Publisher’s version / Version de l'éditeur:
Journal of Colloid and Interface Science, 38, 1, pp. 75-83, 1972-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Phase transitions of adsorbates. III. Heat effects and dimensional
changes in nonequilibrium temperature cycles
Litvan, G. G.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site
LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=2805cc51-771b-4744-b932-bead045b8d8e https://publications-cnrc.canada.ca/fra/voir/objet/?id=2805cc51-771b-4744-b932-bead045b8d8e
Reprinted from J o n ~ < r . ! ~ 01. Cor.r,o~ri .!XI, ISTEIIF.!CIP SCIESCF:, VOIUIIIC 38, No. 1. January 1072 Copyl.iglit @ 1572 11). z\m~ilc~nie Prcss, Inc. l+inled i n U . S . A .
Phase Transitions of Adsorbates
Ill. Heat Effects and Dimensional Changes in Nonequilibrium Temperature Cycles
iValionnl Research Cortncil o j Canadtc Division of Builrling Researcll, Ollawc~, Canccda, K l a OR6
Received llecember 5, 1070; accepted February 0, 1071
Changes in thc dinleilsioils :111d heal coillellt of the porous, 0 6 ' ~ ~ si1ic:t glass-water system were determined as temperature was cycled between +5" and -40°C a t a rate of 0.33"C/min. The results arc esplt~iiled by the fact; t h a t the inaxinlurn esperi- nlentally realizable relative pressure, based oli that of uildercoolecl water, becomes less t h a n unity belo~y O°C and diminishes with declining temperature. As a result, the adsorbate cont;ent decreases by migration of water t o the outer surface, where i t freezes between 0" and -8°C. At lower temperat>ures (-20°C) water drt~iiis from the smaller capillaries into larger voids and solidifies. An accorlllt is given of the aliomalies of the extension isosteres, including hysteresis. I n contrast with prcviolls studies, the rcs~llts obtained with porous glass and organic adsorbates are similar t o those of the glass-watcr system and can be esplairled by the same theory.
Tlle porous, (36 % silica glass-water system llas in several ten1per:~ture regions belon- 0°C anomalous coefficient of tllernlal ex- pansion values aisulned to be clue to pliase clianges of the :idsorbate (1-3). Evide~lcc to this effect llaq been obtained for only one :inoinaly, that occurring on warming arourld
- 10°C and provccl bg calorin~etric nlcans to be caused bj- illeltirig (4, 3 ) .
Other points of paqt studies may bc sum- n~arized :is follo~vs: S o anomaly is obscrv- able ~ i t h an anlount of water less than the equivalent of two layers (3, 5), but at higher concentrations abnormal behavior is ex- hibited, on cooling, at approximately - 7"
and -22°C and, on warming, at approxi- mately - l l ° C . Jlelting is of tlie slion~alous first-order type and the latent heat of fusion ~zpproxin~ately two-thirds the bulk value (3). The freezing point L L ~ - 2YC ancl t hc melting
point a t - l l ° C appear t o be, above the critical value, incleperident of conceixtrzliion. Study of the behavior of otlier zdsorbates (2, 3) shotved differences \{-it11 respect to that of water; on cooling only one length anonlaly
was observecl 2nd it dependecl oil concentra- tion.
Only limited success has beell acllieved iu relating peculiz~rities (exp:insioils, change in slope, 1ij.steresis) to pllgsical processes. Tllc present irivestig:~tions were undertakeu be- cause the results of the lon-temperature, isothermal :~clsorption studies of the porous glass-n-atcr system (6) suggested that 11011-
equilibriun~ experiments might be ot value totvard this end.
A p p a ~ a t u s . The exterlsioll isosteres t~iicl differential thermograms were determined simultaneously. A specimcn :~pproximately 3.0 cn1 long was held between the clamp of the invar framenrorli and that of the movable iron core of a differential linear t,ransformer. The transformer \\*as ~ L S te1llp(>ratur(~-co11-
trollccl (A0.05"C) ancl supplicd with con- stant voltagc ( ~ 0 . 0 0 2 %). Biasing t11~ out- put mad(> it possible to mcasurc thc changcs in tllc 50-mV range of a recording potcnti- omctcr with a precision of A L / L = 1 X Copyri,nl~t @ 1072 I)y Aczldc~nic Press, lnc. . I o ~ ~ r i z n l of Colloid a n d Inlerface Scie,ice, Vol. 38, No. I , J:rrlu:rry 1972
A 23-g.1 ige copper-constantan~t~ thermopile i~iid n 0.1-nlV full-span potentiometer served for the measurement, of the temperature clifferelice between the sample and a nou- poroub glass reference block. The absolute value of the temperature was determined i\it,h the aid of a thern~ocouule soldered onto
:-L copper br~ncl fitted around the specimen.
The assenlbly was hermetically sealed in
a brass cell and placed in a 50 cm deep Dewar vessel above liquid nitrogen. Constant rate of temperature change was acllieved by programmed compensation of the cooling effect. The adsorpt,ion : ~ n d extension iso- therms were determined concurrently on coinpanion s:xmples in a conventional gravi- metric adsorption apparatus incorporating :l Tuckern~aa optical extensometer.
JIaterials. Porous 96 per cent silica glass (Conling 7930) was in the form of plat,e 2
and 5 mm thick; B E T nitrogen surface areas were 191.2 and 100.0 mygm, respectively. T h e large difference in surf:ice area is sur- prising a ~ l d c o n t r n q to the present vie\\-, accorcli~lg t o which little variation irl struc- ture exists in specimens of this type of glass. I t has been f o u ~ l d (7) that the surface area ~, of porous glass plat,es is n function of thicli- ness, presumably a result of incomplete leachiilg during the manufacturing process. Adsorbates were of spectroscopic purity grade clien~icnls.
I'rocet1z~re.s. The suecimerl was vacuum saturated in a glass cell 2nd transferred to the extensometer. Care n-as talien l o avoid desiccation during the n~oulltiilg operation. I n some runs (so indicated) the specimen
\\as almost, completely immersed in the licluid, but to avoid iliterference nitli the n~ovement of the exte~iso~neter the portion of the solid held in the upper clamp \\-as left ill the vnpor phase. Length-change readings have been corrected for the tliermnl espa~iision of the :tssembly (2.0 X mm,i "C).
IiESULTS AN11 DISCUSSION
Lerl!/th ilnolnaly in tile Vici~z~ly of 0°C.
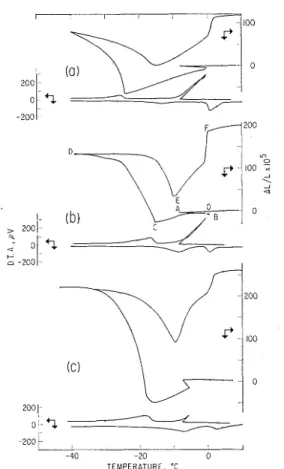
r l
I he csteiisiorl isosteres and thermograms of t l ~ e hnturated samples (Fig. 1) show that :Ln anomi~lous change iri the dime~isions, oc- curring in the region bet\veen -0.2 and
TEMPERATURE, "C
FIG. 1. I)imcusio~lal changes mid thermogram
of the porous glass-water system: ( a ) 2 mm thick glass, water saturatcd, cooling rate 0.25"C/1nin; (b) 5 mm thick glass, water saturated, 0.25"C/inin; (c) 5 inm thick glass, wi~ter saturated, 0.33"C/min.
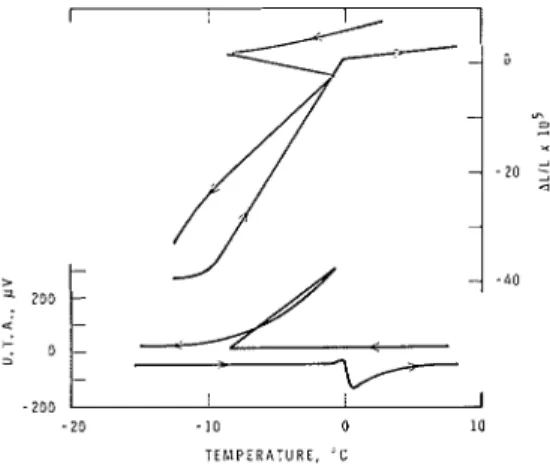
- S°C, n-:ls :~ccompanied by n sudden re- lease of heat. This proves tlie solidificatioil of a t least n portioii of the adsorbed water. Because (a) on visual inspection :xt - 10°C the sample was covered u i t h ice, ( 6 ) the establislled melting poiilt of the ice formed a t -S.O°C n-as 0°C (Fig. 2), and (c) on freezing tlie released heat ~vnrmed tlle system to about O°C, i t may be concluded that ice forms on the outside surface of the specimen and is in the bulk state.
When t h e glass n-:~s cooled ivhile immersed in water, the surrounding water froze a t 0°C although the surface water solidified only a t -0.4"C, as iildicated by the sudden change in the length (Fig. 3). As tlie glass wils in contact nit11 ice cryht:~ls, this indi-
P H A S E T l t A N S I T I O N S O F A1)SOIIBATES '77 cates tliat the lo~ver freezing point cannot
be attributed to undercooling.
The experimentally establisllecl facts that ice formed (a) on the outer surface, (b)
belo\\- 0 ° C temperature, and (c) in the absence of undercooling suggest that water failed to solidify at 0°C because it \\-as con- tained in pores \rhere it could not freeze; and that as tlle tenlperature became colder thali 0°C tlle water illigrated to the surface, nrl~ere
F I G . 2. lli~nensional changes and thermogr:~tn of 5 mm thick poro11s glass saturated with water during a +5O to -15OC temperature cycle (0.33OC/ min).
it formed ice. Tlie causes for this n~cclinriism can be given by examining the aclsorptioll cliaracteristics of porous systems belon- the bulli triple point of the adsorbate.
Adsorbed water remains in a liquid-lilre state well below O°C, and the amount acl- sorbed a is consequently a function of p/p l o
,
the relative pressure based on tllevapor pressure of undercooled water ( p l O )
rather than or1 that of ice (pAo)(G). Became p1° exceeds p," by an increasing margin 3s the trmpcraturc d(~crc~:zscs, and bccausc in cqui- librium thc prevailing pressure p cannot bc grpatclr than p,", the. system is csposcd, 011
cooliiig, to rc.lativc prcssurcs of lcbs tlian unity. Ipiugrc, 4 shows tlic. limiting p/pln vnlucls as :L function of t('mperatur~ T. l2r01n
this it follows that at tc.mpcraturcs belo\\-
0°C anrnx, the maximum amount adsorbed,
dccrc.ascbr and Icacls t o tlic cxudatiorl of water.
The temperature at ~vhich the \v:zter on the external surface freezes depends es- sentially on two factors: the temperature a t
~vhich a becomes equal to a ,,,,,
,
i.e., the shape of the isothern~, and the rate of nucleation. If a = a,,,,, at O°C, the11 migra-tion of water starts immediately belon this temperature; if not undercooled, it promptly freezes (Fig. 3). On the other hand, if the specimen is satur:~ted : ~ t 1'
>
O°C, then at- 4 0 - 30 - 2 0 - 1 0 0 10
T E A I P E R A T U R E , " C
FIG. 3 . Dimensional changes of a 5 lnrn thick p r o ~ ~ s glass sample irnmersed in water.
78 LITVAK
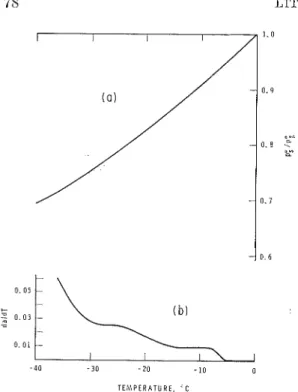
FIG. 4. ((0 Masimum realizable vapor pressure of watm as n function of ternpernture; ( 6 ) Aa/Al'
us. T for 5 mm t,hicli p:)roiLs glass-wnt,er system based on the isotherm of +0.5"C. 0rdin:~te label sllould correct,ly rc:ltl A : L / A T X 100.
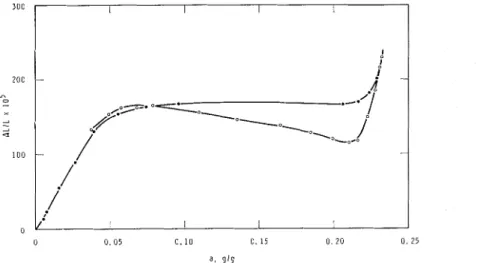
FIG. 6. Adsorptio~l isothern1 of :L 5 null thick
porous glass-water system at +0.5OC.
T
= 0, tve have a i a,,,,, and a becomesequal to a,,,,, only ~vhen 1'
<
0°C. If a<<
a ,,,,, at O°C, we have a Z a ,,,,, in the region between 0" and -20°C and 110 freezing on
the surface occurs (Fig. 5).
Figure 4(a) sho~vs values of the li~lliti~lg
p / p I o as a function of 1' and the isotherm (Pig. 6) li~lks a to p / p L o so that a,,,,, can be related to
1
'
for this system. The derivative of a \\-it11 respect to T is also given (Fig.4(b)), and it can be seer1 that because of the sh:~pe of tlie isotherm a ~ignificant amount of water is exuded only belo\\- -5°C. This agrees well with the previously observed
-
freezing t emperatnre of - 7°C.
I n view of the dynamic nature of the factors causing expansion, it is to be ex- pected that if the cooliilg rate is very slo~\-
110 noma ma lo us dimen.;ional changes call be
observed. T l ~ i s , in fact, was found nit11 the cement-water system (S) at 0.0417°C/mi~i cooling rate.
L
The suggested explanation is not specific10 20 I; to water as adsorbate. The extension "iso- T E ' l P Z R i T .1 "L
steres" of porous glass co~itairli~lg ndsorb:ites
FIG. 5. l~imellsiorlnl ch:Lnges and tllermograms ~ L I c ~ aS ~ > ~ c l ~ h ~ x l l n e , cllloroform, xylene,
of ( a ) 2 mm nlld ( 6 ) 5 mm thlcli glass sample paltly carbon tetrachloride, benzene, and oct:inol ss~turated wibh water. esliibit similar features (Fig. 7). I t can be
PI-IASl': TRANSITIONS OF ADSORBATES
seen th:it, in every system there are at least two freezing ranges. The existence of the anomaly a t high temperature remained 1111- detected in most former studies, either be- cause of insufficient,ly lolv experimental tem- perature (2) or because of erroneous inter- pret ation (3).
A remarlrably unifornl behavior is ex- hibited by the values given in Table I. It, appears that the first freezing occurs betlveen
L " "
-120 8 0 -40 0
TEMPERATIIRE, 'C
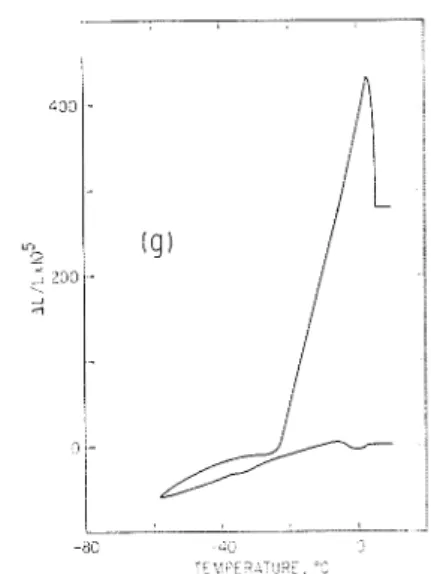
FIG. 7. I>irne~lsio~ial changes and thermogralns of 5 mm thick porous glass with various adsor- bates: ( a ) chloroform, mp -63.5"C; (b) )n-xylem,
mp -47.9"C; (c) carbon tetrachloride, mp
-22.96"C; ((1) octanol, mp -16.7"C; ( e ) benzenc, n1l1 f5.5"C; (f) cyclohesane, n ~ p f6.5"C; and (g) bellzene immersed.
0.92 and 0.98 T f , 1,
,
where I'f, b is the bulk freezing point of the adsorbate in degrees I<elvin. The suggested mechanism is sup- ported also by the fact that melting of the adsorbate occurs sl~vays at T f , I,.
F~,eesinq at L o w Te,nperat1~7.es. I n the tr:in~ition occurring at lonrer temperatures the adsorbate solidifies in the pores. The process is associated \\-it11 an exothermic heat effect, and cspansion, both extending over a tcmpcraturc range of approximately 15 degrees. Despite the gradual nature of the transitio~i for a given type of specinlen (e.y., 5 mnl thick porous glass) the inception temperature of the process is co~istarlt (f 0.3"C).
The sign of the specific volunle chiinge on freezing in the bulk state has 110 bearing
on t,he dimensional cllarlges of t,he adsorbent,- adsorbate syst,em. Exp:insion occurred nit11 all systems (Vig. 7), :ilthough water is the only subst,ance in the group that expnrlcls 011
freezing in the bull< stut,e.
I n thc isot~hermal adsorptior~ studies (6) solidification ~ v a s found neither ilt -3S°C,
arrrrLs = 0.12 gm/gnl, nor at -25"C, a ,,,,, =
0.17 gm/gm. This is surprising because even at -3s0C, p/pn = 0.7 was attained and at
m m m m m m - 5 2 8 2 % i Z % * *
2
,s
z
0 m 0 P4 a a a2
9
G z3
a 5 ill m 3 0 I, <+
H"
0g
z"
4 05
* m w m m a m t . m m m . . . m m m m 0 0 0 0 0 0 0Jo7crnnl a/ Colloid a n d 1 1 1 1 i ~ r j ~ ~ c e Scierrec, Vol. 38, No. 1 , January 1972
-
0 0 0 0 C E 2 u C C 4 @ M . - z E215
a - E * : L O -g;.'
. "ne E r.:, 212 E " $ 2 2 a t.- ?3y 0- E ? i f 2,L-
g n ps; 22: p z g-
E L e , % Z o l m m + + o y y y 0 0 0 - * a m o m A m m m C: 0 % APHASE T l ~ , ~ S S I T I O S S OF A1)SOtiBhTES 8 1
this value of tlle relative pressure, accordiiig deternlinecl by tlie c1lar;~cteristics of tlie to tohe Iielvin equatiori, pores with less than formed ice.
66 A diameter are all filled n-it11 n-ater which This hypothesis is supported by tlle results has :an estimated freezing point as high as
- 18.j°C (9). On the other hand, trnnsition took place on "isosteric" coolirig nit11 a =
0.12 gm/gm ( 5 ) arid even n-ith a = 0.084
gm/gm (3). These findings, if tlle llypothesis that coolirlg brings about d e s ~ r p t ~ i o r ~ is cor- rect, imply that freezing takes place only in states defined by the desorption branch of the i~ot~herm. The theory that the nleriiscus effect is the sole cause of the freezing point Ion-ering, the magnitude of n-hich is assumed t o be inversely proportional t o tlle extent of the vapor pressure reduction, appears to be contradicted by this; for a given value of a the adsorbate has a Ion-er vapor pressure on desorption than on adsorption, so that the latter should have :a higher freezing point. The opposite, ho~vever is found.
The Aa/Al' curve (Fig. 4(b)) increases its value at about -5°C and - 15°C in temper-
ature regions that coincide n-it11 the freezing mnges. The causal relation between the tn-o phenomena a t tlie higher temperature llas been demonstrnted in t h e preceding section. A similar mechanism may explain the findings relnted t o freezing a t low tempera- tures.
One may argue t h a t because n-ater re- mains in t,he liquid state (being unable to freeze in t,he pores it filled on isothern~al adsorption) it drains into the partly lilled larger pores n-hen, on cooling, the nxixi- mum realizable relative pressure becomes less than its vapor pressure. The sharp rise of the Aa/AT curve indicates that below
-
15°C a large amount of water has to leave the porous system. This is probably seldom possible a t this ion- temperature because of the lorr vapor pressure, the high viscosity, nrld the partly blocked pores. Equilibiium can be attained, ho~vever, if the tmpped n-ater freezes, thus reducing the vapor pres- sure to that of ice so that equilibrium con- ditions are attained. The well-established difference betn-een the freezing and melting points of the adsorbate can then be expected because freezing occurs at the temperature :at which 11-nter is transported to a pore in nhicli it can form ice; the melting poirit isof X-ray diffraction studies sho\\-ing that all but tn-o molecular layers of water moved from bet,\\-een the saturated bentonite crys- tals when cooled t o liquid nitrogen temper- ature (10). I11 addition, the dynamic aspect of tlle pehnoinenorl is apparent in the effect of the cooling rate on the magnitude of tlle exparlsion a t about -20°C: 270 X 10-"at 0.38"C/min (Fig. l(c)), 70 X 10-5 a t
0.020S0C/min (Fig. S), and 10 X 10-j a t 2"C/day (5).
I n Table I the absolute temperatures of the transition.; of various adsorbates oc- curring at lon- temperatures are related to TJ, 6 ; it c;an be seen that the values range from 0.76 to 0.91 for freezing and from 0.8'7 to 0.95 for melting. Water appears to be an extreme case.
I t may also be significant that for most inert molecules a third freezing range was observed at very lox temperature. Only after confirnlation of these results, however, can their implications as t o the mechanism be considered.
Interpretatio?~ of tlre I$atensio~z Isosteres. On the basis of the preceding hypothesis a n attempt \\-ill be made t o specify the physicnl processes causing tlle anomalies in the length changes. The specimen saturated a t 7'
>
O°C, point 0 (Fig. l(b)), contracts on cooling o~ving t o thermal effect. At the temperature represented by il (because a>
a,,,,,),freezing on the surface occurs nnd the latent heat of freezing rrarnls the system to 0°C. Poilit R reveals a net contraction for this temperature cycle resulting from loss of witter on cooling to A. The system contracts between B and C again because of thermal contraction and ~ont~inuous loss of \rater. At C the Ivater in the pores commences to solidify-n process that, extends t o point
D.
The large expansion betweerl C and D is associated with freezing and nlav be tlle result of either pressure exerted by tlle water flon- or of the changed interaction betn-een the glass and the solidified adsor- bate. This expansion appears to be partly reversible; tlie system contracts t o E on n-arming, where the low temperature melting
FIG. 9. Estelisioii isotherm of 5 m m thick porous glass-waber systcm a1 f 0.5'C I I 1 I I
I
- 2L - 0 3 - -20 - -40process is completed. Tllc net increase of lengtli a t this temperature is a measure of the damage suffered between C and D. I t must be recognized, however, t h a t the di- nlellsions of the adsorbent a t coristant cover- age depend also on tlieir being i n an adsorp- tion or desorption state, according to the extension isotherms (Fig. 9), a~icl this may well be true lor the nonisothermal collcli- tions as n.as discussed previously (3).
Expansion bet,n-een E :~ncl F is due to ndsorpt,ion brought, about by the increasing relative pressure and the loss of mecllar~csl
1
> : +
- 0
-- 5 0
strength of the glass. This manifests itself
1 ~ 1 1 ~ 1 1 the ice in the crevices melts.
I11 tx recent publication Feldmanl coil- cluded that in the porous glass-water system solid nlenisci exist and freezing of water in the pores involves volun~etric expansion in the bulk state. Because our efforts to re- produce these results have remained so far unsuccessful the conclusions of the work \\-ere not utilizcd in tllc intcrprctation of thc prrsent results.
:
J
I I 1
'
R. F. Feldmaii, Can. J . C l ~ e m . 48, 287 (1070). J o u r n a l 4t' Colloid and I ~ ~ l e r j ~ i c c Science, Vol. 38. So. 1 J ~ m u a ~ . y 1972- 4 0 - 30 - 20 - 1 0 0
T E I \ \ P E R A I U R E , C
FIG. 8. Ilimeiisioi~al changes and thermogrnm of 5 mrn thick porous glass s a t ~ ~ r n t e d w i t h water during n +5' to -45'C tempernlurc cycle, 0.0208"C/mii1 rntc.
3 0 0 2 0 0 ," m
-
2-
2 d 1 0 0 I I 1 I I ," - r" .L! -.-.'I
, / , 0 7 * o%oI
- - / I I IPI-IASE TILAXSITIONS OF AIISORBATES 83 CONCLUSIONS
7 7
I he inevitable dccrease of tllc nlasinlum realizable relative prcssure on cooling ud- sorptioll systems belo\\- tjhe triple point of the aclsorbnt,e leads t o clcsorption. i l s a result, the :~dsorbate migrates from the Inrge voids to the outcr surface, giving rise to high- temperature freezing, and from t,he small pores to thc larger ollcs, causing lower temperature freezing. 011the basis of the
suggested mechanism the features of t . 1 ~ extension isosteres call be interpreted in physical terms. The mechanism is valid fol. all invest,igatccl liquids adsorbed 011 porous
glass, nnd it is expected t,hat it \\-ill be ap- plicable to any other system comprising :I
porous solid and a n adsorbed liquid. The mechanism causing mechanical destruction of such systems on cooling, a phenomenon called frost action, nil1 be discussed in a subsequent public a t' 1011.
ACKNOWLEDGMENTS
The author is indebted t o P. J. Sereda for helpful discussions, to B. Ilurley for assistance in
the design and construction of the apparatus and for carrying out the initial measurements, and to 11. Schultz for performing most of the experiments. This paper is a contributiou from the Division of Building Research, National Research Council of C a ~ l a d a , and is published with the approval of the Director of the IIivision.
REFERENCES
1. IIODGSON, C., A N D MCINTOSI~, R., Can. J.
Chrrn. 37, 1278 (1959).
2. HODGSON, C., A N D ~\ICIXTOSII, R., Can. J.
Chem. 38, 958 (1960).
3. L I ~ v ~ I N , G., A N D ~\/ICINTOSH, R., c a n . J . Chem.
41, 3095 (1963).
4. ANTONIOU, A. A., J. P I L ~ s . CI~ern. 68, 2751 (1964).
5. LITVIN, G., Can. J . CI~enl. 44, 2617 (1966). 6. S I D E U O ~ ~ ' T O ~ ~ , E . W., .\ND LITVAN, G., Trans.
Fararlay Soc. 67, '27% (1971).
7. LITV.\N, G. G., A N D Y IV ISIICI, R . S., t o be
published.
8. LITV-IN, G. G., J. A m . C ~ I . C I ~ I L . SOC., in press. 9. HIYXES, J. h i . , in J. Ilawthorne, Ed., "Low
Temperature Biology of Foodstuffs" p 91, Pergamon Press, 1968.
10. AHLRICI~S, J. L., A N D WIIITE, J. L. Science 136, l l l G (1962)).