Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Technical Translation (National Research Council of Canada), 1948-10-26
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=a8437836-ec36-44a8-81f6-8e8c198e0c31 https://publications-cnrc.canada.ca/fra/voir/objet/?id=a8437836-ec36-44a8-81f6-8e8c198e0c31
NRC Publications Archive
Archives des publications du CNRC
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/20331576
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Supercooling capacity of water and the linear crystallization velocity of ice in aqueous solutions
Ref Ser 421 ~ 2 t h no. TT-79 BLDG COPY NO.
NATIONAL
RESEARCH
COUNCIL
OFCANADA
DIVISION OF MECHANICAL ENGINEERING A IRC PUB
!
1 4TECHNICAL TRANSLATION NO. TT
-
79THE
SLIPERCOOLING CAPACITY OF WATER AND THE LINEAR
CRYSTALLIZATION VELOCITY OF ICE IN AQLlEOLlS SOLUTIONS
KRISTALLlSATlONSGESCHWlNDlGKElT DES EISES IN WASSR~GEN LOSUNGEN
II
BY G. TAMMANN AND A. BUCHNER
OTTAWA
NATIONAL RESEARCH LABORATORIES Ottawa, Canada TECHNICAL TRANSLATION D i v i s ion. of Mechanical E n g i n e e r i n g Pages
-
P r e f a c e-
2-
Text-
9 F i g u r e s-
8 Tech, T r a n s , TT-79 Date-
26 October, 1948,Lab, Order No,
-
5424AF i l e
-
12-R4-22T i t l e : Die U n t e r r n l u n g s f l t h i g k e i t d e s Wassers und d i e
l i n e a r e Kristallisationsgeschwindigkeit des
E i s e s i n wgssrigen L8sungen0 By: G o Tammann and A o ~ f l c h n e r ,
Reference : Ze i t s c h r i f t f u r a n o r g a n i s c h e und a l l g e m e i n e Chemie ( J o u r n a l f o r I n o r g a n i c and General
C h e m i s t r y ) , Vol, 222, 1935, pp, 371-381, Report o f t h e I n s t i t u t e f o r P h y s i c a l Chemistry,
G8 t t i n g e n ,
S u b j e c t : THE SUPERCOOLING CAPACITY OF WATER AND TIE
L I N E A R CRYSTALLIZATION VELOCITY OF ICE I N AQUEOUS SOLUTIONS, S u b m i t t e d b y : J o Lo O r r , T r a n s l a t e d by: He ad, H o A o G o Nathan, Low Temperature L a b o r a t o r y , Approved by: J o H , P a r k i n , D i r e c t o r , SYNOPSIS Very l i t t l e i s known a b o u t t h e c r y s t a l l i z a t i o n v e l o c i t y of w a t e r and i t s s o l u t i o n s o This r e p o r t s e r v e s t o draw a t t e n t i o n t o a f i e l d of s c i e n c e which h a s s c a r c e l y been touched upon by r e s e a r c h ,
Page
-
( i f ) Te ch, Trans, TT- 99 TABLE OF C O N T E N 2 ----
P The S u p e r c o o l f n g C a p a c i t y of 'JVaterP
1, The S u p e r c o o l i n g Capa- c i t y o f Pure Water 2 2 , The I n f l u e n c e of F o r e i g n S u b s t a n c e s on t h e S u p e r - c o o l i n g C a p a c i t y of Water 3 P I The L i n e a r C r y s t a l l i z a t i o n V e l o c i t y of I c e i n Aqueous Sol.ut io n s 5Page
-
lTech, T r a n s , TT-79 THE SUPERCOOLING CAPACITY OF WATER AND TKE
LINEAR CRYSTALLIZATION VELOCITY OF
IC" I N AQUEOUS SOLUTIONS
TH5 SUPERCOOLING CAPACITY OF' WATER
The c r y s t a l l i z a t i o n c e n t r e s of i c e a r i s l n g i n s u p e r c o o l e d w a t e r have
-
even a t s m a l l s u p e r c o o l i n g s-
such g r e a t l i n e a r c r y s t a l P f z a t f o n v e l o c i t i e s t h a t w i t h i n a s h o r t time d e n d r i t f c i c e n e e d l e s a r e s e e n throughout t h e e n t i r e o b s e r v a t i o n v e s s e l , For t h i s r e a s o n t h e observat.ion of t h e f o r m a t i o n of f ' u r t h e r c r y s t a l l i z a t i o n c e n t r e s i s fmpossible, Hence i t i s n o t p o s s i b l e ( b y analogy w i t h o t h e r f l u i d s ) t o determine the s u p e r c o o l i n g c a p a c i t y o f w a t e r a s a f u n c t i o n of t h e s u p e r c o o l i n g , by countfng t h e c r y s t a l l i z a t i o n c e n t r e s which form s p o n t a n e o u s l y w i t h i n a g i v e n time a t a g i v e ns u p e r c o o l i n g , I t i s o n l y p o s s i b l e t o determine t h e appear- ance of t h e f i r s t c r y s t a l l i z a t i o n c e n t r e a s a f u n c t i o n o f t h e time and temperature, This may be done i n two ways, e i t h e r by c o o l i n g t h e w a t e r s l o w l y and determinfng t h e tem- p e r a t u r e a t which t h e c r y s t a l l i z a t i o n b e g i n s o r by c o o l i n g a small q u a n t i t y o f water a s r a p i d l y as p o s s i b l e t o a known temperature and by measuring t h e time u n t i l t h e f i r s t
c r y s t a l l i z a t i o n c e n t r e forms, Observations were made u s i n g b o t h methods, The r e s u l t s a r e g i v e n below,
For the slow c o o l i n g t e s t s g l a s s t u b e s having an i n n e r d i a m e t e r of 0 , 8 mm, and a w a l l t h i c k n e s s o f 0,4 mm, were u s e d , Each tube was f i l l e d w i t h 0,4 c c , of water and
s e a l e d a t b o t h e n d s , The tubes were p u t i n a b a t h which was c o o l e d by a copper p i p e f i l l e d w i t h d r y i c e , A mechanical s t i r r i n g rod ensured uniform temperature d i s t r i b u t i o n , I n
t h i s b a t h t h e t u b e s were cooled w i t h c o n s t a n t v e l o c i t y b e g i n n i n g a t t h e m e l t i n g p o i n t , The t e m p e r a t u r e a t which
spontaneous c r y s t a l l f z a t f o n began was observed, Normally t h e c o o l i n g r a t e was approximately 0,7 t o 0080C p e r minute, For t h e t e s t s u s i n g t h e r a p i d c o o l i n g method t h e tubes were s i m i l a r b u t heda s m a l l e r w a l l t h i c k n e s s o f o n l y 0 , l mm, The t u b e s were p l a c e d i n a b a t h o f a l c o h o l whose t e m p e r a t u r e was m a i n t a i n e d c o n s t a n t t o w i t h i n O , l e C , The time was measured
from t h e immersion of t h e tube t o t h e b e g i n n i n g o f c r y s t a l - l i z a t i o n ,
Pag?
-
2T e c h Trans. TT-79
1,
The Su-1~ C a p a c i t y of Puce W a t e ~The r e a d i n g s t a k e n fn t h e t e s t s w i t h pure water a r e g i v e n i n Table I ,
Tubes 1 t o 4 c o n t a i n e d o r d i n a r y d i s t i l l e d watek while t u b e s a and b were f i . Z l e w i t h heavy w a t e r c o n t a i n i n g moye than
X P
99,s p e r c e n t D20 The t a b l e g i v e s t h e t e m p e ~ a t u r e s of' the b e g i n n i n g of c r y ~ t a l l i z a t i o n a t v a r i o u s c o o l f n g r a t e s , There a r e d i f f e r e n c e s of s e v e r a l degrees be tween t h e v a r f ous t ~ l b o s , whereas, on r e p e a t i n g t h e measurement f o r e a c h f n d f v i d u a l
t u b e , t h e v a r i a t i o n s amount o n l y t o a few t e n t h s o f a d a g r e e ,
d e s p i t e t h e f a c t t h a t t h e formatlorn o f a c s y s % a l l i a a t l o n c e a t r e IS 8 chance occurInence and conseqilently a P a ~ g c r
v a r i a t i o n would have been expected,
The t e m p e r a t u r e of t h e b e g i n n i n g of c r y s t a l - l i z a t i o n was independent o f tho c o o l i n g r a t e o v e r a wide r a n g e , The formatfon of an i c e n u c l e u s was n e v e r t h e l e s s favoured by sudden c o o l i n g o I n f i v e t e s t s o f tube 1, f o r
example, d u r i n g the c o o l i n g from +20° t o -12°C c r y s t a l l i z a t i o n o c c u r r e d a f t e r 12,0 9 0,8 seconds, and i n t u b e 3, a f t e r
'7,S 2 0,4 seconds, A t slow c o o l i n g , t h e s e two tubes remained a t t e m p e r a t u r e s s e v e r a l d e g r e e s below -12OC f o r 1 6 and 9
minutes r e s p e c t i v e l y , b e f o r e c r y s t a l l f z a t f on s e t i n , I n t h e s e t e s t s , too, an u n e x p e c t e d l y good r e p r o d u c i b i l f t y was n o t e d ,
The s u p e r c o o l i n g c a p a c i t y o f heavy w a t e r w a s found t o be s l i g h t l y l e s s t h a n that. of o r d i n a r y w a t e r , I t w a s a l s o found t h a t t h e dependence of t h e b e g i n n i n g s f cpys-
i.narg w a t e r . Z e i t s c h r i f t f a r anorganfsche und
Page -=. 3 Tech,, T r a n s , TT-79 2 , qnf@aloloeFuSoocr2 C a p a c i t y o f Water The i n v e s t f g a t f o n o f t h e s u p e r c o o l i n g c a p a c i t y o f w a t e r , w!-lich y i e l d e d t o t a l l y d i f f e r e n t v a l u e s f o r t h e v a r i o u s t u b e s b u t gave r e s u l t s t h a t c o u l d e a s i l y b e reprao- duced f o r e a c h i n d i v i d u a l t u b e , seemed t o s u g g e s t t h a t t h e v a r i a t i o n s may b e a t , t r f b u t e d t o t h e i n f l u e n c e o f f o r e i g n substances, e s p s c f a l l y s f n c e t h e t e s t s showed t h a t , a s a r u l e , c r y s t a l l f z a t i o n s e t i n a t t.he same p o i n t s o f t h e t u b e s , It i s a known f a c t t h a t , u n d e r c t h e r w i s e i d e n t i c a l c o n d i t l o n s , f o r e i g n s u b s t a n c e s i n s o l u t i o n have a s t r o n g e f f e c t on t h e number o f c r y s t a l l i z a t i o n c e n t r e s b e i n g formed, But e v e n i n s o l u b l e f o r e i g n s u b s t a n c e s , , s u c
a s powdered s t o n e and m e t a l w i r e s , i n f l u e n c e t h i s numherxf; however, n o d i r e c t dependence o f c r y s t a l l i z a t i o n c e n t r e f o r m a t i o n on t h e p r e s e n c e of s o l i d f o r e i g n s u b s t a n c e was o b s e r v e d , , F u r t h e r m o r e , t h e t e s t s o f J, Meyer and W., p f a f f u ) r e v e a l e d t h a t i n m a t e r i a l s w i t h low n u c l e a r numbers ( s u c h a s thymoP, s ~ l o P , benzophenone, and o t h e r s ) t h e number o f c r y s t a l l i z a t i o n c e n t r e s may b e r e d u c e d c o n s i d e r a b l y b y f i l t r a t i o n ,
The t e s t s u p t o t h o p r e s e n t were made w i t h s u b s t a n c e s h a v i n g Bow c r y s t a l l i z a t i o n v e l o c f t i e s , Water h a s n o t been examined a s y e t f n t h i s r e s p e c t b e c a u s e o f i t s h i g h c r y s t a l l i z a t i o n v e l o c i t y , F o r t h a t r e a s o n t h e i f i -
f l u e n c e o f i r o n wf r e s , powders o f r o c k c r y s t a l , q u a r t z
g l a s s , and corondum was i n v e s t i g a t e d I n f i v e t u b e s for. e a c h c a s e . I n t h e t e s t s w i t h i r o n w i r e s ( T a b l e 1 1 ) t h e tempcsa- t u r e o f t h e b e g i n n i n g o f c r y s t a l l f z a t i o n i n p u r e w a t e r was f f r s t of a l l d e t e r m f n e d , Then e l e c t r o h y t i c i r o n w i r e s ,
5 cm, l o n g and 0.2 rnm, i n d i a m e t e r , a f t e r b e i n g c l e a n e d
w i t h w a t e r and a l c o h o l , were p l a c e d i n t h e w a t e r and r e a d i n g 9 o f t h e t e m p e r a t u r e werd a g a i n t a k e n a t whfch c ~ y s t a l l f z a t f o n began s p o n t a n e o u s l y , F i n a l l y t h e w i r e s were kemoved and t h e
t e m p e r a t u r e o f t h e f o r m a t i o n o f t h e f f r s t n u c l e f was a g a i n d e t e r m i n e d , I t becomes c l e a r that; t h e i r o n w i r e r a f s e s t h e t e m p e r a t u r e of t h e b e g i n n i n g o f i ce f o r m a t i o n c o n s i d e r a b l y t h e r e b y i n c r e a s i n g t h e number o f c r y s t a % l i z a t f o n cen- t r e s , A f t e r t h e removal o f t h e w i r e s t h e ~ e ~ c o o l f n ~ ._4-_1-_Y__
-- -
x ) G, Tammann, 2 , p h y s C h e m , 2 5 (18981, p. 456; P , O t h e r , 2 , a n o r g . Chern, 9 1 (99151, p , 24Boxr) J,
Meyer and W, P f a f f 2 , a n o r g , u , a l l g , Chern. 217 ( 1 9 3 4 ) ~ p. 257.Page
-
4Tech,? T r a n s , TT-79
c a p a s i t y i n c r e a s e d s u b s t a n t i a l l y , i n c o n t r a s t wf t h p i p e r o n a l , d l e ~ e , a c c o r d i n g t o P , Othmer" t e s t t s n l , t h e i n c r e a s e d num- b e r o f r l u c l e l remained even a f t e r removal o f t h e w i r e s ,
TABLE
--
I1The e f f e c t s f t h e powdelps s f r o c k c ~ y s t a l , q u a r t z - g l a s s , and corundum on t h e s u p e r c o o l f n g c a p a c i t y is shown i n Table 111, B o t h q u a r t z powders cause a consLderable incrbeaee I n t h o nurnbe~ o f n u c l e i , whereas t h e a d d i t i o n o f corundum i s w i t h o u t e f f e c t ,
TABLE
--
I11-z=zz.--
P u ~ o water fl O C
T h e tAir En s o f u t f o n also afP'er:t-s t h e super-
c o o l i n g c apacf tg o f w a t e r , Water c o n t a i n i n g af r c r y s t a l l l i a e s
on an a v e r a g e a t a t e m p e r a t u r e two d e g r e e s h i g h e ? 'than w a t e r
Page tJ 5
Tech, Trans, TT-~79 The r e s u l t s of t h e s e t e s t s make i t seem probable t h a t a s a g e n e r a l r u l e the spontaneous c r y s t a l l i z a t i o n observed was caused by f o r e i g n s u b s t a n c e s , Consequently i t s h o u l d b e p o s s i b l e , a f t e r removfng t h e s e f o ~ e f g n s u b s t a n c e s , t o superq-
c o o l t h e w a t e r t o a temperature lower t h a n -20°C, t h e l o w e s t temperature r e p e a t e d l y observed, The g r e a t e s t s u p e r c o o l i n g e v e r observed t o d a t e f s 2 9 , 5 ° ~ ~ ) ,
I1 THE LINEAR CRYSTALLIZATION VELOCITY -.OF -- ICE I - N - & U u -
l o The measurement o f t h e c r y s t a l l i z a t i o n v e l o c i t y was
c a r r i e d o u t i n U-tubes which had an i n n e r d i a m e t e r of 1,2 mm, and a w a l l t h i c k n e s s o f 0,s mrn, The temperature b a t h c o n s i s - t e d of a l c o h o l cooled by d r y i c e and c o n t a i n e d i n a t r a n s - p a r e n t Dewar f l a s k , A s p i r a l s t i r r i n g rnecha~lism ensured uniform temperature d i s t r i b u t i o n i n t h e b a t h , , The s t a r t o f
c r y s t a l l i z a t i o n was e f f s c t e d by i n o c u l a t i o n w i t h i c e , The p r o g r e s s of t h e c r y s t a l l i z a t i o n was timed between two marks vrhfch had been made on each arm of t h e U-tube 80 mu- a p a r t
thus e n s u r i n g a s t r a i g h t - l i n e measuring d i s t a n c e , A t low v e l o c i t i e s t h e time measurement was made b y means of a 0,2
second watch, and a t v e l o c i t i e s h i g h e r t h a n 1 , 0 0 0 mm, p e r minute, by a Q,02 second stop-watch, The time taken by t h e
c r y s t a l l f z a t f o n boundary t o p a s s upward and downward through t h e d i s t a n c e between two marks, was d e t e r m i r ~ e d , For v e r y low c r y s t a l l i z a t i o n velocf t i e s ( a few m i l l i n ~ e t r e s p e r minute and l e s s ) t h e U-tube was mounted on a g l a s s s c a l e by means of which t;he p r o g r e s s ~ f t h e c r y s t a l l i z a t i o n was 9abserved, The t e m p e r a t u r e o f t h e b a t h waa maintafned c o n s t a n t d u r i n g the measurement t o w i t h f n k O,OEiO, The i c e was added a f t e r t h e U-tubes had remained a t the e x p e r i m e n t a l temperature
f o r a t l e a s t t h r e e m i n u t e s , To p r e v e n t b u r s t i n g o f the t u b e s , t h e y wepa removed from t h e b a t h b e f o r e t h e l e s t few drops of t h e water c o u l d c r y s t a l l i z e ,
2 , The p r e c f p i t a t i n g i c e c r y s t a l l i z e d i n t h e form o f well- developed d e n d r i t e s , The main growth was i n t h e d i r e c t i o n of t h e stem of t h e d e n d r f t e , As a r u l e , t h e r e f o r e , t h e
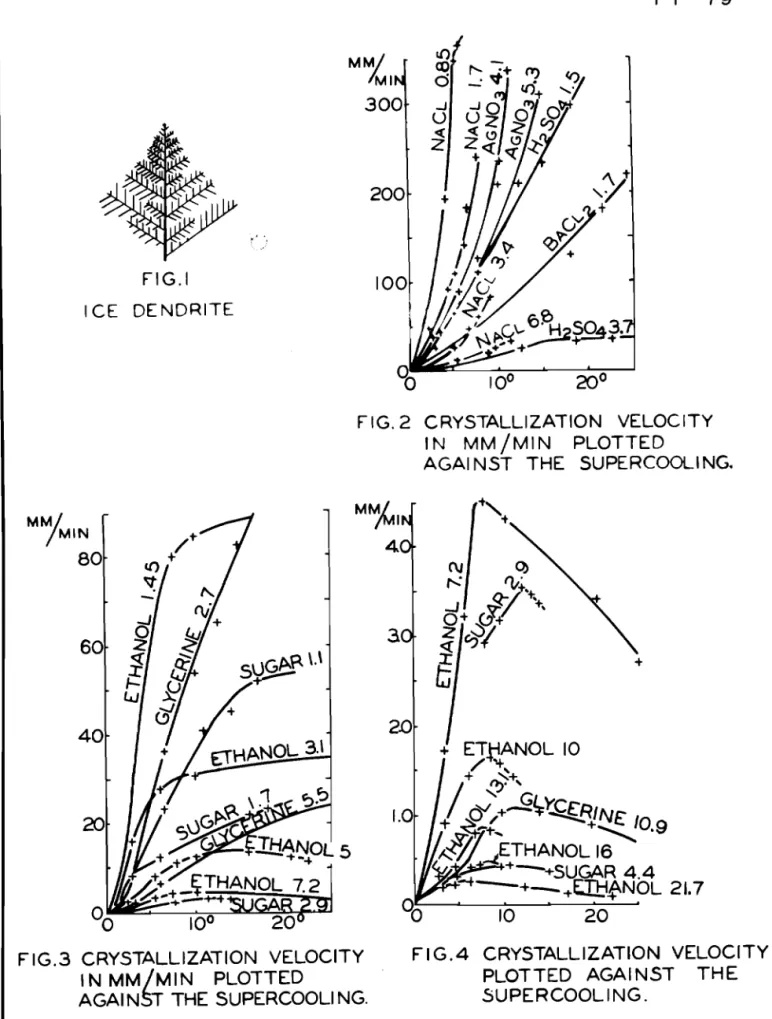
growth of t h e stem c o i n c i d e d w i t h t h e a x f s o f t h e t u b e , The s t r u c t u r e o f t h e d e n d r i t e i s e a s i l y recognized, e s p e c i a l l y a t t h e c r y s t a l l i z a t i o n boundary formed by one o r s e v e r a l d e n d r f t i c t i p s , F i g u r e 1 shows t h e appearance of such a d e n d r i t f c t i p , The branches make a n angle of 60 d e g r e e s
t h i c k - I n s t y t u t u ~ e t a l u $ ~ j f
Page .- 6 Tech, Trans, TT-5'9 n e s s o f t h e s t e m and t h o b r a n c h e s v a r f e s cor',sfdarzabl.y v i f t k ~ t h e c o n c e n t r a t i o n of t h e s o l u t i o n a n d t h e s u p e ~ ~ c o ~ l f n g , A t s l i g h t s u p e r c o o l f n g , t h i n , b a r e l y r e c o g n i z a b l e f o r m a t i o n s a r f s e ,> A t c o n s i d e r a b l e s u p e r c o o l i n g t h e b ~ a n c h e s a r e o f ten s o t h i c k t h a t t h e y c o l l i d e and t h e d e t a i l s arde no l o n g e r d i s t i n c t , The c r y s t a l l i z a t i o n f r o n t t h e n c o n s i s t s o f one o r more I c e t i p s which, however, always have t h e c l i a r a c t e r i s t i o c u t l i n e of t h e d e n d r i . L i c tip:;, w i t h arL m g l e ( i f appx 3 x l m E t e l y
60 d e g r e e s a t t h e t o p , I n p u r e w a t e r d e n d r i t e s t e m s h a v i n g
a t i i i c k n e a s u p t o 0 , 5 nna,, were o b s o ~ v e d , . 1Vlicroscopic measure- ment o f i n d i v i c i u n l d e r i d r i t e s , which c r y s t a l l f z e d a t losv
c r y s t a l l f z a t i o n v e l o c f t y from a 58 p e r c e n t g l y c e r i n e sc#lu-
t i o n , ; r e v e a l e d a t h i c k n g s s o f a p p c o x f m a t e l y 0,02 mm,, for t h e s t e m s , 0,005 m n i , f o r t h e b r a n c h e s , and 0,001 rim, f o r t h e
f i n e s t twigs, 'The p l a n e o f t h e d e n d r - f t e s lies i n t h e hexa.5 g o n a l g r o u p of i c e , hence t21 d e n d r i t e s c o r r e s p o n d t o one o f
t h e s i x r a y s o f a
snow flak ex^,
I n p u r e wateri a t slight s u p e r z c o o l i n g , . bvf de d e v f a t t o n s f ' m m t h e t u b e a x i s wexye o b s e r v e d i n t h e d i r e c t i o n o f t h e d e r l u r i t e stem&, r o s u l t , I n g i n R con-s i d e r a b l y d e c r e a s e d c r y s t s l l i z a t i o n v e l c c i t y x x ) , I n d i l i ~ t e s u c r o s e s o l u t i . o n s t h e dendrite s t e m and t h e b r b s i e k i e s ~ o ~ ~ l o - tfmes showed a c u r v a t u r e w i t h a r a t i i u s o f a few t e n t h s c s f a
m f l l i m e t r e which r e s u l t e d i n a r e d u c e d c r y s t a % l i z a t f o n v e l o c i t y , , T h i s e x p l a i n s t o a c e r t a i n e x t e n t t h e Z i f f e r e ~ l c e s in t h e v a l u e s found i n t h o l i t e r a t u r e f o r t h e c r g s t a b l i z a t f - o n v e l o c i t y o f f c o from p u r e w a t e r , , U p t o t h e p r a e s e n t t k i s e e d i f f e r e n c e s were a t t r i b u t e d t o v a r y i n g t e s t c o n d . l . t i o n s ( tube d i a m e t e r , t h i c k n e s s of t h e t u b e w a l l s ) ,; ~ i l i c n i : h a l ~ ) , who
made hi8 measurements uricier a1:aost t h e stme c o r l r . l l ? ; i ~ ~ i s ss
t h e a ~ t h o r s o f t h e p r a e s e n t r e p o r t , f c u n d v a l u e s ~ I i f c l i w6re s 1 ~ 1 ~ ~ l P e r by a p p p o x f n l a t e l y s e v e n p e r c e n t , s i n c e h i s t-ubea w e r e b e n t t h r e e t i m e s i n ordon. t o o b t a i n a borlger m e a s u r i n g d l ss=. t a n c e , 3 , F l ~ u r e s 2 t o 4 s h c j w the d e p e n c l e n c ~ o f t h e c r y s t a 2 . l l z a t i o n v e 7 , o ~ ~ i t y o f i c e from v a r i o u s so3ut.Fa3ns on t h e s u p e r c c c ~ l i n g , The numbors g i v e n i n t h e f i g u r e s a r e t h e mo4s s f t h e s u b - s t a n c e s d i s s o l v e d i n 1,000 gm, w a t e r , The c ~ y s t a l l i z a t f o n v o l s c f t y was g e n e r a l l y f o l l o w e d u p a s f a r a s the e r g s t a l - B i z a t i s n c a p a c i t y ~ e m i i t t e d , , The d e c r e a s e i n c r v s t a l l i z a t f o n
xm) G , 'Tamrnann and A , ~ a c h n e r , Yoc, e i t ,
Page -- 7
Tech, ' P r u l s - TT-79
t h e v a r i o u s s u b s t a n c e s a t i d e n t i c a l mol c o n c e n t r b t f on, A s
y e t t h e a v a i l a b l e d a t a a r e n o t s u f f i c i e n t t o g i v e % n f o m ~ a t i o n concerning t h e e f f e c t s of t h e s e v a r i o u s s u b s t a n c e s , The de- c r e a s e i n t h e c r y s t a l l i z a t i o n v e l o c i t y of i c e caused b y
s u c r o s e i s p a r t i c u l a r l y l a r g e , Sucrose f a r exceeds the o t h e r s u b s t a n c e s i n m o l e c u l a r weight and t h e r e f o r e h s s a p a r t i c u - l a r l y low r a t e of d i f f u s i o n , , This probably e x p l a i n s t h e s t r o n g e f f e c t ,
I t was o n l y pcissible t o a t t a i n t h e maximum c r y s t a l l i z a t i o n v e l o c i t y when t h i s was l e s s t h a n 100 mm, p e r minute With s m a l l values of t h e maximum c r y s t a l a b l z a t i o n
v e l o c i t y , l e s s t h a n 1 5 rnm, p e r m i n u t e , marked maxima of t h e c r y s t a l l f z a t f o n velocf t y o c c u r , These maxima l i e , i n t h e p r e s e n t c a s e , between 7" and l b O of s u p e r c o o l i n g f n agreement w i t h t h e f i n d i n g s f o r c h e m i c a l l y pure s u b s t m c e a ,
4 , The dependence of t h e c r y s t a l l f z a t i a n v e l o c i t y on the terriperature may b e r e p r e s e n t e d Tor pure s u b s t a n c e s l i k e o t h e r r e a c t i o n v e l o c f t i e s by an e q u a t i o n of the form:
where T1 and
T2
a r e t h e temperatures a t t h e c r y s t a l l i z a t i o n boundary, For s m a l l v a l u e s of the c r y s t a l l i z a t i o n v e l o c f t yt h e s e temperatures do n o t d i f f e r from t h e t of t h e b a t h , I f t h f s formula i s a p p l i e d t o t h e dependence of t e m p e ~ a t u r e on t h e c r y s t a l l i z a t i o n v e l o c i t y o f s o l u t i o n s , then t h e f a c t must kje t a k e n i n t o account t h a t t h e c ~ y s t a l l i z a t i o n v e l o c i t y i e i n f l u e n c e d n o t o n l y by t h e temperature a t t h e c r y s t a l l i z a t i o n boundary, f ,,e,
,
t h e e q u i l i b r i u m t e m p e r a t u r e , b u t a l s o by t h e r a t e of d i f f u s i o n of the d i s s o l v e d s u b s t a n c e , The r a t e o f d i f f u s i o n , however, d e c r e a s e s v e r y subs t a n t i a l l y w i t h de-c r e a s i n g temperature, thus r e d u c i n g the c r y s t s l l i z a t - i o n velos= c i t y , For t h i s r e a s o n t h e formula does n o t h o l d f o r l a r g e concent,ration and temperature i n t e r v s l a , For s m a l l ones however, i t i s i n agreement w i t h t h e observations, The l o g a r i t h m of t h e c r y s t a l l f z a t i o n v e l o c i t y i s a l i n e a r f u n c t i o n of t h e c o n c e n t r a t i o n o r of t h e temperature a t t h e c r y s t a l l i z a t i o n boundary, The a d d i t i o n o f f o r e i g n m a t t e r u s u a l l y lowers t h e m e l t i n g p o i n t , The same a p p l i e s t o t h e c r y s t a l l f z a t f o n v e l o c i t y of t h e c r y s t a l s of t h e s o l v e n t a t i d e n t i c a l s u p e r - coolfngs, although t h i s d e c r e a s e i s s u b s t a n t i a l l y l a r g e r and s a t i s f i e s o t h e r laws, In a bilnsry s e r i e s of mfxtukes t h e c r y s t a l l i z a t i o n v e l o c i t y d e c l i n e s w i t h t h e d i s t a n c e from t h e two components, hence f o r f d e n t f c a i i s u p e r c s o l f n g s t h e
Page
-
8Tech, T r a n s , TT-79
two c u r v e s o f c r y s t a l l i z a t i o n v e l o c i t y i n dependence on t h e c o n c e n t r a t i o n must i n t e r s e c t e x a c t l y a s t h e c u r v e s o f f u s i o n do. However, i t i s n o t requIr3ed t h a t t h e p o i n t o f i n t e r - s e c t i o n o f t h e t w o c u r v e s o f c r y s t a l l f z a t i o n v e l o c i t y s h o u l d l i e a t t h e e u t s c t i c c o n c e n t r a t f o n , A t t h i s p o i n t of' inter- s e c t i o n t h e c r g s t a l l i z a t i o n v e l o c i t i e s sf t h e two components a r e e q u ~ l i f each component c r y s t a l l f z e s s e p a r a t e l y , However, i f b o t h c r y s t u l l i z e t o g e t h e r , t h e n t h e z r y s t a l l l z a t f o n v e l o c i t y
7 1 1 1 g e n e r a l 1 3 i r ~ c r e n u e ~ ? . i z h t l y , coinpared wit21 tth ovvlile of
t,rle ifidividl-l#1 compsnent, 'fie r e a s o n f o r tllis i s Zhht tk?e s e p a r a t i o n o f t h e two s u b s t a n c e s due t o d f f f u s i o n Is f e s i l f - tat;ed b y t h e ex' s t e n c e o f b o t h t y p e s o f c r y s t a l a t tile c r y s t a f =
1 1 z a t l o n frorlt'j, With a m i x t u r e o f t w o components which,
f n t h e pQre ~ t i ~ f ; e have v e r y d f f f c ~ ~ e n t ; c r ~ y s t a l l l z a t i o n velo- c i t i e s , t h e r e a r e c o n c e n t r a t i o n s from whfch e v e n the com- p o n e n t of P a r g s c r y s t a l l f z a t i o n v e l o c i t y c r y s t ; a l l i z e s a t a Power r a t e t h a n t h e p u r e compi>nent o f Power c r y s t a l l i z a t i o n
v e l o c i t y , The i n v e s t i g a t i o n o f g l y c e ~ i n e - w a t e r s o l u t i o n s s e r v e d a s an e x a m p l e , The m a x i m u m c r y s t a l l i z a t i o n vein i t y o f p u r e g l y c e r i n e i s a p p r o x i m a t e l y 0 , l rnm.. p e r m i n u t s m y , Plaom a s o l u t i o 1 1 c o n i ; a l l z l n g 6 0 p e r cant g l y c e r i r i c t h e i c e c r y s t a l l i z e s a t H ~naximui:: c r y s t a l l i z a t i o n v e l o c i t y o f 0,093 rnm, = J e r m i n u t e However, t h e c r y 3 t a l l i z a t i o n v e l o c i t y o f f c o , as
ell as t h a t o f .Qycerint: from 6:) $and 85 p e r c e n t s o 2 u t i s n s , i s a t l e a s t t e n t i m e s s m a k l e ~ ,
5 , Tkle m a g n i t ~ i d e o f t h e c r y s t a l l i z a t i o n v e l o c i t y i s i m p o r t a n t
f o r dec!ding w h e t h e r a s o l u t f o n can b e t ~ a ~ s f o r m e d t o t h e g l a s s - - lf ke s t a t e by r a p i d c o o l i n g , , Small q u a n t f t i e s ( a p p r o x i m a t e l y
Q,'i c e , ) o f e u t e c t i c s o l u t i o n s o f KOH, WaOH, TICB, and CaC12, - whose maximum c r y s t a l l f z a t i o r l v e l o c i t y i s l o w e r than a p p l a ~ x i ' ~ m a t e l y 20 rnrn, p e r m f n u t s , c o u l d b e m a i n t a i n e d g l a s s - l i k e by c o o l i n g i n l i ~ ~ i f d a i r , , 6 , I n t h e concerltzaatforls i n v e v t f g ~ t e d t o d a t e a d d i t i o n s o f d i s s o l v e d for-eii;n r r i 2 t t e ~ r e d u c e t h e c r y s t a l l i z a t i on v e l o c f t y sf i c e , I f , how eve^, t h e e f f e c t of s m a l l q u a n t i t i e s o f d i s - s o l v e d m a t t e r ( s a y l e s s t h a n 0,OB mol p e r 1 S t r e ) i s i n v e s t i - g a t e d , i t i s found that, a s m a l l a d d i t i o n does n o t have a de- c r e a s i n g e f f e c t b u t , on t h e c o n t r a r y , a n i n c r e a s i n g e f f e c t on t h e c r y s t a l l i z a t i o n v e l o c i t y o f i c e , Consequently, a t i d e n t i c a l s u p e r c o o l i n g s , r n a ~ k a d maxima can be f o u n d on t h e c u r v e s r e p r e s e n t i r l g t h e c r y s t a l l i z a t i o n v e l o c i t y a s a f u n c t i o n of t h e c o n c e n t r a t L o n , These c u r v e s f o r s o l u t i o n s o f NaOH,, H C 1 , N a C 1 , and s u c r o s e a r a v e n i n : z a r e s 5 t o 8, The r e -
-
=- ,--= ---=.
p
Tamann and A , fSotschwak,Z i a n o r g , u , a l l g o C h e m , 1 5 9 ( 1 9 2 6 ) , p , 27,
G o Tmilann and E , J e n c k e l , 2, a n o r g , u , a l l g . Chem. 193 ( l g a o ) , P O 7 6 ,
Page
-
9 Tech, Trans, TT-79 s p e c t i v e s u p e r c o o l i n g s a r e g i v e n a t t h e r i g h t - h a n d s i d e of each curve, The i n c r e a s e i n t h e c r y s t a l l i z a t f on velocf t y of i c e which i s e v i d e n t d u r i n g t h e f i r s t a d d i t i o n t o t h e water may be interplaeted i n t h e f o l l o w i n g manner, Due t ot h e f o r m a t i o n of i c e t h e c o n c e n t r a t i o n of t h e i c e forming type of molecule w i l l be reduced, The t r a n s i t i o n of the o t h e r molecule types t o t h e i c e forming type i s a c c e l e r a t e d by t h e a d d i t i o n s , I n d i l u t e s o l u t i o n s t h e r e i s a s l i g h t l y h i g h e r c o n c e n t r a t i o n of t h e i c e forming type of molecule
than i n pure w a t e r , causfng an i n c r e a s e i n t h e c r y s t a l l i z a t i o n v e l o c f t y , I n t h e c a s e o f l a r g e r a d d i t i o n s , however, t h e
d e c r e a s e of the c r y s t a l l i z a t i o n v e l o c i t y caused b y t h e de- c r e a s e i n the e q u i l i b r i u m temperature p r e v a i l s , With i n - c r e a s i n g s u p e r c o o l i n g the maximum c r y s t a l l i z a t i on v e l o c i t y becomes more d i s t i n c t , The r a t e of t ~ a n s i t i o n of t h e
molecules of water i n t o the i c e forming type d e c r e a s e s w i t h dropping temperature and t h e a c c e l e r a t i n g e f f e c t of the added substance becomes more pronounced, For t h e a d d i t i o n of NaCl t h e i n c r e a s i n g e f f e c t i s s m a l l e r t h a n f o r a d d i t i o n s o f H C 1 and NaOH, t h u s s h i f t i n g t h e maximum t o s m a l l e r NaCP
c o n c e n t r a t i o n s , Thi s s h i f t i n g i s more pronounced f n t h e c a s e of sucrose,,
FIG.1 ICE DENDRITE v FIG. 1 - 4 T T - 7 9
+
20°FIG. 2 CRYSTALLIZATION VELOCITY
I N MM/MIN PLOTTED
AGAl NST THE SUPERCOOLING.
F IG.3 CRYSTALLIZATION VELOCITY F I G .4 CRYSTALLIZATION VELOCITY
MIN PLOTTED PLOTTED AGAINST THE
t F I G . 5 - 8 T T - 7 9 2 0 0 0 0
1
0 0.1 0.2 0.3 04 0.5 FIG. 5 F I G . 6 MM N ACL+
-+-i\t
O 001 0.02 0.03 0.040
0-01 0.02 003 0.04 0.05 FIG.7 FIG. 8CURVES OF CRYSTALLIZATION VELOCITY AS A FUNCTION OF SOLUTION CONCENTRATION.