Publisher’s version / Version de l'éditeur:
ECS Transactions, 33, 1, pp. 823-838, 2010-10-10
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1149/1.3484576
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Investigation of advanced hybrid PEM based on sulfonyl fluoride PFSA and grafted inorganic nanoparticles
Mokrini, A.; Siu, A.; Robitaille, L.; Gonzalez, L.; Sanchez, F.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=004c75df-b4e9-4e52-9bc0-0c4842977442 https://publications-cnrc.canada.ca/fra/voir/objet/?id=004c75df-b4e9-4e52-9bc0-0c4842977442
Investigation of Advanced Hybrid PEM Based on Sulfonyl Fluoride PFSA and Grafted Inorganic Nanoparticles
A. Mokrini*a, A. Siua, L. Robitaillea L. Gonzalezb, F. Sanchezb a
Industrial Materials Institute – National Research Council of Canada 75, De Mortagne, Boucherville (Qc) J4B6Y4 Canada
b
Instituto de Química Orgánica General - Consejo Superior de Investigación Científica C/ Juan de la Cierva, 3, 28006 Madrid (Spain)
Nano-composite polymer electrolyte membranes were prepared by melt processing of sulfonyl fluoride form of perfluorosulfonic acid (PFSA) ionomer and two families of inorganic nanofillers, spherical nanoparticles of silicon dioxide prepared by sol-gel, and clay sepiolite ungrafted and grafted with alkyl-sulfonic acid moieties. This approach produces hybrid electrolyte membranes with substantially better properties when compared to reference (neat polymer) and commercial Nafion. The melt-extruded nano-composite electrolytes showed very efficient dispersion of the inorganic fillers, an overall improvement in thermal and dimensional stability, and improved conductivities between 10-2 to 10-3 S.cm-1 at 80°C over the entire humidity range from 100 to 30%.
Introduction
Perfluorosulfonic acid resins (PFSA) are today’s benchmark materials for proton exchange membranes (PEMs) for fuel cells. These materials exhibit outstanding stability and a proton conductivity of 0.1 S/cm at 80°C when fully humidified. However, their proton conductivity is highly dependent on the membrane’s water content. Their conductivity and performance drops dramatically at higher temperatures and/or low humidification. Operating a PEMFC system at low relative humidity would simplify the design and at the same time reduce costs related to an external humidification system.
Organic-inorganic proton exchange membranes have been widely used during the last decade as a means of conferring improved properties. The presence of an inorganic phase is effective in enhancing interaction between components, in limiting dimensional change and in improving conductivity and fuel cell performance under high temperature and/or low relative humidity conditions (1). Organic-inorganic hybrid membranes based on perfluorosulfonic acid polymers (PFSAs) and inorganic natural and synthetic particles are the most studied systems (2-5). The reasons for the improvement in properties and performance are principally due to i) lower gas cross over, ii) higher water retention in the PEM due to the hygroscopic character of the inorganic additive, iii) higher durability due to the increased dimensional stability and lower swelling, and iv) improved electrode kinetics due to the increased water retention in the modified PEM.
While, hybrid membranes are commonly generated by in situ formation of the inorganic material within an ionomer preformed membrane or in a polymer solution (1), melt-extruded PEMs are a new class of membrane materials rarely reported with many desirable properties for fuel cell technologies. It has been demonstrated that extruded PEM show outstanding durability compared to solvent-cast and reinforced membranes (10,000 humidity cycles for melt-extruded Ion Power™ N111-IP, vs, 4,500 cycles for the solvent-cast DuPont™ NR-111, and 6,000-7,000 cycles for the micro-reinforced Gore™ Primea® membrane)(6). The approach presented in this work focuses on the unique combination of functional inorganic fillers with melt-processing methods with the objective to manufacture, advanced nanocomposite PEMs with improved properties for operation at low relative humidity conditions. Furthermore, the use of solvent-free, melt-processing technologies allows a much more uniform dispersion of the inorganic fillers, easier and continuous membrane production, significantly reducing manufacturing time and cost. Two varieties of inorganic fillers have been used in this work; synthetic nanoparticles of silicon dioxide, and sepiolite natural clay grafted with alkyl-sulfonic acid moieties.
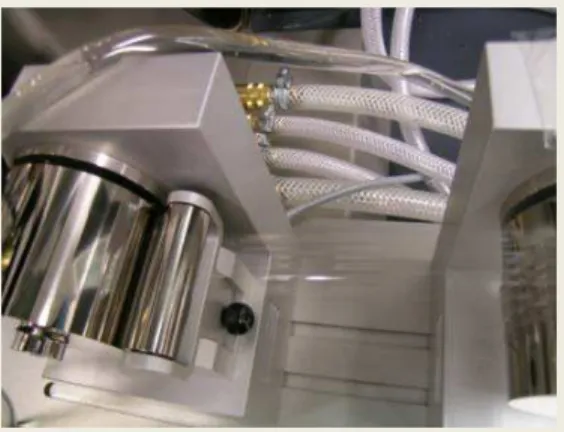
Figure 1. Picture of melt-extruded sulfonyl fluoride based PFSA nanocomposites using a bench-top twin screw micro-extruder equipped with a film line.
Experimental
Fillers preparation
Silica nanoparticles. Spherical silica nanoparticles 60, 80 and 120 nm in diameter were synthesized via the sol-gel method. Briefly, ethanol (100%) and ammonium hydroxide (28%) were stirred together in a closed container for 15 min. MilliQ water was added and the solution thoroughly mixed for another 15 min. Tetraethyl orthosilicate (TEOS) (99.99 % , Aldrich) was added and the reaction mixture was allowed to proceed at room temperature for at least 8 hours under constant stir rate of 300 rpm. The concentrations of reagents used are presented in Table 1. Different particle sizes were obtained by varying the amount of water used in the hydrolysis of TEOS. The workout consists of a series of precipitation with a centrifuge and re-suspension under sonication in fresh solvent (ethanol). Depending on particle size, centrifugation is normally carried at 9000 rpm for 30 to 45 min.
TABLE I. Concentrations of reagents used for the synthesis of mesoporous silica with different particle sizes.
Reagent (vol.%) Nanoparticle size
60 nm 80 nm 120 nm
Ethanol 91.4 90.3 89.6
Ammonium hydroxide 28% 3.4 3.4 3.4
MilliQ water 1.5 2.5 3.2
TEOS 3.8 3.8 3.8
Alkyl grafted sepiolite. Sepiolite is a mineral clay, a complex of magnesium silicate with a typical formula Mg4Si6O15(OH)2·6H2O. The one used for this work is in a fibrous form (Figure 3(a)) with a relatively high surface area of 600-650 m2/g. Sepiolite grafted with heterogenized alkylsulfonic acids through covalent bonds (Si-O-Si) were prepared according to the two following steps:
a. Preparation of a heterogenized alkylmercaptane supported on sepiolite inorganic solid. Sepiolite (10 g) was added to a 100 mL of a 1:1 mixture of aqueous HCl (12N)/ 2-propanol at 65-70ºC and was stirred for 1 hour, then, 3-mercaptopropyltriethoxysilane (MPTES) (3.63g, 1.5 mmol/g of sepiolite) was added after dissolution in 10 mL of 2-propanol. The reaction mixture was stirred at that temperature for 14-16 hours. The solids was filtered under vacuum, thoroughly washed with methanol, methanol/water (1:1) and finally with water. The obtained solid was dried at 90ºC under reduced pressure for at least 24 h prior to analysis.
b. Oxidation of alkylmercaptane groups to alkylsulfonic acids. A suspension of a heterogenized alkylmercaptane on sepiolite was stirred at 60ºC for 14-20h in a mixture of acetonitrile/ hydrogen peroxide (33% v) (9:6 in volume) (15 mL of mixture/ 1 g solid) The resultant solid was filtered, thoroughly washed with acetonitrile, water, 0.1N aqueous perchloric acid, water, ethanol and finally diethyl ether. The obtained solid with alkylsulfonic acids was dried under vacuum at 50ºC prior to characterization.
Fillers Characterization
Scanning electron microscope (SEM) images of the fillers were obtained using a Hitachi S-4700 instrument, on samples previously coated with platinum.
Thermogravimetric analysis (TGA) of mesoporous silica nanoparticles was done on a Setaram TG96 heated at 10oC.min-1 from room temperature to 1000 oC under nitrogen atmosphere. TGA of sepiolite based fillers were measured using a TA Instruments TGAQ500 . The samples were heated to 600ºC at a rate of 10ºC/min in air atmosphere.
Attenuated total reflectance - Fourrier transform infrared (ATR-FTIR) spectra were measured on silica nanoparticles using Nicolet Magna IR 860 between the frequencies of 400 cm-1 and 4000 cm-1.
IEC of sepiolite fillers have been determined by Elemental Analysis (EA) and by titration. For elemental analysis, a Lecco CHNS-932 apparatus was used to determine C, H, N, and S content of the samples. IEC expressed in mmol H+/g of solid was calculated from elemental analysis (EA) on the basis of the percentage of sulfur content (IEC-EA) as an input to the following expression:
3 1000 %S IEC-EA(mmols SO H/g of solid)= 32 100 × × [1]
For titration, 400 mg of sample was stirred in 50 mL of KCl 0.1M for 48 hours, to exchange acid protons with K+ cations of the salt. The solution is then titrated with NaOH 0.0025M, which has been previously standardized by titration with 0.0025 M phthalic acid monopotassium salt, using phenolphthalein as an indicator using following expression: 3 mL NaOH NormNaOH 1000 IEC-T(mmols SO H/g of solid)= 400 × × [2]
Composite PEMs preparation
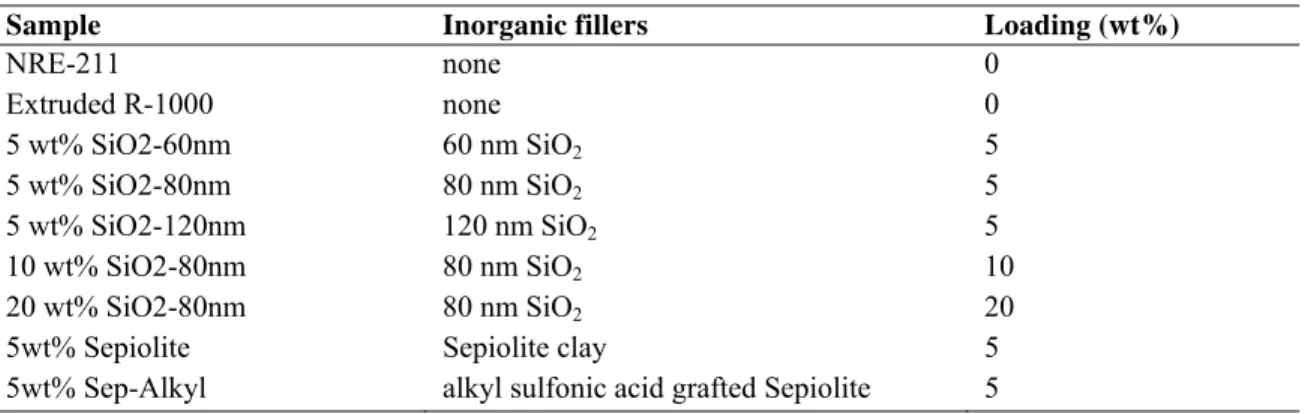
Melt extrusion. Nanocomposite PEMs were prepared using melt-processing technologies, by blending the inorganic nanoparticles and an extrusion grade of Nafion®. Thermolastic perfluorinated precursor resins in the sulfonyl fluoride (-SO2F) form purchased from Ion-Power was used (Nafion R1000, EW=1000 g.eq-1, IEC=1 meq.g-1). Melt processing of nanocomposites was carried out at 240°C, using a 5cc micro-extruder (DSM-Explore) equipped with a film line (Figure 1). The die used for thin film preparation has an opening gap of 0.1mm and a width of 3.5cm. The screws RPM, the calender rolls speed and torque was varied to achieve the required thickness. The strips of composite membranes obtained had a final width of approximately 2.5 cm and a thickness in the range of 25 to 50 microns. Table II lists the compositions of all the samples prepared.
Hydrolysis and activation. The protocol used for conversion of melt-extruded composite membranes from sulfonyl fluoride form to the acid form includes three chemical treatment steps:
1. Hydrolysis reaction where the film is converted to the salt form (K+), this step involves hydrolysis in a solution of KOH/DMSO/DI water. (15% KOH / 35% DMSO / 50% de-ionized (DI) water at 80°C for one hour)
2. Acid conversion process where the hydrolyzed polymer is converted to the acid (H+) form by exchanging the K+ for H+ ions using a solution of nitric acid (HNO3).
TABLE II. Compositions of series of nanocomposite PEMs
Sample Inorganic fillers Loading (wt%)
NRE-211 none 0 Extruded R-1000 none 0 5 wt% SiO2-60nm 60 nm SiO2 5 5 wt% SiO2-80nm 80 nm SiO2 5 5 wt% SiO2-120nm 120 nm SiO2 5 10 wt% SiO2-80nm 80 nm SiO2 10 20 wt% SiO2-80nm 80 nm SiO2 20
5wt% Sepiolite Sepiolite clay 5
3. Activation by alternating treatments with solutions of H2O2 and H2SO4, and boiling in water.
Hybrid membranes characterization
Transmission electron microscopy (TEM) was used to examine the morphology of nanocomposite membranes. The samples were prepared by placing the thin films into epoxy resin. The cured epoxies containing the membranes were then microtomed at room temperature into thin slices of 50-80 nm using a diamond knife. TEM of ultrathin sections of the polymer/silica hybrid samples were obtained with a Philips CM 200 instrument with an acceleration voltage of 200 kV.
Water uptake (WU) was determined by equilibrating the membranes in deionised water at room temperature after the activation treatment. They were then removed from the water container, quickly dry wiped and immediately weighed. Subsequently, they were dried overnight under vacuum at 80°C temperature and weighed again. The water content was calculated from the weight difference of the membrane (in H+ form) in its hydrated and dry state. Three measurements were carried out for each formulation.
Wet mass-Dry mass
Water Uptake (%)= ×100
Dry mass
[3]
Volume change or dimensional swelling was determined by measuring the thickness, width and length of wet and dried membranes (in H+ form). Dimensions of dried membranes were determined by drying them in a vacuum oven at 80°C overnight. Four measurements were carried out for each formulation.
Volume of wet membrane-Volume of dry membrane
Volume Change (%)= ×100
Volume of dry membrane [4]
Conductivity at reduced humidity: A Bekktech sample punch was used to cut a piece from the membranes for testing. The dimensions of the samples to be tested were approximately 5mm x 25 mm. In-plane proton conductivities were measured using a Solartron Impedance Analyser 1260 and a Bekktech in-plane conductivity cell with platinum electrodes. A strip of membrane (in H+ form) was set between 2 platinum (Pt) electrodes and an alternating current was passed through the plane of the sample. The cell was placed in an ESPEC-SH261 environmental chamber with controlled humidity and temperature. Nyquist plots between 5 MHz to 10 Hz were collected and membrane resistances were extrapolated by fitting the semi-circle part of the data to Randles equivalent circuit. Proton conductivities were calculated from the equation:
L σ=
R×A [5]
where σ is proton conductivity, L is the distance between the Pt electrodes, R is membrane resistance and A is the cross-sectional area of the strip. The samples were allowed to equilibrate in the chamber with monitoring. The membranes took 30min to 1 hour to equilibrate.
For thickness measurements, a high precision Mitutoyo Gauge which applies a constant pressure of 3.5 Newtons to the sample using a non-rotating stem was used. Rated accuracy is ±3 microns.
Thermal stability of nanocomposite membranes was studied on a TA Instrumets TGAQ500 Thermogravimetric Analyzer. The samples were heated to 950ºC at a rate of 10ºC.min-1 in air atmosphere.
Differential Scaning Calorimetry (DSC) technique was used to study the state of water in the nanocomposite membranes was studied using a TA Instruments Thermal Analyzer. A wet sample was hermetically sealed in a pan, rapidly cooled inside the DSC chamber at ‐95ºC and then heated to 100ºC using a scan rate of 15ºC/min. Freezable water percentage is determined form the melting enthalpy peak observed at around 0ºC in the DSC curves using the following equation: (334 j/g being the enthalpy of fusion of pure water) DSC sample ΔH (mj) Freezable Water(%)= /334(j/g) ×100 W (mg) ⎧⎛ ⎞ ⎫ ⎪ ⎪ ⎜ ⎟ ⎨⎜ ⎟ ⎬ ⎪⎝ ⎠ ⎪ ⎩ ⎭ [6]
Results and discussion
The synthetic method used for spherical mesoporous silica nanoparticles involves a base catalyzed condensation reaction of silanol groups of hydrolyzed TEOS according to the following steps (7):
Hydrolysis: -Si-(OEt)+ H2O ↔ -Si-(OH)+EtOH Condensation: 2 -Si-(OH) ↔ (-Si-O-Si-) + H2O
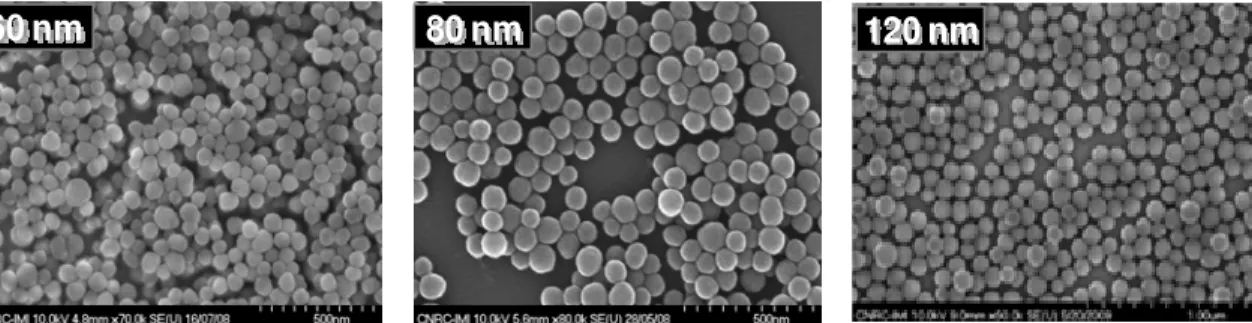
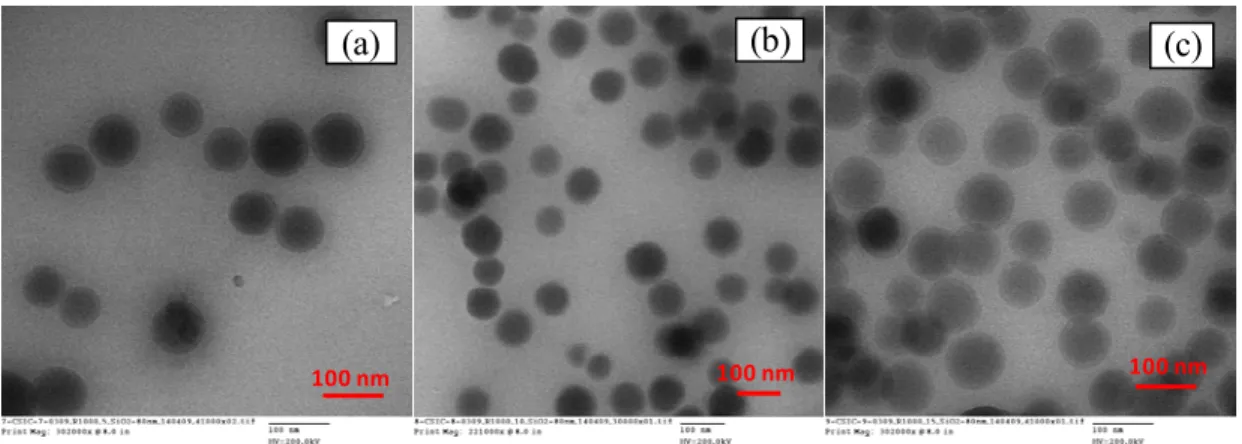
The basic hydrolysis is strongly solvent dependant (8). The shape and growth of the nanoparticles can be influenced by the ionic strength (9). Therefore, the nature of the counter-ion of the base used, influenced the ionic strength and as a consequence the size of the particles. Since the condensation of silanol (Si-OH) groups is a fast reaction in a basic medium, the rate of particle synthesis is dependant on the first step (i.e. ratio of water content/TEOS in the mixture) (4). After the addition of reagents, the solution is clear during hydrolysis of TEOS. When the condensation starts, the solution becomes turbid. The turbidity becomes maximal overnight and remains constant. The final turbidity of the solution is a function of the particle size: the turbidity increases in general with particle size. SEM images of the nanoparticles are illustrated in figure 2. The silica nanoparticles have a spherical shape and diameters ranging between 60-120 nm, depending on the content of water in the reaction mixture (Table I).
60 nm 80 nm 120 nm
Figure 2. SEM micrographs on silica nanoparticles synthesized by base catalyzed sol-gel reaction.
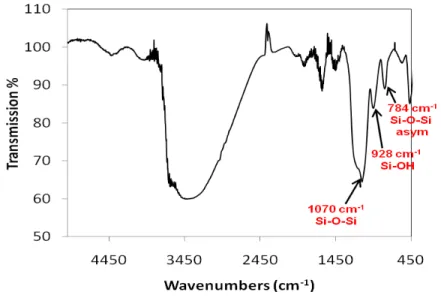
Infrared spectra recorded on 60 nm nanoparticles is illustrated in Figure 3. Si-O-Si antisymmetric , Si-OH and Si-O-Si asymmetric vibrational bands are observed at 1070, 928 and 784 cm-1, respectively. These values agree with those published in the literature (4, 10). The presence of Si-OH groups on the surface of silica nanoparticles is the responsible of its hydrophilic character, and of its water retention properties; however, some ethoxysilane groups (-Si-OEt) are expected to remain non-hydrolyzed on particle surface (4), as a weak shoulder bands between 1100 to 1180 cm-1 corresponding to -Si-OEt is observed.
Figure 3. ATR-FTIR spectra recorded on 60 nm SiO2 nanoparticles.
Thermogravimetric analysis on silica nanoparticles (thermograms not shown in this paper) shows a weight loss around 6 wt.% below 250°C corresponding to the loss of physically adsorbed water in the nanoparticles; and a second weight loss of 8wt.% between 250 – 600°C is attributed to loss of silanol ((i.e. OH) and ethoxy groups on the silica surface to form siloxane Si-O-Si bonds. Given that melt-extrusion of hybrid membranes is performed around 240°C (below the onset of ethoxy and silanol groups) minimal loss of surface hydrophilicity is expected from the silica fillers that will be recovered during hydrolysis and activation steps in the final hybrid membranes. Some interactions are expected, such as hydrogen bond interactions between the silica filler and the ionic phase of the Nafion ionomer through the silanol groups on the silica surface. Sepiolite with heterogenized alkylsulfonic acids through covalent bonds (Si-O-Si) were prepared in two steps according to the following reaction scheme:
OH OH OH OH OH O O Si OH SH OH OH O O Si OH S O O OH OH SH (RO)3Si H2O2 / CH3CN Toluene/100ºC /24h S u p p o rt 60ºC / 24-48h S u p p o rt S u p p o rt
Figure 4. Scheme of the grafting reaction of sepiolite clay with alkyl-sulfonic acid groups.
The heterogenization of an alkylmercaptanesilane to a hydrophilic inorganic solid by means of one or several covalent Si-O-Si bonds be done according to two alternative approaches: 1) by grafting, where a 3-mercaptopropyltrialkoxysilane reacts with the silanol on the surface of the support in presence of traces of water by partial hydrolysis and condensation forming Si-O-Si-R bond with simultaneous removal of alcohols or 2) incorporation of organic component in the sol-gel process, where a mixture of tetramethoxysilane and a 3-mercaptopropyltrialkoxysilane was co-polymerized in presence of water using ammonium fluoride as condensation catalysts; the incorporation of a selected short-chain aliphatic alcohol could modulate the size and distribution of pore and after several preliminary attempts 2-propanol have been selected in this work (11-12). In a second step, the oxidation of mercaptane groups supported on inorganic solid to sulfonic groups was achieved with hydrogen peroxide solution (33%) in acetonitrile at 60ºC for 24-48h with efficient stirring. Ion exchange capacity, expressed in mmol H+/g of solid calculated from elemental analysis (EA) and titration are shown in Table III. Unmodified sepiolite does not contain significant sulfur or acidic sites, while Sep-Alkyl presents acidic sites stronger than hydrochloric acid that could be interchanged with KCl and titrated; the analogous values obtained from sulfur contents and active H+ indicate a quantitative oxidation of mercaptane groups supported on sepiolite surface.
TABLE III. IEC of clay sepiolite before and after grafting with alkyl sulfonic acid groups as determined by elemental analysis and titration
Sample IEC-EA (mmol/g) IEC-T (mmol/g)
Sepiolite 0.08a 0.07a
Sep-Alkyl 0.68 0.62 a
From refrence (13).
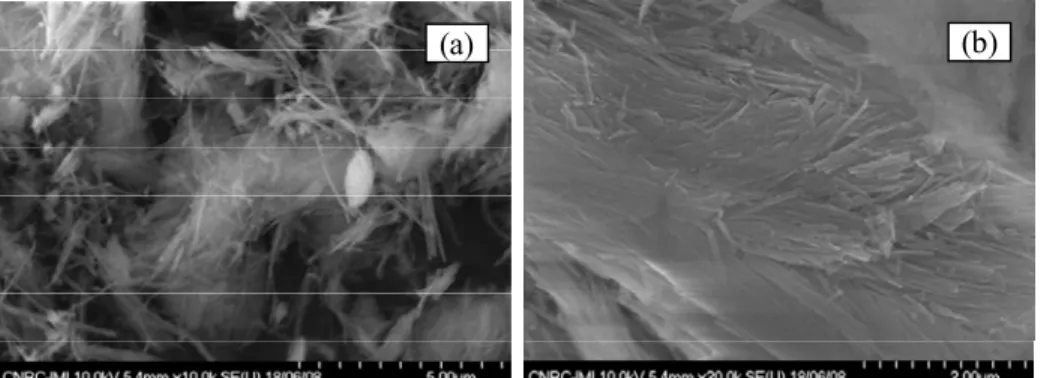
Figure 5 shows SEM micrographs on sepiolite clay before and after grafting with alkyl sulfonic acid groups.
(a) (b)
Figure 5. SEM micrographs on (a) natural sepiolite Clay (b) Sepiolite grafted with alkyl sulfonic acid moieties.
Thermogravimetric analysis on sepiolite clay before grafting shows a weight loss below 200°C, corresponding to the reversible loss of water content (zeolitic water). Above 200°C, there is an additional water loss (coordination water) accompanied with an irreversible crystal structure change. Sepiolite lattice starts to degrade upon heating at temperatures higher than 400°C (14). After grafting the TGA thermogram (not presented in this paper) shows an additional step starting at 250°C corresponding to the loss of
sulfonic acid and grafted organic moieties. Nevertheless, at 240°C, the processing temperature used of hybrid membrane melt processing, both materials show minor weight loss, about 10 wt% for sepiolite and 7wt% for Sep-alkyl, corresponding basically to reversible loss of zeolitic water.
Hybrid melt-extruded proton exchange membranes
The TEM technique represents a powerful analysis tool to examine the morphology of melt-extruded polymer nanocomposites. The dispersion of the filler as well as the presence of agglomerated clusters or defects can be assessed. Figure 6 shows representative TEM micrographs for the series of nanocomposites studied using two magnifications. Nanocomposite membranes based on 5wt% SiO2-80nm spherical particles (Figure 6(a)), typically showed a very good and efficient dispersion of nanoparticles, individual nanoparticles can be distinguished. The composite membranes based on 5wt% of ungrafted sepiolite, shows a very good dispersion and exfoliation of the clay platelets. Individual platelets with high aspect ratio can be observed in the high magnification image (Figure 6(b)). The TEM image of the 5wt% sepiolite-g-Alkylsulfonic acid composite membrane shows a good dispersion of the fillers, however, the high magnification image (Figure 6(c)) shows a higher agglomeration of the clay platelets compared to the ungrafted clay. This is due to the tendency of sulfonic acid moieties to form ionic clusters.
(a) 500 nm (b) 500 nm (c) 500 nm (a) 100 nm 100nm (b) (c) 100 nm
Figure 6. TEM micrographs with two magnifications obtained on PFSA extruded nanocomposites containing (a) 5wt% SiO2-80nm (b) 5wt% sepiolite and (c) 5wt%
Figure 7 shows TEM micrographs obtained for the series of nanocomposite membranes based on SiO2-80nm spherical particles with different loadings. The images show a very good and efficient dispersion of nanoparticles through the entire thickness of the thin membranes. At higher loading (20wt%), nanoparticles seem to almost form a connected network. (a) 100 nm (b) 100 nm (c) 100 nm
Figure 7. TEM micrographs of extruded nanocomposites containing (a) 5wt% SiO2-80nm (b) 10wt% SiO2-80nm and (c) 20 wt% SiO2-80nm.
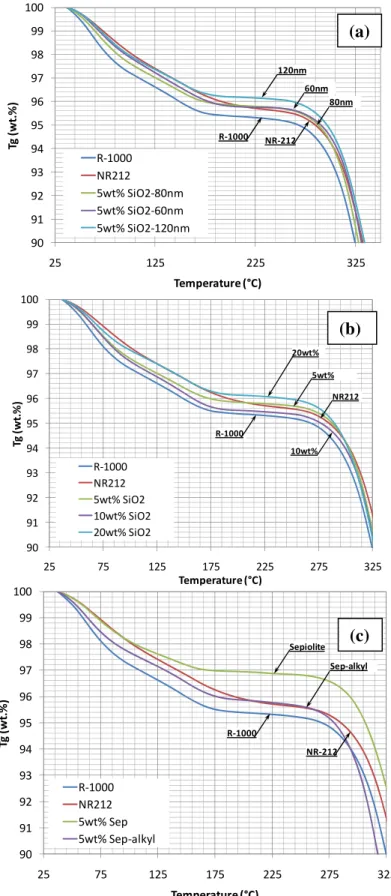
Thermal stability of the melt-extruded nanocomposite electrolyte membranes containing spherical silica nanoparticles as well as grafted and ungrafted sepiolite clay has been studied by TGA. Independently of the type of the fillers, thermograms obtained show three weight losses typical of sulfonated polymers in general; the first weight loss below 275°C can be attributed to the dehydration, the second from 275 to 400°C to the degradation of sulfonic acid groups, and the last one above 400°C to the degradation of the polymer backbone. Some of the TGA results in Figure 8 show that overall the addition of the inorganic fillers had enhanced the thermal stability of the polymer as is evidenced by a reduction in weight loss. Nanocomposite membranes with SiO2 nanoparticles show that improved stability for particles with higher size, and for higher loading. For sepiolite based composites, ungrafted sepiolite showed improved stability compared to the alkyl-sulfonic acid grafted.
90 91 92 93 94 95 96 97 98 99 100 25 125 225 325 Tg (w t.% ) Temperature (°C) R‐1000 NR212 5wt% SiO2‐80nm 5wt% SiO2‐60nm 5wt% SiO2‐120nm 120nm R‐1000 60nm 80nm NR‐212 90 91 92 93 94 95 96 97 98 99 100 25 75 125 175 225 275 325 Tg (w t. %) Temperature (°C) R‐1000 NR212 5wt% SiO2 10wt% SiO2 20wt% SiO2 20wt% R‐1000 5wt% 10wt% NR212 90 91 92 93 94 95 96 97 98 99 100 25 75 125 175 225 275 325 Tg (w t. % ) Temperature (°C) R‐1000 NR212 5wt% Sep 5wt% Sep‐alkyl Sepiolite R‐1000 Sep‐alkyl NR‐212
Figure 8. TGA results obtained for the series of extruded nanocomposite PEMs in H+ form and reference membranes. (a) series with spherical SiO2 nanoparticles with different sizes (60, 80 and 120nm) (b) series with 80nm SiO2 with diferent loadings (5,
10, and 15wt%) (c) series with sepiolite before and after grafting with Alkyl-sulfonic acid.
(a)
(b)
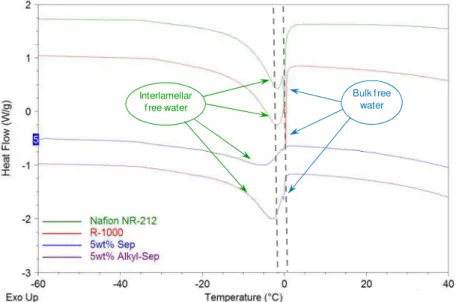
Proton conductivity depends on the water absorption, the hydration of the polymer and the transport of protons in PEM are known to be critical factors. The state of water in hydrated membranes can be broadly classified into two groups, which are respectively freezable water and bound water. Water equilibrated membranes have been analyzed by DSC. From the melting enthalpy peak observed in the thermograms around 0ºC, the percentage of free water can be approximated according to the equation described in the experimental part. The double peak suggests two states of freezable water, free water that occurs at 0°C, and interlamellar water at slightly lower temperature as shown in Figure 9 for sepiolite based series of membranes. The melting temperature of the interlamellar water shifts to lower temperature for some membranes. The lower the melting temperature implies the increased interaction between water and the polymer matrix. The bound water in the membranes is calculated from the difference between total water and free water contents.
Bulk f ree water
Interlamellar f ree water
Figure 9. DSC thermograms obtained for the series of extruded nanocomposites PEMs with sepiolite clays.
From the results shown in Table IV, the amount of bound water is higher for composite membranes in all cases, as a consequence of the incorporation of hydrophilic fillers to the polymer. Since bound water will evaporate at higher temperatures compared to free water, the results suggest that the fillers contribute to retain more water molecules in the membrane’s structure.
TABLE IV. Water content in nanocomposite proton exchange membranes determined from DSC measurements
Sample Total Water
(wt%) Bound Water (wt%) Freezable Water (wt%) R-1000 32.92 17.53 15.39 5wt% SiO2-60nm 29.60 20.80 8.79 5wt% SiO2-80nm 41.79 23.83 17.95 5wt% SiO2-120nm 35.61 21.54 14.10 5wt% Sepiolite 26.68 21.29 5.39 5wt% Sep-Alkyl 32.87 21.50 11.36
Dry/wet volume change and water uptake results the nanocomposite PEMs are presented in Figure 10. Hybrid PEMs based on sepiolite clays present slightly lower volume change and water uptake than reference materials (Figure 10(a)). The series based on 80nm SiO2 spherical nanoparticles with different loadings show reduced volume change and water uptake for 10wt% loading (Figure 10(b)). For the series based on SiO2 spherical nanoparticles with different particle sizes, the higher the particle size, the higher the dimensional stability and lower the water uptake of hybrid membranes; volume change was reduced 35% when 5wt% of 120nm SiO2 nanoparticles was incorporated. 0 20 40 60 80 100 120 140
Nafion NR‐211 R‐1000 5wt% Sepiolite 5wt% Sep‐Alkyl
(% ) Volume Change Water uptake 0 20 40 60 80 100 120 140 Nafion NR‐ 211 0 5 10 20 (% ) Loading (wt%) 0 20 40 60 80 100 120 140 Nafion NR‐ 211 0 60 80 120 (% ) Nanoparticles size (nm)
Figure 10. Dry/wet volume change and water uptake measured for nanocomposite PEMs series. (a) Sepiolite clays (b) SiO2 spherical nanoparticles with different particle size (60,
80 and 120nm). (c) 80nm SiO2 with different loadings (5, 10, and 15wt%)
(a)
(b)
Conductivities measured at 80°C at two relative humidities; 50 and 30% are presented in figure 11. All composite PEMs show an improvement in conductivity compared to extruded R-1000 (internal reference 21.1 mS/cm at 80ºC-50%RH and 5.3 mS/cm at 80ºC-30%RH) and NRE-211 (commercial solvent cast external reference 10.1 mS/cm at 80ºC-50%RH and 7.9 mS/cm at 80ºC-30%RH). For the series with sepiolite clays, alkyl sulfonic acid-grafted sepiolite based composite membranes shows higher conductivity than ungrafted sepiolite. Conductivities measured were 30 mS/cm at 80ºC-50%RH and 10.4mS/cm at 80ºC-30%RH. The series with SiO2 spherical 80 nm nanoparticles, the proton conductivity of composite membranes increased with the increase in additive loading from 5% to 10%. However, further increase of additive content caused a decrease in proton conductivity. Conductivities measured at 10 wt% optimum loading were 25.7 mS/cm at 80ºC- 50%RH and 16.4 mS/cm at 80ºC-30%RH. It should be noted that the proton conductivity of silica composite membranes at all loading levels was still higher than that of commercial Nafion and extruded R-1000. When the filler loading was maintained constant at 5wt%, 120 nm SiO2 was the optimum particle size. Conductivity measured for 5 wt% composite PEM is 41.6 mS/cm at 80ºC-50%RH and 13.1 mS/cm at 80ºC-30%RH. 0.000 0.005 0.010 0.015 0.020 0.025 0.030 0.035 C ondu c tiv it y ( S /c m ) 80oC, 50%RH 80oC, 30%RH 0 0.005 0.01 0.015 0.02 0.025 0.03 0.035 0.04 0.045 C o nd uct ivi ty ( S /c m ) 80oC, 50% RH 80oC, 30%RH
Figure 11. Ex-situ in-plane conductivity of nanocomposite PEMs with different inorganic fillers. (a) Sepiolite clays (b) SiO2 spherical nanoparticles with different particle sizes.
(a)
This increase in conductivity is related to the fact that silicon dioxide nanoparticles synthesized in this study are hydrophilic amorphous silica. Their surface chemistry is important. During the formation of the nanoparticles, hydroxyl groups become attached to the silicon atoms on the particle surface. This makes the surface hydrophilic and capable of hydrogen bonding with suitable molecules of materials in vapor, liquid, or solid form. The three groups which can be identified are: 1) the isolated hydroxyl group, 2) hydrogen-bonded hydroxyl groups, and 3) the siloxane group (15) . The silica surface hydroxyl (OH) groups are the main centers of adsorption of water molecules. Water can be associated by hydrogen bonds to any type of surface silanols and sometimes to internal silanol groups. The OH groups in ultramicropores, into which only water molecules can penetrate, were not classified as surface silanol groups but as structurally bound water, as confirmed by DSC measurements previously discussed.
Conclusions
This work has shown that melt processing technologies can be used efficiently to produce a new class of hybrid electrolyte membranes with substantially improved ex-situ properties than reference material and commercially available Nafion. Different families of fillers have been incorporated in order to further improve the properties of PEMS, specially in terms of conductivity at low RH and dimensional stability, which will translate to increased durability of the membranes. The nano-composite electrolytes showed very efficient dispersion of the inorganic fillers. Independently from the nature of the filler, an overall improvement in dimensional stability, and improved conductivities between 10-2 to 10-3 S.cm-1 at 80°C over the entire humidity range from 100 to 30% was measured. The most promising materials are being evaluated in-situ.
Acknowledgments
Financial support from the collaboration project NRC-CSIC is gratefully acknowledged. The authors thank J. A. Esteban-Perales from IQOG-CSIC (spain) for assistance in the synthesis of sepiolite based fillers.
References
1. D.J. Jones, J. Rozière, Adv. Polym. Sci., 215, 219 (2008).
2. F. Pereira, K. Vallé, P. Belleville, A. Morin, S. Lambert and C. Sanchez, Chem.
Mater., 20, 1710 (2008).
3. H. Uchida, Y. Ueno, H. Hagihara and M. Watanabe, J. Electrochem. Soc., 150(1), A57 (2003).
4. K.T. Adjemian, S.J. Lee, S. Srinivasan, J. Benziger and A.B. Bocarsly, J.
Electrochem. Soc., 149(3), A256 (2002).
5. Y. Kim, Y. Choi, H.K. Kim, J.S. Lee, J. Power Sources, 195(15), 4653(2010). 6. T. Greszler, DOE Hydrogen Program review meeting, September 2006. 7. B. Nair, J. Colloid and Interface Sci., 178, 565 (1996).
8. E.G. Rochow, The chemistry of silicon, Pergamon Press, New York (1973).
9. A. Van Blaaderen, J. Van Geest and A. Vrij, J. Colloid Interface Sci., 154, 481 (1992).
10. P. Innocenzi, P. Falcaro, I. Meccanica, U. Padova, D. Grosso, and F. Babonneau,
11. A. Corma, and H. García, Adv. Synth. Catal., 348, 1391 (2006). 12. A. Corma, and H. García, Top. Catal., 48, 8 (2008).
13. F.J. Fernandez-Carretero , V. Compan, and E. Riande, J. Power Sources, 173, 68 (2007).
14. C. Serna, J. L. Ahlrichs and J. M. Serratosa, Clays and Clay Minerals, 23, 452 (1975).
15. Zhuravlev et al., Colloids and Surfaces A: Physico-chem. Eng. Aspects, 173, 1 (2000).