Seleno-independent glutathione peroxidases
More than simple antioxidant scavengers
Ste´phane Herbette1, Patricia Roeckel-Drevet1and Joe¨l R. Drevet2
1 UMR 547-PIAF, INRA⁄ Universite´ Blaise Pascal, Aubie`re Cedex, France
2 UMR 6547-GEEM, CNRS⁄ Universite´ Blaise Pascal, Aubie`re Cedex, France
Introduction
Glutathione peroxidase (GPX; EC 1.11.1.9) catalyses the reduction of H2O2 or organic hydroperoxides to
water or the corresponding alcohols using reduced glutathione. GPX was discovered in 1957 [1] as an enzyme that protects erythrocytes against oxidative damage. Later, several additional types of mammalian GPX were isolated, and those enzymes were shown to be expressed in a wide range of organisms. In mam-mals, together with superoxide dismutases (EC 1.15.1.1) and catalases (EC 1.11.1.6), GPX constitutes the enzymatic antioxidant system which recycles active oxygen species (AOS) and limits their toxicity. The mammalian GPX family is divided into six clades according to their amino-acid sequence, substrate
specificity and subcellular localization (Table 1): classi-cal or cytosolic (GPX1), the first mammalian GPX to be identified [1–3]; gastrointestinal (GPX2); plasma (GPX3); phospholipid hydroperoxide (PHGPX or GPX4); epididymal (GPX5); olfactory epithelium (GPX6).
Except for GPX5 and GPX6, all mammalian GPX proteins contain a selenocysteine (SeCys) residue instead of a Cys residue (Table 1). SeCys is considered to be the 21st amino acid. Its cotranslational incorpor-ation into protein is mediated by a SeCys tRNA, the presence of a stem loop structure located downstream from its UGA codon and designated as the SECIS ele-ment (SeCys insertion sequence), and by the recruit-ment of a specific elongation factor. This SeCys insertion system recognizes the opal codon UGA as a
Keywords
free-radical scavenger; glutathione
peroxidase; oxidative stress; selenocysteine; thioredoxin
Correspondence
J. Drevet, Universite´ Blaise Pascal, CNRS UMR 6547 GEEM, 24 avenue des Landais, 63177 Aubiere cedex, France
Fax: +33 4 73 40 52 45 Tel: +33 4 73 40 74 13
E-mail: joel.drevet@University-bpclermont.fr
(Received 16 January 2007, revised 2 March 2007, accepted 7 March 2007)
doi:10.1111/j.1742-4658.2007.05774.x
Glutathione peroxidases (GPXs, EC 1.11.1.9) were first discovered in mam-mals as key enzymes involved in scavenging of activated oxygen species (AOS). Their efficient antioxidant activity depends on the presence of the rare amino-acid residue selenocysteine (SeCys) at the catalytic site. Nonse-lenium GPX-like proteins (NS-GPXs) with a Cys residue instead of SeCys have also been found in most organisms. As SeCys is important for GPX activity, the function of the NS-GPX can be questioned. Here, we highlight the evolutionary link between NS-GPX and seleno-GPX, particularly the evolution of the SeCys incorporation system. We then discuss what is known about the enzymatic activity and physiological functions of NS-GPX. Biochemical studies have shown that NS-GPXs are not true GPXs; notably they reduce AOS using reducing substrates other than glutathione, such as thioredoxin. We provide evidence that, in addition to their inefficient scavenging action, NS-GPXs act as AOS sensors in various signal-transduction pathways.
Abbreviations
AOS, activated oxygen species; GPX, glutathione peroxidase; NS-GPX, nonselenium GPX; PHGPX, phospholipid hydroperoxide GPX; SeCys, selenocysteine; TPx, thioredoxin peroxidase.
signal to insert a SeCys residue. Selenoproteins have been found in archaebacteria, eubacteria and eukaryo-tes, but not in all organisms. Although SeCys is rarely used in protein synthesis, it appears to be essential for selenoprotein function, and is found at the catalytic sites of many selenoenzymes [4]. This has been demon-strated, for instance, by point mutations of SeCys to Cys in GPX1 as well as in GPX4, which both led to a dramatic fall in enzymatic activity [5,6]. SeCys and Cys differ in a single chalcogen atom (Se versus S). The selenol group is entirely ionized at physiological pH (pKa¼ 5.2), whereas the thiol group of Cys is only
partially ionized under similar conditions (pKa¼ 8).
Once ionized, the thiol or selenol group is able to react with H2O2 or hydroperoxides. The catalytic triad of
amino-acid residues, i.e. Trp, Glu, Cys or SeCys, is common to all GPXs (Fig. 1, alignment).
Although GPX-like proteins have been found in most organisms studied, expression of SeCys-contain-ing GPX is restricted to only a few of them. Besides mammals, SeCys-containing GPXs have been found in other vertebrates such as Gallus gallus [7] and Danio rerio [8]. Outside the vertebrate clade, SeCys-containing GPXs have been reported in the parasitic helminth Schistosoma mansoni [9], the nematode Setaria cervi [10], the arthropod Boophilus microplus [11], the alga Chlamydomonas reinhardtii [12], and also in the DNA sequence of the virus HIV-1 [13]. In addition to the seleno-dependent GPXs, nonselenium GPXs (NS-GPXs) are found in these organisms and are widely represented in mammals [14,15]. NS-GPXs have been found in higher plants [16–19], yeast [20], the protozoan Trypanosoma cruzi [21], the nematode Brugia pahangi [22] and the cyanobacterium Synecho-cystis [23]. To our knowledge, neither selenoproteins nor SeCys insertion systems have been found in these organisms. In the prokaryotes Escherichia coli and Neisseiria meningitidis and in the eukaryote Plasmo-dium falciparum, only NS-GPXs have been found, although these organisms can express selenoproteins [24,25].
Although many physiological functions of the thor-oughly investigated seleno-dependent GPXs have been elucidated, especially in mammals [26,27], NS-GPXs have, so far, been the subject of few studies. This is essentially because they have been found to be a lot less efficient at detoxifying AOS and peroxides than seleno-dependent GPXs. In this study, we conducted an analysis of the evolutionary relationships between GPXs, particularly the evolution of the SeCys incor-poration system. We then focused on the enzymatic activity and proposed physiological functions of the NS-GPXs. Table 1. Characteristics of GPX proteins from mammals. The tissue expression given for GPX1, GPX3 and GPX4 are the most representative, as the protein or the mR NA has been found in all tissues investigated. For GPX6, the presence of SeCys was confirmed in man and pig [50], and invalidated in rat and mouse [15]. Numeric nomenclature GPX1 GPX2 GPX3 GPX4 GPX5 GPX6 Literature denomination cGPX (cytosolic or classical) GI-GPX (gastro-intestinal) pGPX (plasmatic) PHGPX (phospholipid hydroperoxide) eGPX (epididymal) OMP (olfactory-metabolizing protein) Tissue distribution Red cells, liver, lung, kidney, … Stomach, intestine, liver Kidney, lung, epididymis, vas deferens, placenta, seminal vesicle, heart, muscle, … Testis, spermatozoa, liver, kidney, heart, brain, … Epididymis, spermatozoa Olfactory epithelium Cellular localization Cytosol, nucleus, mitochondria Cytosol, nucleus Secreted, cytosol Nucleus, cytosol, mitochondria, membrane-bound Secreted Molecular mass (kDa) 21 22 22.5 19 24 22.5 Multimerization 4 4 4 1 2 4 SeCys Yes Yes Yes Yes No Yes ⁄No First reference Mills, 1957 [1] Chu et al. 1993 [144] Takahashi et al. 1987 [145] Ursini et al. 1985 [35] Ghyselink et al. 1989 [146] Dear et al. 1991 [15]
Phylogenetic evaluation of NS-GPXs
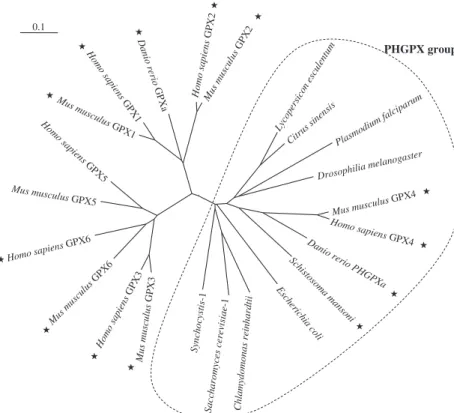
Most NS-GPXs belong to the PHGPX group We compared GPX amino-acid sequences from var-ious organisms (Fig. 2). This analysis was carried out independently of the presence⁄ absence of SeCys in the proteins. The tree shows that the GPX family can be subdivided into three broad groups. One group includes the mammalian GPX1 and GPX2 and iso-forms from other vertebrates such as the zebra fish D. rerio[8]. Another group comprises the mammalian GPX3, GPX5 and GPX6 proteins. A third group, the PHGPX group, includes the mammalian GPX4 and most of the GPXs from various organisms. This analy-sis is in agreement with previous phylogenetic evalua-tions based on gene structures of mouse GPX1, GPX3, GPX4 and GPX5 [28] or based on amino-acid sequences from various mammalian GPXs [28,29].Except for some higher vertebrate GPXs, most of the GPX proteins investigated belong to the PHGPX group. An exception to this situation is, for example, a GPX protein from the parasitic nematode B. pahangi (persists in the human lymphatic system and is
respon-sible for lymphatic filariasis), which shows the highest similarity to GPX3 from Homo sapiens [22]. Proteins similar to this B. pahangi GPXs have also been found in other parasitic nematodes [30,31]. One possible explanation is that the secreted GPX3 has been trans-ferred from vertebrates to nematodes by horizontal gene transfer. Genetic analyses have revealed the importance of horizontal gene transfer between organ-isms, especially between a host and its parasitic or symbiotic organism [32]. Another observation in favour of the hypothesis of horizontal gene transfer is the close similarity of GPX from HIV-1 to human GPX3 [13].
In mammals, it has been postulated that the GPX gene family has evolved from a common gene ancestor by duplication events followed by random integration in the genome [28,29]. The ancestor was proposed to be represented by the GPX4⁄ PHGPX sequence. After a first duplication event, the ancestral PHGPX would have diverged into two groups, one group represented by genes encoding the intracellular GPX1 and GPX2 proteins, respectively, and the second represented by genes encoding the secreted GPX3, GPX5 and GPX6 proteins. On the basis of the low similarities found
Fig. 1. Alignment of GPX amino-acid sequences from various organisms. The sequences were compared and aligned
usingCLUSTALWsoftware [143]. Nucleotide⁄
protein sequence accession numbers
(GenBank⁄ SWISS-PROT): C. reinhardtii
(AB009083), C. sinensis (Q06652), E. coli (P06610), L. esculentum (Y14729), Mus musculus GPX1 (P11352), GPX2 (BC054848), GPX3 (U13705), GPX4 (O70325), GPX5 (P21765) and GPX6 (NP_663426), P. falciparum (Z68200), S. cerevisiae-1 (P30614), Sch. mansoni (Q00277), Synechocystis PCC 6803 (NP401201). Amino-acid residues of the catalytic triad are marked with an asterisk. Residues common to all proteins are indica-ted by white letters on a black background, whereas those shared by more than seven proteins are shadowed.
between PHGPXs and other GPX proteins, the phylo-genetic divergence of the PHGPX gene and the genes encoding other GPX types has been estimated to have happened approximately one billion years ago [33]. In fact, mammalian PHGPX appears to be more closely related to GPXs from various organisms than to all mammalian GPX types. Hence, the PHGPX group must be rated as a phylogenetically old achievement of the GPX family. This suggests that PHGPX proteins, including most NS-GPXs, would fulfil an important function conserved in various organisms and distinct from other mammalian GPX functions. PHGPX can also be distinguished from other GPX proteins, as they are the only ones that are monomeric (Table 1). PHGPX proteins do not possess the subunit interac-tions sites identified by X-ray crystallography, for example, in the bovine GPX1 protein [34]. Large gaps in the PHGPX sequences are observed when aligned with sequences of other GPX types (Fig. 1). Accord-ingly, PHGPX proteins have been shown to be expressed as monomers in several species including mammals, plants and nematodes [9,35,36]. However, a PHGPX from Populus trichocarpa was recently shown to be expressed as a homodimer [37], despite the pres-ence of gaps in its sequpres-ence. Determination of the structural components responsible for the dimerization of this plant PHGPX would help to clarify the extent
of oligomerization in the PHGPX group. Taken together, these data support the fact that most NS-GPXs form a separate clade from the mammalian PHGPX.
Although the GPX4⁄ PHGPX gene is present as a single copy in mammals and organisms such as E. coli, several PHGPX genes have been found per haploid genome in many species. For instance, seven and six PHGPX genes were isolated from Arabidopsis thaliana and P. trichocarpa [37], respectively, and were the only GPX type found. For Arabidopsis, comparison of their structures showed that the number of exons is similar (five to seven), the exon–intron structure is well con-served between some gene pairs, and neighbouring genes are also conserved between these pairs [38]. These observations support the idea of duplication events leading to the A. thaliana PHGPX gene family. The seven Arabidopsis PHGPX genes have been shown to be differently regulated at the transcriptional level, and, on the basis of sequence criteria, the proteins have been proposed to display different cellular locali-zations in A. thaliana [38]. Functionality of the differ-ent PHGPX isoforms has been investigated in some species. In Saccharomyces cerevisiae, three PHGPX-encoding sequences have also been found to be differ-entially regulated and to encode functional enzymes [20]. The existence of two PHGPX genes and the
Fig. 2. Phylogenetic tree of GPX proteins from mammals and various organisms. The amino-acid sequences were compared using
CLUSTALWsoftware [143], and thePHYLIP
program was used to construct the tree.
Nucleotide⁄ protein sequence accession
numbers (GenBank⁄ SWISS-PROT):
B. pahangi (X69128) C. reinhardtii (AB009083), C. sinensis (Q06652), D. rerio GPXa (AY215589) and PHGPXa (AY216590), D. melanogaster (AAO41409), E. coli (P06610), Homo sapiens GPX1 (P07203), GPX2 (P18283), GPX3 (P22352), GPX4 (P36969), GPX5 (NM_001509) and GPX6 (NM_182701), L. esculentum (Y14729), Mus musculus GPX1 (P11352), GPX2 (BC054848), GPX3 (U13705), GPX4 (O70325), GPX5 (P21765) and GPX6 (NP_663426), P. falciparum (Z68200), S. cerevisiae-1 (P30614), Sch. mansoni (Q00277), Synechocystis PCC 6803 (NP401201). A bar (0.1) indicates branch lengths. Proteins containing a SeCys are shown by a star.
biochemical properties of the corresponding proteins have also been reported in Synechocystis [23]. In Try-panosoma cruzi, the two existing PHGPX genes have been shown to encode proteins with different cell local-ization [39]. Together these data suggest that each indi-vidual PHGPX gene has evolved with specific functions via tissue-specific and cell-specific expression. In support of this hypothesis, a PHGPX isoform often displays more similarities to GPX isoforms from other species than to another isoform from the same species, provided that the two species are closely related. This has recently been illustrated in plants by a phylo-genetic analysis of the amino-acid sequences of the PHGPX gene family from several species [40]. For example, a PHGPX from Oryza sativa shares more homology (up to 98%) with PHGPX proteins from several monocotyledons and eudicotyledons than with any other rice PHGPX (65%).
It should be mentioned that, although most NS-GPXs belong to the PHGPX family, a few, such as GPX5 in mammals and a GPX in nematodes, are not included in this gene family but are in fact closer in sequence to the GPX3 subgroup. Conversely, some seleno-dependent GPXs belong to the PHGPX family (Fig. 2). This raises the question of the evolution of seleno-dependence or seleno-independence in the GPX family. In other words, have GPXs evolved by the acquisition of SeCys or by loss of SeCys?
No SeCys in GPX – remnant or innovation? Sequences that encode putative GPX-like proteins can be found in eukaryotes and eubacteria, suggesting that GPX evolved before the separation of eukaryotes and eubacteria. Although most NS-GPXs belong to the PHGPX gene family, some, such as GPX5, GPX6 in rodents and a GPX in nematodes, are not included in this gene family, and conversely some seleno-depend-ent GPXs belong to it (Fig. 2). Hence, the evolution of GPX seleno-dependence is unclear. NS-GPXs are pre-valent in living organisms, although SeCys is import-ant for the activity of SeCys-containing GPXs. This paradox led to the question of whether GPXs have lost SeCys or whether others have gained it.
Selenoproteins have been found in archaea, eubacte-ria and eukaryotes [4] with common SeCys incorpor-ation features, such as the use of the same UGA codon and the use of a tRNA for SeCys that is ini-tially aminoacetylated with a serine [4]. In addition, SeCys tRNA and selenophosphate synthase, which provides the selenium donor, are conserved in all selenoprotein-encoding genomes. These observations suggest that the insertion of SeCys in the genetic code
occurred before the separation of archaea, eubacteria and eukaryotes. As several organisms appear to lack selenoproteins, it has been proposed that SeCys may be a relic of the primordial genetic code [41,42]. According to these authors, UGA was initially a sense codon for SeCys, which was used in many enzymes in the primordial world. Later, when oxygen concentra-tion increased in the atmosphere, evoluconcentra-tionary proces-ses selected against the use of SeCys because of the sensitivity of this amino acid to oxidation. The use of SeCys progressively decreased and UGA became a nonsense codon. In contrast, other authors argue that SeCys was added to the genetic code and that its use increased during evolution culminating in vertebrates [43]. Selenoproteins, such as GPX, formate dehydroge-nase and iodothyronine deiodinase, would take advantage of the redox properties of SeCys, superior to those of Cys, for their specific functions [44,45]. Finally, an independent origin of the prokaryotic and eukaryotic selenoproteomes has been proposed as there is no direct relation between the two selenoproteomes [46]. With the exception of selenophosphate synthetase, no homology can be found between prokaryote and eukaryote selenoproteins. Eubacterial and archaeal selenoproteins are primarily involved in catabolic pro-cesses, whereas eukaryote selenoproteins participate in antioxidant and anabolic processes. A study based on a comparative genomic approach has revealed a scat-tered phylogenetic distribution of selenoproteins in eukaryotes [47], suggesting a dynamic SeCys⁄ Cys evo-lutionary exchange instead of the contradictory images of the SeCys evolution described above. That some organisms prefer selenoproteins whereas others prefer Cys-containing homolog proteins suggests a different history for each protein and for each species, in which evolutionary events and functional constraints play a key role.
Several selenoproteins including two PHGPX pro-teins have been identified in the plant C. reinhardtii [12,48]. These selenoproteins, as well as the SeCys insertion system, were found to be similar to those of mammals, indicating a common origin for plant and animal selenoproteins⁄ SeCys insertion systems. Cys-containing homologues of these selenoproteins have also been found in higher plants and other animals. Phylogenetic analyses led to exclusion of horizontal gene transfer between C. reinhardtii and mammals, suggesting that the selenoproteins evolved early and were independently lost from higher plants and some animals [48]. Recently, an analysis of sequences derived from several marine organisms supports the hypothesis that SeCys utilization has been lost by many groups of organisms during evolution [49].
In mammals, NS-GPXs and SeCys-containing GPXs, both originating from a unique gene ancestor as dis-cussed above, are expressed. The GPX5 protein, a NS-GPX, shows the closest similarities to GPX3, a SeCys-containing protein derived from the GPX1 gene, which also encodes a selenoprotein [28]. These authors also showed that GPX1 is probably derived from the GPX4 gene also encoding a selenoprotein. It seems unlikely that GPX1, GPX3 and GPX4, but not GPX5, have independently acquired the SeCys, as incorpor-ation of this amino acid requires a rather complicated system. It appears more probable that GPX5 originated from a gene that encoded a SeCys, which later lost the SeCys residue. In the fish D. rerio, the GPX family comprises four selenoproteins: two GPX1 and two GPX4. These proteins are phylogenetically related to mammalian GPX1 and GPX4 [8]. It appears most unli-kely that the GPX1 and GPX4 genes independently acquired SeCys in fishes and mammals. This observa-tion supports the idea that the mammalian GPX5 was first a selenoprotein, which evolved after the separation of fishes and mammals, by the replacement of SeCys by Cys. Another example is GPX6, which is a NS-GPX in rodents but a selenoprotein in other mammals [50]. Like GPX5, GPX6 shows the greatest similarities to the SeCys-containing GPX3 protein.
Taking into account these observations, we propose that SeCys has been lost during evolution in some GPXs in mammals and many eukaryotes. One may ask why this is so, when this residue greatly increases potential GPX activity, and what could be the function of NS-GPXs.
Enzymatic activities of NS-GPXs
Do NS-GPXs display GPX activity?Global GPX activities have been detected in crude extracts from several higher plants [51–53], P. falci-parum [54] and yeast [55]. However, this total GPX activity also takes into account the activity of conta-minating glutathione S-transferases or peroxiredoxins which can metabolize GPX substrates [56]. In addition, PHGPX activity has to be distinguished from GPX activity. The latter is more active in reducing H2O2
and various organic hydroperoxides such as t-butyl hydroperoxide, and the former is more efficient at reducing phospholipid and lipid hydroperoxides. In most studies, both activities were measured by enzy-matic characterization of NS-GPXs, although the authors related only the PHGPX activity.
A partly purified GPX from Citrus sinensis has been shown to display PHGPX activity as low as the
activ-ity of a mammalian GPX in which SeCys was replaced by Cys [35]. We have shown that two recombinant plant NS-GPXs, expressed in E. coli and purified by affinity chromatography, also have low PHGPX activ-ity similar to that of the citrus GPX [57]. These complementary approaches demonstrate that plant NS-GPXs have a rather low PHGPX activity. Similar results have also been found with two recombinant NS-GPX proteins from T. cruzi produced in E. coli [21,58]. With the use of the same experimental design, i.e. tagged protein production in E. coli and affinity purification, other investigations showed that a recom-binant NS-GPX from C. reinhardtii express PHGPX activity that is 36 times higher than those from land plants [59]. In addition, PHGPX activity of the three yeast NS-GPXs has been investigated by mutant ana-lyses and biochemical characterization showing that at least one of these yeast NS-GPXs is a major PHGPX enzyme [20,60]. Nevertheless, the specific activities of yeast, algal and plant NS-GPXs remain remarkably low compared with the specific activity of mammalian PHGPX. This observation raised the question of the physiological importance of their activity in detoxify-ing hydroperoxides.
The absence of SeCys from the catalytic site is not the only reason to question the capacity of NS-GPXs to behave in vivo as expected for a GPX enzyme. Bri-gelius-Flohe´ et al. [29] suggested that PHGPX may be misnamed, as all residues invoked to bind glutathione are mutated or deleted in PHGPX and PHGPX-like proteins. Compared with GPX1, PHGPX⁄ GPX4 has a lower affinity for glutathione and its activity is also lower by more than one order of magnitude in most tissues [61,62]. We have shown that two plant NS-GPXs exhibit weak affinity for glutathione because their apparent Kmvalues for glutathione correspond to
supra-physiological concentrations of glutathione [57]. P. falciparum NS-GPX also showed weak affinity for glutathione [63]. Other NS-GPXs distinct from the PHGPX group, such as mammalian GPX5 and the NS-GPX from B. Pahangi, also lack four of the five amino acids involved in the binding of glutathione [64]. The GPX from B. Pahangi has been shown to exhibit a low affinity for glutathione [64] together with low GPX activity [30,64].
Some NS-GPXs can use thioredoxin as reducing substrate
The question of an alternative reducing substrate to glutathione was first addressed for mammalian seleno-GPX. Human GPX3 is secreted into the plasma, although the plasma glutathione concentration is very
low (< 0.5 lm [65]), suggesting that the function of GPX3 might be completely dependent on other elec-tron donors. Thioredoxin and glutaredoxin have been shown to be efficient electron donors for human GPX3 [66]. Thioredoxins are small ubiquitous proteins with a redox-active dithiol⁄ disulfide in their active site. Reduced thioredoxin operates together with thioredox-in reductase and NADPH as a general protethioredox-in disul-fide-reducing system [67]. Glutaredoxins have similar conformation and function to thioredoxins, but they constitute a distinct protein family as they show no sequence similarity to thioredoxin and they are reduced by glutathione [68]. In contrast with GPX1 and GPX2, the mammalian GPX4 can use various thiol-containing reducing substrates such as cysteine, dihydrolipoamide and dihydrolipoic acid in addition to glutathione [6]. Moreover, a thiol oxidase activity of GPX4 has been demonstrated on different proteins [69].
The weak affinity of NS-GPX for glutathione has led many authors to reconsider their reducing sub-strate. A NS-GPX from P. falciparum was the first GPX shown to use thioredoxin to reduce H2O2 or
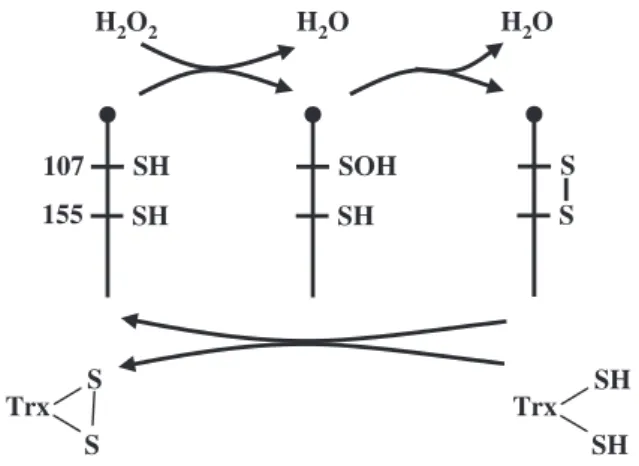
organic hydroperoxides, leading the authors to reclas-sify it as a thioredoxin peroxidase (TPx) [63]. We, and others, have demonstrated that NS-GPXs from land plants, yeast and Drosophila can also exhibit TPx activity [37,57,70–73]. For these proteins, a strong affinity for thioredoxin was revealed, compatible with in vivo concentrations. The Cys residues involved in enzymatic activity were identified [70–72] and an enzy-matic mechanism proposed (Fig. 3). The Cys forming
the catalytic triad of GPX is oxidized by H2O2 or by
an organic hydroperoxide, and, when oxidized, it reacts with another Cys from the well-conserved GPX PCNQF motif (Figs 1 and 3). The resulting disulfide bridge is then reduced by thioredoxin. Supporting this mechanism, Cys-mediated interactions between endog-enous plant thioredoxin and NS-GPX were observed in vivo in two distinct studies [74,75]. Moreover, the redox state of the yeast NS-GPX in vivo was found to be linked to that of endogenous thioredoxin [71].
It thus seems that NS-GPXs may act similarly to some peroxiredoxins, especially peroxiredoxin of the Q and II types [76]. Conversely, some peroxiredoxins, known for their TPx activity, can use glutathione to reduce hydroperoxides [56,77]. This link between GPX and peroxiredoxin can be explained by common struc-tural features. Both enzymes belong to the thioredoxin fold superfamily [78]. This superfamily also comprises glutathione S-transferase, thioredoxin, glutaredoxin and DsbA proteins generating disulfide bridges. All these proteins possess the CxxC motif, in which two Cys residues are separated by two other amino acids, or a derivative of this motif: CxxS, SxxC, CxxT or TxxC [79]. They also possess similar secondary struc-tures with a-helices and b-sheets. For NS-GPXs, the Cys residue identified in the CxxT motif is one involved in both GPX and TPx activity. We have shown that plant NS-GPXs reduce phospholipid hydroperoxides using thioredoxin [57], whereas peroxi-redoxin exhibits weak activity towards these substrates [80]. The PHGPX structure allows access to these hydrophobic compounds. Therefore, GPXs and peroxi-redoxin appear complementary to reduce various peroxides using thioredoxin as a common electron donor. In agreement with this statement, the functional relationship between GPX and peroxiredoxin had previously been suggested by Rouhier & Jacquot [81].
TPx activity is, however, not a characteristic of all NS-GPXs, as the NS-GPX from B. pahangi has GPX activity but not TPx activity [64], in contrast with that documented for its GPX3 human homologue [66]. In addition, reducing substrates other than thioredoxin have been found for some NS-GPXs. Two NS-GPX proteins from Synechocystis PCC 6803 are NADPH-dependent peroxidases, but they have no GPX activity [23]. It has been shown that a NS-GPX from S. cere-visiae reduces H2O2 using the transcription factor
YAP1 as a reducing substrate [82]. In vivo interac-tion between the NS-GPX and YAP1 has also been demonstrated.
These recent results help to clarify the enzymatic function of NS-GPXs. On the one hand, they demon-strate that NS-GPXs have antioxidant activities of
Fig. 3. Peroxide-reduction mechanism of NS-GPXs. The mechanism
of H2O2reduction and GPX regeneration by thioredoxin is proposed
for the NS-GPX from Brassica napus (GenBank accession number AF411209). The position of the catalytic cysteines is given accord-ing to the results of Jung et al. [70]. The protein is represented by pins with the N-terminus as a knob.
physiological relevance. On the other, they suggest that NS-GPXs may be involved in protein thiol–disulfide exchanges. The next challenge will probably be identifi-cation of the reducing substrates of NS-GPXs, i.e. the proteins targeted in thiol–disulfide exchanges. This will not be a simple task because the reducing substrate is expected to be specific to the organism investigated, as underlined above, and to the NS-GPX isoforms in the same organism. In addition, the in vivo reducing and peroxide substrates may depend on cellular localiza-tion, as it has been shown that the different NS-GPX isoforms are expressed in different cell compartments [38,83,84]. Therefore, one cannot rule out the idea that several physiological reducing substrates are used by the same NS-GPX. We have recently shown in tomato stem that a NS-GPX was localized in the cytoplasm or apoplast depending on the cell type [84]. In the apo-plast, it is likely that the NS-GPX uses a reducing sub-strate distinct from thioredoxin or glutathione to reduce peroxides, as these thiols are either not expressed or are expressed at very low level. In support of this, a set of disulfide bond (Dsb) proteins belong-ing to the thioredoxin fold superfamily has been char-acterized in the periplasm of prokaryotes [85]. These proteins could be potential reducing substrates for NS-GPX, and other extracellular reducing substrates can be expected in eukaryotes. Alternatively, the NS-GPX could have different functions in these differ-ent localizations.
NS-GPXs – more than antioxidant
enzymes
Protection against oxidative damage
Several authors proposed that NS-GPXs could be involved in protecting the cell from oxidative damage by scavenging peroxides. This function has been inves-tigated in several organisms. In C. sinensis cells, salt-induced gene expression of a NS-GPX has been shown to depend on AOS accumulation, and oxidative stress is sufficient to induce gene expression [86]. Various AOS have been shown to be able to up-regulate gene expression of several NS-GPXs [87–89]. Overexpres-sion of GPX5 in mammal cells also rendered them more tolerant to oxidative stress [90]. In addition, it has been proposed that an increase in the expression level of this mammalian NS-GPX would compensate for a decrease in expression of seleno-GPX isoforms in mice fed a selenium-deficient diet, in order to cope with failing seleno-dependent GPX activity [91]. Transgenic tobacco plants overexpressing a NS-GPX from Chlamydomonas were also found to be more
tolerant to oxidative stress generated by paraquat [92]. Similarly, the expression of a tomato NS-GPX in S. cerevisiae prevented H2O2-induced cell death [93].
Conversely, a streptococcus strain mutated for a NS-GPX was more sensitive to oxidative treatments than the wild-type strain [94].
Considering their homology with the mammalian membrane-bound GPX4, most NS-GPXs have been proposed to be involved in reducing membrane peroxi-dation. We and others have recently demonstrated that NS-GPXs from various organisms efficiently reduced lipid peroxides as well as a broad range of peroxide in vitro [23,57,59,60,70], but the question of the in vivo substrate of NS-GPX has rarely been addressed. Recently, the ability of the two NS-GPXs from Synechocystis to scavenge lipid hydroperoxides in vivo has been examined [95]. It has been shown that the GPX knock-out mutants have a lower fatty acid hydroperoxidase activity and a higher concentration of lipid hydroperoxides under normal conditions as well as after oxidative treatment. In mammals, Utomo et al. [96] demonstrated that a NS-GPX is essential to avoid cell death after polyunsaturated fatty acid treat-ment. These results clearly indicate that plant and ani-mal NS-GPXs protect cells from oxidative injury at the membrane level.
Phospholipid hydroperoxidase activity has been clearly demonstrated in vivo for a yeast NS-GPX [97]. This function appeared to be linked to the PHGPX structure, supporting the idea that most NS-GPXs would fulfil the same physiological function, i.e. preser-ving membrane integrity. In this report, the authors added GPX1 sequences allowing multimerization into a functional NS-GPX. They confirmed that the lack of these sequences in the PHGPX protein is responsible for the in vitro phospholipid hydroperoxidase activity as well as for their in vivo role in the protection against lipid peroxidation.
Signalling function
AOS and peroxides are not only considered to be toxic molecules but they are also known to be key players in signalling pathways of several physiological processes. By regulating their accumulation, NS-GPXs like any antioxidant enzyme would interfere with these signal-ling pathways. For example, the mammalian GPX4 has been demonstrated to regulate the production of leukotrienes [98] and prostaglandins [99], which are key mediators of inflammation processes, as well as to reduce the interleukin-1-dependent stimulation of NF-jB [100]. Another example is the antiapoptotic function of GPX4 in the mitochondrial death pathway
[101]. These events depend on lipoxygenase activities, which are inhibited by GPX4 [102–104]. Analogous functions can be expected for NS-GPXs of the PHGPX group.
A signalling function has been clearly demonstrated for a S. cerevisiae NS-GPX. Delaunay et al. [82] repor-ted that the NS-GPX called ScGPX3 functions as an H2O2receptor and as a redox transducer for the
tran-scriptional activator YAP1. ScGPX3 interacts in vivo with YAP1 and oxidizes two Cys residues using H2O2.
Oxidation of these residues leads to the nuclear accu-mulation of YAP1 [105], which can activate transcrip-tion of defence genes such as antioxidants [106]. In contrast, reduction of the Cys residues of YAP1 by thioredoxin leads to its inactivation by cytoplasmic sequestration. This regulatory function of ScGPX3 has been demonstrated to depend on its PHGPX structure, especially the ‘gap sequences’ distinguishing mono-meric PHGPX proteins from the multimono-meric GPXs [97]. The phospholipid hydroperoxidase and the YAP1-mediated signalling activities have been shown to be independent. ScGPX3 was also recently shown to interact in vivo through the formation of an inter-molecular disulfide bond with a methionine sulfoxide reductase [107]. This interaction, inhibiting the activity of methionine sulfoxide reductase, was compromised by treatment with H2O2, leading the authors to suggest
that ScGPX3 functions as a redox-dependent regulator of enzyme activity.
Hence, ScGPX3 has at least two independent func-tional roles: protection from membrane peroxidation and signalling of oxidative stress.
A NS-GPX from A. thaliana has also been shown to function as a redox transducer in response to drought stress and abscisic acid [108]. The NS-GPX was shown to interact physically with a 2C-type protein phospha-tase from the abscisic acid signalling pathways and to regulate its phosphatase activity. For this, NS-GPX modulated the redox state of the protein phosphatase using H2O2.
Regarding these recent data, we speculate that most NS-GPXs could fulfil such signalling activities based on thiol redox exchange with protein partners. As dis-cussed above, such interactions between NS-GPXs from various organisms and thioredoxins have been shown in vivo and in vitro. In addition to their partici-pation as electron donors in the fight against oxidative stress, thioredoxins are involved in redox regulation of several physiological processes. Therefore, it is plaus-ible that NS-GPXs participate in the regulation of these physiological processes by acting on thioredoxin-mediated signalling pathways. In plants, several types of thioredoxin exist and participate in seed
germina-tion, cell division, reproducgermina-tion, cell communication and photosynthesis [109,110]. In animals, thioredoxins regulate the activity of very basic stress-response tran-scription factors such as NF-jB and AP1 [111]. They also fulfil a specific function in the inhibition of apop-tosis, immunomodulation, and pregnancy [112]. In prokaryotes, thioredoxins are necessary for DNA syn-thesis or sulfate reduction and are required for the assembly and export of invasive phages such as T7, f1 or M13 [112].
A PHGPX-like protein from the hymenopteran endoparasitoid Venturia canescens has been shown to lack the conserved Cys or SeCys catalytic residue found in GPX [113]. Except for this residue, this pro-tein displays all the conserved regions characteristic of GPX proteins and shows high homology to the NS-GPX from Drosophila melanogaster. This extracel-lular NS-GPX is not an active enzyme but may retain the capacity to interact with membrane lipids. Given its high expression levels in the calyx lumen, the authors proposed that this NS-GPX binds to oxidized phospholipids on the membrane, thereby masking or otherwise removing their potential immune-eliciting properties. This study indicates the capacity of PHGPX protein, i.e. most NS-GPXs, to function in a way other than as a simple antioxidant.
We undertook a proteomic analysis of transgenic tomato plants overexpressing a NS-GPX to determine whether this overexpression would interfere with gene expression [114]. The accumulation of two proteins involved in the Calvin cycle and the signalling protein, RanBP1, was found to be affected in NS-GPX-over-expressing plants, suggesting that the NS-GPX interferes with the photosynthetic process and the GTPase-medi-ated signalling pathways. In addition, in the same study, we showed that NS-GPX-overexpressing plants exposed to chilling conditions had greater photosyn-thetic activity because of greater activity of the enzymes involved in this process. Similarly, Yoshimura et al. [92] reported that transgenic tobacco plants overexpressing a NS-GPX from Chlamydomonas had higher photosynthetic activity in chilling conditions than control plants.
Function in structural organization
A structural function has clearly been demonstrated for the seleno-dependent GPX4 in mammals. GPX4 has been found to be expressed as an enzymatically inactive, oxidatively cross-linked, insoluble protein in mature spermatozoa [115]. It has been found to be responsible for the polymerization of a sperm mito-chondrion-associated cysteine-rich protein (SMPC), a
major component of the sperm mitochondrial capsule [116,117]. During this polymerization process, GPX4 catalyzed the formation of cystine from adjacent SMPC cysteine residues, followed by a reshuffling [117]. Sperm cell GPX4 was also found to be associ-ated with sperm nuclei where it promotes disulfide bridging on thiol-containing protamines, allowing increased compaction of the sperm nuclei [118]. Although, this was first demonstrated for a seleno-GPX, a function in structural organization through disulfide bridging can also be expected for some NS-GPXs. As discussed above, several NS-GPXs were able to oxidize thioredoxin through the formation of a disulfide bridge from two adjacent cysteine residues (Fig. 3). All these results argue for a function of NS-GPXs in disulfide bridging. Protein targets of NS-GPXs remain to be discovered to understand clearly the role of NS-GPX in disulfide bridge-mediated structural remodelling of cell structures.
Physiological processes for which NS-GPXs have been proposed
Role in defence⁄ response to adverse conditions
In many organisms, especially plants, NS-GPXs have been shown to be involved in the response to environ-mental stress. Numerous studies have reported that various stress conditions alter the steady-state level of mRNA encoding NS-GPXs in plant species, including tobacco [16,119], Arabidopsis [120], tomato [18], sun-flower [19], pea [83], citrus [86] and barley [121]. Tested stress conditions included osmotic pressure, gentle mechanical stimulation, wounding, salt and herbicide treatments, and exposure to ozone, UV, sulfur dioxide, heat and strong light. In some cases, the increase in mRNA accumulation was confirmed by an increase in accumulation of NS-GPX protein [17,84].
In a few reports, transgenic approaches have been used to investigate the role of NS-GPXs in plant response to environmental stress. Transgenic tobacco plants overexpressing a Chlamydomonas NS-GPX were found to be more tolerant to chilling and salt [92]. A NS-GPX from Lycopersicon esculentum expressed in S. cerevisiae cells has been shown to function as a cytoprotector, preventing Bax-induced and heat stress-induced cell death and delaying yeast senescence [93]. Transient expression of this NS-GPX in Nicotiana tabaccum also produced tolerance to salt and chilling and suppressed the apoptotic-like features associated with these stress conditions. In addition, we have shown that, after chilling, the photosynthetic activity of transgenic tomato plants overexpressing a NS-GPX was not affected, whereas it was decreased in control
plants [114]. A S. cerevisiae strain with mutations in the three NS-GPXs was found to be more sensitive to aluminium treatment than the wild-type or any single NS-GPX mutant, indicating that the NS-GPX genes may collectively contribute to tolerance to aluminium [122]. Taken together, these results support the idea that NS-GPXs are definitively involved in resistance to various environmental stress conditions.
More precisely, the different NS-GPX isoforms are likely to have different functions in the stress response, as suggested by differences in gene regulation. In L. esculentum, we observed that the highest transcript level of the GPXle-1 isoform was observed 1–2 h after rubbing of an internode, whereas a significant accumu-lation of mRNA of the GPXle-2 isoform was observed later, 2–6 h after stimulation [18]. In fact, the GPXle-2 transcript started to accumulate when the concentra-tion of GPXle-1 mRNA was back to normal. More-over, GPXle-1 mRNA also accumulated in the roots of rubbed plants, whereas GPXle-2 mRNA did not, underlying differences in terms of inducibility between the two isoforms. Furthermore, we have shown that messengers of two NS-GPXs from Helianthus annuus accumulated differentially in response to various com-ponents of stress signalling pathways [89]. In Hordeum vulgare, the expression of two isoforms was shown to be induced by salt and osmotic stress and by paraquat treatment, whereas expression of a third isoform was repressed in these conditions [121]. According to the authors, these results could be explained by the differ-ent subcellular localizations of the NS-GPXs. Simi-larly, another report indicates that the genes of the GPX family of A. thaliana were differently regulated through diverse signalling pathways and that the pro-teins would be localized in distinct cell compartments [38]. A specific response was observed for a C. rein-hardtii NS-GPX gene that was shown to be transcrip-tionally up-regulated by the oxygen singulet O1 2
produced in photosystem II, suggesting a special func-tion for this GPX in protecfunc-tion against O1
2 [88,123].
Taking into account these observations, we suggest that the NS-GPX isoforms fulfil different functions, especially in response to stress, and that this speci-ficity is closely related to their regulation pathways and⁄ or their tissue restriction and ⁄ or their subcellular localization.
From the data collected to date, it seems that NS-GPXs are involved in stress responses as well as in specific functions in normal conditions. Although NS-GPXs are known to be induced and expressed in stress conditions, their expression has often been shown to be constitutive, suggesting that they also have basal functions in nonstress situations. Supporting this idea
are our observation that a tomato NS-GPX was expressed in the cytoplasm of collenchyma in rubbed internode, whereas it was expressed in the cytoplasm of the same cell type in an unstressed internode [84].
Function in sexual reproduction
In mammals, a NS-GPX (GPX5) was first discovered in the male genital tract [124]. Recently, its expression and putative functions in the epididymis and spermato-zoa have been reviewed [125]. Its expression is tightly controlled by androgen and is exclusively restricted to epididymis [126]. The protein was found to be both free in the epididymal fluid and associated with transi-ting spermatozoa in the epididymal lumen [127–129]. GPX5 association with spermatozoa is limited to the acrosome region [128]. This is another example of a NS-GPX that is part of the antioxidant strategies in the epididymis, more particularly involved in protec-tion of spermatozoa from oxidative damage [130]. Spermatozoa are highly sensitive to oxidative attack because of the high polyunsaturated fatty acid content of their plasma membrane. Spermatozoa themselves are known to produce AOS, which are involved in the regulation of their ultimate maturation (i.e. capacita-tion and acrosomic reaccapacita-tion). The secreted NS-GPX (GPX5) plays a role in the fine tuning of AOS in the sperm environment during epididymal maturation.
Sex-specific expression and specific tissue distri-bution in sex organs have also been observed for other NS-GPXs, suggesting a role in reproduction. A NS-GPX from Sch. mansoni has been shown to be spe-cifically expressed in vitteline cells of female worm and absent in male worm tissues [131]. Its expression requires a mature reproductive system and the protein has therefore been proposed to be involved in the formation of eggs. Conversely, the expression of a NS-GPX from D. melanogaster was found to be greater in male than in female flies, probably because of its strong expression in testes [113]. In the male reproductive tissues, the protein was detected in the testicular duct whereas expression was low in other tissues. Although lower in female flies, specific expres-sion of the NS-GPX was also observed in the follicle and nurse cells from ovaries [113].
Function in host–pathogen interactions
In plants and animal phagocytic cells, the generation and release of AOS are thought to be important com-ponents of the host’s immunity against bacterial infec-tions. Pathogens have developed effective systems to counteract the resulting oxidative stress (for review, see
[132]). For example, mutant bacteria defective in resist-ance to oxidative stress have been shown to be aviru-lent [133]. Neisseria meningitidis and Stretptococcus pyogenes mutant strains defective in a NS-GPX were more sensitive to oxidative stress than the respective wild-type strains, suggesting that NS-GPXs are import-ant components of the bacterial import-antioxidimport-ant system [94]. The contribution of NS-GPXs to bacterial virulence has been investigated in a St. pyogenes mutant strain defective in the unique NS-GPX [134]. In this study, it was demonstrated that the NS-GPX was essential for bacterial pathogenesis in several murine models of streptococcal diseases. However, the NS-GPX was not necessary for the St. pyogenes viru-lence in a zebrafish model of streptococcal disease, characterized by the absence of inflammatory process. Taken together, these observations suggest that bacter-ial pathogenesis requires NS-GPX for defence against the oxidative stress that accompanies the inflammatory response of the host. Furthermore, in B. pahangi, a lymphatic nematode parasite, a NS-GPX was found to be the major cuticular glycoprotein [22]. This NS-GPX was expressed at low level in mosquito-derived infec-tion larvae, whereas after infecinfec-tion of the mammalian host, its expression was up-regulated and the protein was secreted in the parasite cuticle. Once again, the NS-GPX may protect the parasite against the AOS produced by the host phagocytes.
Similarly, a plant nematode parasite expressed a secreted form of NS-GPX in addition to an intracellu-lar isoform [31]. This secreted isoform showed anti-oxidant activity restricted to the hypodermis, which may protect the parasite from host defences.
The involvement of NS-GPX in the mechanism of host defence has also been investigated, especially in plants. One of the earliest responses to avirulent pathogen attack is the generation of an oxidative burst that can trigger cell death, also called the hypersensi-tive response. This is thought to deprive the pathogens of food and confine them to the infection site. We have demonstrated that two NS-GPXs from H. annuus were differently regulated in leaves infected with either a virulent or an avirulent race of the obligate parasite Plasmopara halstedii, suggesting that NS-GPX gene expression level is important for establishment of the hypersensitive response developed to limit spreading of the avirulent pathogen [89]. Both NS-GPXs were up-regulated during the compatible interaction with the virulent race, whereas they were down-regulated during the incompatible interaction with the avirulent strain. Similar results have been obtained with a rice NS-GPX after infection with the blast pathogen [135]. Interestingly, modification of the expression of the
sunflower NS-GPX is temporally correlated with the Plasmopara-induced symptoms in plants for both com-patible and incomcom-patible interactions [89,136].
A decrease in gene expression during an incompat-ible interaction has also been observed for an ascor-bate peroxidase, another antioxidant enzyme [137]. This down-regulation of antioxidant genes favours AOS accumulation, which is necessary to promote the hypersensitive response. More precisely, down-regula-tion of NS-GPXs favours the lipoxygenase-mediated accumulation of lipid hydroperoxides, an enzymatic peroxidative process that is a major feature of the hypersensitive response [138]. In contrast, up-regula-tion of NS-GPX expression would help in fighting against oxidative damage generated during the disease progression. Our hypothesis about the function of NS-GPXs in the hypersensitive response could be tes-ted quite easily in transgenic plants known to overex-press a NS-GPX. In agreement with such behaviour are the data of Yoshimura et al. [92], who have shown that overexpression of a tomato NS-GPX in tobacco conferred protection against the phytopatho-gen Botrytis cinerea. Although this result seems to be contradictory to our hypothesis, it actually completes it because this fungus is a necrotrophic pathogen which utilizes dead plant tissue originating from an induced oxidative burst [139]. For this special host– pathogen interaction, we propose that overexpression of NS-GPXs could reduce AOS or lipid peroxides, limiting host cell death and hence the propagation of the pathogen.
Thus, in our opinion, the expression level of the NS-GPX gene of the host is differently adjusted depending on the pathosystem, to help mount the appropriate defence response. Further studies of NS-GPX gene expression (i.e. level and subcellular localization of proteins) in the host as well as in the pathogen would help to clarify NS-GPX function in the interaction.
Conclusions
Although seleno-GPXs appear to be an efficient means of AOS scavenging, especially because of the presence of the SeCys residue, NS-GPXs are prevalent in the living world, and some of them probably evolved from seleno-GPXs. The weak GPX⁄ PHGPX activity found for most of the NS-GPXs and the fact that they use substrates other than glutathione, especially thio-redoxin, led us to conclude that NS-GPXs are not true GPXs and hence are misnamed. Thioredoxin is prob-ably not the only potential physiological substrate, as demonstrated for a yeast NS-GPX which oxidizes
in vivo a transcriptional factor. Hence, these proteins could be included in a thiol peroxidase (TPx) family. A reclassification of the NS-GPXs away from the GPX family has also been proposed by other authors [81,140]. In support of this reclassification, NS-GPXs with the mammalian PHGPX form a separate clade from the other seleno-GPXs (Fig. 2).
We can speculate that NS-GPX⁄ TPxs, using a pro-tein as reducing substrate, do not compete with effi-cient AOS-scavenging enzymes such as catalase and seleno-GPXs in animals and ascorbate peroxidase in plants. The concentration of the NS-GPX⁄ TPx-redu-cing substrate is lower than the concentrations of ascorbate and glutathione, which accumulate in the millimolar range, and this is probably not enough to scavenge AOS during oxidative stress. Instead, NS-GPX⁄ TPx would use AOS for protein disulfide exchange in signal-transduction pathways as proposed for yeast and Arabidopsis NS-GPX⁄ TPx [82,108] or in structural organization processes. This is in line with the re-evaluation of the concept of oxidative stress as an ‘oxidative signalling’ proposed by Foyer & Noctor [141], that is, a process by which cells sense the envi-ronment and make appropriate adjustments to gene expression, metabolism and physiology. By such sig-nalling functions, NS-GPX⁄ TPx would be involved in defence⁄ response to adverse conditions, in protection against AOS induced by adverse conditions, and in host–pathogen interactions. In most organisms, several genes encode NS-GPX⁄ TPx (there are, for example, seven NS-GPX-encoding genes in A. thaliana), and they can be expressed in the same cell. The idea that all these peroxidases might simply be backup systems of other and more efficient antioxidant systems can be questioned. More likely, they have evolved to play highly specialized parts in cellular processes. This view is also based on the emerging knowledge on the speci-fic roles of the mammalian seleno-GPX4 [142]. To clarify the precise role of individual NS-GPXs, in vivo analyses will have to be performed, especially with regard to their tissue and cell localization. Phenotypic and functional analyses of knockout organisms must also be conducted to determine the true roles of these proteins. Further challenges will be to identify the thiol protein substrates of the NS-GPXs. The identification of the YAP1 transcription factor and methionine sulf-oxide reductase as thiol protein partners in yeast [82,107] suggests that many other protein partners in addition to thioredoxin can be expected in various organisms. In plants, several NS-GPX isoforms are expressed, and their respective protein partners remain unknown as well as the components of any signal-transduction pathways based on disulfide exchange.
Such investigations will not only help in understanding NS-GPX function but will also provide new insights into the role of AOS in cell physiology.
Acknowledgements
We are indebted to Felicity Vear for correction of English grammar and syntax.
References
1 Mills GC (1957) Hemoglobin catabolism. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem 266, 20752–20760.
2 Flohe´ L, Gunzler WA & Schock HH (1973) Glu-tathione peroxidase: a selenoenzyme. FEBS Lett 32, 132–134.
3 Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG & Hoekstra WG (1973) Selenium: bio-chemical role as a component of glutathione peroxi-dase. Science 179, 588–590.
4 Stadtman TC (1996) Selenocysteine. Annu Rev Biochem 65, 83–100.
5 Rocher C, Lalanne JL & Chaudie`re J (1992) Purifica-tion and properties of a recombinant sulfur analog of murine selenium-glutathione peroxidase. Eur J Biochem 205, 955–960.
6 Maiorino M, Aumann KD, Brigelius-Flohe R, Doria D, Van den Heuvel J, McCarthy J, Roveri A, Ursini F & Flohe L (1995) Probing the presumed catalytic triad of selenium-containing peroxidases by mutational ana-lysis of phospholipid hydroperoxide glutathione peroxi-dase (PHGPx). Biol Chem Hoppe Seyler 376, 651–660. 7 Kong BW, Kim H & Foster DN (2003) Cloning and
expression analysis of chicken phospholipid-hydroper-oxide glutathione peroxidase. Anim Biotechnol 14, 19–29. 8 Thisse C, Degrave A, Kryukov GV, Gladyshev VN,
Obrecht-Pflumio S, Krol A, Thisse B & Lescure A (2003) Spatial and temporal expression patterns of sele-noprotein genes during embryogenesis in zebrafish. Gene Expr Patterns 3, 525–532.
9 Maiorino M, Roche C, Kiess M, Koenig K, Gawlik D, Matthes M, Naldini E, Pierce R & Flohe L (1996) A selenium-containing phospholipid-hydroperoxide glu-tathione peroxidase in Schistosoma mansoni. Eur J Bio-chem 238, 838–844.
10 Singh A & Rathaur S (2005) Identification and charac-terization of a selenium-dependent glutathione peroxi-dase in Setaria cervi. Biochem Biophys Res Commun 331, 1069–1074.
11 Cossio-Bayugar R, Miranda E & Holman PJ (2005) Molecular cloning of a phospholipid-hydroperoxide glutathione peroxidase gene from the tick, Boophilus
microplus(Acari: Ixodidae). Insect Biochem Mol Biol 35, 1378–1387.
12 Fu LH, Wang XF, Eyal Y, She YM, Donald LJ, Standing KG & Ben-Hayyim G (2002) A selenoprotein in the plant kingdom. Mass spectrometry confirms that an opal codon (UGA) encodes selenocysteine in Chla-mydomonas reinhardtiiglutathione peroxidase. J Biol Chem 277, 25983–25991.
13 Zhao L, Cox AG, Ruzicka JA, Bhat AA, Zhang W & Taylor EW (2000) Molecular modeling and in vitro activity of an HIV-1-encoded glutathione peroxidase. Proc Natl Acad Sci USA 97, 6356–6361.
14 Ghyselinck NB, Jimenez C & Dufaure JP (1991) Sequence homology of androgen-regulated epididymal proteins with glutathione peroxidase in mice. J Reprod Fertil 93, 461–466.
15 Dear TN, Campbell K & Rabbitts TH (1991) Molecu-lar cloning of putative binding and odorant-metabolizing proteins. Biochemistry 30, 10376–10382. 16 Criqui MC, Jamet E, Parmentier Y, Marbach J, Durr
A & Fleck J (1992) Isolation and characterization of a plant cDNA showing homology to animal glutathione peroxidases. Plant Mol Biol 18, 623–627.
17 Holland D, Baen-Hayyim G, Faltin Z, Camoin L, Strosberg AD & Eshdat Y (1993) Molecular characteri-zation of salt-stress associated protein in citrus: protein and cDNA sequence homology to mammalian glu-tathione peroxidases. Plant Mol Biol 21, 923–927. 18 Depe`ge N, Drevet J & Boyer N (1998) Molecular
clon-ing and characterization of tomato cDNAs encodclon-ing glutathione peroxidase-like proteins. Eur J Biochem 253, 445–451.
19 Roeckel-Drevet P, Gagne G, Tourvieille de Labrouhe D, Dufaure JP, Nicolas P & Drevet JR (1998) Molecu-lar cloning, organ distribution and stress-mediated induction of two glutathione peroxidase-encoding mRNAs in sunflower (Helianthus annuus). Physiol Plant 103, 385–394.
20 Inoue Y, Matsuda T, Sugiyama KI, Izawa S & Kimura A (1999) Genetic analysis of glutathione peroxidase in oxidative stress response of Saccharomyces cerevisiae. J Biol Chem 274, 27002–27009.
21 Wilkinson SR, Meyer DJ & Kelly JM (2000) Biochem-ical characterization of a trypanosome enzyme with glutathione- dependent peroxidase activity. Biochem J 352 Part 3, 755–761.
22 Cookson E, Blaxter ML & Selkirk ME (1992) Identifi-cation of the major soluble cuticular glycoprotein of lymphatic filarial nematode parasites (gp29) as a secre-tory homolog of glutathione peroxidase. Proc Natl Acad Sci USA 89, 5837–5841.
23 Gaber A, Tamoi M, Takeda T, Nakano Y & Shigeoka S (2001) NADPH-dependent glutathione peroxidase-like proteins (Gpx-1, Gpx-2) reduce unsaturated fatty
acid hydroperoxides in Synechocystis PCC 6803. FEBS Lett 499, 32–36.
24 Aho EL & Kelly LP (1995) Identification of a glu-tathione peroxidase homolog in Neisseria meningitidis. DNA sequence 6, 55–60.
25 Mourier T, Pain A, Barrell B & Griffiths-Jones S (2005) A selenocysteine tRNA and SECIS element in Plasmodium falciparum. RNA 11, 119–122.
26 Brigelius-Flohe R (1999) Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med 27, 951–965.
27 Arthur JR (2000) The glutathione peroxidases. Cell Mol Life Sci 57, 1825–1835.
28 Dufaure JP, Lareyre JJ, Schwaab V, Matte´i MG & Drevet JR (1996) Structural organisation, chromosomal localization, expression and phylogenetic evaluation of mouse glutathione peroxidase encoding genes. C R Acad Sci Paris 319, 559–568.
29 Brigelius-Flohe R, Aumann K, Blocker H, Gross G, Kiess M, Kloppel K, Maiorino M, Roveri A, Schuckelt R & Ursini F (1994) Phospholipid-hydroperoxide glutathione peroxidase. Genomic DNA, cDNA, and deduced amino acid sequence. J Biol Chem 269, 7342–7348.
30 Tripp C, Frank RS, Selkirk ME, Tang L, Grieve MM, Frank GR & Grieve RB (1998) Dirofilaria immitis: molecular cloning and expression of a cDNA encoding a selenium-independent secreted glutathione peroxidase. Exp Parasitol 88, 43–50.
31 Jones JT, Reavy B, Smant G & Prior AE (2004) Glu-tathione peroxidases of the potato cyst nematode Globodera rostochiensis. Gene 324, 47–54.
32 Koonin EV, Makarova KS & Aravind L (2001) Hori-zontal gene transfer in prokaryotes: quantification and classification. Annu Rev Microbiol 55, 709–742. 33 Schuckelt R, Brigelius-Flohe R, Maiorino M, Roveri
A, Reumkens J, Strassburger W, Ursini F, Wolf B & Flohe L (1991) Phospholipid hydroperoxide glutathione peroxidase is a selenoenzyme distinct from the classical glutathione peroxidase as evident from cDNA and amino acid sequencing. Free Radic Res Commun 14, 343–361.
34 Epp O, Ladenstein R & Wendel A (1983) The refined structure of the selenoenzyme glutathione peroxidase at 0.2-nm resolution. Eur J Biochem 133, 51–69.
35 Ursini F, Maiorino M & Gregolin C (1985) The sele-noenzyme phospholipid hydroperoxide glutathione per-oxidase. Biochim Biophys Acta 839, 62–70.
36 Beeor-Tzahar T, Ben-Hayyim G, Holland D, Faltin Z & Eshdat Y (1995) A stress-associated citrus protein is a distinct plant phospholipid hydroperoxide glutathione peroxidase. FEBS Lett 366, 151–155.
37 Navrot N, Collin V, Gualberto J, Gelhaye E, Hirasawa M, Rey P, Knaff DB, Issakidis E, Jacquot JP & Rouh-ier N (2006) Plant glutathione peroxidases are
func-tional peroxiredoxins distributed in several subcellular compartments and regulated during biotic and abiotic stresses. Plant Physiol 142, 1364–1379.
38 Rodriguez Milla MA, Maurer A, Rodriguez Huete A & Gustafson JP (2003) Glutathione peroxidase genes in Arabidopsisare ubiquitous and regulated by abiotic stresses through diverse signalling pathways. Plant J 36, 602–615.
39 Wilkinson SR & Kelly JM (2003) The role of glu-tathione peroxidases in trypanosomatids. Biol Chem 384, 517–525.
40 Kang SG, Jeong HK & Suh HS (2004) Charac-terization of a new member of the glutathione peroxidase gene family in Oryza sativa. Mol Cells 17, 23–28.
41 Leinfelder W, Zehelein E, Mandrand-Berthelot MA & Bock A (1988) Gene for a novel tRNA species that accepts 1-serine and cotranslationally inserts selenocys-teine. Nature 331, 723–725.
42 Bock A, Forchhammer K, Heider J, Leinfelder W, Sawers G, Veprek B & Zinoni F (1991) Selenocysteine: the 21st amino acid. Mol Microbiol 5, 515–520. 43 Gladyshev V & Kryukov GV (2001) Evolution of
sele-nocysteine-containing proteins: significance of identifi-cation and functional characterization of
selenoproteins. Biofactors 14, 87–92.
44 Axley MJ, Bock A & Stadtman TC (1991) Catalytic properties of an Escherichia coli formate dehydrogenase mutant in which sulfur replaces selenium. Proc Natl Acad Sci USA 88, 8450–8454.
45 Berry MJ, Maia AL, Kieffer JD, Harney JW & Larsen PR (1992) Substitution of cysteine for selenocysteine in type I iodothyronine deiodinase reduces the catalytic efficiency of the protein but enhances its translation. Endocrinology 131, 1848–1852.
46 Hatfield DL & Gladyshev VN (2002) How selenium has altered our understanding of the genetic code. Mol Cell Biol 22, 3565–3576.
47 Castellano S, Novoselov SV, Kryukov GV, Lescure A, Blanco E, Krol A, Gladyshev VN & Guigo R (2004) Reconsidering the evolution of eukaryotic selenopro-teins: a novel nonmammalian family with scattered phylogenetic distribution. EMBO Rep 5, 71–77. 48 Novoselov SV, Rao M, Onoshko NV, Zhi H, Kryukov
GV, Xiang Y, Weeks DP, Hatfield DL & Gladyshev VN (2002) Selenoproteins and selenocysteine insertion system in the model plant cell system, Chlamydomonas reinhardtii. EMBO J 21, 3681–3693.
49 Copeland PR (2005) Making sense of nonsense: the evolution of selenocysteine usage in proteins. Genome Biol 6, 221.
50 Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo´ R & Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300, 1439–1443.
51 Drotar A, Phelps P & Fall R (1985) Evidence for glu-tathione peroxidase activities in cultured plant cells. Plant Sci 42, 35–40.
52 Kuroda H, Sagisaka S & Chiba K (1992) Collapse of peroxide-scavenging systems in apple flower-buds asso-ciated with freezing injury. Plant Cell Physiol 33, 743–750.
53 Navarry-Izzo F & Izzo R (1994) Induction of enzyme activities and antioxidant production in barley plants as a result of SO2fumigation. Plant Sci 96, 31–40.
54 Fairfield AS, Abosch A, Ranz A, Eaton JW & Meshnick SR (1988) Oxidant defense enzymes of Plas-modium falciparum. Mol Biochem Parasitol 30, 77–82. 55 Galiazzo F, Schiesser A & Rotilio G (1987)
Glu-tathione peroxidase in yeast. Presence of the enzyme and induction by oxidative conditions. Biochem Biophys Res Commun 30, 1200–1205.
56 Singh AK & Shichi H (1998) A novel glutathione per-oxidase in bovine eye. Sequence analysis, mRNA level, and translation. J Biol Chem 273, 26171–26178. 57 Herbette S, Lenne C, Leblanc N, Julien JL, Drevet JR
& Roeckel-Drevet P (2002) Two GPX-like proteins from Lycopersicon esculentum and Helianthus annuus are antioxidant enzymes with phospholipid hydroperox-ide glutathione peroxidase and thioredoxin peroxidase activities. Eur J Biochem 269, 2414–2420.
58 Wilkinson SR, Taylor MC, Touitha S, Mauricio IL, Meyer DJ & Kelly JM (2002) TcGPXII, a glutathione-dependent Trypanosoma cruzi peroxidase with substrate specificity restricted to fatty acid and phospholipid hydroperoxides, is localized to the endoplasmic reticu-lum. Biochem J 364, 787–794.
59 Takeda T, Miyao K, Tamoi M, Kanaboshi H, Miya-saka H & Shigeoka S (2003) Molecular characterization of glutathione peroxidase-like protein in halotolerant Chlamydomonassp. W80. Physiol Plant 117, 467–475. 60 Avery AM & Avery SV (2001) Saccharomyces
cerevi-siaeexpresses three phospholipid hydroperoxide glu-tathione peroxidases. J Biol Chem 276, 33730–33735. 61 Zhang L, Maiorino M, Roveri A & Ursini F (1989)
Phospholipid hydroperoxide glutathione peroxidase: specific activity in tissues of rats of different age and comparison with other glutathione peroxidases. Bio-chim Biophys Acta 1006, 140–143.
62 Weitzel F, Ursini F & Wendel A (1990) Phospholipid hydroperoxide glutathione peroxidase in various mouse organs during selenium deficiency and repletion. Bio-chim Biophys Acta 1036, 88–94.
63 Sztajer H, Gamain B, Aumann KD, Slomianny C, Becker K, Brigelius-Flohe R & Flohe L (2001) The putative glutathione peroxidase gene of Plasmodium fal-ciparumcodes for a thioredoxin peroxidase. J Biol Chem 276, 7397–7403.
64 Tang L, Gounaris K, Griffiths C & Selkirk ME (1995) Heterologous expression and enzymatic properties of a
selenium-independent glutathione peroxidase from the parasitic nematode Brugia pahangi. J Biol Chem 270, 18313–18318.
65 Wendel A & Cikryt P (1980) The level and half-life of glutathione in human plasma. FEBS Lett 120, 209–211. 66 Bjornstedt M, Xue J, Huang W, Akesson B &
Holmg-ren A (1994) The thioredoxin and glutaredoxin systems are efficient electron donors to human plasma glu-tathione peroxidase. J Biol Chem 269, 29382–29384. 67 Holmgren A (1985) Thioredoxin. Annu Rev Biochem
54, 237–271.
68 Eklund H, Cambillau C, Sjoberg B, Holmgren A, Jornvall H, Hoog J & Branden C (1984) Conforma-tional and funcConforma-tional similarities between glutaredoxin and thioredoxins. EMBO J 3, 1443–1449.
69 Godeas C, Tramer F, Micali F, Soranzo M, Sandri G & Panfili E (1997) Distribution and possible novel role of phospholipid hydroperoxide glutathione peroxidase in rat epididymal spermatozoa. Biol Reprod 57, 1502–1508.
70 Jung BG, Lee KO, Lee SS, Chi YH, Jang HH, Kang SS, Lee K, Lim D, Yoon SC, Yun DJ, Inoue Y, Cho MJ & Lee SY (2002) A chinese cabbage cDNA with high sequence identity to phospholipid hydroperoxide glutathione peroxidases encodes a novel isoform of thioredoxin-dependent peroxidase. J Biol Chem 277, 12572–12578.
71 Tanaka T, Izawa S & Inoue Y (2005) GPX2, encoding a phospholipid hydroperoxide glutathione peroxidase homologue, codes for an atypical 2-Cys peroxiredoxin in Saccharomyces cerevisiae. J Biol Chem 280, 42078– 42087.
72 Maiorino M, Ursini F, Bosello V, Toppo S, Tosatto SC, Mauri P, Becker K, Roveri A, Bulato C, Benazzi L, et al. (2007) The thioredoxin specificity of Dro-sophilaGPx: a paradigm for a peroxiredoxin-like mech-anism of many glutathione peroxidases. J Mol Biol 365, 1033–1046.
73 Iqbal A, Yabuta Y, Takeda T, Nakano Y & Shigeoka S (2006) Hydroperoxide reduction by thioredoxin-speci-fic glutathione peroxidase isoenzymes of Arabidopsis thaliana. FEBS J 273, 5589–5597.
74 Balmer Y, Vensel W, Tanaka C, Hurkman W, Gelhaye E, Rouhier N, Jacquot J, Manieri W, Schu¨rmann P, Droux M, et al. (2004) Thioredoxin links redox to the regulation of fundamental processes of plant mitochon-dria. Proc Natl Acad Sci USA 101, 2642–2647. 75 Wong J, Cai N, Balmer Y, Tanaka C, Vensel W,
Hurkman W & Buchanan B (2004) Thioredoxin targets of developping wheat seeds identified by complemen-tary proteomic approaches. Phytochemistry 65, 1629– 1640.
76 Horling F, Konig J & Dietz KJ (2002) Type II peroxi-redoxin C, a member of the peroxiperoxi-redoxin family of Arabidopsis thaliana: its expression and activity in
comparison with other peroxiredoxins. Plant Physiol Biochem 40, 491–499.
77 Chen JW, Dodia C, Feinstein SI, Jain MK & Fisher AB (2000) l-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2
activities. J Biol Chem 275, 28421–28427.
78 Schro¨der E & Pointing CP (1998) Evidence that perox-iredoxins are novel members of the thioredoxin fold superfamily. Protein Sci 7, 2465–2468.
79 Fomenko DE & Gladyshev VN (2003) Identity and functions of CxxC-derived motifs. Biochem 42, 11214– 11225.
80 Konig J, Lotte K, Plessow R, Brockhinke A, Baier M & Dietz K (2003) Reaction mechanism of plant 2-Cys peroxiredoxin. Role of the C terminus and the quatern-ary structure. J Biol Chem 278, 24409–24420.
81 Rouhier N & Jacquot JP (2005) The plant multigenic family of thiol peroxidases. Free Radic Biol Medical 38, 1413–1421.
82 Delaunay A, Pflieger D, Barrault MB, Vinh J & Tole-dano MB (2002) A thiol peroxidase is an H2O2
recep-tor and redox-transducer in gene activation. Cell 111, 471–481.
83 Mullineaux PM, Karpinski S, Jimenez A, Cleary SP, Robinson C & Creissen GP (1998) Identification of cDNAS encoding plastid-targeted glutathione peroxi-dase. Plant J 13, 375–379.
84 Herbette S, Brunel N, Prensier G, Julien JL, Drevet JR & Roeckel-Drevet P (2004) Immunolocalization of a plant glutathione peroxidase-like protein. Planta 219, 784–789.
85 Stirnimann CU, Grutter MG, Glockshuber R & Capitani G (2006) nDsbD: a redox interaction hub in the Escherichia coli periplasm. Cell Mol Life Sci 63, 1642–1648.
86 Avsian-Kretchmer O, Eshdat Y, Gueta-Dahan Y & Ben-Hayyim G (1999) Regulation of stress-induced phospholipid hydroperoxide glutathione peroxidase expression in citrus. Planta 209, 469–477.
87 Levine A, Tenhaken R, Dixon R & Lamb C (1994) H2O2from the oxidative burst orchestrates the plant
hypersensitive disease resistance response. Cell 79, 583– 593.
88 Leisinger U, Ru¨fenacht K, Fischer B, Pesaro M, Spengler A, Zehnder AJB & Eggen RIL (2001) The glutathione peroxidase homologous gene from Chlamy-domonas reinhardtiiis transcriptionally up-regulated by singlet oxygen. Plant Mol Biol 46, 395–408.
89 Herbette S, Lenne C, Tourvieille de Labrouhe D, Drevet JR & Roeckel-Drevet P (2003) Transcripts of sunflower antioxidant scavengers of the SOD and GPX families accumulate differentially in response to downy mildew infection, phytohormones, reactive oxygen spe-cies, nitric oxide, protein kinase and phosphatase inhi-bitors. Physiol Plant 119, 418–428.
90 Vernet P, Rigaudie`re N, Ghyselinck NB, Dufaure JP & Drevet JR (1996) In vitro expression of a mouse tissue-specific glutathione peroxidase-like protein lacking the selenocysteine can protect stably transfected mamma-lian cells against oxidative damage. Biochem Cell Biol 74, 125–131.
91 Vernet P, Rock E, Mazur A, Rayssiguier Y, Dufaure JP & Drevet JR (1999) Selenium-independent epididy-mis-restricted glutathione peroxidase 5 protein (GPX5) can back up failing Se-dependent GPXs in mice subjected to selenium deficiency. Mol Reprod Dev 54, 362–370.
92 Yoshimura K, Miyao K, Gaber A, Takeda T, Kanaboshi H, Miyasaka H & Shigeoka S (2003) Enhancement of stress tolerance in transgenic tobacco plants overexpressing Chlamydomonas glutathione per-oxidase in chloroplasts or cytosol. Plant J 37, 21–33. 93 Chen S, Vaghchhipawala Li W, Asard H & Dickman
M (2004) Tomato phospholipid hydroperoxide glu-tathione peroxidase inhibits cell death induced by Bax and oxidative stresses in yeast and plants. Plant Physiol 135, 1630–1641.
94 King K, Horenstein J & Caparon M (2000) Aerotoler-ance and peroxide resistAerotoler-ance in peroxidase and PerR mutants of Streptococcus pyogenes. J Bacteriol 182, 5290–5299.
95 Gaber A, Yoshimura K, Tamoi M, Takeda T, Nakano Y & Shigeoka S (2004) Induction and functional analy-sis of two reduced nicotinamide adenine dinucleotide phosphate-dependent glutathione peroxidase-like pro-teins in Synechocystis PCC 6803 during the progression of oxidative stress. Plant Physiol 136, 2855–2861. 96 Utomo A, Jiang X, Furuta S, Yun J, Levin D, Wang
Y, Desai K, Green J, Chen P & Lee W (2004) Identifi-cation of a novel putative non-selenocysteine contain-ing phospholipid hydroperoxide glutathione peroxidase (NPGPx) essential for alleviating oxidative stress gener-ated from polyunsaturgener-ated fatty acids in breast cancer cells. J Biol Chem 279, 43522–43529.
97 Avery A, Willetts S & Avery S (2004) Genetic dissec-tion of the phospholipid hydroperoxidase activity of yeast gpx3 reveals its functional importance. J Biol Chem 279, 46652–46658.
98 Imai H, Narashima K, Arai M, Sakamoto H, Chiba N & Nakagawa Y (1998) Suppression of leukotriene for-mation in RBL-2H3 cells that overexpressed phospholi-pid hydroperoxide glutathione peroxidase. J Biol Chem 273, 1990–1997.
99 Sakamoto H, Imai H & Nakagawa Y (2000) Involve-ment of phospholipid hydroperoxide glutathione perox-idase in the modulation of prostaglandin D2 synthesis. J Biol Chem 275, 40028–40035.
100 Brigelius-Flohe R, Friedrichs B, Maurer S, Schultz M & Streicher R (1997) Interleukin-1-induced nuclear fac-tor kappa B activation is inhibited by overexpression of
![Fig. 1. Alignment of GPX amino-acid sequences from various organisms. The sequences were compared and aligned using CLUSTALW software [143]](https://thumb-eu.123doks.com/thumbv2/123doknet/14090846.464738/3.892.340.792.113.583/alignment-sequences-various-organisms-sequences-compared-clustalw-software.webp)