AJH 1991; 4:131S-134S
Isradipine Twice Daily Lowers Blood Pressure
Over 24 H
Arie J. Man in't Veld, David G. Holmes, Antonius Lasance, and Peter de Zw art
T h e o b j e c t i v e of t h i s s t u d y w a s to c o m p a r e t h e ef fects of i s r a d i p i n e a n d p l a c e b o o n b l o o d p r e s s u r e (BP) at t h e e n d of t h e d o s i n g i n t e r v a l ('trough'). F o l l o w i n g a t h r e e - w e e k p l a c e b o p e r i o d , 187 p a t i e n t s w h o h a d p r e v i o u s l y s h o w n a r e s p o n s e t o t r e a t m e n t w i t h i s r a d i p i n e ( b a s e d o n office BP m e a s u r e m e n t s ) w e r e r a n d o m i z e d to d o u b l e - b l i n d t r e a t m e n t w i t h 2.5 m g i s r a d i p i n e t w i c e d a i l y or p l a c e b o for six w e e k s . F o u r of t h e s e p a t i e n t s w i t h d r e w f r o m t h e s t u d y d u r i n g t h e d o u b l e - b l i n d p h a s e b e c a u s e of a d v e r s e e v e n t s (one t a k i n g i s r a d i p i n e a n d t h r e e t a k i n g p l a c e b o ) . Blood p r e s s u r e d u r i n g t h e d o u b l e - b l i n d s t u d y w a s a l w a y s m e a s u r e d 12 h after d r u g a d m i n i s t r a t i o n ( t r o u g h v a l u e s ) . T h e r a t e of n o r m a l i z a t i o n
From the Department of Internal Medicine, Erasmus Hospital, Rot terdam, The Netherlands; Department of Clinical Research, S a n d o z Pharma, Basle, Switzerland; Department of Clinical Research, S a n d o z BV, U d e n , The Netherlands.
Address correspondence and reprint requests to David G. Holmes, MB, ChB, D C H , Department of Clinical Research, Sandoz Pharma, Lichtstrasse 35, C H - 4 0 0 2 , Basle, Switzerland.
[defined a s d i a s t o l i c BP (DBP) < 90 m m H g ] w a s 5 2 / 96 (54%) i n t h e i s r a d i p i n e - t r e a t e d g r o u p c o m p a r e d w i t h 3 0 / 8 7 (33%) i n t h e p l a c e b o g r o u p . A f u r t h e r 1 2 / 9 6 (12%) p a t i e n t s t a k i n g i s r a d i p i n e s h o w e d a fall i n D B P of > 10 m m H g , a l t h o u g h t h e i r D B P w a s still n o t < 90 m m H g , c o m p a r e d w i t h 5 / 8 7 (6%) p a t i e n t s r e c e i v i n g p l a c e b o . T h i s d i f f e r e n c e w a s s t a t i s t i c a l l y significant (P = .003). T h u s , i s r a d i p i n e i n a d o s e of 2.5 m g t w i c e d a i l y l o w e r s b l o o d p r e s s u r e o v e r 24 h . A m J H y p e r t e n s 1991;4:131S-134S KEY W O R D S : I s r a d i p i n e , t r o u g h b l o o d p r e s s u r e , d u r a t i o n of a c t i o n , p l a c e b o . additional a n t i h y p e r t e n s i v e benefit w a s a p p a r e n t w i t h doses a b o v e 7.5 m g twice daily. T h u s , using doses h i g h e r t h a n t h o s e required to lower blood p r e s s u r e served only to increase t h e frequency a n d / o r severity of side effects.
Most of these results, h o w e v e r , w e r e b a s e d on b l o o d pressure m e a s u r e m e n t s t a k e n 4 to 6 h after d r u g a d m i n istration (office m e a s u r e m e n t s , as is t h e usual practice). Relatively few data are available from studies in w h i c h b l o o d pressure w a s m e a s u r e d at t h e e n d of t h e 12 h dosing interval (trough m e a s u r e m e n t s ) , a l t h o u g h w h a t data there are did s h o w t h a t t h e therapeutic effect lasts for 12 h w i t h isradipine at a d o s e of 2.5 m g twice daily. Because t h e inclusion of patients n o t r e s p o n d i n g to t r e a t m e n t w a s t h o u g h t to m a k e t h e evaluation of t h e d u r a t i o n of effect less accurate, t h e s t u d y w a s carried out d o u b l e - b l i n d a n d placebo-controlled in p a t i e n t s w h o h a d already s h o w n a clinical r e s p o n s e to 2.5 m g isradipine twice daily.
P A T I E N T S A N D M E T H O D S
P a t i e n t S e l e c t i o n T h e following w e r e t h e criteria for entry into t h e placebo p h a s e of t h e study. Patients h a d
I
sradipine is a d i h y d r o p y r i d i n e characterized as a p o t e n t inhibitor of calcium c h a n n e l influx w i t h a h i g h specificity for vascular s m o o t h muscle as o p p o s e d to cardiac muscle. In anesthetized cats a n d dogs, isradipine p r o d u c e s vasodilatation a n d increased coronary, cerebral, a n d skeletal blood flows. Cardiac o u t p u t a n d m y o c a r d i a l contractility a r e i n c r e a s e d w h e r e a s myocardial oxygen c o n s u m p t i o n is r e d u c e d . Isradipine h a s b e e n evaluated in several r a n d o m i z e d , double-blind, parallel-group trials in t h e t r e a t m e n t of patients w i t h essential h y p e r t e n s i o n . These trials u s e d doses of 1.25 to 10 m g twice daily. T h e results of these trials indicated t h a t 1.25 m g twice daily is effective in reducing blood pressure a n d t h a t a d o s e - r e s p o n s e rela tionship applies w i t h doses u p to 7.5 m g twice daily. N o132S M A N I N T V E L D ET AL AJH-FEBRUARY 1991-VOL 4, NO. 2, PART 2
to h a v e received t r e a t m e n t w i t h 2.5 m g isradipine twice daily as m o n o t h e r a p y for at least four w e e k s a n d h a d to h a v e a d o c u m e n t e d diastolic blood pressure (DBP) < 95 m m H g or a reduction in DBP of at least 10 m m H g d u r i n g t h e isradipine t r e a t m e n t . Patients w e r e to s h o w n o clinically relevant a b n o r m a l laboratory test results. To qualify for e n t r y into t h e d o u b l e - b l i n d p h a s e , p a tients w e r e to h a v e a resting, sitting DBP of at least 95 m m H g , b u t n o t > 115 m m Hg, at t h e e n d of t h e placebo p h a s e .
S t u d y D e s i g n A multicenter, double-blind, parallel-g r o u p , placebo-controlled s t u d y desiparallel-gn w a s used. Total d u r a t i o n of t h e s t u d y per patient w a s n i n e w e e k s as follows: w e e k s 1 to 3, placebo w a s h - o u t p h a s e ; w e e k s 4 to 9, 2.5 m g isradipine twice daily or placebo. Patients w h o s e r e s p o n s e to isradipine h a d b e e n d e m o n s t r a t e d in a p r e c e d i n g clinical study directly e n t e r e d t h e placebo p h a s e of t h e comparative study. Patients w h o h a d n o t already s h o w n a response w i t h 2.5 m g isradipine twice daily initially received placebo for t w o w e e k s to estab lish t h e diagnosis of h y p e r t e n s i o n ; this w a s followed b y 2.5 m g isradipine twice daily for four w e e k s to establish t h e r e s p o n s e to t r e a t m e n t before entering t h e placebo p h a s e .
Patients eligible for t h e s t u d y received o n e placebo tablet twice daily (at 9 AM a n d at 9 PM) for t h r e e w e e k s . If, after three weeks of placebo, t h e blood pressure h a d still n o t reached the level required for inclusion, t h e p l a c e b o period w a s e x t e n d e d b y t w o w e e k s to a maxi m u m total duration of five w e e k s . Patients w h o con formed to t h e admission criteria at t h e e n d of t h e pla cebo p h a s e were r a n d o m i z e d to receive t r e a t m e n t w i t h either 2.5 m g isradipine or placebo twice daily. This w a s given for six weeks.
B l o o d P r e s s u r e M e a s u r e m e n t Sitting pulse a n d blood p r e s s u r e recordings w e r e t a k e n after t h e patient h a d b e e n in t h e clinic for at least 20 m i n a n d h a d rested in a sitting position for at least 3 m i n . Systolic b l o o d pressure (SBP) w a s recorded w i t h a m e r c u r y s p h y g m o m a n o m e ter at Korotkoff p h a s e I a n d DBP w a s recorded at Korot-koff p h a s e V. T h r o u g h o u t t h e study, blood pressure m e a s u r e m e n t for each patient w a s p e r f o r m e d w i t h t h e s a m e cuff size (appropriate to t h e circumference of t h e arm), from t h e s a m e a r m a n d , if possible, b y t h e s a m e investigator.
For t h e examinations at t h e e n d of t h e placebo period a n d after four a n d six w e e k s of t h e d o u b l e - b l i n d treat m e n t , t h e p a t i e n t a t t e n d e d t h e clinic in t h e m o r n i n g b e t w e e n 8:30 a n d 9:30 AM, 12 h ( ± 3 0 m i n ) after t h e previous e v e n i n g medication at 9 PM. Blood pressure w a s m e a s u r e d (trough values), followed b y administra tion of t h e m o r n i n g dose of isradipine. T h e effect o n b l o o d pressure at p e a k w a s n o t recorded. If, o n t h e d a y s of these t h r e e visits t h e m o r n i n g dose h a d i n a d v e r t e n t l y b e e n t a k e n before a t t e n d i n g t h e clinic, or t h e previous
e v e n i n g dose h a d b e e n taken m o r e t h a n 13 or less t h a n 11 h before, t h e examination w a s p o s t p o n e d until t h e next d a y to e n s u r e that true t r o u g h values w e r e o b tained.
Statistical M e t h o d s T h e n u l l - h y p o t h e s i s to b e tested w a s : there are n o differences in t r o u g h blood p r e s s u r e -lowering effects b e t w e e n t r e a t m e n t w i t h 2.5 m g isradi p i n e twice daily a n d placebo twice daily after six w e e k s of treatment. T h e Wilcoxon signed-rank test w a s u s e d to c o m p a r e t h e c h a n g e s from baseline w i t h i n each treat m e n t g r o u p , a n d t h e Utest according to M a n n W h i t -ney-Wilcoxon w a s u s e d to assess t h e c h a n g e s from b a s e line b e t w e e n t h e t w o t r e a t m e n t groups.
In addition, a categorical analysis of t h e blood p r e s sure results in évaluable patients w a s also c o n d u c t e d , using t h e following categories of DBP r e s p o n s e at w e e k 9 (six w e e k s of treatment) a n d t h e m i n i m u m discrimina tion statistic (2 I test): 1. < 9 0 m m Hg; 2. > 9 0 m m Hg, b u t w i t h reduction > 10 m m Hg; 3. > 9 0 m m H g , b u t w i t h reduction < 1 0 a n d ^ 5 m m Hg; 4. > 9 0 m m H g a n d w i t h reduction < 5 m m Hg.
R E S U L T S
A total of 188 patients w e r e recruited into t h e study. O n e w i t h d r e w d u r i n g t h e placebo p h a s e b e c a u s e of gas trointestinal bleeding, leaving 187 patients to b e r a n d o m i z e d . Four patients discontinued t h e d o u b l e - b l i n d t r e a t m e n t because of adverse events. T h e placebo g r o u p comprised 90 patients (46 m e n , 44 w o m e n ; m e a n age 50 years, r a n g e 27 to 64 years), a n d t h e active t r e a t m e n t g r o u p included 97 patients (53 m e n , 44 w o m e n ; m e a n age 49 years, r a n g e 23 to 65 years). O n e h u n d r e d a n d ten patients received isradipine in t h e four-week selection p h a s e ; t h e r e m a i n i n g 77 patients h a d already received isradipine for periods of u p to 127 w e e k s before entering t h e study.
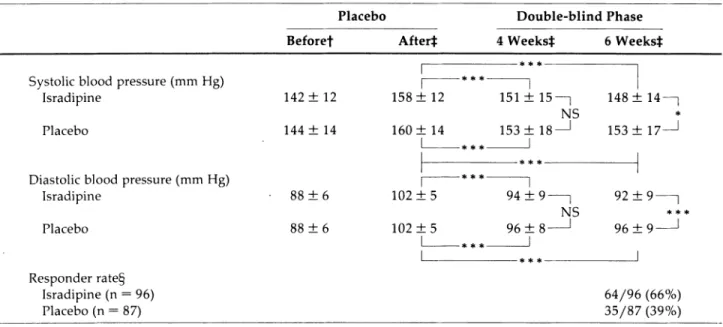
Table 1 s h o w s t h e average blood pressure values for t h e 183 patients w h o completed t h e d o u b l e - b l i n d study. T h e t w o t r e a t m e n t g r o u p s w e r e c o m p a r a b l e for b o t h SBP a n d DBP at baseline, a n d b o t h g r o u p s s h o w e d a statistically significant reduction in DBP after four a n d six w e e k s of treatment. H o w e v e r , t h e isradipine-treated patients s h o w e d a further fall in blood pressure b e t w e e n t h e fourth a n d sixth w e e k s of t r e a t m e n t w h e r e a s , in the placebo-treated patients, there w a s n o relevant c h a n g e in blood pressure after t h e fourth w e e k of treatment. T h e reduction in DBP from baseline w a s statistically significantly greater (P = .001) in t h e isradipine-treated patients after six w e e k s t h a n in t h e placebo-treated p a tients.
T h e percentage of patients achieving a s u p i n e DBP of 90 m m H g or less w a s greater ( 5 2 / 9 6 , 5 4 % ) in t h e active-t r e a active-t m e n active-t g r o u p active-t h a n in active-t h e placebo-active-treaactive-ted g r o u p ( 3 0 / 87, 3 3 % ) . Also greater w a s t h e n u m b e r of patients w h o did n o t attain a DBP of 90 m m H g or less, b u t did
AJH-FEBRUARY 1991-VOL 4, NO. 2, PART 2 I S R A D I P I N E O V E R 24 H O U R S 133S
TABLE 1. AVERAGE BLOOD PRESSURE VALUES AND NORMALIZATION RATE BEFORE, DURING, AND AFTER TREATMENT WITH 2.5 MG ISRADIPINE OR PLACEBO TWICE DAILY
Placebo Double-blind Phase Beforef After* 4 Weeks}: 6 Weeks* Systolic blood pressure (mm Hg)
Isradipine Placebo
Diastolic blood pressure (mm Hg) Isradipine Placebo Responder rate§ Isradipine (n = 96) Placebo (n = 87)
Γ
142 ± 12 144 ± 14 88 ± 6 88 ± 6I
158 ± 12 160 ± 14I
151 ±15—] 148: NS 153 ±18-I
153: 102 ±5 102 ± 51
94 ± 9 -96 ±8-I
NSL
92 ± 96 ±I
14- 179 9 -64/96 35/87 (66%) (39%)f Office' blood-pressure measurement. f Blood pressure measured 12 h after dosage.
§ Response = diastolic blood pressure ^ 90 mm Hg or a fall of^lO mm Hg. *F = .034; *** Ρ < .001; NS = not significant.
achieve a reduction of at least 10 m m H g in DBP [ 1 2 / 9 6 (12%) ν 5 / 8 7 (6%)]. T h u s , t h e total n u m b e r of patients w i t h either a normalization of blood pressure or a g o o d blood pressure r e s p o n s e w a s 6 4 / 9 6 (66%) in t h e isradi pine g r o u p a n d 3 5 / 8 7 (39%) in t h e placebo g r o u p . T h e difference in categorical r e s p o n s e b e t w e e n t h e t w o g r o u p s w a s statistically significant (P = .003).
T h e m o s t c o m m o n adverse event r e p o r t e d d u r i n g t h e s t u d y in b o t h t r e a t m e n t g r o u p s w a s h e a d a c h e (six occur rences w i t h isradipine a n d n i n e w i t h placebo). A l t h o u g h t h e p e r c e n t a g e of all patients entering t h e d o u b l e - b l i n d s t u d y w h o r e p o r t e d adverse events w a s similar for b o t h t r e a t m e n t g r o u p s ( 1 6 / 9 7 , 1 6 % w i t h isradipine a n d 1 3 / 90, 1 4 % w i t h placebo), t h e total n u m b e r of adverse e v e n t s w a s greater in those receiving isradipine (n = 26) t h a n placebo (n = 18). There w e r e t w o reports of flush ing a n d three of e d e m a in patients receiving active treat m e n t , b u t n o reports of either in patients taking placebo. Four patients discontinued t h e d o u b l e - b l i n d t r e a t m e n t b e c a u s e of adverse events: o n e because of e d e m a (isra dipine) a n d three because of h e a d a c h e (placebo).
D I S C U S S I O N
T h e blood pressure-lowering effect of isradipine u n d e r n o r m a l clinic conditions is well e s t a b l i s h e d .1 - 5 In those
studies, h o w e v e r , blood pressure w a s m e a s u r e d w h e n ever t h e patient a t t e n d e d t h e clinic, a n d t h e r a p y w a s taken twice daily, once in t h e m o r n i n g a n d again in t h e evening. T h u s , t h e blood pressure m e a s u r e m e n t s re
flected t h e effects of t h e d r u g b e t w e e n its p e a k period a n d its trough. This is t h e blood pressure v a l u e generally u s e d by physicians in their u s u a l care of patients, b u t it does n o t clearly establish t h a t t h e d r u g h a s a blood p r e s sure-lowering effect t h a t persists t h r o u g h o u t t h e dosing interval. T h a t t h e effect is still p r e s e n t at t h e e n d of t h e 12 h dosing interval h a s b e e n s h o w n in o t h e r s t u d i e s .6'7
Those studies w e r e , h o w e v e r , either s i n g l e - b l i n d6 or
w e r e carried out in elderly p a t i e n t s .7
T h e s t u d y r e p o r t e d h e r e w a s p e r f o r m e d to assess t h e 12 h d u r a t i o n of action using a placebo-controlled s t u d y design in y o u n g e r patients. T h e results s h o w e d a statis tically significantly greater lowering of DBP w i t h isradi p i n e t h a n w i t h placebo. In this study, t h e r a p y w a s given at 9 PM on t h e e v e n i n g before t h e next m o r n i n g ' s clinic visit, w h i c h w a s at 9 AM ± 30 m i n . T h u s , t h e b l o o d pressure values recorded w e r e true t r o u g h values a n d t h e blood pressure-lowering effect w a s t h a t at t h e e n d of t h e dosing interval.
It can, therefore, b e c o n c l u d e d t h a t t h e blood p r e s s u r e -lowering effect of isradipine is p r e s e n t t h r o u g h o u t t h e dosing interval a n d t h a t a twice-daily regimen is a p p r o priate. T h e fact t h a t t h e blood pressure fell in t h e isradi pine-treated patients b e t w e e n t h e fourth a n d sixth w e e k s of t r e a t m e n t while r e m a i n i n g stable, or e v e n ris ing slightly, in t h e placebo-treated patients is further evidence t h a t t h e reduction in b l o o d pressure is a n effect of t h e t r e a t m e n t a n d n o t a s p o n t a n e o u s c h a n g e . This is also a n indication t h a t t h e effect of isradipine is n o t m a x i m u m at, for example, t w o w e e k s of t r e a t m e n t a n d ,
134S M A N IN'T V E L D ET A L AJH-FEBRUARY 1991-VOL. 4, NO. 2, PART 2
therefore, conclusions as to t h e m a g n i t u d e a n d d u r a t i o n of a n t i h y p e r t e n s i v e effect b a s e d o n studies in w h i c h t h e effects are assessed after only t w o w e e k s are of limited value.
A l t h o u g h t h e s t u d y w a s n o t i n t e n d e d to assess t h e tolerability of isradipine but, rather, to e x a m i n e t h e d u r a tion of action in patients w h o h a d already s h o w n a re s p o n s e to t r e a t m e n t , these results also d e m o n s t r a t e t h a t isradipine is well tolerated. A l t h o u g h t h e n u m b e r of a d v e r s e e v e n t s ( b u t n o t t h e n u m b e r of patients report ing a d v e r s e events) w a s h i g h e r in t h e isradipine-treated patients t h a n in t h e placebo-treated patients, t h e overall safety w a s a d j u d g e d b y t h e investigators to b e t h e s a m e for b o t h t r e a t m e n t s .
R E F E R E N C E S
1. Sundstedt CD, Rüegg PC, Keller A, Waite R: A multicenter evaluation of the safety, tolerability and efficacy of isradi pine in the treatment of essential hypertension. Am J Med 1989;86(suppl 4A):98-102.
2. Shepherd AMM, Carr AA, Davidov M, et al: Efficacy and safety of isradipine in hypertension. J Cardiovasc Phar macol 1989;13:580-585.
3. Simonsen K, Sundstedt CD: Dose-response relationship and incidence of adverse drug reactions with isradipine in patients with essential hypertension. Am J Med 1989; 86(suppl 4A):91-93.
4. Kirch W, Burger KJ, Weidinger G, Welzel D: Efficacy and tolerability of the new calcium antagonist isradipine in essential hypertension. J Cardiovasc Pharmacol 1990: 15(suppl 1):S55-S59.
5. Miller H: Isradipine: overall clinical experience in hyper tension in the United States. J Cardiovasc Pharmacol 1990;15(suppl 1):S60-S64.
6. De Keyser P, Bouvé J, Clement D, et al: Isradipine in es sential hypertension: the Belgian General Practitioners' Study. Am J Med 1989;86(suppl 4A):103-109.
7. The British Isradipine Hypertension Group: Evaluation of the safety and efficacy of isradipine in elderly patients with essential hypertension. Am J Med 1989;86(suppl 4A):110-114.