HAL Id: hal-02376421
https://hal.archives-ouvertes.fr/hal-02376421
Submitted on 22 Nov 2019HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires
Cloning and Regulation of Expression of the Rat Kidney
Urea Transporter (rUT2)
Craig Smith, Wen-Sen Lee, Sonia Martial, Mark Knepper, Guofeng You, Jeff
Sands, Matthias Hediger
To cite this version:
Craig Smith, Wen-Sen Lee, Sonia Martial, Mark Knepper, Guofeng You, et al.. Cloning and Regulation of Expression of the Rat Kidney Urea Transporter (rUT2). Journal of Clinical Investigation, American Society for Clinical Investigation, 1995, �10.1172/JCI118194�. �hal-02376421�
Cloning
and
Regulation of
Expression
of the Rat Kidney Urea
Transporter (rUT2)
Craig P. Smith,* Wen-Sen Lee,* Sonia Martial,* Mark A. Knepper,§ Guofeng You,* Jeff M. Sands,* and Matthias A. Hediger*
*RenalDivision, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts 02115; tRenal Division, Department of Medicine,Emory UniversitySchool of Medicine, Atlanta,Georgia30322; and§Laboratoryof Kidney andElectrolyte Metabolism, National Heart, Lung, and Blood Institute, NationalInstitutesof Health, Bethesda, Maryland 20892
Abstract
Inmammals,ureais the predominant end-product of
nitro-gen metabolism and plays a central role in the
urinary-concentratingmechanism. Urea accumulation in the renal
medulla is critical to theabilityof thekidneyto concentrate urine toanosmolalitygreaterthan systemic plasma.
Regula-tion ofureaexcretion and accumulationinthe renal medulla dependsonthefunctionalstateof specialized
phloretin-sen-sitive urea transporters. To study these transporters and their regulation of expression we isolated a cDNA which
encodes the rathomologue (rUT2)of rabbit UT2(You,G.,
C. P. Smith, Y. Kanai, W.-S. Lee, M. Stelzner, and M. A.
Hediger,etal. Nature(Lond.). 1993.365:844-847). Rat UT2 has 88% amino acid sequenceidentity to rabbit UT2 and 64%identitytotherecentlycloned humanerythrocyteurea
transporter, HUT1l (Olives, B., P. Neav, P. Bailly, M. A.
Hediger, G. Rousselet, J. P. Cartron, and P. Ripoch J.Biol.
Chem. 1994. 269:31649-31652). Analysis of rat kidney
mRNA revealed twotranscriptsof size 2.9 and 4.0 kb which had spatially distinct distributions. Northern analysis and insituhybridization showed that the 4.0-kbtranscriptwas
primarily responsive to changes in the protein content of the diet whereas the 2.9-kb transcript was responsive to
changesinthe hydrationstateof the animal. These studies reveal that theexpressionlevels of the two rUT2transcripts
aremodulatedby differentpathways to allow fluid and ni-trogen balance to beregulated independently.Our data
pro-videimportant insightsinto theregulationof the renalurea
transporterUT2 andprovideabasisonwhich to refineour
understandingof theurinary concentratingmechanism and its regulation. (J. Clin. Invest. 1995. 96:1556-1563.) Key
words: urea * membrane transport protein * nitrogen
me-tabolism * urinary concentratingmechanism * colon
Introduction
Carrier-mediatedureatransportplaysanimportantrole in
nitro-gen balance and water conservation (1). Urea formed in the
AddresscorrespondencetoMatthias A.Hediger,RenalDivision, Brig-ham and Women's Hospital, 75 Francis Street, Boston, MA 02115. Phone:617-732-5850;FAX: 617-732-6392. Wen-Sen Lee'scurrent ad-dress is CardiovascularBiology Laboratory,Harvard School of Public Health, Boston,MA02115.
Receivedfor publication23 December 1994 andacceptedin revised form2June 1995.
liver via theureacycleentersthecirculation and is freely filtered by the kidney. Theamountof filteredureaexcreted is regulated and depends on the physiological status of the animal. Protein restriction causes a decrease in the fraction of filtered urea excreted(2-4). This response reduces the loss of nitrogen from the body and serves to maintain plasma urea concentration, which would otherwise decrease in direct proportion to the lowering of nitrogen intake (2, 3, 5-7). Micropuncture studies have suggested that the decrease in urea excretion with low protein intake isaresult of increased urea absorption from the collecting ducts (6, 8, 9). Inagreement, measurementsin iso-latedperfused inner medullary collecting ducts
(IMCD)'
showan increase in phloretin-sensitive urea transport in the initial IMCD afterdietary protein restriction (7, 10).
Regulation of urea transport forms an integral part of the urinary concentrating mechanism. During antidiuresis, in-creasedplasma vasopressin stimulates arapid increase inurea
transport in the IMCD allowing urea to enter the medullary interstitium (11). Increasing urea transport in the IMCD in conjunction withNa,K,2CI cotransport in the thick ascending limb of the loop of Henle (12) enables the establishment and maintenance of thehypertonic medulla which, in turn, provides the osmotic gradient requiredforwaterreabsorption (13, 14). Urea absorbed in the IMCD is furthermore secreted into thin ascendinganddescending limbs anddescending vasa rectain
aprocess calledurearecycling. Thisrecycling pathway limits
urea dissipation from the inner medulla allowing the
mainte-nanceof thecorticopapillary osmotic gradient. The IMCD (15) and the descending vasa recta (16, 17) have been recently shownto containphloretin-sensitive urea transporters.
We haveisolatedbyexpressioncloninginXenopus oocytes
acDNA codingfor the vasopressin-regulatedurea transporter (18) which had been previously characterized by Knepper and colleagues using in vitroperfusedtubules( 1,11, 15 ).Recently, Ripoche and colleagues isolated acDNAfrom ahumanbone
marrow cDNA librarycoding for the human erythrocyte urea
transporter(HUTI 1)(19). Thelibrary screening strategythey
used toisolate this cDNA was based on the cDNA sequence of rabbit UT2. HUT11 has63% amino acididentitywith rabbit UT2 and has functional characteristics which are similar
toUT2.
Since little is known about theregulationofurea
transport-ers although they play a central role in nitrogen and water
homeostasis amajor goal of thisstudywas toisolate a cDNA
encodingtheratisoform ofrabbit UT2 andto usethisas a
probe
forNorthern analysisand in situhybridizationtodeterminethe1. Abbreviations used in thispaper: IMCD,innermedullary collecting duct; ISOM,innerstripeof the outermedulla.
J. Clin. Invest.
©TheAmericanSocietyfor Clinical Investigation,Inc. 0021-9738/95/09/1556/08 $2.00
regulation of rat UT2 (rUT2) mRNA expression in kidneys from animals maintainedatdifferentphysiologicalstates.
Methods
Clones were isolated from a nonamplified rat inner medulla Xgt22a cDNA library. Approximately 300,000 clones were screened using a gel-purified,32P-labeled(r7QuickPrime;PharmaciaBiotech Inc., Pisca-taway, NJ) rabbit UT2 cDNA. Hybridization was at370C (50% for-mamide) and the final wash was in 0.1XSSC-0.1% SDSat400C.Initial screeningyielded32clones,outof whicha2.9kb cDNAwasisolated and subcloned into the NotlISal1 site ofpSPORTL. Both strands of the cDNA clone were sequencedby the dideoxy-sequencing method using Sequenase V2.0 DNAsequencing kit(USB Biologicals, Cleve-land, OH).
cRNA wassynthesizedin vitro andmicroinjected(50 ng)into colla-genase treated and manually defolliculated Xenopus oocytes, as pre-viously described (20).AfterincubationinBarth's solutionfor3 d at 180C,oocytes werepreincubated in 200 mM mannitol, 2 mMKCI, 1 mMMgCl2, 1 mM CaCl2, 10 mM Hepes buffer,5 mMTris, pH7.4, for 1 h. To measure ureauptake,2.71Ci['4C]urea/mland 1 mMurea were addedto thepreincubation solution. Unlabeledurea wasfreshly deionized before use by passing through an ion-exchange column (AG501-X8(D), 20-50 mesh; Bio-Rad Laboratories, Richmond, CA). After uptake,oocytes werewashedwithice-cold uptake solution con-taining1 mM unlabeled urea,dissolvedin10%SDS,and the radioactiv-itywasmeasuredbyscintillationcounting. Inhibition of[14C]urea up-takeby0.35 mM or0.70 mMphloretinwasmeasuredbyaddition of phloretin to thepreincubationsolution 15 min before addition of[14C] -urea aspreviously described (18). Uptakeof[14C]urea(1 mM) was alsomeasuredin the presenceof 50mMmannitoland 150 mM thiourea (18). cRNA was translated in vitro using arabbit reticulocyte lysate system(PromegaCorp., Madison, WI) aspreviously described(21). Analysis wasby SDS-PAGEusinga 10%acrylamidegel.
To study the regional distribution ofrUT2 mRNA inthe kidney, polyA' RNAwasisolatedfrommicrodissectedregions ofthekidney (see Fig. 3) by the guanidine isothyocyanate method using cesium-trifluoroaceticacidfollowedbyoligo (dT)-cellulose column chromatog-raphy (Collaborative Biochemical Products,Bedford, MA). Poly A' RNA(3
tig/lane)
wasseparated in a 1% agarose gel in the presence of2.2 M formaldehyde and transferred to nylon filters. Filters were probedusinga32P-labeledfulllengthrandomprimedrUT2 cDNAprobe andhybridizedat42°C (50%formamide).Finalwashingwasin0.1x SSC-0.1% SDSat650C.Manipulation of dietary protein content. To study the effect of changing the dietary protein content onthe pattern of UT2 mRNA expression, groups ofpathogen-free male Sprague-Dawley rats were givenfreeaccessfor 4 wkto waterandeither a normal dietcontaining 18% protein (National Institutes ofHealth [NIH]-31 diet) or a low proteindietcontaining 8% protein (NIH-31LPdiet). The lowprotein dietwasisocaloric with the 18% proteindietandwas supplemented withmethionine, lysine,calcium,andphosphatetomatchlevelsin the normaldiet. The lowproteindiet was the same as that usedbyIsozaki etal. (7, 10) and has been shown to cause a significant decrease in bloodureanitrogen, urinaryureaexcretion rate, and prevent malnutri-tion(10).
Manipulation ofthehydrationstate.Tostudythe effectofchanging thehydrationstate onthe pattern ofexpression of UT2 mRNAs in the kidney, groups of pathogen-free male Sprague-Dawley rats were treated asfollows: (a)waterrestricted;ratshadfree access to food but water wasrestricted to 10 ml/day for 3 d. (b) Water diuretic; rats hadfree accessto watercontaining 10% glucose without additional food for 3 d.(c) Water diuretic then water restriction, rats had free access to water containing10%glucosewithout additional food for 3 d, then no water butfreeaccess tofood for 2 d. Control Rats weregiven free access to foodandwater. Thesetreatmentshave beenpreviously foundtoresult
insignificant changes inurine osmolality andnot to cause glucosuria
or
hyperglycemia
(22).Kidneys which were to be usedfor Northern analysis were removed andimmediatelyfrozen inliquid nitrogen. Kidneysdestined to beused for in situ hybridization were fixed with 4% paraformaldehyde and stored at-80'C. Total RNAwas isolated from whole kidneys by the guanidine isothyocyanate methodusing cesium-trifluoroacetic acid, or from innermedullaby acid phenol extraction (Tri-reagent; Molecular ResearchCenter, Inc. Cincinnati, OH) and separated as describedabove. Equal loading of total RNA (8
itg/lane)
for Northern analysis was achievedby ensuring that theintensity ofthe28S and18Sribosomal RNAbands,when stainedwith ethidium bromide and visualized under UV light, were constant between samples. Filterswere hybridizedat 480C(50%formamide)with a32P-labeled full length rUT2cDNAprobe andwashed in 0.1x SSC-0.1% SDSat650C.In situ hybridization ofrat kidney was performed as previously described(23).Briefly,7-pm slices wereprobedwith35S-labeledsense and antisense RNA probes synthesized fromthe full-lengthclone(in pSPORTl)afterlinearization of theplasmidDNAwithNotl orSall, using T7 (sense) or SP6 (antisense) RNApolymerase, respectively. RNA probeswerehydrolysed for50-raintogenerateprobes of 100 nucleotides. Afterhybridization,the dried tissuesectionswereexposed overnight to Hypermax film(AmershamCorp.,Arlington Heights,IL). For eachtreatment, in situ hybridization wasperformedon thekidney fromthreeseparateanimals.
Results
Lowstringency screening ofa ratkidney medullacDNAlibrary
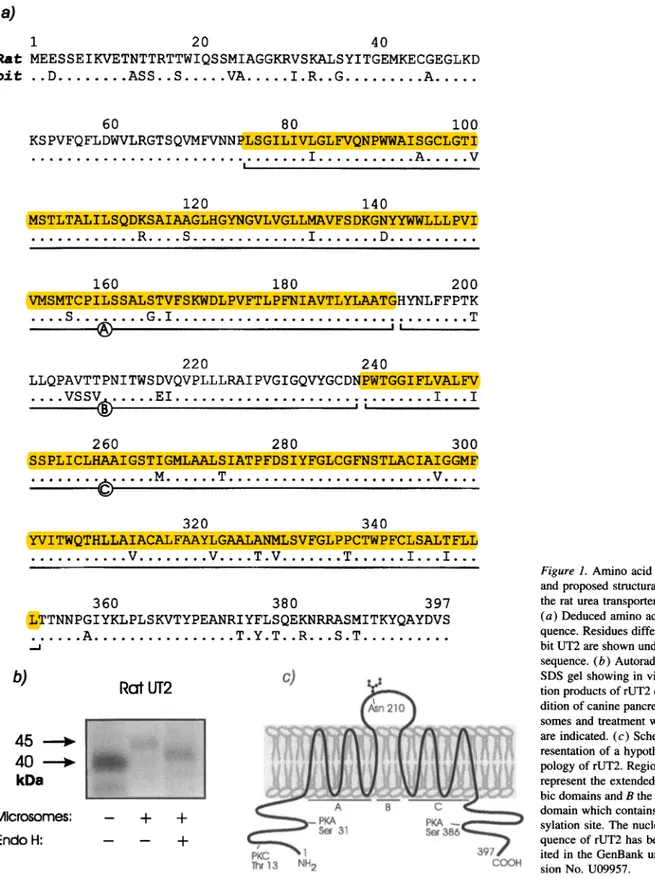
with thefull-length rabbit UT2-cDNA probe yieldeda 2,940-bpcDNA. Expression studiesinXenopus oocytes (see below) showedthat this cDNAencodesaphloretin-sensitive urea trans-porter. rUT2 cDNA has a polyadenylation sequence (AT-TAAA)atposition 2898,anopenreading frame from nucleo-tides 928to 2118 and encodes a397 residueproteinwhich has 88.1% amino acididentitywith rabbitUT2(Fig. 1 a)and64% identity with HUTi 1.
In vitro translation ofrUT2cRNAusing rabbit reticulocyte lysates gave a protein with an apparent Mr of 40 kD in the absence of, and 45 kD in the presence of canine pancreatic microsomes (Fig. 1 b). This 5-kD shiftinMrwasreversedby
treatmentwithendoglycosidase-H andisindicative of glycosyl-ation atasingle site. Fig. 1 cis aschematic representation of rUT2 showing a hypothetical topology and the predicted
N-glycosylation, cAMP phosphorylation and protein kinase C sites. rUT2 has two characteristic extended hydrophobic do-mains (18) shown in Fig. 1 c.
Injection ofrUT2 cRNA into Xenopusoocytes induced an
11-fold increase inthe uptake (3 min) of [14C]urea (1 mM) comparedtowater-injected control oocytes (data notshown). Uptake (90 s) of ureaby rUT2 was completely inhibited by
0.70 mM phloretin, 71% by 0.35 mM phloretin, and 62% by 150mM thiourea(Fig. 2). These properties are consistent with those of the vasopressin-regulated urea transporter UT2 (11, 15, 18).
HighstringencyNorthern analysis showedhybridization of rUT2 cDNA to species at 2.9 and 4.0 kb in kidney. These
transcripts were not detected in colon, ileum, jejunum,
duode-num,stomach, skeletal muscle, heart, liver, lung,orbrain(data
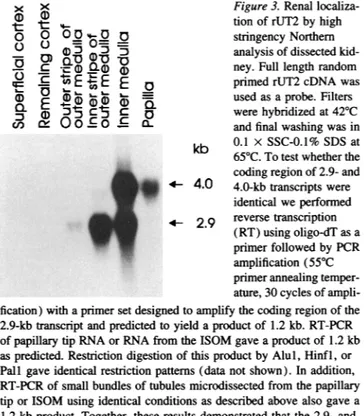
not shown). Fig. 3 shows thedistribution of rUT2 mRNA in
dissected ratkidney. Strong hybridization is seen at4.0 kbin
theinner part of the innermedulla, whereas the innerstripeof
the outermedulla(ISOM) showshybridization at2.9 kbonly.
In theouterpartof the inner medulla both the2.9 and 4.0 kb transcriptsarepresent. Insitu hybridization(Fig. 4c, control)
a)
1 20 40
Rat MEESSEIKVETNTTRTTWIQSSMIAGGKRVSKALSYI TGEMKECGEGLKD Rabbit . .D... ASS. .S...VA...I.R.G...A.
60 80 100 KSPVFQFLDWVLRGTSQVMFVNNPLSGILIVLGLFVQNPWWAISGCLGTI .I .A.V 120 140 MSTLTALILSQDKSAIAAGLHGYNGVLVGLLMAVFSDKGNYYWWLLLPVI . ...R. S.I.D. 160 180 200 VMSMTCPILSSALSTVFSKWDLPVFTLPFNIAVTLYLAATGHYNLFFPTK . S... G.I. T 220 240 LLQPAVTTPNITWSDVQVPLLLRAIPVGIGQVYGCDNPWTGGIFLVALFV ... VSSV .E ... E I I 260 280 300 SSPLICLHAAIGSTIGMLAALSIATPFDSIYFGLCGFNSTLACIAIGGMF .M.T.V.... 320 340 YVITWQTHLLAIACALFAAYLGAALANMLSVFGLPPCTWPFCLSALTFLL . V . T.V. T. I .I... 360 380 397 LTTNNPGIYKLPLSKVTYPEANRIYFLSQEKNRRASMITKYQAYDVS .
...A.
T.Y.T. .R...S.T. -Jb)
45
.-40 -*
kDa
Microsomes: Endo H:Rat
UT2
C)
A - ++
PKA__----r
S 31 - - + 1 lhrl13 NH2 21g B C PKA _ Se386''__* 397, COOHFigure1. Amino acid sequence andproposed structural model of therat ureatransporter(rUT2). (a)Deducedaminoacid se-quence.Residues different in rab-bit UT2areshown underneath the sequence.(b) Autoradiograph of SDSgel showinginvitro transla-tionproductsof rUT2 cRNA. Ad-dition of caninepancreatic micro-somesandtreatmentwith EndoH areindicated.(c) Schematic rep-resentation of a hypothetical to-pologyof rUT2.RegionsAand C representthe extended hydropho-bic domains andBthehydrophilic domainwhich contains the glyco-sylation site. The nucleotide se-quence of rUT2 has been depos-ited in the GenBank under acces-sion No.U09957.
theinner medullacorrespondingtothe2.9and 4.0 kbtranscript, respectively. A weakersignalwas seenin theouterpartof the inner medulla and probably represents a composite of the 2.9 and 4.0 kbtranscripts.
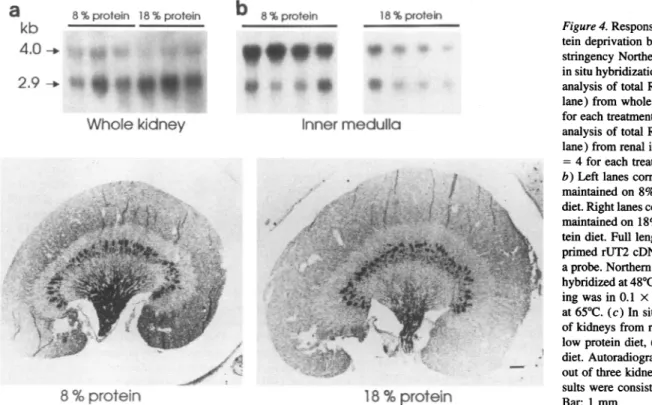
Thelevels ofexpression ofthetworUT2transcripts differed considerablybetween thekidneysofrats fedalowproteindiet
(8% protein) comparedtokidneys fromrats fedanormal diet
(18%protein). The same pattern was evident in different ani-mals that received the same treatment. Northern analysis showed that low protein diet caused anincreasein the4.0-kb transcriptin the innermedulla.Therewas nodetectablechange
in the levels of the2.9-kbtranscriptsinthisregion ofthekidney (Fig. 4,aandb;TableI).Theincreaseinthe 4.0-kbtranscript
was notdetected intheRNAfrom the wholekidney. Thiswas
ml
-ff-w
AA
v
-
_
--U) 0 0o 0 E q 0'l
02C
,:0 11 Rat UT2 c Inhibitor: none 0.35mM 0.70mM phlorelin phioretin Figure 2.Expression ofUT2 inXenopusoocytes.Fille' oocytesinjectedwithrUT2 cRNA,open bars represent oocytes. Bars representmean±SEM(n = 5-7).(a) U[14C]urea. (b) Inhibitionof1 mM [14C]ureauptake
by
mMphloretin. (c) Inhibitionof1 mM['4C]urea uptake thiourea. Alluptakeswerefor 90 s.
most probably due to dilution of the signal by other mRNA species.Insituhybridizationrevealed thattheincreasedamount
of the 4.0-kbtranscriptwas associated withanincrease in and spreading towards theoutermedulla of thehybridization signal inthe inner part of the inner medulla (Fig.4c).
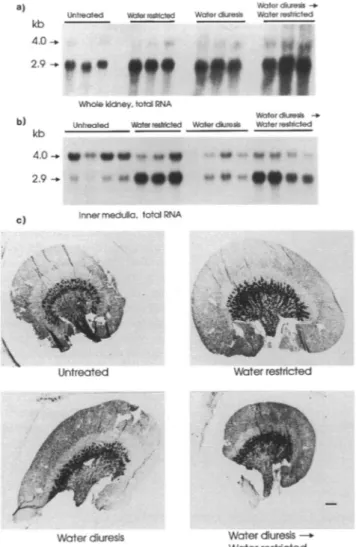
Largechanges in the amountof rUT2transcriptswere ob-served in responsetoalterations in thehydrationstate(Fig. 5; TableI). After 3 d ofwaterrestriction, the level of the2.9-kb transcript in the inner medulla and the wholekidneyincreased (Fig. 5, aandb). Incontrast,the level of the 4.0-kbtranscript
wasconsiderably decreased in the inner medulla whereas there
was no discernable change in the level of this transcript in whole kidney RNA, again most probably due to dilution by other mRNA species. In situ hybridization showed that the changes revealed by Northernanalysis corresponded tothe ap-pearanceofastrongsignal in theouterpartof the innermedulla,
an increase in the signal in the ISOM and a decrease in the signal in the papillary tip. The same pattern was evident in different animals that received thesame treatment.
Waterdiuresis, inducedby withdrawing access tofood and adding 10% glucose tothedrinkingwater, causedanincrease in theamountof the2.9-kb transcript in whole kidney, but had
noeffect on the levels of this transcript in the inner medulla (Fig. 5; Table I). No detectable change was observed in the
4.0-kbtranscriptin wholekidney,but a decrease in the amount
of thistranscript was evident in innermedullaryRNA. In situ hybridization revealed that thesechangeswereassociated with anincreasein thehybridization signalin the ISOM anda de-creaseinthesignal intheinner medulla. This pattern was con-sistentindifferentanimals thatreceivedthe sametreatment. We conclude that the increased hybridization signal in the ISOM representsthe observed increase in the 2.9-kb transcript and the decreasedhybridization signal in the inner medullarepresents the observed decrease inthe4.0-kb transcript.
x x
Figure
3. Renallocaliza-t
t(D
tionof rUT2by higho 0 0°0 - D stringency Northern
- 0
A)J
J23
7 analysis of dissectedkid-0 -a D)
a)
a) ney. Fulllength
randomH20
v-
E E primed rUT2 cDNAwasORNA- (3 ~ k i~ _ usedas aprobe. Filters :a)
31
Cj
C , werehybridized
at420C
n 00 C0 C and final washing was in kb 0.1 x SSC-0.1% SDS at 650C.To test whether the coding region of 2.9- and
I-.:X._ + 4.0 4.0-kb transcripts were identical we
performed
f--2.9- reversetranscriptionI
_ $- -- -. -0 7(RT)using oligo-dTas aprimer
followedby
PCR amplification(550C
primerannealingtemper-150 mM ature, 30 cycles of
ampli-thlourea
fication)withaprimersetdesignedtoamplifythecodingregionof the 2.9-kb transcriptandpredicted toyieldaproduct of 1.2 kb. RT-PCR dbarsrepresent of papillary tip RNA or RNA from the ISOM gave a product of 1.2 kbwater-injected as predicted.Restriction digestion of this product by Alul,Hinfl, or [ptake of1 mM Pall gave identical restriction patterns (data not shown). In
addition,
y0.35and0.70 RT-PCR of small bundles of tubules microdissected from the papillary We by 150 mM tip or ISOM using identical conditions as described above also gave a 1.2-kbproduct. Together, these results demonstrated that the 2.9- and 4.0-kbtranscriptshave identical or almost identicalcoding regions.
Waterdiuresis followed by waterrestriction causedan
in-creasein the amountof the 2.9-kbtranscriptin inner medulla and whole kidney (Fig. 5; TableI). A decrease in the level of the4.0-kb transcriptwasobserved in innermedulla, butnotin wholekidney.In situhybridization revealed the appearance of
a signal in the outerpart of the inner medulla anincrease of thesignal in the ISOM andareduction in thesignalin the inner part of the inner medulla. The same pattern was evident in different animals that received the same treatment.
Discussion
Central to the urinary concentrating mechanism are urea and
watertransporterslocated in theplasma membranes of collect-ing duct cells. Vasopressin stimulates ureaandwatertransport inthe terminal IMCD ( 11). Aconsiderable amountis known about thestructureandfunction of thepredominant vasopressin-regulatedwaterchannelAQP-CD (previously called WHC-CD) (24), whereas,relatively little is known about the vasopressin-regulatedureatransporter. IntheIMCD this transporter allows
urea to enterthemedullaryinterstitiumbyfacilitated diffusion (11)(Fig. 6). This process, together with the
Na,K,2C1
cotrans-port inthickascendinglimbsof thekidney nephron (12)(Fig. 6), helpstoestablish andmaintainsthecorticopapillaryosmotic
gradient whichprovides the driving force forwater
reabsorp-tion (25).
Of equal importance is the role of carrier-mediated urea
transport in reabsorption of urea. Feeding rats a diet low in
protein isknown to causethefraction of filteredureaexcreted bythekidneyto decrease. Accompanying thischangethere is
induction ofphloretin-inhibitable urea transport in IMCD (7, 10).Salvagingof ureanitrogenfrom thelargeintestine is also
8 % protein 18 % protein
Whole
kidney
8 %
proteli
b 8%protein18%protein
nner medul
a
*inner
medulla
In
18 %
protein
Figure 4.Responsetodietary pro-teindeprivationbasedonhigh stringencyNorthernanalysis and in situhybridization. (a)Northern analysisof total RNA(8 utg per
lane)from wholekidney(n = 3 for eachtreatment).(b)Northern analysisof total RNA(8jsgper lane)from renal inner medulla(n =4 for eachtreatment).(aand
b)Leftlanescorrespondto rats maintained on8%(low) protein diet.Rightlanescorrespondto rats maintained on 18%(control) pro-tein diet.Fulllengthrandom primedrUT2cDNA was usedas aprobe.Northern blotfilterswere hybridizedat480Cand final wash-ingwasin0.1 x SSC-0.1%SDS at65TC.(c)In situhybridization ofkidneysfromratsfed;(left)
lowprotein diet, (right) control diet.Autoradiographsshow one outofthreekidneys analyzed; re-sults were consistent in all three. Bar: 1 mm.
animals thatnormallyeat adiet low in protein and in protein-deprivedanimals(26,27). Phloretin-inhibitableurea
transport-ers areimplicatedin this process.
Previously, weisolateda cDNAencoding the vasopressin-sensitiveureatransporterfromrabbit kidney (UT2) (18). Here we report the cloning of the rat kidney isoform (rUT2), the tissue distribution of rUT2 and the effect of dietary protein restriction andmanipulationof thehydrationstate onregulation of rUT2 mRNA.
Structure andfunction ofrUT2. Rat and rabbit UT2 are
passive transporters characterized by a low affinity for urea.
The lowaffinities of these proteins (18, 28, 29) together with their unusual kinetic behavior(28) suggest thatthey constitutea
uniqueclassofproteins whichconceivablyrepresent atransition between facilitated transporters and channels. The predicted
structureof UT2 isfurthermore unusual.In contrast tothe char-acteristic pattern of several distincthydrophobictransmembrane segments interspersed withhydrophilicregions asobserved in
mostplasmamembrane transportproteins, orthecharacteristic ,8-barrelstructureof theporinsof the bacterialoutermembrane (30),UT2has twolargeextended hydrophobicdomains (Fig.
Table I. Summary of Changes in Levels of the 2.9 and 4.0-kb TranscriptsinWholeKidney and in Inner Medulla
Wholekidney Inner medulla
Transcript 2.9 4.0 2.9 4.0
Lowproteindiet + +
Water restricted 1 4
Waterdiuretic T '- + I
Waterdiuretic-water restricted It I
I,increase; 1, decrease; A,nochange.
1 c). It is conceivable that a major portion of the protein is entirely embedded in the membrane.
Cellular localization of rUT2 in kidney. Northern analysis
performed on rat tissueclearly demonstrated that thereare two
different rUT2 transcripts of 2.9 and 4.0 kb and that these are restricted to thekidney medulla. Twotranscripts of 3.0 and 4.0 kb wereobserved in rabbit kidney medulla and in contrastto rat a4.0-kbtranscriptwasalsodetected in rabbit colon (18).
Thepresenceofasignal in rabbit colon and the absence of
a signal in rat colon is of interest. Mammals do not possess urease,theenzymewhichcatalyzesthehydrolysisof ureainto NH3 and CO2. However, the bacteriathat reside in the large intestine have urease. It has beenproposed that ureadiffuses from the blood into the colon where it is broken down by
bacteria(26, 27).TheNH3released isreabsorbedandused as anitrogen source forresynthesis of amino acids. This mecha-nism isthoughttobeimportantinherbivorousmammals,such asrabbits,becausetheyeat adietlow inprotein.Incomparison,
rats are omnivorous and consume a diethigherinprotein. Our findingsindicatethat rabbitspossessafacilitated urea transport system in colon whereas rats do not. This may represent an
adaptationinresponsetothedifferentamountsofdietary protein
consumed bythese species.
In thekidneythe two transcripts areconfined tothe renal medulla andoccupy spatiallydistinctregions.The 2.9-kb
tran-scriptispredominantly expressedin theISOM whereas the
4.0-kbtranscript isconfined to the inner medulla. Bothtranscripts
arepresentinthe outerpart of the innermedulla.
This pattern ofrUT2 mRNA expression is similar tothat found inthe rabbitkidneywhich has a3.0-kbtranscriptin the outer medulla and a 4.0-kb transcript in the inner medulla.
Amplificationof the4.0-kbtranscriptinratkidney byPCRwith
primers flanking the complete coding region of the 2.9-kb cDNAyielded productsof thesame size withidentical
restric-tion mapssuggesting thatthe twotranscripts havethesameor
a
kb
4.0
-_
2.9
-_
C
-II. bkh,- V.. ..Untreated wrrewced Watercdresl
kb
4.0_- t is ~*
2.9 _ _
Wholekidney.totldRNA
Vordrubs
b) Untreated wateredtcted Waerdluress Waterredlueed
kb
4.0.
2.9
-.o-Innermedula.totci RNA
-.i
-X
v Untreated ws IFdW
Ir
W
IR Waterdiuresis Waterrestricted z. f I ' Water duresis-. WaterrestrictedFigure 5. Responsetoalterationof thehydrationstatebasedonhigh stringencyNorthernanalysisand in situhybridization. (a) Northern analysis oftotal RNA (8 Mgperlane)fromwhole kidney(n= 3for
eachtreatment). (b) Northern analysis of total RNA (8Mgperlane) fromrenal inner medulla (n =3-4 for each treatment). (a and b)From
lefttoright lanes correspondtokidneysfrom control, water-restricted, water-diuretic, andwater-diuretic then water-restrictedrats.Fulllength randomprimed rUT2 cDNAwasusedas aprobe. Northern blot filters
werehybridizedat480C and finalwashingwasin0.1 xSSC-O. I% SDS
at65TC.(c)In situhybridization ofkidneysfromratstreatedas indi-cated.Autoradiographsarerepresentativeofoneoutof thethreekidneys analyzedforeachtreatment;resultswereconsistentinall threekidneys.
Bar: 1 mm.
avery similar coding region and probably differ at 3' and/or 5' untranslated regions (see Fig. 3 legend). This relationship
was also found to be true for the two rabbit UT2 transcripts (18).
Basedon the presentdata and those of rabbit UT2 (18) it isreasonable to assume that thehybridization signal revealed
byin situ hybridization in the deeppart of the inner medulla (4.0-kb transcript) resides in the IMCD (Fig. 6). Physiological studies in the ratIMCD have demonstrated thepresence ofa
phloretin-sensitive transporter on both apical and basolateral plasma membranes of terminal IMCDcells (15, 31). As indi-catedabove, Pallone andcolleagues recently reported phloretin-sensitive urea transport in the descending vasa recta of the
outer medulla (16, 17). Studies by Imai and colleagues have demonstrated moderately high urea permeabilities in the thin descending limbs of the short loop nephrons of the outer me-dullasuggesting the presence of urea transporters ( 1, 32).
How-ever, phloretin sensitivity in this segment has not been estab-lished. The presence of the 2.9-kb transcript in the outer medulla agrees well with these findings and suggeststhatrUT2resides in the descending vasa recta and/or in thin descending limbs of short loops of henle(Fig. 6). Single segment localization of UT2 mRNA and immunocytochemistry will be necessary to confirm the actual sites of rUT2 expression.
Response todietary protein restriction. Consistent with the findings thatdietaryprotein restriction causes a decreasein the
fraction of filtered urea excreted and anassociated increase of phloretin-inhibitable urea transport in the IMCD, our data show thatfeeding a low protein diet induces an increase in the 4.0-kbtranscript which is associated with an increase in intensity andaspreading towards the outer medulla of the hybridization signal revealed by in situ hybridization (Fig. 4 c) (7). We
conclude therefore that the increase in the 4.0-kb transcript in
theinnermedulla is associated with an increase of functional
ureatransporters in IMCDcells. Dietaryprotein restriction has been found to affect in acoordinated manner the mRNA and protein levels of several enzymes involved in or associated with ureasynthesis such as hepatic glutaminase and carbamyl phosphate synthetase (33, 34). Factors such as glucagon and dexamethasone have been found to bring about coordinated induction of urea cycle enzymes (35). Whole animal studies suggested that the effect of dietary protein restriction on urea
excretion is due to glucocorticoids (2). Thus, glucocorticoids may contributeto long-termregulation of rUT2.
Thephysiological role of the adaptive changes of mamma-liankidneyinresponse to lowprotein diet are not clear although they appear tobe present in the few species that have been tested (2,3). Since in rats we found no evidence of urea transport in
colon, implying that salvaging ofureanitrogenfrom colon does
not occur, it is unlikely that the renal changes represent an adaptation gearedatpreserving nitrogen balance. The changes may, however, function to preserve blood ureaconcentrations
so as tomaintainareserveofurea toenableurinetobe
concen-trated, should the need arise.
Responses tomanipulation of the hydration state. Littleis
known about the regulation of urea transport in response to chronic changes in the hydration state. In the present study,
water restriction was found to induce an increase in the level of the 2.9-kb transcript. This correspondedto a largeincrease in thehybridization signal in theinner medulla and the ISOM. Furthermore, the amount of 4.0-kb transcript decreased in the inner medulla.Incontrast tothese results is the increase of the 4.0-kb transcript in theinner medulla induced by low protein diet. Recently, the expression levels of several other proteins
andtheir mRNAs have been found to beregulatedin response
to restriction ofwaterintake. For example, large increases in
expression ofthevasopressin-regulatedwaterchannelAQP-CD
occurs in ratcollectingducts inresponse tothirsting (36) and
vasopressininfusion(37). Furthermore,inresponseto hyperto-nicity,there isup-regulationofproteinssuch asaldosereductase andosmolyte transporters whichregulate thecellular levels of organic osmolytes in the renal medulla(22, 38). The
mecha-nismsof theseregulatoryprocessesareunderinvestigationand remainlargelyunknown. However, it seems likelythat
mecha-a)
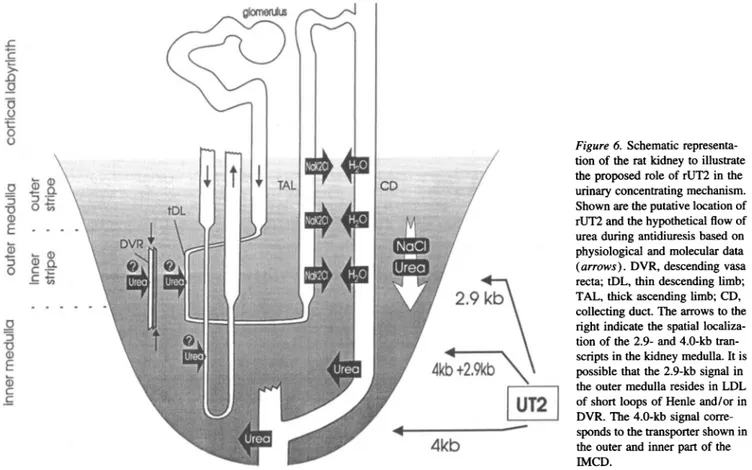
Figure6. Schematic
representa-tion of theratkidneytoillustrate theproposed role of rUT2 in the urinaryconcentrating mechanism. Shownaretheputative location of rUT2 and the hypothetical flow of
ureaduringantidiuresis basedon
physiological and molecular data (arrows).DVR,descendingvasa
recta;tDL, thindescending limb; TAL,thick ascending limb; CD, collecting duct. Thearrowstothe right indicate thespatial localiza-tionof the2.9- and 4.0-kb
tran-scriptsinthe kidneymedulla. It is possible that the 2.9-kb signalin theoutermedulla resides in LDL ofshort loopsof Henle and/or in DVR. The4.0-kb signal
corre-spondstothetransportershownin theouterand innerpartof the IMCD.
nisms will bediscovered thataresharedamong these proteins
andrUT2 whichorchestrate theantidiureticresponse.
It isofinterestthatwaterdiuresis increasedtheamountof
the2.9-kb transcript in RNA isolated from the whole kidney, butnotin RNA isolated from the inner medulla. Thischange
wasassociated withanincrease in theintensityof the
hybridiza-tionsignalinthe ISOM. We conclude from this that the level of the2.9 kbtranscriptincreasedspecificallyintheISOM. This is in contrastto theresponse to antidiuresis wherethe 2.9-kb transcriptincreased in both inner andoutermedulla. This
sug-gests differential segmental regulationof the 2.9-kbtranscript, possibly by multiplehormones and/or due to the presenceof
differentreceptorsorsensorsexpressedincellsofkidneyinner andouter medulla.
Proposeddifferential regulatorymechanismsofrUT2
tran-scripts. Protein dietary restriction was found to cause an
in-creaseinthe4.0-kbtranscriptwhereasthe2.9-kbtranscriptwas
sensitive to dehydration. This indicates that regulation of the 4.0-kb transcript is independent and distinct from thatof the
2.9-kb transcript. Thus, theprotein restrictionresponse maybe
primarily dueto hormones, e.g., glucocorticoids, whereas the responsetodehydrationmaybemediatedbydifferent hormones
such as vasopressin. The factors which regulate thetwo
tran-scripts and confersegmentally distinct responseswill need to beidentified as well astheirmechanism of action. Moreover,
like the urea cycle enzymes, two of which are regulated by
transcriptionalcontroland threeby stabilizationofmRNA(35),
rUT2 mRNA levels mayberegulated byone orbothofthese
mechanisms.
In conclusion, rUT2 is an unusually hydrophobic, highly
conserved(88.1%identical torabbitUT2), structurally unique
transporterwhichplaysacentral role intheurinary
concentrat-ing mechanism and regulation of nitrogen balance. Itspattern of tissuedistribution isspeciesspecificandprobablyassociated withtheproteincontentof the diet.rUT2mRNA isextremely responsive to changes in the physiological state ofthe animal
and the pattern of theresponses are specific to thefunctional
state of the kidney. This regulation at the mRNA level is in contrasttothat of othertransporterssuchasintestinal and renal
Na+/glucose cotransporters which are regulated primarily at
the translational orposttranslational levels (39). The 2.9- and
4.0-kbtranscriptsof rUT2areindependently regulatedand their message levelsmost likely are controlledby multiple factors.
In conjunction with future localization ofrUT2 in thekidney
innerandoutermedulla,thesedata will leadtoabetter
under-standingof thedynamicsandregulationofrenalurea
reabsorp-tion andurinaryconcentration.
Acknowledgments
C.P. Smith isaNationalKidneyFoundation fellow and W.-S. Lee isa
Juvenile Diabetes Foundation International fellow. J.M. Sands
per-formed this workduringtenureofanEstablishedInvestigatorship from American Heart Association. This work was supported by National Institute of Health grants R29-DK41707 and RO1-DK45688 to J.M. Sands andRO1-DK707844toM.A.Hediger.
References
1.Marsh,D.J.,and M. A.Knepper.1992. Renalhandlingofurea.InRenal
Physiology. E. E.Windhager, editor. OxfordUniversity Press,Oxford. 1317-1347.
2.Knepper,M.A.,R.A.Danielson,G.M.Saidel,and K. H. Johnston.1975.
-c t
n
D O -0 a) 0 OC_) 4-0. 0 0) c aEffects ofdietary proteinrestriction andglucocorticoid administration on urea excretion in rats Kidney Int. 8:303-315.
3. Schmidt-Nielsen, B., J. M. Barrett, B.Graves, and B. Crossley. 1985. Physiological and morphological responses of the rat kidney to reduceddietary
protein. Am. J. Physiol. 248:F31 -F42.
4.Peil, A. E., H. Stolte, and B.Schmidt-Nielsen. 1990. Uncoupling of glomer-ularandtubular regulation of urea excretion in rat. Am. J.PhysioL
258:F1666-F1674.
5. Schmidt-Nielsen, B. 1958. Urea excretion in mammals. Physiol. Rev. 32:139-168.
6. Lassiter, W. E., M. Mylle, and C. W. Gottschalk. 1966. Micropuncture
studyof urea transport in rat renal medulla. Am. J.Physiol. 210:965-970. 7.Isozaki, T., J. W. Verlander, and J. M. Sands. 1993. Lowproteindiet alters ureatransportand cell structure in the rat initial innermedullary collectingduct. J.Clin. Invest. 92:2448-2457.
8.Ullrich,K.J., G. Rumrich, and B.Schmidt-Nielsen. 1967. Urea transport in the collecting duct of rats on normal and lowproteindietPflugersArch.
295:147-156.
9.Danielson,R. A., B.Schmidt-Nielsen,and C.Hohberger. 1970.
Micropunc-turestudyoftheregulationof ureaexcretion bycollectingducts in rats onhigh
andlowproteindiets. In Urea and theKidney.B.Schmidt-Nielsen and D. W. S. Kerr, editors.Excerpta Medica, Amsterdam.375-384.
10.Isozaki,T., A.G. Gillan,C. F. Swanson, and J. M. Sands. 1994. Protein restriction sequentially induces new urea transport processes in rat initial IMCD. Am.J.Physiol. 266:F756-F761.
11.Sands, J. M., H.Nonoguchi, and M. A. Knepper. 1987. Vasopressin effects onureaandH20transport in innermedullary collecting duct subsegments. Am. J.Physiol.253:F823-F832.
12.Gamba, G., A. Miyanoshita,M. Lombardi, J. Lytton, W.-S. Lee, M. A.
Hediger,and S. C. Hebert. 1994. Molecular cloning, primary structure and charac-terization of two members of the mammalian electroneutral sodium-
(potassium)-chloridecotransporterfamily expressedin kidney. J. Biol. Chem. 269:17713-17722.
13.Sands, J. M., and J. P. Kokko. 1990. Countercurrent system. Kidney Int. 38:695-699.
14.Roy, D. R., H. E. Layton, and R. L. Jamison.1992.Countercurrent mecha-nism and itsregulation.InTheKidney:PhysiologyandPathophysiology.D. W. Seldin and G.Giebisch,editors. RavenPress,Ltd. NewYork. 1649-1692.
15.Chou,C. L., and M. A. Knepper. 1989. Inhibition of urea transport in inner medullarycollectingductby phloretinand ureaanalogues.Am. J.Physiol. 257:F359-F365.
16.Pallone, T.L., J.Work,R. L.Mayers, and R. L. Jamison. 1994. Transport of sodium and urea in outermedullary descendingvasa recta. J. Clin. Invest. 93:212-222.
17.Pallone, T. L. 1994. Characterization of the urea transporter in outer
medullary descendingvasarecta. Am.J.Physiol. 267:R269-R267.
18. You, G., C. P. Smith, Y. Kanai, W.-S. Lee, M. Stelzner, and M. A.
Hediger. 1993. Cloningand characterization of the vasopressin-regulated urea transporter. Nature(Lond.). 365:844-847.
19.Olives, B., P. Neau, P. Bailly, M. A. Hediger, G. Rousselet, J.-P. Cartron, and P. Ripoche. 1994. Cloningandfunctionalexpressionofaureatransporter from human bone marrow cells. J.Bio.Chem. 269:31649-31652.
20. Hediger, M. A., M. J. Coady, S. I.Tyson, and E. M. Wright. 1987.
Expression cloning and cDNA sequencing of theNa+/glucose co-transporter. Nature(Lond.).330:370-381.
21. Wells, R.G., A. M. Pajor, Y. Kanai, E. Turk, E. N. Wright, and M. A. Hediger.1992. Cloning of a human kidney cDNA with similarity to the sodium-glucose cotransporter. Am. J. Physiol. 263:F459-F465.
22. Sands, J. M., and D. C. Schrader. 1990. Coordinate response of renal medullary enzymes regulating net sorbitolproductionindiuresis andantidiuresis.
J. Am. Soc.Nephrol. 1:58-65.
23. Kanai, Y., M. G. Stelzner, W.-S.Lee,R.G.Wells,D.Brown, and M. A. Hediger. 1992.ExpressionofmRNA(D2) encodingaproteininvolved in amino acid transport in S3proximaltubule. Am. J.Physiol. 263:F1087-F1093.
24.Fushimi, K., S. Uchida, Y. Hara, Y.Hirata,F.Marumo, and S. Sasaki. 1993.Cloning and expression ofapicalmembrane waterchannel of ratkidney collectingtubule Science(Wash.DC).361:549-552.
25. Knepper, M. A., and F.C. Rector, Jr. 1991. Urinaryconcentrationand dilution. In The Kidney. B. M. Brenner and F. C. Rector, Jr.,editors. W. B. SaundersCo.,Philadelphia,445-482.
26. Moran, B. J., and A. A. Jackson. 1989. l5N-ureametabolism in the functioning human colon: luminal hydrolysis and mucosal permeability. Gut. 31:454-457.
27. Langran, M., B. L. Moran, J. L. Murphy, and A. A. Jackson. 1992. Adaptation to a diet low in protein:effect of complex carbohydrate upon urea kineticsin normal man.Clin.Sci.82:191-198.
28. Chou, C. L., J. M. Sands, H. Nonoguchi, and M. A. Knepper. 1990. Concentration dependence of urea and thiourea transport in rat innermedullary collectingduct. Am. J.Physiol. 258:F486-F494.
29. Toon, M. R., and A. K. Solomon. 1991. Transport parameters in the human red cell membrane: solute-membrane interactions ofamides and ureas. Biochim.Biophys. Acta. 1063:179-190.
30. Cowan,S. W.,T. Schirmer, G.Rummel, M. Steiert, R. Ghosh,R. A. Pauptit, J. N. Jansonius, and J. P. Rosenbush. 1992. Crystal structuresexplain
functionalproperties of two E. coliporins.Nature(Lond.).358:727-733. 31.Star,R. A.1990.Apicalmembrane limits ureapermeationacross the rat inner medullary collectingduct. J. Clin. Invest. 86:1172-1178.
32.Imai,M., M.Hayashi,and M. Akari. 1984. Functionalheterogeneityof the descending limbsof Henle's loopPlugersArch.402:393-401.
33.Watford,M., N. Vincent, Z. Zhan, J. Fannelli, T. Kowalski, and Z. Kova-cevic. 1994. Transcriptional control of rathepatic glutaminase expression by dietary proteinlevel andstarvation. J. Nutr. 124:493-499.
34.Meijer,A.J., W. H. Lamers, and A. F. M.Chamuleau. 1990.Nitrogen
metabolism and ornithinecycle function.Physiol.Rev.70:701-748.
35.Ulbright, C.,and P. J.Snobgrass. 1992. Coordinate induction of the urea
cycleenzymesby glucagonand dexamethasoneisaccomplished bythreedifferent mechanisms. Arch. Biochem.Biophys.301:237-243.
36.Nielsen, S., S. R.DiGiovanni,E. I.Christensen, and M. A. Knepper. 1993. Cellular and subcellular immunolocalization ofvasopressin-regulatedwater channelin ratkidney.Proc. Natl.Acad. Sci. USA. 90:11663-11667.
37.DiGiovanni, S. R., S.Nielsen,E. I. Christensen, and M. A. Knepper. 1994.Regulationofcollectingduct water channelexpression by vasopressinin Brattleboro rat. Proc.Natl.Acad. Sci. USA. 91:8984-8989.
38.Burg,M.B.,and A.G. Garcia-Perez. 1992. Howtonicityregulatesgene
expression. J. Am.Soc.Nephrol. 3:121-127.
39.Hediger,M.A., and D. B. Rhoads. 1994. Molecular physiology of sodium-glucose transport. Physiol.Rev.74:993-1026.