont confirmé que l'augmentation
de l‟activité catalytique est évidemment liée à la plus grande surface spécifique et a la plus grande quantité d‟oxygène adsorbée des pérovskites développées. Les résultats ont montré une proportionnalité entre les vitesses des réactions et la
IV
surface spécifique du catalyseur. Dans une étude suivante, l‟intérêt de la recherche est porté sur reformage du méthane à sec, comme cette réaction est très pertinente pour l‟industrie du fait qu‟elle consiste en la conversion de deux gaz à effet serre (CH4 et CO2) en gaz de synthèse (CO + H2). Des résultats
prometteurs ont été obtenus dans ce cas aussi en utilisant les matériaux développés de type LaNiO3 comme un pré-catalyseur. De meilleures efficacité et
stabilité ont été observées pour Ni/La2O3, catalyseurs dérivés des LaNiO3, par
V
ABSTRACT
Perovskites are mixed metal oxides that can be represented by the general formula ABO3. Since the initial report regarding their catalytic activity, these
materials have received immense research attention worldwide. Perovskites are proven to be cost effective and efficient alternatives to noble metals for various total/partial oxidation as well as synthetic chemical reactions. Additionally these mixed metal oxides are well known for their high temperature stability, high mobility of oxygen and the stabilization of unusual cation oxidation states. For these reasons various strategies were developed for the synthesis of these materials. However perovskites synthesized using conventional methods generally result in low specific surface area materials, which is a major drawback as far as catalytic applications are concerned. This pertinent lower value of surface area is resulting from the high temperature treatment involved in the synthesis of these materials. This issue was taken up and in the present project the first goal was to obtain perovskite structured mixed metal oxides with high specific surface area. Nanocasting is a recently developed solid templating method that is proven to be efficient for the synthesis of various chemical compositions with extremely high values of specific surface area. By applying this method a series of LaBO3 (B = Mn,
Ni, Co, Fe) perovskites were synthesized and these materials were found to posses extremely high values of specific surface areas (up to 150 m2g-1).
Initial tests for the total oxidation of methanol as a probe molecule confirmed that these novel materials are highly active catalysts, especially LaMnO3. Further
studies confirmed that the enhanced activity was obviously related to the higher specific surface areas and higher amount of adsorbed oxygen species obtained for the nanocast perovskites in comparison with the bulk. Our results demonstrated the proportionality of reaction rates to the specific surface area of the catalyst. In a following study, we chose dry reforming of methane, since this reaction involves the conversion of two green house gases (CH4 and CO2) into syngas (CO + H2),
which is more industrially relevant. Promising results were obtained in this case also using nanocast LaNiO3 as a pre-catalyst. Enhanced efficiency and stability
VI
were observed for Ni/La2O3 catalysts derived from nanocast LaNiO3 in comparison
to its bulk counterpart. In particular, these materials were found to be coke resistant for 48 hours under the conditions of dry reforming.
VII
Table of contents
RÉSUMÉ ... III ABSTRACT ... V LIST OF TABLES ... XI LIST OF FIGURES ... XIII ABBREVIATIONS ... XVII ACKNOWLEDGEMENTS ... XIX PREFACE ... XXI
Chapter 1– Introduction ... 1
1.1 Perovskites ... 1
1.1.1 Structure and properties ... 1
1.1.2 Synthesis and catalytic properties ... 3
1.2 Nanocasting ... 5
1.2.1 Principles of nanocasting ... 5
1.2.2 Ordered mesoporous silica templates ... 8
1.2.3 Overview nanocasting ... 12
1.3 Catalytic tests ... 18
1.3.1 Total oxidation of methanol ... 18
1.3.2 Dry reforming of methane ... 21
Chapter 2 – Experimental methods ... 25
2.1 Synthesis of ordered mesoporous silica templates ... 25
2.2 Synthesis of mesoporous perovskites ... 26
2.3 Characterization ... 27
2.3.1 Powder X-Ray Diffraction (XRD) ... 27
2.3.2 N2 – physisorption ... 29
2.3.3 Elemental analysis ... 31
2.3.4 Electron Microscopy (TEM and SEM) ... 31
2.3.5 X-ray photoelectron spectroscopy (XPS) ... 32
2.3.6 Temperature programmed reduction (TPR-H2) ... 32
2.3.7 Temperature programmed desorption (TPD-O2,) ... 33
2.3.8 Thermogravimetric / Differential thermal analysis (TG/DTA) ... 33
2.4 Catalytic tests ... 34
2.4.1 Total oxidation of methanol ... 34
VIII
Chapter 3 - Kinetics of methanol oxidation over mesoporous perovskite catalysts 39
3.1 Résumé ... 40
3.2 Abstract ... 41
3.3 Introduction ... 42
3.4 Experimental ... 43
3.4.1 Synthesis of ordered mesoporous KIT-6 silica ... 43
3.4.2 Nanocasting of mesoporous perovskites ... 43
3.4.3 Characterization ... 44
3.4.4 TPR/TPD ... 44
3.4.5 Catalytic tests ... 45
3.4.6 Kinetic studies ... 45
3.5 Results and discussion ... 45
3.5.1 Synthesis and characterization of mesoporous perovskites ... 45
3.5.2 Temperature programmed reduction by hydrogen ... 47
3.5.3 Temperature programmed desorption of oxygen ... 49
3.5.4 Catalytic tests ... 51
3.5.5 Stability ... 52
3.5.6 Kinetic studies ... 53
3.6 Conclusions ... 56
3.7 References ... 57
Chapter 4 - Pore structure effects on the kinetics of methanol oxidation over nanocast mesoporous perovskites ... 59
4.1 Résumé ... 60
4.2 Abstract ... 61
4.3 Introduction ... 62
4.4 Experimental ... 63
4.4.1 Synthesis of ordered mesoporous SBA-15 silica ... 63
4.4.2 Nanocasting of mesoporous perovskites ... 64
4.4.3 Characterization ... 65
4.4.4 Temperature programmed reduction (TPR) and desorption (TPD) ... 65
4.4.5 Catalytic tests ... 65
4.4.6 Kinetic studies ... 66
4.5 Results and discussion ... 66
4.5.1 Synthesis and characterization of mesoporous perovskites ... 66
IX
4.5.3 Temperature programmed desorption of oxygen ... 73
4.5.4 Catalytic tests ... 74
4.5.5 Kinetic studies ... 75
4.6 Conclusions ... 78
4.7 References ... 80
Chapter 5- Surface properties of nanocast mesoporous perovskites ... 83
5.1 Résumé ... 84
5.2 Abstract ... 85
5.3 Introduction ... 86
5.4 Experimental ... 87
5.4.1 Synthesis of ordered mesoporous KIT-6 silica ... 87
5.4.2 Nanocasting of mesoporous perovskites ... 88
5.4.3 Characterization ... 89
5.5 Results and discussion ... 89
5.5.1 Synthesis and characterization of mesoporous perovskites ... 89
5.5.2 SEM-EDS analysis ... 95
5.5.3 XPS analysis ... 96
5.6 Conclusions ... 101
5.7 References ... 103
Chapter 6 - Coke resistant nanostructured Ni/La2O3 catalyst for dry reforming of methane ... 105
6.1 Résumé ... 106
6.2 Abstract ... 107
6.3 Introduction ... 108
6.4 Experimental ... 110
6.4.1 Synthesis of ordered mesoporous SBA-15 silica ... 110
6.4.2 Synthesis of LaNiO3 perovskites ... 110
6.4.3 Characterization ... 111
6.4.4 Catalytic tests ... 112
6.5 Results and discussion ... 113
6.5.1 Physicochemical characterization... 113
6.5.2 Catalytic studies ... 119
6.5.3 Stability tests ... 121
6.6 Conclusion ... 125
X
Chapter 7 – General conclusions and perspectives ... 129 Bibliographic references ... 133 APPENDIX ... 137
XI
LIST OF TABLES
Table 1.1 General methods used for the synthesis of perovskite structured mixed metal
oxides. SBET is the specific surface area determined by the BET method …...4
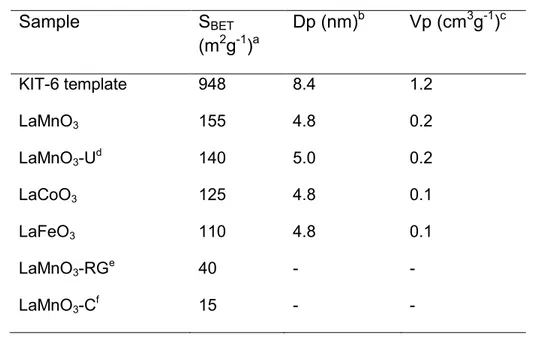
Table 3.1 Structural parameters of the KIT-6 template and nanocast perovskites obtained
by performing N2 physisorption analysis at -196 ºC………….………...48
Table 3.2 Amount of H2 consumed during TPR-H2………...50
Table 3.3 Amount of O2 desorbed during TPD-O2…………...51
Table 4.1 Structural parameters of the SBA-15 templates and nanocast perovskites
obtained by performing N2 physisorption analysis at -196 ºC…………...68
Table 4.2 Amount of H2 consumed during TPR-H2………...72
Table 4.3 Amount of O2 desorbed during TPD-O2.………...74
Table 4.4 Kinetic parameters obtained for total oxidation of methanol over nanocast
perovskites………..78
Table 5.1 Structural parameters of the KIT-6 template and nanocast perovskites obtained
by performing physisorption analysis at -196 ºC.………...92
Table 5.2 Surface and bulk elemental composition of the nanocast mesoporous
perovskites.………...101
Table 6.1 Structural parameters obtained for LaNiO3 perovskites and Ni/La2O3 obtained by
performing reduction at 700 ºC……..………...114
Table 6.2 Amount of H2 consumed during TPR-H2………...118
Table 6.3 Structural parameters obtained for Ni/La2O3 catalysts after performing the
XIII
LIST OF FIGURES
Figure 1.1 Representations of the ideal perovskite structure with BX6 octahedron forming
the centre of the cube (left) and BX6 octahedron occupying the corners of the cube with A
site atom at the centre. (right)…...………..1
Figure 1.2 Schematic representation of the steps involved in the mesostructure evolution
in the nanocasting technique using ordered mesoporous silica as hard template…...………..6
Figure 1.3 Mechanistic pathway showing the self assembly of formation of mesoporous
materials ………..………...9
Figure 1.4 Pore models of mesostructures with symmetries (A) p6mm, (B) Ia3d, (C) Pm3n, (D) Im3n, (E) Fd3m, (F) Fm3m…...……..10 Figure 1.5 The effect of the hydrothermal aging temperature on the pore structure of
SBA-15-type materials: (a) low emperature aging (35-60 ºC), main mesopores 5-6 nm, wall thickness 4 nm, micropore volume = 0.2 mLg-1; (b) aging at 80-100 °C, main mesopores
7-9 nm, wall thickness 3.2 nm, micropore volume 0.1 mLg-1; (c) high temperature aging
(>120 °C), main mesopores >9 nm, wall thickness 2 nm, no micropores.……...…...11
Figure 1.6 TEM image showing hexagonal arrangement of carbon rods in CMK-3 (left)
and CMK-5 carbon tubes templated from SBA-15.…...………...14
Figure 1.7 TEM images of (1) LiCoO2 (2) MgO and (3) Co3O4 synthesized using hard
templating method………...15
Figure 1.8 Schematic representation of the main reaction products obtained from
methanol oxidation as a function of acidic-basic character of the catalyst surface.…...19
Figure 2.1 Wide angle X-Ray diffraction patterns of various perovskite oxides (left) and
small angle XRD patterns of mesoporous MgO along with parent templates. RG represents samples synthesized by reactive grinding method and CIT represents sample synthesized using citrate method.…...………28
Figure 2.2 IUPAC classifications of adsorption isotherms (left) and hysteresis loops
(right)...………..30
Figure 2.3 TEM images of mesoporous hematite, α-Fe2O3, templated using ordered
mesoporous silica KIT-6…...…………..…32
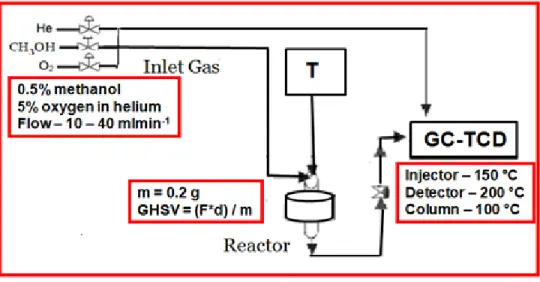
Figure 2.4 Schematic representation of the experimental set up used for performing the
total oxidation of methanol ………...35
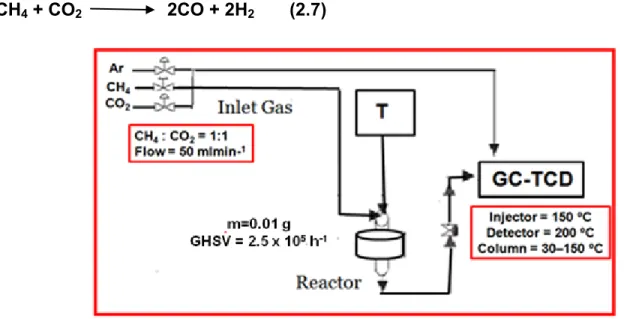
Figure 2.5 Schematic representation of the experimental set up used for the dry reforming
of methane……...37
Figure 3.1 N2 physisorption isotherm and pore size distribution (inset) of the parent KIT-6
silica hard template (left). Wide-angle powder XRD patterns of mesoporous perovskite oxides synthesized by use of ordered mesoporous KIT-6 silica aged at 100 ºC as a hard template. The lowest curve corresponds to the LaMnO3 perovskite synthesized without
using citric acid (right)………...…46
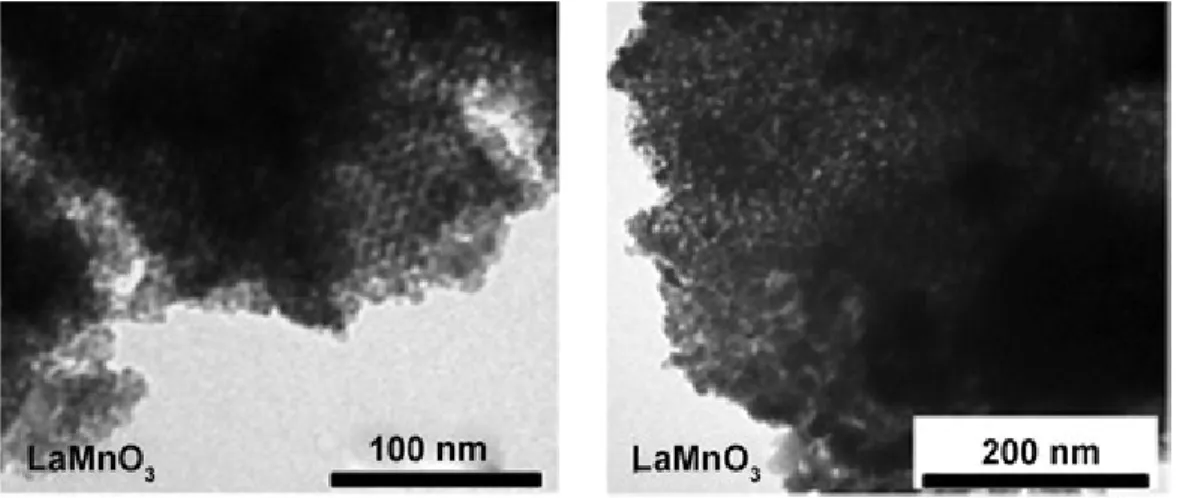
Figure 3.2 TEM images of nanocast LaMnO3 synthesized by use of ordered mesoporous
XIV
Figure 3.3 (A) N2 physisorption isotherms and b) the corresponding pore size distributions
of nanocast perovskite oxides synthesized by use of ordered mesoporous KIT-6 silica aged at 100 ºC as a hard template. The isotherms of LaCoO3 and LaFeO3 are plotted with
an offset of 60 and 110 cm3g-1, respectively, for clarity. (B) Pore size distributions were
calculated from the adsorption branch of the isotherm by using the NLDFT method………...………..……48
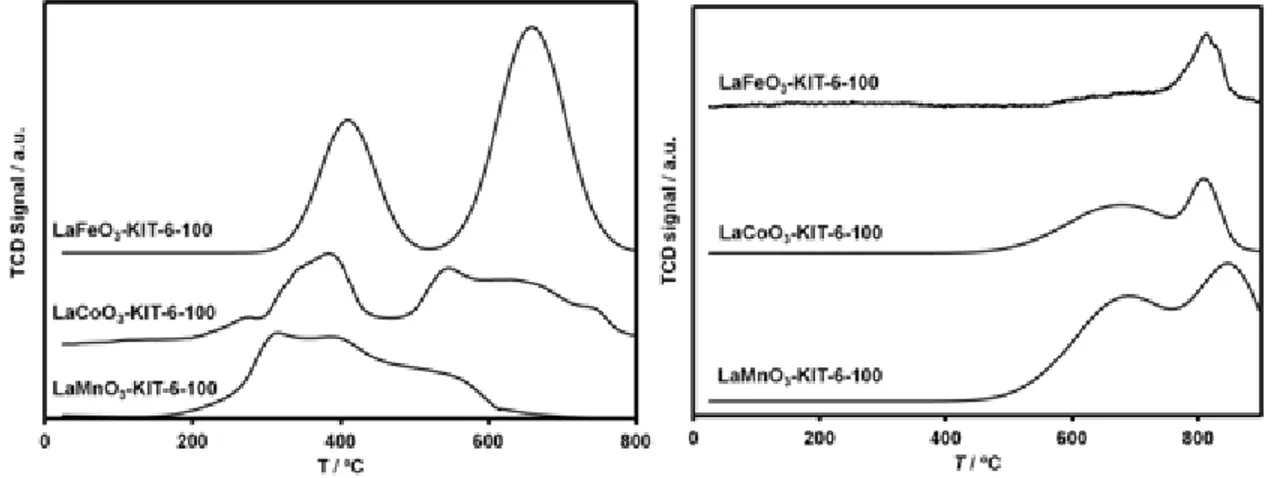
Figure 3.4 TPR-H2 (left) and TPD-O2 (right) profiles of nanocast mesoporous perovskite
oxides synthesized by use of ordered mesoporous KIT-6 silica aged at 100 ºC as a hard template………...49
Figure 3.5 Methanol conversion profiles as a function of temperature over LaMnO3,
LaCoO3, and LaFeO3 perovskites synthesized by using the nanocasting method (left).
Methanol conversion profiles as a function of temperature over LaMnO3 perovskites
synthesized by using the nanocasting method, the reactive grinding method (RG), and the conventional citrate process (C). The empty symbols represent the recalculated values of conversion for LaMnO3 synthesized by using the nanocasting method (Calculated KIT-6),
the reactive grinding method (Calculated-RG), and the conventional citrate process (Calculated-C)…...………52
Figure 3.6 A comparison of the N2 physisorption isotherms (A) and wide angle XRD
patterns (B) of the nanocast LaMnO3 perovskite before and after the catalytic
test……...…53
Figure 3.7 Comparison between fresh catalyst and used catalyst for methanol conversion
over LaMnO3 perovskites synthesised using nanocasting. The open symbols represent the
calculated value of conversion……...53
Figure 3.8 (A) Methanol conversion over nanocast LaMnO3 at different space velocities.
The values of the space velocities increase at rates of 19500, 39100, 58600, and 78200 h -1from left to right. (B) Cross-plotting the values of experimental conversions obtained for
nanocast LaMnO3 at selected temperatures as a function of pseudo-contact time. Points
represent experimental data, and lines are calculated by using Equation (4.1). (C) The numerical values of rates obtained are represented as a function of the partial pressure of methanol; 1 atm=101325 Pa and (D) Arrhenius plot of the rate constant k obtained for nanocast LaMnO3…………...55
Figure 4.1 N2 physisorption isotherms (-196 °C) and the corresponding pore size
distributions (right) of ordered mesoporous SBA-15 silica hard templates. Pore size distributions were calculated from the adsorption branch of the isotherm using the NLDFT method.……….……...67
Figure 4.2 Wide angle powder XRD patterns of mesoporous LaMnO3 perovskite oxides
synthesized by use of ordered mesoporous SBA-15 as the hard template. The numbers denote the aging temperature of the template……….……….68
Figure 4.3 TEM images of LaMnO3-35 (a, b), LaMnO3-100 (c, d) and LaMnO3-140 (e,
f)………..69
Figure 4.4 N2 physisorption isotherms (-196°) and the corresponding pore size
distributions (right) of nanocast perovskite oxides synthesized by use of ordered mesoporous SBA-15 silica hard templates. Pore size distributions were calculated from the adsorption branch of the isotherm using the NLDFT method.……….70
XV
Figure 4.5 N2 physisorption isotherms (-196 °C) and the corresponding NLDFT theoretical
isotherms (colour) of nanocast LaMnO3 perovskite oxides synthesized by use of ordered mesoporous SBA-15 silica hard templates. …...71
Figure 4.6 (A) TPR-H2 and (B) TPD-O2 profiles of nanocast mesoporous perovskite oxides
synthesized by use of ordered mesoporousSBA-15 silica hard templates aged at different temperatures………...73
Figure 4.7 Methanol conversion profiles as a function of temperature over nanocast
LaMnO3 perovskites synthesized using SBA-15 silica hard template aged at different
temperatures (GHSV = 39100 h-1).………...75
Figure 4.8 Temperature dependent methanol conversion profiles for the total oxidation of
methanol over nanocast LaMnO3-35 (left) LaMnO3-100 (middle) and LaMnO3-140 (right) at
different space velocities ………...76
Figure 4.9 Cross-plotting the values of experimental conversions at selected temperatures
as a function of pseudo-contact time obtained for nanocast LaMnO3. Points represent
experimental data, and lines are calculated by using Equation (6.1). The numerical values of rates obtained in each case are represented as a function of the partial pressure of methanol (right).………...77
Figure 4.10 Arrhenius plots for the rate constant k obtained for nanocast LaMnO3-35,
LaMnO3-100 and LaMnO3-140. The linear correlation between the pre-exponential factor
and the specific surface area shown on the right.….……...78
Figure 5.1 N2 physisorption isotherms (-196 °C) and the corresponding pore size
distributions (bottom) of ordered mesoporous KIT-6 silica hard templates. Pore size distributions (inset) were calculated from the desorption branch of the isotherm using the NLDFT method.………90
Figure 5.2 Wide-angle powder XRD patterns of mesoporous perovskite oxides
synthesized by use of ordered mesoporous KIT-6 silica aged at 100 ºC as a hard template. A comparison of the wide angle XRD pattern of nanocast LaMnO3 with its bulk counterpart
synthesized by the citrate process is given on the right.………...90
Figure 5.3 TEM images of nanocast LaFeO3, LaCoO3 and LaMnO3 perovskites
synthesized using ordered mesoporous KIT-6 aged at 100 ºC as hard tem………...91
Figure 5.4 (A) N2 physisorption isotherms and b) pore size distributions of nanocast
perovskite oxides synthesized using ordered mesoporous KIT-6 silica aged at 100 ºC as a hard template. The isotherms of LaCoO3 and LaFeO3 are plotted with an offset of 60 and
110 cm3g-1, respectively, for clarity. (B) Pore size distributions were calculated from the
adsorption branch of the isotherm using the NLDFT method.…………...93
Figure 5.5 N2 physisorption isotherms (left) and pore size distributions (right) LaMnO3
-KIT-6 composite after each step of impregnation. The materials were calcined at 500 and 700 ºC after the first and second impregnation respectively. Pore size distributions were calculated from the adsorption branch of the isotherm using the NLDFT method.………...94
Figure 5.6 N2 physisorption isotherms (left) and pore size distributions (right) of nanocast
LaMnO3 during each step of NaOH treatment. The isotherm of LaMnO3-NaOH-1 is plotted
with an offset of 30 cm3g-1, for clarity. Pore size distributions were calculated from the
adsorption branch of the isotherm using NLDFT method.……...94
Figure 5.7 29Si MAS NMR spectra of LaMnO
XVI
Figure 5.8 Representative SEM images of the nanocast perovskites. LaMnO3-C
represents the bulk sample synthesized using the citrate process.………...95
Figure 5.9 Representative SEM images of the nanocast LaMnO3 perovskites during each
step of template removal ………...96
Figure 5.10 O 1s, La 3d and Si 2s XPS spectra of nanocast mesoporous perovskites after
removal of the KIT-6 hard template………97
Figure 5.11 Fe 2p, Co 2p and Mn 2p core level XPS spectra of nanocast mesoporous
perovskites after removal of the KIT-6 hard template………..98
Figure 5.12 Mn 2p, La 3d, O 1s and Si 2s core level XPS spectra of nanocast mesoporous
perovskites during each step of template removal step using 2M NaOH………100
Figure 6.1 N2 physisorption isotherm and the corresponding pore size distribution (inset)
of ordered mesoporous silica SBA-15 aged at 100 ºC. The pore size distribution was calculated from the adsorption branch of the isotherm using NLDFT method (left). Wide angle XRD patterns of LaNiO3 and Ni/La2O3 obtained by performing reduction at 700 ºC
(right). (a) LN-NCR, (b) LN-NC, (c) LN-CR and (d) LN-C...114
Figure 6.2 TEM images of LN-C (a,b), LN-CR (c,d), LN-NC (e,f) and LN-NCR
(g,h)...115
Figure 6.3 N2 physisorption isotherms of as synthesized and reduced forms of nanocast
and bulk LaNiO3 perovskites (left). Pore size distributions calculated from the adsorption
branch using NLDFT method for as synthesized and reduced nanocast LaNiO3 are given
on the right...116
Figure 6.4 TPR-H2 profiles of nanocast and bulk LaNiO3 perovskites (left). Ni (3p) XPS
spectra of nanocast LaNiO3 and Ni/La2O3 catalysts obtained from nanocast LaNiO3 are
given on the right……….………117
Figure 6.5 Temperature dependent conversion profiles of CH4 and CO2 over nanocast and
bulk LaNiO3 perovskites (left) and Ni/La2O3 catalysts obtained from nanocast and bulk
LaNiO3 perovskites are given on the right (GHSV = 2.1 x 105 h-1)...120
Figure 6.6 Temperature dependent variation of experimental product ratios obtained for
methane dry reforming over nanocast and bulk LaNiO3 perovskites (inset) (GHSV = 2.1 x
105 h-1)...120
Figure 6.7 CH4 and CO2 conversions as a function of time on stream at 700 ºC over
Ni/La2O3 catalysts derived from nanocast and bulk LaNiO3 (GHSV = 2.1 x 105 h-1).
Variation of experimental product ratios under the same conditions are shown on the right………..122
Figure 6.8 Thermogravimetric analysis and Raman spectra (inset) of the catalysts after
stability tests for 48 hours (left). N2 physisorption isotherms and wide angle XRD patterns
(inset) of Ni/La2O3 cataysts obtained from nanocast and bulk LaNiO3 after 48 hours on
stream at 700 ºC (right). (* represents La silicates and ● represents La(OH)
XVII
ABBREVIATIONS
BET – Brunauer Emmett Teller
CMC – Critical Miscelle Concentration CMT – Critical Miscelle Tempearture CMK – Carbon Mesostructures from Korea KIT-6 – Korea Institute of Technology No.6 MCM-41 – Mobil Composition of Matter No. 41 NLDFT – Non-Local Density Functional Theory PEO – Poly Ethylene Oxide
PPO – Poly Propylene Oxide SBA-15 – Santa Barbara No. 15 SEM – Scanning Electron Microscopy TEM – Transmission Electron Microscopy TPR – Temperature Programmed Reduction TPD – Temperature Programmed Desorption XPS – X-ray Photoelectron Spectroscopy XRD – X-Ray Diffraction
XIX
ACKNOWLEDGEMENTS
First of all I would like to express my gratitude towards my supervisor Prof.
Freddy Kleitz for giving me the opportunity to work in his research group. Not least,
I would like to express my gratefulness towards my co-supervisor Prof. Serge
Kaliaguine for giving me the occasion to explore the exciting theme of catalysis. I
am deeply grateful towards both my supervisors for all the advices, motivation and guidance I received during the entire period of my research. I am indebted to them for the freedom I received, to explore various aspects of material science, apart from my dissertation topic.
I would like to thank Mr. Gilles Lemay, Dr. Bendaoud Nohair and Dr. Zahra
Sarshar for the assistance I received from them on various laboratory aspects in
Chemical Engineering, especially for the training to handle various experimental setups.
Further I would like to express my gratefulness to Dr, Pascale Chevallier,
Mr. Jean Frenette, Mr. Richard Janvier, Mr. Alain Adnot and Mr. André Ferland for
their assistance in various characterization techniques such as XPS, XRD and electron microscopy.
Also I would like to gratefully acknowledge Dr. Francois Berubé, Dr. Benoit
Levasseur and Mr. Remy Guillet-Nicolas for their kind friendship, stimulating
discussions and laboratory training during the initial period of my stay in Québec. I wish to thank all my colleagues belonging to Kleitz group and Kaliaguine
group for all the help that I received.
Finally, I express my sincere gratitude to all the Professors, research professionals, technicians, administrative staff and all other members of the Department of Chemistry and Department of Chemical Engineering, for all the valuable help that I received, during the entire period of my dissertation in Université Laval.
I wish to thank my family and friends for their valuable support, understanding and patience.
XXI
PREFACE
For the present time the major issues and challenges that we face are the ones concerning environment and energy. Most of our energy demands are currently dealt with using fossil fuels. On one hand the progressive depletion of the fossil fuel reserves puts forward the need for alternative renewable and sustainable energy resources. On the other hand the harmful effects produced from the expulsion of green house gases and volatile organic compounds inherent with the burning of hydrocarbons contained in the fossil fuels is taking place on a large scale. Technologies that can tackle this pollution resulting from the industrial and automobile emissions are highly demanding and the need of the hour. The development of such technologies depends on high throughput catalytic conversions. Since catalysts can accelerate the degradation or transformation reactions, a wide range of studies were performed in the past regarding the synthesis of durable, cost effective and efficient catalytic systems for the development of sustainable chemical processes. The objective of this doctoral thesis will be to develop novel catalysts for various end-of-the-stream processes with an environmental perspective as well as synthetic chemical reactions leading to important feedstock which can be further processed to value added chemicals. Perovskite-structured mixed metal oxides are well known cost effective and efficient alternatives to noble metals for various catalytic processes, resulting from their high temperature stability and interesting physical and surface properties. As of now, most of the existing synthetic methods for this class of materials rely on high temperature treatments for the formation of the crystalline phase. These high temperature exposures lead to particle sintering, resulting in materials with lower values of specific surface area. Since specific surface area is one of the most important parameters influencing the efficiency of a catalyst, it is challenging to develop novel strategies to synthesize perovskite oxides with enhanced values of specific surface area. This issue was taken up and in the present project the first goal was to obtain mesoporous perovskite structured mixed metal oxides with high specific surface area.
XXII
Initial investigations regarding the catalytic efficiency of these materials were performed for the removal of volatile organic compounds. In this study, methanol, a comparatively simple molecule was used as the model compound. Efficiency of these mesoporous perovskites as catalysts will be discussed for the total oxidation of methanol along with the deeper insights obtained from the kinetic data processing. Correlation of the observed catalytic activity was made with respect to the specific surface area and amount of oxygen species. Comparisons were performed by varying the B site metallic cation in the perovskite structure (Mn, Fe and Co) as well as with similar bulk compositions synthesized using the conventional citrate process and reactive grinding method. Further a more industrially relevant reaction designated as dry reforming (also called CO2
reforming) of methane was chosen for the second stage. This reaction involves the conversion of two green house gases (CH4 and CO2) into syngas (CO + H2), which
is a valuable feedstock that can be further processed into various value added chemicals. Ni based perovskite compositions were chosen for this study. In this case mesoporous perovskite is presented more as a catalyst precursor than as a catalyst itself. Also, comparisons were performed with the bulk counterpart synthesized by the conventional citrate process regarding reactant gas conversion and long term stability.
Briefly, besides the present introductory chapter, this thesis includes the following ones:
Chapter 1 gives an introduction about perovskite structured mixed metal oxides,
an overview of the nanocasting strategy which is the synthetic protocol used in this study and a short review of the two catalytic reactions performed, i.e., total oxidation of methanol and dry reforming of methane.
Chapter 2 discusses the specific experimental methods employed for the
synthesis of mesoporous perovskites by nanocasting followed by a presentation of the characterization methods used in this study. Also details of the specific experimental set up regarding the catalytic reactions used for this study are depicted.
XXIII
Chapter 3 gives the summary of initial results obtained for total oxidation of
methanol over nanocast mesoporous perovskites synthesized using ordered mesoporous silica KIT-6 as hard template, along with the results obtained from kinetic data processing
Chapter 4 describes the effects of variation of the template (SBA-15) parameters
on the nanocast perovskites with emphasis on catalytic and kinetic results
Chapter 5 gives a brief account of the surface characterization of mesoporous
perovskite compositions synthesized by nanocasting.
Chapter 6 illustrates the utility of the Ni/La2O3 catalyst obtained from mesoporous
LaNiO3 perovskite synthesized using ordered mesoporous silica SBA-15 as hard
template, as catalyst precursor for dry reforming of methane.
Chapter 7 summarizes the general conclusions obtained followed by some
1
Chapter 1– Introduction
1.1 Perovskites
1.1.1 Structure and properties
Perovskites are mixed metallic compounds represented by the general formula ABX3, where A and B represent a large cation and a medium sized cation
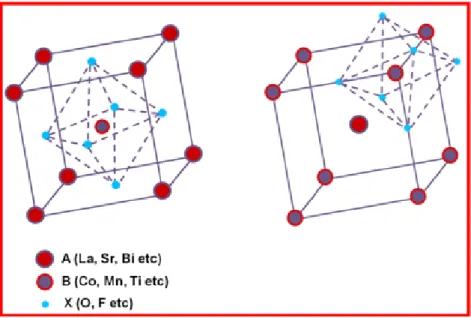
respectively whereas X represents an anion.1 Based on the anion in the structure, the final compound could be an oxide, a sulphide, a hydride or a halide (mostly fluoride). Anions can also be a mixture of elements; oxynitrides being the most well known examples. Figure 1.1 depicts the structure of an ideal ABX3 perovskite.
Figure 1.1 Two different representations of the ideal perovskite structure with BX6 octahedron forming the
centre of the cube (left) and BX6 octahedron occupying the corners of the cube with A site atom at the centre of
the cube (right).
The perovskite structure can be viewed as the BX6 octahedron forming the
centre of a cube with the larger A atoms occupying the corners or as a cube consisting of corner sharing BX6 octahedron with A atoms incorporated at the
2
an ideal perovskite structure, the following relation between ionic radii holdswhere rN is the ionic radii of atoms.2
rA+rX = √2 (rB+rX) (1.1)
Ideal structure, however, was found to be retained in very few cases. Orthorhombic and rhombohedral distortions are commonly found along with less common distortions – tetragonal, monoclinic and triclinic. The measure of deviation from the ideal structure can be represented by the “Tolerance factor” as introduced by Goldschmidt.3 For an ideal perovskite structure, the „t‟ value is found to be unity. However those with lower t values (0.75 < t ≤ 1) are found to exist.4
t = (rA+ rX) / √2 (rB+rX) (1.2)
Oxides form the most common and interesting compounds with perovskite structure. Almost all the metallic natural elements in the periodic table are found to be stable in the perovskite structure. The wide range of properties shown by perovskite type oxides finds applications in catalysis, magnetism, solid oxide fuel cells, superconductivity, etc. Proper combination or partial substitution of the A and/or B site atoms introduces abnormal valences or lattice defects which in turn gives rise to modifications in their chemical properties. The ready availability of a family of isomorphic solids with controllable physical properties makes them attractive for basic research in catalysis.5
Apart from the requirements imposed by the tolerance factor, electroneutrality is another important property that needs to be maintained for a perovskite structure. As a rule, the total cationic charge must be balanced by the total anionic charge. However cationic or anionic nonstoichiometry is often encountered in this class of materials leading to the formation of lattice defects in the structure. Oxygen excess nonstoichiometry in perovskite oxides is not as common as oxygen deficiency because of thermodynamic limitations. The best-characterized perovskite showing oxidative nonstoichiometry is LaMnO3+λ.6,7
Neutron diffraction studies revealed that oxygen excess in LaMnO3+λ could be
3
(as La2O3) or by the presence of active metal in an unusual oxidation state (Mn4+
for example).8 On the other hand an example for oxygen deficient perovskite is the brownmillerite structured LaNiO2.5. In this case, octahedral layers alternate with
one square planar layer to achieve oxygen vacancy ordering.9 Also, tetragonal, orthorhombic and monoclinic forms of LaCuO3-λ can be obtained by performing
calcinations under inert atmosphere.10 Small size and large charge of the B site cations induces thermodynamic limitations for the formation of B site vacancies. However since BO3 array forms a stable network, the structure can tolerate the
absence a minor percentage of A site cations. 1.1.2 Synthesis and catalytic properties
Various strategies were developed for the synthesis of perovskite-structured oxides. Out of these the choice of a particular method depends on the type of application expected. Generally, perovskite oxides for catalytic applications contain a lanthanide ion in the A-site and a transition metal cation in the B-site. For catalytic applications specific surface area and crystal structure play crucial roles. For this reason, the synthesis of these materials for catalytic applications always focused on obtaining crystalline materials with high values of specific surface area. Broadly, the synthesis methods can be classified into the solid state routes and liquid – solid routes (Table 1.1).
1.1.2.1 Solid state routes
The oldest method developed for the synthesis of perovskite structured mixed metal oxides is the ceramic method. In this method, thoroughly mixed precursors (oxides, hydroxides or carbonates) of the metals are calcined at elevated temperature required for the crystallization of the perovskite phase (1000 ⁰C) for many hours. However, the surface areas of thus synthesized perovskites were found to be less than 5 m2g-1.11,12 The high temperature used in solid state
reactions, for perovskite crystallization, results in the sintering of particles which in turn leads to a large grain size and low surface area. Microwave method is another solid state method developed in the last decade by Rao et al. This method involves microwave irradiation of the precursors for a few minutes (10 minutes). Even
4
though nanometer sized crystalline perovskites were obtained by this method; no information regarding the specific surface area was reported by the authors.13 Recently another method, which involves grinding additives developed by Kaliaguine et al. produced perovskite oxides with surface areas >100 m2/g. Here thermal energy required for the crystallization of the perovskite structure is replaced by mechanical energy and thereby limiting the sintering of particles.14,15 High surface area materials were obtained when low temperature was used for the formation of the perovskite phase. High surface concentrations of OH groups were observed in perovskites prepared by the reactive grinding method. This method has the advantage of providing high density of catalytically active sites. However, in this case also the values of specific surface area are found to decrease at higher calcination temperatures.
Table 1.1 General methods used for the synthesis of perovskite structured mixed metal oxides. SBET is the
specific surface area determined by the BET method.
Method Composition SBET (m2g-1)
Ceramic11, 12 LaAMnO 3 (A = Na, K, Rb) < 3 Microwave13 LaBO 3 (B = Cr, Co, Ni) - Reactive grinding14,15 LaCoFeO3 10 – 105 Coprecipitation16 LaMnO 3 15 Complexation17,18 LaCoO 3 20 Dispersion on a support20 LaCoO3/MCM-41 340
5
1.1.2.2 Liquid – solid routes
Since high surface area is a requirement for perovskite oxides synthesized for catalytic applications, various low temperature synthesis methods were developed. Among these, co-precipitation16 is a simple method for preparing agglomeration free powders with an average particle size of 80 nm using hydroxides as precursors. Complexation and precipitation is another commonly used method developed to synthesize perovskites with enhanced surface area. Citric acid is the most common complexing agent used to obtain a highly homogeneous precursor which on calcination at comparatively low temperature leads to crystalline perovskite oxides.17,18 Freeze drying and spray drying are other common liquid – solid routes followed to synthesize perovskites.19 However in all these cases the specific surface areas of the final products were found to be less than 25 m2g-1. Attempts were also made to disperse the perovskites on a support matrix having high specific surface area. For this a high surface area support is chosen (most commonly silica or alumina) and impregnated with the precursor solution. Here, the sintering of the particles during high temperature calcination process is restricted by the support. Kaliaguine et al. reported the synthesis of LaCoO3 perovskites supported on mesoporous silica MCM-41.20 The sample with
47.5% LaCoO3 possessed a BET surface area of 340 m2g-1. The supported
catalysts showed higher catalytic activity and resistance to SO2 poisoning in the
complete methane oxidation compared to bulk LaCoO3 catalysts. However mass
transfer limitations were observed. Another concern that needs to be taken care of while using a support is the compatibility of the support with the desired phase. 1.2 Nanocasting
1.2.1 Principles of nanocasting
Nanocasting21 is a procedure in which a mold with relevant structures in the nanometer scale is filled with another material and the mold is afterwards removed. In this technique the structure and properties of the templates play a crucial role. Relatively precise negative replica of the template is created. An advantage of using the hard template is the fact that the synthesis is relatively easy to control,
6
since the template structures are fixed. Inorganic porous solids such as ordered mesoporous silica or carbon are mostly used as nanoscale hard templates. The pore structure of these parent materials is transferred into the solid structure of the generated porous materials while the walls of the parent become voids. One of the most versatile materials to be used as hard templates is ordered mesoporous silica which can be prepared in diverse pore structures and particle morphologies. Since a three dimensional structure is necessary in the mold to maintain a stable replica, only silicates having interconnected pores are successful, leading to nanowire arrays or 3-D frameworks with tunable mesostructures.
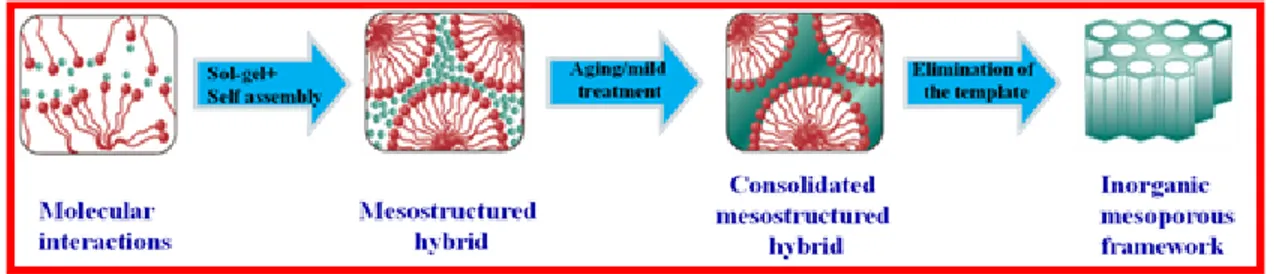
Figure 1.2 Schematic representation of the steps involved in the mesostructure evolution in the nanocasting
technique using ordered mesoporous silica as hard template.
Choice of the precursor is of particular importance in nanocasting. A precursor which does not chemically react with the template needs to be selected. The conversion of the precursors into a material of desired composition inside the pores should be simple and with as little volume shrinkage as possible. In order to facilitate easy diffusion into the mesopores, the precursor needs to be in the gaseous or liquid form under ambient conditions. The degree of precursor loading also affects a faithful replication. The interaction between the pore surface and the precursor generally includes weak interactions like hydrogen bonding, coulombic or van der Waals interactions.22 Higher pore filling can be achieved by enhancing the
hydrophilicity of the surface. The presence of an OH group in the precursor can be necessary, since hydrogen bonds can be easily generated with the silanol groups of the templates. Also it needs to be noted that a very strong interaction between
7
the precursor and the template results in deposition and accumulation of the precursors near the pore entrances resulting in pore blocking. Much attention should be paid for selecting the solvent. For silica pore surface with free silanol groups present, a polar solvent is suitable. This will enhance the diffusion of the precursor through the pores. The need for a polar solvent is inevitable since the solubility of the metal salts is high in polar solvents. Ethanol serves as one of the preferred solvent in the nanocasting process because of its low boiling point and high solubility of most of the inorganic precursors. Ethanol also has the amphiphilic property compatible to the silica pore wall surface, which enhances the capillary force. For the replica to be homogeneous, decomposition products should leave the samples preferentially via gas phase.
Four different methods of impregnation have been used for the synthesis of ordered mesoporous non siliceous materials.
1. Wet impregnation - In this method, the template is dispersed as a powder in a dilute solution (water or ethanol) solvent. The precursor species dissolved in the solvent is added with stirring and will get diffused into the pores where they are adsorbed to the pore walls. This often results in a limited loading of the precursor solution inside the pores and hence requires several impregnation cycles. After subsequent removal of the solvent, the formation of the desired phase takes place at high temperature.23
2. Incipient wetness - A saturated solution of the precursor of the same volume as the pore volume of the template, is used for impregnation. The solution is drawn into the pores by capillary forces. Since the amount of the precursor is determined by the pore volume of the template, no precursor material is expected to be deposited on the outer surface. Incipient wetness technique usually leads to higher loadings than wet impregnation24.
3. Dual-solvent - Here two different solvents are to be used to disperse the silica mold and to dissolve the metal precursors. Generally, the silica template is suspended in a non polar solvent and an aqueous solution of the metal precursor with respect to the pore volume of the template has to be
8
slowly added. The precursor is expected to move into the pores during stirring. This method helped to improve the filling efficiency of the porous channels and homogeneity of the products. Anne Davidson and coworkers utilized this method for the nanocasted synthesis of Co3O4 and β-MnO2
using water and dry hexane as solvents.25,26
4. Solid-liquid - In this method, a mixture of the template and the precursor in the solid phase, without using any solvents, is subjected to heat treatment. The essential condition for this method is that the melting point of the precursor should be lower than its decomposition temperature. On reaching the melting point, the precursor gets converted to the liquid phase and enters the pores through capillary action. As the temperature is raised further, the precursor will start decomposing to reach the final state. Yue and Zhou synthesized porous single crystals of Co3O4, NiO, CeO2, and
Cr2O3 using this method.27
In order to obtain the final replica, the template needs to be removed without affecting the cast. In the case of mesoporous silica templates, leaching with different agents (HF or NaOH) depending on the stability of the final product can be used. Using HF will facilitate the complete removal of silica. However because of the severe hazardous nature of HF, NaOH is preferred for those situations where both these leaching agents can be applied. However a small amount of residual silicon is sometimes observed when NaOH is used as the leaching agent. When carbon is used as the hard template, simple heat treatment under an oxidizing atmosphere is used to remove the template.
1.2.2 Ordered mesoporous silica templates
Considerable milestones were achieved regarding the synthesis, characterization and textural parameter control of ordered mesoporous silica (pore size: 2 – 50 nm according to IUPAC28) during the past two decades, starting from the initial discovery of MCM-41 in early nineties.29 The syntheses of these materials rely on the formation of miscellar aggregates of molecular surfactants or block copolymers. This self assembly process is followed by the deposition of
9
inorganic phase where a cooperative self assembly takes place between the template and the inorganic precursor. Finally, selective removal of the template results in the formation of ordered mesoporous silica materials.30
A wide variety of surfactants including ionic (cationic and anionic), neutral and non-ionic block polymers can be used. Non-ionic surfactants which are available in a wide variety of chemical structures will be a suitable choice owing to their biodegradability, low price and non toxicity. Also, it is much easier to obtain ordered mesoporous silica with interconnected pore systems when non-ionic surfactants such as triblock copolymers are used as soft templates. Generally, the synthesis of mesoporous silica under non-ionic surfactants takes place under acidic conditions. The mesophase formation can be controlled by varying the hydrophilic/hydrophobic ratio of the structure directing agent, solution pH and temperature. Moreover, the use of non- ionic surfactants results in improved hydrothermal stability and enables more straightforward tailoring of the pore parameters. A silica source is added to a dilute micellar solution and polymerization of the silica is induced by an acid. The van der Waals or hydrogen bonding interactions between the polymerizing silica and the amphiphile result in the precipitation of silica-amphiphile mesophase composite. Porous structure results when the structure directing agent is removed by calcination, solvent extraction or microwave treatment.31 Calcination is the most common method in which the material is treated at high temperature (generally 550 ºC) to burn away the organic species.
Figure 1.3 A simplistic mechanistic pathway showing the self assembly of formation of mesoporous materials
As a result of tremendous research efforts, a large variety of ordered structures were developed by the soft templating method through the non-covalent
10
intermolecular interactions, including 2D hexagonal32,33 (p6mm), 3D cubic34-37 (Im3m, Ia3d, Fm3m), etc (Figure 1.4). Another advantage of this method is that the resulting periodic structure is restricted to the nanometer scale. The pore structure, pore width and wall thickness can be fine tuned by varying one or more of the synthesis parameters. Varying the concentration of the templating agent or changing the composition of the copolymers are found successful to fine tune the pore size and wall thickness of the material.32,38
Changes in the molecular geometry and the polymer chain length also allow the fine tuning of the pore size. Pore size control of mesoporous materials can be achieved by varying the time and temperature of the synthesis and hydrothermal treatment. An increase in the synthesis temperature leads to an increase in hydrophobicity of the micelle core volume which leads to an increase in pore size and decrease in wall thickness.39 An increase in aging temperature also leads to
an increase in pore diameter and pore volume and modifies the intra-wall porosity.
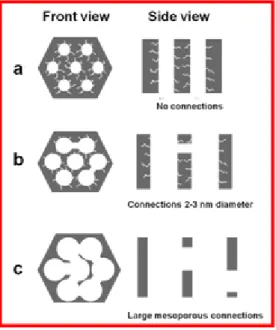
Figure 1.4 Pore models of mesostructures with symmetries (A) p6mm, (B) Ia3d, (C) Pm3n, (D) Im3n, (E) Fd3m, (F) Fm3m32
A longer period of hydrothermal treatment also induces a similar effect.40 The ratio between the silica source and surfactant can be varied to change the degree of pore connectivity. Increasing the silica content in the reaction mixture has been found to decrease the pore size, pore volume and surface area. The
11
variation in network connectivity with respect to the change in silica/surfactant ratio has been evidenced by imaging platinum replicas of SBA-15, with TEM.33 The thickening of the pore walls of SBA-15 and KIT-6 has been observed as a result of increasing the silica/surfactant ratio.41
Addition of acid or base during the synthesis leads to the modification of the pH of the solution. Since the degree of condensation and polymerization of the inorganic species is pH dependent, the modification of the pore dimensions is obvious.42 Salt addition also affects the properties of the resulting mesoporous solids. Both Critical Micelle Concentration (CMC) and Critical Micelle Temperature (CMT) of the block polymer can be decreased on salt addition.30 The solubilization of hydrophobic additives inside the core of the micellar assembly can be employed to increase the pore size. Mesitylene (trimethylbenzene) is the most widely used additive. Variations of cohesive properties of the solvent by performing the synthesis in mixed solvents can also be used to fine-tune the pore size. The addition of butanol as cosurfactant coupled with a low HCl concentration leads to the formation of cubic Ia3d KIT-6 mesophase.34
Figure 1.5 The effects of the hydrothermal aging temperature on the pore structure of SBA-15-type materials:
(a) low temperature aging (35-60 ºC), main mesopores 5-6 nm, wall thickness 4 nm, micropore volume = 0.2 mL/g; (b) aging at 80-100 °C, main mesopores 7-9 nm, wall thickness 3.2 nm, micropore volume = 0.1 mL/g; (c) high temperature aging (>120 °C), main mesopores >9 nm, wall thickness 2 nm, no micropores.36
12
As mentioned above, calcination is the most common method used to remove the organic surfactant templates. As-synthesized materials are heated under oxygen or air at a temperature above 550 ⁰C to decompose the organic surfactants. However the condensation of surface silanol groups that occur during calcination results in the lowering of the unit cell parameter and an increase in the surface hydrophobicity of the final material. The framework contraction has direct influence on mesopore size and the complementary intra-wall porosity.43 Extraction is another method to remove the surfactants. This can be achieved by liquid extraction, acid treatment, oxygen plasma treatment or supercritical fluid extraction. The framework-surfactant interactions are weak in SBA-type materials. Such weak interactions are favorable for solvent extractions. The advantages of this method are that the physicochemical properties are not modified and the framework contraction is limited. However solvent extraction does not remove the templating species completely and as a result the intrawall microporosity is not often completely developed.30 Methods were also developed to liberate the main mesopores first and then the intrawall porosity.44 Ether cleavage with sulfuric acid solution removes the accessible polymer located in the large mesopore system, where as the poly ethylene oxide (PEO) groups should remain essentially unaffected. A mild calcination at 200⁰C then allows the removal of the remaining PEO groups in the walls and makes the micropores accessible. This acid treatment prevents any pronounced shrinkage of the mesostructure which in turn affords materials with larger pore dimensions than the regularly calcined SBA-15. More importantly, the strongly acidic medium not only induces a more pronounced condensation of the silica but also permits the generation of a high density of silanol groups on the surface.
1.2.3 Overview nanocasting
One of the major applications of ordered mesoporous silica is their use as hard templates for the synthesis of various other mesostructured compositions. This method designated as nanocasting or hard templating is widely used by research groups around the world for the synthesis of ordered mesoporous non
13
silicious materials as an alternative to the soft templating strategies. This approach enables to overcome limitations of the direct templating processes with soft micellar surfactant aggregates especially in the case of carbons or transition metal-based materials that are very sensitive to thermal treatment conditions and redox reactions. Nanocasting is comparatively easier and predictable since the nanoscale pore structure is fixed.
Knox et al. were the first to synthesize porous carbon via nanocasting method, using silica gel or porous glass as templates.45 However, the templates as well as replicas contained a disordered pore structure. The first successful synthesis of nanocast mesoporous carbon with an ordered pore structure was reported in 1999, by Ryoo et al., where MCM-48 was used as a template. CMK-1 thus obtained was not an exact replica of MCM-48 since its symmetry was somewhat lower.46 This is because the pore architecture of the MCM-48 template
consists of two different channel systems which are not interconnected. After template removal, the carbon sub networks formed inside the disconnected silica channels are joined by displacement with one another. Similarly when MCM-41, consisting of linear mesopores arranged parallel to each other was replicated, bundles of linear rods of the respective product were obtained.
Many studies were carried out to synthesize mesoporous carbons with ordered structures designated as CMK-n series, using different silicas. Generally, the synthetic procedure for nanocast ordered mesoporous carbon involves impregnation of mesoporous silica as a template with carbon precursor. Subsequent polymerization followed by carbonization of the precursor in the pore system results in a carbon-silica composite. Finally, the mesoporous replica can be obtained after removal of the silica template by HF or NaOH leaching. One should keep in mind that carbon precursors that have high carbon yield and do not just decompose during the carbonization step should be selected. Suitable carbon precursors were found to be sucrose, furfuryl alcohol, phenolic resin, mesophase pitch, etc.41,47-49 Sucrose is a convenient precursor, but its polymerization is difficult to control with H2SO4 as catalyst. Also sucrose based carbon materials have
14
systematically smaller lattice parameters and less dense frameworks. Mesophase pitch consisting of well stacked layers of carbon rings is interesting since it results in the formation of graphitized carbon materials. Furfuryl alcohol is attractive because it is a liquid at room temperature and is miscible in many organic solvents. It can be easily polymerized especially in presence of an acid catalyst.
Figure 1.6 TEM image showing hexagonal arrangement of carbon rods in CMK-3 (left) and CMK-5 carbon
tubes templated from SBA-15.21,50
The symmetry of the templates was retained when silica materials with interconnected pore system were used. The hexagonal p6mm symmetry of SBA-15 was preserved in its replica CMK-3 and the cubic Ia3d symmetry of mesoporous silica, KIT-6 was found to be preserved in CMK-8.46 CMK-3 consists of hexagonal arrangement of 1D carbon rods as shown in Figure 1.6. Interestingly, by varying the filling degree of the carbon precursor, the structure of the mesoporous carbon replica can easily be varied. If the pore system of the Al-containing SBA-15 is only coated by the carbon precursor, rather than being completely filled, a surface-templated mesoporous carbon, named CMK-5, with an array of hollow carbon tubes can be obtained.50 A TEM image and structure model of CMK-5 is presented in Figure 1.6. Two different types of pores are observed in CMK-5, one generated in the inner part of the channels which are not filled with carbon precursor and the other one is obtained from where the silica walls of the SBA-15 template had previously been. For this reason CMK-5 shows extremely large surface areas and large pore volumes, which gives this material great potential in adsorption and as a catalyst support.51
The successful synthesis of a large variety of ordered mesoporous carbon encouraged the extension of this method for the synthesis of other non siliceous
15
compositions. In particular, a broad range of scientific endeavor reflects a growing interest in ordered mesoporous metal oxides. As in the case of carbon the choice of a hard template is essential for nanocast mesoporous oxides. So far KIT-6 and SBA-15 mesoporous silica are frequently used as the templates and metal salts are used as the precursors. There are few reports on nanocast mesoporous metal oxides applying cage-type cubic mesoporous silica SBA-16. The use of a single metal precursor will result in the formation of a single metal oxide where as the use of different metal salts as precursors in the correct stoichiometry can result in the formation of high surface area mixed metal oxides such as spinels or perovskites. A major factor which determines the resulting structure is the filling degree of the voids and template removal conditions. Materials with higher surface areas are obtained for sufficient loadings of the precursors.
Crystalline mesoporous CeO2 with high thermal stability was synthesized
using SBA-15 or KIT-6 as the hard templates and inorganic salt CeCl3.7H2O as the
precursor. The products have very narrow pore-size distribution, large surface area and pore volume.52 Zhao et al. reported the synthesis of nanoarrays of In2O3,
Cr2O3, Fe2O3, Co3O4, CeO2 and NiO using microwave digested SBA-15, SBA-16
and FDU-1 silica materials as hard templates.53 All oxides synthesized at a temperature of 650⁰C, exhibited long range order except Cr2O3 and NiO. Very high
surface areas up to 137 m2g-1, determined using the BET method and pore
volumes up to 0.4 cm3g-1 were obtained. Wang et al. reported the synthesis of Co3O4 using vinyl functionalized KIT-6 as hard template with BET surface area 122
m2g-1 and pore volume 0.2 cm3g-1.54
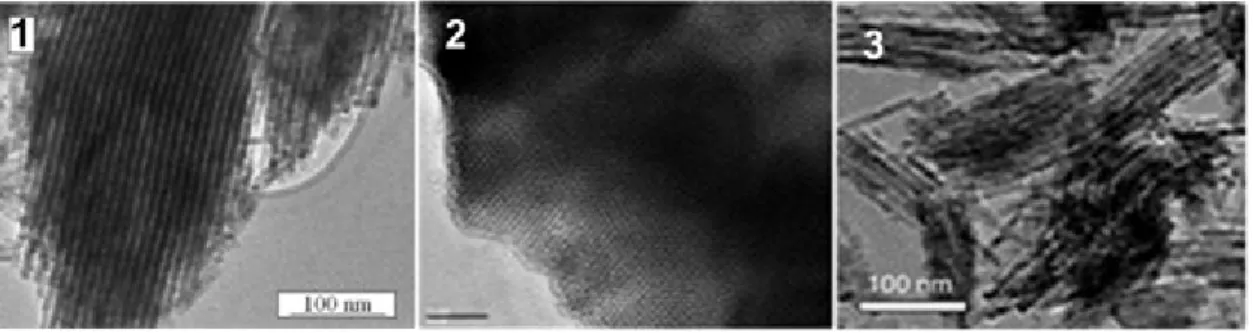
Figure 1.7 TEM images of (1) LiCoO256 (2) MgO64 and (3) Co3O458 synthesized using the hard templating
16
The influence of the network interconnectivities of the parent SBA-15 and KIT-6 silica materials and the loading of the precursors for the tuning of the nanocast materials were studied by Schuth and coworkers.55 One of the limitations of the nanocasting method is that the precursor should be selected such that it should not react with the silica template, a problem which renders it difficult to synthesize compounds containing alkali metals. Jiao et al. has reported that post synthesis reactions can be applied to incorporate alkali metals while retaining the nanostructured morphology.56 Mesostructured Co3O4 has been synthesized using
SBA-15 and KIT-6 as hard templates. After template removal, Co3O4 was treated
with LiOH to obtain mesostructured LiCoO2 (Figure 1.7). The BET surface areas of
the samples were found to be 70 and 92 m2g-1 for the samples templated from
SBA-15 and KIT-6 respectively. It was also shown that by varying the calcination temperature during the synthesis of hard templates, the pore sizes and wall thickness of the mesoporous oxides can be tuned.
Regarding the synthesis of mixed metal oxides using multiple precursors, various compositions with spinel and perovskite structure are reported. Cabo et al. synthesized NiCo2O4 spinels using ordered mesoporous silica SBA-15 and KIT-6
as hard templates.57 Hoang et al. reported the synthesis of NiFe
2O4 and CuFe2O4
spinels by the method of nanocasting using ordered mesoporous silica KIT-6 and SBA-15 as hard templates.58 These authors also reported the morphology controlled synthesis of spherical mesostructures using MCM-48 nanospheres as hard templates. The first synthesis of mesoporous perovskites were reported by Schwickardi et al.59 These authors reported the synthesis of LaFeO3 with
disordered pore structure using carbon as a hard template. More recently ordered mesostructures of LaCoO3 perovskites were reported by Wang et al.60 using vinyl
functionalized KIT-6 as hard template.
Nanocast materials are found to be highly efficient for a large number of catalytic applications, either as catalysts or as supports. Regarding catalytic oxidation reactions, CO oxidation is the most studied one using nanocast catalysts. Shen et al. synthesized mesoporous CeO2 using ordered mesoporous KIT-6 as
17
hard template. These materials with a specific surface area 112 m2g-1 showed excellent CO conversion efficiency in comparison with conventional CeO2
catalysts. These authors also showed that by loading 20 % CuO on these nanocast CeO2 replicas, T50 (the temperature required to attain 50 % conversion) for the
reaction was decreased to 115 ºC.61 Among other ordered mesoporous oxides studied for CO oxidation, Co3O4, β-MnO2 and NiO showed considerable extent of
conversion below 0 ºC.62 Zhu et al. used SBA-15 as hard template to synthesize mesoporous CuCo2O4, MnCo2O4 and NiCo2O4. In their study it was observed that
CuCo2O4 and MnCo2O4 exhibit high activities and good stability in CO oxidation.63
Tuysuz et al. synthesized mesoporous Co3O4, with highly ordered pore structure
and enhanced specific surface area using ordered mesoporous KIT-6 silica as hard
template. 100 % conversion of CO to CO2 at a space velocity of 18 000 mL gcat-1 s
-1, was observed around room temperature. The activity was found to be dependent
on the textural properties of the catalysts in which the catalyst with the highest specific surface area and the most open pore system was found to be the best.
However about 50 % of activity loss was observed over 4 h during the reaction.64
Roggenbuck et al. synthesized mesoporous CeO2 using CMK-3 carbon as a
hard template. This material with a specific surface area of 148 m2g-1 when used as a catalyst for methanol decomposition showed substantially higher performance than that of a commercial CeO2 with a low surface area of 6 m2g-1.65 Wang et al.
synthesized a series of mesoporous metal oxides by using vinyl-functionalized
KIT-6 as a hard template and demonstrated that mesoporous Cr2O3 (surface area 113
m2g–1) exhibits higher activity than commercial Cr2O3 (10 m2g–1) in toluene
combustion.66 Xia et al. demonstrated the application of mesoporous Cr2O3 in the
combustion of toluene, ethyl acetate, formaldehyde, acetone, and methanol,
mesoporous α- Fe2O3 in the oxidation of acetone and methanol and mesoporous
Co3O4 in the oxidation of toluene and methanol.67 Also, LaCoO3 perovskites
synthesized using vinyl functionalized KIT-6 as hard template was found to show extremely high value of specific surface area (96 m2g-1) and excellent activity for the total oxidation of methane in comparison with the bulk catalyst synthesized using the citrate process.60 Nanocast mesoporous oxides were found to be efficient