Dose-Response Relationships Following Oral
Administration of DuP 753 to Normal Humans
Y. Christen, B. Waeber, J. Nussberger, R.J. Lee, P.B.M.W.M. Timmermans, and H.R. Brunner
W e a s s e s s e d t h e i n h i b i t o r y effect of D u P 753, a n o r a l l y a c t i v e a n g i o t e n s i n II r e c e p t o r a n t a g o n i s t , o n t h e p r e s s o r a c t i o n of e x o g e n o u s a n g i o t e n s i n I a n d II i n h e a l t h y v o l u n t e e r s . I n a s i n g l e d o s e s t u d y , d o s e s of 2.5, 5,10, 20, a n d 40 m g of D u P 753 or p l a c e b o w e r e t e s t e d s e r i a l l y at o n e w e e k i n t e r v a l s . I n t h e m u l t i p l e d o s e s t u d y , t h e a d m i n i s t r a t i o n of p l a c e b o or D u P 753 (5, 10, 20, or 40 m g , p e r os o n c e d a i l y ) for e i g h t c o n s e c u t i v e d a y s w a s e v a l u a t e d . T h e b l o o d p r e s s u r e r e s p o n s e t o a n g i o t e n s i n I a n d II w a s i n h i b i t e d i n a d o s e - d e p e n d e n t f a s h i o n w i t h a b l o c k i n g effect still p r e s e n t 24 h p o s t d r u g . D u P 753 also i n d u c e d a d o s e - d e p e n d e n t c o m p e n s a t o r y r i s e i n p l a s m a r e n i n . T h i s n e w c o m p o u n d w a s w e l l t o l e r a t e d b y t h e s e n o r m a l v o l u n t e e r s . T h u s , D u P 753 a p p e a r s to b e a w e l l t o l e r a t e d , o r a l l y a c t i v e , p o t e n t a n d l o n g - l a s t i n g a n t a g o n i s t of a n g i o t e n s i n II i n h u m a n s . A m J H y p e r t e n s 1991;4:350S-354S KEY W O R D S : A n g i o t e n s i n II, D u P 753, a n g i o t e n s i n II a n t a g o n i s t .
S
aralasin (sar^ala8-angiotensin II) w a s t h e firstreceptor antagonist of angiotensin (ANG) II administered to h u m a n s .1 - 3 This p e p t i d e a n
tagonist w a s n o t orally active a n d h a d to b e a d ministered parenterally. Therefore, long-term anti h y p e r t e n s i v e t r e a t m e n t w a s n o t possible. F u r t h e r m o r e , t h e antagonist w a s also a partial agonist.4 C o n s e q u e n t l y ,
t h e majority of patients did n o t r e s p o n d w i t h a fall in blood pressure.
F u r u k a w a a n d his coworkers h a v e synthetized s o m e imidazole derivatives t h a t specifically block t h e A N G II-induced vasoconstriction.5 I m p o r t a n t chemical m o d i
fications of these initial molecules h a v e led to t h e syn thesis of D u P 7 5 3 .6 7 This c o m p o u n d is a p o t e n t a n t a g o
nist of A N G II w h e n administered orally to a n i m a l s .8'9
From the Hypertension Division and Cardiovascular Research Group, University Hospital, Lausanne, Switzerland (YC, BW, JN, HRB), and Ε. I. du Pont de N e m o u r s & Company, Medical Products Dept., Wilmington, Delaware, USA, and Geneva, Switzerland (RJL, PBMWMT).
This work w a s supported by grants from the Cardiovascular Re search Foundation, the Swiss National Science Foundation, and Ε. I. du Pont de N e m o u r s & C o m p a n y .
Address correspondence and reprint requests to Hans R. Brunner, M D , Division d'Hypertension, CHUV, 1011 Lausanne, Switzerland.
We h a v e assessed for t h e first time t h e pharmacoki netic a n d p h a r m a c o d y n a m i c profile of D u P 753 after single a n d r e p e a t e d oral administration to normal h u m a n subjects. T h e results suggest t h a t D u P 753 is a p o t e n t a n d long-acting antagonist of A N G II n o t only in animals, b u t also in h u m a n s .1 0
M E T H O D S
A total of 37 male subjects aged 20 to 38 years were recruited to carry out t h e t w o consecutive studies. All subjects w e r e considered h e a l t h y on t h e basis of a medi cal history, a physical examination, a routine blood and urine analysis, a n d an electrocardiogram. T h e study protocols w e r e a p p r o v e d by t h e institutional ethics committee.
In order to quantitate the pressor effect of exogenous A N G , blood pressure w a s m e a s u r e d at t h e finger using a p h o t o p l e t h y s m o g r a p h (Finapres, O h m e d a , Englewood, CO). T h r o u g h o u t t h e studies, t h e volunteers w e r e al l o w e d to continue their usual free s o d i u m intake. They w e r e asked to come to our research facility at 7 AM on t h e first d a y of t h e investigation after fasting over night. T h e y w e r e immediately placed in a s u p i n e posi tion a n d v e n o u s catheters w e r e inserted in a vein in each forearm.
AJH-APRIL 1991-VOL 4, NO. 4, PART 2 D u P 753 I N H U M A N S 351S
S t u d y O n e ( S i n g l e D o s e S t u d y ) After a 30 m i n resting period to reach a steady baseline b l o o d pressure a n d h e a r t rate, a d o s e - r e s p o n s e curve to bolus injections of A N G I w a s established. Angiotensin I (Senn Chemicals, Dielsdorf, Switzerland) w a s dissolved in 0.9% N a C l to achieve a concentration of 1 / / g / m L . T h e bolus injec tions w e r e started at a dose of 10 n g / k g a n d increased thereafter every 10 to 15 m i n b y steps of 10 n g / k g until a systolic b l o o d pressure increase of 25 to 40 m m H g w a s r e a c h e d . This test dose w a s t h e n r e p e a t e d at least twice. T h e systolic b l o o d pressure responses to these three con secutive injections of t h e test dose w e r e a v e r a g e d a n d t h e resulting m e a n u s e d to define t h e baseline r e s p o n s e to A N G I.
D u P 753 is t h e p o t a s s i u m salt of 2nbutyl4chloro5 h y d r o x y m e t h y l l [ ( 2 ' 4 H tetrazol 2nbutyl4chloro5 yl)biphenyl 4 -yl)methyl] imidazole (Ε. I. d u P o n t d e N e m o u r s & C o m p a n y , Wilmington, D E ) .1 1 T h e dose to b e a d m i n i s t e r e d
w a s dissolved in 20 m L of t a p water. T h e effect of t h e d r u g w a s t h e n m o n i t o r e d using bolus injections of t h e A N G I test dose at times 1 5 , 3 0 , a n d 45 m i n a n d 1, IV2,2, 2 V2, 3 , 4 , 5 , 6 , 7 , 1 3 , 2 3 , 2 5 , a n d 27 (up to 33 for t h e 40 m g
dose) h after d r u g intake. Between t h e h o u r s 7 a n d 12 a n d again b e t w e e n t h e h o u r s 13 a n d 22, t h e subjects were allowed to leave t h e clinic. This t w o d a y protocol was r e p e a t e d four times in each v o l u n t e e r (except for one v o l u n t e e r w h o only h a d 3 administrations) a n d at each p h a s e a different dose of d r u g or placebo w a s given in single-blind fashion.
S t u d y T w o ( M u l t i p l e D o s e S t u d y ) D u r i n g t h e multi ple dose study, each subject received, in t h e m o r n i n g s on 8 consecutive days, o n e dose of D u P 753 (either 5 , 1 0 , 20, or 40 mg) or placebo. T h u s , each dose or placebo w a s given in single-blind fashion to six subjects, w i t h t h e exception of t h e 20 m g dose, w h i c h w a s t a k e n b y only five volunteers. In this second investigation, A N G II was u s e d as t h e agonist. Angiotensin II (Senn C h e m i cals, Dielsdorf, Switzerland) w a s dissolved in 0.9% NaCl to achieve a concentration of 1 / / g / m L . T h e d o s e -response curve to establish t h e test dose of A N G II w a s carried out d u r i n g t h e w e e k preceding t h e first a d m i n i s tration of D u P 753 using t h e s a m e p r o c e d u r e described above. D u r i n g s t u d y d a y s 1 to 9, t h e subjects c a m e every day at 8 AM a n d 8 PM to t h e research facility for m e a s u r e ment of h e a r t rate, weight, a n d blood pressure b y a sphygmomanometer (sitting a n d standing). O n s t u d y days 1 to 8, t h e dose of D u P 753 or placebo w a s a d m i n istered at 8 AM in a similar fashion to t h e single-dose study. O n d a y s 1, 4, a n d 8, a n A N G II challenge w a s performed before a n d again 6 a n d 12 h p o s t - d r u g in take. Additional test doses w e r e injected 24 h post d r u g on day 1 as well as 24, 30, a n d 36 h after t h e last dosing on day 8.
Aldosterone w a s d e t e r m i n e d by a direct r a d i o i m m u noassay.1 2 For t h e m e a s u r e m e n t of p l a s m a renin activity
(PRA), g e n e r a t e d A N G I w a s t r a p p e d a n d q u a n t i t a t e d b y h i g h affinity a n t i b o d i e s .1 3'1 4 For t h e q u a n t i t a t i o n of
i m m u n o r e a c t i v e A N G II, a n e w m e t h o d u s i n g m o n o c l o nal antibodies against A N G II w a s u s e d .1 5 N o r e p i n e p h
rine w a s m e a s u r e d b y a radioenzymatic a s s a y .1 6'1 7 S u b
jects r e m a i n e d in a s u p i n e position for 30 m i n prior to blood sampling.
T h e d a t a are p r o v i d e d as m e a n ± SEM u n l e s s o t h e r wise indicated. T h e y w e r e e v a l u a t e d w i t h a o n e w a y A N O V A m o d e l . A Ρ < .05 w a s considered significant. T h e correlation coefficients w e r e calculated, w h e n indi cated, b y t h e m e t h o d of least squares.
R E S U L T S
D u P 753 h a d n o effect o n resting b l o o d p r e s s u r e or p u l s e rate, neither after single administration n o r d u r i n g t h e 8 d a y treatment. T h e c o m p o u n d w a s well tolerated a n d n o clinically significant side effect w a s observed in ei ther of t h e t w o studies. Also, b l o o d counts, t h e results of routine laboratory tests a n d u r i n e analysis, a n d electro cardiogram m e a s u r e m e n t s w e r e n o t modified by D u P 753.
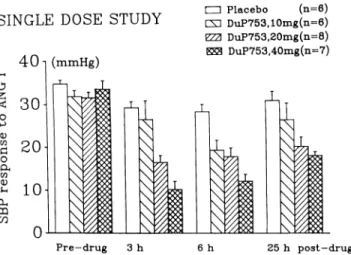
T h e c h a n g e s in systolic blood pressure i n d u c e d b y t h e test dose of A N G I w e r e calculated as t h e difference b e t w e e n post- a n d prechallenge values for each v o l u n teer at each dose a n d at each time. C o m p a r e d to placebo, n o clear effect w a s observed w i t h t h e doses of 2.5 a n d 5 m g . Doses of 10, 20, a n d 40 m g i n d u c e d h o w e v e r a d o s e - d e p e n d e n t inhibition of t h e r e s p o n s e to A N G I. Figure 1 s h o w s t h e dose-related inhibition of t h e r e s p o n s e to A N G I expressed in p e r c e n t of t h e baseline r e s p o n s e . T h e p e a k inhibitory effect w a s r e a c h e d 7, 5, a n d 3 h after intake of t h e 10, 20, a n d 40 m g doses of D u P 753, respectively.
C o m p a r e d to placebo, t h e 8 d a y t r e a t m e n t w i t h D u P 753 taken once a d a y resulted in a d o s e d e p e n d e n t r e
-S I N G L E D O -S E -S T U D Y 4 0 1 ( m m H g ) • Placebo (n=6) L X ] DuP753,10mg(n=6) ΥΖΆ DuP753,20mg(n=8) DuP753,40mg(n=7) -drug 25 h p o s t - d r u g
FIGURE 1. Effect of single doses of oral DuP 753 (10, 20, or 40
mg) or placebo on the systolic blood pressure (SBP) response (mean ± SEM) to test doses of ANG I in healthy volunteers.
a u c t i o n in t h e systolic b l o o d pressure r e s p o n s e to A N G II t h r o u g h o u t t h e t r e a t m e n t period. T h e degree of blockade achieved w i t h t h e 20 a n d 40 m g doses w a s similar 6 h p o s t - d r u g intake o n d a y s 1,4, a n d 8 (P < .01
for effect of D u P 753 ν placebo). O n d a y s 4 a n d 8,
h o w e v e r , t h e subjects receiving 40 m g of D u P 753 s h o w e d a clear t r e n d t o w a r d s a r e d u c e d r e s p o n s e to e x o g e n o u s A N G II immediately before t h e m o r n i n g d o s e of t h e d r u g (Figure 2).
P l a s m a aldosterone levels fell after administration of single doses of D u P 753, b u t a similar decrease w a s also seen after placebo. T h e single doses of D u P 753 p r o d u c e d a d o s e d e p e n d e n t increase in p l a s m a A N G II, w h i l e n o c h a n g e w a s observed after placebo administra tion. As early as 30 m i n after t h e highest dose of 40 m g , a small rise w a s already a p p a r e n t . N o significant c h a n g e in p l a s m a n o r e p i n e p h r i n e w a s observed after single a d ministration of D u P 753.
Figure 3 depicts t h e PRA a n d p l a s m a A N G II levels m e a s u r e d at t h e b e g i n n i n g a n d at t h e e n d of r e p e a t e d administration of placebo or D u P 753. Both PRA a n d A N G II s h o w e d a m a r k e d d o s e - d e p e n d e n t increase 6 h p o s t - d r u g intake, a n d this rise w a s clearly m o r e p r o n o u n c e d o n d a y 8 t h a n o n d a y 1. Both variables h a d already increased significantly 6 h after 20 m g of D u P 753 o n t h e first day, (P < .05 ν placebo). While t h e b a s e line results o n d a y 1 w e r e all very similar, o n d a y 8 there w a s a t r e n d for a d o s e - d e p e n d e n t increase e v e n of t h e p r e - d r u g values.
D I S C U S S I O N
These data d e m o n s t r a t e t h a t D u P 753 is a p o t e n t orally active A N G II antagonist w i t h a relatively long d u r a t i o n of action. At p e a k effect, t h e 40 m g dose i n d u c e d a n
MULTIPLE DOSE STUDY
4 0 - 1 (mmHg) tZU P l a c e b o ( n = 6 ) m D u P 7 5 3 , 1 0 m g ( n = 6 ) 7ΖΔ D u P 7 5 3 , 2 0 m g ( n = 5 ) W D u P 7 5 3 , 4 0 m g ( n = 6 ) P r e - d r u g 6 h p o s t - d r u g DAY 1 P r e - d r u g 6 h p o s t - d r u g DAY 8
FIGURE 2. Effect of eight consecutive days of treatment with
daily oral doses of DuP 753 (10, 20, or 40 mg) or placebo on the systolic blood pressure (SBP) response (mean ± SEM) to test doses
of ANG II in healthy volunteers. Adapted from Christen et al.10
MULTIPLE DOSE STUDY
1 0
ξ
8 • P l a c e b o ( n = 6 ) m D u P 7 5 3 , 1 0 m g ( n = 6 ) ΥΖΔ D u P 7 5 3 , 2 0 m g ( n = 5 ) W D u P 7 5 3 , 4 0 m g ( n = 6 ) *<
6 4 ^ 2 0 P r e - d r u g 6 h p o s t — d r u g DAY 1 P r e - d r u g 6 h p o s t - d r u g DAY 8 6 0Ί Οe
<
Β P r e - d r u g 6 h p o s t - d r u g DAY 1 P r e - d r u g 6 h p o s t - d r u g DAY 8FIGURE 3. Effect of different doses of daily oral DuP 753 (10,
20, or 40 mg) or placebo (n = 6 for each dose except the dose of 20 mg where n = 5) on plasma renin activity and immunoreactive ANG II measured before and 6 h post-drug intake on days 1 and 8.
Values are given as mean ± SEM. *P < .05 ν placebo. Adapted
from Christen et al.10
approximately 7 0 % reduction in t h e systolic pressure r e s p o n s e to A N G I or II. With this highest dose, even 24 h p o s t - d r u g a definite effect w a s still present. Plasma renin activity a n d A N G II levels also r e s p o n d e d with a d o s e - d e p e n d e n t c o m p e n s a t o r y rise as o n e m i g h t have expected b a s e d o n earlier observations m a d e w i t h sara
lasin,2 as well as o n t h e extensive experience accumu
lated with angiotensin converting e n z y m e (ACE) inhibi tors. This c o m p e n s a t o r y rise w a s d o s e - d e p e n d e n t and m o r e p r o n o u n c e d o n d a y 8 of d r u g administration than o n d a y 1. This also is in a g r e e m e n t w i t h findings ob
tained after administration of ACE i n h i b i t o r s .1 8 The
long d u r a t i o n of t h e blocking effect of D u P 753 is also reflected in t h e p l a s m a renin activity a n d A N G II levels. T h u s , while o n d a y 1 all p r e d r u g m e a s u r e m e n t s pro vided similar results, o n d a y 8, p l a s m a renin activity as well as p l a s m a A N G II w e r e clearly increased before the administration of 20 a n d 40 m g as c o m p a r e d to the p r e - d r u g results o b t a i n e d before placebo.
AJH-APRIL 1991-VOL 4, NO. 4, PART 2 D u P 753 I N H U M A N S 353S
N o significant effect of t h e A N G II antagonist on p l a s m a aldosterone could b e detected. There are m a n y possible reasons w h y there w a s n o net effect of t h e d r u g o n p l a s m a aldosterone. Most importantly, t h e 40 m g dose did n o t p r o v i d e maximal receptor blockade at t h e vascular receptors. It is also conceivable t h a t t h e re p e a t e d bolus injections of A N G I or II in t h e face of incomplete receptor blockade m a y h a v e exerted a stimu lating effect o n aldosterone secretion.
T h e clinically m o s t relevant question is of course h o w D u P 753 as a therapeutic agent c o m p a r e s to t h e various agents already available for blockade of t h e r e n i n - a n giotensin cascade. Obviously, t h e p r e s e n t s t u d y c a n n o t provide a n y conclusive a n s w e r to this question since t h e d r u g w a s a d m i n i s t e r e d only to n o r m a l volunteers. N e v ertheless, it is already evident t h a t this A N G II a n t a g o nist exhibits features w h i c h a p p e a r particularly p r o m i s ing as a future therapeutic agent. C o m p a r e d to saralasin it h a s t h e evident a n d decisive a d v a n t a g e of being orally active. F u r t h e r m o r e , while t h e present data c a n n o t rule out t h e existence of a n agonistic effect of D u P 753 b e cause relatively small doses w e r e administered, it s e e m s likely t h a t n o such effect will occur in h u m a n s , since in various a n i m a l studies n o n e w a s observed at very h i g h
doses of d r u g .7 These preliminary data obtained in n o r
mal volunteers d o n o t allow u s to predict t h e a n t i h y p e r tensive p o t e n c y a n d efficacy of D u P 753. Nevertheless, the findings obtained w i t h D u P 753 in h y p e r t e n s i v e animals a n d t h e earlier observations m a d e w i t h sarala sin suggest t h a t D u P 753 will likely b e c o m e a n effective c o m p o u n d to treat h y p e r t e n s i o n a n d possibly conges tive heart failure. C o m p a r e d to angiotensin converting enzyme inhibitors, it w o u l d h a v e t h e a d v a n t a g e of greater specificity. It shares w i t h t h e ACE inhibitors, as well as w i t h t h e renin inhibitors, t h e u n e s c a p a b l e fact that the closed regulatory loop of renin secretion is fully operative, a n d as a consequence, blockade at a n y point beyond t h e r e n i n secreting process automatically in duces a c o m p e n s a t o r y rise in renin a n d A N G II. Whether this c o m p e n s a t o r y rise h a s a n y clinical conse quences r e m a i n s to be seen.
R E F E R E N C E S
1. Pals DT, Masucci FD, Sipos F, Denning CS, Jr.: A specific competitive antagonist of the vascular action of angio tensin II. Circ Res 1971;29:664-672.
2. Brunner HR, Gavras H, Laragh JH, Keenan R: Angioten
sin II blockade in man by sar1-ala8-angiotensin II for
understanding and treatment of high blood pressure. Lancet 1973;ii:1045-1048.
3. Brunner HR, Gavras H, Laragh JH, Keenan R: Hyperten sion in man, exposure of the renin and sodium compo nents using angiotensin II blockade. Circ Res 1974;34(suppl l ) : 3 5 - 4 3 .
4. Streeten DHP, Anderson GH, Fruiberg JM, Dalakos TG: Use of an angiotensin II antagonist (saralasin) in the rec ognition of angiotensinogenic hypertension. Ν Engl J Med 1975;292:657-662.
5. Furukawa Y, Kishimoto S, Nishikawa K: Hypotensive imidazole derivatives. US Patent 4,340,598 issued to Ta keda Chemical Industries, Ltd., Osaka, Japan, 1982. 6. Timmermans PBMWM, Carini DJ, Chiu AT, et al: An
giotensin II receptor antagonists. Am J Hypertens 1990;3:599-604.
7. Wong PC, Price WA, Chiu AT, et al: Nonpeptide angio tensin II receptor antagonists: VIII. Characterization of functional antagonism displayed by DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther 1990;252:719-725.
8. Wong PC, Price WA, Chiu AT, et al: Hypotensive action of DuP 753, an angiotensin II antagonist in spontane ously hypertensive rats. Nonpeptide angiotensin II re ceptor antagonists: X. Hypertension 1990;15:459-468. 9. Wong PC, Price WA, Chiu AT, et al: Nonpeptide angio
tensin II receptor antagonists: studies with EXP 9270 and DuP 753. Hypertension 1990;15:823-834.
10. Christen Y, Nussberger J, Waeber B, et al: Oral adminis tration of DuP 753, a specific angiotensin II receptor an tagonist, to normal volunteers; inhibition of the pressor response to exogenous angiotensin I and II. Circulation (in press).
11. Chiu AT, McCall DE, Price WA, et al: Nonpeptide angio tensin II receptor antagonists. VII. Cellular and biochemi cal pharmacology of DuP 753, an orally active antihy pertensive agent. J Pharmacol Exp Ther 1990; 252:711-718.
12. Nussberger J, Waeber B, Brunner HR, et al: Highly sensi tive microassay for aldosterone in unextracted plasma: comparison with two other methods. J Lab Clin Med 1984;104:789-796.
13. Poulsen K, Jorgensen J: An easy radioimmunological mi croassay of renin activity, concentration and substrate in human and animal plasma and tissues based on angio tensin I trapping by antibody. J Clin Endocrinol Metab 1974;39:816-825.
14. Nussberger J, Fasanella d'Amore T, Porchet M, et al: Repeated administration of the converting enzyme inhib itor Cilazapril to normal volunteers. J Cardiovasc Phar macol 1987;9:39-44.
15. Nussberger J, Keller I, Waeber B, Brunner HR: Angioten sin II measurement with high-affinity monoclonal anti bodies. J Hypertens 1988;6(suppl 4):S424-S425. 16. Peuler JD, Johnson G A: Simultaneous simple isotope
radioenzymatic assay of plasma norepinephrine, epi nephrine and dopamine. Life Sei 1977;21:625-636. 17. Laurent S, Juillerat L, London GM, et al: Increased re
sponse of brachial artery diameter to norepinephrine in hypertensive patients. Am J Physiol 1988;255:H36-H43.
18. Mooser V, Nussberger J, Juillerat L, et al: Reactive hy-perreninemia is a major determinant of plasma angio tensin II during ACE inhibition. J Cardiovasc Pharmacol 1990;35:276-282.