Plant, Cell and Environment (2006) 29, 36–47
36
Blackwell Science, LtdOxford, UKPCEPlant, Cell and Environment0016-8025Blackwell Publishing Ltd 2005? 2005 29?3647
Original Article
Cloning and expression of a putative xylem sucrose transporter M. Decourteix
et al.
Correspondence: Soulaiman Sakr. Fax: +330473407916; e-mail: Soulaiman.Sakr@univ-bpclermont.fr
JrSUT1
, a putative xylem sucrose transporter, could mediate
sucrose influx into xylem parenchyma cells and be
up-regulated by freeze–thaw cycles over the autumn–winter
period in walnut tree (
Juglans regia
L.)
MÉLANIE DECOURTEIX1 , GEORGES ALVES1 , NICOLE BRUNEL1 , THIERRY AMÉGLIO2 , AGNÈS GUILLIOT1 , RÉMI LEMOINE3 , GILLES PÉTEL1
& SOULAIMAN SAKR1
1UMR 547-PIAF, site des Cézeaux, Université Blaise Pascal, 24 avenue des Landais, 63177 Aubière Cedex, France, 2UMR
547-PIAF, site INRA de Crouelle, 234 avenue du Brézet, 63039 Clermont-Ferrand Cedex 2, France and 3ESA CNRS 6161,
6161-CNRS, Bâtiment Botanique, Laboratoire de Physiologie et Biochimie Végétales, 40 avenue Recteur Pineau, 86022 Poitiers, France
ABSTRACT
Sucrose has been reported to play multiple roles in the winter biology of temperate woody species. However, no report on the molecular basis of sucrose transport in xylem tissue has yet been made. In the walnut tree, it is demon-strated that during the autumn–winter period, active absorption of sucrose from xylem vessels to parenchyma cells (sucrose influx) is much higher when samplings were taken shortly after a period of freezing temperatures. Here, the question of whether this increased sucrose influx mir-rors a regulation of sucrose transporters in xylem tissue was tested. A putative sucrose transporter cDNA (JrSUT1:
Juglans regia sucrose transporter 1) was isolated. Over the
autumn–winter period, JrSUT1 transcripts and respective proteins were present in xylem parenchyma cells and highly detected when samplings were performed shortly after a freeze–thaw cycle. This up-regulation of JrSUT1 level was confirmed in controlled conditions and was not obtained in bark. Immunolocalization studies showed that JrSUT1 and plasma membrane H++++-ATPase (JrAHA) were colocalized
to vessel-associated cells (VACs), which control solute exchanges between parenchyma cells and xylem vessels. We propose that JrSUT1 could be involved in the retrieval of sucrose from xylem vessel. All these data are discussed with respect to the winter biology of the walnut tree.
Key-words: freezing; influx; sucrose transporter;
vessel-associated cells; walnut tree; xylem.
INTRODUCTION
In temperate woody species, it has been reported that sucrose has multiple functions during the autumn–winter period. In xylem parenchyma cells (symplast), sucrose is
involved in the acquisition of cold or freeze tolerance (Sau-ter 1988) and the maintenance of basal respiration (Höll 1985). In xylem vessels (apoplast), high sucrose concentra-tions are required for the local recovery of winter embolism in walnut tree due to freeze–thaw cycles (Juglans regia L., Améglio et al. 2001, 2002; Ewers et al. 2001).
Over the autumn–winter period, sucrose can reach more than 60 mg mL-1 (about 175 m
M) and account for more
than 60% of the total xylem sap soluble sugars. Sap sucrose concentration has been found to inversely vary with tem-peratures (Améglio et al. 2004), and this variation has been proposed to reflect the relative importance of two opposite movements of sucrose between symplast and apoplast. The first is referred to as sucrose efflux (sucrose passage from the symplast to the apoplast) and the second, as sucrose influx (sucrose re-absorption into the symplast). These transport phenomena occur between xylem vessels and ves-sel associated cells (VACs), which are the only parenchyma cells connected with the xylem vessels by means of large and numerous pits (Czaninski 1977; Alves et al. 2001). Hence, they could constitute a regulatory checkpoint for the exchanges between vessels and parenchyma cells.
According to Valentin et al. (2001) and Améglio et al. (2004), sucrose efflux could, in part, occur via a facilitated diffusion. In contrast, sucrose influx into parenchyma cells, which occurred with rising temperatures, has been pro-posed to be an active process mediated by H+/sugar sym-porters (Sauter 1983). It thus would depend upon the proton-motive force generated by the plasma membrane H+-ATPase (Axelsen & Palmgren 2001; Arango et al. 2003). In willow tree (Salix smithiana), sucrose influx was assessed by perfusing shoot sections with a 5% sucrose solution, which is about the maximum level that has been found at low temperatures in this species (Sauter 1983). This author showed that the sucrose content in sap significantly decreased (about 50%) at 21 ∞C, whereas a much smaller loss was measured at 2 ∞C, indicating that the sucrose influx into symplast is temperature-dependent. A similar
conclu-Cloning and expression of a putative xylem sucrose transporter 37
sion was recently drawn for the walnut tree, where
absorp-tion of perfused radiolabelled sucrose (14C-suc) by
parenchyma cells also proved to be much higher at 15 ∞C than at 1 ∞C (Valentin et al. 2001). There is also a certain amount of circumstantial evidences supporting the pres-ence of an H+/sugar symporter: (1) VACs are symplastically connected to other parenchyma cells and apoplastically connected to vessels, which implies that sucrose influx needs a membrane transporter (Catesson 1983; Czaninski 1987); (2) like companion cells, VACs exhibit a high respi-ratory activity which is a characteristic of cells specialized in the apoplastic transport of assimilates (Catesson & Cza-ninski 1967; Sauter 1972; Alves et al. 2001); (3) plasma
membrane H+-ATPase, that generates the proton-motive
force required to drive the secondary active transport, was
localized to plasma membrane of VACs (Fromard et al.
1995; Arend et al. 2002). However, no sucrose transporter cDNA has so far been cloned and characterized in xylem tissue of woody species.
In herbaceous species, many sucrose transporters have been isolated and well-characterized during the last decade (Lemoine 2000; Williams, Lemoine & Sauer 2000; Lalonde
et al. 2003). They are expressed in source (Stadler et al.
1995; Stadler & Sauer 1996; Kühn et al. 2003) as well as in sink organs, e.g. tobacco pollen (Nicotiana tabacum, Lem-oine et al. 1999), broad bean seeds (Vicia faba, Weber et al. 1997), plantain young ovules (Plantago major, Gahrtz et al. 1996). Their activity can be transcriptionally (Vaughn, Har-rington & Bush 2002), post-transcriptionally (Sakr et al. 1997; Chiou & Bush 1998; Matsukura et al. 2000; Yao Li, Weiss & Goldschmidt 2003) and post-translationally
(Rob-lin et al. 1998) controlled. This kind of study has thus far
been quite limited in woody species, where only few sucrose/H+ cotransporters have been characterized. These include the VvSUTs from berry grape (Vitis vinifera:
Davies, Wolf & Robinson 1999; Ageorges et al. 2000), the
BpSUC1 from birch root (Betula pendula, Wright et al.
2000) and more recently SUT1 and 2 from citrus root ( Cit-rus sp., Yao Li et al. 2003). However, none of them has been studied during the autumn–winter period.
Sucrose can play multiple roles throughout the autumn– winter period, such as acquisition of freezing tolerance, local winter embolism recovery and carbon and energy resources. We present here a study of the molecular basis of sucrose transport in xylem in search of the first clues concerning its role and regulation during this period.
MATERIALS AND METHODS
Plant material, control freezing experiments and
isolation of xylem and bark tissues
Thirteen-year-old-walnut trees (Juglans regia cv. Fran-quette scions on wild walnut root stocks) grown in an orchard near Clermont-Ferrand (south-central France) were used for this study. One-year-old twigs were collected throughout the autumn–winter period (from October to March just before the growing season) in 2001–02 and
2002–03. Twenty-centimetre-long segments were sampled in the apical part of the stem. To prevent any contamination of the xylem by bark (all tissues outside xylem) and
vice-versa, the bark was peeled and the outside xylem was
scraped with a scalpel (Sakr et al. 2003; Karlson et al. 2004). Indeed, since the scrapped material contains the cambium, xylem tissue cannot be contaminated with the phloem or any tissue external to xylem. The xylem and the bark were harvested separately and between October and March, every month. For RNA extraction, both tissues were frozen immediately in liquid nitrogen.
In addition, 3-year-old potted plants of the same variety were used for controlled freezing experiments. The grafted plants were grown in individual 33 L, well-drained contain-ers filled with a mixture of peat (33%) and clay soil (67%). The potted trees were grown outdoors until early October, and then put into a ‘cool’ greenhouse, where the tempera-tures were normally kept the same as outdoors. Air tem-peratures in the greenhouse were continuously recorded and a heating system was automatically turned on when temperatures dropped to 0 ∞C, warming the greenhouse to 2 ∞C. Thus the trees were exposed to cold temperatures, allowing winter acclimation, but not to freezing tempera-tures prior to the controlled freezing experiments.
A large cooling chamber was designed to hold up to four potted trees that were up to 2 m in height. Data loggers (DL2e; Delta T devices, Cambridge, UK) recorded temper-ature fluctuations as 5-min averages and averaged at 1-min intervals.
In late November the container-grown trees (aerial part) were progressively cooled from 5 ∞C to -10 ∞C at a rate of -5 ∞C h-1 and held for 2 h at -10 ∞C. They then were warmed to 5 ∞C at a rate of 5 ∞C h-1. This freezing–warming cycle (5 ∞C; -10 ∞C; 5 ∞C) was repeated two times. In paral-lel, the other trees (control) were maintained at about 5 ∞C. Xylem was then collected at the end of this treatment as
described above. The temperature -10 ∞C was chosen
because it is a daily minimal air temperature encountered each year in the orchard in winter and is inferior to -4 ∞C, a threshold temperature that initiates the extracellular ice formation in walnut tree stems (Améglio et al. 2001). To test whether freezing treatment alone is sufficient to affect
JrSUT1 transcripts, portions of twigs of container-grown
trees were progressively cooled from +5 ∞C to -5, -10 or -15 ∞C at a rate of -5 ∞C h-1. They were then maintained for 4 h at the imposed temperature before xylem was col-lected. As a control, twigs were maintained at 5 ∞C.
Sucrose influx
Twenty-centimetre-long segments were cut in the apical part of 1-year-old stems (same trees than those used for ARN and protein extractions) and preconditioned at 15 ∞C for 48 h in a temperature controlling refrigerator (Liebherr 650 L), according to Valentin et al. (2001). During the pre-conditioning, the shoot segments were enclosed in plastic bags in order to avoid dessication. Thereafter, tissues out-side xylem were scraped out from the ending sections to
38 M. Decourteix et al.
avoid contamination by phloem constituents (Fromard
et al. 1995; Valentin et al. 2001; Améglio et al. 2004). Each segment was first perfused with 3 mL of the control solution (MgCl2 0.1 mM, KCl 0.1 mM, CaCl2 0.1 mM and MES 10 mM adjusted to pH 5.5 with HCl) under moderate pressure (0.1 MPa), and then with 2 mL of a radioactive solution
[control solution supplemented with 1 mM sucrose and
70 kBeq mL-1 14C-sucrose (NEN Life Science Products,
Boston, MA, USA) with or without addition of 1 mM
HgCl2]. HgCl2 blocks sucrose transport activity by dissipat-ing the proton electrochemical gradient that drives active sucrose absorption (Bush 1993). Thus, active sucrose uptake values are obtained by deducting the passive uptake value from the total one. After 1 h of incubation at 15 ∞C, xylem vessels were rinsed with 6 mL of control solution and shoot segments were frozen and conserved at -20 ∞C until sugar extraction and radioactivity counting.
Stem segments used for expression analysis were har-vested within 24 h before those used for sucrose influx, so that they all had the same temperature prehistory.
Means and standard errors were computed and the sig-nificance (P < 0.05) of the difference of 14C-sucrose absorp-tion between the November–December and the February– March periods was evaluated by the non-parametric test of Mann and Whitney (Sprent 1992).
Extraction of total RNA
Plant material was ground to a fine powder in liquid nitrogen using a mortar and a pestle. Total RNA was extracted from xylem, bark (all tissues outside xylem), leaf and fine root according to the method previously described by Gévaudant
et al. (1999). RNA was quantified spectrophotometrically
and its quality was checked by gel electrophoresis.
Construction and screening of xylem-derived
cDNA Library
Total RNA was extracted from xylem tissue as described
above. Poly(A+)RNA was isolated from RNA (1 mg) using
PolyATract mRNA isolation system III (Promega, Madison, WI, USA). A stem xylem complementary DNA library was constructed (Sakr et al. 2003) using the ZAP-cDNA kit and instructions provided by Stratagene (La Jolla, CA, USA).
A xylem sucrose transporter probe was obtained by reverse transcriptase (RT)-polymerase chain reaction (PCR). Reverse transcription was carried out using 1 mg of total RNAs extracted from xylem tissue in February and the Ready-To-Go™ T-Primed First-Strand Kit (Amersham, Piscataway, NJ, USA). For the PCR reaction the following degenerate primers were used: SutA (sense) 5¢- T(A/T)(C/
T)G(A/G)(C/T)AC(A/T)GA(C/T)TGGATGG-3¢ and
SutB (antisense) 5¢-CC(C/G/T)(A/G)(G/T)(G/T)GA(C/G/ T)A(A/G)(A/C/T)CCTTGGCC-3¢, which were deduced from conserved regions of plant sucrose transporter cDNAs (EMBL data library).
Amplification was carried out in a thermal cycler (Gene Amp PCR system; Perkin Elmer Applied Biosystems,
Fos-ter City, California, USA) using standard protocols. The amplified fragment (440 bp) was sequenced and then used as a SUT homologous probe to screen the cDNA library under low stringency conditions. Five clones were obtained and completely sequenced. DNA sequence information showed that they originated from one gene, which was referred to as JrSUT1 (Juglans regia SUcrose Transporter 1). Its sequence has the following EMBL/GenBank/DDBJ accession number: AY504969.
Semi-quantitative RT-PCR
One microgram of total RNAs was extracted from xylem tissue as described above, reverse transcribed using the Ready-To-Go™ T-Primed First-Strand Kit (Amersham), and 3 mL of the synthesized first-strand cDNA was ampli-fied by PCR. Amplification of JrSUT1 and JrAct (Juglans
regia Actin) was performed with the following specific
primers: Sut11 (sense), 5¢-AGATGCAATATCCGCAGT TCCC-3¢ and Sut12 (antisense), 5¢-GCATGGCTTTTG TAGCATTGGG-3¢ for the amplification of JrSUT1, and Act11 (sense), 5¢-GATGAAGCCCAATCAAA GAGGGGT-3¢ and Act12 (antisense), 5¢-TGTCCATCAC CAGAATCCAGCAC-3¢ for the amplification of JrAct. Sut11 and Sut12 primers of JrSUT1 cDNA clone were designed based on JrSUT1 3¢UTR. PCR reaction was car-ried out as follows: 2 min at 94 ∞C for denaturation, 20 s at 94 ∞C, 20 s at 59 ∞C and 45 s at 72 ∞C, for 33 (JrSUT1) and 25 (JrAct1) cycles, and 10 min at 72 ∞C for final extension. The optimal numbers of PCR cycles were established to generate unsaturated (linear phase) but detectable signals. These PCR were performed in parallel on the same cDNA and under the same conditions.
We also used another primer couple Sut1A (sense), 5¢-TGTTCGACACAGATTGGATGGG-3¢ and Sut1B
(anti-sense) 5¢-CCCAGAGAAAGTCCTTGGCC-3¢, which
were, respectively, designed from the highly conserved LFDTDWMG and GQGLSLG sequences, and therefore susceptible to amplify different sucrose transporter iso-forms in walnut tree. RT-PCR amplification was carried out as described above performing 27 cycles.
For the amplification of JrAHA (Juglans regia
Autoinhi-bated H+-ATPase) (EMBL, accession number AY347715),
the following primer couple: AHA11 (sense): 5¢-GCT TGGGATCTTGTCATTGAAA-3¢ and AHA12 (anti-sense): 5¢-AGGGGTCCAAAAACACAAAAG-3¢ was chosen (Alves 2003). AHA11 and AHA12 primers were designed from JrAHA. PCR reaction was carried out as follows: 2 min at 94 ∞C for denaturation, 20 s at 94 ∞C, 20 s at 59 ∞C and 45 s at 72 ∞C, 25 cycles, and 10 min at 72 ∞C for final extension.
Preparation of anti-JrSUT antibody
Anti-JrSUT1 antiserum was raised in rabbits (Eurogentec, Herstal, Belgium) against a synthetic peptide (MEVE-SANKDMRAAQR) coupled to a carrier protein (KLH). Its sequence was derived from the specific N-terminal
region of the JrSUT1 protein. Antibodies were then affin-ity-purified against the synthetic peptide (Eurogentec), and we checked it cross-reacted with the N-terminal region of JrSUT1 (data not shown).
The purified anti-JrAHA antiserum was raised against a conserved synthetic peptide (CDPKEARAGIREVHF) of plasma membrane H+-ATPase (Alves et al. 2004).
Isolation of microsomal fraction, sodium
dodecyl sulphate-polyacrylamide gel
electrophoresis and western blot
Isolation of xylem microsomal fractions was performed according to Alves et al. (2001). Protein samples (30 mg) were subjected to sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis (PAGE) according to Laemmli (1970). The gel system consisted of a 5% stacking gel and a 10% resolving gel. After gel electrophoresis, polypeptides were electroblotted onto a nitrocellulose
membrane (Transfer Membrane, BioTraceTM NT; Pall
Cor-poration, New York, NY, USA). The transfer buffer con-tained 25 mM Tris, 193 mM glycin, 0.1% (w/v) SDS and 20% (v/v) methanol. The transfer was performed at a constant current of 400 mA for 2 h at room temperature. Tris-buff-ered saline (TBS) was used as the basic medium for immu-noblotting. Dry milk 10% (w/v) and Tween 20 [0.1% (v/v)] were used to block nitrocellulose filters. Immunodetection was achieved with either the JrSUT1 or the JrAHA anti-body diluted 1/3000 and 1/1000, respectively, as primary
antibody and antirabbit IgG (H + L) (P.A.R.I.S.,
Com-piègne, France) as peroxydase labelled secondary antibody,
diluted 1/10 000. The protein-antibody complex was
detected with the ECL western blotting analysis system (Amersham Pharmacia Biotech, Little Chalfont, UK).
JrSUT1 and JrAHA immunolocalization in
xylem tissue
Samples, 3–5 mm in length, were cut from 1-year-old-twigs and fixed for 45 min at 4 ∞C with 1.5% (v/v) formaldehyde and 0.5% (v/v) glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) containing 0.5% (v/v) dimethylsulfoxide. They were abundantly washed in 0.1 M phosphate buffer pH 7.4
and then in PBS pH 7.2 (containing 8 mM NaH2PO4,
1.5 mM KH2PO4, 140 mM NaCl and 2.7 mM KCl). Samples were cut to 15 mm sections with a cryomicrotome. Sections were rinsed in PBS pH 7.2 and were laid for 15 min in PBS supplemented with 0.1% (v/v) Triton X-100, 0.2% (w/v) Glycin (pH 7.2). After these washings, non-specific sites were saturated for 45 min by normal goat serum in PBS pH 7.2, 0.2% (v/v) Triton X-100, 0.2% (v/v) Tween, 0.1% (w/v) bovine serum albumin (BSA) and incubated over-night, with either 1/100 diluted antibody against JrSUT1 sucrose transporter, 1/10 diluted antiboby against JrAHA
H+-ATPase, or the same antibodies saturated with the
KLH-coupled synthetic peptide (as control). After washing in PBS pH 7.2 and 0.1% (v/v) Triton X-100 (2 ¥ 20 min) and then in TBS pH 8.2, 0.2% (v/v) Triton X-100 (2 ¥ 15 min),
the sections were laid for 45 min in TBS pH 8.2, 0.2% (v/v) Triton, 0.2% (v/v) Tween, 1% (w/v) BSA and 5% (v/v) normal goat serum. Sections were then incubated for 3 h with goat antirabbit antibody conjugated to alkaline phos-phatase (Sigma, St Louis, MO, USA) diluted 1/40 in block-ing solution. Tissue sections were washed with TBS and then incubated with chromogenic substrates, NBT and BCIP (Bio-Rad, Hercules, CA, USA). Color development was carried out at room temperature and was stopped by washing in H2O. Sections were then mounted onto micro-scopic slides, air-dried and covered with cover-slips for microscopy analysis using Eukitt (Kindler GmbH & Co., Freiburg, Germany) as a mounting media. A mouse anti-rabbit antibody conjugated to fluorescein isothiocyanate (FITC) (g-chain specific; Sigma) diluted 1/20 in PBS, BSA 10% was also used to detect bound primary antibodies. After rinsing with PBS (3 ¥ 10 min), Evans blue was used to reduce autofluorescence. Sections were then mounted in Citifluor (AF1, Agar Scientific Ltd, Cambridge, England) and observed using an Axioplan 2 microscope (Zeiss, Jena, Germany) equipped with a 455- to 495-nm bandpass exci-tation filter and a 505- to 555-nm bandpass barrier filter. In parallel, the alkaline phosphatase approach was chosen to investigate JrAHA immunolocalization in the same shoot.
RESULTS
Active sucrose uptake
Active sucrose absorption was estimated at +15 ∞C by sub-tracting the passive uptake (with HgCl2) to the total uptake (without HgCl2) of sucrose. This active sucrose absorption from xylem vessels to xylem parenchyma cells (sucrose influx) was estimated in one-year-old apical stem segments for two periods: November–December and February– March (2001–02). These two periods differ by the minimum and maximum temperatures recorded 24 h before sampling (Fig. 1). Active sucrose absorption measured at +15 ∞C was more than five-times higher for the November–December period, when outdoor minimum temperatures were <-3.8 ∞C, than in February–March, when they were >+1.1 ∞C (Fig. 1).
Cloning of JrSUT1 cDNA
In order to check whether the increased sucrose uptake is related to the regulation of sucrose transporters, we decided to isolate a cDNA encoding a putative sucrose transporter. The screening of a xylem cDNA library with the walnut xylem sucrose transporter PCR-amplified frag-ment (see Materials and methods) resulted in the isolation of a full-length cDNA of 1887 bp, designated JrSUT1 (Juglans regia Sucrose Transporter 1; EMBL accession number AY 504969).
The open reading frame of JrSUT1 is 1549 bp long and encodes a 516 amino-acid polypeptide, with a calculated molecular mass of 55 kDa and a computer calculated iso-electric point (pI) of 9.37, which is similar to the pIs of many
40 M. Decourteix et al.
sucrose transporters (Lemoine 2000). JrSUT1 shares between 72 and 39% identical amino acids with the sucrose transporters already identified in other species (Fig. 2). The highest identities were found with the sucrose transporters from Ricinus communis (71%) (RcSUT1, EMBL accession number AJ224961) and from Euphorbia esula (72%) (EeSUC1, EMBL accession number AF242307). JrSUT1 deduced amino acid sequence also contained the histidine residue (at position 61) conserved in all proton-sucrose symporters (Lemoine 2000) and was shown to play a key role in the sucrose transport reaction (Lu & Bush 1998). Hydropathy analyses of the JrSUT1 protein predict 12 transmembrane domains, another feature common to all plant sucrose transporters (Lemoine 2000). Thus, JrSUT1 presents all the characteristics of plant SUcrose Transport-ers (SUTs) and belongs to the major facilitator superfamily (MFS).
Expression analysis of JrSUT1 in
different organs
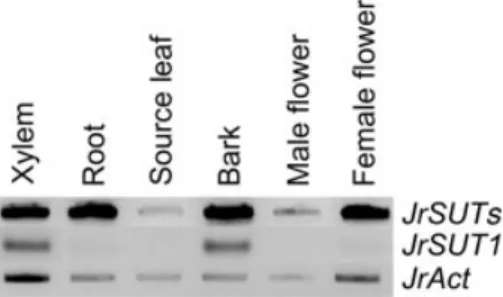
Transcript level analysis was carried out by a semi-quanti-tative RT-PCR approach, using RNAs extracted from leaves, fine roots, xylem, bark (all tissues outside xylem) and flowers (male and female). Sut1A and Sut1B designed from conserved coding sequences of JrSUT1 were used as primers, and therefore the PCR-amplified band (440 pb) is designated JrSUTs (Fig. 3). JrSUTs transcripts were highly expressed in stem tissues (xylem and bark) and in other sink organs (fine roots, female flowers) (Fig. 3). In addition,
JrSUTs were hardly detected in source organs (leaves),
suggesting that their expression could be confined to sink organs.
A specific couple of primers (Sut11/Sut12) was designed based on the 3¢UTR of JrSUT1. As 3¢UTR sequences are reported to be the most divergent regions within genes (Matsukura et al. 2000; Duval et al. 2002; Marin-Olivier
et al. 2002; Sakr et al. 2003), this couple of primers should
allow us to isolate gene-specific probes by PCR. JrSUT1 transcripts were mainly detected in xylem and bark tissues (Fig. 3). Hardly any JrSUT1 transcript was found in the other tested organs (flowers, leaves and fine roots), indicat-ing that JrSUT1 may be a stem-specific sucrose transporter.
Effects of freezing temperatures and
freeze–thaw cycles on the JrSUT1 level in
controlled conditions
We wanted to check whether JrSUT1 transcripts are respon-sive to freezing temperatures only, or to freeze–thaw cycles
Figure 1. Comparison of active sucrose uptake by xylem parenchyma cells between two periods: November–December (Nov–Dec) and February–March (Feb–March). Twigs were first preconditioned for 48 h at +15 ∞C. They were rinsed with the control solution and then perfused with 2 mL of a radioactive solution [control solution supplemented with 1 mM sucrose and 70 kBeq mL-1 14C-sucrose with (passive uptake) or without (total
uptake) addition of 1 mM HgCl2]. The uptake took place for 1 h at
+15 ∞C. Values are means ± SE (n = 5 for November–December and n = 3 for February–March). According to the non-parametric test of Mann and Whitney (Sprent 1992), means are significantly different with a P-value <0.04. Minimum (Min) and maximum (Max) air temperatures are given for each sampling date (in degrees Celsius).
Figure 2. Comparison of amino acid sequences of sucrose/H+
symporters from mono- and dicotyledonous plants. The dendrogram was generated by comparison of the sequences by the
CLUSTAL W 1.74 software (Thompson, Higgins & Gibson 1994). PmSUC3 (accession no. AJ534442); LeSUT2 (accession no. AF166498); VvSUC12 (accession no. AF021809); AtSUC3 (accession no. AJ289165); ZmSUT1 (accession no. AB008464); LeSUT4 (accession no. AF176950); VvSUC11 (accession no. AF021808); DcSUT1 (accession no. Y16766); OsSUT2 (accession no. AB091672); PmSUC1 (accession no. X84379); DcSUT2 (accession no. Y16768); RcSUT1 (accession no. AJ224961); EeSUC1 (accession no. AF242307); VfSUT (accession no. Z93774); VvSUC27 (accession no. AF021810); CsSUT1 (accession no. AY098891); AtSUC2 (accession no. X75382). The walnut tree polypeptide is marked by an asterisk. Percentages of identity are noted on the right.
as can be encountered in the orchard in Clermont-Ferrand. For this purpose, twigs artificially exposed to a range of freezing temperatures (-5, -10 and -15 ∞C) without subse-quent warming were used. Whatever the imposed temper-ature, the level of JrSUT1 transcripts was constant and similar to that of control (twigs exposed to +5 ∞C) (Fig. 4a). We next artificially exposed container-grown trees to two freeze–thaw cycles (-10 ∞C/+5 ∞C), as described in the material and methods section. Compared to the control (exposed to +2 ∞C), xylem tissue harvested from freeze– thaw-treated-trees contained a higher pool of JrSUTs and
JrSUT1 transcripts (Fig. 4b). In order to check whether this
effect could extend to other membrane protein-related genes, we examined in parallel the expression pattern of
plasma membrane H+-ATPase (JrAHA). Unlike JrSUT1,
no difference in the JrAHA transcript level was found between these two conditions (Fig. 4b).
To check whether we could obtain the same regulation of JrSUT1 for outdoor-grown trees as for controlled condi-tions, JrSUT1 level was estimated in xylem tissue over 2 years.
JrSUT1 transcripts in xylem tissue over the
autumn–winter period, in 2001–02
The highest level of JrSUT1 transcripts was detected in November and December, when minimum temperatures recorded within 24 h before the samplings were -5.1 and -5.6 ∞C, respectively, and maximum temperatures were >+1.8 ∞C (Fig. 5). In January, when twigs were not submit-ted to any freeze–thaw cycle within 48 h before sampling (minimum temperatures were >1 ∞C), JrSUT1 level was much lower than in November and December (Fig. 5). Sim-ilar data were obtained in February and March.
JrSUT1 transcript and protein patterns in xylem
tissue collected over the autumn–winter period,
in 2002–03
To check whether the observed seasonal pattern of JrSUT1 transcripts level is mainly due to the temperature regime,
we decided to follow JrSUT1 transcripts along the autumn– winter period of another year, 2002–03 (Fig. 6a).
In contrast to 2001–02, JrSUT1 level was constant and weak in xylem tissue from October to December when sampling did not follow any freeze–thaw event. Indeed, minimum temperatures recorded 48 h (or more) before sampling were >+1.8 ∞C (Fig. 6a). Similar data were also found in February and March, when minimum tempera-tures were >+2 ∞C for the 48 h preceding sampling. The highest level of JrSUT1 transcripts was detected in January, where xylem samples were collected after some freeze– thaw events. Indeed, minimum temperatures were -1.8 and -4.4 ∞C within 24 and 48 h before sampling, respectively, and maximum temperatures were +5.8 and +7.5 ∞C
(Fig. 6a). The difference in transcript accumulations
between January and the other months was also observed, when the Sut1A and Sut1B primers (designed from SUTs conserved coding sequences) were used.
The relative JrSUT1 abundance was also followed using an affinity-purified antibody raised against the specific N-terminal region of the JrSUT1 protein. Samples used to extract RNAs and proteins were harvested the same day and from the same shoots. Preliminary data of immunoblot analysis showed that the antibody cross-reacted with a 55-kDa protein in microsomal fraction, which is in accordance with the computer-predicted molecular weight and with the classical range of molecular weight for SUTs (Lemoine 2000). In addition, the antibody did not cross-react with
Figure 3. Expression pattern of JrSUT1 in xylem tissue, fine roots, source leaves, bark tissues, and male and female flowers of walnut tree. Semi-quantitative RT-PCR was performed either with JrSUTs non-specific primers (sut1A/sut1B), JrSUT1 specific primers (Sut11/Sut12), or JrAct primers (Act11/Act12). At least three individual sets of RT-PCR reactions were carried out in each case.
Figure 4. JrSUT1 transcripts levels in xylem tissue harvested from container-grown trees subjected to freezing temperatures. (a) Twigs were subjected to freezing without warming. Semi-quantitative RT-PCR was performed either with JrSUTs non-specific primers (sut1A/sut1B), JrSUT1 non-specific primers (Sut11/ Sut12) or JrAct primers (Act11/Act12). At least three individual sets of RT-PCR reactions were carried out in each case, and data from one of them are presented here. (b) Trees were subjected to two freeze–thaw cycles (-10 ∞C) or not (control).
Semi-quantitative RT-PCR was performed with either JrSUTs non-specific primers (sut1A/sut1B), JrSUT1 non-specific primers (Sut11/ Sut12), JrAHA specific primers (AHA11/AHA12) or JrAct primers (Act11/Act12). At least, three individual and independent sets of RT-PCR reactions were carried out in each case, and data from one of them are presented here.
42 M. Decourteix et al.
other walnut sucrose/H+ symporters since JrSUTs tran-scripts were detected in fine roots whereas no signal was detected by the antiserum in microsomal fractions from leaves and fine roots (unpublished results) (Fig. 3). Not-withstanding these data, we cannot completely exclude that the affinity-purified antibody directed against the quite spe-cific N-terminal region of JrSUT1 might recognize other
sucrose transporters. To simplify, we chose to refer to JrSUT1 to indicate proteins cross-reacting with this poly-clonal antibody.
Accumulation patterns of JrSUT1 transcripts and pro-teins correlated well, except in February (Fig. 6a & b). JrSUT1 amount was weak and nearly constant over the autumn period (from October until December),
signifi-Figure 5. Time course of JrSUT1 transcripts in walnut xylem tissue collected over the autumn–winter period 2001–02. Semi-quantitative RT-PCR was performed with JrSUT1 specific primers (Sut11/Sut12) or JrAct primers (Act11/Act12). At least, five individual sets of RT-PCR reactions were carried out, and one of them is presented here. Minimum (Min) and maximum (Max) air temperatures preceding sampling within 24 and 48 h are given for each sampling date (in degrees Celsius).
Figure 6. Time course of JrSUT1 transcript and protein accumulation in walnut stem tissues collected over the autumn–winter period 2002–03. (a) Semi-quantitative RT-PCR was performed on xylem tissue, using JrSUTs non-specific primers (sut1A/sut1B), JrSUT1 specific primers (Sut11/Sut12), JrAHA (AHA11/AHA12) and JrAct primers (Act11/Act12). At least five individual sets of RT-PCR reactions were carried out, and data from one of them are presented here. (b) Western blot analyses were run on microsomal fractions extracted from xylem tissue. Nitocellulose membranes were hybridized with the affinity-purified anti-JrSUT1 antibody, raised against the specific N-terminal region, and with the anti-JrAHA antibody, raised against a conserved region. Four independent experiments yielded similar results, and data from one of them are presented here. (c) Semi-quantitative RT-PCR was carried out on bark tissues. The bark and xylem tissues were sampled from the same shoot. Five individual sets of RT-PCR reactions were carried out and data from one of them are presented here. Minimum (Min) and maximum (Max) air temperatures preceding sampling within 24 and 48 h are given for each sampling date (in degrees Celsius).
cantly enhanced in January, reached its maximum in Feb-ruary and dropped in March Unlike JrSUT1, JrAHA transcript and protein levels were constant and insensitive to this temperature regime (Fig. 6a & b).
The freezing temperature-related abundance of JrSUT1 messenger in xylem tissue raised the question of its tissue-specificity. To answer this, RNAs were isolated from bark taken from the same shoots in 2002–03, and the pattern of
JrSUT1 level was assayed. As shown in Fig. 6c, JrSUT1
transcripts were also expressed in bark tissues, which is in accordance with our previous data (Fig. 3). However, in contrast to xylem tissue, freezing temperatures in January did not enhance JrSUT1 transcripts in bark (Fig. 6c). Sim-ilar data were obtained with JrAHA (Fig. 6c).
Immunolocalization of JrSUT1 and JrAHA in
xylem tissue
VACs belong to the xylem parenchyma cell type but repre-sent a very special kind of parenchyma cells. Moreover they are the only cells that surround xylem vessels. Therefore, they play a pivotal role in the nutrient exchanges between xylem vessels and the other parenchyma cells, which are involved rather in storing carbohydrates (Alves et al. 2001; Alves 2003).
Tissue localization of JrSUT1 was achieved by in situ immunolocalization using two different immunodetection methods: alkaline phosphatase conjugated to the secondary antibody (Fig. 7a & b) and immunofluorescence labelling
Figure 7. Immunolocalization of JrSUT1 and JrAHA in walnut xylem sampled in January 2003, using two different techniques. JrSUT1 (a, b) and JrAHA (e, f)
immunolocalization using secondary antibody conjugated to alkaline phosphatase. Negative controls (g, h). The signal was visualized by blue staining. JrSUT1 immunolocalization (d) with secondary antibody conjugated to FITC. Negative control (c). Immunofluorescence is visible as a strong green colour, mainly in VACs (black arrows). Black and white arrows indicate the localization of VACs. r, radial parenchyma cells; vac, vessel-associated cells; xv, xylem vessels; f, fibres. Scale bars = 50 mm
44 M. Decourteix et al.
(Fig. 7c & d). Immunolocalization experiments were car-ried out using stem sections from 1-year-old twigs sampled in January 2003, in which higher transcript and protein amounts were already observed (Fig. 6a & b).
With the alkaline phosphatase method, a strong specific labelling was visible predominantly in VACs, and in both axial and radial parenchyma cells, particularly those near VACs (Fig. 7a & b). Neither with saturated antibody (neg-ative control, data not shown) nor in the absence of the primary antibody (Fig. 7g & h) was any signal detected. Similar data were obtained with the immunofluorescence labelling. The immunofluorescence was detectable as a strong green signal at the periphery of the cells, mainly close to xylem vessels (VACs) (Fig. 7d), while autofluores-cence of the lignified cell walls, such as fibres and vessels, was slightly visible in the control (Fig. 7c).
The tissue localization of JrAHA, which is required for H+-cotransport of nutrient, was also investigated using the alkaline phosphatase method. As for JrSUT1, a strong labelling was observed in living cells of xylem parenchyma, particularly VACs (Fig. 7e & f). No signal was detected with saturated antibody (negative control, data not shown). These data reveal, for the first time, a co-immunodetection of JrSUT1 and JrAHA to VACs, which are considered to be specialized in nutrient exchange between xylem vessels (apoplast) and xylem parenchyma cells (symplast).
DISCUSSION
JrSUT1 encodes a putative stem
sucrose symporter
To identify a sucrose transporter putatively expressed in xylem tissue during the autumn–winter period, a xylem-derived cDNA library was screened and the first entire cDNA clone encoding a putative stem-specific sucrose transporter was obtained (EMBL accession number AY504969). JrSUT1 was highly similar to (Fig. 2) and has all of the characteristics of plant sucrose transporters. The greatest identity was seen with RcSUT1 (Ricinus communis Sucrose Transporter) and EeSUC1 (Euphorbia esula Sucrose transporter) (more than 70%). Therefore, JrSUT1 was referred to as a putative sucrose transporter.
The expression pattern of JrSUT1 was studied by semi-quantitative PCR, as recently used in the walnut tree (Sakr
et al. 2003). JrSUT1 transcripts were predominantly
detected in both xylem and bark tissues and not found in the other tested organs (leaf, root, male and female flow-ers). JrSUT1 thus encodes a stem-specific, and therefore sink-specific, putative sucrose transporter (Fig. 3). Other sink-specific sucrose transporters have been reported such as PmSUC1, the flower-specific sucrose transporter from
P. major (Garthz et al. 1996), VfSUC1, the seed-specific
transporter from V. faba (Weber et al. 1997) and DcSUT2, the sink-specific transporter from Daucus carota (Shakya & Sturm 1998). Moreover, JrSUT1 is seemingly not the sole walnut sucrose transporter since another cDNA was detected in the organs when the RT-PCR experiments were
carried out with (Sut1A/Sut1B), a couple of primers designed from conserved regions. This is consistent with sucrose transporters being encoded by a multigene family in higher plants (Barker et al. 2000; Yu, Hu & Wang 2002).
JrSUT1 is localized to VACs
During the autumn–winter period, sucrose concentration in walnut xylem sap (apoplast) has been shown to decrease as temperature increases (Améglio et al. 2004), due to its active re-absorption by xylem parenchyma cells (symplast), presumably via a sucrose/H+-cotransporter (Valentin et al. 2001) localized to VACs (Fig. 7). VACs, which are the only parenchyma cells directly connected to xylem vessels (Fig. 7b; Czaninski 1977; Alves et al. 2001; Sakr et al. 2003), allow nutrient exchanges between apoplast and symplast. The absence of plasmodesmatal connections between ves-sels and VACs (Catesson 1983; Czaninski 1987), implies that nutrients must cross their plasma membrane as they do towards companion cells in the phloem (Bonnemain & Fromard 1987; Oparka & Santa Cruz 2000). In Robinia xylem tissue, Fromard et al. (1995) showed that the plasma
membrane H+-ATPase, which generates the driving force
for active transport of nutrients, was located in VACs. Inter-estingly, our data from two independent immunolocaliza-tion studies shows that JrSUT1, a putative sucrose transporter, and JrAHA, an H+-ATPase are both localized to VACs. This observation is in perfect accordance with the idea that JrSUT1 plays a major role in sucrose influx into xylem parenchyma cells, via VACs.
Xylem-localized JrSUT1 is up-regulated by
freeze–thaw cycles
Previous investigations have reported that mRNA levels of sucrose transporters could be controlled at the transcript level by sucrose itself (Chiou & Bush 1998), salt stress (Noiraud, Delrot & Lemoine 2000), light (Matsukura et al. 2000), or wounding (Meyer et al. 2004). This is the first report on the up-regulation of SUT transcripts by freeze– thaw cycles in higher plants. This conclusion is based upon data obtained in both controlled and field conditions (Figs 4, 5 & 6). No increase in the transcript levels of
JrSUT1 was found when twigs were artificially submitted
to a range of freezing temperatures only (-5 to -15 ∞C), indicating that accumulation of JrSUT1 transcripts was not induced during the freezing phase. However, a significant and a specific increase in JrSUT1 transcripts was detected when two freeze–thaw cycles (-10 ∞C/+5 ∞C) were imposed to twigs (Fig. 4), suggesting that JrSUT1 transcripts rather respond to freeze–thaw events. The data obtained in field conditions tend to support this idea. Indeed, in November and December 2001 (24 h prior to sampling dates, mini-mum temperatures were <-5 ∞C and maximini-mum tempera-tures were >2 ∞C), the level of JrSUT1 transcripts was highest, compared with other months of the same year (minimum and maximum temperatures were both >+1 ∞C) (Fig. 5). It is noteworthy that this pattern is completely
different from that in November and December 2002
(min-imum temperatures 24 h before sampling date were
+1.8 ∞C), where the JrSUT1 level was low and weaker than in January 2003 (minimum temperatures 24 and 48 h prior to sampling were -1.8 and -4.4 ∞C, respectively, and maxi-mum temperatures were >5.8 ∞C) (Fig. 6). Taken together, these data show that JrSUT1 expression depends on the daily temperature alternation during the autumn–winter period.
On the other hand, with the exception of February 2003, there is a positive correlation between the increased level of JrSUT1 transcripts and their respective proteins (Fig. 6a & b). In February 2003, sampling was carried out about 96 h after trees had been subject to freeze–thaw cycles (-5.1 ∞C as minimum temperature and 12.7 ∞C as maximum temper-ature), which normally should lead to a stimulation of both transcripts and proteins of JrSUT1. In this context, the lack of relation between JrSUT1 transcript levels and respective protein levels may be explained by assuming a rapid turn-over of JrSUT1 transcripts.
The relationship between the temperature regime and the abundance of JrSUT1 is tissue-specific, since no change in JrSUT1 transcripts was found in bark tissues (Fig. 6c). In addition, the levels of H+-ATPase transcripts and corre-sponding proteins in xylem changed neither under
con-trolled conditions (Fig. 4), nor in response to the
temperature regime in the field (Fig. 6a & b). Thus, the freeze–thaw-related JrSUT1 accumulation cannot merely result from a general stimulation of all plasma membrane proteins, but is rather specific to putative xylem-localized sucrose transporters.
Physiological significance of the accumulation
of xylem-localized JrSUT1 in response to freeze–
thaw cycles
Freeze–thaw cycles are encountered daily in Clermont-Ferrand in winter. The up-regulation of JrSUT1 due to such alternations could be, at least in part, related to the rare ability of Juglans trees to repair xylem embolism after its freeze–thaw induction. Most tree species recover hydraulic conductivity in spring by the production of new and func-tional conduits (Cochard et al. 2001) and/or refilling of air-filled conduits by positive root pressure (Ewers et al. 2001). However, Juglans has one more specific mechanism: an active refilling of embolized vessels through the creation of winter stem pressure (Améglio et al. 2001, 2002).
According to the ‘vitalistic’ models, this would involve activities of living xylem parenchyma cells and high sucrose concentrations in xylem sap. For cold temperatures, the influx is very low and there is a high release of sugars (mainly sucrose). The subsequent increase in sugar concen-tration in xylem vessels could lead to an increased stem osmotic pressure, and thus permit embolism repair (Améglio et al. 2001, 2004). Xylem sap sucrose concentra-tion then decreases progressively as temperatures increase (Améglio et al. 2004). The lowering of sap sucrose concen-tration during warmer phases has been attributed to
increased sucrose influx into xylem parenchyma cells (Val-entin et al. 2001; Améglio et al. 2004). Since these cells actively absorb more sucrose and contain a larger amount of JrSUT1 after a freeze–thaw cycle, and since JrSUT1 and JrAHA1 are colocalized to VACs, we conclude that JrSUT1 could allow the VACs to recover the sucrose lost in the vessel after thawing and for mild temperatures. This sucrose retrieval from xylem sap (apoplast) to parenchyma cells (symplast) may be compared with the retrieval of sucrose from the phloem along the path from source to sink organs as proposed for StSUT1 (Riesmeier, Hirner & Frommer 1993) or for AgSUT (Noiraud et al. 2000).
The sugar exchanges between xylem vessels and the neighbouring storage parenchyma tissues suggests that the xylem pathway is involved in carbohydrates movements in winter, when phloem is considered non-functional (Aloni 1991; Aloni & Peterson 1997). This is in agreement with other results obtained on Juglans regarding carbon imported into the branches during winter but also in spring outgrowth (Lacointe et al. 2004).
ACKNOWLEDGMENTS
This work was supported by the Ministère de la Recherche et de l’Education Nationale and the INRA (Environment and Agronomy department). We thank Brigitte Saint-Joa-nis, Sylvaine Labernia and Marc Vandamme as contribu-tors.
REFERENCES
Ageorges A., Issaly N., Picaud S., Delrot S. & Romieu C. (2000) Characterization of an active sucrose transporter gene expressed during the ripening of grape berry (Vitis vinifera L.). Plant Physiology and Biochemistry 38, 177–185.
Aloni R. (1991) Wood formation in deciduous hardwood trees. In Physiology of Trees (ed. A.S. Raghavendra), pp. 175–197. Wiley, New York, USA.
Aloni R. & Peterson C.A. (1997) Auxin promotes dormancy cal-lose removal from the phloem of Magnolia kobus and calcal-lose accumulation and earlywood vessel differentiation in Quercus robur. Journal of Plant Research 110, 37–44.
Alves G. (2003) Les cellules associées aux vaisseaux (CAVs) chez Juglans regia: étude de l’ATPase-H+ du plasmalemme. PhD
The-sis, Université Blaise Pascal, Aubière, France.
Alves G., Ameglio T., Guilliot A., Fleurat-Lessard P., Lacointe A., Sakr S., Petel G. & Julien J.-L. (2004) Winter variation in xylem sap pH of walnut trees: involvement of plasma mem-brane H+-ATPase of vessel-associated cells. Tree Physiology
24, 99–105.
Alves G., Sauter J.J., Julien J.-L., Fleurat-Lessard P., Ameglio T., Guilliot A., Petel G. & Lacointe A. (2001) Plasma membrane H+-ATPase, succinate and isocitrate dehydrogenases activities
of vessel-associated cells in walnut trees. Journal of Plant Phys-iology 158, 1263–1271.
Améglio T., Bodet C., Lacointe A. & Cochard H. (2002) Winter embolism, mechanisms of xylem hydraulic conductivity recov-ery and springtime growth patterns in walnut and peach trees. Tree Physiology 22, 1211–1220.
Améglio T., Decourteix M., Alves G., Valentin V., Sakr S., Julien J.-L., Petel G., Guilliot A. & Lacointe A. (2004) Temperature effects on xylem sap osmolarity in walnut trees: evidence for a
46 M. Decourteix et al.
vitalistic model of winter embolism repair. Tree Physiology 24, 785–793.
Améglio T., Ewers F.W., Cochard H., Martignac M., Vandame M., Bodet C. & Cruiziat P. (2001) Winter stem xylem pressure in walnut trees: effects of carbohydrates, cooling and freezing. Tree Physiology 21, 387–394.
Arango M., Gevaudant F., Oufattole M. & Boutry M. (2003) The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta 216, 355–365.
Arend M., Weisenseel M., Brummer M., Osswald W. & Fromm J. (2002) Seasonal changes of plasma membrane H+-ATPase and
endogenous ion current during cambial growth in poplar plants. Plant Physiology 129, 1651–1663.
Axelsen K. & Palmgren M. (2001) Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiology 126, 696–706. Barker L., Kühn C., Weise A., Schulz A., Gebhardt C., Hirner B., Hellmann H., Schulze W., Ward J.M. & Frommer W.B. (2000) SUT2, a putative sucrose sensor in sieve elements. Plant Cell 12, 1153–1164.
Bonnemain J. & Fromard L. (1987) Physiologie comparée des cellules compagnes du phloème et des cellules associées aux vaisseaux. Bulletin de la Société Botanique de France 134, 27–37. Bush D.R. (1993) Inhibitors of the proton-sucrose symport.
Archives of Biochemistry and Biophysics 307, 355–360. Catesson A. (1983) A cytochemical investigation of the lateral
walls of Dianthus vessels. Differentiation and pit-membrane for-mation. IAWA Bulletin 4, 89–101.
Catesson A. & Czaninski Y. (1967) Mise en évidence d’une activité phosphatasique acide dans le reticulum endoplasmique des tis-sus conducteurs de robinier et de Sycomore. Journal of Micros-copy 6, 509–514.
Chiou T. & Bush D.R. (1998) Sucrose is a signal molecule in assimilate partitioning. Proceedings of the National Academy of Sciences of the USA 95, 4784–4788.
Cochard H., Lemoine D., Ameglio T. & Granier A. (2001) Mech-anisms of xylem recovery from winter embolism in Fagus sylvat-ica. Tree Physiology 21, 27–33.
Czaninski Y. (1977) Vessel-associated cells. IAWA Bulletin 3, 51–55. Czaninski Y. (1987) Généralité et diversité des cellules associées aux vaisseaux (cellules de contact sensus lato). Bulletin de la Société Botanique de France 134, 19–26.
Davies C., Wolf T. & Robinson S.P. (1999) Three putative sucrose transporters are differentially expressed in grapevine tissues. Plant Science 147, 93–100.
Duval F., Renard M., Jaquinod M., Biuo V., Montrichard F. & Macherel D. (2002) Differential expression and functional anal-ysis of three calmodulin isoforms in germinating pea (Pisum sativum L.) seeds. Plant Journal 32, 481–493.
Ewers F.W., Ameglio T., Cochard H., Beaujard F., Martignac M., Vandame M., Bodet C. & Cruiziat P. (2001) Seasonal variation in xylem pressure of walnut trees: root and stem pressures. Tree Physiology 21, 1123–1132.
Fromard L., Babin V., Fleurat-Lessard P., Fromont J., Serrano R. & Bonnemain J. (1995) Control of vascular sap pH by the vessel-associated cells in woody species (physiological and immunolog-ical studies). Plant Physiology 108, 913–918.
Gahrtz M., Schmelzer E., Stolz J. & Sauer N. (1996) Expression of the PmSUC1 sucrose carrier gene from Plantago major L. is induced during seed development. Plant Journal 9, 93–100. Gévaudant F., Samson I., Guilliot A. & Pétel G. (1999) An
improved method for isolating polyphenol-free RNA from woody plant tissues. Journal of Trace and Microprobe Tech-niques 17, 445–450.
Höll W. (1985) Seasonal fluctuation of reserve materials in the trunkwood of spruce (Picea abies (L.) Karst.). Journal of Plant Physiology 117, 355–362.
Karlson D.T., Xiang Q.Y., Stirm V.E., Shirazi A.M. & Ashworth E.N. (2004) Phylogenetic analyses in cornus substantiate ances-try of xylem supercooling freezing behavior and reveal lineage of desiccation related proteins. Plant Physiology 135, 1654–1665. Kühn C., Hajirezaei M.-R., Fernie A.R., Roessner-Tunali U., Czechowski T., Hirner B. & Frommer W.B. (2003) The sucrose transporter StSUT1 localizes to sieve elements in potato tuber phloem and influences tuber physiology and development. Plant Physiology 131, 102–113.
Lacointe A., Deleens E., Améglio T., Saint-Joanis B., Lelarge C., Vandame M., Song G.C. & Daudet F.A. (2004) Testing the branch autonomy theory: a 13C/14C double-labelling experiment
on differentially shaded branches. Plant, Cell and Environment 27, 1159–1168.
Laemmli U. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. Lalonde S., Tegeder M., Throne-Holst M., Frommer W.B. & Patrick J.W. (2003) Phloem loading and unloading of sugars and amino acids. Plant, Cell and Environment 26, 37–56.
Lemoine R. (2000) Sucrose transporters in plants: update on func-tion and structure. Biochimica et Biophysica Acta 1465, 246–262. Lemoine R., Bürkle L., Barker L., Sakr S., Kühn C., Regnacq M., Gaillard C., Delrot S. & Frommer W.B. (1999) Identification of a pollen-specific sucrose transporter-like protein NtSUT3 from tobacco. FEBS Letters 454, 325–330.
Lu J. & Bush D. (1998) His-65 in the proton-sucrose symporter is an essential amino acid whose modification with site-directed mutagenesis increases transport activity. Proceedings of the National Academy of Sciences of the USA 95, 9025–9030. Marin-Olivier Chevalier T., Fobis-Loisy I., Dumas C. & Gaude T.
(2002) Aquaporin PIP genes are not expressed in the stigma papillae in Brassica oleracea. Plant Journal 24, 231–240. Matsukura C., Saitoh T., Hirose T., Ohsugi R., Perata P. &
Yamagu-chi J. (2000) Sugar uptake and transport in rice embryo. Expres-sion of companion cell-specific sucrose transporter (OsSUT1) induced by sugar and light. Plant Physiology 124, 85–93. Meyer S., Lauterbach C., Niedermeier M., Barth I., Sjolund R. &
Sauer N. (2004) Wounding enhances expression of AtSUC3, a sucrose transporter from Arabidopsis sieve elements and sink tissues. Plant Physiology 134, 684–693.
Noiraud N., Delrot S. & Lemoine R. (2000) The sucrose trans-porter of celery. Identification and expression during salt stress. Plant Physiology 122, 1447–1455.
Oparka K.J. & Santa Cruz S. (2000) The great escape: phloem transport and unloading of macromolecules. Annual Review of Plant Physiology and Plant Molecular Biology 51, 323–347. Riesmeier J.W., Hirner B. & Frommer W.B. (1993) Potato sucrose
transporter expression in minor veins indicates a role in phloem loading. Plant Cell 5, 1591–1598.
Roblin G., Sakr S., Bonmort J. & Delrot S. (1998) Regulation of a plant plasma membrane sucrose transporter by phosphoryla-tion. FEBS 424, 165–168.
Sakr S., Alves G., Morillon R., Maurel K., Decourteix M., Guilliot A., Fleurat-Lessard P., Julien J.-L. & Chrispeels M. (2003) Plasma membrane aquaporins are involved in winter embolism recovery in walnut tree. Plant Physiology 133, 630–641. Sakr S., Noubahni M., Bourbouloux A., Riesmeier J., Frommer
W., Sauer N. & Delrot S. (1997) Cutting, ageing and expression of plant membrane transporters. Biochimica et Biophysica Acta 1330, 207–216.
Sauter J.J. (1972) Respiratory and phosphatase activities in contact cells of wood rays and their possible role in sugar secretion. Zeitschrift für Planzenphysiology 67, 135–145.
Sauter J.J. (1983) Efflux and reabsorption of sugars in the xylem II. seasonal changes in sucrose uptake in Salix. Zeitschrift für Planzenphysiology 111, 429–440.
Sauter J.J. (1988) Temperature-induced changes in starch and sug-ars in the stem of Populus ¥ canadensis ‘robusta’. Journal of Plant Physiology 132, 608–612.
Shakya R. & Sturm A. (1998) Characterization of source- and sink-specific sucrose/H+ symporters from carrot. Plant Physiology
118, 1473–1480.
Sprent P. (1992) Méthodes pour trois échantillons ou plus. Pratigue des statistiques non paramétriques (Eds INRA), pp. 129–150. Paris, France.
Stadler R., Brandner J., Schulz A., Gahrtz M. & Sauer N. (1995) Phloem loading by the PmSUC2 sucrose carrier from Plantago major occurs into companion cells. Plant Cell 7, 1545–1554. Stadler R. & Sauer N. (1996) The Arabidopsis thaliana AtSUC2
gene is specifically expressed in companion cells. Botanica Acta 109, 299–306.
Thompson J., Higgins D. & Gibson T. (1994) CLUSTAL W: improving the sensitivity of progressive mutiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Research 22, 4673–4680.
Valentin V., Améglio T., Lacointe A., Julien J.L. & Pétel G. (2001) Sugars exchanges between vessels associated cells and xylem vessels, in relation with the temperature, in walnut. Acta Horti-culturae 544, 509–515.
Vaughn M., Harrington G. & Bush D. (2002) Sucrose-mediated transcriptional regulation of sucrose symporter activity in the phloem. Proceedings of the National Academy of Sciences of the USA 99, 10876–10880.
Weber H., Borisjuk L., Heim U., Sauer N. & Wobus U. (1997) A role for sugar transporters during seed development: molecular characterization of a hexose and a sucrose carrier in fava bean seeds. Plant Cell 9, 895–908.
Williams L.E., Lemoine R. & Sauer N. (2000) Sugar transporters in higher plants-a diversity of roles and complex regulation. Trends in Plant Science 5, 283–290.
Wright D.P., Scholes J.D., Read D.J. & Rolfe S.A. (2000) Changes in carbon allocation and expression of carbon transporter genes in Betula pendula Roth. colonized by the ectomycorrhizal fun-gus Paxillus involutus (Batsch) Fr. Plant, Cell and Environment 23, 39–49.
Yao Li C., Weiss D. & Goldschmidt E.E. (2003) Effects of carbo-hydrate starvation on gene expression in Citrus root. Planta 217, 11–20.
Yu J., Hu S. & Wang J. (2002) A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296, 79–92.
Received 24 February 2005; received in revised form 26 May 2005; accepted for publication 1 June 2005