HAL Id: hal-03190818
https://hal.archives-ouvertes.fr/hal-03190818
Submitted on 26 May 2021
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Dynamics of task-related electrophysiological networks:
a benchmarking study
Judie Tabbal, Aya Kabbara, Mohamad Khalil, Pascal Benquet, Mahmoud
Hassan
To cite this version:
Judie Tabbal, Aya Kabbara, Mohamad Khalil, Pascal Benquet, Mahmoud Hassan. Dynamics of
task-related electrophysiological networks: a benchmarking study. NeuroImage, Elsevier, 2021, 231,
pp.117829. �10.1016/j.neuroimage.2021.117829�. �hal-03190818�
ContentslistsavailableatScienceDirect
NeuroImage
journalhomepage:www.elsevier.com/locate/neuroimage
Dynamics
of
task-related
electrophysiological
networks:
a
benchmarking
study
Judie
Tabbal
a,b,∗,
Aya
Kabbara
a,
Mohamad
Khalil
b,c,
Pascal
Benquet
a,
Mahmoud
Hassan
da Univ Rennes, LTSI - U1099, F-35000 Rennes, France
b Azm Center for Research in Biotechnology and Its Applications, EDST, Lebanese University, Beirut, Lebanon c CRSI Lab, Engineering Faculty, Lebanese University, Beirut, Lebanon
d NeuroKyma, F-35000 Rennes, France
a
r
t
i
c
l
e
i
n
f
o
Keywords:
Magneto-encephalography (MEG) Electrophysiological brain networks Dynamic functional connectivity Dimensionality reduction Source separation
a
b
s
t
r
a
c
t
Motor,sensoryandcognitivefunctionsrelyondynamicreshapingoffunctionalbrainnetworks.Trackingthese rapidchangesiscrucialtounderstandinformationprocessinginthebrain,butchallengingduetothegreat varietyofdimensionalityreductionmethodsusedatthenetwork-levelandthelimitedevaluationstudies. Us-ingMagnetoencephalography(MEG)combinedwithSourceSeparation(SS)methods,wepresentanintegrated frameworktotrackfastdynamicsofelectrophysiologicalbrainnetworks.WeevaluatenineSSmethodsappliedto threeindependentMEGdatabases(N=95)duringmotorandmemorytasks.Wereportdifferencesbetweenthese methodsatthegroupandsubjectlevel.WeseektohelpresearchersinchoosingobjectivelytheappropriateSS methodwhentrackingfastreconfigurationoffunctionalbrainnetworks,duetoitsenormousbenefitsincognitive andclinicalneuroscience.
1. Introduction
Evolvingevidenceshowthatmotor,sensory,emotionaland cogni-tivefunctionsemergefromdynamicinteractionsbetweencorticaland subcorticalbrainstructures.Specificrhythmsofneuralnetworksallow synchronization andlong-rangecommunication between distant and distributedbrainareas.Thisphenomenawasshowncrucialduring vi-sual(BolaandSabel,2015;Hassanetal.,2015;Mheichetal.,2018), auditory(Fontolanetal., 2014),sensorimotor (Pomperet al., 2015; Wilkins andYao, 2020) andcognitive (Negrón-Oyarzo etal., 2018; Rouhinenetal.,2020)tasks. Thisbraincommunicationisvery tran-sientandthereisadynamicreorganizationoffunctionalbrainnetworks duringbehavioraltasks,evenatsub-secondtimescale(Vidaurreetal., 2018b).Therefore,theanalysisofwhole-braindynamicfunctional con-nectivity(dFC)hasbecomeaburgeoningfieldofresearchincognitive neuroscience(BassettandSporns,2017;Bullmore andSporns, 2009; Irajietal.,2020;Kabbaraetal.,2020).Inthisregard, Magneto/Electro-encephalography(MEG/EEG)provides a unique directand noninva-siveaccesstotheelectrophysiologicalactivityof thewhole brain,at the millisecond scale. Benefiting from the excellent time resolution of the MEG/EEG (~millisecond), current methods allow of estimat-ingsub-secondtime-varyingfunctionalbrainnetworks inthecortical space through sensor-level signals(Hassan et al., 2014; Hassan and
∗Correspondingauthor.
E-mailaddress:judytabal95@gmail.com(J.Tabbal).
Wendling,2018).Thekeychallengehere ishowtocharacterize and quantifytheserapidlychangingnetworks.
In this context, several frameworks have been used toexplicitly model/capture dynamics over time such as Hidden Markov Model (HMM)(Bakeretal.,2014;Vidaurreetal.,2018a,2018b,2016), Au-toregressivemodel(AR)(Casorsoetal.,2019)andGeneralLinearModel (GLM)(Friston,1994).Forexample,HMMdescribesthebrainactivity asasequenceof districtstates;each representsauniquepattern ob-tainedfromanobservationmodel,andastatetimecourseindicatingthe pointsintimeatwhichthatstateisactive.Otherapproachesanalyzethe timevaryingsignalusingdata-driventechniques,where‘brainnetwork states’arederiveddirectlyfrom thedatawithoutapriorihypothesis on thefittingmodel. Thesemethods haveshowedpromising results, despitethefactthattheselectionoftheusedalgorithmislargely em-pirical.Thesemethodsarebasedontwomainsteps:(1)slidingwindow approach,thatformsaseriesoftemporalnetworks,(2)adimensionality reductionorclusteringapproachincludingKmeans(Allenetal.,2014; Ciricetal.,2017;Duetal.,2016;Fongetal.,2019;LiuandDuyn,2013; Mheichetal.,2015;O’Neilletal.,2015),componentanalysissuchas temporalIndependentComponentAnalysistICA(O’Neilletal.,2017), PrincipalComponentAnalysis(PCA)(Leonardietal.,2013)and Non-negativeMatrixFactorization(NMF)(Chaietal.,2017).Althoughthe conceptualdifferencebetweenthesemethods(andwithineachfamily ofmethodssuchasdifferentICAalgorithms)istheoreticallyobvious(as
https://doi.org/10.1016/j.neuroimage.2021.117829
Received13November2020;Receivedinrevisedform25January2021;Accepted29January2021 Availableonline5February2021
theyarebasedondifferentassumptions),thestudiesthatinvestigatethe differencesbetweenthemremainedveryfew.Theexistingcomparative studiesaremainlylimitedtoconfirmingresultsofdifferencesbetween twoconditions(Leonardietal.,2013)ortoprovethatobtainedresults areunaffectedbythemethod’schoice(Milleretal.,2016).However,a throughoutquantitativeandqualitativecomparativestudyusingboth simulation-basedanddata-drivenapproachesisstillmissingandthere isnoclearconsensusaboutthe‘best’(ifany)sourceseparationor clus-teringmethodtobeusedtoadequatelytrackingdFC,whichisthemain objectiveofourstudy.
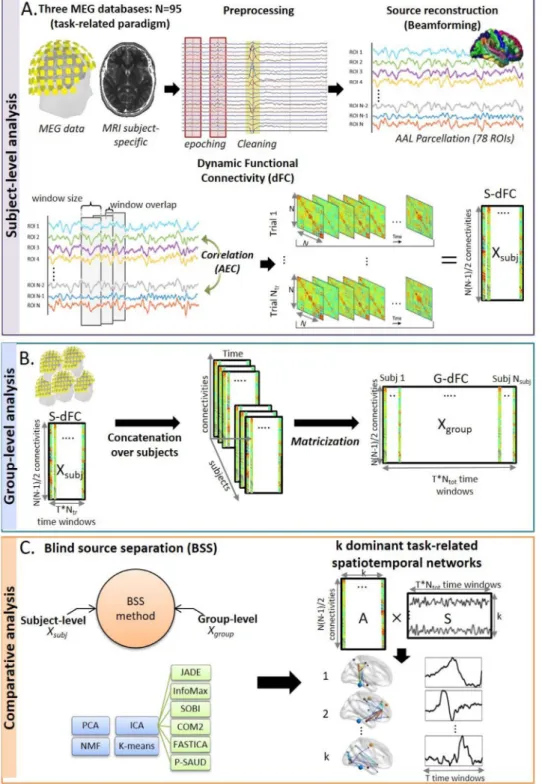
Here,weevaluatetheperformanceofnine dimensionality reduc-tionmethodsusedtotrackfunctionalconnectivitystatesatbothgroup andindividuallevels.This wasdoneusing simulationsandthree in-dependentMEGdatasets(N=95)recordedduringmotorandworking memorytasks(seeFig.1).Thedynamicbrainnetworks were recon-structedusingMEGsourceconnectivitymethodcombinedwitha slid-ingwindowtechnique.Thedimensionalityreductionalgorithmswere comparedintermsoftheirtemporalandspatialaccuracy.These meth-odsincludePCA,NMF,KmeansandsixvariousversionsofICA(Joint ApproximationDiagonalizationof Eigen-Matrices (JADE),INFOMAX, Second-OrderBlind Identification(SOBI), fixed-pointalgorithm (Fas-tICA),COM2andPenalizedSemi-AlgebraicUnitaryDeflation(P-SAUD). The motivationbehind using several ICA subtypes is that each one hasitsowndefinitionof statisticalindependenceandseveralstudies showedconceptualdifferencesbetweenthem(Kachenouraetal.,2008; Sahonero-AlvarezandCalderon,2017).Wealsoanalyzedtheoptimal numberofsubjectsneededforeachmethodtorevealsignificantresults. Thisstudyaimsatprovidingaframeworkforresearchersinterestedin studyingreconfigurationoffunctionalbrainnetworksduringcognitive processes.
2. Materialsandmethods 2.1. Data
2.1.1. Dataset1:‘self-pacedbuttonpresstask’
Thisdatasetincludes15healthyrighthandedparticipants(9male and6female,aged25±4years(mean±SD)).Theywereaskedtopress abuttonwiththeindexfingeroftheirnon-dominanthand,onceevery 30seconds,andshouldnotcountthetimebetweenpresses.Moredetails aboutthisdatasetcanbefoundin(Kabbaraetal.,2019;O’Neilletal., 2017).
2.1.2. Dataset2:‘HCPlefthandmovementTask’
61healthyparticipants(28maleand33female,aged22-35) com-pletedtheMEGMotortaskprovidedbytheHumanConnectomeProject (HCP) (MEG-1 release) (Van Essen et al., 2012). The correspond-ingexperimentalprotocolwas adaptedfrom Bucknerandcolleagues (Buckneretal.,2011;ThomasYeoetal.,2011).Itwasperformedin twosessionsof14mineach,withasmallbreakbetweenthem.Each sessionconsistedof42totalblocksrandomlydistributed;32ofthem werepartitionedinto16handmovementsblocks(8rightand8left), and16-footmovementsblocks(8rightand8left),andtheremaining 10blockswereinterleavedresting/fixationblocks.Eachmotoreffector blockwasprecededbya3secvisualcuethatpromptsparticipantsto eithertaptheirleftorrightindexandthumbfingersorsqueezetheir leftorrighttoes.Theblocklastedfor12secandconsistedof10 sequen-tialmovements,eachinitiatedwith150mspacingstimulifollowedby 1050msblackscreenfortaskexecution.Here,forsimplicity,wewere in-terestedinthetrialsrelatedtothelefthandmovesonly.MEGdatawas recordedatSaintLouisUniversityat508.6275Hzsamplingfrequency andco-registeredwiththeavailablesubjectspecificMRI.EMGactivity wasalsorecordedfromeachlimb.
2.1.3. Dataset3:‘sternbergworkingmemorytask’
19 healthyparticipants(10maleand9female, aged25±3years (mean±SD))performedSternbergtask,inwhichtwoexamplevisual stimuli,mainlyabstractgeometricshapes,weresuccessivelypresented onascreen;eachfor0.6secandseparatedby1sec.Then,amaintenance periodof7secwasleftbeforethepresentationofathirdprobe stimu-lus.Consequently,subjectswereaskedtopressabuttonwiththeirright indexfingeronlyiftheprobestimulusmatchedeitherofthetwo ex-amplestimuliandanimmediatefeedbackwillbegiventoshowtheir responsecorrectness.30trialswerepresentedseparatedby30secofrest. Inbothdatasets1and3,MEGdatawererecordedusinga275-channel CTFMEGsystemat600Hzsamplingfrequencyandco-registeredwith subject-specificMRI.BothdatasetswereapprovedbytheUniversityof NottinghamMedicalSchoolResearchEthicsCommittee(O’Neilletal., 2017;Vidaurreetal.,2018a).
2.2. Methodology 2.2.1. Preprocessing
Bothdatasets 1and3werereceivedalready preprocessedas de-scribed in (O’Neillet al., 2017).Briefly, bad segmentsproducedby muscles,eyeor headmovementwerealready visuallyinspectedand removed. Fordataset 2, we used the preprocessingpipeline offered bytheHCPconsortium,which includesremoving badchannels, seg-mentsandbadindependentcomponentsfromtaskdata.Segmentswere retrievedfromthedataset1intheinterval[-15;+15sec]relativeto thebuttonpressonset,andfromthedataset3intheintervalof[-16; +28sec]relativetostimuluspresentation.InHCPanalysis, wechose dataepochstime-lockedtoEMGonsetaswewereconcernedin explor-ingbrainnetworksinvolvedduringmovementexecution.Thus,trials weresegmentedin[-1.2;+1.2sec]relativetoEMGonset.Then,as func-tionalconnectivitywasprovedtobefrequency-dependent(Bakeretal., 2014;Hippetal.,2012),eachdatasetwaspreprocessedinits appro-priatefrequencybandactivelyinvolvedinthecorrespondingcognitive task.Whilebetaband[13-30Hz]wasusedforself-pacedandHCPleft handmotortask,workingmemorydatawasfilteredinabroaderband [4-30Hz]asitishasbeenshowntoinvolvemultiplefrequencybands, accordingtopreviousstudies(Brookesetal.,2012;O’Neilletal.,2017). Afterthesepreprocessingsteps,anaverageof34,150and29persubject werekeptfromdataset1,2and3,respectively.
2.2.2. Sourcereconstructionandfunctionalconnectivity
Inordertolocalizebrainsourcesandreconstructtheiractivities,we usedtheLinearlyConstrainedMinimumVarianceBeamforming(LCMV) (ROBINSON, 1999) approach on parcellated cortex using AAL atlas (N=78regionsofinterests-ROIs-(Gongetal.,2009))(Hillebrandetal., 2016).Thiswasdonebyregisteringeachsubject’sanatomicalMRIto anMNItemplate(Smithetal.,2004)followedbyaninverse registra-tiontotheanatomicalsubjectspace. Datacovariance wascomputed withinthespecificfrequencybandusedandatimewindowspanning thewholeexperiment(Brookesetal.,2008)witharegularization pa-rameter(5%)usingTikhonovmethod.Theforwardmodelwasbased uponadipoleapproximation(Sarvas,1987)andamultiplelocalsphere headmodelfittedtothesubject-specificMRIscalpsurface.Dipole ori-entationwasdetermined usinganon-linear searchforoptimum ‘sig-nal tonoiseratio’(SNR)(SekiharaandNagarajan,2008).Following this,weestimated thefunctionalconnectivity bycomputingthe am-plitude envelope correlations(usingHilberttransformation) between all ROIs(Brookeset al.,2012; Hippet al.,2012).In ordertoavoid spurious estimates of functionalconnectivity, we performed leakage correction on the reconstructed sources signals. We used the multi-variateapproach based onsymmetric orthogonalisationproposed by (Brookesetal.,2012;Colcloughetal.,2015)fordatasets1and3,while pair-wiseorthogonalization(Brookesetal.,2016;Tewarieetal.,2019b) wasappliedtodataset2duetotheshorttimeperiodofthetask.
Fig. 1. Illustration of the investigation structure for each of the three task-related paradigms. A. The fundamental processing pipeline applied on each subject data from sensor-level (using non-invasive MEG tech-nique) to cortical-level (using beamforming astheinverseproblemsolution) todynamic functional connectivity computation (S-dFC) (usingtheslidingwindowapproach).B. Con-catenationofS-dFCofallsubjectsalongtime axistoformagroupdatareferredtoasG-dFC, C.Comparativeanalysisbetweenninedifferent sourceseparation(SS)methods(sixvariantsof ICA,PCA,NMFandKmeans)appliedonboth group-level (Xgroup) and subject-level (Xsubj) datainordertoderivekdominanttask-related spatiotemporal components (the mixing ma-tricesrepresent brainspatialmapswhilethe extracted sources represent corresponding temporalweightsfluctuations).
2.2.3. Dynamicfunctionalconnectivityanalysis(dFC)
Toestimatethedynamicfunctionalbrainnetworks,weadoptedthe widelyusedapproachofslidingwindowsfordatasets1and3.Tothis end,atimewindowoflength6secwith0.5secwasusedfordatasets 1and3asappliedby(O’Neilletal.,2017).Concerningthedataset2 (HCPdataset),thefasttimescaleofthetaskimposesaverysmalltime windowwidththatmaybetoonoisytoextractmeaningful informa-tion(Liuzzietal.,2019).Thus,weavoidedtoapplytheslidingwindow approach,andusedinsteadthehightemporalresolutionversionof am-plitudeenvelopecorrelationmetric;the‘InstanteneousAmplitude Cor-relation’(IAC)alreadyvalidatedinarecentworkforthesamedataset
(Tewarieetal.,2019b).Asaresult,weobtained,foreachsubjecttrial,a ‘dynamicfunctionalconnectivity(dFC)’matrixofdimension[NxNxT], whereTreferstothenumberofwindowsfordatasets1and3,and num-beroftotaltimesamplesfordataset2(T=49,1221and75fordatasets 1,2and3respectively).Next,duetosymmetry,weunfoldedthismatrix intoa2-D[Nx(N−1)/2×T]matrixbyremovingtheredundant connec-tionsineachtimewindow.Then,themeanofeachrowofthismatrix issubtractedfromthedata.Finally,allsubjects’trialsdFCwere con-catenated alongthetemporaldimension.Wedefined thismatrixasa ‘Groupdynamicfunctionalconnectivitymatrix(G-dFC)’,denoted‘X’. Notethatfordataset2,weaveragedconnectivitymatricesofalltrials
relativetoeachsubject(Zhuetal.,2020)duetomemorylimitationin Matlabregardinghighdimensionaldataofthetemporallyconcatenated ‘sample-by-sample’dFCofallsubject’strials.
2.2.4. Task-relatedfunctionalbrainnetworks
2.2.4.1. Problem statement. The resultant G-dFC matrix representing thetime-varyingfeaturescanbeexpressedasasalinearmixtureof ele-mentarybrainnetworksthatfluctuatedynamicallyovertime.Suchissue isthemainconcernofSourceSeparation(SS)approachaimingat recov-ering‘k’hiddensourcesfromasetofobservationswithminimalpriori knowledgeaboutthesesources.Inthiscontext,theSSproblemcanbe formulatedasfollows:
X=A × S (1)
Where:
• ‘X’isthecomputedG-dFCmatrixofdimension[qxm]:
○ q=Nx(N−1)/2withN=78,representingconnectivitiesbetween allROIs.
○ m=TxNtotwithTisthenumberoftimewindowsandNtotisthe totalnumberoftrialsforallsubjects.
• ‘A’is the mixingmatrixof dimension [qxk]illustrating the con-tributionweightsofeachindividualconnectiontothecomponents sources,thusthespatialmapsofbrainnetworks(k<min(q,m)).
• ‘S’isthesourcesmatrixofdimension[kxm]representingtemporal sourcessignaturesofG-dFC,collapsedacrossallconnections. AmongexistingSSalgorithms,wechoseninepopular/well-known methods: six different variants of temporal Independent Component Analysis(tICA),PrincipalComponentAnalysis(PCA),Non-negative Ma-trix Factorization(NMF) andKmeans as a state-of-the-art clustering method.Theyalltransformthedesiredmatrixfactorizationintospatial mapsandtimeseries.However,theydifferprimarilyintheconstraints imposedondecomposed components.Below,wewillgiveasuccinct descriptionaboutthesemethods.
2.2.4.2. Independent component analysis: ‘temporal statistical indepen-dence’. ICAtendstolinearlytransformmultivariateobservationsinto asetof‘statisticallymutuallyindependent’latentvariablesunderthe hypothesisthatthesevariablesareas‘non-Gaussian’aspossible.Inour study,we examinetemporalICA (tICA)adoptedbyseveral previous studies(O’Neilletal.,2017;Yaesoubietal.,2015)inordertoobtain statesthatfluctuateindependentlyintime.Inthiscontext,decomposed signals‘S’consistofthe‘k’sourcetimecoursesandtheassociatedmixing matrix‘A’illustratesthecontributionoftemporallyindependentmaps. Thereareseveralcriteria tomeasure independencesuchas mini-mizationofmutualinformationandmaximizationofnon-Gaussianity. Hence,differentalgorithmsareproposedtoperformICAdecomposition, eachyieldingtodifferentICAmodelwithspecificcharacteristics.Here, weevaluatetICAusingsixdifferentpopularandprominentmethods: (1)JADE,(2)InfoMax,(3)SOBI,(4)FastICA,(5)CoM2and(6)PSAUD. Thesemethodsarechoseninsuchawaytocovervariousstatistical inde-pendencedefinitions,statisticalorderandcomputationalprocess tech-niques.Briefly,InfoMaxandFastICAarebasedoninformationtheory, whileallotherselectedmethodsoptimizecontrastfunctionsbasedon cumulantsofthedata.Amongthem,SOBIusesonlySecondOrder(SO) cumulantsincontrasttoothersthatexploitbothSOandFourthOrder (FO)cumulants.Inaddition,FastICAandPSAUDuseadeflationprocess fordecompositionwhileotherICAvariantsjointlyseparatesources. De-tailsaboutICAsubtypesusedcanbefoundinsupplementarymaterials. 2.2.4.3. Principalcomponentanalysis:‘variancemaximization’. PCAisa basiclineartechniquewidelyusedfordatadimensionalityreduction. Itinvolvesamathematicalprocedurethattransformsasetof observa-tionsofpossiblycorrelatedvariablesintosmallernumberoforthogonal, hencelinearly uncorrelatedvariablescalled principal componentsor ‘eigenvectors.Thisprocedureisdefinedinsuchawaythatthevariance
or‘eigenvalues’ofthedataismaximized.Then,afixednumber‘k’of eigenvectorsandtheirrespectiveeigenvaluescanbechosentoobtaina consistentrepresentationofthedata.Here,weapplytheSingularValue Decomposition(SVD)algorithmofPCA(GolubandReinsch,1970)on ourpredefinedinputmatrix‘X’.Defining‘A’and‘S’matricesfromSVD outputsismoreclarifiedinSupplementaryMaterials.
2.2.4.4. Non-negativematrixfactorization:‘positivity’. Nonnegative ma-trixfactorization(NMF)isanunsupervisedmachine-learningtechnique (LeeandSeung,1999)thatimposes‘non-negativity’constraintonthe decomposedfactorswhensolvingSSproblem.WhenappliedtoG-dFC data‘X’,NMFleadstoparts-basedrepresentationthatcapturesadditive combinationofbasissubgraphs‘A’ateachtimewindowwith tempo-ralcoefficients‘S’eliminatingnegativesignalvariations.Amongseveral existingNMFapproaches,weselectedAlternatingLeastSquares(ALS) algorithmthathaspreviouslyshowngoodperformanceinfMRIcontext (Dingetal.,2013)with100timesreplications.
2.2.4.5. Kmeansclustering:‘sparsity’. Kmeansisoneofthesimplest un-supervisedlearningalgorithmsthatsolvetheSSproblemthrough clus-teringapproach(Lloyd,1982).Thealgorithmworksiterativelytoassign eachpointtoonlyoneofthe‘k’groupsbasedonfeaturesimilarity. Math-ematicalcomputationofKmeansclustersisdefinedinSupplementary Materials.Inourframework,thesparsecodingadoptedbyKmeans re-strictsasingletimepointtohaveauniqueactivatednetworkstate.The computedclusters‘A’representsthestructureofcommonconnectivity patternsacrosssubjects.Foragiventrial,eachtimewindowisassigned withthecorrespondingclusterindex.Then,thematrix‘S’iscalculated asthefrequencyofreoccurrenceofeachclusterateachtimewindow acrossalltrialsandsubjects.Here,weadaptedthesameprocedureof KmeansusedbyAllenetal.(Allenetal.,2014):L1(Manhattan)distance isused,asitwassuggestedtobemoreeffectivethanL2(Euclidiean) dis-tanceforhigh-dimensionaldata(Aggarwaletal.,2001).Thealgorithm isreplicated100timestoincreasechancesofescapinglocalminima, andcentroidpositionswererandomlyinitialized.Then,Kmeansreturns thesolutionwiththelowest‘SUMD’(within-clusterSumsof points-to-centroidsDistances).
2.2.5. Comparativeanalysis 2.2.5.1. MEGgroup-levelanalysis.
2.2.5.1.1. Selectionofoptimalnumberofcomponents(NCopt). Inthe
contextofdimensionalityreductionmethods,thechoiceoftheoptimal numberofcomponents(NCopt)tobeextractedisstillachallengingissue.
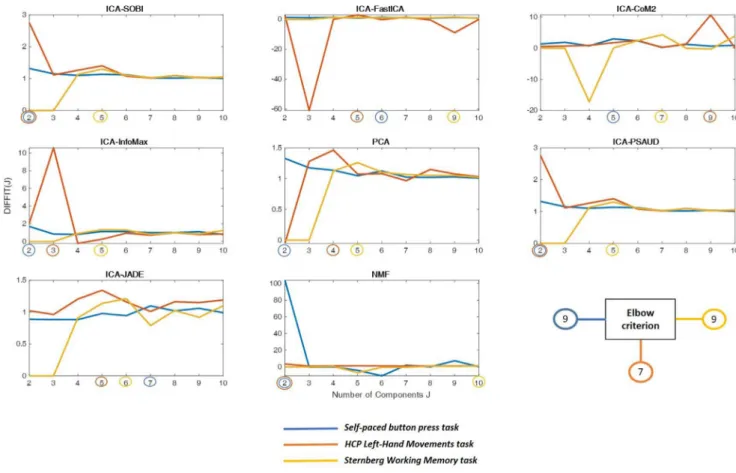
Here,weusedthewell-knownapproach:‘Elbowcriterion’(Allenetal., 2014)forKmeansmethod(withmaximumnumberofclusters=10).For allotherSSmethods,weestimatedNCoptbasedonthegoodnessoffit
approach(TimmermanandKiers,2000;Wangetal.,2018)previously usedbymanyrecentworks(Tewarieetal.,2019b;Zhuetal.,2020). Weperformedthe‘DIFFIT’methodthatreferstothedifferenceindata fittingwitharangeofinputNCvariedfrom2(formotortasks)and4 (forworkingmemorytask)to10components,andselectedtheNCthat givesthelargestDIFFITvalueastheNCopt.Technicaldetailsaboutthese approachescanbefoundintheSupplementaryMaterials.
2.2.5.1.2. Selectionofsignificantcomponents. AmongtheNCopt
ex-tractedcomponents,identifyingthosethatreflectgenuinebrainactivity relatedtothetaskiscritical.Inthispaper,wefollowedatesting pro-cedure adoptedby(O’Neilletal., 2017)andpreviouslydescribed in (Huntetal.,2012;Winkleretal.,2014)todeterminesignificant com-ponentsmodulatedbythetasks.Thetestingreliesontheconstruction ofempiricalnulldistributionbasedona‘signflipping’permutation ap-proach.Therefore,acomponentwasconsideredsignificantif,atany timepoint,thecorrespondingtimesignal,averagedovertrials,fell out-sideathresholddefinedat0.05withcorrections.Foralldatasets, 2-taileddistributionwasallowed,andBonferronicorrectionswereapplied formultiplecomparisonsacrosstheNCoptcomponentsandacross
tem-poraldegreeoffreedom.Moredetailsabout‘sign-flipping’approachand thresholdvaluessettingcanbefoundinSupplementaryMaterials. 2.2.5.2. MEG subject-levelanalysis. Besides group-levelanalysis, itis crucialtotesttheperformanceofeachmethodwhenapplieddirectly onindividualdFC.Tothisend,insteadofconcatenatingtrialsfromall subjectsasinthefinalstepof‘G-dFC’computation,weperform,foreach subject,adFCconcatenationofalltrialsrelatedonlytothissubjectto formasubjectspecificdFC,denoted‘S-dFC’.Then,allselectedSS meth-odswereapplied on‘S-dFC’matrixtoextractsubject-specificspatial andtemporalsignatures(k=10).Inordertoquantitativelyevaluateand comparemethodsstrengthatsubject-levelcontext,wemeasure,foreach method,bothspatialandtemporalsimilaritiesbetweeneachextracted S-dFCcomponentandsignificantG-dFCcomponents.Theseparameters are:
(1)AverageDistance(AD)for‘spatialsimilarity’: 𝐴𝐷= ∑ 𝑠𝑑(𝑛𝑠,𝑛𝑔) 𝑁𝑠 𝑠∈ [ 1,𝑁𝑠];𝑔∈[1,𝑁𝐺] (2) Where𝑑(𝑛𝑠,𝑛𝑔)istheEuclidiandistancebetweenthenode𝑛𝑠ofS-dFC
networkandthenearestnode𝑛𝑔fromthesignificantG-dFCnetwork.𝑁𝑠
isthetotalnumberofnodesinS-dFCnetwork,and𝑁𝐺denotesthetotal
numberofnodesinG-dFCnetwork.Allnetworkswere70%thresholded. LowervaluesofADindicatestrongerspatialsimilaritybetweenS-dFC andG-dFCnetworks.
(2)CorrelationSignals(CS)for‘TemporalSimilarity’: 𝐶𝑆(𝑇𝑆,𝑇𝐺)= ∑ 𝑠∑𝑔(𝑇𝑆𝑠𝑔−𝑇𝑆)(𝑇𝐺𝑠𝑔−𝑇𝐺) √(∑ 𝑠∑𝑔(𝑇𝑆𝑠𝑔−𝑇𝑆) 2)(∑ 𝑠∑𝑔(𝑇𝐺𝑠𝑔−𝑇𝐺) 2) 𝑠∈[1,𝐿𝑠];𝑔∈[1,𝐿𝐺] (3)
Where𝑇𝑆 isthetemporalsignalof eachS-dFCcomponentof length 𝐿𝑠and𝑇𝐺 representstemporalsignalsofG-dFCsignificantcomponent
oflength𝐿𝐺.HighervaluesofCSrevealstrongertemporalsimilarity
betweenS-dFCandG-dFCsignals.
Weperformthisanalysisoneachsubjectamongthe15subjectsof theMEGdataset1(Motortask).Therefore,foreachmethod,wecounted thenumberofsubjectsthatshowsatisfactoryresultsperformanceinthe contextofS-dFC,basedonthepreviouslyexplainedmeasures.Then,to approximatethenumberofsubjects/trialsneededforeachSSmethodto givesignificantresults,wefollowthesameprocedureexplainedabove, butinsteadofsinglesubjectS-dFCcomputation,weincreasedthe num-berofconcatenatedsubjectsindFCcomputationfromNsubj=2to14,
progressively.Inordertohavegeneralizedandreliableresults,we con-sideredallpossiblecombinationsrelativetoeachNsubj(𝐶𝑁15𝑠𝑢𝑏𝑗),where
differentsetsofNsubjsubjectswereselectedamongthe15existingdata
subjects. 3. Results
In the following, we present our evaluation study on real MEG data, however our methodology was also tested on simulated data. Theseresultscanbefoundinthesupplementarymaterial.Briefly,the simulation-basedanalysisshowedthatallmethodsprovidesatisfactory resultsintermsofspatialandtemporalsimilaritybetweenreconstructed andsimulatedcomponentswiththebestperformanceforNMFmethod andthe worst for SOBI. All methods, except for FastICA, NMFand Kmeans,providedconsistentresults.PSAUDandPCAwerethefastest. ResultsrevealedthatSOBI,NMFandKmeansconvergemoreslowlythan otherswiththeincreasedvalueofSNR.Readercanreferto supplemen-tarymaterialtoseethedetailedquantitativeanalysisonsimulateddata. WefirstlyraneachalgorithmateachvalueofNCandcalculatedthe correspondingDIFFITvaluesinordertoselecttheoptimalnumberof components(NCopt)relativetotheseSSmethods.Resultsareshownin
Fig.2forthethree empiricaldatasets.Hereinafter,we setNCtothe computedNCoptvaluerelativetoeachmethodandtask.
ResultsofdifferentSSmethodsappliedonempiricaldataare illus-tratedinFigs.3–5.IneachFig.,wepresentedonlythecomponentsthat demonstratedsignificanttaskmodulationbasedontheappliednull dis-tributionapproach(describedin themethodssection).Thenetworks werethresholdedonlyforvisualizationpurpose(70%fordataset1and 3,85%fordataset2).Correspondingdynamicreconfigurationofeach significant networkwereplotted together.Thetemporalfluctuations representcomponenttimesignalsaveragedovertrialsandsubjects. 3.1. Self-pacedbuttonpresstask
Inthistask,participantswereaskedtopressabuttonwiththeindex oftheirnon-dominanthandevery30seconds.
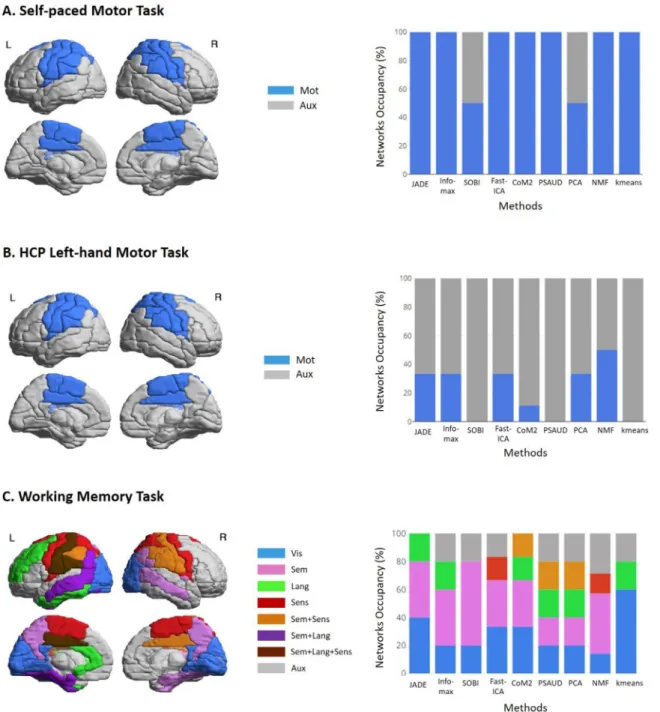
Basedonliteraturefindings (seetableS1insupplementary mate-rials),wewereinterestedinquantifyingSSmethodsabilitytoextract asensorimotornetworkfromsignificantcomponents.Tothisend,we definedabrainnetworkwithactivatedAALregionsinbothmotor cor-tex(includingprecentral,paracentral,rolandicandsupplementary mo-torareas)andsomatosensorycortex(includingpostcentral,parietaland supramarginalareas)servingasamasktemplateforournetworkof in-terest(sensorimotornetwork),illustratedinFig.7.Then,weselected eachsignificantnetworkandcomputedthestrengthofeachactivated nodeinthatsignificantnetwork(definedasthesumofalledgesweights connectedtothatnode).TheratioofthestrengthofactivatedAALnodes thatbelongstosensorimotormaskiscalculatedrelativetothestrength of allactivatednodesinthatsignificantnetwork.Incasetheratiois greaterthanacertainthresholdvalue,thenetworkisconsideredasa sensorimotornetworkdenotedas‘mot’intheFig.3.Otherwise,the net-workisdenoted‘Aux’referringtoauxiliarynetwork.Aftermanytrials (threshold=0.5,0.6,0.7),thresholdvaluewassetto0.6asithasshown moreconvenientresults,whenvisuallyinspectingcomponents classifi-cation(falsepositiveandfalsenegative).Thereadercanreferto sup-plementarymaterials(Fig.S8)formoredetailsaboutthecomputation oftheratiovaluesforallcomponents.
Fig.3showsthatallSSmethodswereabletoextractatleastone sig-nificant‘Mot’network.Allsignificantcomponentsextractedfromthe fiveICAmethods(JADE,InfoMax,FastICA,CoM2andPSAUD),NMF and Kmeans methodswere categorizedas‘Mot’ networkdue to the strongparticipationofsensorimotornodesinthesenetworks (sensori-motorstrength ratio>0.6) althoughsome ofthemmayinvolve addi-tionalfewconnectionstootherregions.Ontheotherhand,SOBIand PCAmethodsshowed‘Aux’networks(sensorimotorstrengthratio<0.6) besides‘Mot’networks,withremarkableactivationsinfrontalregions. Temporalvariationwassimilarforalmostall significantcomponents overallmethodsshowingapeakvalueat0sec,thebuttonpresstime, withslightdifferencesinamplitudevalues,indicatingsignalintensities relativetoeachcomponent.Notethatnegativeconnectivity,referredto asblueconnectionsinspatialnetworksandnegativetemporalvaluesin temporalsignals,representsdesynchronizationbetweenbrainregions. Therefore,allstudiedSSmethodswereabletoextractatleastone sig-nificantcomponentthathighlightstrongconnectionsbetweensensory andmotorregionsmodulatedsignificantlybythetaskattheexact but-tonpressinstant(‘Mot’).
3.2. Left-handmovementtask
Thistaskisalsomotorbutdifferentthanthepreviousone.Herethe participantswereaskedtorapidlyandsuccessivelytaptheirleftindex andthumbfingers.Similarlytotheprevioustask,thesamesensorimotor maskwasappliedtoquantifyresultantnetworkstodiscriminate‘Mot’ from‘Aux’networks.Thereadercanrefertosupplementarymaterials (Fig.S9)formoredetailsaboutthecomputationoftheratiovaluesfor allcomponents.
Fig.2.OptimalNumberofComponents(NCopt)results.DIFFITvaluesareplottedagainstnumberofcomponent‘J’forallICAmethods,PCAandNMF.Theblueplot
correspondstotheself-pacedbuttonpresstask,theorangeplotforHCPleft-handmovementtaskandtheyellowoneforWorkingMemorytask.TheoptimalNCthat givesthehighestvalueofDIFITrelativetoeachtaskismarkedbyasmallcircleonthex-axis.ResultsofoptimalNCrelativetoKmeansusingtheelbowcriterionis alsoshown.(Forinterpretationofthereferencestocolourinthisfigurelegend,thereaderisreferredtothewebversionofthisarticle.)
Fig.4showsthatnotallSSmethodswereabletoextracta sensori-motor‘Mot’network.Forexample,noneofthesignificantcomponents ofSOBI,PSAUDandKmeanshassurvivedthethresholdimposedforthe strengthofactivatedsensorimotornodes(Fig.S9)andaretherefore con-sideredas‘Aux’networksasindicatedinFig.4.Inthesemethods,‘Aux’ networksconsistofeitheravisualnetworksignificantlymodulated di-rectlyaftertheonsetofmovementinKmeansand0.35secbeforeonset inSOBIandPSAUD,oranetwork(oneinSOBIandPSAUDandsixin Kmeans)involvingstrongconnectionsbetweenalmostallbrainareas modulatedat0.2secbeforeandafteronset.Itshouldbenotedthat al-thoughthisnetworkshowsstrongactivationoftherightprecentraland postcentralnodes,itfailedtobequantifiedasasensorimotornetwork duetothehighcoverageofthebrain.
AllremainingSS methodswereabletoextract one‘Mot’network amongallsignificantcomponents.Thespatialrepresentationof‘mot’ networkinvolvessensorimotorwithsomecingulatenodesfromtheleft cortexinJADE, rightcortexinInfoMaxandbothleft andright cor-ticesinotherSSmethods.These‘Mot’networksshowsignificantdrop inconnectivityaround0.2secfollowingthemovementonset.Significant increasedmodulationwasalsoobservedat-0.2secinInfoMax,FastICA, CoM2,PCAandNMFmethods.Exacttimesofcomponentssignificance areindicatedwithstarsonFig.4.FromthisFig.,wecanseethat‘Aux’ networksshowvariousspatialpatternsbetweenSSmethods(suchasthe integrationofareasfromvisual,motor-frontal,motor-visual,temporal lobes…).
Regardingtemporalevolution,thehandmovementherearemuch morefrequentthantheprevioustask.Clearly,thefastneuralactivity duetotheshorttimebetweensuccessivebuttonpressesis expressed asanoscillatorybehaviourofbrainnetworkactivityaroundthezero timebuttonpress,asshowedalsopreviously(Vidaurreetal.,2018a).
Thisyieldstotheobtainedtemporalvariationwherethemotornetwork stateseemstohavehighconnectivitybeforebuttonpressandbeginto haveadrop-inconnectivitytoreachitssignificantpeakafter~0.2sec (referredtoasadesynchronizationinhighfrequencies(Vidaurreetal., 2018a)).
3.3. Workingmemorytask
Thistaskismuchmorecomplexcomparingtotheothertwotasks. Subjectsherewereaskedtovisualizeandmemorizetwovisualshapes andrespond toa thirdprobe stimulusbyabutton press(withtheir rightindexfinger)in caseofmatching.Theincreasedcognitiveload evoked by the Sternberg task is expected to induce variations in a greater numberofsignificantbrainnetworksincludingstimulus visu-alisation(visualnetwork),semanticprocessingandpatternrecognition (semantic,languagenetworks)andbuttonpressresponse(sensorimotor network).
Inasimilarcontextofprevioustasks,fourmasksweredefinedhere relatedtothemostrelevantworkingmemoryrelatednetworksfound in literature. These masks are also illustrated in Fig. 7. The visual mask consistsoftheactivationof primaryvisualcortex(occipital ar-eas,cuneus,calcarineandlingual)andisdenotedas‘Vis’intheFig.5. Thesemanticmaskinvolvesconnectionsbetweenbilateraltemporal (in-cludingfusiform,heschl,parahippocampal)andparietallobe (postcen-tral,supramarginal,angular,precuneus).Thelanguagemask(denoted ‘Lang’)isdefinedasaleftlateralisednetworkwithactivationofnodes fromtemporal,frontalandparietalregionsfromtheleftcortex.The sen-sorimotor mask(‘Mot’)ispreviouslydefinedinmotortasks.Detailed ratiovaluesofthefourmasksforallcomponentscanbefoundin de-tails insupplementary materials(Fig.S10).Inthistask,byapplying
Fig.3.Self-pacedmotortaskresults.SpatialandtemporaldistributionofallsignificantcomponentsderivedfromallcomparedSSmethodsappliedonG-dFCin theself-pacedmotortask(N=15subjects).Allbrainnetworkswerethresholdedforvisualization;lineswidthindicatesconnectivitystrengthbetweenregions.Red linesrepresentpositiveconnectivityvalueswhiletheblueonesrepresentnegativevalues.IntegratedAALnodesarerepresentedbyspheresofdifferentsizesthat revealconnectivityweights(strength)betweenthatregionandtherestofbrain.Correspondingtemporalevolutionisaveragedacrossalltrialsandsubjects.Time valuesonthex-axisrepresentthepositionoftheslidingwindow’scenter,relativetothebuttonpressatt=0sec(asillustratedbyaverticalline).Acolorcodeis attributedforeachcomponentinspaceandtime.ForeachSSmethod,onlysignificantcomponents(pcorrected<0.05)thatappearoutsidethe‘sign-flip’basednull
distribution(asdescribedinmethodssections)areshownhere.AllNCoptextractedcomponentswithcorrespondingnulldistributionareshowninSupplementary
Fig.S4foranexampleofICA-JADEmethod.NotethatsensorimotornetworkisclearlyactivatedatthebuttonpressinstantinallSSmethods.InthisFig.,‘Mot’ referstosensorimotornetworkand‘Aux’referstoallothers‘non-sensorimotor’networks.AninteractiveversionofICA-JADEresultscanbefoundonourgithub https://github.com/judytabbal/dynbrainSS.gitusingrotatableMATLABfigures.(Forinterpretationofthereferencestocolourinthisfigurelegend,thereaderis referredtothewebversionofthisarticle.)
simultaneouslyfourmasksonthesamenetworkcomponent,thereisa possibilitythattheratiostrengthofmorethanonemasksurvivesthe threshold(0.6).Inthiscase,thenetworkbelongstothemaskthatgives thehighestratiostrengthvalue.Incaseofequalitybetweentwomasks, weconsiderthatthenetworkbelongstobothmasks.
Fig.5illustratesthe spatialdistributionof all significant compo-nents for all SS methods, and temporalvariation for three of these methods(JADE,NMFandKmeans)forvisualisationclarity.The tem-poralevolutionofother SSmethodscan be foundinSupplementary Fig.S7.
Startingfromt=0sec,twovisualstimulus(shapes)werepresented successively,eachfor0.6sec.Duringthisperiod,allmethods,exceptfor PCA,wereabletoextractoneormoresignificant‘Vis’network.Wecan noticefewadditionalconnectionsfromoccipitaltoparietalortemporal regionsinthisnetwork.Timevariationofthisnetworkshowssignificant peakduringthefirsttwosecondsperiod.
Followingstimuluspresentation,subjectsshouldretaintheobserved shapesinworkingmemory.Duringthisperiod,knownasthe mainte-nancephase,allmethods,exceptKmeans,showsignificantdecreased modulationat[4-6sec]ofatleastone‘Sem’network.Thebreakdownin
Fig.4.HCPlefthandmovementstaskresults.SpatialandtemporaldistributionofallsignificantcomponentsderivedfromallcomparedSSmethodsappliedon G-dFCintheleft-handmotortask(N2=61subjects).Allbrainnetworksarethresholdedforvisualization.Timevaluesonthex-axisrepresentthepositionofthe slidingwindow’scenter,relativetothebuttonpressatt=0sec(asillustratedbyaverticalline).Acolorcodeisalsoattributedforeachcomponentinspaceandtime. ForeachSSmethod,onlysignificantcomponents(pcorrected<0.05)thatappearoutsidethenulldistributionareshownhere.Exacttimesofsignificancerelativeto eachcomponentareindicatedwithstars.,revealinganoscillatorytemporalactivationofmotorcomponentforallSSmethods.AllNCoptextractedcomponentswith
correspondingnulldistributionareshowninSupplementaryFig.S5foranexampleofICA-JADEmethod.InthisFig.,‘Mot’referstosensorimotornetworkand‘Aux’ referstoallothers‘non-sensorimotor’networks.AninteractiveversionofICA-JADEresultscanbefoundonourgithubhttps://github.com/judytabbal/dynbrainSS.git usingrotatableMATLABFigs..Reproducingtheseresultsisalsopossible/availableusingtheMATLABinterfaceongithub.
thisnetwork’sconnectivitywaspreviouslydemonstrated(O’Neilletal., 2017).Duringthesameperiod,wecannoticeadrop-inconnectivityin ‘Sens’networkrevealedonlybyNMFmethod.
At[10-12sec]period,manynetworksseemtobesignificantly mod-ulatedamongallmethods:(1)‘Vis’isre-activatedattheprobestimulus presentationinallmethodsexceptforPSAUD,(2)‘Sens’shows signifi-cantincreasewithFastICAmethod.Thisnetworkbecomesmoststrongly connectedaroundthetimebuttonpressresponse,(3)‘Lang’ is com-monlyderivedbyJADE,InfoMax,andPCA,andexhibitsanincreased connectivitypeakingduringprobepresentation.Wecannoticethatthis networkisalsosignificantlydecreasedinCoM2,PSAUDandKmeans around5sec.(4)Threemethods(JADE,InfoMaxandPCA)alsoshowed significantincreasedmodulationof‘Sem’network.(5)Anetworkthat belongsequallytoboth‘Sem’and‘Sens’masks(denotedas‘Sem+Sens’
network)isstronglyactivatedduringthisperiodinCoM2andPSAUD. Notethatthesetwomaskshavecommonbrainregionsmainlyinparietal loberesponsibleforsensoryprocessingwhichiscoherentwiththetask evolution.This‘Sem+Sens’networkisalsomodulatedinPCAmethod. Besidesthesecomponents,few‘Aux’networkswerealsoconsidered sig-nificantasshowninFig.5.
In summary,the three SS methods(CoM2,PSAUD andPCA) suc-ceededtoderiveallexpectedcomponentswiththeirappropriate tem-poral significant modulation. JADE and InfoMax were able to ex-tract visual, semantic and language but not the sensorimotor net-work. FastICA and NMF missed the language component. How-ever, SOBI was unable to show both sensorimotor and language networks and Kmeans failed to extract semantic and sensorimotor components.
Fig.5. Sternbergworkingmemorytaskresults.SpatialandtemporaldistributionofallsignificantcomponentsderivedfromallcomparedSSmethodsappliedon G-dFCintheworkingmemorytask(N3=19subjects).Allbrainnetworksarethresholdedforvisualization.Timevaluesonthex-axisrepresentthepositionofthe slidingwindow’scenter,relativetothefirstvisualstimuluspresentationatt=0sec(asillustratedbyaverticalline).Thefirsttwoverticallinesillustratetheinstant ofsuccessivevisualexamplespresentationatt=0sand1.6secandthethirdverticallineatt~9secseparatesbetweenthemaintenanceperiodthatlastsfor7secand theprobepresentationfollowedbyapossiblebuttonpressandfeedback.Acolorcodeisattributedforeachcomponentinspaceandtime.ForeachSSmethod, onlysignificantcomponents(pcorrected<0.05)thatappearoutsidethenulldistributionareshownhere.Exacttimesofsignificancerelativetoeachcomponentare
indicatedwithstars.TemporalvariationofonlyJADE,NMFandKmeansisillustrated,whereastherestareshowninSupplementaryFig.S7.AllNCoptextracted
componentswithcorrespondingnulldistributionareshowninSupplementaryFig.S6foranexampleofICA-JADEmethod.Notethatinthistask,muchlargervariety ofsignificantnetworksareextractedamongSSmethods,includingvisual,sensorimotor,language,semantic,andothernetworksatdifferenttemporalactivation.In thisFig.,‘Vis’referstoVisualnetwork,‘Sem’toSemantic,‘Sens’toSensorimotor,‘Lang’toLanguageand‘Aux’toothernetworks.AninteractiveversionofICA-JADE resultscanbefoundonourgithubhttps://github.com/judytabbal/dynbrainSS.gitusingrotatableMATLABfigures.
Fig.6. TypicalexampleofthespatiotemporalreconfigurationofbrainnetworksduringworkingmemorytaskusingICA-JADE.Allsignificantnetworksextracted fromJADEarecollectedandpresentedsequentiallyrelativetoeacheventandperiodtime.Thenominationandtheexacttemporalperiodofsignificantactivation ofeachnetworkisclearlyindicated.Correspondingcognitivefunctionsarealsospecified.Inthisfigure,‘Vis’referstoVisualnetwork,‘Sem’toSemantic,‘Sens’to Sensorimotor,‘Lang’toLanguageand‘Aux’toothernetworks.
Forthethreetasks,spatialandtemporaldistributionof theNCopt componentsderivedfromJADEmethod,withcorrespondingnull dis-tribution,canbefoundinSupplementaryFigs.S3,S4,S5.Forfurther clarityinvisualisationandinterpretation,weillustrated,inFig.6,the spatiotemporalreconfigurationofthefunctionalbrainnetworksas ob-tainedbyICA-JADE.
Furthermore,wediscriminateddifferentSSmethodsperformancein termsoftheactivationofrelevantbrainnetworksineachtask.For ex-ample,inmotortasks,wecalculatedthe‘Mot’networkoccupancy per-centagedefinedasthenumberof‘Mot’networks(quantitativelydefined bythemaskasexplainedabove)dividedbythetotalnumberof signif-icantcomponentsfoundinthecorrespondingSSmethod.Similarly,for workingmemorytask,theoccupancypercentageofvisual, semantic, language,sensorimotorandauxiliarynetworkswereevaluatedforeach method.ResultsareshowninFig.7withthespatialrepresentationof thecorrespondingrelevantbrainregions.Therefore,Fig.7resumesthe overallperformanceofeachSSmethodshowingvariabilityinthe meth-ods’abilitytodirectlyextracttheappropriatetask-relatedcomponents. 3.4. PerformanceofeachSSmethodsatsubject-level
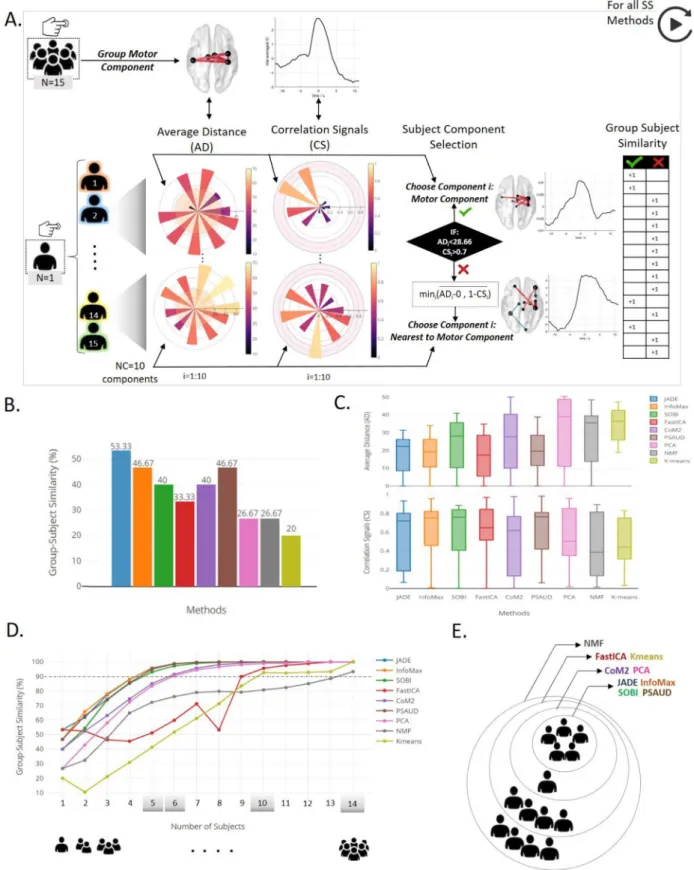
Hereourobjectiveistoevaluatetheperformanceofthemethods atthesubject-level.Wetesti)thecapacityofeachmethodtoextract significantcomponentsrelatedtothetask:todoso,wecomputedthe correlationbetweenthecomponentsobtainedbyeachmethodoneach subjectwiththesignificantnetworkobtained atthegroupleveland ii)thenumberofsubjectsneededforeachmethodtodetect‘expected’ networks:herewetestedtheoverallperformanceofeachmethodby increasingthenumberofsubjects,goingfrom1to15asweperformed subject-levelanalysisontheself-paceddata.Fig.8.Asummarizesthe subject-levelanalysisscenario.Foreachmethod,wechoseoneofthe significantmotorcomponentsderivedfromthedecomposition ofthe
group-level(N=15subjects),mostlytheoneshowinglittleintervention fromregionsotherthansensorimotor(‘Mot’)andhavinghightemporal coefficientsamplitude(supposedtobethebestforeachmethod).This componentillustratesa‘group’motornetworkwithtemporal modula-tionatthebuttonpresstime.Itwill,eventually,serveasa‘mas’ compo-nentforsubject-levelanalysis,asweareconcernedinmotorcomponent extraction.
ForeachSS method,NC=10 componentswerederivedfromeach subjectdata.Then,AverageDistance(AD)andCorrelationSignals(CS) betweeneachofthesecomponentsandthe‘group’motorcomponent rel-ativetotheSSmethodwerecomputedinordertoquantifytheabilityof themethodtoextract,fromasinglesubject,atask-relatedcomponentin space(motornetwork)andtime(temporalmodulationatbuttonpress time)respectively.Followingthis,onlyoneofthese10componentsis selectedforresultscalculation.Thisselectionisbasedontwoconditions criteriaonADandCSvalues.InthecasewhereADcomponentisless thanathreshold(setastheaverageofADvaluesofallcomponentsfor allsubjectsandSSmethods),andCSishigherthan0.7(chosenasa trade-off betweenmoderateandhighcorrelation),thenthecomponent isconsideredtobeamotorcomponent.Bysettingthesethresholds,we consideredtheexistenceofinter-subjectdifferences,thus,allowing sub-jectstohavedifferentbutnearspatialdistributionofmotornetwork. Therefore,ifatleastoneoftheextractedcomponentspassthese condi-tions,correspondingADandCSvaluesaredenotedandthenumberof subjectsthatgivesimilarresultstogroup-levelisraisedby1.Otherwise, weselectedcomponent𝑖asthenearestcomponenttothegroup-level re-sult,withacompromisebetweenspatialandtemporalsimilarities.
AtypicalexampleisillustratedinFig.8.AshowingthatPCA decom-positionwasabletoextractamotorcomponentfromsubject2 (com-ponent5),whereasnomotorcomponentwasderivedfromsubject14, ADandCSvaluesofcomponent7weredenotedinthiscase.Spatialand
Fig.7. SSmethodsperformanceevaluationforrealMEGtasks.Foreachtask,brainregionsinvolvedineachrelevantnetworkareillustratedontheleftsideusing theAALatlas,whilebrainnetworksoccupancyareshownontherightside.Theoccupancypercentagerepresentsthepresencepercentageofthesedefinedbrain networksrelativetoallsignificantextractedcomponents.Formotortasks,motornetwork(‘Mot’)wasemphasizedwithauxiliary(‘Aux’)networksrelativetoall significantcomponents,whilevisual(‘Vis’),semantic(‘Sem’),language(‘Lang’),sensorimotor(‘Sens’)and‘Sem+Sens’networksarehighlightedincontrasttoother auxiliary(‘Aux’)networks.Referringtotheserepresentations,capabilitiesofdifferentSSmethodsinextractingrelevanttask-relatedcomponentscanbeevaluated.
temporaldistributionsof selectedsubjects’componentsin bothcases areshowninFig.8.Resultsoftheremainingcomponentsforthese2 subjects’examplesareshowninSupplementaryFig.S11.
Asaresult,twoparameterswerecollectedandrepresentedinFig.8.B and8.C respectively. Group-subject similaritypercentage was calcu-latedasthenumberofsubjectsthatgivesamotorcomponentsimilarto thegroup-levelresultrelativetothetotalnumberofsubjects(N=15). Fig.8.BillustratesthisparameterforallSSmethods.Wecanseethat JADEwasabletoextract atask-relatedcomponentfrom8outof15 subjects(53.33%),InfoMaxandPSAUDfrom7subjects(46.67%),SOBI andCoM2from6(40%),FastICAfrom5(33%),PCAandNMFfrom 4(26.67%)andKmeansfrom3(20%).TheFig.8.Cshowsthe distri-butionsofADandCSvaluesofselectedcomponentsfromeachsubject
overallSSmethods.Methodswithhighersubject-groupsimilarity per-centagehavelowermedianvaluesofADandhighermedianvaluesof CS.Inaddition,wecannoticefromADandCSmedianvaluesthat sim-ilarityinspacewasmucheasiertobesatisfiedthantemporalsimilarity formostSSmethods.Interquartilerangevaluesshowtheexistenceof inter-subjectvariabilityresults.However,somemethodsshowedhigher interquartilerangeofADvalues(CoM2andPCA),orCSvalues(JADE, CoM2andNMF)relativetoothermethods.
3.5. TheoptimalnumberofsubjectsofeachSSmethod
Then,thesameprocedurewasappliedwithincreasingthenumberof subjectsfromonesubject(single-subject)to14subjects.ADandCSare
Fig.8. Subject-levelanalysisandresultsrelativetotheself-pacedmotorexperiment.A.descriptionofsubjectcomponentselectionprocedurebasedonAverage Distance(AD)andCorrelationSignals(CS)valuesbetweensubject’scomponentandthegroupmotorcomponent,whenSSmethodsareappliedonS-dFCdata. GroupmotorcomponentisshownforPCAexample.ADandCSvaluescomputedforallcomponentsarepresentedfortwosubjects.BasedonADandCScondition limitshighlightedineachpolarbar,successandfailureinextractingmotorcomponentarebothillustratedbysubjects2and14respectively.Spatialandtemporal distributionwithcorrespondingADandCSvaluesforallcomponentsofbothsubjects2and14areillustratedinSupplementaryFig.S11.Asmalltableontheright illustratesresultsofsuccessandfailureforallsubjectsinPCA.B.ResultsofthenumberofsubjectsthatsuccessfullyextractedamotorcomponentineachSSmethod relativetothetotalsubject’snumber,denotedGroup-SubjectSimilarity,areshown.C.distributionsofADandCSvaluesofallselectedcomponentsforthe15subjects areillustrated.D.GeneralizationstudywithincreasingnumberofsubjectsandthecorrespondingresultsofGroup-Subjectsimilaritypercentage(whenconsidering allpossiblecombinations).E.Greyshadedvalues(5,6,10and14)representthecriticalnumberofsubjectsrequiredforeachSSmethodtohavea90%precisionin extractingthetaskrelatedcomponent.
computedforallpossiblecombinations.Thenumberofpossible com-binationsiscalculated.Forexample,7-subjectsanalysisrequires6435 combinations,hence6435valuesof ADandCS.Foreach numberof subjects,wecalculatedsubjecttogroupsimilarityastheratiobetween numberofcombinationsthatsucceededinextractingamotor compo-nentrelativetothetotalnumberofpossiblecombinations.Resultsare illustratedinFig.8.E,F.Asexpected,thepercentagesimilarityincreases withincreasingnumberofsubjectsforallSSmethods.Fluctuationsin similarityresultsareobservedinsomemethods(asFastICA,NMFand Kmeans)duetothenon-consistencycharacteristicofthesemethods(as previouslyproved).Somemethodsrequiredasmallernumberof sub-jectsfordataanalysistoprovidesatisfactoryresults(motorcomponent atbuttonpresstimeinourcase)thanothers.Forexample,thefourICA versions(JADE,InfoMax,SOBIandPSAUD)required5subjectsinorder toattainaminimumsimilaritylevelof90%betweensubjectandgroup levelresults.CoM2andPCArequired6subjects,whilemuchmore sub-jectswereneededforothers(10subjectsforFastICAandKmeansand 14forNMF)asshowedinFig.8.E.Overall,ICAmethodsandspecially thosebasedonthehighorderstatistics(suchasJADE)outperformother methodsinextractingnetworksatthesubject-level.
4. Discussion
Inthisstudy,wehaveevaluatedtherobustnessofthemost popu-larSSmethodsappliedtoextractthemainbrainnetworksfluctuating duringtimeinordertohelpresearchersmakearationalchoice(ifany) amongthemultitudeofavailablemethods.Specifically,ninealgorithms havebeencomparedusingsimulateddata(seesupplementary materi-als)andthreeindependentMEGdatasets(N=95)recordedduringmotor andmemorytasks.Thediscrepancyinthedatasetssizeandbehavioral tasksperformed allowstesting SS methodsperformance ondifferent scenarios.Astheevokedresponses(analyzedhere)last forhundreds ofmilliseconds,weconductedourcomparativeanalysisbasedonMEG datasetstobenefitfromtheexcellenttemporalresolutionofthis tech-nique.However,thesamepipelinestudycanbeappliedintask-related fMRIcontext.
Overall,ourresultsshowvariabilitybetweentheevaluatedSS meth-odsandevenbetweenICAsubtypes.Theperformanceofthesemethods dependsonthenatureofthetask(simplevscomplex,slowvsfasttime scaletasks).Inasimpleandrelativelyslowtimescaletask(asself-paced buttonpresstask),allmethodssucceededintrackingspatiallyand tem-porallythedynamicbrainactivity.However,whenitcomestomuch fastertask(HCPmotortask)ormorecomplextask(WorkingMemory), somemethods(SOBIandKmeansforinstance)showedlower perfor-manceinextractingrelevantbrainnetworks(asdefinedbyourmasks). Resultsrelativetoeachtaskwillbediscussedlaterindetails.
First,thequantitativecomparisonperformedonsimulateddynamic networksshowedthatallSSmethodshavesuccessfullyseparated func-tionalnetworksbasedontheirconnectivitytimecourses.However, spa-tialandtemporalsimilaritiesinSOBIweresignificantlylowerthanother SSmethods,especiallyforthefourthsimulatedstate(P4)andthe sec-ondstate(P2)asshowninSupplementaryFig.S3,whichinvolvesmore complexspatiotemporalactivitythanotherstates.Asexpected,FastICA, NMFandKmeansmethodswereprovedinconsistentwithmultipleruns. Thisiscausedbythenatureofthesealgorithmsthatisbasedonrandom inputinitialisationsuntilsolutionconvergence.Thenoiseeffectonthe resultsobtainedwasalsotestedandshowedanincreasedperformance forallmethodswithhigherSNRvaluewithslowerconvergencetothe optimalaccuracyforsomemethods(SOBI,NMFandKmeans)relative toothers.Regardingcomputationtime,CoM2,PCAandPSAUDwere thefastestwhereasInfoMaxandJADEweretheslowest.Still,the ex-ecutedtimeofthese algorithmsissensibletodataset’sfeatures(size, complexity,type…).Othermetricssuchasthenumberoffloating-point operations(FLOPs)requiredforthealgorithmcompletioncouldbealso tested.Wealsosuggestforfuturestudiestoexploreotherdata simula-tionapproachesthatbuildthedesiredground-truthbrainstatesbased
onmorerealisticmodeling(usingNeuralMassforinstance),however, thismayintroducetheeffectofotherparametersinthecomparison (for-wardproblem,inversesolution…).Besidessimulationapproach,some studiesattempt toconsiderfMRIdata asagroundtruth toquantify andcompareSSmethodsperformanceinthecontextofM/EEG stud-ies(Colcloughetal.,2016;Jetal.,2020).
The method’s performance were evaluated on three real MEG datasetsalreadypublishedandtestedbypreviousstudies(Casorsoetal., 2019;O’Neilletal.,2017;Tewarieetal.,2019a;Vidaurreetal.,2018a; Zhuetal.,2020).Accordingtoself-pacedmotortask,resultsshowed thatallSSmethodshavesuccessfullyextractedoneormoresignificant networkthatinvolvestrongconnectivitybetweensensorimotorregions (‘Mot’). ForHCP data, a similar sensorimotornetwork wasrevealed amongsignificantcomponentsinallSSmethodsexceptforSOBI,PSAUD andKmeans.Integratedregionsinthisnetworkmainlyincludenodes fromcentralandparietalgyrus.Thesensorimotornetworkisstrongly coherentwiththetask(Melniketal.,2017;Yousry,1997)sinceit re-quiresbothmovement(throughbuttonpressorhandmovement)and tactileresponse(asthesubjectwillfeelthebuttonorfingerstape).The effectofright-handednessofallparticipantsofself-paceddatasetisalso revealed bythepresencestrongerimplication ofsensorimotor nodes fromtherightcortexrelativetotheleftoneasrevealedbythesphere sizesandconnectionsinFig.3.Itisnoteworthytomentiontheexistence ofanetworkthathighlightedsignificantconnectionsinthevisuallobe inJADEforself-pacedbuttonpresstaskandmostSSmethodsforHCP task.ThisnetworkwaspreviouslynoticedbyOneilletal.studyingthe samebuttonpresstask(O’Neilletal.,2017).Thiscanbeinterpretedasa crossmodalsynchronizationbetweenvisualandsensorimotorcortexas previouslystudied(Baueretal.,2020).Regardingtemporalevolution, itisclearthatallnetworksmodulatesignificantlywiththeexactbutton presstimeforself-pacedtask.Differently,thetemporalvariationrelated toHCPmotortasktakesanoscillatoryshape,whichwasalsoreported byotherstudiesdealingwiththesamedataset(Vidaurreetal.,2018a; Zhuetal.,2020).Apossiblereasonforthisactivitywassuggestedby (Vidaurreetal.,2018a)consideringaleakageeffectoftemporalactivity ofpreviousbuttonpressintothenexttrialduetothefastsuccessive tri-als.Inbothtasks,thereexistsauxiliary‘Aux’networksthatsignificantly modulatedwiththetaskbutnotdirectlyrelatedtothemotorcortex ac-tivity.Theoccupancypercentageof‘Aux’networksincreasesforallSS methodsinHCPresultsmainlyinCoM2andKmeans.Thepresenceof thesenetworkscanberelatedeithertotherobustness/sensitivityofthe SSmethodrelativetospuriousnetworksortothereliabilityofthe tech-niquesusedforselectionofoptimalnumberofcomponents(DIFFIT)or significantcomponents(nulldistribution)thatwillbefurtherdiscussed later.
InordertoevaluatethespatialandtemporalaccuracyofSS meth-odsathigherlevelsofcomplexity,wetestedthemethodsonSternberg workingmemorytask.AllSSmethodsdetectedvisualnetwork,which is consistent withthe presentationof visual stimuliattwo different times.Regionsintheprimaryvisualloberelatedtostimulus visualisa-tion(Grill-Spectoretal.,1998)andlateraloccipitalcortexresponsible forobject/shaperecognition(Corbettaetal.,1991;Grill-Spectoretal., 2001; KourtziandKanwisher,2001)werepresentinthese networks. Thebuttonpressresponseisreflectedbysensorimotorconnections con-sistentlywithpreviousworkingmemorystudies(Metzaketal.,2011; Yamashitaetal.,2015)onlybyusingCoM2,PSAUDandPCAmethods. Inordertoprocessandmaintainobservedstimuliasawayto memo-rizethem,ahigherlevelofcognitionisillustratedbya‘semantic net-work’,whichmainlyencompassesbilateralparietalandtemporalareas activationinallSSmethods,exceptforKmeans.Thisiscoherentwith previousstudiesthatdemonstratetheevidentroleofparietalcortexas aworkspacefor sensoryandperceptualprocessingin working mem-oryframework(Chaietal.,2018)throughangular(Frackowiak,1992; Vandenbergheetal.,1996),precuneus(CavannaandTrimble,2006), and hippocampal (Baddeley et al., 2011) areas. Bilateral inferior temporal regions also play important role in semantic processing
(Nestoretal.,2006;Vigneauetal.,2006).Fusiformgyri,strongly mod-ulatedinourresults,hasalsoshownaparticularconcerninthis con-text(Mion etal., 2010). The detectionof the‘language’ networkby JADE,InfoMax,CoM2,PSAUD,PCAandKmeansmethods,was compat-iblewithpreviousfindings(Brookesetal.,2011b;O’Neilletal.,2017). Temporalandparietallobeswereremarkablyactivatedbythese meth-ods,mainlytheparahippocampalandsupramarginalgyrirespectively. Theseregionsarecriticalinmemoryencodingandretrievaland seman-ticcognition(Axmacheretal.,2008;Caminitietal.,2015;Dembetal., 1995; Derrfusset al.,2004; Deschamps etal., 2014; Vigneau et al., 2006).Inasimilar (abstractshapebased)workingmemorytask,the interpretation ofthis networkwas relatedtoaverbalisationnaming strategyemployedbyparticipantsasawaytoaidinmemoryencoding (Caminiti etal.,2015;O’Neilletal.,2017).Therefore,thisnetwork’s activationmaybepossiblewiththetaskasitmodulatesstronglywith theprobepresentationandresponsetime.
Itisimportanttopointhere thattheresultantnetworkswere de-noted objectivelyin this studyusing a quantification approach. For a better interpretation of the functional significance of results, we built template brain masks, referring to the literature (see table S1 insupplementarymaterials),fromAAL corticalregions.These masks areused for seedingour networks of interest: ‘Mot’ in motor tasks, ‘Vis’/’Sem’/’Lang’/’Sens’inworkingmemorytask.Networkswerethen classifiedbasedontheiractivatednodesstrengthrelativetoeachmask. Inaddition,theusedmaskshavedistinctspatialdistribution,withsome sharedregions mainlybetween ‘Sem’and‘Sens’networks.However, whenweaimtodeeplyinvestigatetask-relatedsub-networks,the‘mask’ techniqueseemstohavemuchmorecomplexityrelatedtothespecificity oftheintegratedbrainregionsandtheprecisionofanaccuratethreshold inordertoappropriatelyclassifynetworksresults.
Althoughweperformedourstudyoncognitivetasks,itisatopicof greatinteresttoapplythismethodologypipelineonresting-state exper-imentssincemanystudieshaveshownthedynamicreconfigurationof thebrainduringrestaswell(Kabbaraetal.,2017;Liégeoisetal.,2019). Regardingmethodologicalconsiderations,first,theoptimalnumber ofcomponentstobederivedwasstillachallengingquestionforallSS methodsratherthanadirectlimitationofouralgorithms.Inthisstudy, weappliedtwowell-knownalgorithms(DIFFITandelbowcriterion)for arangeofnumberofcomponentstoautomaticallyselecttheoptimal numberofcomponentsrelativetoeachSSmethod.Theevaluatedrange ofNCvalues wasupperlimitedby10 componentsin ordertoavoid spuriousnetworks.Moreover,wetriedtofixthenumberofcomponents to10forallSSmethodsintheself-pacedmotortaskatthegroup-level, asalreadysetinapreviouswork(O’Neilletal.,2017)thatusedFastICA algorithmandlittledifferencewasobservedfortheoverallresults.
Second,weshouldpointthatnotallthesecomponentsare neces-sarilyessential,especiallyinthecaseofsimpletasksasmotortasks.To thisend,wefollowedtheapproachofthenulldistributionbasedonsign flippingalgorithm(Huntetal.,2012;O’Neilletal.,2017;Winkleretal., 2014;Zhuetal.,2020)toselectonlycomponentswhosetemporal dy-namicssignificantlymodulatewiththetask.Inthisway,weensurethat braindynamicsrelativetothestudiedbehaviouraltaskscanbe summa-rizedanddescribedbytheretainedcomponentsthroughanautomatic waythatallowsustoobjectivelycomparetheSSmethodsperformance. Inaddition,thefactthatthistechniqueisapurelydata-driven proce-durethatdoesnotrequireanypriorhypothesisorconditions manip-ulationmakesitlikelyadaptedtothespecificexamineddataset.Two pointsregardingsignificantcomponentsselectionareimportantto men-tionhere.First,intheappliednulldistribution,networksweredefined tobesignificantiftheyfelloutsidethenulldistributionineither posi-tiveornegativesidesbecausetheyreflecttrial-onset-lockedthateither increasesordecreasesinconnectivityacrosssubjects(asamplitude en-velopecorrelationwasadopted).Second,itshouldbenotedthat com-ponentssignificancewasevaluatedrelativetothespecifictaskduration. Forexample,temporaldurationoftheentireanalysisforself-pacedand workingmemorytaskscanincludedynamicsthatshouldbeexcluded
fromtheanalysis.Inthiscontext,welimitedoursignificance interpreta-tion/assessmentintheintervalof[-2;+2sec]and[-0.5;+0.5sec]relative tothebuttonpressinstantinthecaseofself-pacedandHCPmotortasks respectively,and[-2;+16sec]relativetothevisualstimuluspresentation formemorytask.Inaddition,fewlimitationsaretobediscussedwhen dealingwiththisselection.First,itwasnotconvenienttorelyonthis techniquewhenthenumberoftrialsandsubjectswaseithertoosmall ortoobig.Asmallnumberwillnotallowtobuildareliablenull distri-butionwhileahugeonewillhaveitscomputationalcostregardingall possiblesubjects’combinationsforsign-flippingprocedure,asalready executedintheHCPanalysis.Moreover,thereisnoconsensusaboutthe thresholds/marginsthatdefinewellalimitlevelforcomponent’s am-plitude.Forinstance,thereexistsnetworkswhosetemporalvariation peaksatthelimitofnulldistributionenvelope.Theseareconsideredto becriticalcomponentsthatmaybeintegratedinthetaskbut consid-erednottobefollowingtheautomaticcriteriaofthisnulldistribution. Futureworksshouldthereforeinvestigatemoreaboutthis methodolog-icalapproachintheframeworkofcognitivetasks,inadditiontoresting stateexperiments.Also, itis crucialtoseekmoremethodstouseor combinewiththeappliedtechniqueinordertohavemorerobustbasis forsignificantcomponentsselection.Itisnoteworthytoreportthatnull distribution-basedtechniquewasapplieduniformlyforallSSmethods, thusourmainobjectiveofcomparisonwasbuiltonaunifiedevaluation framework.
Third,weusedthesamepipelinesupportedbytheprevious stud-iesdealingwiththesamedataset(corticalparcellation,source recon-struction,functionalconnectivitymetricandsourceleakagecorrection, frequency bandsandslidingwindow settings)(Kabbaraet al.,2019; O’Neilletal.,2017).Byapplyingalreadytestedandvalidated method-ologicalapproaches,we avoidinfluencingfactorsonthecomparison performed.However,wepointoutthatothermethodologicalsolutions couldbeexploitedbyotherresearchesusingthesamepipelineadopted inthiswork.Regardingcorticalparcellation,wechoseAALatlasbased onitssuccessfuluseinpreviousMEGinvestigations(O’Neilletal.,2017; Tewarieetal., 2016).Thisatlasalsoprovidesgoodbasisfor the or-thogonalisationprocedureadoptedsinceitsnumberofregionsis suffi-cientlylow(78ROIs)andwellseparated(Colcloughetal.,2015).The beamformerspatialfilteringwasselectedastheinverseproblem solu-tion duetoits demonstrated efficiencyin themeasurementof static (Brookeset al., 2011a) anddynamic (Bakeret al., 2014) functional connectivity. Functionalconnectivity between ROIsregions was esti-matedthroughAmplitudeEnvelopeCorrelation(AEC).Thistechnique hasbeensuccessfulinelucidatingelectrophysiologicalnetworksof func-tionalconnectivity (Colcloughet al.,2016). Othermethods, such as phasecouplingscanbeconsideredasanalternativewaytoprobe dif-ferent typeof functionalconnectivity(Lachauxetal.,1999). Sliding windowsettings(lengthandstep)wereselectedcarefullyasatrade-off betweentemporalresolutionandtheaccuracyofthederivedadjacency matrices(length=6sec, step=0.5secforself-pacedandworking mem-orytasks)(O’Neilletal., 2017).However,accordingtorecentworks (Fraschinietal.,2016;Liuzzietal.,2019),itcanbeseenthatmetrics (in-cludingamplitudeenvelopecorrelation)performpoorlyforveryshort statedurationswhencombinedwiththeslidingwindowapproachbelow fewseconds,providingnoisyresultsoflowcorrelationwithgroundtruth insimulations.Forthisreason,wefollowedtheworkof(Tewarieetal., 2019b)toestimatedynamicfunctionalconnectivitybytakingsample by sampletimeseriesratherthanwindowedaggregated samples us-ingtheInstantaneousAmplitudeCorrelation(IAC).Thishightemporal resolutionmeasureofFChasshowngreatsensitivitytogenuine fluc-tuationsinfunctionalconnectivityappliedinthesamecontextofour study.
Itwouldbeinterestingtotestdata-drivenwindowsapproachinthis context using therecurrence plots of theamplitude envelopesas in (Tewarieetal.,2019b)insteadof averagingtrialsindataset2to re-duceheavydFCmatrices.Itcouldbe alsotestedagainstfixedsliding windowapproachasfordatasets1and3.
Concerning frequency bands, it was crucial to preprocess each datasetinitsappropriatebandwidth.Forexample,brainsignalsin self-pacedandHCPmotortaskswereprovedtobemoreactiveinthebeta band,whilebroaderrangeoffrequencybandsareintegratedin com-plexcognitivetasksasworkingmemory(O’Neilletal.,2017;Zhuetal., 2020).
5. Conclusion
Decipheringofdynamicsofelectrophysiologicalbrainnetworksis oneofthemostimportantgoalsinneuroscience.Inthispaper,we eval-uatedandcomparedninepopularsource separation(SS)methodsto identifydominantnetworksofconnectionswithcorresponding tempo-raldynamicsatgroup-levelaswellassubject-level, usingsimulation andempiricalMEGdata(N=95subjects)recordedduringthreedifferent tasks:(1)simplebuttonpresstask,(2)fastfingermovementtask(HCP) and(3)Sternbergworkingmemorytask.Resultsshowcloseconsistency forall SS methodsin successfully identifyingatransientnetworkof connectionslinkingsomatosensoryandprimarymotorregions inthe relativelyslowandsimplebuttonpresstask.Variabilitybetweenthese methods’performanceisrevealedinrapidtasksofsub-secondtimescale (HCPmotortask)andin amorecomplex task(Sternberg).TheSOBI andKmeansalgorithmsshowedtheweakestperformanceamongtested methods.CoM2,PSAUDandPCAshowedpromisingresultsinworking memorytask,revealingtheformationanddissolutionofmultiple net-worksthatrelatetosemanticprocessing,patternrecognitionand lan-guageaswellasvisionandmovement.Atthesubjectlevelanalysis,ICA methodsusinghighstatisticalorder(JADE,InfoMax,CoM2andPSAUD) outperformothermethods.Ourmainmessageisthatresearchersshould beawaretoselecttheappropriateSSmethodsandotherrelated param-eters(epochlength,taskcomplexityanddatasetsize)whenanalyzing dynamicsofbehavioraltasks.
Dataavailability
Data supporting the findings of this study are available in the link(https://github.com/judytabbal/dynbrainSS.git).AllHCPdataare available on https://www.humanconnectome.org/software/hcp-meg-pipelines.Thedatasets1and3areavailableuponrequest.
Codeavailability
Codes supporting the findings of this study are available in the link(https://github.com/judytabbal/dynbrainSS.git).Allanalysiscodes necessarytoproducetheresultsherewereperformedinMATLAB soft-ware,usingFieldTripToolboxhttp://www.fieldtriptoolbox.orgfordata segmentation,filteringandsourcereconstructionsteps,EEGLAB tool-boxforsomeSSmethodsasJADE,InfoMaxandSOBIandother MAT-LABimplemented functionsasdetailedandprovidedin theprevious link.Agraphicaluserinterfaceismadefreelyavailableallowingother researcherstotestthemethodsonour(ortheir)simulatedandrealdata. Creditauthorstatement
JT,AK,MHandPBcontributedtothedesignandimplementation oftheresearch,totheanalysisoftheresultsandtothewritingofthe manuscript.MKandPBwereinvolvedinprovidingfundingsourcesfor thestudyandsupervisedthework.Allauthorsprovidedcritical feed-backandhelpedshapethestudy.
Acknowledgement
ThisworkwasfinancedbytheRennesUniversityandtheInstitute ofClinicalNeuroscienceofRennes(projectnamedEEGCog).Thestudy wasalsofundedbytheNationalCouncilforScientificResearch(CNRS) in Lebanon. The authorswould alsolike tothank thethe Lebanese
University, theLebanese Associationfor ScientificResearch (LASER) andCampusFrance,ProgrammeHubertCurienCEDRE(PROJECTNo. 42257YA),forsupportingthisstudy.
Supplementarymaterials
Supplementarymaterialassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.neuroimage.2021.117829. References
Aggarwal, C.C., Hinneburg, A., Keim, D.A., 2001. On the surprising behavior of distance metrics in high dimensional space. In: Van den Bussche, J., Vianu, V. (Eds.), Database Theory — ICDT 2001, Lecture Notes in Computer Science. Springer, Berlin, Heidel- berg, pp. 420–434. doi: 10.1007/3-540-44503-X_27 .
Allen, E.A., Damaraju, E., Plis, S.M., Erhardt, E.B., Eichele, T., Calhoun, V.D., 2014. Track- ing whole-brain connectivity dynamics in the resting state. Cereb Cortex 24, 663–676. doi: 10.1093/cercor/bhs352 .
Axmacher, N., Schmitz, D.P., Wagner, T., Elger, C.E., Fell, J., 2008. Interactions between medial temporal lobe, prefrontal cortex, and inferior temporal regions during visual working memory: a combined intracranial eeg and functional magnetic resonance imaging study. J. Neurosci. 28, 7304–7312. doi: 10.1523/JNEUROSCI.1778-08.2008 .
Baddeley, A., Jarrold, C., Vargha-Khadem, F., 2011. Working memory and the hippocam- pus. J. Cogn. Neurosci. 23, 3855–3861. doi: 10.1162/jocn_a_00066 .
Baker, A.P., Brookes, M.J., Rezek, I.A., Smith, S.M., Behrens, T., Probert Smith, P.J., Wool- rich, M., 2014. Fast transient networks in spontaneous human brain activity. eLife 3, e01867. doi: 10.7554/eLife.01867 .
Bassett, D.S. , Sporns, O. , 2017. Network neuroscience. Nature Neurosci. 20, 353 .
Bauer, A.-K.R., Debener, S., Nobre, A.C., 2020. Synchronisation of neural os- cillations and cross-modal Influences. Trends Cognit. Sci. 24, 481–495. doi: 10.1016/j.tics.2020.03.003 .
Bola, M. , Sabel, B.A. , 2015. Dynamic reorganization of brain functional networks during cognition. Neuroimage 114, 398–413 .
Brookes, M.J., Hale, J.R., Zumer, J.M., Stevenson, C.M., Francis, S.T., Barnes, G.R., Owen, J.P., Morris, P.G., Nagarajan, S.S., 2011a. Measuring functional connectivity using MEG: methodology and comparison with fcMRI. Neuroimage 56, 1082–1104. doi: 10.1016/j.neuroimage.2011.02.054 .
Brookes, M.J., Tewarie, P.K., Hunt, B.A.E., Robson, S.E., Gascoyne, L.E., Liddle, E.B., Lid- dle, P.F., Morris, P.G., 2016. A multi-layer network approach to MEG connectivity analysis. NeuroImage 132, 425–438. doi: 10.1016/j.neuroimage.2016.02.045 .
Brookes, M.J., Vrba, J., Robinson, S.E., Stevenson, C.M., Peters, A.M., Barnes, G.R., Hille- brand, A., Morris, P.G., 2008. Optimising experimental design for MEG beamformer imaging. NeuroImage 39, 1788–1802. doi: 10.1016/j.neuroimage.2007.09.050 .
Brookes, M.J., Wood, J.R., Stevenson, C.M., Zumer, J.M., White, T.P., Liddle, P.F., Morris, P.G., 2011b. Changes in brain network activity during working mem- ory tasks: a magnetoencephalography study. Neuroimage 55, 1804–1815. doi: 10.1016/j.neuroimage.2010.10.074 .
Brookes, M.J., Woolrich, M.W., Barnes, G.R., 2012. Measuring functional connectivity in MEG: a multivariate approach insensitive to linear source leakage. Neuroimage 63, 910–920. doi: 10.1016/j.neuroimage.2012.03.048 .
Buckner, R.L., Krienen, F.M., Castellanos, A., Diaz, J.C., Yeo, B.T.T., 2011. The organiza- tion of the human cerebellum estimated by intrinsic functional connectivity. J Neu- rophysiol 106, 2322–2345. doi: 10.1152/jn.00339.2011 .
Bullmore, E., Sporns, O., 2009. Complex brain networks: graph theoretical anal- ysis of structural and functional systems. Nat. Rev. Neurosci. 10, 186–198. doi: 10.1038/nrn2575 .
Caminiti, S.P., Siri, C., Guidi, L., Antonini, A., Perani, D., 2015. The neural correlates of spatial and object working memory in elderly and Parkinson’s disease subjects. Behav. Neurol. 2015, 1–10. doi: 10.1155/2015/123636 .
Casorso, J., Kong, X., Chi, W., Van De Ville, D., Yeo, B.T.T., Liégeois, R., 2019. Dy- namic mode decomposition of resting-state and task fMRI. NeuroImage 194, 42–54. doi: 10.1016/j.neuroimage.2019.03.019 .
Cavanna, A.E., Trimble, M.R., 2006. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583. doi: 10.1093/brain/awl004 .
Chai, L.R., Khambhati, A.N., Ciric, R., Moore, T.M., Gur, R.C., Gur, R.E., Satterth- waite, T.D., Bassett, D.S., 2017. Evolution of brain network dynamics in neurode- velopment. Network Neurosci. 1, 14–30. doi: 10.1162/NETN_a_00001 .
Chai, W.J., Abd Hamid, A.I., Abdullah, J.M., 2018. Working memory from the psycho- logical and neurosciences perspectives: a review. Front Psychol. 9. doi: 10.3389/fp- syg.2018.00401 .
Ciric, R., Nomi, J.S., Uddin, L.Q., Satpute, A.B., 2017. Contextual connectivity: a frame- work for understanding the intrinsic dynamic architecture of large-scale functional brain networks. Sci. Reports 7, 1–16. doi: 10.1038/s41598-017-06866-w .
Colclough, G.L., Brookes, M.J., Smith, S.M., Woolrich, M.W., 2015. A symmetric mul- tivariate leakage correction for MEG connectomes. Neuroimage 117, 439–448. doi: 10.1016/j.neuroimage.2015.03.071 .
Colclough, G.L., Woolrich, M.W., Tewarie, P.K., Brookes, M.J., Quinn, A.J., Smith, S.M., 2016. How reliable are MEG resting-state connectivity metrics? Neuroimage 138, 284–293. doi: 10.1016/j.neuroimage.2016.05.070 .
Corbetta, M. , Miezin, F.M. , Dobmeyer, S. , Shulman, G.L. , Petersen, S.E. , 1991. Selective and divided attention during visual discriminations of shape, color, and speed: func- tional anatomy by positron emission tomography. J. Neurosci. 11, 2383–2402 .
Demb, J.B., Desmond, J.E., Wagner, A.D., Vaidya, C.J., Glover, G.H., Gabrieli, J.D., 1995. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional