Bulletin of Entomological Research (1998) 88, 149-163 149
Evidence for the occurrence of sibling
species in Eubazus spp. (Hymenoptera:
Braconidae), parasitoids of Pissodes spp.
weevils (Coleoptera: Curculionidae)
M. Kenis
1* and NJ. Mills
2'International Institute of Biological Control, European Station, Chemin
des Grillons 1, 2800 Delemont, Switzerland:
2Insect Biology, Wellman
Hall, Department of Environmental Science, Policy and Management,
University of California, Berkeley, CA 94720-3112, USA
Abstract
Comparative studies were made on three presumed sibling species of the
genus Eubazus, parasitoids of European Pissodes spp. weevils, to clarify their
taxonomy and define diagnostic characters. Several populations of £.
semirugo-sus (Nees), E. robustus (Ratzeburg) and Eubazus sp. were compared with respect
to their morphology (mainly through morphometric analyses), fecundity,
isoenzyme patterns and host preference. Crosses were made to assess the
genetic and behavioural compatibility of the populations. In addition, the North
American E. crassigaster (Provancher), a parasitoid of Pissodes strobi (Peck), was
compared to E. semirugosus, a species selected for introduction against P. strobi
in Canada. The ratio of the length of the ovipositor sheath to the fore wing
length was the most discriminating morphometric variable, but discriminant
analyses including several measurements were needed to completely separate
European species. A canonical discriminant function provided a total separation
between males of E. crassigaster and E. semirugosus, but not between females.
Eubazus crassigaster and E. semirugosus were totally separated by the banding
pattern of the enzyme phosphogluconate dehydrogenase whereas hexokinase
and esterase provided a diagnostic separation between Eubazus sp. and E.
robustus. Eubazus sp. differed from all the other species by having a greater
number of ovarioles and, consequently, a higher potential fecundity. In a
two-choice oviposition test, E. semirugosus and Eubazus sp. showed a significant
preference for their natural host, P. castaneus De Geer and P. piceae (Illiger),
respectively. A similar test made with their progenies reared under standard
conditions showed that the difference in host preference was genetically fixed.
Males and females of different species did not mate readily, in contrast to
individuals from the same species. All attempts to interbreed E. robustus
and Eubazus sp. failed, but a few crosses between E. semirugosus and the
two other European species produced fertile offspring. These observations
strongly suggest that the complex of Eubazus spp. parasitoids attacking Pissodes
spp. in Europe is composed of at least three sibling species, two of which
appear to have specialized on distinct host species that occupy exclusive
microhabitats.
*Fax: 41 32 422 4824 E-mail: m.kenis@cabi.org
150 M. Kenis and N.J. Mills Introduction
There is general agreement as to the importance of biosystematic studies on natural enemies in classical biological control (e.g. Caltagirone, 1985; Gauld, 1986; Powell & Walton, 1989; DeBach & Rosen, 1991). Selecting the most appropriate species or biotype for introduction against a pest is often considered an essential component in a biological control programme (Diehl & Bush, 1984; Ehler 1990; Hopper et al, 1993). Unfortunately, parasitic Hymenoptera, the most frequently used biological control agents, are among the most difficult insects to identify and classify. Even in the well-studied western Palaearctic region, many groups of parasitic Hymenoptera need taxonomic revision, as some described species represent complexes of sibling or sister species while others, on further examination, lead to synonomies. Because of the frequent occurrence of sibling species and intraspecific variability, classical morphological criteria are often insuffi-cient to clarify the taxonomy of parasitic Hymenoptera, and additional observations on quantitative morphometrics and comparative studies on genetic, ecological and behavioural differences are needed (Gauld, 1986; Powell & Walton, 1989).
Species of Eubazus (Hymenoptera: Braconidae) are egg-prepupal parasitoids of Pissodes spp. weevils (Coleoptera: Curculionidae), whose larvae live in trunks and cones of conifers. In Europe, there has been some confusion in the taxonomy of this parasitoid genus. In the past, several names were used for parasitoids of Pissodes spp.: semirugosus Nees, atricornis Ratzeburg, firmus Ratzeburg, robustus Ratzeburg and mucronatus Thunberg. These have been included in the genera AUodorus Foerster, Brachistes Wesmael, Calyptus Haliday, Eubadizon Nees, and Sigalphus Latreille (Ratzeburg, 1844, 1848; Marshall, 1888, 1891; Laidlaw, 1933; Fahringer, 1934). The more recent literature, however, has considered that only a single parasitoid species of this group attacks all Pissodes hosts in Europe (Haeselbarth, 1962; Annila, 1975; Roques, 1975; Alauzet, 1982; Mills & Fisher, 1986), but there is no agreement on either the generic or specific nomenclature. In a recent revision of the genera of the subtribe Brachistina, van Achterberg (1990) collapsed the former genera AUodorus, Brachistes, Calyptus and Eubazus Nees to sub-genera of the genus Eubazus s.l. According to his key, all the Eubazus species parasitizing Pissodes hosts belong to the sub-genus AUodorus. This genus, however, is still in need of revision, which is currently being undertaken by C. van Achterberg, Leiden. The Eubazus spp., parasitoids of Pissodes spp., will be revised in a forthcoming paper by C. van Acterberg and M. Kenis.
Recently, parasitoids of European Pissodes spp. were surveyed (Mills & Fisher, 1986; Kenis & Mills, 1994) as part of a biological control programme against the white pine weevil, Pissodes strobi (Peck), a native pest of pines and spruces in North America. For each of the five host species investigated, a Eubazus species was found to be the most abundant parasitoid. It quickly appeared from morphologi-cal and ecologimorphologi-cal observations that several species were probably involved. Until further investigations on the taxonomy of the genus are made, C. van Achterberg (personal communication) proposed separation of the European Eubazus parasitoids of Pissodes hosts into three
species living in different microhabitats (Kenis & Mills, 1994). The species attacking P. validirostris (Sahlberg) in pine cones is considered to be E. robustus (Ratzeburg). E. semirugosus (Nees) (= atricornis Ratzeburg) is supposed to be the species parasitizing P. castaneus De Geer (= notatus (Fabricius).), P. pini (Linnaeus.) and P. piniphilus (Herbst) in pine trunks, while the species attacking P. piceae (Illiger) in fir trunks is regarded as an undescribed species, to be described in van Achterberg and Kenis (in preparation). Lovaszy (1941) has also reported a Eubazus species from the rare P. harcyniae (Herbst) in spruce trunks, but the identity of the parasitoid remains uncertain.
We have previously reported differences in the phe-nology of the Eubazus populations, reared under standard conditions, which support the separation of three distinct species (Kenis et al., 1996). This study also identified a high altitude biotype of E. semirugosus, differing from other Eubazus populations by having an obligatory diapause, as the best candidate for introduction against P. strobi in North America due to synchronization of its phenology with that of the target host. A similar diapausing biotype of £. robustus was also found during this study but it is not yet clear whether these mountain, diapausing, populations belong to the same species as the lowland, non-diapausing populations.
Here we present comparative observations on the morphology, fecundity, isoenzyme composition, host pref-erence and cross-matings of populations of E. semirugosus, E. robustus and Eubazus sp. When possible, investigations also included Eubazus ( = AUodorus) crassigaster Provancher, a North American parasitoid of P. strobi, and Eubazus parasitoids from P. harcyniae. Eubazus crassigaster is mor-phologically very similar to the European species and, as E. semirugosus is likely to be introduced into North America for the control of P. strobi, it is of primary importance to define a clear method to distinguish between the European and the American species.
Material and methods Collection and rearing
Several populations of Pissodes hosts and their respective Eubazus parasitoids were obtained from field collected material as detailed in Kenis & Mills (1994). Eubazus semirugosus was reared from P. castaneus, P. pini and P. piniphilus in pine trunks, E. robustus was obtained from P. validirostris in pine cones, and Eubazus sp. was reared from P. piceae in fir trunks. Two populations of a Eubazus species emerged from P. castaneus and P. piniphilus were suspected to be E. robustus because they emerged later from their hosts and they showed a longer development time than E. semirugosus when reared on P. castaneus in the laboratory (Kenis et al, 1996). Eubazus crassigaster was reared from Sitka spruce leaders in British Columbia, Canada, by M.A. Hulme, Pacific Forestry Centre, Victoria, British Columbia, Canada and sent as adults to the European Station of the International Institute of Biological Control at Delemont, Switzerland. In addition, 15 pinned females of a Eubazus species obtained from P. harcyniae in spruce trunks were received from W. Grodzky, Forest Research Institute, Krakow (Poland), and added to our morphometric analyses. Site and collection characteristics are described in table 1.
Occurrence of sibling species in Eubazus spp. 151
able 1. Characteristics of the collections sites, length of the ovipositor sheath (OVIP) and ratio of length of the ovipositor sheath to fore ing length (OVIP/WL) measured in females of Eubazus spp. from different populations.
jurce host Site (region, country1, elevation) Host tree
N
Measurements + S.E. (min-max) OVIP (10-2 mm) OVIP/WL (xlOOO) semmigosus Pissodes castaneus P. P. P. P. P. P. P. P. P. P. castaneus castaneus castaneus castaneus castaneus pini pini pini pini piniphilus . robustus P. validirostris P. P. P. P. P. validirostris validirostris validirostris validirostris validirostris ubazus sp. P. piceae P. P. piceae piceae . crassigaster P. strobi P. strobi Jncertain species P. harcyniae P. P. piniphilus castaneus Beaumotte (Haute-Saone, F, 300 m) Fontainebleau (Seine-et-Marne, F, 100 m) Lorris (Loret, F. 100 m) Paimpont (Ille-et-Vilaine, F, 150 m) De Koog (Texel Isl., NL, 10 m) Thetford (Norfolk, GB, 50 m) Delemont (Jura, CH, 500 m) Zernez2 (Graubunden, CH, 1900 m) Val-d'Ajol (Vosges, F, 550 m) Brusson2 (Valle D'Aosta, I, 1500 m) Grissheim (Baden-Wuttemberg, D, 200 m) St-Crepin (Hautes-Alpes, F, 900 m) Fontainebleau (Seine-et-Marne, F, 100 m) Nevache2 (Hautes-Alpes, F, 1700 m) Leuk (Valais, CH, 900 m) Olivet (Loiret, F, 100 m)
Sacele (Transilvania, ROM, 700 m)
Val-d'Ajol (Vosges, F, 550 m) Leuk-Albinen (Valais, CH, 1250 m) Delemont (Jura, CH, 500 m)
Port McNeill (Vancouver Isl., CAN, 20 m) Fair Harbour (Vancouver Isl., CAN, 20 m)
Szklarska Poreba (Sudety Mts., POL, 800 m) Delemont1 (Jura, CH, 500 m) Fontainebleau3 (Seine-et-Marne, F, 100 m) Pinus sylvestris P. sylvestris P. nigra P. sylvestris P. nigra P. sylvestris P. sylvestris P. uncinata P. sylvestris P. sylvestris P. sylvestris P. sylvestris P. sylvestris P. uncinata P. sylvestris P. nigra P. sylvestris Abies alba A. alba A. alba Picea sitchensis P. sitchensis P. abies Pinus sylvestris P. sylvestris 20 20 20 20 20 9 20 20 13 12 20 20 20 20 20 20 20 20 20 5 20 20 15 20 9 194 + 2 e,f,g (170-206) 177 + 4 c,d,e (154-218) 186 + 2 c,d,e,f (161-204) 170 + 3 b,c (139-197) 199 ± 2 f,g (182-214) 188 + 5 (163-202) 183 + 3 c,d,e,f (156-199) 199 + 3 f,g (180-221) 188 ± 4 c,d,e,f,g (156-204) 210 + 3 g (197-230) 188 + 3 c,d,e,f,g (149-206) 144 + 2 a (130-161) 148 + 1 a (139-156) 146 + 2 a (120-161) 145 ± 1 a (134-151) 152 + 1 a,b (144-163) 145 + 1 a (139-154) 271 + 3 h (249-290) 260 + 3 h (232-278) 271 + 5 (255-284) 171 + 3 b,c,d (139-192) 178 + 2 c,d,e (156-192) 192 + 4 d,e,f,g (168-223) 141 + 1 a (130-156) 153 + 1 (149-156) 459 + 5 b (424-520) 470 + 4 b (432-507) 454 + 5 b (414^88) 457 + 7 b (399-503) 457 ± 4 b (430-486) 475 + 9 (440-511) 466 + 5 b (414-503) 461 ± 5 b (409-196) 456 + 7 b (423-508) 461 ± 7 b (430-509) 461 + 6 b (402-521) 376 + 5 a (334^39) 370 + 3 a (342-390) 366 ± 5 a (300-114) 373 ± 3 a (345-407) 380 + 3 a (352^09) 378 + 4 a (350-120) 540 ± 5 c (490-571) 526 + 4 c (500-554) 536 + 8 (517-557) 437 + 4 b (409-178) 443 ± 4 b (418^85) 464 + 9 b (394-522) 387 + 4 a (357^14) 383 + 6 (358^08)
CAN, Canada; CH, Switzerland; D, Germany; F, France; GB, Great Britain; I, Italy; NL, The Netherlands; POL, Poland; ROM, Romania. Diapausing biotypes of E. semirugosus and E. robustus.
Blow developing populations.
Iqual letters in columns indicate homogeneous groups at 0.05 significance level, Scheffe multiple range test. Sample sizes of less than 0 were not tested statistically.
152 M. Kenis and NJ. Mills
Table 2. Characters selected for measurements on 20 males (M) and/or 20 females (F) of 17 Eubazus spp. populations. Abbreviation AL3 MSH MSL OVIP T2 T3 T4 T5-7 SI S2 FL FW TL TW BTL BTW WL W3SR HH OOL POL Measurement
Length of 3rd antennal segment Mesosoma height
Mesosoma length
Length of ovipositor sheath Length of 2nd tergite Length of 3rd tergite
Apparent length of 4th tergite in dorsal view Apparent length of tergites 5 to 7 in dorsal view Length of 1st abdominal suture
Length of 2nd abdominal suture Length of hind femur
Width of hind femur Length of hind tibia Width of hind tibia Length of hind basitarsus Width of hind basitarsus Fore wing length
Vein 3-SR + SRI of fore wing Height of head Ocullar-ocellar line Postocellar line Sex M M F M F F M F M F M M M F M M F F M F F M F F M F M M M F M F
Unless otherwise mentioned, all parasitoid material used in this study emerged from field collected material.
Eubazus parasitoids and P. castaneus were reared in the laboratory as described in Kenis (1994). Pissodes piceae was reared using the same methods as for P. castaneus, except that the rearing host was silver fir, Abies alba, and adults were collected in the field rather than reared from collected trunks.
Morphometric analyses
A preliminary analysis was made of 43 morphometric parameters from five male and five female specimens from five Eubazus populations: E. semirugosus from P. castaneus (collected at Beaumotte) and from P. pint (Zernez), E. robustus from P. validirostris (St.-Crepin), Eubazus sp. from P. piceae (Val-d'Ajol), and E. crassigaster from P. strobi (Port McNeill). The morphometric parameters were selected as those most commonly used in taxonomic studies of the Braconidae and were measured according to van Achterberg (1988), except the ocular-ocellar line which we defined as the shortest distance between the posterior ocellus and eyes.
Although commonly used in insect morphometric analyses, ratios are sometimes criticized (e.g. Reyment et al., 1984). However, as insect parasitoids vary in absolute size according to the quality of the host species (e.g. Kishi, 1970; Mendel, 1986), all 43 morphometric measurements were divided by the hind femur length and the fore wing length to provide two sets of ratios for preliminary analysis. Additionally, ratios commonly used in braconid taxonomy were calculated (e.g. van Achterberg, 1990). All ratios were analysed using a one-way ANOVA. Measurements of ratios that exhibited a significant level of variation between Eubazus spp. populations (P<0.01) were selected for further study (table 2). Each of these characters were measured on 20 male and 20 female parasitoids from 17 Eubazus populations. The same ratios were calculated and analysed using a one-way ANOVA. Finally, the most discriminating ratios were measured on an additional eight Eubazus populations. When
ratios failed to provide a complete separation between Eubazus spp., discriminant analyses were performed on the individual measurements and morphometric characters were compounded into canonical discriminant functions using the SPSS (1993) procedure.
Five populations, one of each of the four presumed Eubazus spp. (three Palaearctic and one Nearctic) plus a population of the diapausing biotype of E. semirugosus, were reared on P. castaneus for one generation and specimens of the Fl generation were compared using morphometric measurements found most discriminating in the parent generation.
In addition to morphometric analyses, Eubazus para-sitoids were carefully examined for other non-morphometric taxonomic characters.
Specimens of Eubazus spp. used in this study are maintained in the collections of the IIBC European Station in Delemont, Switzerland, and the Nationaal Natuurhistorisch Museum in Leiden, The Netherlands.
Fecundity
The potential fecundity of the Eubazus parasitoids was compared by counting the number of ovarioles per female, a trait which has been shown to be closely correlated with the number of eggs available for oviposition (Price, 1975). Between 5 and 41 females from several populations of E. semirugosus, E. robustus, Eubazus sp. and E. crassigaster were dissected and the number of ovarioles was counted. For each female, the hind femur length was measured as an indication of adult size.
Isoenzyme analyses
Six Eubazus populations were compared using isoenzyme electrophoresis: E. semirugosus from P. pini collected at Delemont and Zernez, E. robustus from P. validirostris at St. Crepin and Fontainebleau, Eubazus sp. from P. piceae
Occurrence of sibling species in Enbazus spp. 153
at Val-d'Ajol and E. crassigaster from P. strobi at Fair Harbour.
Horizontal starch gel electrophoresis was performed using the standard procedures and modified protocols from Murphy et al. (1990). Five males and five females from each population were assayed for 13 enzyme systems. These were: aspartame aminotransferase (AAT), catalase (CAT), cytosol aminopeptidase (CAP), esterase (EST), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), hexokinase (HK), 1-iditol dehydrogenase (IIDH), malate dehydrogenase [MDH), malic enzyme (ME), phosphoglucomutase (PGM), phosphogluconate dehydrogenase (PGDH), trehalase (TRE), and xantine dehydrogenase (XDH). Of these, six could not be stained, or staining was not clear enough for reliable interpretation (CAT, CAP, GAPDH, IIDH, TRE and XDH) whilst three loci stained and resolved well but showed no difference in band pattern (AAT, ME and MDH-2). Five loci (EST, HK, MDH-1, PGDH and PGM) showed variation between Eubazus populations and were tested on 18 to 50 specimens from the same populations.
Given the small number of populations and enzyme systems studied, no serious attempt was made to study the genetic variation between populations. Instead, the isoen-zyme analyses were performed to estimate their potential as identification tools for Eubazus spp.
Cross-mating experiments
Males and females of different Eubazus populations and biotypes were crossed to assess their genetic compatibility. One to three virgin females less than 1 day old were placed in 5.5x2 cm vials with males that were 0-10 days old. For each experiment, the sex ratio was one female to two males. The mating behaviour was observed in the vial for 30 min and copulations were monitored. Then, the insects were put in 50 x 30 x 30 cm gauze covered wooden cages for further matings. Two days later, Scots pine logs containing fresh eggs of P. castaneus were offered to the females for oviposition over a period of 2 days. The logs were then kept at 23°C and the emergence of male and female parasitoids was monitored daily. At 23°C, Eubazus spp. emerged between 30 and 75 days after oviposition. After the emergence period, logs from crosses involving the diapaus-ing biotype of E. semirugosus were placed in a humid chamber at 2°C for three months and incubated again for parasitoid emergence. To assess the fertility of the female offspring, these were mated with their brothers and reared for a third generation. Rates of diapause observed in male and female offspring from crosses between the diapausing and the non-diapausing biotypes of E. semirugosus were compared with the rates of diapause observed in laboratory rearing of both biotypes as described by Kenis et al. (1996).
Host preference
Only two host species, P. castaneus and P. piceae, were reared in the laboratory because it was too difficult to rear P. validirostris in pine cones and P. strobi could not be reared in Europe because of the quarantine risk. Thus host preference investigations were restricted to two parasitoid species, E. semirugosus from P. pini, collected at Delemont, and Eubazus sp. from P. piceae, collected at Val-d'Ajol.
A cut section (c. 30x7 cm) of a trunk of Pinus sylvestris Linnaeus and one of Abies alba Miller (Pinaceae), containing
a minimum of 20 eggs of P. castaneus and P. piceae, respectively, were placed vertically in opposite corners of a gauze covered wooden cage (50x30x30 cm). A naive, mated, female parasitoid, at least 2 days old, was placed in the middle of the cage and allowed to select one of the two hosts. The first oviposition attempt was monitored. Then the female was removed from the cage and the logs were swapped. For both species, an Fl generation was reared on P. castaneus and the female offspring were tested using the same procedure as for the parent generation. Between 18 and 49 females per species and per generation were tested.
Results Morphometrics Females
Several measurements and ratios provided significant differences between Eubazus spp. when analysed by ANOVA. For most of them, however, groups largely overlapped. Only the length of the ovipositor sheath could be considered as a diagnostic character. Table 1 shows, for several populations of all Eubazus spp., the variation in the absolute length of the ovipositor sheath and in the ratio ovipositor sheath length to fore wing length (OVIP/WL), the fore wing length being used as an indicator of the body size. Similar results were obtained when the ovipositor was divided by other measurements, such as hind femur length, hind tibia length, or mesosoma length. The ovipositor is shorter for E. robustus and longer from Eubazus sp. There was no overlap in the ovipositor length between Eubazus sp. and the other species. The ratio OVIP/WL provided a discrete separation between E. robustus and Eubazus sp., and between E. crassigaster and Eubazus sp., but E. semirugosus tended to overlap with all other species, although the overlap with E. robustus and Eubazus sp. was weak. There was no significant geographic variation in the ratio OVIP/WL between populations from the same species, including the diapausing and the non-diapausing biotypes of E. semirugosus and E. robustus, while sympatric populations of different species were as different as allopatric populations. The two parasitoid populations that emerged from P. castaneus and P. piniphilus, which were suspected to be E. robustus because of their longer development time in comparison to E. semirugosus, indeed had similar OVIP/WL ratios to the populations of E. robustus from P. validirostris.
Differences in the OVIP/WL ratio were maintained when Eubazus species were reared under standard conditions on P. castaneus in the laboratory (table 3). Ratios appeared to be better taxonomic characters than absolutes sizes. Indeed, for all species there was no difference in the relative size of the ovipositor between the parent and the Fl generation, while the absolute size of the ovipositor of Eubazus sp. from P. piceae significantly decreased when reared on the smaller P. castaneus.
The two sympatric populations of £. semirugosus and E. robustus from Fontainebleau were totally separated using the ratio OVIP/WL. However, when all the populations of E. semirugosus and E. robustus were included in the analysis, a discrete separation between the two species was obtained only by performing a discriminant analysis including 15 morphometric characters (fig. 1). When the discriminant function was applied to the doubtful, 'late emerging'
154 M. Kenis and N.J. Mills
Table 3. Morphometric measurements of parent and Fl generations in females of Eubazus spp. Parent generations (P) emerged from field collected hosts, Fl generations emerged from Pissodes castaneus in the laboratory.
Source host (collection site)
E. semirugosus P. castaneus (Paimpont) P. pini (Zernez)1 E. robustus P. validirostris (Fontainebleau) Eubazus sp. P. piceae (Val-d'Ajol) E. crassigaster
P. strobi (Port McNeill) P Fl P Fl P Fl P Fl P Fl OVIP (10-2 170 + 3 b (139-197) 168 + 2 b (144-180) 199 ± 3 d (180-221) 186 + 2 c,d (158-204) 148 + 1 a (139-156) 146 + 2 a (125-156) 271 + 3 f (249-290) 230 + 3 e (194-254) 171 + 3 b,c (139-192) 175 + 3 b,c (144-202) Measurements + S.E. mm) WL ( l O2 mm) 373 + 7 a,b (319-435) 359 + 7 a (302-412) 432 + 7 d (383^87) 405 + 7 b,c,d (336-458) 401 + 3 b,c,d (377-423) 377 + 6 a,b (302^35) 503 + 6 e (447-563) 420 ± 8 c,d (348^64) 391 ± 5 a,b,c (331^23) 403 + 8 b,c,d (313-158) (min-max) OVIP/WL (xlOOO) 457 + 7 b,c (399-503) 470 + 5 c (432-517) 461 + 5 b,c (409-496) 459 + 5 b,c (431-515) 370 + 3 a (342-390) 387 + 3 a (359-115) 540 + 5 d (490-571) 550 + 5 d (502-610) 437 ± 4 b (409^78) 436 + 5 b (392^80) 'Diapausing biotype of E. semirugosus.
Equal letters in columns indicate homogeneous groups at 0.05 level. Scheffe multiple range test. N = 20 for each population and generation.
S E. semirugosus I • Eubazus sp. from P. piniphilus I
I E. robustus
I Eubazus sp. from P. castaneus
-3 0 3 Canonical discriminant function
Fig. 1. Histogram of the canonical discriminant function for females of all populations of Eubazus semirugosus from Pissodes pini and P. castaneus, and E. robustus from P. validirostris. The same function was later applied to the doubtful, late emerging Eubazus sp. populations reared from P. piniphilus from Delemont, and P. castaneus from Fontainebleau. Function: -0.030 MSH + 0.126 OVIP-0.011 SI - 0.011 WL - 0.338 POL + 0.346 BTW + 0.097 FL + 0.004 TL + 0.114 TW - 0.025 OOL - 0.238 BTL - 0.087 FW + 0.104 T2 + 0.051 T3 - 0.050
Occurrence of sibling species in Eubazus spp. 155
-15 -10 -5 0
Function 1
10 15
Fig. 2. Plots of first versus second canonical discriminant functions for females of all populations of Eubazus semirugosus (open circles) from Pissodes castaneus and P. pint, E. robustus (triangles) from P. validirostris and Eubazus sp. (squares) from P. piceae. The same two functions were later applied to the 15 undetermined specimens from P. harcyniae (solid circles). Function 1: -0.008 MSH-0.024 MSL + 0.130 OVIP +0.033 SI-0.002 T3-0.018 WL-0.016 OOL-0.393 POL-0.155 BTL +0.304 BTW +0.086 FL-0.085 FW-0.025 PL + 0.014 TW + 0.061 T2 - 9.531; Function 2: +0.025 MSH + 0.038 MSL - 0.015 OVIP + 0.075 SI - 0.087 T3 - 0.015 WL + 0.007 OOL + 0.093
POL+ 0.223 BTL-0.218 BTW +0.095 FL-0.091 FW-0.123 TL-0.044 TW-0.032 T2-2.734.
populations from P. piniphilus a n d P. castaneus, these clearly clustered with the E. robustus g r o u p rather than with £. semirugosus.
The diapausing biotypes of E. semirugosus and E. robustus were morphologically identical to their respective non-diapausing biotype. For both species, not a single ratio was found significantly different between the two biotypes.
Figure 2 shows the graph of the two canonical discriminant functions that maximize the separation be-tween the three European Eubazus spp. When plotted in this
graph, the Eubazus population from P. harcyniae clearly clustered with E. semirugosus. In multiple range tests involving all Eubazus spp. populations, no significant difference was found in any ratio between the population from P. harcyniae a n d a E. semirugosus population.
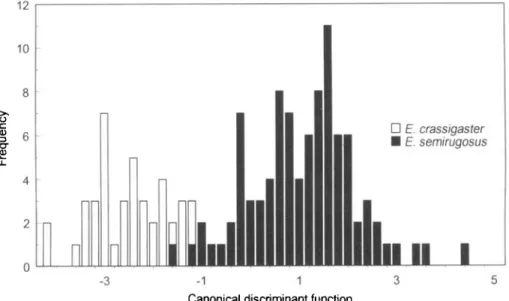
Eubazus semirugosus a n d E. crassigaster largely overlapped for all ratios tested, and no discriminant analysis provided a complete separation between the females of these two species. The best result in separating E. crassigaster from E. semirugosus was achieved using a canonical discriminant
12 10 3 • E. crassigaster • E. semirugosus
I
-3 -1 1Canonical discriminant function
Fig. 3. Histogram of the canonical discriminant function for females of all populations of Eubazus semirugosus from Pissodes pini and P. castaneus, and E. crassigaster from P. strobi. Function: 0.129 OOL + 0.452 POL+ 0.059 BTL-0.102 BTW-0.071 FL + 0.353 FW-0.003
156 M. Kenis and N.J. Mills
Table 4. Most significant ratios observed in males of Eubazus spp. from different populations. Host (site)
£. semirugosus
Pissodes castaneus (Beaumotte) P. castaneus (Fontainebleau) P. castaneus (De Koog) P. castaneus (Thetford) P. pini (Zernez)1 P. pini (Delemont) P. pini (Brusson)1 P. pini (Val-d'Ajol) P. piniphilus (Grissheim) E. robustus P. validirostris (St-Crepin) P. validirostris (Fontainebleau) P. validirostris (Nevache)1 P. validirostris (Leuk) P. validirostris (Sacele) Eubazus sp. P. piceae (Val-d'Ajol) P. piceae (Leuk-Albinen) P. piceae (Delemont) E. crassigaster
P. strobi (Port McNeill) P. strobi (Fair Harbour) Uncertain species P. piniphilus (Delemont)2 P. castaneus (Fontainebleau)2 N 20 12 20 7 20 20 12 7 20 20 20 20 20 15 20 17 4 20 20 20 6
Ratios + S.E. (min-max) x 100 AL3/BTL 58 + 1 c,d,e,f (52-65) 58 ± 1 b,c,d,e,f (50-64) 60 + 1 e,f (56-65) 61 + 2 (55-67) 60 + 1 d,e,f (54-68) 60 + 1 e,f (52-67) 62 + 1 e,f (57-65) 58 + 1 (54-61) 58 + 1 c,d,e,f (52-65) 56 + 1 a,b,c,d,e (43-63) 56 + 1 a,b,c,d,e (50-64) 55 + 1 a,b,c,d (50-62) 53 + 1 a,b,c (43-59) 56 + 1 a,b,c,d,e (52-59) 52 + 1 a,b (44-57) 54 + 1 a,b,c,d (46-60) 46 + 0 (45-46) 63 + 1 f (58-71) 60 + 1 e,f (52-67) 52 + 1 a (47-57) 54 + 1 (50-57) S l / F L 68 + 1 a,b (56-80) 61 ± 1 a (57-69) 68 + 1 a,b (63-73) 68 + 2 (56-70) 66 + 1 a,b (58-78) 66 + 1 a,b (61-78) 65 ± 1 a,b (61-72) 66 + 1 (61-70) 62 + 1 a (56-70) 78 + 1 c,d (70-85) 79 + 1 c,d (57-86) 82 + 1 d,e (76-93) 78 + 1 c,d (75-83) 85 + 1 d,e (79-91) 72 + 1 b,c (60-89) 71 + 1 b,c (61-81) 71 + 1 (67-73) 86 + 1 e (79-95) 83 + 1 d,e (76-87) 73 + 1 b,c (67-83) 90 + 2 (83-98) OOL/POL 159 ± 3 a,b,c,d,e,f (141-179) 167 + 3 c,d,e,f,g (154-183) 161 + 2 b,c,d,e,f (143-183) 152 + 3 (140-160) 166 + 3 e,f,g (144-192) 163 + 3 d,e,f (150-192) 165 + 2 b,c,d,e,f,g (150-177) 162 + 4 (146-175) 167 + 2 e,f,g (150-192) 148 + 3 a,b,c,d,e (129-177) 140 + 2 a (120-150) 146 + 2 a,b,c,d (127-167) 144 + 2 a,b,c (129-157) 142 + 2 a,b (129-158) 177 + 4 f,g,h (153-221) 174 + 4 f,g,h (154-220) 182 + 14 (150-217) 192 + 3 h (171-217) 187 + 3 g,h (167-200) 146 + 3 a,b,c,d (120-167) 139 + 4 (125-150) 'Diapausing biotypes of E. semirugosus and E. robustus.
2
Slow developing populations.
Equal letters in columns indicate homogeneous groups at 0.05 level, Scheffe multiple range test. Sample sizes of less than 10 were not tested statistically.
function including 15 characters, in which case 97% of the individuals were classified correctly (fig. 3).
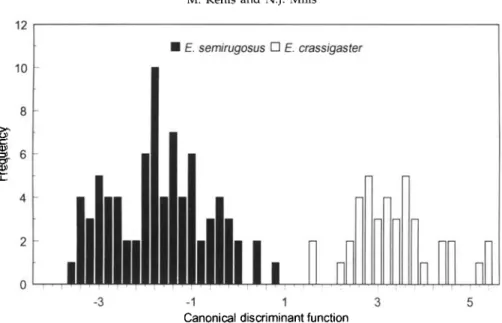
Males
No single ratio of male measurements provided a discrete separation between Eubazus spp., but the ratio of first metasomal suture to hind femur length (Sl/FL) was the most discriminating, with E. semirugosus having a conspicuously shorter relative suture length than E. crassigaster and E.
robustus (table 4). Eubazus crassigaster w a s significantly different from Eubazus sp. and E. robustus in the ratio AL3/BTL and the OOL/POL ratio was significantly higher in E. crassigaster a n d Eubazus sp. than in £. robustus. The two doubtful, late emerging populations from P. piniphilus and P. castaneus had similar ratios to the populations of £. robustus.
As observed in females, the absolute size of Eubazus sp. decreased when reared on P. castaneus in the laboratory (table 5). In contrast, the Sl/FL ratio was constant between the field and laboratory generation. Differences in the Sl/FL
Occurrence of sibling species in Eubazus spp.
Table 5. Morphometric measurements of parent and Fl generations in males of Eubazus spp. Parent generations (P) emerged from field collected hosts, Fl generations emerged from Pissodes castaneus in the laboratory.
157
Source host (collection site)
£. semirugosus P. castaneus (Beaumotte) P. pini (Zernez)1 E. robustus P. validirostris (Fontainebleau) Eubazus sp. P. piceae (Val-d'Ajol) E. crassigaster
P. strobi (Port McNeill)
P Fl P Fl P Fl P Fl P Fl Measurements ± S.E. FL (10-2mm) 101 + 2 b (94-118) 86 + 2 a (72-94) 108 + 2 b (82-125) 97 + 2 a,b (74-113) 102 ± 2 b (86-115) 100 + 2 b (84-115) 137 + 2 c (118-154) 107 + 2 b (86-122) 100 + 1 b (84-108) 97 + 3 a,b (79-115) SI (10-2mm) 69 + 2 b,c (58-91) 55 + 1 a (41-62) 7 1 + 2 b,c,d (55-86) 66 ± 2 a,b (50-84) 80 + 2 c,d,e (58-91) 81 + 2 d,e (62-103) 99 + 2 f (79-115) 76 + 2 b,c,d,e (65-89) 87 ± 1 e (74-96) 83 + 3 e (63-103) (min-max) Sl/FL (xlOO) 68 + 1 a,b (56-80) 63 + 1 a (57-70) 66 + 1 a,b (58-78) 68 + 1 a,b (62-74) 79 ± 1 c,d (57-86) 81 + 1 d,e (68-90) 72 ± 2 b,c (60-89) 71 + 1 b (66-78) 86 + 1 e (79-95) 86 + 1 e (76-95) 'Diapausing biotype of E. semirugosus.
Equal letters in columns indicate homogeneous groups at 0.05 level, Scheffe multiple range test. N = 20 for each population and generation.
ratio observed between Eubazus spp. in the parent generation were maintained in the laboratory generation reared on P. castaneus.
Two canonical functions maximizing the differences between European Eubazus spp. separated Eubazus sp. from E. robustus but E. semirugosus slightly overlapped with the
-2
Fig. 4. Plots of first versus second canonical discriminant functions for males of all populations of Eubazus semirugosus (circles) from Pissodes castaneus and P. pini, E. robustus (triangles) from P. validirostris and Eubazus sp. (squares) from P. piceae. Function 1: 0.030 S2 - 0.001 T2 - 0.026 T3 - 0.258 OOL - 0.302 POL - 0.002 W3SR + 0.007 WL - 0.019 SI - 0.011 MSL + 0.046 BTL + 0.058 FL + 0.003 TL - 0.020 HH + 0.089 MSH - 0.088 AL3 - 5.905; Function 2: 0.015 S2 - 0.134 T2 + 0.041 T3 - 0.247 OOL + 0.278 POL - 0.026 W3SR - 0.016 WL + 0.085
158 M. Kenis and N.J. Mills
E. semirugosus • E. crassigaster
- 1 1 3 5
Canonical discriminant function
Fig. 5. Histogram of the canonical discriminant function for males of all populations of Eubazus semirugosus from Pissodes pini and P. castaneus, and E. crassigaster from P. strobi. Function: 0.009 S2- 0.083 T2 +0.023 T3 +0.248 OOL-0.699 POL+ 0.057 W3SR-0.023
WL +0.180 SI+ 0.034 MSL- 0.025 BTL-0.145 FL +0.060 TL-0.003 HH-0.036 MSH-0.038 AL3 +2.056.
two other species (fig. 4). E. crassigaster and E. semirugosus were totally separated using a discriminant function including 15 morphometric characters (fig. 5).
An important variation was also found in the length of the fourth to seventh abdominal tergites. In E. crassigaster males, the apparent length of tergites five to seven is shorter than that of the fourth tergite, while it is longer in the European species. However, as the apparent length of the fourth to seventh abdominal tergites depends on the killing method, relative tergite lengths cannot be used as fixed taxonomic characters, but they certainly provide the easiest method for separating males of E. crassigaster and E. semirugosus when all specimens have been killed using the same method.
Non-morphometric variation
The only consistent morphological variation found among the Eubazus spp. is the clypeal tooth which, in both male and female E. crassigaster, is absent or reduced compared to the European species (fig. 6). However, in both groups there are some specimens that cannot be assigned with certainty to one of the two groups.
Isoenzyme analyses
Eubazus crassigaster was totally separated from all European Eubazus spp. by the banding pattern of the enzyme PGDH. It could also be separated from E. robustus by the banding pattern of HK and from Eubazus sp. by that of EST (table 6). Among the European species, HK and EST patterns
(a)
Fecundity
The four Eubazus spp. varied in the number of ovarioles per female (ANOVA: F = 132.88, d.f. = 3, P< 0.001). Eubazus sp. from P. piceae had almost twice as many ovarioles (mean = 38.0, S.D. = 4.4, min-max = 30-47, n = 41) as E. semirugosus (mean = 23.0, S.D. = 3.1, min-max = 11-28, n = 37), E. robustus (mean = 24.1, S.D. = 2.8, min-max = 18-29, n = 49) and E. crassigaster (mean = 21.4, S.D. = 1.8, min-max = 19-23, n = 5), suggesting a much greater fecundity. The number of ovarioles was correlated with the body size, estimated by the length of the hind femur, in Eubazus sp. (r = 0.426, n = 41, P = 0.005), but not in E. semirugosus (r =-0.048, n = 37, P = 0.798) or E. robustus (r = 0.271, n = 49, P = 0.086).
(b)
Fig. 6. Clypeus: (a) Eubazus semirugosus; (b) E. crassigaster; scale line = 0.1 mm.
Occurrence of sibling species in Eubazus spp. 159 Table 6. Allele frequenties for isoenzyme loci in populations of Eubazus spp.
Locus HK MDH-1 PGDH PGM EST Allele n2 A B n A B C n A B C n A B C D n A B C D E. St-Crepin 51 0.00 1.00 52 1.00 0.00 0.00 51 0.98 0.00 0.02 51 0.00 0.37 0.59 0.04 49 0.00 0.00 1.00 0.00 robustus Fontainebleau 42 0.00 1.00 42 0.98 0.02 0.00 42 1.00 0.00 0.00 42 0.00 0.26 0.71 0.02 42 0.00 0.00 0.98 0.02 E. semirugosus Zernez1 60 0.87 0.13 60 1.00 0.00 0.00 60 1.00 0.00 0.00 60 0.00 0.98 0.00 0.02 O3 Delemont 26 1.00 0.00 26 1.00 0.00 0.00 26 1.00 0.00 0.00 26 0.00 1.00 0.00 0.00 03 Eubazus sp. Val-d'Ajol 60 1.00 0.00 60 0.90 0.00 0.10 60 1.00 0.00 0.00 60 0.00 1.00 0.00 0.00 58 1.00 0.00 0.00 0.00 E. crassigaster Fair Harbour 77 1.00 0.00 77 0.79 0.01 0.19 77 0.00 1.00 0.00 77 0.01 0.99 0.00 0.00 57 0.00 0.12 0.00 0.88 'Diapausing biotype. 2
Number of alleles scored (2xno. of females + no. of males).
3EST patterns did not resolve satisfactorily and are not included in the table.
Alphabetical order in allelic designations indicates rapidity of migration to the anode during electrophoresis (e.g. A migrates faster than B).
differentiated E. robustus from Eubazus sp., but E. semirugosus shared common HK bands with both species and EST could not be resolved satisfactorily for £. semirugosus. There was very little difference in the banding patterns between the diapausing and the non-diapausing biotypes of E. semirugo-sus, as well as between the two E. robustus populations tested.
Cross-mating experiments
The results of cross-mating studies are shown in table 7. In crosses between males and females of the same species, matings were usually observed within the first 5 min. Almost all these crosses gave rise to fertile females. In contrast, Eubazus males did not show much interest in females of other species. Only one interspecific mating was observed within the 30 min of observation, between a male of E. semirugosus and a female of Eubazus sp. However, one crossing between E. semirugosus and Eubazus sp. and two between E. semirugosus and E. robustus produced fertile females. All attempts to cross E. robustus and Eubazus sp. failed.
Most crosses between the diapausing and the non-diapausing biotypes of E. semirugosus resulted in fertile female offspring. When females from the non-diapausing biotype were crossed with males of the diapausing biotype, 69% of their female offspring entered into diapause while all males emerged. Crosses between females of the diapausing biotype and males of the non-diapausing biotype gave rise to all males and a majority of females entering into diapause (fig. 7). These results show that the diapause is genetically based and carried by both sexes.
Host preference
When given a choice for oviposition between eggs of P. castaneus and P. piceae in pine and fir logs, respectively, E. semirugosus females showed a strong preference for P. castaneus while females of Eubazus sp. from P. piceae significantly preferred their original host (fig. 8). When reared on P. castaneus for one generation, the offspring of E. semirugosus still preferred P. castaneus, but Eubazus sp. did not show a preference for either of the two proposed hosts. The difference in host preference between the two species was highly significant in the parent generation as well as in the Fl generation.
Discussion
According to Mayr's (1942) general definition, to which most systematists still adhere, species are groups of interbreeding populations that are reproductively isolated from one another. However, reproductive isolation in nature is not easy to verify. It is particularly difficult to determine whether two host-associated biotypes are just host races or sibling species, because host races are usually a step toward speciation and the limit between the two categories is rather arbitrary (Bush, 1975; Diehl & Bush, 1984). There are several examples in the literature showing that what were believed to be host races appeared to be distinct species after careful studies (e.g. Vet et al., 1984; Holler, 1991). We consider, from the evidence provided, that the complex of Eubazus spp. parasitoids attacking Pissodes hosts in Europe is composed of at least three sibling species, each of them being largely specialized in a different microhabitat, as previously
160 M. Kenis and N.J. Mills suggested in earlier studies (Kenis & Mills, 1994; Kenis et al.,
1996). Eubazus semirugosus is found only on P. castaneus, P. pini, and P. piniphilus in pine trunks and, perhaps, on P. harcyniae in spruce trunks. Eubazus sp. is specific to P. piceae in fir trunks and E. robustus is primarily a parasitoid of P. validirostris in pine cones, but occasionally attacks Pissodes spp. in pine trunks.
Eubazus robustus and Eubazus sp. appear to be totally reproductively isolated, as all attempts to interbreed these two species failed, while successful reproduction was easily demonstrated within each species. Interbreeding between E. semirugosus and the two other European species is still possible, but difficult, apparently because adults of different species do not mate as readily as individuals from the same species. Preliminary studies on isoenzymes supported the idea of an intermediate position of E. semirugosus, as
hexokinase banding patterns clearly separate E. robustus from Eubazus sp., while E. semirugosus shares bands of both species.
The ratio of ovipositor length to fore wing length provided the best morphometric character to separate the three European species, with E. robustus having the shortest, and Eubazus sp. the longest relative ovipositor length. Here again, E. semirugosus tended to overlap with both species, and only discriminant functions including several measure-ments provided a full separation between E. semirugosus and its two sibling species. The differences in morphology were not host-induced but genetically fixed as they were maintained when the Eubazus species were reared on a common host.
The intermediate position of E. semirugosus observed in cross-mating experiments, isoenzyme analyses and
Table 7. Results of crosses. Species crossed1 (mxf) Intrapopulation sem(c) x sem(c) rob x rob esp x esp Interpopulation sem (p)xsem(p) sem(p) x sem(c) sem(p) x sem(p) sem(c) x sem(p) sem(p) x sem(p) sem(c) x sem(p) sem(c) x rob sem(p) x rob sem(c) x rob sem(p) x rob sem(c) x rob rob x sem(c) rob x sem(c) rob x sem(c) rob x sem(p) rob x sem(c) sem(p) x esp sem(p) x esp sem(p) x esp sem(p) x esp esp x sem(p) esp x sem(p) esp x sem(p) rob x esp rob x esp rob x esp esp x rob Unmated females sem(c) sem(p) rob esp Collection sites (mxf) Beaumotte FxBeaumotte F St-Crepin Fx St-Crepin F Val-d'Ajol Fx Val-d'Ajol F Brusson CHx Val-d'Ajol F Zernez CHxLorris F Zernez CH x Delemont CH Lorris Fx Zernez CH Delemont CHx Zernez CH Fontainebleau Fx Zernez CH Beaumotte F x Fontainebleau F Delemont CH x Leuk F Fontainebleau F x Fontainebleau F Delemont CHx St-Crepin F Fontainebleau FxLeuk CH Fontainebleau FxBeaumotte F Fontainebleau FxBeaumotte F Fontainbleau FxBeaumotte F Leuk CHx Delemont CH Fontainebleau F x Fontainbleau F Val-d'Ajol Fx Val-d'Ajol F Val-d'Ajol Fx Val-d'Ajol F Zernez CHx Val-d'Ajol F Delemont CH x Val-d'Ajol F Val-d'Ajol Fx Val-d'Ajol F Val-d'Ajol Fx Delemont CH Val-d'Ajol Fx Zernez CH St-Crepin Fx Val-d'Ajol F St-Crepin Fx Val-d'Ajol F Fontainebleau Fx Val-d'Ajol F Val-d'Ajol x St-Crepin F Lorris F Val-d'Ajol F Fontainebleau F Val-d'Ajol F Mating observed2 Y Y Y Y Y Y Y Y Y N N N N N N N N N N N Y N N N N N N N N N Offspring in Fl (m/f) 14/8 9/4 10/12 4 / 4 34/10 25/21 13/23 13/0 8/6 10/0 19/0 10/0 3/6 9/0 24/0 33/0 11/0 4 / 6 16/0 15/0 13/0 37/0 16/0 11/18 41/0 10/0 26/0 10/0 4/0 14/0 12/0 12/0 8/0 18/0 in F2 (m/f) 0/7 7/3 9/6 0 / 1 8/8 NR3 6/8 9/5 1/3 43/4 6/7
'sem(c), Eubazus semirugosus from Pissodes castaneus; sem(p), E. semirugosus from P. pini; sem(p), diapausing biotype of E. semirugosus from P. pini; rob, E. robustus from P. validirostris; Esp, Eubazus sp. from P. piceae. 2
YES (Y), at least 1 mating observed during the first 30 minutes of contact; NO (N), no mating observed during the first 30 minutes of contact.
3
NR, Fl not reared for a second generation.
Occurrence of sibling species in Eubazus spp. 161 M F NDxD M F DxND Crosses (M M NC xF) M F DxD ;
ig. 7. Percentages of diapause (solid bars) and emergence (open jars) in male and female offspring resulting from crosses between the diapausing (D) and the non-diapausing (ND) jiotypes of Eubazus semirugosus, and percentages of diapause ind emergence observed in laboratory rearing of both biotypes.
Numbers above the bars are sample sizes.
Tiorphometric analyses suggests that Eubazus sp. and z. robustus have evolved separately from E. semirugosus, or
:
rom a common ancestor living in pine trunks. This lypothesis is supported by the fact that the majority of °issodes species live in pine trunks (Kudela, 1974; O'Brien, L989a,b). Very few species attack fir trunks and P. validirostris s the only Pissodes species living in cones.
Speciation is not complete among the European para-iitoids, as E. semirugosus is still genetically compatible with :he two other species when forced to cross in the laboratory, in the field, however, pre-mating barriers probably limit the Dossibilities for interbreeding. The most important factor :
avouring isolation is most certainly the fact that adults tend
£ 40
-P F1
E. semirugosus
p F1 Eubazus sp.
Fig. 8. Preference for first oviposition in Eubazus semirugosus 'reared from Pissodes pini) and Eubazus sp. (reared from P. piceae) .vhen given a choice between P. castaneus eggs in pine logs 'hatched bars) and P. piceae eggs in fir logs (open bars). Parents 'P) emerged from their original host while the Fl generation emerged from P. castaneus in rearing. Numbers in the bars are iample sizes. Stars in the bars indicate a significant preference :
or one of the two hosts (test for binomial distribution, P < 0.05). Different letters above two bars indicate a significant difference
in host preference (2-tailed Fisher exact test, P < 0.05).
to mate with siblings at the emergence site, as observed in Eubazus sp. (Haeselbarth, 1962), E. robustus (Roques, 1975) and E. semirugosus (M. Kenis, unpublished data). We also observed that males show more interest in conspecific females than in females of other species. Other isolating factors include host preference, as shown with E. semirugosus and Eubazus sp., and temporal differences in the timing of adult emergence. Adult emergence periods overlap in the field, because E. semirugosus can emerge from April to October (M. Kenis, unpublished data), but the peak emergence periods are not synchronized, which reduces the possibility of interspecific mating. In the Swiss Jura, E. semirugosus has two peaks of emergence, in late April-early May and in July (Kenis et al., 1996). Roques (1975) observed, in a similar climatic region in central France, that E. robustus emerges in late May and, to a lesser extent, in late August and in September, and Haeselbarth (1962) showed that in Bavaria Eubazus sp. mainly emerges from late May to late June.
Eubazus robustus was found to emerge from P. castaneus and P. piniphilus in pine trunks, and thus, mating with E. semirugosus could potentially occur between these two species. However, we observed that when E. robustus and E. semirugosus parasitize the same population of P. castaneus, E. robustus emerges later than E. semirugosus, which, again, limits the possibility of interbreeding (M. Kenis, unpublished data).
As shown by Annila (1975) and Roques (1975), about 25% of the E. robustus emerge from P. validirostris in late summer when there are no eggs of P. validirostris available for oviposition. Thus P. castaneus, P. piniphilus and perhaps P. pini can be used as alternate hosts for this late summer generation. Pissodes piniphilus appears to be a particularly suitable host for E. robustus. It is usually found in the crown of mature trees, i.e. in the vicinity of cones, while P. castaneus prefers to attack young trees and P. pini prefers dying or freshly cut trees. Furthermore, P. piniphilus, being the smallest Pissodes species, lays its eggs closer to the bark surface rendering them more accessible to E. robustus whose ovipositor is shorter than that of E. semirugosus. More generally, the length of the ovipositor in Eubazus spp. is related to the oviposition behaviour of their respective hosts. Pissodes piceae is the largest host and its long rostrum allows it to dig oviposition holes deep in the bark. Furthermore, it lays up to 15 eggs in the same hole (Haeselbarth, 1962), and thus, to reach all the eggs, Eubazus sp. needs a longer ovipositor than its sister species whose hosts lay eggs in groups of one to five and closer to the bark surface.
In the laboratory, E. semirugosus and Eubazus sp. preferred to oviposit in their respective hosts, and this difference was maintained in a second generation after rearing on P. castaneus under standard conditions, which suggests that host preference has a genetic basis. Neverthe-less, for Eubazus sp., rearing on P. castaneus significantly increased the acceptability of this unnatural host compared to the parent generation originating from P. piceae, suggesting that host preference is also influenced by the host, or host habitat, experienced during the pre-emergence period, a phenomenon often observed in insect parasitoids (e.g. Kudon & Berisford, 1980; Turlings et al., 1993).
Eubazus sp. differed from all the other species by having a greater number of ovarioles and, consequently, a higher potential fecundity. The variation observed in the fecundity of Eubazus spp. probably results from a variation in the
162 M. Kenis and N.J. Mills spatial distribution of their respective hosts. Most solitary
parasitoids evolve a fecundity that matches the number of hosts they are likely to encounter in their lifetime (Godfray, 1994). Eggs of P. piceae are usually found in high numbers in mature, isolated fir trees. Once a Eubazus sp. female has located an attacked tree, she has the opportunity to meet hundreds of host eggs at the same site. Thus, the fitness of Eubazus sp. is maximized by a greater fecundity. In contrast, the spatial distribution of the other Pissodes hosts is more scattered because a cone, a leader or a young tree is likely to contain less hosts than a mature fir. Thus, compared to Eubazus sp., E. robustus, E. crassigaster and E. semirugosus will probably encounter less hosts in their lifetime. For these species, the number of hosts parasitized will largely depend on their searching efficiency. As a result, instead of allocating resources to increased fecundity, they probably allocate resources to increased searching and flying capacity.
There is no reason to believe that, in E. semirugosus and E. robustus, the mountain, diapausing biotype and the lowland, non-diapausing biotype are different species. The two biotypes of E. semirugosus mate and interbreed as well as specimens from the same biotype, and there are no morphometric or enzymatic characters which separate them. Diapause is merely an adaptation to synchronize their phenology to that of their hosts in different environments (Kenis 1994; Kenis et ah, 1996). Nevertheless, diapause in Eubazus spp. is genetically based, as it can be transmitted from father to offspring. This observation shows the potential of hybridization in the improvement of Eubazus parasitoids and of biological control agents in general. The diapausing biotype of E. semirugosus was selected as the best agent for the biological control of the white pine weevil, because the diapause characteristic allows the parasitoid to synchronize its life cycle with that of the target host (Kenis, 1994; Kenis et ah, 1996). However, this strain might be less effective in other traits, such as host location. Indeed, the diapausing biotype is reared from pine Pissodes spp., while most of the damage by the white pine weevil is observed on spruce (Alfaro, 1982; Lavallee & Benoit, 1989). Transmitting the genes responsible for diapause by hybridization into a biotype or species which naturally locates spruce could improve the potential for biological control. Genetic improvement of natural enemies has often been proposed to enhance their effectiveness as biological control agents (DeBach, 1958; Beckendorf & Hoy, 1985; Hoy, 1990), but, until now, the only satisfactorily results were achieved in the improvement of pesticide resistance in phytoseiid mites (Hoy, 1990).
It is not yet possible to be sure of the identity of the Eubazus species that attacks P. harcyniae, as only morpho-metric data are available for comparison. Morphomorpho-metrics suggests that it is E. semirugosus, but live material is needed to carry out cross matings, isoenzyme analyses, or host location comparisons.
Eubazus crassigaster is morphometrically very similar to E. semirugosus, but the presence or absence of a tooth on the clypeus separates most individuals, and for the doubtful specimens, separation can be achieved using multivariate discriminant functions. Should E. semirugosus be introduced into North America for the biological control of P. strobi, the best method to distinguish the two parasitoid species is electrophoresis of the PGDH enzyme, as it provides total separation and has the added advantage of detecting natural hybridization between the native and the exotic species.
Nonetheless, additional analyses of different populations of E. crassigaster should be made to be sure that the European allele is not present in North America.
Acknowledgements
We would like to thank M. Hulme for his continuous support during this study, and for the shipment of live specimens of £. crassigaster. We are also most grateful to A. Roques, J.P. Raimbault and V. Mihalciuc for the collection of pine cones in France and Romania, and W. Grodzki for providing us with the dry specimens of Eubazus sp. from P. harcyniae. We thank C. van Achterberg (Nationaal Natuurhistorisch Museum, Leiden, The Netherlands) for his help with Eubazus taxonomy and J. Pasteels for allowing us to use the electrophoresis facilities at the Universite Libre de Bruxelles. Electrophoresis analyses could not have been carried out without the precious help of P. Mardulyn. M. Seier, S. Leroy, K. Kock, C. Lopez Vaamonde, P. Chalon, A. Van averbeke and D. You helped in field collections and laboratory experiments, and K. Carl, J.-C. Gregoire, H. Daly, C. van Achterberg and D. Quicke kindly revised the manuscript. This work was supported by the Canadian Forest Service.
References
Achterberg, C. van (1988) Revision of the subfamily Blacinae Foerster (Hymenoptera, Braconidae). Zoologische Verhan-delingen 249, 1-324.
Achterberg, C. van (1990) Revision of the genera Foersteria Szepligeti and Polydegmon Foerster (Hymenoptera: Bra-conidae), with the description of a new genus. Zoologische Verhandelingen 257, 1-32.
Alauzet, C. (1982) Biocenose de Pissodes notatus F. ravageur des pins maritimes en foret de Bouconne (Haute-Garone: France). Nouvelle Revue d'Entomologie 12, 81-89.
Alfaro, R.I. (1982) Fifty-year-old Sitka spruce plantations with a history of intense weevil attack. Journal of the Entomological Society of British Columbia 79, 62-65.
Annila, E. (1975) The biology of Pissodes validirostris Gyll. (Col., Curculionidae) and its harmfulness, especially in Scots pine seed orchards. Communicationes Instituti Forestalis Fenniae 85, 1-95.
Beckendorf, S.K. & Hoy, M.A. (1985) Genetic improvement of arthropod natural enemies through selection, hybridization or genetic engineering techniques, pp. 167-187 in Hoy, M.A & Herzog, D.C (Eds) Biological control in agricultural IPM systems. Orlando, Academic Press.
Bush, G.L. (1975) Sympatric speciation in phytophagous parasitic insects, pp. 187-207 in Price, P.W. (Ed.) Evolution-ary strategies of parasitoids. New York, Plenum.
Caltagirone, L.E. (1985) Identifying and discriminating among biotypes of parasites and predators, pp. 189-200 in Hoy, M.A & Herzog, D.C. (Eds) Biological control in agricultural IPM systems. Orlando, Academic Press.
DeBach, P. (1958) Selective breeding to improve adaptations of parasitic insects. Proceedings of the X International Congress of Entomology 4, 759.
DeBach, P. & Rosen, D. (1991) Biological control by natural
enemies. 2nd edn. 440 pp. Cambridge University Press. Diehl, S.R. & Bush, G.L. (1984) An evolutionary and applied
perspective of insect biotypes. Annual Review of Entomology 29, 471-504.
Occurrence of sibling species in Eubazus spp. 163
Ehler, L.E. (1990) Introduction strategies in biological control of
insects, pp. 111-134 in Mackauer, M., Ehler, L.E. & Roland, J. (Eds) Critical issues in biological control. Andover, Intercept.
Fahringer, J. (1934) Opuscula Braconologia, palaearktische region
Bd. 2. pp. 321-594. Wien.
Gauld, I.D. (1986) Taxonomy, its limitations and its role in
understanding parasitoid biology, pp. 1-21 in Waage, J. & Greathead, D.J. (Eds) Insect parasitoids. London, Academic Press.
Godfray, H.C.J. (1994) Parasitoids. Behavioral and evolutionary
ecology. 473 pp. Princeton University Press.
Haeselbarth, E. (1962) Zur Biologie, Entwicklungsgeschichte
und Oekologie von Brachistes atrkornis Ratz. als eines Parasiter von Pissodes piceae. Zeitschrift fiir Angewandte Entomologie 49, 233-289.
Holler, C. (1991) Evidence for the existence of a species closely
related to the cereal aphid parasitoid Aphidius rhopalosiphi De Stefani-Perez based on host ranges, morphological characters, isoelectric focusing banding patterns, cross-breeding experiments and sex pheromone specificities (Hymenoptera, Braconidae, Aphidiinae). Systematic Ento-mology 16, 15-28.
Hopper, K.R., Roush, R.T. & Powell, W. (1993) Management of
genetics of biological control introductions. Annual Review of Entomology 38, 27-51.
Hoy, M.A. (1990) Genetic improvement of arthropod natural
enemies: becoming a conventional tactic? pp. 405-415 in Baker, R.R. & Dunn, P.E. (Eds) New directions in biological control. New York, Alan R. Lyss.
Kenis, M. (1994) Variations in diapause among populations of
Eubazus semirugosus (Nees) (Hym.: Braconidae), a para-sitoid of Pissodes spp. (Col.: Curculionidae). Norwegian Journal of Agricultural Sciences, Supplement 16, 77-82.
Kenis, M. & Mills, N.J. (1994) Parasitoids of European species
of the genus Pissodes (Col: Curculionidae) and their potential for the biological control of Pissodes strobi (Peck) in Canada. Biological Control 4, 14-21.
Kenis, M., Hulme, M.A. & Mills, N.J. (1996) Comparative
developmental biology of populations of three European and one North American Eubazus spp. (Hymenoptera: Braconidae), parasitoids of Pissodes spp. weevils. Bulletin of Entomological Research 86, 143-153.
Kishi, Y. (1970) Difference in sex ratio of the pine bark weevil
parasite, Dolichomitus sp. (Hymenoptera: Ichneumonidae) emerging from different host species. Applied Entomology and Zoology 5, 126-132.
Kudela, M. (1974) Curculionidae, Pissodini. pp. 299-310 in
Schwenke, W. (Ed.) Die Eorstschadlinge Europas 2 Band. Hamburg, Paul Pareys.
Kudon, L.H. & Berisford, C.W. (1980) Influence of brood hosts
on host preference of bark beetle parasites. Nature 283, 288-289.
Laidlaw, W.B.R. (1933) Two British parasites of Pissodes. Scottish
Forestry journal 47, 24-31.
Lavallee, R. & Benoit, P. (1989) Le Charancon du pin Blanc.
Forets Canada, Feuillet d'information, CLF 18, 1-13.
Lovaszy, P. (1941) Zur Kenntnis der Schlupwespen einiger
schadlicher Russelkafer. Annales Entomologica Fennici 7, 194-204.
Marshall (1888) Species des hymenopteres d'Europe et d'Algerie,
tome quatrieme. 609 pp. Beaune (France).
Marshall (1891) Species des hymenopteres d'Europe et d'Algerie,
tome cinquieme. 635 pp. Gray (France), Bouffaut Freres.
Mayr, E. (1942) Systematics and the origin of species, from the
viewpoint of a zoologist. New York, Columbia University Press.
Mendel, Z. (1986) Hymenopterous parasitoids of bark beetles
(Scolytidae) in Israel: relationships between host and parasitoid size, and sex ratio. Entomophaga 31, 127-137.
Mills, N.J. & Fisher, P. (1986) The entomophage complex of
Pissodes weevils, with emphasis on the value of P. validirostris as a source of parasitoids for use in biological control, pp. 297-305 in Roques A. (Ed.) Proceedings of the 2nd International Conference IUFRO Cone and Seed Insects Working Party, Briancon, September 1986. Olivet (France), INRA.
Murphy, R.W., Sites, J.W., Buth, D.G. & Haufler, C.H. (1990)
Proteins 1: isozyme electrophoresis. pp. 45-126 in Hillis, D.M. & Moritz, C. (Eds) Molecular systematics. Sunderland, Massachussets, Sinauer.
O'Brien, C.W. (1989a) Revision of the weevil genus Pissodes in
Mexico with notes on the neotropical Pissodini (Coleoptera, Curculionidae). Transactions of the American Entomological Society 115, 415^32.
O'Brien, L.B. (1989b) A catalog of the Coleoptera of America
north of Mexico. Family Curculionidae, subfamily Pissodinae Bedell 1888. Agriculture Handbooks. 529-143d,
1-8.
Powell, W. & Walton, M.P. (1989) The use of electrophoresis in
the study of hymenopteran parasitoids of agricultural pests, pp. 343-365 in Loxdale, H.D. & Den Hollander, J. (Eds) Electrophoretic studies on agricultural pests. Oxford, Clarendon Press.
Price, P.W. (1975) Reproductive strategies of parasitoids.
pp. 87-111 in Price, P.W. (Ed.) Evolutionary strategies of parasitoids. New York, Plenum.
Ratzeburg, J.T.C. (1844) Die Ichneumonen der Forstinsekten, Bd. 1.
226 pp. Berlin.
Ratzeburg, J.T.C. (1848) Die Ichneumonen der Forstinsekten, Bd. 2.
238 pp. Berlin.
Reyment, R.A., Blackith, R.E. & Campbell, N.A. (1984)
Multivariate morphometrics. 233 pp. London, Academic Press.
Roques, A. (1975) Etude de la merocenose des cones de pins sylvestres
en foret de Fontainebleau. 164 pp. These 3e cycle, Paris VI.
SPSS (1993) SPSS for Windows, advanced statistics, Release 6.0.
578 pp. Chicago, SPSS Inc.
Turlings, T.C.J., Wackers, F.L., Vet, L.E.M., Lewis, WJ. & Tumlinson, J.H. (1993) Learning of host-finding cues by
hymenopterous parasitoids. pp. 51-78 in Lewis, A.C. & Papaj, D.R. (Eds) Insects learning: ecological and evolutionary perspectives. New York, Chapman and Hall.
Vet, L.E.M., Janse, C.J., van Achterberg, C. & van Alphen, J.J.M. (1984) Microhabitat location and niche segregation in
two sibling species of drosophilid parasitoids: Asobara tabida (Nees) and A. rufescens (Foerster) (Braconidae: Alysiinae). Oecologia 61, 182-188.
(Accepted 20 November 1997) L; CAB INTERNATIONAL, 1998