\
OST.OF
TE'C,
s
FEB
3
1965
BEHAVIOR OF LIQUID LUBRICANTS IN VACUUM
by
STEPHEN MALKIN
S.B., Massachusetts Institute of Technology
(1963)
Submitted in Partial Fulfillment of The
Requirements For the Degree of Master of Science
at the
Massachusetts Institute of Technology February, 1965
Signature of Author
Signature
redacted
Department of Mec1inical Engineering, January 18 ,
Certified by
Accepted by
Signature redacted
Thesis Superviscr
Signature redacted
Chairman, Department Committee on Graduate Students
ABSTRACT
BEHAVIOR OF LIQUID LUBRICANTS IN VACUUM
by
Stephen Malkin
Submitted to the Department of Mechanical Engineering on January
18, 1965 in partial fulfillment of the requirements for the
Degree of Master of Science.
Studies with a crossed-cylinder and pin-on-disk apparatus with silicone, mineral and diester base oils and EP additives are
compared for tests run in vacuum and air on 52100 steel specimens. The oxygen dependent behavior of sulfur and chlorine additives suggests that their EP effectiveness is due to promotion of oxide reactions. Electron microprobe studies attribute T.C.P. action to both phosphate and oxide formation on the surfaces. The joint reaction of lubricant, environment, and surface is discussed for silicone, mineral, and diester oils.
Thesis Supervisor: George S. Reichenbach
TABLE OF CONTENTS Page iv ii iv Title Page Abstract Table of Contents List of Figures Acknowledgements Sections: I. Introduction II. Apparatus
III. Experimental Results and Discussion
1. Crossed-Cylinder Tests
2. Pin-on-Disk Results IV. Further Discussion
V. Conclusions
VI. Future Work References 1 4 10
10
26 31 34 36 37LIST OF FIGURES Figure 1. Figure 2. Figure 3. Figure 4. Figure 5. Figure 6. Figure 7. Figure 8. Figure 9. Figure 10. Figure 11. Figure 12. Figure 13. Figures 14-16 Figure 17.
Photograph of Crossed-Cylinder Test Apparatus Schematic Drawing of Apparatus
Photograph of Vacuum Test Apparatus
Photograph of Test Chamber and Dynamometer Crossed-Cylinder Test-Mineral Oil
Crossed-Cylinder Test-Mineral Oil plus 2% Sulphur Crossed-Cylinder Test-Mineral Oil plus 5%
n-Hexadecyl Chloride
Crossed-Cylinder Test - Mineral Oil plus 5%
T.C.P.
Crossed-Cylinder Test - Di(2-Ethylhexyl Azelate) Diester
Crossed-Cylinder Test - Diester plus,5% Phenothiazine
Crossed-Cylinder Test - Diester plus.5% Phenothiazine plus 5% T.C.P.
Crossed-Cylinder Test - Silicones Pin-on-Disk Test - F-50 Silicone
Pin-on-Disk Test - Mineral Oil at Different Speeds
Viscosity - Temperature Chart - F-50, F-44 and SAE 20. - iv-Page 5 9 9 9 11 13 14 17 19 21 .2 23 27 28 29
ACKNOWLEDGEMENT
The author is especially indebted to Professor George Reichenbach for supervising his thesis and offering numerous helpful suggestions. The author also thanks Mr. J.B. Van Saun
for his work with the dectron microprobe, Mr. R.G. Foster for
designing and building the pin-on-disk machine, Mr. S. Marcolongo for performing numerous machining jobs, Dr. R.S. Fein of Texaco for analyzing the sludges, Miss R. Messoumian for typing the thesis, and other members of the Surface Laboratory for helpful discussions and suggestions.
The author gratefully acknowledges thc financial support of the Pratt and Whitney Division of United Aircraft Corporation and the Instrumentation Laboratory of the Massachusetts Institute of Technology.
1. INTRODUCTION
The use of vacuum to study the boundary lubrication process is important for two reasons. First of all it generates practical information concerning the behavior of lubricants for space applications such as satellites, rockets, moon probes, etc. Secondly, and equally important, it serves as a tool in the
investigation of the basic phenomena of boundary lubrication by providing an important variable, namely the environment. In
the Surface Laboratory at M.I.T. it has been extensively used in the latter application for examining the behavior of extreme pressure (EP) additives, silicone fluids, and related features of boundary lubrication.
Problems involving the use of liquid lubricants in a vacuum may arise due to rapid evaporation of the lubricant or the lack of oxygen.
The evaporation problem can often be met by supplying large amounts of lubricant and/or using lubricants with low evaporation rates. If this is not sufficient it is usually necessary to employ solid lubricants.
The lack of oxygen can often strongly affect boundary lubrication. Reichenbach and Foster (1) have shown that load carrying capacity can be reduced by more than 50% in the absence of oxygen and eve-in the presence of EP additives. Similar results have been obtained
by Vinogradov et al (2). Klaus and Bieber (3) have shown that
-1-an optimum oxygen concentration exists which will result in minimal wear.
In the present investigation the results from
crossed-cylinder wear tests and pin-on-disk friction tests were compared for runs made in vacuum and air. A wide range of lubricants
which were studied included mineral, diester, and silicone base oils. E.P. additives studied individually in compound with
U.S.P. mineral oil included elemental sulfur, n-hexadecyl
chloride, and tricresyl phosphate (T.C.P.). Diester oils tested included di (2 - ethylhexyl) azelAte blended both with and
without phenothiazine (antioxidant) and T.C.P. The silicone oils include General Electric F-50 (chlorophenyl methyl silicone), F-44 (the same as F-50 but with a small amount of antioxidant), and Dow Corning DC 200 50 censtitokes (dimethyl silicone).
In conjunction with the crossed-cylinder testing, studies of the chemical composition and optical appearance of the rubbing surfaces were undertaken as a bachelor's thesis by Mr. John B. Van Saun (4). Wear scars on the crossed-cylinder specimens were examined with the electron microprobe,, a device which is
used to bombard a sample with an electron beam. Resultant X-ray
emissions of 2-3 p diametercan be sampled with spectrometers using
a standard of known compositions. Quantitative determination of elemental concentrations to within .01 weight per cent can be achieved. Wear scars were scanned parallel and perpendicular
to the direction of relative sliding velocity producing a rectangular gridwork of traces a few microns in width.
In some cases, these results were correlated with microscopic observations of the wear scars. One important disadvantage of the electron microprobe is that the depth of penetration of the electron beam was greater than the film thickness and could not be accurately determined. Therefore the reading of element concentration often included material below the surface film.
-3-Ii. APPARATUS
The equipment used for the crossed-cylinder tests was the same as was described in (1). The vacuum pumping station is a standard commercial unit with a 4-inch oil diffusion pump. The stainless steel test chamber attached to the pumping station can obtain a pressure of 1 x 10 torr if the chamber is clean. Blanked off, the pump can reach 8 x 109 torr. The pressures obtained in the present series of tests varied
-5 -4
between 10 - 10 torr depending on the lubricant being tested. Figure 1 shows a picture of the equipment.
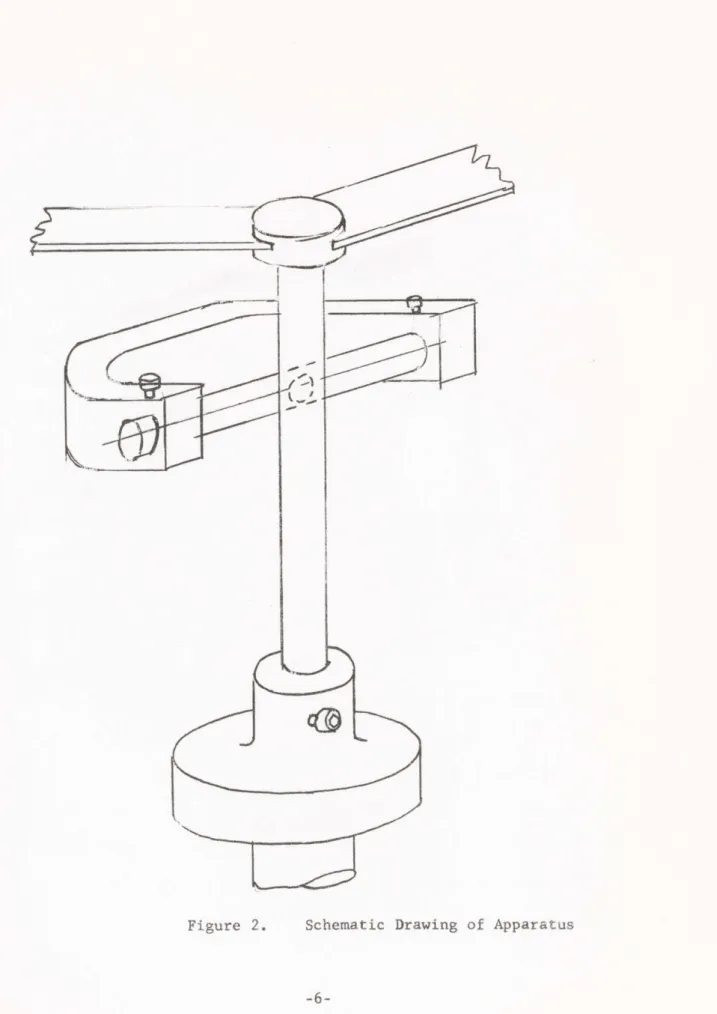
The crossed-cylinder test specimens were 1/4 inch 52100 steel bearing rollers of hardness 60-62 Rockwell C. One roller is rotated through the magnetically driven spindle while the other is held stationary against it at a right angle producing a circular wear scar (See figure 2). Loads
from 10 to 140 kilograms can be transmitted from outside the chamber through a bellows assembly to the contact area. Oil was supplied by a wick which was thoroughly soaked in
the lubricant prior to testing, such that there was always a meniscus at the contact between the two specimens. The
test specimens were prepared by ultrasonic cleaning in an acetone bath. All lubricants were degassed overnight in a
Pnotograph of crossed cylinder
test apparatus.
5
Figure 2. Schematic Drawing of Apparatus
-6-AIL cf the previous tests described in (1) were run for
a duration of 1 hour at a speed of 150 R.P.M. At the low pressures obtained in the vacuum tests, heat generated at the friction zone must be dissipated either by conduction through the specimen and retainer or radiation through the environment. At ambient conditions, however, heat could also be dissipated by convection through the atmosphere. Therefore it is expected that tests in vacuum would run hotter than comparable tests in air since friction heat would be more difficult to remove. The temperature difference between a comparable vacuum and air test was measured adjacent to the wear scar with a thermocouple arrangement and found to be as much as 250F. A question
arising from this is whether the generally poorer behavior of lubricants in a vacuum could be attributed to higher operating temperatures.
To resolve this problem all present tests were performed at 30 R.P.M. for 5 hours duration. This reduced the rate of
friction heat input to approximately 20% of its former value
and minimized the temperature difference between vacuum and
air testing to only a few degrees. Many trends observed in the previous higher speed tests were still present confirming
-7-the earlier results.
The pin-on-disc machine is shown in the photographs Figures 3 and 4. The pumping station utilizes a mercury diffusion pump which can pump the chamber, when clean, down to about 107 torr. A variable speed motor capable of speeds up to 2000 R.P.M. is magnetically coupled to the disk from outside the chamber. The pin, of 1/4 inch diameter with a spherical end of 1/2 inch diameter is held in an arm
instrumented with a strain ring assembly to measure friction force. This arrangement is pictured in Figure 4. Load is applied by dead weights attached to the arm above the pin. In the present tests, the pin rubbed against the disk on a 2 inch diameter. Both pin and disk specimens were 52100 steel heat treated to 60-62 R hardness. A micrometer control bleed
c
value was connected to the chamber to allow air to flow into the chamber from a partially evacuated second chamber. This gave very fine control on the pressure obtained and ensured the composition of the environment in the test chamber to be mostly air.
The pins were ultrasonically cleaned in an acetone bath
before use. The disks were ground, lapped, and finally polished on 4/0 energy paper prior to flooding with the test lubricant.
-8-Photograph of vacuum test apparatus
Photograph of test chamber and dynamometer.
9
itL
Figure 3.
EXPERIMENTAL RESULTS AND DISCUSSiON
1. Crossed-Cylinder Tests
The results of the crossed-cylinder tests are shown in Figures
5-12 where the wear scar diameter is plotted against contact load.
The range of loads considered was from about 10 to 140 kilograms. In many tests a limit load was observed corresponding to a sharp increase in wear with increasing load. This limit load phenomenon is a general feature of boundary lubrication and EP additives are chosen for their ability to raise the limit load. In many
instances the limit loads were apparently above 140 kilograms and could not be exhibited with the present apparatus at 30 R.P.M. The behavior of each of these lubricants will now be considered individually.
a) U.S.P. Mineral Oil
The results of the mineral oil tests in air and vacuum are shown in Figure 5. At loads below approximately 97 kilograms wear resistance is slightly improved in vacuum. However at about
100 kilograms the vacuum tests show a sharp limit load. This is
apparently due to the exclusion of oxygen which can chemically
react with both the lubricant and the rubbing steel surfaces.
Oxidation of the lubricant increases the polarity or acidity of mineral oil by presumably forming organic peroxides which, in turn, oxidize the remaining oil to such products as fatty acids and
oxyacids which can readily react with the surfaces. Metal reaction
LU61kICAKJT: MINERAL OIL
30
k.p.M
0V/\ c
OURS
V/\CUUM
h~IK
...
4
11 1 a I a k a I a a a A I Izo
5o
4
05
0k4
'0 60
30
too
110
Izo
130
1+0
150
NTACT
LOADKILOGRAM5
Crossed-Cylinder Test-Mineral Oil
-11-2.4
#2.2.1
2Z.
6
I-
1.2.4-it
Oh-
0-0
1 C
co
Figure 5.i xygen can lead to the formation of a protective oxide fili.
b) U.S.P. Mineral Oil plus 2% Sulfur
The behavior of sulfur as an EP additive has been generally attributed in the past to the formation of a protective sulphide
layer. More recent work by Godfrey (5), however, has shown the major constituents of films on steel surfaces lubricated with sulphurized oils to be iron oxide Fe304 with only a minor fraction of iron sulfides. He concluded that the presence of sulphur serves to promote oxidation of the steel under EP conditions.
Figure 6 shows the results of the crossed-cylinder tests for sulphurized mineral oil as compared with pure mineral oil. The sulphurized oil does not behave as well in vacuum as in air. The improvement of the sulphurized oil as compared to the plain mineral oil in vacuum suggests that what little oxygen was available in the vacuum tests was more effectively used in the presence of sulphur. The oxygen dependent behavior of this lubricant supports
Godfrey's conclusions.
c) U.S.P. Mineral Oil plus 5% n-Hexadecyl Chloride
Figure 7 shows the EP behavior of chlorinated mineral oil
as compared to pure mineral oil. The lubricant works wells in air
to the extent that above approximately 80 kilograms load the wear vs. load curve actually begins to show decreasing wear with load. While the full significance of this unusual behavior is not yet understood, it is apparently due to the onset of a beneficial
3.,
LUBKCANT'MINERAL (
L
+
)4ULFW
$
'30
RPM
5-H0 URS
W
2.4-
A
--
VAC-UUM
o
AIR
tu'
REF. MINERAL OIL
2
--
I-
VAcUu(A
UI
-uJl
t1.6-C--.
0 1o
?0
30 40
50
0 70 60 90
100
110 ZO 130
K-0 150
CONTACT
LOADI K
ILO
GRAM
S
Figure 6. Crossed-Cylinder Test-Mineral Oil plus 2% Sulphur
-13-LUKkCANT
:
MIN
EKAL
O
L +
5%
/NJ-HE)(A
DECVL CHLORIDE
3ORPM'
-51-OUk$
y
-
VAc.UUM
A I
.. u
REF:
MINEkAL
OIL
VA CUUM
AIRP..
0 -Lii24
02
-JI2
-U
U1.0
/
If
I/
-~ -1-~~'~~'
a a -- -~ I U A fl i..LO
10 ?O30 4-6
50 to'
CO NTA
CT
70 80
s0o
LOAD, KI
100
11012-0
130
140
LOGR.AMS
Crossed-Cylinder Test-Mineral Oil plus 5% n-Hexadecyl Chloride
-14-
I-3%
0.4
0
Figure 7.J50
a
9
A A _
-r
O,..N
chemical reaction between oxygen and chloride which is activated
by the increasing temperature with higher loads. This phenomenon
was also observed with diester oils.
The EP behavior of chlorine on steel is generally believed to result from the formation of an easily sheared iron chloride film on the friction surface. A simple test for the presence of
iron chloride was performed. A few drops of distilled water were
deposited on the wear scar followed by a few drops of 0.1 normal silver nitrate solution. If an iron chloride film is present,
it should go into solution and a white silver chloride precipitate should form. Indeed this was the case with the tests run in vacuum where increasing amounts of silver chloride were found with increasing loads. However in no instance was there any
trace of silver chloride precipitate on any of the specimens run in air. Yet the EP action was effective in the air test but not the vacuum test.
Therefore, the EP action of chlorinated oil is not simply due to the formation of a protective iron chloride film. The dependence of oxygen for effective lubrication strongly suggests
that, like sulphur, the presence of chlorine promotes oxide forma i4on. This statement is supported by the work of Brummage (6) who, using electron diffraction techniques, identified oxychloride films on
surfaces run with chlorinated oils.
-15-U.S.P. Mineral OivI pi.s T.C.P.
Figure 8 shows the EP action in vacuum and air for mineral oil plus 5% T.C.P. as compared to pure mineral oil. Unlike the sulfur and chloride additives, T.C.P. exhibits good wear
resistance in vacuum and air, giving almost identical results. Studies with the electron microprobe indicate that for tests run in vacuum there was good correlation between phosphorous and oxygen content on the surface with increasing amounts at
higher loads. Peaks in phosphorous concentration corresponded to peaks in oxygen concentration almost exclusively with the
proportionality factor of about 2-3 by weight. On the other hand tests run in air show a peak phosphorous concentration at
intermediate loads. The phosphorous content of air formed films was .9-1.0%, whereas vacuum formed film phosphorous content varied
from 1.4% up to 4% on the most heavily loaded sample. Phosphorous peaks appear as streaks on the surface running in the direction
of relative velocity with each streak containing little spots of phosphorous. The range of oxygen concentration was about 7% except in the highest loaded air tests where concentrations up to 30%
occurred.
Thi; strongly suggests that, with the exclusion of oxygen fromthe environment, the phosphate group enters directly into the surface reactions. This could also be the situation with the tests in air, when in addition, there is a competing oxide formation on the surface.
LUE5IcANTMINE
RAL
'0RP
M
-
-HoURS
*-
\ACLUIVMo
AI
R.
REF. MINERAL
OIL
/--VACOUVM
--AIR
OIL +5
0/oTC, P,
/- ______ - - -.~---I I ~ I I I
o
o
o30 40
50
10
70 80
CONTACT LOAD
90 100
110 Jzo 130
I'+0 ISU,
KILOGRAM5
Crossed-Cylinder Test-Mineral Oil plus 5% T.C.P.
2.41-j14. 1.2 1.00 ~8
o.z-0
Figure 8.Recent work by Codfrey (7) and Barcroit and. Daniel (8) attribute the effectiveness of organic phosphate additives to the formation of metal phosphates or metal organophosphates on the friction surface. Such a reaction could occur by the organic phosphate hydrolyzing in the presence of water vapor to acid phosphate which would ultimately result in metal phosphates.
At first glance this mechanism would not be expected to work in vacuum in the absence of water vapor, but the cross
cylinder tests showed good results for T.C.P. additive in vacuum. Klaus and Bieber (9) have emphasized the importance of polar impurities in commercially obtained T.C.P. This would indicate
that the effectiveness of T.C.P. additive in vacuum is due to the acid phosphate impurities present prior to testing. This idea correlates well with the electron microprobe studies.
e) Diester Oils
The diester oils are employed as base fluids in the lubricants used for the military specification series Mil-L-7808. These oils usually consist of a diester base, phenothiazine antioxidant, and T.C.P. (10).
The first oil tested was di(2-ethylhexyl azel'ate). The results in Figure 9 indicate that over the entire range of loads, wear resistance was better in vacuum than in air. This effect
persisted with all diesters tested and will be further discussed at a later point.
LUBRICANT.' DI-Z ETHYLH
'30 RPM
-5H
OUR.5
0_-
VA CUUM
o
AIR
E)CY L AZ
F..LAT
E
a a a a I * I I I I I a II
O
10
LO
30
40b 4 0
10 8o
itoo
110
.IZO
'0 140
CONTACT LOAD, k ILO&RAIMS
Crossed-Cylinder Test - Di(2-Ethylhexyl Azelate) Diester.
2.4j-I.4
1.0-
0.8-o,
4
0.22
0
Figure 9.3,0
A negative slope region on the wear vs. 3oad plot.
which occured with chlorinated oil, Figure 7, also appeared with this lubricant in the air tests. It is interesting to
note that with the addition of only .5% phenothiazine antioxidant, Figure 10, this effect is diminished in air while the results in vacuum remain essentially unchanged. The beneficial surface reactions which are brought on by higher temperatures due to increased loads are inhibited in the presence of antioxidant.
Figure 11 shows the effect of adding 5% T.C.P. to the lubricant. The additive is effective in reducing wear in both vacuum and air with less wear observed in the vacuum.
f) Silicone Oil
Three silicones investigated were General Electric F-50 (chlorophenyl methyl silicone), F-44 (the same as F-50 but with a small amount of antioxidant), and Dow Corning DC 200 (dimethyl silicone). All of these silicones have a kinematic viscosity
of 50 centistokes at 770F.
The test results appear in Figure 12 for the crossed-cylinder vacuum and air tests. The lubricants ranked according
to their effectiveness in reducing wear are in the order F-50,
F-44, and DC 200. In all cases the wear in vacuum was comparable or greater than 'hat in air, the difference increasing with
increasing load.
In a recent paper Tabor and Winer (10) have demonstrated that silicones exhibiting a larger pressure coefficient of viscosity
2 .4
LUBRIcAN
T:
DI-2 E THYLH
EXYL
AZELATt-+
.
5-
%
PH ENO
T H
IAZINE
30LPM
-6HOUR5
'A- -
-VACUUM
-
A
IR.
1 .6 10'o,4
02
V
o.L
5 a I I I I ~I A A A S I i A0
10
Z0
50
40
'~0
'c
*70
80
90 100
1O iZO
130 140
ISO
CONTACT
Figure 10.LOAD,
KILOGRAMS
Crossed-Cylinder Test - Diester plus.5% Phenothiazine
-21-2-8
2.2-L UbK
IC4A
NT U
l -Z E TH'L H EY'L
A
CELATE
t-05%
PHENO TH
IAlINf
+ 5-0o
CP
30 RPM
-SHOUR-S
X-
-
VA
CUUM
0
-
AIR-KEF. DUI -Z
ET
HYL HEM LAELATE
+
.
g*/o PHNENOCTHIA7 N
-/
-VA CU UM
2.1
2141
0.,
0.4
0.1
o
(0
zo
t3o
40
5o
4.070
RD 30
100
110
t0 130140
15o
CO NTACT
Figure 11.LOADr KILO&KAMS
Crossed-Cylinder Test - Diester plus
.5% Phenothiazine plus 5% T.C.P.
-22-2.6
-
-~ i 'I I * I II
a a ~ I S 051LI CON E 5
30 RpM
F- 5-o
F- 44
D
C-'
zoo
0
A4
2+4
-f4 oURS
VAcouv\
0
A
a
'II
u.-2
Ui
(V_
uJ
(/A0o-04
o'Z.k
I a0
o
'3o
40
50
(CoN
TAC
T
Figure 12.'70
80
90
t0
110
JZ(
130
140
LoAD;
K
I LO
Ck-AMI S
Crossed-Cylinder Test - Silicones
.22-
Iz-0
1510
QIU
'L-72
can frm thin hydrodynamic films a, higher loadS. Ma.4emaia1l
the viscosity of most lubricants can be described by the expression
O(1)
where "W" is the viscosity at pressure "p", and is the
viscosity at zero pressure, generally taken at ambient pressure.
The symbol "k " can be taken as a constant for a particular lubricant, and therefore the larger the value of "P", the greater is the
viscosity increase under pressure. Silicone lubricants with more phenyl groups have a larger "o(" than the dimethyl groups.
The loads encountered in the crossed-cylinder apparatus are well above the load which can be completely supported by a hydrodynamic film. Since the silicones are relatively inert they would not tend to form solid chemical films; therefore, large loads should be carried by both a thin fluid film and direct metallic contact. The increased wear in vacuum would be due to insufficient protective oxide on the parts of the interface which come into
direct metal to metal contact. The silicone lubricants with smaller values of "ow" should not be as effective in preventing wear since less of the load would be carried by the not so viscous
fluid film and more by metallic contact. Indeed this was the case with the DC 200 as compared to the F-50.
A common feature of these silicone tests are the appearance
of brown-black sludges which may be formed by a chemical reaction on freshly exposed highly reactive metal surfaces. In the presence of an antioxidant these reactions might be expected to be modified since the sludge may be either oxidation products or polymers formed with whatever oxygen may be dissolved in the lubricant or otherwise present in the contact zone. The antioxidant would serve to use up some of the available oxygen for this reaction. Less sludge did form with the F-44 than with the F-50.
Samples of sludges formed in both air and vacuum tests were sent to Dr. R.S. Fein at Texaco Research Center for analysis. Using an infrared absorption technique he found that in addition to small amounts of black ferromagnetic wear particles, the sludge for the most part consisted 6f silicone compounds essentially
similar to the base oils except in a highly polymerized complex form.
All sludge was organic in nature and would be expected to act like
a grease. Air and vacuum sludges showed little variation. Although the F-44 seemed to generate more sludge, there was little difference in the wear resistance of these two lubricants
in air. The sludge formation then would not seem to be an important factor for boundary lubrication with silicones. The adverse
behaviors of the F-44 in vacuum would be due to the antioxidant
which uses up what little oxygen is available for oxide formation.
-25-2. Pin on Disk Results
Tests with the pin-on-disk machine are currently being performed and the results offered herein represent a very small part of the
total effort. Friction tests with F-50 appear in Figure 13 for a speed of 175 R.P.M. and load of 1600 kilograms where the pressure was varied from 7 x 10-6 to 760 torr. As pressure is decreased below 10~4 torr the friction coefficient begins to rise. If the
load is supported by both a fluid film and direct metallic contact, as postulated, the rise in friction could be attributed to increased metallic friction.
Earlier tests performed by Mr. R.G. Foster on this apparatus studied the effect of friction coefficient on speed using mineral oil as a lubricant. The disk in this case was at a hardness of
80 RB in contrast to the above results with disks of hardness
60-62 RC. His results, Figures 14, 15, and 16 show a sharp increase in friction at a critical speed for a given load and
pressure. If the speed was lowered after reaching this transition, the friction would drop to its lower value. This speed phenomena as of yet has not been able to be demonstrated with F-50 as the lubricant. At the time of these tests this phenomenon was not satisfactorily explained.
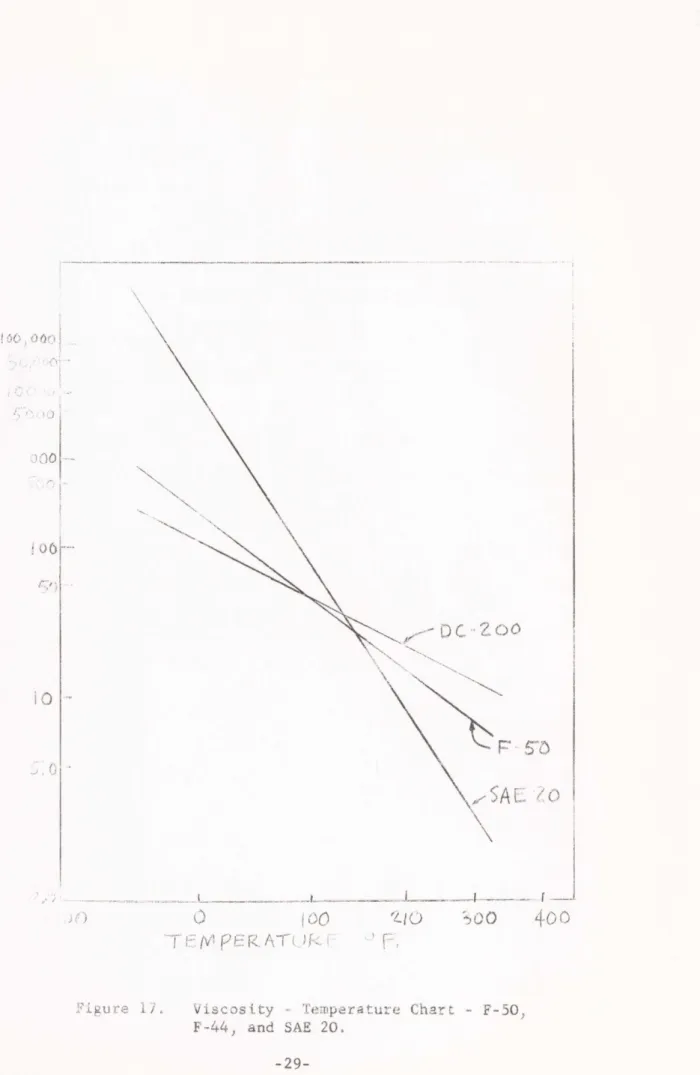
With reference to Figure 17 it is apparent that an oil with viscosity specification SAE 20, which closely approximates the mineral oil used here, shows much greater changes with temperature than silicone oils. Even though the viscosity of the SAE oil has
LOAP =Ito
~,:.
A
N
Pf= p
-=
fl/t)
K
vIM
I00 II/.MIN.
-',
-5'-4 30
10
(6
16
p R S I
Figure 13. -Zi -I o a 10to
10
10
S U
RE
1T
O K
Pin-on-DiskTest - F-50 Silicone-27-~L
-'
U.L.(U
0
U10
.05
01
IC
7
I0
2gO
S010
4I
4 -I~
0
INITIAL
PASS,
55i~4 TOh.
C-1 N &W
TRAC.
S~10-4
-TO
R
oII?
I
1418 GrZAMS
MINERAL
OIL
Q~tj-
--
--I
I I I II
I I II
1 1
AL
PAS
S
A M
r TKAC
K
'3 xto --
o re.
-1 x ~ T RS EcoND
PASS
Z.
1170
/430GRAMS
AINEZAL OIL
I I a aI
I
I
I
I
0
IR0
& RLAMSMINEKAL OIL
0o
2Q1o5 To
RR.
I
I
I I I3
$UfZFACF
Figures 14-16S
PEFD
) -4
IN cHES PEK.
MI WUTE
Pin-on-Disk Tests - Mineral Oil at Different Speeds
OCDIj
c:j7
0.11-20.4
0&
IL
U
0.1
0
+
J
1,5
b
I
III
4ra
O
O
I
-00
iO
SAE
O
J2
C
'.oo
4o
0
7-E 10t
E K A T f<
F",
Figure 17. Viscosity - Temperature Chart - F-50, F-44, and SAE 20.
-29-more than twice the viscosity of F-50 or DC-200 50 censtitoke fluid at room temperature (770F), its viscosity at 3000F is only
about 3 censtitokes as compared to 7 censtitokes for F-50 and
12 censtitokes for PC 200. Therefore this transition observed
with mineral oil but not with silicone might be attributed, at least in part, to high temperatures encountered at high sliding velocity with simulatneous thinning of the oil. Other rheological
effects concerning the effects of shear rate and pressure on viscosity complicate the picture.
Comparing Figures 14 and 16, where the loads are comparable, this effect occurs at lower sliding velocity at harder vacuum. This is, in general, consistent with the above ideas since the friction values are greater at the low pressure and therefore the contact zone becomes hotter. Further work will be undertaken
to study this effect in more detail.
-30-IV. FURTHER DISCUSSION
One interesting aspect of the crossed-cylinder tests is seen from the behavior of the three base oils considered - silicone, mineral, and diester oils. The silicone oil is the least
corrosive followed respectively by mineral and diester oils. With the silicone oils the wear is greater in.vacuum than in air
over the entire range of loads while just the opposite is true for diester oil. The mineral oil shows a transition at about
97 kilograms below which wear is greater in air and above which
is greater in vacuum. This suggests that with less available oxygen, good boundary lubrication can be obtained from the corrosive action of lubricants, the more corrosive lubricants being more effective at higher loads. Where the wear is
greater in air than in vacuum, the reaction generated more than an optinum or sufficient quantity of products.
Corrosiveness in this sense represents the ability of the lubricant to react with the metal surface in the presence of whatever oxygen is available. Since the silicones tested always
result in greater wear in vacuum than in air, approaching the same wear at low load, the corrosive action of the lubricant and
environment taken together appears unimportant as far as wear is concerned. The pin-on-disk tests with F-50 silicone as discussed above suggest the differences in behavior in vacuum and air are attributable to changes in metallic friction components independent
of the lubricant. The crossed-cylinder tests with both the mineral
-31-aAd die :er, 'cwever, indicate that currosivenees, as explained
above, is also important. At higher loads increasing wear rates require that corrosive products from faster to replenish the more rapidly wearing surface film. This is of course a self-accelerating process since the increased temperature associated with higher loads will result in more rapid chemical reaction. The crossed-cylinder
limit load observed with the mineral oil in vacuum suggests that the increased temperature could not produce enough of a corrosive film fast enough to overcome the increased wear with greater loads. This transition represents a change to the type of lubrication obtained with the silicone. The noncorrosive nature of the silicone
exhibited in these tests throughout the load spectrum investigated explains why there is not a limit load transition but rather a gradual increase in wear with load. Since the diester oil does not show a limit load transition and its wear resistance is better
in air than in vacuum the corrosive action of the lubricant is sufficient even with little oxygen present to replenish worn away surface films.
The transition phenomenon observed with the pin-on-disk machine at high sliding speed are similar in nature to those observed
by Fein (12). Using a four-ball machine he found that the flash temperatures at transition either increase with viscosity and speed and decrease with load, or are independent of load or speed,
depending on the hydrocarbon tested. This was explained by proposing a boundary lubrication mechanism involving "viscous
trapping
of iubricant between interacting load-supporting asperities" It is interesting to note that the viscosity at the transitiontemperature for all lubricants tested covered a very narrow range despite a wide range of transition temperatures observed, thus implying that viscosity plays a fundamental role in the
transition.
Silicone oils show a flatter viscosity-temperature behavior than mineral oils. On the other hand the pressure coefficient of viscosity for the silicones is generally lower than for mineral
oils. Therefore under load the silicone would not be expected
to form thin hydronamic film as readily as minerals oils. As the sliding speeds are raised, viscosity-temperature effects would become increasingly significant and the relative behavior of the
silicones should improve.
-33-V. CONCLUSIONS
The use of vacuum has produced much enlightening information concerning boundary lubrication. EP action was studied in vacuum and air with crossed-cylinder tests. The behavior of sulfur as an EP additive has been attributed to promotion of oxide formation. Results with chlorinated mineral oil suggest a similar behavior. Although the proseace of oxygen does not seem to affect the wear behavior of phosphated mineral oil, studies with the electron microprobe indicate both a phosphate and oxide reaction on the friction surface.
The role of oxygen in combined reaction with the surface and lubricant has been stressed in tests with silicone, mineral and diester oils. The limit load transition with mineral oil is
attributed to the inability of the corrosive action of the lubricant to replenish worn away surface films. This problem does not arise with more corrosive diesters. Relatively inert silicones function as boundary lubricants by supporting the contact load with a fluid film and direct metallic contacts they show no limit
load transition and increased wear in vacuum is due to direct oxygen reaction with the surface. The formation of sludges is apparently a secondary effect.
Viscosity seems to be an important parameter in boundary lubrication. Speed transitions with the pin-on-disk machine
poor behavior of silicones under heavy load conditions can be
partly attributed to their inherently low pressure coefficient of viscosity as well as their relative inertness towards the surface.
-35-VI. FUTURE WORK
Future work is planned using the pin-on-disk machine to study, in more depth, speed transitionsat various loads. One complication arising in such tests is due to the changing contact area of the hemispherical pins. Future tests will probably be run with cone shaped specimens to slow down the rate of increase of contact area.
The frictional behavior above the transition speed will also
be investigated. If the frictional behavior in this zone proves
to be comparable to that observed with dry friction, this would
support the idea that the transition occurred by the thinning and squeezing out of lubricant from the contact area.
REFERENCES
1. G.S. Reichenbach, R. Shaw,Jr., and R.G. Foster, "Lubricant
Behavior in High Vacuum", Trans. A.S.L.E., Vol. 7, 83-90,
1964.
2. G.V. Vinogradov, N.T. Pavlosvskaya, and Yu, Ya. Podolsky, "Investigation of the Lubrication Process under Heavy Friction Conditions", Wear, Vol. 6, 202-225, 1963.
3. E.E. Klaus and H.E. Bieber, "Effect of Some Physical and
Chemical Properties of Lubricants on Boundary Lubrication", Trans. A.S.E.E., Vol. 7, 1-10, 1964.
4. J.B. Van Saun, "Boundary Lubrication: An Elucidation of
the Nature of Extreme Pressure Lubricated Friction Surfaces", S.B. Thesis, M.I.T., May 1964.
5. D. Godfrey, "Chemical Changes in Steel Surfaces During Extreme
Pressure Lubrication", A.S.L.E. Trans., Vol. 5, 56-66, 1962.
6. See F.P. Bowden and D. Tabor, The Friction and Lubrication of Solids, p. 233, Oxford, 1950.
7. D. Godfrey, "The Lubrication Mechanism of Tricresyl Phosphate
on Steel", A.S.L.E. Preprint No. 64 LC-1.
8. F.T. Barcroft and S.G. Daniel, "The Action of Neutral Organic Phosphates as EP Additives", A.S.M.E. Paper No. 64-Lub-22, 1964.
9. E.E. Klaus and H.E. Bieber, "Effects of P Impurities on the Behavior of Tricresyl Phosphate-32 as an Antiwear Additive",
A.S.L.E. Preprint No. 64 LC-2, 1964.
10. See R.C. Gunderson and A.W. Hart, Synthetic Lubricants.
Chapter 5, 1962.
11. D. Tabor and W. 0. Winer, "Silicone Fluids: Their Action as Boundary Lubricants", A.S.L.E. Preprint No. 64 LC-8, 1964. 12. R.S. Fein, "Effects of Lubricants on Transition Temperatures",
A.S.L.E. Preprint No. 64 LC-7, 1964.