HAL Id: hal-01760591
https://hal.archives-ouvertes.fr/hal-01760591
Submitted on 1 Feb 2021HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
(Nd/Pr)2NiO4+δ: reaction intermediates and redox
behavior explored by in situ neutron powder diffraction
during electrochemical oxygen intercalation
Monica Ceretti, Olivia Wahyudi, Gilles André, Martin Meven, Antoine
Villesuzanne, Werner Paulus
To cite this version:
Monica Ceretti, Olivia Wahyudi, Gilles André, Martin Meven, Antoine Villesuzanne, et al.. (Nd/Pr)2NiO4+δ: reaction intermediates and redox behavior explored by in situ neutron powder diffraction during electrochemical oxygen intercalation. Inorganic Chemistry, American Chemical Society, 2018, 57 (8), pp.4657-4666. �10.1021/acs.inorgchem.8b00393�. �hal-01760591�
(Nd/Pr)
2
NiO
4+
: reaction intermediates and redox
behavior explored by in situ neutron powder
diffraction during electrochemical oxygen
intercalation
Monica Cerettia,*, Olivia Wahyudia,b, Gilles Andréc, Martin Mevend , Antoine Villesuzanneb and
Werner Paulusa
a. Institut Charles Gerhardt Montpellier, UMR 5253 CNRS-UM-ENSCM, Université de
Montpellier, Place Eugène Bataillon, 34095 Montpellier Cedex 5, France.
b. CNRS, Université de Bordeaux, ICMCB, UMR5026, 87 Av. Dr. A. Schweitzer, 33608
Pessac Cedex, France
c. Laboratoire Léon Brillouin, UMR12, CEA-CNRS, CEA Saclay, 91191 Gif Sur Yvette,
France
d. Institute of Crystallography, RWTH Aachen University and Jülich Centre for Neutron
ABSTRACT. Oxygen intercalation/deintercalation in Pr2NiO4+δ and Nd2NiO4+δ was followed by
in situ neutron powder diffraction during electrochemical oxidation/reduction, in a dedicated
reaction cell at room temperature. For both systems three phases, all showing the same
line-width, were identified. The starting phases, Pr2NiO4.23 and Nd2NiO4.24, considered with an
average orthorhombic Fmmm symmetry, although both show a slight monoclinic distortion, get
reduced in a 2-phase reaction step to tetragonal intermediate phases with 0.07 ≤ ≤ 0.10 and
P42/ncm space group, which on further reduction transform, again in a 2-phase reaction step,
towards the respective stoichiometric (Pr/Nd)2NiO4.0 phases, with Bmab space group.
Electrochemical oxidation does, however, not proceed fully reversibly for both cases: while the
re-oxidation of Nd2NiO4+δ is limited to the tetragonal intermediate phase with δ = 0.10, the
homologous Pr2NiO4+δ can be re-oxidized up to δ = 0.17, showing orthorhombic symmetry. For
the intermediate tetragonal phase, we were able to establish for Pr2NiO4.09 a complex anharmonic
displacement behavior of the apical oxygen atoms, as analyzed by single crystal neutron
diffraction and Maximum Entropy Analysis, in agreement with a low-T diffusion pathway for
oxygen ions, activated by lattice dynamics.
KEYWORDS. Non-Stoichiometric Oxides, in-situ, neutron diffraction, electrochemistry, oxygen
ionic conductors, Ruddlesden-Popper oxides.
1. Introduction
Pr2NiO4+δ (PNO) and Nd2NiO4+δ (NNO) adopt the K2NiF4 structure-type (Figure 1) and are
today among the most promising candidates for oxygen membranes or electrolytes in solid oxide
Figure 1. Crystal structures of (Pr/Nd)2NiO4+. (a) The orthorhombic average Fmmm structure
for 0.23, showing the interstitial (Oint), the apical (Oap) and the equatorials (Oeq) oxygens.
For the idealized stoichiometry corresponding to = 0.25, one tetrahedra out of eight is occupied. (b) the stoichiometric phase with 0.00 and orthorhombic symmetry in Bmab
Combining inelastic neutron scattering together with first-principles lattice dynamical
simulations, we have recently shown the capability of the apical oxygen atoms to diffuse towards
the interstitial sites down to ambient temperature in Nd2NiO4+δ, following an energetically
shallow oxygen diffusion pathway.4 For both title compounds, NNO and PNO, we could
evidence a large and anharmonic displacement behaviour of the apical oxygen atoms, suggesting
a strong impact of lattice dynamics on the diffusion mechanism, which is supposed to be phonon
consequence, push the apical oxygen atoms towards adjacent interstitial sites.7-10 For PNO, with δ 0.23, the ortho/tetragonal phase transformation at around 365°C, induces the rock salt type Pr2O2 layer to become wholly anharmonic, showing a double well potential for the apical oxygen
and the Pr atoms. This anharmonic behaviour thus naturally amplifies the highly anisotropic 2D
oxygen diffusion inside the rock salt layer for these types of frameworks.
Both compounds are able to uptake important quantities on interstitial sites upon high
temperature synthesis, reaching δmax. 0.23. Together with the fact that i) interstitial oxygen
atoms induce a phonon-assisted diffusion mechanism at moderate temperatures and ii) an
important amount of δ 0.15 is still present at 1000°C, PNO and NNO reveal as obviously promising candidates for oxygen membranes operating at moderate temperature. Finally yet
importantly, PNO and NNO are key candidates to explore oxygen diffusion mechanisms,
triggered by low-energy phonon modes. In order to better explore the oxygen intercalation
reaction mechanism at ambient conditions on both PNO and NNO, and to build the respective
phase diagram as a function of the oxygen stoichiometry , we studied this reaction by in situ neutron diffraction on polycrystalline electrodes. While electrochemical oxygen intercalation has
already been explored for NNO by X-ray diffraction11, no such studies have been reported in
literature for PNO. In this work, we were especially motivated to explore possible oxygen
ordering in course of the intercalation reaction using in situ neutron diffraction, which is more
sensitive to low-Z elements such as oxygen.
2. Experimental Details and Methods
Synthesis and Working Electrode Preparation. Nd2NiO4+δ and Pr2NiO4+δ were prepared by
NiO were thoroughly mixed together in stoichiometric quantities and heated in air at 1250°C for
12 hours. The powder was then pressed into pellets of 13 mm diameter and 1 g mass, which were
again heated in air for 48 hours under the same conditions, with an intermediate regrinding and
pelletizing. The as obtained pellets were used as working electrodes for the structural studies by
in situ neutron powder diffraction (NPD). The electrochemical cell used for NPD was made of
quartz, quite transparent to neutrons, containing three electrodes (one working electrode, Pt
counter electrode and Ag/AgCl reference electrode) in a 1 N KOD electrolyte, as described in
details elsewhere.12 The working electrode (sample) consisted of reshaped pellets (total mass
about 3 g) glued together by a carbon glue and contacted by a Pt wire. The electrolyte was
prepared from equivalent amounts of 40% KOD solution in D2O (99.9% enriched, Aldrich).
Single Crystal Growth. High quality Pr2NiO4+δ single crystals were grown by the traveling
solvent floating zone method (TSFZ) as described elsewhere.13 Compared to polycrystalline
electrodes, which react on a reasonable timescale in thermodynamical equilibrium, the reactivity
of centimeter-sized single crystals - used for neutron diffraction structural investigations - is
strongly reduced due to the smaller active reaction surface. Therefore, in order to obtain the
target oxygen stoichiometry of =0.1 in a reasonable reaction time, the crystals were annealed under a dynamic vacuum ( 10-3 mbar) at 600°C.
Neutron Diffraction.
In situ neutron powder diffraction studies were performed on the G4.1 two-axis diffractometer
of the Laboratoire Léon Brillouin in Saclay (LLB, Orphée reactor), France. The diffractometer is
equipped with an 800-cells multi-detector covering a 2 range of 80°, the used wavelength being 2.42 Å.
Nd2NiO4+ powder diffraction patterns were collected every 2 hours during a period of 3.5
days, achieving a charge transfer of 0.01 electrons/formula unit (e-/f.u.) per diffractogram. Due to
the limitation of neutron beam time, powder patterns for Pr2NiO4+were recorded every hour,
with a charge transfer of 0.015 e-/f.u. per diagram. During all in situ experiments, fresh
electrolyte was pumped at constant flow through the electrochemical cell in order to guarantee
both stable reactions and a constant background during the whole experiment. All the
electrochemical reduction/oxidation reactions were carried out in a galvanostatic mode, applying
a constant current. The applied current density did not exceed 500 A/cm2. NPD experiments were performed during the reduction of the as sintered starting compounds until the
stoichiometric Nd2NiO4.00 and Pr2NiO4.00 are reached. After completing the reduction, both
samples were re-oxidized in order to verify the reversibility of the oxygen intercalation reaction.
Further structural investigations were performed by NPD, using the 3T2 two axes
diffractometer at LLB (=1.225 Å), as well as single crystal neutron diffraction on the four-circle diffractometer HEiDi at MLZ in Garching, Germany ( = 0.79 Å). Rietveld refinements of all NPD data were done using the FULLPROF suite.14 Special care was taken for the background
correction of the in situ diffraction experiment: it was carefully determined from the starting
compound and taken as constant for the whole experiment. Single crystal refinements were
carried out using SHELXL.15 Nuclear densities have been reconstructed in real space through the
maximum entropy method (MEM) via PRIMA (Practice Iterative MEM Analysis);16 scattering
lengths were taken from ref. 11, and nuclear density distributions were visualized by using the
VESTA program.17
3.1 Nd2NiO4+δ electrochemical reduction-oxidation
Nd2NiO4+δ shows a rich sequence of phase transitions and distinct line phases as a function of
oxygen stoichiometry (), even at room temperature. For Nd2NiO4+δ, variation of can been
controlled as a function of temperature and oxygen partial pressure.18, 19 Only ref 11 reports on the
structural evolution of NNO during electrochemical oxygen intercalation/deintercalation at
ambient temperature, studied by ex situ X ray diffraction. The obtained phase diagram shows, in
addition to the orthorhombic Fmmm starting phase, reported as Nd2NiO4.18, and the
stoichiometric end phase Nd2NiO4.00, a non-stoichiometric tetragonal phase Nd2NiO4.06±0.02,
which has been indexed in the tetragonal space group F4/mmm. However, reported lattice
parameters, oxygen stoichiometry as well as symmetry, are not always consistent, showing
problems with the reproducibility as well as the complexity of the system.
In the present work, neutron powder diffraction was chosen to follow the structural evolution
of the title compounds, during the electrochemical reaction. Importantly, neutron diffraction
yields bulk information, while the penetration depth of X-rays is limited to several μm only,
rendering difficult to quantify the different volume fractions of the respective phases formed
during the reaction. This is important when it comes to quantify phase analysis, which is a
difficult task when analyzed with X-ray diffraction, related to inhomogeneities between the
surface and in the bulk of the working electrode, especially for solid-state reactions with slow
kinetics. The second advantage for neutron diffraction is that the coherent neutron scattering
length of oxygen (~5.803 fm) is of the same magnitude of that for Nd/Pr (~ 7.69 fm/4.58 fm) and
Ni (~ 10.3 fm), resulting in a significantly better contrast for (low-Z) oxygen atoms; this allows
NPD patterns of Nd2NiO4+ measured in situ during a complete electrochemical
reduction-oxidation cycle on the neutron diffractometer G4.1 (=2.42Å) are shown in Figure 2. Data were corrected for an unbalanced background, resulting essentially from the quartz cell and KOD
electrolyte. The background was determined with good counting statistics for the first diffraction
pattern, prior to the reduction, and subsequently subtracted from all following diffractograms.
The electrochemical reaction was performed in a galvanostatic mode, allowing a direct
correlation of the structural and magnetic evolution with the charge transfer, i.e. the oxygen
stoichiometry (see Eq. 1). The starting compounds, Nd2NiO4+ and Pr2NiO4+, showing
respectively an oxygen excess stoichiometry of 0.24 and 0.23, are usually reported to be orthorhombic, but reveal in fact a minor monoclinic splitting, the monoclinic angle being 90.06°
and 90.07° respectively. This monoclinic distortion can already be detected on a high resolution
laboratory X-ray diffractometer on a highly crystalline sample with low mosaicity. Although the
used electrodes were monoclinic (space group F112/m, which is a subgroup of Fmmm) with a =
5.3681(4) Å, b = 5.4439(4)Å, c = 12.3514(10)Å, = 90.07(1)° for NNO and a = 5.3945(4) Å, b = 5.4538(4)Å, c = 12.4408(10)Å, = 90.07(1)° for PNO (see Figures S1 and S2), they are considered in the following as orthorhombic with Fmmm symmetry, as the instrumental
resolution of the G4.1 neutron powder diffractometer did not allow discriminating between
orthorhombic and monoclinic symmetry.
For the electrochemical reduction of Nd2NiO4+ an electrode of 40 10 1 mm3 was obtained
using four sintered and reshaped pellets, in order to optimize for intensities. Figure 2 (bottom)
shows the obtained diffractograms as a function of the charge transfer, showing principally 3
intermediate phase with P42/ncm symmetry, (iii) and the fully reduced and stoichiometric phase
Nd2NiO4.0, having the low temperature orthorhombic (LTO) phase with Bmab space group.
The electrochemical reaction can formally be described as:
RE2NiO4.00 +O2- RE2NiO4+δ + 2e- (1)
Thus the oxygen stoichiometry is directly related to the charge transfer (n), expressed in e-/f.u., by the simple relation = 2n. A charge transfer of about 0.48 e-/f.u. was needed to reach the pure stoichiometric phase, corresponding to the intercalation of = 0.24 oxygen atoms. The starting material was thus calculated to be Nd2NiO4.24, in agreement with the value reported in
literature.13
All diffractograms were analyzed by Rietveld refinement using the FullProf package. Here, the
refinements - and especially the identification of the different phases - are not an easy task,
mainly due to the very similar lattice parameters of all present phases. Rietveld refinements for
the three different phases are reported in Figure S3-S5. Since the quality of the in situ
diffractograms does not allow precise occupancies and thermal displacements, and to avoid
incoherent divergences in the refinements, we kept the structural models in Fmmm, P42/ncm and
Bmab unchanged, while essentially refining the volume fraction and lattice parameters only. In
this way we could obtain a reasonable approach of the phase relations during the electrochemical
reactions. Refined lattice parameters and scale factors are illustrated in Figure 3(a) and 3(b),
Figure 2. Evolution of the NPD patterns obtained in situ during the electrochemical
reduction-oxidation of Nd2NiO4+δ (G41, = 2.422(2) Å). The yellow ellipsoid shows the incommensurate
superstructure reflections of the starting compound. In the upper right part, zoom-in view of the
diffraction patterns between 40° and 56° in 2.For comparison, the different diffraction patterns are reported in the bottom part: the pattern of the starting phase with Fmmm symmetry and = 0.24, the intermediate P42/ncm phase with = 0.10 and the fully reduced stoichiometric
compound with Bmab symmetry ( = 0.00) as well as the maximum re-oxidized phase. Peaks indicated with (*) arise from the carbon glue, while the reflection indicated by (+), in the
For the Nd2NiO4.24 starting phase, we observed in addition to those corresponding to the Fmmm
space group two reflections at 2=65° and 67° (Figure 1). Both reflections are not detectable by X-ray diffraction, even at good counting statistics, and are supposed to be superstructure
reflections, related to an incommensurate superstructure due to oxygen ordering, as already
discussed in ref. 7. While the reflection at 2 = 67° completely disappears after a charge transfer of 0.15 e-/f.u., the one at 2 = 65° can be further indexed as (212) for the tetragonal and the stoichiometric Nd2NiO4.00 phase, for which the corresponding orthorhombic (122) reflection is
absent due to the extinction rules of space group Bmab. A careful inspection of the lattice
parameters and respective scale factors (Figure 2) reveals the starting and end phase to be both
stoichiometric line phases, since their lattice parameters are constant over the whole range of the
reaction and only their respective scale factors vary. Inspection of the evolution of the scale
factors, identifies the transformation of the Nd2NiO4.24 starting phase towards the tetragonal
intermediate phase as a two-phase region step (see Figure 3(b)). However, the c lattice parameter
of the tetragonal phase is slightly but continuously decreasing, which seems somehow to be
Figure 3. Nd2NiO4+: evolution of the unit cell parameters a, b, c, (a) and of the refined scale
factor parameter (b), as a function of the charge transfer n (and thus of the oxygen excess ), obtained from Rietveld refinements. The errors bars in the a, b, c, parameters are within the
dimensions of the dots. Refinements were done for the Fmmm orthorhombic starting phase (red
dots), the P42/ncm tetragonal intermediate phase (light blue dots) and the stoichiometric
orthorhombic phase with Bmab symmetry (dark blue dots). Oxygen content was derived from
The space group of the tetragonal intermediate phase does, however, not correspond to F4/mmm,
as reported earlier from X-ray diffraction studies in ref. 11, but to P42/ncm, as indicated by the
presence of P-type reflections, e.g. the (104) reflection at 2 = 53°; its intensity increases linearly with the charge transfer in the beginning of the reduction, while the incommensurate reflection at
67° weakens simultaneously. The appearance of P-type reflections, whereas their intensity is
close to zero in X ray diffraction, is an interesting observation, as all tetrahedral sites of the
interstitial oxygen atoms are symmetrically equivalent for F4/mmm, but split into two different
sites in P42/ncm, with different point symmetries. The 4b Wyckoff site in (¾ ¼ ¾) shows a site
symmetry of , while a 2.mm site symmetry is obtained for the (¼ ¼ z) site, with 4e Wyckoff notation. As discussed below for Pr2NiO4.09 , only the 4b sites are occupied by interstitial oxygen
atoms. This is at first sight surprising, as a lower structural flexibility of the Oint(Oap)4
tetrahedron centered at (¾ ¼ ¾) is expected, related to the site symmetry and associated symmetrical increase in case of oxygen occupation (as outlined below, section 3.3), and the
resulting short Oint-Oap oxygen distances of about 2.2 Å. The situation seems, however, to be
different for the 4e position, allowing an asymmetric opening of the (Oap)4 tetrahedron and thus
enabling an easier structural response when it comes to avoid steric constraints.
In this context it is also interesting to note that thermally prepared Nd2NiO4.08 has been reported
to show a slight deviation from the tetragonal to orthorhombic symmetry with the Pccn space
group, as explored by X-ray diffraction.20 While for structure refinements in ref 20 all positions of
the basic structure were constraint to the equivalent positions in the P42/ncm space group, it was
P42/ncm space group becomes an important finding, as a corresponding site symmetry is absent
for the Pccn space group. This suggests that the true symmetry of Nd2NiO4.08 could also be the
tetragonal P42/ncm rather than the postulated Pccn.
Following the scale factor (Figure 3(b)), we can clearly define a single-phase region for the
P42/ncm phase for 0.07 0.10, rendering Nd2NiO4+ to be non-stoichiometric. Below =
0.07 the reduction proceeds as a two-phase reaction, where the volume fraction of Nd2NiO4.07
continuously decreases, while the amount of stoichiometric Nd2NiO4.00 accordingly increases. It
goes along with a significant increase of the orthorhombicity (a-b)/(a+b) = 0.0182, compared to
0.0069 for the Nd2NiO4.24 starting phase. From Figure 2, the orthorhombic splitting of the basic
reflections as (200/020) and (202/022) becomes evident, while the (212) and also the
antiferromagnetic (011) reflections have no orthorhombic counterparts, as they both are
forbidden by symmetry. The zoomed section from 49°-56° shows, in addition to the (200/020)
splitting, the evolution of the (104) reflection, which is characteristic of the P42/ncm and the
Bmab phases only. Refinements carried out for the nuclear/magnetic structure of Nd2NiO4.00 in
space group Bmab yielded a magnetic moment of x = 0.686 μB, showing G-type
antiferromagnetic ordering similar to that reported in ref. 21
After obtaining the stoichiometric Nd2NiO4.00 phase, the current polarization was reversed to
start the re-oxidation. As shown in the evolution of the diffraction patterns, the
deintercalation/intercalation reaction turns out as not fully reversible, since the re-oxidation stops
when reaching the tetragonal phase, corresponding to Nd2NiO4.10. Although the current density
was the same as the one used during the reduction, the slope of the scale factor during
re-oxidation, in the region 0 n 0.08 e-/f.u., is steeper compared to the reduction reaction. Beyond n = 0.08 e-/f.u., a significantly reduced slope is observed, probably related to a change in
the kinetics of the oxidation reaction. A transformation from the orthorhombic Nd2NiO4.00 phase
to the tetragonal phase is clearly accompanied by the vanishing of the splitting of orthorhombic
reflections like the (202/022). The separation of the strongest reflections (200/020) cannot easily
be used for a quantitative phase identification due to the almost perfect overlap of the (020)
reflection of the Bmab phase with the (113) reflection of both phases. Further attempts to
electrochemically oxidize the tetragonal phase of Nd2NiO4+ beyond 0.09, to access the
Fmmm initial phase Nd2NiO4.24, did not succeed.
3.2 Pr2NiO4+δ electrochemical reduction-oxidation
The electrochemical reduction/oxidation of as sintered Pr2NiO4.23 pellets was done in the same
way as described above for Nd2NiO4.24. Thereby, the indicated oxygen stoichiometry is the result
of TG analysis, carried out under hydrogen, as well as the electrochemical titration outlined
below. The diffractograms, obtained by in situ NPD are given in Figure 4, as a function of the
charge transfer. All patterns were corrected for background as described above for NNO.
In analogy to NNO, the starting phase is orthorhombic and all reflections can be indexed in the
Fmmm space group, except the one situated at 67.5° in 2 which is again attributed to an incommensurate superstructure reflection. Its intensity is, however, much lower compared to
NNO, as already mentioned in ref. 22. Following the refined lattice parameters and scale factor as
depicted in Figures 5(a) and 5(b), we conclude on a very similar phase diagram compared to
Nd2NiO4+. The reduction proceeds in a two-phase reaction mechanism from the orthorhombic
Pr2NiO4.23 to the tetragonal Pr2NiO4.10 phase (refinement is given in Figure S6), which gets
further reduced to Pr2NiO4.07 in a single-phase reaction mechanism. The formation of
antiferromagnetic G-type ordering, as deduced from the (011) reflection, the magnetic moment
of Ni2+ pointing along the a-axis with Mx = 0.550 μB.23, 24
Figure 4. Evolution of the NPD patterns obtained in situ during the electrochemical
reduction-oxidation of Pr2NiO4+δ (G41, = 2.422(2)Å). In the upper right part, zoom-in view of the
diffraction patterns between 40° and 56° in 2 For comparison, the different diffraction patters are also reported in the bottom: the starting pattern Fmmm ( = 0.23), the intermediate P42/ncm
for = 0.10, the stoichiometric fully reduced compound ( = 0.00) Bmab, once again the intermediate P42/ncm phase ( = 0.10) and the final Fmmm phase reached upon re-oxidation. The
reflection indicated by (+) in the stoichiometric phase is magnetic. The yellow ellipsoid shows the incommensurate superstructure reflections of the starting compound.
Figure 5. Pr2NiO4+: (a) evolution of the unit cell parameters a, b, c, and (b) of the refined scale
factor parameters as a function of the charge transfer n (and thus of the oxygen excess ). The errors bars in the a, b, c, parameters are inside the dimensions of the dots. Refinements were
done for the Fmmm orthorhombic starting phase (red dots), the P42/ncm tetragonal intermediate
phase (light blue dots) and the stoichiometric orthorhombic Bmab phase (dark blue dots). Oxygen content was derived from the electrochemical titration data.
Contrary to the NNO system, the re-oxidation reaction seems at first sight to be fully reversible
for PNO: the final oxidized phase shows again the Fmmm symmetry with the same scale factor
as the starting phase. However, the phase purity during the oxidation is not so nicely defined as
for the reduction, as it can be seen from the individual patterns shown in Figure 4 (bottom). This
might be due, at least partially, to grain contact problems related to significant changes in the
volume, i.e. lattice parameters. The evolution of the scale factors shows that the re-oxidized
phase is already reached at a charge transfer equivalent to = 0.17 only. Moreover, it does not show any incommensurate reflections, and the orthorhombicity is slightly larger compared to the
starting phase, the lattice parameters being a = 5.3945(4)Å, b = 5.4748(6)Å, c = 12.4281(8)Å.
These values, however, perfectly match with those of the Pr2NiO4.16 phase, prepared by classical
solid state reaction, and which has been reported as a line phase with Fmmm symmetry.25
Since oxygen formation was observed at the counter electrode during the electrochemical
oxidation when reaching a charge transfer corresponding to = 0.16, it becomes evident that further oxidation towards = 0.23 is hindered, for reasons unclear so far. We anyway counterchecked the symmetry and lattice parameters of this maximum re-oxidised phase by ex
situ high resolution X-ray diffraction, in order to verify or not a possible monoclinic symmetry,
as obtained for the starting phase Pr2NiO4.23 (Figure S8). Its pattern matches also here nicely with
an orthorhombic Fmmm structure, although a significant broadening of the reflection profiles is
observed, probably related to reduced mosaicity induced by the reduction/oxidation reaction. We
therefore conclude that the maximum electrochemically oxidized phase shows a stoichiometry
The reversibility of the reaction is clearly enhanced for PNO when comparing to the homologue
NNO system, as oxidation continues far beyond the tetragonal intermediate phase. Nevertheless,
a full reversibility remains incomplete for both systems. The question, whether the Fmmm-phase
corresponding to = 0.16 already appears during the reduction from the as sintered Pr2NiO4.23
electrodes, is difficult to answer, due to similarities in the lattice parameters and limited
instrumental resolution. From the refinements (see Figures 5(a) and 5(b)), it can be seen that the
c-axis and a-axis parameters of the re-oxidized phase are almost identical to the starting phase
parameters, while the b-axis parameter corresponds closely to the (a, b)-parameter of the
intermediate tetragonal phase.
Further inspections of the evolution of all P-type reflections, i.e. reflections that are forbidden in
Fmmm, reveal a steady increase in their intensities from the beginning of the reduction and
which go through the stoichiometry range corresponding to = 0.16. This clearly suggests the absence of this Fmmm phase with = 0.16 during the electrochemical reduction.
3.3 Intermediate tetragonal phase
As for NNO, a non-stoichiometric tetragonal phase Pr2NiO4+ with 0.07 ≤ ≤ 0.10 was obtained
during the electrochemical reduction/oxidation, although there is no obvious reason for this
specific reaction intermediate to appear. In addition, the P42/ncm space group, implying an
octahedral tilting around the [110] axis with respect to the Fcell (low temperature tetragonal
-LTT - phase), usually occurs only for the low-T modification of nickelates with Ruddlesden
Popper frameworks with close to 0, as is the case for RE2NiO4.0 (RE = La, Nd, Pr).21 The
formation of this LTT-type phase with a significant interstitial oxygen stoichiometry, during the
This astonishing formation of this tetragonal non-stoichiometric phase, for both Nd2NiO4+ and
Pr2NiO4+ with 0.07 ≤ ≤ 0.10, motivated us to further investigate related structural changes,
with a special focus on the occupation of the two non-equivalent tetrahedral sites and related
oxygen ordering. To this end we first investigated the maximum electrochemically re-oxidized
Nd2NiO4.09 sample, used for the in situ experiment, by high resolution powder diffraction on the
3T2 diffractometer at LLB. The refinement, which is given in the Supplementary Information
(Figure S9 and Tables S1-S2), was non-conclusive with respect to a preferred occupation of the
two tetrahedral sites. We checked both tetrahedral site occupation separately, and were able to
refine a stoichiometry corresponding to = 0.09 for both cases, and underlining the difficulty to differentiate between both tetrahedral sites even when neutron powder diffraction is employed.
To obtain a higher structural resolution, we attempted to study the homologous PNO phase by
single crystal neutron diffraction. The reaction kinetics for single crystals of suitable size is,
however, strongly reduced for electrochemical reactions, and the as grown Pr2NiO4.23 single
crystal was instead reduced for 30 minutes at 600°C and 10-3 mbar vacuum yielding the
tetragonal phase Pr2NiO4.09.
Its crystal structure was subsequently investigated on HEIDI, the neutron single crystal
diffractometer installed at the hot source of the FRM II reactor of MLZ. Structure factors were
collected up to high q-values, i.e. up to sin = 0.87 Å-1. The refinements were done in the space group P42/ncm. No absorption correction was applied due to the negligible absorption
Table 1. Pr2NiO4.09 : structure data obtained from single crystal neutron diffraction data,
collected on diffractometer HEiDI@MLZ with = 0.79 Å
Atom x y z Occ U11 U22 U33 U23 U13 U12 Pr 0.9909(3) 0.9909(3) 0.3618(2) 2.00 0.0093(6) 0.0093(6) 0.0105(8) - - 0.0018(5) Ni 0 0 0 1.00 0.0029(5) 0.0029(5) 0.0143(7) 0.0003(2) 0.0003(2) -0.0004(2) O1 ¼ ¼ 0.9797(2) 1.00 0.0058(7) 0.0058(7) 0.019(1) - - -0.0023(5) O2 ¾ ¼ 0 1.00 0.007(7) 0.007(7) 0.032(1) - - 0.0016(6) O3 0.0380(3) 0.0380(3) 0.1772(2) 1.92(9) 0.0222(8) 0.0222(8) 0.0102(9) 0.0006(4) 0.0006(4) -0.0065(7) O4 ¾ ¼ ¼ 0.09(2) 0.013(2)
Refinements were carried out in SG P42/ncm with unit cell parameters a = b = 5.476(9) Å; c =
12.317(5) Å, 996 measured reflections (sin = 0.79 Å-1), (465 unique) Rint(hkl) = 2.8%, Rw=5.6%,
GooF=1.23. (Uij are in Å2). The special positions (xxz) of Pr and O3 refer to the second setting of SG P42/ncm.
The under-occupation of the equatorial O3 is probably due to part of the density "escaping" from the harmonic description.
The structure corresponds to the LTT structure-type, reported previously for different
stoichiometric RE2MO4.0 phases at low temperature.24 It is characterized by the tilt of the
octahedra around the [110]-axis, with respect to the F-cell. From a difference Fourier analysis, it
turns out that the interstitial oxygen atoms are located in the (4b) tetrahedral site only, in (¾ ¼
¼), resulting into an unreasonable short distance of 2.18 Å (4x) between the Oint and Oap.
Thereby the shift of the apical oxygen atoms results in a rotation of the (4b) tetrahedra around
the c-axis, which corresponds here to 10° (see Figure 6). As further outlined below, the short
Oint-Oap distances do not reveal the real but a more average structure approach, not taking into
account the local Oap displacements, induced by the presence of Oint. At this stage it is, however,
not clear whether the better localisation of the interstitial oxygen atoms obtained from single
data have been collected up to sin = 0.87 Å-1, whereas the NPD data are limited to sin = 0.71 Å-1.
As already reported for the average high-temperature tetragonal (HTT) F4/mmm structure of
Pr2NiO4.23 at 400°C, significant anisotropic displacement factors were found for the apical and
the equatorial oxygen atoms.6 The anisotropy is almost four times larger along the c-axis for the
equatorial oxygen atoms O(1) and O(2) while, for the apical O(3) atom, displacements are two
times larger in the (a,b) plane compared to the c-axis. This has been reported for a series of
K2NiF4 type oxides and is not only restricted to nickelates.26, 27 These anisotropic displacements
along [110] direction with respect to the F-cell, have been interpreted as dynamically activated
libration-modes of the MO6-octahedra, induced by the presence of interstitial oxygen atoms,
pushing away the coordinating Oap atoms, and thus creating an oxygen diffusion pathway along
the [110]-direction inside the Pr2O2 rock-salt layer, towards the interstitial sites.6, 8, 28, 29 A similar
behaviour is also evidenced here by the Maximum Entropy reconstruction of the nuclear
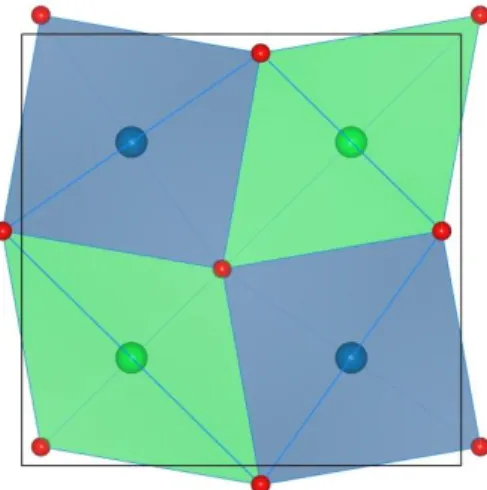
Figure 6. The two different tetrahedral sites with 4b (green) and 4e (blue) Wykoff notation
showing 2.mm and site symmetry respectively. The 4b tetrahedra are rotated by 10° around the
c-axis.
The phased data obtained for Pr2NiO4.09 from neutron diffraction were used for the reconstruction
of the scattering densities by the maximum entropy method (MEM), giving the most probable
unconstrained and unbiased scattering distribution. This is equivalent to an anharmonic
description of all constituents, which is model free, the only constraint being the symmetry given
by the space group. The MEM approach has also the advantage to consider strong and weak
reflections with equal importance, resulting in a much better defined background in the nuclear
scattering maps compared to the classical Fourier-methods, particularly interesting when the
description of the extra oxygen atoms or some disorder is needed.
The nuclear density distribution of Pr2NiO4.09 obtained by MEM is reported in Figure 7, where
sections from 0.16 ≤ z ≤ 0.34 clearly evidence the influence of the interstitial oxygen atoms on
the displacements of the coordinating Oap atoms. In addition to their equilibrium positions, extra
polyhedron at least on a local scale. We note, however, that the selective (4b) tetrahedral site
occupation is only 10%, not allowing a cooperative long-range order of the Oint. The increase for
the partially occupied (4b) tetrahedra goes along with the reduction of the other remaining
tetrahedra in the unit cell, reflecting both displacement directions of the Oap as confirmed by the
MEM data. The displacements of all apical oxygen atoms are thus highly anharmonic, putting a
general problem on classical structure refinement using harmonic displacement ellipsoids. This,
consequently, allows understanding the refinement qualities (see Table 1) resulting in an
R-factor above 5%, while the Rint of 2.8 % confirms the excellent data quality of the measured
intensities.
The steric effects due to the presence of the interstitial oxygen atoms here is thus completely
equivalent to the behaviour found for the displacements of the apical oxygen atoms in Pr2NiO4.23,
following an intersticialcy push-pull diffusion mechanism, as found for K2NiF4 type oxides but
commonly occurring at more elevated temperatures.8, 30
Beside a better structural understanding of the intermediate phase with respect to the oxygen
diffusion mechanism, we also checked for possible magnetic reflections such as
(100)/(101)/(011), as reported for Pr2NiO4.06.31 In agreement with the powder diffractograms
obtained during the in situ studies reported above, we confirm the absence of these reflections
[100] [110]
Figure 7. Illustration of the anharmonic displacements of the apical oxygen atoms inside the
Pr2O2 rock-salt layer, as reconstructed by the MEM method. Left: isosurfaces of the Oap (green)
tetrahedrally coordinated by Oint atoms (blue). Middle: projection of the nuclear densities for
0.16 z 0.34; the F-unit cell is outlined. Right: [110]/[001] projection cut along [1-10] with an integration thickness (x,x) = 0.07 showing the oxygen diffusion pathway along [110], outlined
with a blue dashed line.
4. Conclusion
The electrochemical reduction in aqueous alkaline electrolyte and subsequent oxidation of as
sintered Nd2NiO4.24 and Pr2NiO4.23 electrodes appear to be very similar for both compounds in
terms of structural evolution with the charge transfer. In both cases, a two-phase reaction step
transforms the starting phases into a non-stoichiometric tetragonal phase (0.07 ≤ ≤ 0.10), which subsequently transforms, again in a two-phase reaction, into the respective stoichiometric
RE2NiO4.00 phases. The oxidation processes are, however, significantly different, showing
tetragonal Nd2NiO4.10 and orthorhombic Pr2NiO4.17 as the maximum re-oxidised phases, while
reaching oxygen gas formation at the electrode. It seems that, in both cases, the phases with the
[0 01 ] [0 10 ]
NNO, are avoided. One explanation for this not fully reversible oxidation might originate from
the extra strain, necessary to induce a monoclinic distortion and associated formation of oxygen
ordering, as described in ref.6.
It is thereby intriguing to see that the phase diagram obtained by electrochemical reactions at
ambient temperature is essentially corresponding to the phases obtained by classical solid-state
reactions under controlled oxygen partial pressure at high temperatures. In this context, it is
remarkable to observe that a selective occupation of only one out of two tetrahedral sites is
achieved during the formation of the tetragonal intermediate phase. The formation mechanisms
and stability for these phases, showing a narrow composition range of 0.07 ≤ ≤ 0.10, are not clear yet. Electrochemical cycling of structurally closely related La2CuO4+ and La2CoO4+ only
shows orthorhombic symmetry for max = 0.07 and 0.25 respectively, while La2NiO4.0 becomes
tetragonal for already small values of .32, 33 It is clear that we are still far away from an understanding of the subtle structural deformations induced by small changes in values in Ruddlesden-Popper type oxides. It would be of particular interest to explore oxygen intercalation
reactions in non-aqueous electrolytes, allowing to significantly increase both reduction and
oxidation potentials, and presumably allowing to access higher oxygen doping concentrations.
ASSOCIATED CONTENT
Supporting Information.
The following files are available free of charge.
AUTHOR INFORMATION
Corresponding Author
monica.ceretti@umontpellier.fr
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval
to the final version of the manuscript.
Funding Sources
This work was financially supported by the French National Agency Research ANR projects “Assisted Mechanisms for Oxygen Ionic conduction in non-Stoichiometric oxides” (AMOXIS,
No. ANR-14-CE05-0016-02) and “Structural induced Electronic Complexity controlled by low
temperature Topotactic Reaction” (SECTOR No. ANR-14-CE36-0006-01).
ACKNOWLEDGMENT
We would like to thank Dr. F. Porcher of the Laboratoire Léon Brillouin (LLB, Saclay, France)
for having given us the possibility to perform a data collection on the 3T2 diffractometer. X-rays diffraction measurements have been performed at the “Plateforme d’Analyse et de
Caractérisation du Pôle Chimie Balard”, Montpellier, France. Single crystal neutron diffraction
data have been measured at the single crystal diffractometer HEiDi operated jointly by RWTH
Aachen and Forschungszentrum Jülich GmbH (JARA cooperation) at the Heinz Maier-Leibnitz
Zentrum (MLZ, Garching, Germany).
1. Kovalevsky, A. V.; Kharton, V. V.; Yaremchenko, A. A.; Pivak, Y. V.; Tsipis, E. V.; Yakovlev, S. O.; Markov, A. A.; Naumovich, E. N.; Frade, J. R. Oxygen permeability, stability and electrochemical behavior of Pr2NiO4 + -based materials. J. Electroceram. 2007, 18, 205-218.
2. Takahashi, S.; Nishimoto, S.; Matsuda, M.; Miyake, M. Electrode Properties of the Ruddlesden–Popper Series, Lan+1NinO3n+1 (n=1, 2, and 3), as Intermediate-Temperature Solid
Oxide Fuel Cells. J. Am. Ceram. Soc. 2010, 93, 2329-2333.
3. Ishihara, T. Oxide ion conductivity in defect perovskite, Pr2NiO4 and its application for
solid oxide fuel cells. Journal of the Ceramic Society of Japan 2014, 122, 179-186.
4. Perrichon, A.; Piovano, A.; Boehm, M.; Zbiri, M.; Johnson, M.; Schober, H.; Ceretti, M.; Paulus, W. Lattice Dynamics Modified by Excess Oxygen in Nd2NiO4+: Triggering
Low-Temperature Oxygen Diffusion. J. Phys. Chem. C 2015, 119, 1557-1564.
5. Paulus, W.; Schober, H.; Eibl, S.; Johnson, M.; Berthier, T.; Hernandez, O.; Ceretti, M.; Plazanet, M.; Conder, K.; Lamberti, C. Lattice Dynamics To Trigger Low Temperature Oxygen Mobility in Solid Oxide Ion Conductors. J. Am. Chem. Soc. 2008, 130, 16080-16085.
6. Ceretti, M.; Wahyudi, O.; Cousson, A.; Villesuzanne, A.; Meven, M.; Pedersen, B.; Bassat, J. M.; Paulus, W. Low temperature oxygen diffusion mechanisms in Nd2NiO4+ and
Pr2NiO4+via large anharmonic displacements, explored by single crystal neutron diffraction. J. Mater. Chem. A 2015, 3, 21140-21148.
7. Paulus, W.; Cousson, A.; Dhalenne, G.; Berthon, J.; Revcolevschi, A.; Hosoya, S.; Treutmann, W.; Heger, G.; Le Toquin, R. Neutron diffraction studies of stoichiometric and oxygen intercalated La2NiO4 single crystals. Solid State Sciences 2002, 4, 565-573.
8. Parfitt, D.; Chroneos, A.; Kilner, J. A.; Grimes, R. W. Molecular dynamics study of oxygen diffusion in Pr2NiO4+. Phys. Chem. Chem. Phys. 2010, 12, 6834-6836.
9. Villesuzanne, A.; Paulus, W.; Cousson, A.; Hosoya, S.; Le Dreau, L.; Hernandez, O.; Prestipino, C.; Houchati, M. I.; Schefer, J. On the role of lattice dynamics on low-temperature oxygen mobility in solid oxides: a neutron diffraction and first-principles investigation of La2CuO4+. J. Solid State Electrochem. 2011, 15, 357-366.
10. Li, X.; Benedek, N. A. Enhancement of Ionic Transport in Complex Oxides through Soft Lattice Modes and Epitaxial Strain. Chem. Mater. 2015, 27, 2647-2652.
11. Bhavaraju, S.; DiCarlo, J. F.; Scarfe, D. P.; Yazdi, I.; Jacobson, A. J. Electrochemical Intercalation of Oxygen in Nd2NiO4+x (0 < x <.0.18) at 298 K. Chem. Mater. 1994, 6, 2172-2176.
12. Le Toquin, R.; Paulus, W.; Cousson, A.; Prestipino, C.; Lamberti, C. Time-resolved in situ studies of oxygen intercalation into SrCoO2.5, performed by neutron diffraction and X-ray
absorption spectroscopy. J. Am. Chem. Soc. 2006, 128, 13161-13174.
13. Wahyudi, O.; Ceretti, M.; Weill, I.; Cousson, A.; Weill, F.; Meven, M.; Guerre, M.; Villesuzanne, A.; Bassat, J. M.; Paulus, W. Growth of high quality single crystals of strontium doped (Nd,Pr)-nickelates, Nd2-xSrxNiO4+ and Pr2-xSrxNiO4+ Crystengcomm 2015, 17,
6278-6285.
14. Rodríguez-Carvajal, J. Recent developments of the program FullProf. Commission for Powder Diffraction. IUCr Newsletter 2001, 26, 12-19. The complete FULLPROF suite can be obtained from: http://www.ill.eu/sites/fullprof/index.html.
15. Sheldrick, G. A short history of SHELX. Acta Crystallographica Section A 2008, 64, 112-122.
16. F. Izumi , R. A. D. Recent Research Developments in Physics; 81-7895-046-4; 2002; pp 699-726.
17. Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272-1276.
18. Zaghrioui, M.; Giovannelli, F.; Brouri, N. P. D.; Laffez, I. Anomalies in magnetic susceptibility of nonstoichiometric Nd2NiO4+δ (δ=0.049, 0.065, 0.077, 0.234). J. Solid State
Chem. 2004, 177, 3351-3358.
19. Ishikawa, K.; Metoki, K.; Miyamoto, H. Orthorhombic–orthorhombic phase transitions in Nd2NiO4+δ (0.067≤δ≤0.224). J. Solid State Chem. 2009, 182, 2096-2103.
20. Ishikawa, K. Crystal structure of Nd2NiO4.08. Solid State Ionics 2014, 262, 682-686.
21. Fernández-Díaz, M. T.; Rodríguez-Carvajal, J.; Martínez, J. L.; Fillion, G.; Fernández, F.; Saez-Puche, R. Structural and magnetic phase transitions in Pr2NiO4. Z. Physik B -
Condensed Matter 1991, 82, 275-282.
22. Fernández-Díaz, M. T.; Martínez, J. L.; Rodríguez-Carvajal, J. High-temperature phase transformation of oxidized R2NiO4+δ (R=La, Pr and Nd) under vacuum. Solid State Ionics 1993,
63–65, 902-906.
23. Fernandez Diaz, M. T.; Martinez, J. L.; Rodriguez Carvajal, J.; Beille, J.; Martinez, B.; Obradors, X.; Odier, P. Metamagnetism in Single-Crystal Pr2NiO4. Phys Rev B 1993, 47,
5834-5840.
24. Fernández-Díaz, M. T.; Rodríguez-Carvajal, J.; Martínez, J. L.; Odier, P. Low temperature phase and magnetic ordering in Pr2NiO4. Physica B: Condensed Matter 1992, 180–
181, Part 1, 122-124.
25. Sullivan, J. D.; Buttrey, D. J.; Cox, D. E.; Hriljac, J. A Conventional and High-Resolution Synchrotron X-Ray-Diffraction Study of Phase Separations in Pr2NiO4+. J. Solid State Chem. 1991, 94, 337-351.
26. Le Toquin, R.; Paulus, W.; Cousson, A.; Dhalenne, G.; Revcolevschi, A. Interstitial and apical oxygen order–disorder in La2CoO4+δ observed by single-crystal neutron and X-ray
diffraction. Physica B: Condensed Matter 2004, 350, E269-E272.
27. Le Dréau, L.; Prestipino, C.; Hernandez, O.; Schefer, J.; Vaughan, G.; Paofai, S.; Perez-Mato, J. M.; Hosoya, S.; Paulus, W. Structural Modulation and Phase Transitions in La2CoO4.14
Investigated by Synchrotron X-ray and Neutron Single-Crystal Diffraction. Inorg. Chem. 2012, 51, 9789-9798.
28. Yashima, M.; Enoki, M.; Wakita, T.; Ali, R.; Matsushita, Y.; Izumi, F.; Ishihara, T. Structural Disorder and Diffusional Pathway of Oxide Ions in a Doped Pr2NiO4-Based Mixed
Conductor. J. Am. Chem. Soc. 2008, 130, 2762-2763.
29. Yashima, M.; Sirikanda, N.; Ishihara, T. Crystal Structure, Diffusion Path, and Oxygen Permeability of a Pr2NiO4-Based Mixed Conductor (Pr0.9La0.1)2(Ni0.74Cu0.21Ga0.05)O4+δ. J. Am.
Chem. Soc. 2010, 132, 2385-2392.
30. Chroneos, A.; Parfitt, D.; Kilner, J. A.; Grimes, R. W. Anisotropic oxygen diffusion in tetragonal La2NiO4+ : molecular dynamics calculations. J. Mater. Chem. 2010, 20, 266-270.
31. Buttrey, D. J.; Sullivan, J. D.; Shirane, G.; Yamada, K. Influence of oxygen nonstoichiometry on structure and magnetism in Pr2NiO4+. Phys Rev B 1990, 42, 3944-3951.
32. Paulus, W. Etude de l'intercalation et du désordre de l'oxygène dans quelques oxydes de basse dimension. Université Paris XI, Orsay, 1998.
33. Paulus, W.; Heger, G.; Rudolf, P.; Schöllhorn, R. In situ neutron diffraction studies on the electrochemical oxidation of polycrystalline La2CuO4. Physica C: Superconductivity 1994,
For Table of Contents Only. Structural evolution of (Pr/Nd)2NiO4+ electrodes was followed by
in situ neutron diffraction during electrochemical reaction, as a function of oxygen