A.B. Bowdoin College
S.M. Massachusetts Institute of
Technology
1956
1958
Submitted in Partial Fulfillment of the Requirements for the Degree of
DOCTOR OF SCIENCE from the
Massachusetts Institute of Technology November, 1960
Signature Redacted
Signature of Author Certified by Accepted by Department of Me4llurgySignature Redacted
Signature Redacted
Signature Redacted
If I,,' z WNt Chairman,Departmental Committee / Graduate Students C3WS.OF TECU 1j 0~0J UN 7 1961
LJB RAPS'
DESULFURIZATION OF VACUUM INDUCTIONMELTED HIGH STRENGTH STEEL: RELATION TO MECHANICAL PROPERTIES
by
DESULFURIZATION OF VACUUM INDUCTION MELTED HIGH STRENGTH STEEL: RELATION TO MECHANICAL PROPERTIES
Submitted to the Department of Metallurgy on November 22, 1960 in partial fulfillment of the requirements for the
Degree of Doctor of Science
ABSTRACT
A method of desulfurizing molten steel during the refining cycle of the vacuum induction melting process has been developed. This method
involves the use of a sintered layer of a CaO - 10% CaF2 mixture on the bottom of a magnesia crucible. Final sulfur concentrations of 0.004 weight per cent were consistently attained using an AISI 4300 series steel in which the carbon contentvaried from 0.40 to 0.60 weight per cent. Two grades of charge material which had initial sulfur concentrations of 0.013 and 0.020 weight per cent were used. The final sulfur contents were found to be
independent of carbon concentrations and initial sulfur concentrations. In heats melted in magnesia crucibles without the CaO - 10% CaF2 lining, limited desulfurization was obtained at low refining pressures as a result of
volatilization.
Tensile properties were determined of the sound cast metal,
heat treated to a high strength level. Data were analyzed statistically, and the analysis showed the ultimate tensile strength and per cent reduction in area could be satisfactorily estimated as linear functions of the carbon and
ii.
sulfur concentrations in the steel. The relationships are: UTS = 273,132 (%C) + 1,075,736 (%s) + 143,889 %RA = 136.82(%C) - 1355.5(%S) + 99.0
The reduction in area of desulfurized 4340 steel varied from 37.0 to 40.4 per cent at tensile strengths of 250,000 to 260,000 pounds per square inch. Tensile strengthsas high as 317,000 pounds per square
inch were obtained at higher carbon contents.
Thesis Supervisors: Merton C. Flemings, Assistant Professor of Metallurgy Howard F. Taylor, Professor of Metallurgy
iii. TABLE OF CONTENTS Section Page ABSTRACT --- i LIST OF TABLES --- v LIST OF FIGURES --- vi ACKNOWLEDGEMENTS --- vii I INTRODUCTION --- I II LITERATURE SURVEY --- 3
A Behavior of Alloying and Impurity Elements in Liquid Iron under Vacuum --- 3
B Segregation --- 7
C Formation of Sulfides --- 11
D Effect of Sulfides on Mechanical Properties --- 14
E Desulfurization Theory --- 17
III EXPERIMENTAL PROCEDURE --- 21
A General Procedure --- 21
8 Equipment --- 21
C Melting, Refining, and Casting --- 23
D Analyses and Testing --- 25
IV RESULTS AND DISCUSSION --- 26
A Desulfurization --- 26
B Control of Alloy Analysis --- 28
C Structure --- 29
D Mechanical Properties --- 30
iv.
TABLE OF CONTENTS (Cont'd.)
CONCLUSIONS
---SUGGESTIONS FOR FURTHER STUDY
---BIBLIOGRAPHY ---APPENDIX ---BIOGRAPHICAL NOTE ---Section V VI VII Page 33 35 36 59 63
V.
LIST OF TABLES
Table Number
Liquid-Vapor Distribution Coefficients
---Solid-Liquid Equilibrium Segregation
Coefficients in Liquid Iron ---Mold Material
-Heat Treatment ---Chemical Analysis ---Gas Analyses ---Treatment and Variables of Each Heat
---Results of Tensile Tests
---Page 4 9 40 41 42 43 44 45 I II III IV V VI VII VIII --- 40000000000000- 4
vi.
LIST OF FIGURES Figure
Number Pa ge
Segregation of sulfur and oxygen in solidifying liquid iron determined for initial concentrations
of 0.01 and 0.001 per cent according to Case B --- 47
2 Experimental Casting --- 48
3 Details of tensile test specimen --- 49
4 Estimated ultimate tensile strength as a function of carbon and sulfur as based on experimental data --- 50
5 Correlation of estimated ultimate tensile strengths with experimental data --- 51
6 Estimated per cent reduction in area as a function of sulfur and carbon contents based on experimental data - 52 7 Correlation of estimated per cent reduction in area with experimental data --- 53
8 Casting #11 --- 5+
9 Casting #114 --- 54
10 Casting #16 --- 55
11 Casting #26 --- 55
12 Casting #11, Air melted, 0.009 per cent sulfur --- 56
13 Casting #15, Vacuum melted, 0.020 per cent sulfur --- 56
14 Casting #16, Vacuum melted, 0.013 per cent sulfur --- 57
15 Casting #17,Vacuum melted, 0.009 per cent sulfur --- 57
vii.
ACKNOWLEDGEMENTS
The author of this thesis wishes to express his sincere appreciation to Professor Merton C. Flemings for his enthusiasm and invaluable advice in all phases of this in-vestigation. He is also indebted to Mr. Richard W. Willard for aid in preparing the statistical analyses. To all others without whose assistance this work could not have been successful, thanks are also extended.
-4
I. INTRODUCTION
Recent work has indicated that many factors have important roles in determining the tensile and impact ductilities of cast, high-strength, low-alloy steel. Variables such as micro-porosity(l), as-cast grain structure(l),
the shape and number of inclusions(2), and the relative amounts of impurities
such as sulfur and phosphorous(3,4,5) have been observed to limit these
pro-perties in air melted steel. Improvement of the ductilities of these steels by vacuum melting and refining is significant and may generally be attributed to (1) a cleaner steel due to a lower oxygen content, (2) less gas-formed micro-porosity due to a lower concentration of residual gases, and (3) a
lower concentration of impurities due to a high purity charge and the removal of substantial amounts of impurities having high vapor pressures. Present vacuum melting techniques, though, are unable to remove any great amount of sulfur(6,7). To achieve optimum quality, therefore, the initial material or charge, must be as free from sulfur as possible(6).
An effective method of desulfurization has been published which does not involve the use of a slag(8'9). Sulfur removal by this method in heats melted in air is not particularly good with respect to final sulfur contents except at high carbon levels. However, under a carbon monoxide atmosphere and by use of stronger deoxidizers such as silicon or aluminum consistent final sulfur concentrations of 0.001 weight per cent and better are reported. As excellent deoxidation under vacuum can be achieved by small amounts of
carbon alone(lOPl,12), application of this method of desulfurization to the
vacuum refining of molten steels should produce results which are equally
-2-Since significant increases in ductilities of air-melted,
high-strength, low-alloy steels have been reported to accompany desulfurization from 0.023 to 0.008 per cent(3) and from 0.010 to 0.003 per cent(5) it may also be possible to produce similar improvements in vacuum melted steels.
Should such effects occur to an appreciable extent the following possibilities present themselves:
1. The ductilities currently attained for vacuum melted steels may be further improved.
2. The sulfur content of the charge may be increased without
sacri-ficing the ductility of the product.
3. Tensile and yield strengths of the steel may be increased through
-3-II. LITERATURE SURVEY
A. Behavior of Alloying and Impurity Elements in Liquid Iron Under Vacuum. Most purification during vacuum refining results from the
distilla-tion of atoms or molecules from the liquid metal surface. The rate at which an element volatilizes from a liquid metal bath is proportional to the
partial pressure of that element. Ideally, the maximum rate of volatili-zation of a pure element is given by:
Wmax = 3.5P (M/T)1/2 (13) Eq. I
where
Wmax = gm/cm2 /sec
P = Vapor pressure of the element ( dynes/cm2 )
M = Molecular weight of the element (grams) T = Temperature (*K)
For iron at 1600*C. this equation gives a value of about 37 grams/cm2/sec which is obviously several orders of magnitude too great. This can in part
be accounted for by the following factors: (1) the supply of heat to the
surface from which the evaporation is occurring is not great enough to maintain this rate of distillation, (2) a partial pressure of gas exists
in the chamber particularly in the space above the liquid which results in a return of atoms to the liquid from the vapor, and (3) the effect of furnace
dimensions(6,13).
Fischer(O) presents a quantitative approach to distillation of impurity and alloying elements from ferrous base alloys. The behavior of various
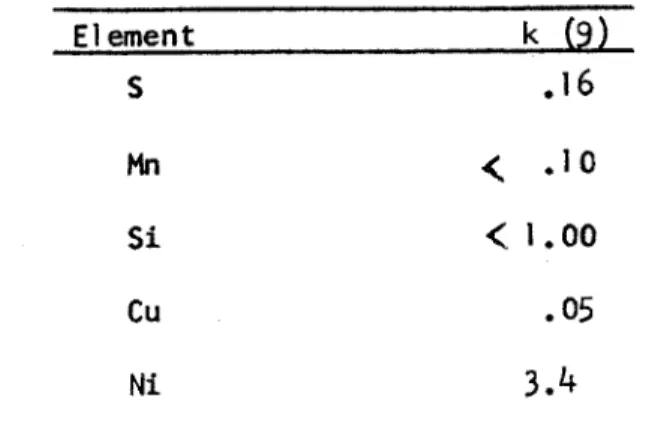
-4-of alloy or impurity element concentration in the liquid to its concentration in the vapor. Values of k for several elements in liquid iron are given in Table I. A value greater than 1 indicates that the concentration of the element will increase during refining while a value less than 1 indicates
Element k (9) S .16 Mn ( .10 Si < 1.00 Cu .05 Ni 3.4
Table I. Liquid-Vapor Distribution Coefficients (9)
the opposite. Decreasing values of k indicate an increasing tendency to volatilize. From the table it can be seen that elements such as manganese
and copper are readily distilled from liquid iron and steel.
The distillation of sulfur is not rapid enough to be of practical
commercial significance(l0,18). Additions of carbon and/or silicon increase
the rate of desulfurization of iron in vacuum. There are two likely reasons
for this; first, additions of carbon lead to the formation of carbon-sulfur
compounds which have higher vapor pressures than gaseous sulfur(lk), and second, both carbon and silicon increase the activity of sulfur in iron(l5a),
so that the vapor pressures of sulfur and carbon-sulfur compounds are
increased. This method severely limits the range of alloys, though, which can be effectively desulfurized.
With regard to the extent of desulfurization in vacuum, there is a good deal of disagreement among individual investigators. Jones states that no change in sulfur concentration is observed in samples taken at intervals during the refining period(7). Samarin on the other hand claims a fifty per cent reduction in the sulfur content of air-melted steel as a result of a ten-minute vacuum ladle treatment(16). However, in view of Fischer's work and consideration of the vapor pressures of sulfur and sulfur-carbon compounds, it must be said that some desulfurization occurs, but that the overall amount due to the direct effects of vacuum is usually slight.
Removal of Dissolved Gases: The exact mechanism of the removal of dissolved gases such as nitrogen, hydrogen, and oxygen is not precisely known(17). When present in large quantities in the charge, one of these gases may, after meltdown, exert such a large pressure that a bubble is formed. The pressure necessary to maintain such a bubble is:
P = P +oh + (18) Eq. 2
r
where
Pi = barometric pressure over melt = density of the liquid metal
h = depth of metal at level of bubble O= surface tension of liquid metal
-6-The presence of suitable nucleation sites such as discontinuities in crucible material is necessary for boiling(li,12). The importance of rapid bubbling or boiling is not only to effect the removal of those elements which initially form the bubble but the mechanical flushing and stirring effects as well.. The stirring is essential in some liquid metal systems for heat transfer, and the flushing removes gases whose partial pressures are such that they cannot form a bubble of their own. However, even under vacuum the boiling cycle is usually short-lived. This does not mean that the removal of dissolved gases ceases at that time. Further
decrease in gas content occurs, but whether this occurs as the distillation of atoms, or as molecules which are formed in the liquid, or after the atoms have escaped from the liquid is not certain.
The difference between actual residual gas content and that predicted by thermodynamics is often significant 0) A case in point is the deoxida-tion of iron by carbon. Experimentally, a limit of about 0.001% 0 is reached, which is considerably greater than the accepted equilibrium value(10,11,12).
Also, this limit is fairly constant over a large range of pressures and carbon contents(l8). This may be due to the influence of the liquid metal surface tension on the pressure required to nucleate carbon monoxide bubbles(12) unavailability of suitable nucleation sites(ll), or the pollution of the
metal with oxygen by the refractory(II>I7). The latter results in equilibrium being established between the oxygen entering the metal from the refractory
-7-B. Segregation.
In air melted steels the amount of inclusions present in the liquid
metal when it is cast is very small; many of the oxides formed when the
metal is killed are skimmed off before pouring. The majority of inclusions form during solidification and are due to segregation which enriches the liquid with impurity and alloying elements. Thus, although an oxide or sulfide might be thermodynamically unstable in the liquid when the metal is cast it may become stable during solidification when segregation occurs.
The actual composition of the liquid at the solid-liquid interface
cannot be precisely determined since it is a function of the diffusion rates of the solute in the solid and liquid alloy as well as a function of the rate
of solidification and the degree of stirring in the liquid. Also, additional
alloying elements affect segregation coefficients by different amounts.
Approximations can be made, though, by consideration of experimental evidence
and by making several assumptions, which give an indication of the concentra-tion of various elements at different stages of solidificaconcentra-tion. From this,
the time of formation of oxides and sulfides can be roughly approximated
by means of thermodynamic data and the laws of mass action.
Assuming "plane-front" solidification, the following basic types of segregation can be defined(19).
Case A: No diffusion of solute in solid or liquid. The equilibrium
segregation coefficient, ko, which is defined as the ratio at a given temperature of the equilibrium solute concentration in the solid to its equilibrium concentration in the liquid,
A
8
-holds throughout solidification. The effective segregation coefficient, k, is unity and no segregation occurs. This
case can be approached under conditions of rapid solidifi-cation and no convection in the liquid.
Case B: Perfect diffusion of solute in liquid, no diffusion of solute in solid. The effective segregation coefficient, k, equals the equilibrium segregation coefficient, ko.
Under these conditions maximum segregation occurs.
Case C: Perfect diffusion of solute in solid and liquid. Effective
segregation coefficient is ko. In this case, diffusion of solute in the solid erases the effects of segregation. This
condition can only be approached under conditions of
ex-tremely low solidification rates.
The solidification of a steel casting occurs in a dendritic manner whether it be by columnar dendrites under unidirectional solidification
conditiorsor by equiaxed dendrites which form under conditions of more equi-directional solidification. Since the previous discusson has assumed the absence of a dendritic type of freezing,its application to steel castings
becomes definitely limited. There is reason to believe, though, that Case B
can be applied with considerable accuracy to individual microscopic regions which are characterized by small, approximately plane surfaces of the dendrites growing in a linear fashion into the interdendritic liquid(20). It is also
likely that the overall freezing rate of the casting has little effect on the growth rate of this small surface since, as the overall freezing rate
- 9
-increases the dendritic surface area is increased to accommodate it. Hence,
even at strongly chilled surfaces the interdendritic segregation may not be significantly changed.
Table II gives equilibrium solid-liquid segregation coefficients for various elements in solidifying iron. These should not be confused with the experimental liquid-vapor distribution coefficients discussed in
con-nection with vacuum refining.
Element ko Al .92 C .13 Cr .95 Mn .84 0 .02 P .13 Si .66 S .02
Table II. Solid-Liquid Equilibrium Segregation Coefficients in Liquid Iron(l5a)
Figure 1 illustrates graphically the segregation of sulfur and oxygen into solidifying liquid iron as determined by Case 8 and the following equation.
In addition to the assumptions already made, it is to be further understood that ko is assumed to remain constant throughout the solidification process.
- 10
-C = CO0 (-) (ko - 1) (19) Eq. 3
where,
C = composition of the liquid at the solidifying interface ko= equilibrium segregation coefficient
Co= original concentration of the solute g = fraction of metal solidified
Also indicated in Figure I are the approximate compositions at which several of the more common inclusion phases would become thermodynamically stable in an AISI 4300 series steel which would contain 0.30 per cent silicon and 0.80 per cent manganese. Complicating factors due to the presence of other alloying elements are neglected. It is interesting to note that manganese sulfide (MnS) is not in equilibrium with the liquid metal until the metal is about 99% solid, whereas silica is always stable
except in the initial stages of solidification of a highly deoxidized steel. Although a quantitative determination of the segregation behavior of
soli-difying steel may not be justifiable here because of the assumptions made, it is worthwhile to note qualitatively some of the more important aspects.
1. Silica becomes stable at an early stage of solidification even in vacuum-melted steels containing only small amounts of oxygen.
2. Due to the low oxygen concentration in vacuum melted steels, the size and number of silicate inclusions is small.
3. Sulfide inclusions do not form until late in the solidification process. Therefore, they must be relegated to the primary grain boundaries.
It is important to remember that the segregation illustrated in Figure 1 applies only to microsegregation, and not to macrosegregation.
C. Formation of Sulfides.
The effects of various alloying elements on distribution, shape, and composition of non-metallic inclusions is extensively treated in the
literature(2,21,22) and will not be discussed in detail here. However, a brief discussion of pertinent factors concerning inclusion formation and the effects of inclusions on the mechanical properties of cast steels is necessary to emphasize the role of inclusions in limiting these properties.
Sims and Dahle have classified sulfides into three categories according to their shape(21). Subsequent investigations have brought out
the fact that the chemical composition of the steel is a major factor in determining shape of sulfides(2,22). Type I sulfides as designated by Sims and Dahle are formed as an immiscible liquid in the liquid portions of the
solidifying steel. They are composed mainly of manganese sulfide but contain substantial amounts of iron, oxygen and some silicon. Type I inclusiors are characteristically round and are usually found in unkilled steels.
Type II sulfides were first attributed to small amounts of residual aluminum(21). These sulfides occur at the primary grain boundaries as thin
212
-that these sulfides are attributable to low oxygen levels (22), resulting from deoxidation by means of small amounts of aluminum, zirconium, titanium, or other elements(22) or by carbon in vacuum(2). Apparently the removal of oxygen increases the solubility of sulfur in the liquid metal so that the
sulfides precipitate in a true eutectic fashion. The transition from Type I to Type II sulfides occurs at approximately 0.01 per cent oxygen(2). Further deoxidation does not change the Type II sulfides. This is of great importance in vacuum melted steels since the usual oxygen concentration is of the order of 0.002 per cent. This indicates that any sulfides which form will have a tendency to be of the most detrimental shape.
Type III sulfides are angular and equiaxed and occur with larger excesses of aluminum or zirconium than cause Type II inclusions. Increased
titanium on the other hand increases the severity of Type II sulfides. Several explanations of the transition of sulfides from Type II to Type III have been
published:
1. Aluminum and zirconium increase the surface tension of the precipitate so that it does not wet the primary grains, while titanium
decreases the surface tension of the sulfide(22).
2. Aluminum sulfide is less soluble than manganese sulfide so that it precipitates earlier and grows to a larger size. Titanium sulfide is very soluble in the liquid metal so that it does not precipitate until the
last metal freezes(21).
13
-steel so that manganese sulfide precipitates at an earlier stage of
solidification. This sulfide is nearly pure MnS since it has a high melting point and a crystalline shape(2).
The second explanation was proposed by Sims and Dahle in 1936(22), but has since given way to the surface tension theory(22,23). However, it is difficult to reconcile this with the third explanation since the former concerns a second phase of low melting point while the latter involves a high melting point phase.
Since the oxygen levels of vacuum melted steels make these steels particularly prone to Type II sulfide inclusions, the control of sulfur
concentrations is necessary to maintain their ductile properties. By plotting the number of sulfide inclusions against the sulfur content in AISI 4340
steel Sims found that 0.003 per cent sulfur is the upper limit at which no sulfide inclusions would be found(2). Just what effect variations in sulfur levels below 0.01 per cent have on ductilities of vacuum melted steels has heretofore been uncertain.
The presence and quantity of several alloying and impurity elements are important factors in determining the behavior of sulfur in steels. As has been already stated the absence of oxygen (sometimes accompanied by
small amounts of residual aluminum, zirconium, or titanium) is responsible for Type II inclusions. Further increases in aluminum and zirconium lead
to Type III inclusions while increased titanium aggravates the severity of the Type II sulfides. Low carbon and silicon contents decrease the tendency
- 14
-to form Type III sulfides(22). This may be due -to a decreased activity of sulfur which would delay the precipitation of sulfides until a later stage of solidification. Further increases of aluminum and zirconium sometimes result in a reversion of Type III inclusions back to Type II. These
con-siderations are further complicated by increased amounts of manganese(22)
so that the overall picture becomes quite complex. It seems evident that
there must be more than three general types of sulfide inclusions if their behavior is to be satisfactorily accounted for. This would resolve the
inconsistency concerning the Type III sulfides which has been previously mentioned. Also, some of the uncertainty over Type II inclusions may be
clarified by considering that in one case the behavior is due to low surface tension whereas the other may be the result of precipitation at the very end of the solidification process as a eutectic.
D. Effect of Sulfides on Mechanical Properties.
Present evidence suggests that inclusions are responsible for
initiating several types of fracture and for preventing attainment of
optimum ductility. Several types of inclusions are responsible for these phenomena and must be separately discussed.
The usual ductile tensile fracture of steel has a typical "cup and cone" appearance. Initial separation of the metal occurs in the interior of the specimen by a process of nucleation, growth, and eventual coalescence of small micro-cracks(24). These micro-cracks are attributed to the fracture of oxide inclusions or the separation of these inclusions from the matrix
- 15
-both important factors with regard to the behavior of the fracture. Larger inclusions are more effective stress raisers as the stresses which must be
carried by the metal adjacent to them increase as the inclusion size increases. Voids in the metal due to microporosity, prior mechanical working, or high residual stresses can also nucleate fibrous fracture but in sound unworked metal these factors are of less consequence than inclusions. As the internal
fracture surface progresses, it unites with micro-cracks already formed ahead of it. If the number of inclusions is significantly reduced more deformation
is necessary to move the fracture surface from one micro-crack to the next. In some cases, this has resulted in reduction in area values approaching 100 per cent(24,25).
The presence of film-type precipitates, such as Type II sulfides, is known to greatly reduce impact and tensile ductility(2,21). Their effect is influenced by their orientation to the applied stress. If the stress is normal to their surface, the internal fracture area is well formed after only a small amount of plastic deformation so that reduction of area and elongation values may be greatly decreased. If these inclusions are oriented parallel to the applied stress such as they are in longitudinal directions of forged or hot-rolled material or in columnar sections of castings, their projected area and influence on properties is considerably less.
Type I sulfides, because of their shape, apparently have an effect similar to oxide inclusions, but are probably less harmful, though, since they are more plastic and have a stronger bond with the metal matrix(2) Type III sulfides may be slightly more harmful because of their angularity.
_j
16
-Fatigue fracture is known to be nucleated by oxide inclusions in many cases. This is particularly true when inclusions are large and are
located at or near the metal surface(24,26). The inclusions apparently act
as stress raisers in much the same manner as notches(26),
In forged or hot-rolled material sulfides are strung out parallel
to the working direction because they are soft and plastic at the working
temperatures. Some of the more brittle oxides are fractured and also become directionally distributed. This is one factor causing the worked material to have planes of weakness parallel to the working direction.
Resultant anisotropy can be reduced by cross-rolling, reduction of sulfur
content, or by additions of cerium to the liquid metal (2). Cerium forms stable sulfides early in the solidification process which are similar to
Type I inclusions in shape and which are not easily deformed.
In order to attain, then, an optimum combination and consistency of mechanical properties, a steel must contain minimum amounts of both
sulfur and oxygen. This is particularly true at the higher strength levels. Other factors, such as microporosity and shrinkage, are also important but
the real advantages of their elimination cannot be fully realized unless the effects of inclusions are also eliminated or reduced to a minimum.
Vacuum induction melting of steel presents a mechanism whereby these optimum properties can be achieved. Improvement of vacuum melted steels over their air-melted counterparts is due to excellent deoxidation without the use of metallic deoxidizers, and the vaporization of dissolved
- 17
-gases and some impurity elements. Further desulfurization in combination with good casting design to insure completely sound metal can then be expected to produce the maximum possible ductilities at critical high
strength levels with presently known techniques. E. Desulfurization Theory.
The problem of removing sulfur from steel has been widely studied. Techniques have been developed which effectively remove sulfur from air-melted steels; these are most effective on small arc and induction air-melted heats where furnace size and atmosphere do not place strong restrictions on the changing of slags and on their compositions. Sixty per cent de-sulfurization in arc melted heats is reported by Zotos(3) using a double slag technique with final values of the order of 0.008 weight per cent sulfur. Colling and Ahearn(5) reduced sulfur to 0.003 weight per cent in small induction furnace heats.
Vacuum desulfurization presents certain difficulties which are not
encountered in air melting and which have necessitated the use of electrolytic materials to maintain satisfactory sulfur concentrations. Slags, as such, cannot be effectively employed under vacuum. Reactions of various slag components with the liquid metal would contaminate the metal. Presence of a slag cover would also hinder distillation of gases and volatile elements, and difficulties of slag removal could lead to entrapment of slag during pouring. Therefore, the use of slags in vacuum melting is not practical. The solution to this problem lies in using a refractory material for the crucible or as a crucible lining or wash which has a strong desulfurizing
- 18
-The deoxidizing power of carbon in liquid iron under vacuum produces an excellent situation for desulfurization since the oxygen is continually
removed from solution in iron by carbon, and the oxidation level of vacuum melted iron is considerably lower than it is in other processes. Therefore, in the presence of a highly basic refractory such as calcium oxide under vacuum, desulfurization might be expected to occur readily. This has not been shown to be so. The difficulty is believed to be due to the formation of a complex calcium-iron oxide which blocks the transfer of sulfur from the liquid metal into the refractory, and additions of small amounts of calcium fluoride to the crucible material apparently flux this oxide so that the desulfurization reaction can proceed(9).
The vacuum desulfurization of molten iron can be thermodynamically expressed. Assuming, 1) CaO present in excess, 2) both CaO and CaS present as pure solids, 3) no reaction or compound formation between CaO and CaS, 4) no solubility of CaO or CaS in the metal, and 5) no formation of CaC2, the reaction of carbon and sulfur with the crucible can be considered.*
This reaction can be expressed by the aquation:
Ca0(solid) + C + S = CaS(solid) + CO(gas) Eq. 4
Using data from the literature(l5ab), the standard free energy change for the reaction is -10850 calories per mole at 16000C. From this, the equilibrium constant is calculated to be:
K = Pco = 18.45 Eq. 5
ac x as
The following reaction is assumed to control the carbon monoxide partial pressure at the metal-crucible interface.
CaO(solid) + C -> Ca(gas) + CO(gas) Eq. 6
The standard free energy change of this reaction at 16000C. is + 48390
calories per mole which results in a value of 2.24 x 10-6 for K. If it is assumed that the partial pressures of Ca and CO are equal andfurthermore, that the activities of carbon, oxygen, and sulfur in a 0.4 per cent carbon steel can be replaced by their respective weight per cent concentrations, a CO partial pressure of 9.5 x 10~4 atmospheres is obtained. Substituting this value into Eq. 5 yields an equilibrium sulfur concentration of 1.3 x 10-4 per cent.
In practice the actual CO partial pressure is considerably greater as seen
by the non-equilibrium concentration of oxygen in the melt. Where a partial lining of the CaO mixture is employed in a magnesia crucible, the polluting action of the latter material may control the actual CO partial pressure.
Substitution of MgO for CaO in Eq. 6 results in a CO partial pressure of
9.35 x 10-3 atmospheres, which in turn yields an equilibrium sulfur concen-tration of 1.3 x 1,0-3 per cent.
Other methods of producing low sulfur vacuum melted steel have been attempted. One such investigation involves a process whereby a slurry of lime and water was employed as a crucible wash with good results(27).
Additions of cerium in the form of misch metal have been reported to reduce sulfur from 0.017 to 0.006 per cent, and calcium can similarly be added in the form of a calcium-iron master alloy(6). However, the rate of vaporization of these desulfurizing elements is quite violent so that a partial pressure
-- 20
-of inert gas is required, the master alloy is fairly expensive, and the reaction product remains in the liquid metal. Vacuum remelting of steel
previously desulfurized in air heats yields sulfur contents down to 0.003
per cent(4). In this case, there is apparently no desulfurization under
vacuum and the additional air melting time with special slags and deoxidation would add considerably to the cost of producing such steel.
-21
-III. EXPERIMENTAL PROCEDURE
A. General Procedure.
Vacuum melting of steels which are ordinarily air melted is only justified when certain critical requirements must be met. In the case of
low alloy steels heat treated to high strength levels, adequate ductility must be maintained. Low impurity concentrations due to a high purity charge, volatilization of some elements, and good deoxidation in vacuum refining yield a material superior to similar steels which have been air melted. These
vacuum melted steels are considerably cleaner and more ductile. In this
investigation, AlSI 4340 steel was used for two specific reasons. First,
this material is commercially produced by both air and vacuum melting processes. Secondly, several recent investigations of the effect of various factors on
the mechanical properties of this alloy have been published which provide a basis and comparison for this work.
The general plan was to produce two series of vacuum-melted castings, one series from a high purity charge and the other from a charge of commercial purity. Several heats in each series were desulfurized. Effort was made in
casting and mold design to produce metal of the highest possible soundness to eliminate microporosity as a variable.
B. Equipment.
The vacuum melted heats were carried out in a fifteen-pound vacuum induction melting unit. Several heats of this size were made but such factors
- 22
-the use of ten-pound heats for -the greater part of -the work. Standard preformed magnesia crucibles were employed for vacuum melted heats. These
were backed with pure Mg0 grain sand. Several attempts to produce
CaO-CaF2 crucibles were unsuccessful. Shrinkage incurred during sintering
and large quantities of gas in the material made these crucibles extremely
fragile. Several attempts to drive off this gas by heating under reduced pressure were not successful because the extremities of the crucible near
the induction coils could not be heated to any appreciable extent. Two heats were melted in one of these crucibles, however, These were strongly
decarburized by the water vapor which was evolved in the crucible and which bubbled continuously through the liquid metal. The rate of gas evolution was not greatly affected by a combined holding time of nine hours for the
two heats.
An alternative method which proved to be successful involved sintering of a layer of the CaO-CaF2 mixture on the bottom of a magnesia crucible. This
technique added considerable life to the crucible used in this investigation. Failure did occur finally above the level of liquid metal due to a ring of metal deposited during the boil which expanded and contracted during subsequent heats. Another advantage of this method is the fact that the sintered layer
can easily be removed and replaced by a fresh layer. This was done after each
heat at first and later after every two heats. The linings were apparently
gas-free after sintering since all the material could be raised to a high heat while under reduced pressure.
The test casting consisted of a central sprue or downgate from which projected three pairs of arms. Each arm was designed to yield one tensile
- 23
-or impact specimen. The total casting weight was fifteen pounds and could furnish up to six test specimens. However, the ten-pound heats
yielded only four test specimens. These test specimens were heavily risered and strongly chilled to promote good directional solidification and feeding.
Details of the casting are illustrated in Figure 2.
The mold material was ethyl silicate bonded mullite. The molds were fired at 1800*F. for three hours before inserting steel chills and assembling. After this, the mold was preheated to 400*F. and placed into the furnace which was immediately evacuated. No difficulties were encountered with gas evolution from the mold. One or two castings showed a few surface
depressions and pinholes but the general quality of the castings did not
warrant a higher preheating temperature.
C. Melting, Refining, and Casting.
Two series of heats were carried out in the vacuum furnace. One series consisted of electrolytic iron plus alloying additions and the other of Armco iron plus additions. The alloying additions were in the form of
ferro-alloys and were the same in each series. The electrolytic iron was first vacuum melted and cast into ingots to facilitate charging and to prevent bridging. The Armco iron was charged as squares which had been sand blasted
to remove the oxide scale. All materials were charged in the crucible except ferromanganese which was added late in the refining cycle.
After the metal and mold were in place, the furnace was evacuated and the charge preheated under low power for about thirty minutes to reduce the thermal shock to the crucible. At this point the melting cycle began
-24
under high power. When the metal began to melt, the chamber was closed off from the vacuum pumps and helium was bled into the system until the pressure was 5 cm. Hg. This procedure prevented a violent boil which had caused difficulties in earlier heats. After meltdown the pressure in the chamber was gradually reduced to maintain a steady moderate boil. This carbon boil usually ceased after several minutes at a pressure of about 200 microns Hg. Two heats were poured at this pressure after the carbon boil had subsided. The remainder of the heats were evacuated to a pressure of 1 to 5 4,tHg. and held at this pressure for twenty minutes for refining.
After the refining period, the pressure was again raised to about
5 cm. Hg. and the ferromanganese was added. The pressure was then reduced
to 100/4 Hg. and held for three minutes. At this point, helium was allowed
to bleed into the chamber and the metal was cast. The helium pressure was allowed to reach 20 cm. Hg. to decrease the possibility of gas pick-up by the metal from the mold. The chamber was not evacuated after this until the metal was completely solid.
Air melted heats were melted in eighty-pound capacity magnesia lined clay-graphite crucibles in an induction furnace. Iron, ferro-chromium, ferromolybdenum and electrolytic nickel squares were placed in the crucible. After meltdown ferrosilicon and ferrocarbon alloys were added. The temperature was then raised to 3200*F. and the ferromanganese addition made. The metal was then reladled into a second preheated magnesia lined crucible. During
this process aluminum and calcium-manganese-silicon alloy were added to deoxidize the metal. The castings were poured at 2850*F. The molding procedure was the same as was used for the vacuum melted castings.
-25-D. Analyses and Testing.
Radiographs of the unmachined test bar blanks and of 1/8 inch machined cross sections were taken to determine if there was any gross
unsoundness in the earlier heats. Chemical analyses for sulfur, phosphorus and all alloying elements were obtained for all heats. Oxygen and nitrogen analyses were carried out on several early heats, and since the results agreed well with published data for vacuum melted steels, this step was
discontinued.
Particular difficulty was encountered in attaining reproducible
carbon concentrations. This is felt to be caused by moisture in the crucible material which decreased with each successive heat after a crucible was
installed. Another factor may have been the difference in initial oxygen concentrations between the Armco and electrolytic charges.
Tensile test blanks were heat treated to develop high strength properties before being machined into tensile bars. Details of the heat treatment are presented in Table IV and the test bar design in Figure 3. The bars were pulled at room temperature in a standard tensile test machine.
Yield strength (0.2 per cent offset), ultimate tensile strength, per cent elongation, and per cent reduction in area were measured.
26
-IV. RESULTS AND DISCUSSION A. Desulfurization.
The desulfurization of heats melted in magnesia crucibles was investigated to verify data reported in the literature(9,lo,18,28) as well as to provide com-parison specimens for tensile testing (Table V lists of analyses of all heats).
One heat formulated using Armco iron (casting #15) and one heat using electrolytic iron (casting #16) were melted and refined for twenty minutes at a pressure of
200,4L- Hg. The final sulfur concentrations of these castings agreed well with
the estimated sulfur content of the two initial charge' indicating essentially
no desulfurization took place (as was anticipated). The final analyses of the
two heats were 0.020 per cent for the Armco heat and 0.013 per cent for the electrolytic material; estimates of amount of desulfurization obtained in sub-sequent heats were made using these figures as reference points.
Heats #17, #24, and #25 are the remaining ones in the control series. Desulfurization of these heats at refining pressures of I - 5,,- Hg. for one-half hour ranged from thirty to slightly over fifty per cent. Minimum sulfur
concentrations of 0.010 per cent and 0.006 per cent were found for the Armco and electrolytic based heats respectively. These results agree well with those of
Fischer and indicate that the mechanism of desulfurization involved in this case
is the distillation of sulfur and carbon-sulfur compounds( 0). The final sulfur
content of 0.006 per cent, obtained for the heat formulated with electrolytic iron, is in excellent agreement with sulfur contents obtained commercially in vacuum melted 4340 steel, using an electrolytic charge(28).
The remainder of the vacuum melted heats in this work were desulfurized
- 27
-crucible. The final sulfur concentrations of all heats were 0.003 to 0.005 per cent after thirty minutes of refining at 1 - 5 4,. Hg. The majority of
values were 0.004 per cent, and the rest were within the experimental error of the analyses. Differences in initial sulfur contents resulting from use of electrolytic iron for some heats and Armco iron for others apparently had no effect on the final sulfur content.
Although significant desulfurization was obtained, this was not as great as would be expected from previous work with CaO - CaF2 crucibles under protective atmospheres and air(8'g). Nor did the final values approach
closely the calculated equilibrium value of 0.0002 per cent. Lower resultant concentrations would be expected if the area of the lime mixture in contact with the melt were increased and if the magnesia, which is less stable and may therefore oxidize the liquid metal at a greater rate than lime, were
removed from the system. However, even with these modifications, it is not likely that sulfur concentrations below 0.001 per cent could be attained with refining times of reasonable length.
Another approach which might be more profitable in obtaining still lower sulfur concentrations would be the use of greater amounts of silicon or other strong deoxidizers to reduce the available oxygen content of the
bath to below the levels obtained by carbon deoxidation. Agitation of the bath or flushing with an inert gas makes carbon a more effective deoxidizer in vacuum refining and thus might make the desulfurization process more
efficient. It is doubtful that these considerations would be of major practical significance, though, because the economic factors involved would probably not
28
-justify the small increase in purity.
It is not likely that further desulfurization would occur in the system investigated with moderate increases in refining times. The time required for completion of the reaction under similar conditions is ex-tremely rapid(8>9). The sulfur content of melts over one hundred pounds
has been shown to reach a constant value in a matter of five minutes(9).
B. Control of Alloy Analysis.
The chemical analyses of all heats are recorded in Table V. The difficulty in obtaining reproducible carbon concentrations is evident from the data. This is felt to be the result of the variation of the quantity
of chemically combined moisture in the refractory materials. The concentration of carbon was found to increase with each successive casting when the initial carbon content was held constant. This indicates that with each heat more of this moisture is driven off so that its decarburizing effect on the melt
decreases.
Control of other alloying elements was good with the exception of
manganese in some instances. Manganese is inherently difficult to control
because of its volatility. Gas analyses of several preliminary heats were
obtained and are presented in Table VI. They are in good agreement with
published data(10,11,12,17,18). These analyses were then discontinued as there seemed to be no reason to suspect any substantial variation in
-29-C. Structure.
Microradiographic analyses of several castings were carried out (1) according to the method developed by Uram, Flemings, and Taylor . In all cases, the observed microporosity was less than 0.2 per cent as
determined by this method which indicated that the castings were structurally sound.
It was hoped to maintain a columnar primary grain structure in all castings to minimize any differences due to structural variation. However,
the structure is apparently quite sensitive to pouring temperature and possibly to carbon content. Thus the primary grain structure varied from completely columnar to completely equiaxed with several castings which exhibited intermediate structures. Representative macrostructures are
illustrated in Figures 8 - 11. As no microporosity could be observed in either the columnar or equiaxed castings, the effect of this variation on the mechanical properties is presumably slight.
Polished sections of several castings were examined to determine the quantity and types of inclusions present. Figures 12 - 16 are photo-micrographs taken from some of these castings. At sulfur concentrations
greater than 0.010 per cent, Type II sulfide inclusions were found. This was expected as it was pointed out in Section IIC that vacuum melted steels are particularly prone to the formation of these film-like inclusions. The
number and severity of these sulfides increased with increasing sulfur
concentrations. In the castings containing less than 0.010 per cent sulfur, only spherical inclusions were observed. There was no distinguishable
- 30
-difference in either the number, size, or shape of the inclusions observed in this material. In view of the theoretical considerations discussed in Sections IIB and IIC, it is believed that these inclusions are nearly pure silica.
D. Mechanical Properties.
The results of the tensile testing are recorded in Table VIII. As the material tested covered a range of carbon and sulfur concentrations, it was desirable to analyze the data by statistical procedures. A method of least squares was used and equations were developed which estimated the per cent reduction in area and the ultimate tensile strength in terms of the two independent variables, carbon and sulfur. These equations define planes which relate the three variables, carbon, sulfur, and either tensile strength or per cent reduction in area. By holding carbon or sulfur constant, the expected value of these properties can be plotted against either variable. This is equivalent to intersecting the plane defined by the equation by a second plane. Thus, straight line relationships must be obtained. Plots of the deviations of experimental data from the estimated values were
con-structed for both the ultimate tensile strength and the per cent reduction in area against both carbon and sulfur. These plots all showed only random scatter and did not detect any apparent relationships other than linear.
The data from several bars were not used in the statistical analyses. The air melted heat, casting #11, was not included as this would introduce a fourth variable. Three other bars were apparently defective and were not included for this reason.
- 31
-It must be emphasized that the approximations of the data by straight line relationships is limited only to the ranges of compositions studied. These relations may, and in some instances must, break down outside these ranges. For instance, negative values of reduction in area are not possible and so the straight lines of Figure 6 cannot continue below the abscissa.
A determination of the effect of carbon on the tensile strength was carried out for carbon concentrations ranging from 0.40 to 0.60 per cent. The following equation relating the estimated tensile strength to the con-centrations of carbon and sulfur was derived. An outline of this derivation can be found in Appendix I.
UTS(est) = 273132(%C) + 1075736(%5) + 143889
Plots of the estimated tensile strength with respect to carbon and sulfur are illustrated in Figure 4. As is indicated in the above equation and in Figure 4, the sulfur content has a small positive effect on the tensile strength. The agreement of the estimated tensile strength with the experimental data is extremely good, as is shown in Figure 5. Statistically, carbon alone is associated with 76 per cent of the variance of the tensile strength. Carbon and sulfur together are associated with 82 per cent of the variability. Therefore, only 18 percent is attributable to random variables and variables such as grain structure, microporosity, and tensile testing.
The effect of sulfur may possibly be a consequence of the least squares procedure coupled with this particular experiment. Repeated controlled
- 32
-An equation relating carbon and sulfur concentrations to the per cent reduction in area was similarly determined.
/RA(est) = -136.82(%C) - 1355.5 (%S) + 99.0
This is plotted in Figure 6 against per cent sulfur for several carbon concentrations. Both carbon and sulfur are shown to have pronounced effects on the reduction in area. There is more scatter in this data than was found for the tensile strength relation as is evident in Figure 7. Here the carbon and sulfur together can be related to 68 per cent of the variance in the reduction in area values. Forty per cent can be attributed to the carbon alone. Thus, 32 per cent must be accounted for by other variables.
Yield strengths, as measured by the 0.2 per cent offset method, were determined and are included in Table VIII. No marked correlation with either carbon or sulfur content or with tensile strength was observed.
This agrees with published data concerning air melted 4300 series steels(28).
Elongation values were measured as the per cent elongation in one inch. These varied from a low of 3.0 per cent to a high of 13.5 per cent and were more or less proportional to the reduction in area values in that
high values for the latter were accompanied by high elongations. Because of the range of the reduction in area values, this measurement was felt
to be more sensitive in indicating the effects of chemical variables on tensile strength and tensile ductility.
33
-V. CONCLUSIONS
1. A study of the desulfurization of vacuum induction melted high strength low alloy steel has been conducted. The relation of this desulfurization
to the mechanical properties of vacuum melted AISI 4300 series cast steel
has also been investigated.
2. In a series of heats melted in magnesia crucibles, desulfurization was negligible at a refining pressure of 200 microns, but at I - 5 microns
sulfur contents were reduced 30 - 50 per cent. In one heat, sulfur was reduced from 0.020 to 0.010 per cent; in another, sulfur was reduced 0.013 to 0.006 per-cent (both after refining at I - 5 microns pressure). These sulfur reductions were attributed to distillation of sulfur and carbon-sul fur compounds.
3. A sintered layer of a CaO - 10% CaF2 mixture on the base of a magnesia
crucible was found to result in significantly greater desulfurization compared ;qith that obtained in magnesia crucibles alone. An additional
advantage of this sintered layer is that it lengthens the crucible life
and does not necessitate any change in normal melting and refining practices.
4. In a series of heats conducted in the partially lined magnesia crucibles
consistent residual sulfur concentrations of 0.003 to 0.005 per cent were
obtained. The final sulfur content was independent of the initial sulfur (in the range 0.013 to 0.020 per cent) and carbon content (in the range
0.40 to 0.60 per cent). The desulfurization obtained was attributed primarily to a crucible-metal reaction.
5. Ultimate tensile strengths up to 317,000 psi were obtained in the de-sulfurized vacuum melted metal. All test bars from fully desulfurized heats containing up to 0.55 per cent carbon exhibited ductile fractures.
34
-6. Values of reduction in area for desulfurized 4340 specimens ranged from a low of 37 per cent to a high of 40.7 per cent. Elongation of the same
specimens ranged from 10.0 to 12.5 per cent in the one inch gauge length. 7. Type II sulfide inclusions were observed in castings containing more than
0.010 per cent sulfur. Silicates were present in all castings and Type III sulfides were found in all but the desulfurized castings. Size, type, and distribution of these inclusions are a major factor in determing the duc-tility at a given strength level.
8. The test castings were completely sound when examined by microradiography; hence microporosity is not believed to be a significant factor affecting
mechanical properties.
9. Statistical analysis of the tensile test data showed that ultimate tensile strength and reduction in area of the castings heat treated to high strength levels can be linearly expressed in terms of carbon and sulfur contents within the composition limits of this investigation.
10. The per cent reduction in area of the material investigated can be estimated by the following equation:
%ORA(est) = -136.82(%C) - 1355.5(%S) + 99.0
This indicates that a linear decrease in the per cent reduction in area results from increases in either carbon or sulfur concentrations.
11. The ultimate tensile strength of these steels can be estimated as a linear function of the carbon and sulfur concentrations:
1. It has been pointed out that the straight line relationships determined in this investigation are valid only within the composition limits studied. Future determinations of the tensile strengths and reductions
in area for compositions outside of these limits would give more
insight into the behavior of the 4300 series low alloy high strength steels. This would provide a basis for the determination of the
non-linear relationships which exist between these two properties and the concentrations of carbon and sulfur.
2. A study of the effects of desulfurization on the impact strength of 4300 series steels at various carbon concentrations would be worthwhile.
3. An investigation of the mechanical properties of desulfurized vacuum melted steel as a function of the quality of the charge material should
be made to determine the feasibility of producing these steels from lower cost materials.
35
36
-VII. BIBLIOGRAPHY
1. Uram, S. Z., Flemings, M. C., Taylor, H. F., "High Strength Cast Steel Structure and Microporosity-Effect on Mechanical Properties", Modern Castings, 18, 1, 1960.
2. Sims, C. E., "The Nonmetallic Constituents of Steel", Trans. A.I.M.E.,
215, 1959, pp. 367-394.
3. Zotos, J., "Effect of Phosphorus and Sulfur Content on the Ductility and Toughness of Cast Low Alloy Steels", Electric Furnace Steel Proceedings, A.I.M.E., jj5 1958, p. 274.
4. Schaffer, P. S., Flemings, M. C., Taylor, H. F., "Vacuum Induction Melting of High Strength Steels", Modern Castings, 18, 4, 1960.
5. Colling, D. A., Ahearn, P. J., "Desulfurization by Calcium Inoculation
Improves Properties of Cast Steel". Presented to A.F.S. Casting Congress,
May, 1960.
6. Askoy, A. M., "Thermodynamics and Kinetics in Vacuum Induction Melting", Vacuum Metallurgy, Chapter 4, Reinhold Publishing Corporation, New York,
1958.
7. Jones, W. E., "Induction Melting of Ingot Products", Vacuum Metallurgy Chapter 12, Reinhold Publishing Corporation, New York, 1958.
8. Fischer, W. A., Engelbrecht, H., "Die Gleichzeitige Entschwefelung und Desoxydation von Stahlschmelzen", Stahl und Eisen, Z5, 1955, pp. 70-75.
- 37
-9. Fischer, W. A., Cohnen, T., "Der Einfluss des Kohlenstoffgehaltes auf die Entschwefelung von Eisenschmelzen durch einen Kalk-Flusspat-Tiegel im Hochfrequenzofen", Archiv fur das Eisenhuttenwesen, 21, pp. 355-365, 1950.
10. Fischer, W. A., "Die Metallurgie des Eisens im Hochvakuum", Archiv fur das Eisenhuttenwesen, 31, 1960, pp. 1-9.
11. Bennett, G.H.J., Protheroe, H. T., Ward, R. G., "The Role of Carbon as a Deoxidizing Agent in the Production of Vacuum-Melted Steel", J.I.S.I,
195, 1960, pp. 174-180.
12. Samarin, A. M., "Deoxidation of Steel in Vacuum", Vacuum Metallurgy, Reinhold Publishing Corporation, New York, 1958.
13. St. Clair, H. W., "Distillation of Metals under Reduced Pressure", Vacuum Metallurgy, Reinhold Publishing Corporation, New York, 1958. 14. Fincham, C.J.B., Bergman, R. A., "Thermo-dynamic Properties of C.S. and Solutions of Sulfur in Carbon-Saturated Liquid Iron", J. Metals, 2, 1957, pp. 690-694.
15. Basic Open Hearth Steelmaking, A.I.M.E., 1951.
a. Chipman, J., "Physical Chemistry of Liquid Steel", Chapter 16. b. Chipman, J., "Physical Chemistry of High Temperature Reactions",
Chapter 14.
16. Samarin, A. M., "Effect of Liquid Steel Treatment in Vacuum on the
Composition and Properties of Some Steels", Vacuum Metallurgy, Chapter 17, Reinhold Publishing Corporation, New York, 1958.
-38-17. Machlin, E. S., "Kinetics of Vacuum Induction Refining-Theory , Trans. A.I.M.E., 218, 1960, PP. 314-326.
18. Fischer, W. A., Hoffmann, A., "Verhalten von Eisen - und Stahlschmelzen im Hochvakuum", Archiv fur das Eisenhuttenwesen, 2, 1958, p. 339.
19. Pfann, W. G., "Redistribution of Solute during Freezing", Liquid Metals and Solidification, A.S.M., 1958.
20. Brown, P. E., "Mass Transport in Dendritic Solidification", Sc.D.
Thesis, M.I.T., 1960.
21. Sims, C. E., Dahle, F. B., "Effect of Aluminum of the Properties of
Medium-Carbon Cast Steel", A.F.A. Trans., 46, 1938, pp. 65-132.
22. Sims, C. E., Saller, H. A., Boulger, F. W., "Effects of Various
Deoxidizers on the Structures of Sulphide Inclusions", A.F.S. Trans.,
Q,
1949, pp. 233-248.23. Smith, C. S., "Grains, Phases, and Interfaces; An Interpretation of Microstructure", A.I.M.E. Metals Technology, T.P. 2387, 1948.
24. Crussard, C., Plateau, J., Tamkankar, R., Henry, G., and Lagennesse, D., "A Comparison of Ductile and Fatigue Fractures", Fracture Technology Press and J. Wiley & Sons, 1959, pp. 524-558.
25. Cottrell, A. H., "Theoretical Aspects of Fracture", Fracture
39
-26. Cummings, H. N., Stulen, F. B., Shulte, W. C., "Relation of Inclusions to the Fatigue Properties of SAE 4340 Steel", A.S.M. Trans., 4_, 1957,
pp. 482-512.
27. Ward, R. G., Hall, R., "Desulfurization of Molten Steel by Solid Lime", J.I.S.I., jj, 1960, pp. 75-78.
28. Larson, H. R., Herlihy, F. B., "Development of Low Alloy Steel Compositions Suitable for High Strength Steel Castings", W.A.D.C. Technical Report 59-63, June, 1959.
- 40
-TABLE III MOLD MATERIAL
Ethyl -Sil icate Solution
Ethyl-silicate 52.5%
Ethyl -al cohol 42%
Water 5%
HC1 (conc.) 0.5%
All per cents by volume.
Molding Slurry
1000 cc. Ethyl-silicate Solution 5 lbs. #100 Mullite Refractory
9.3 grams MgO powder
-41
TABLE IV HEAT TREATMENT
22000F* 2 hours air cool**
1750*F* 2 hours air cool
1600*F 2 hours furnace cool to 1450*F
1450*F 3 hours oil quench
400*F 2 hours water quench
400*F 2 hours water quench
* Carried out in argon atmosphere.