Optimizing economic outcomes in antibiotic therapy of patients with acute bacterial exacerbations of chronic bronchitis
Texte intégral
(2) J.-C. Pechère and L. Lacey differentiating between success and failure. However, a recent meta-analysis involving nine placebo-controlled clinical trials demonstrated that antibiotic therapy produced a small, but statistically significant, improvement in patients with ABECB.8 In addition, a seminal study showed that clinical recovery, at least in patients with the most severe respiratory tract symptoms, is more rapid when carefully selected, effective antibiotics are administered.9 Infection increases airway inflammation and obstruction which in turn may precipitate acute respiratory failure in the most severely affected patients. Thus, effective antibiotic therapy may reduce the risk of decompensation and its corollary, hospitalization, although it must be remembered that patients are hospitalized for reasons other than ABECB. Antibiotics are used widely in the management of patients with ABECB. For example, the National Center for Health Statistics in the USA estimated that, in 1994, 90.9% of patients with chronic bronchitis received antibiotic treatment for acute episodes.3 Owing to the high prevalence of chronic bronchitis in the adult population and its important socio-economic consequences, optimizing the management of patients with ABECB in order to minimize the economic impact of the disease has become an important issue. Increases in antibiotic resistance rates among respiratory pathogens have generated a need for novel oral antibiotics for the treatment of patients with bacterial infections. The introduction of the extended-spectrum fluoroquinolones, in particular, has provided alternative therapeutic modalities for use in patients with lower respiratory tract infections such as ABECB. However, as clinical trials comparing two or more antibiotic regimens are almost always designed to demonstrate only clinical equivalence, as opposed to the superiority of one over the other, it is often not possible for these studies to confirm that newer agents are significantly better in terms of clinical response rates than the comparator drugs. The very large clinical trials that would be necessary to show that novel drugs have significant clinical advantages are simply impractical. However, there are other more easily demonstrable benefits associated with new agents, including superior pharmacokinetic profiles which facilitate simplified and shorter treatment regimens, better tolerability, broader antimicrobial spectra and lower rates of resistance among bacterial pathogens. It has been argued, on the grounds of high acquisition costs and the risks of promoting the emergence of resistance to our most potent weapons, that new antibiotics should be reserved for patients in whom older drugs have failed or are not likely to be effective. The aim of this article is to consider the economic arguments in a discussion of issues associated with the use of old, compared with new, antibiotics in the treatment of patients with ABECB.. Which patient? Which antibiotic? When considering the administration of antibiotics to patients with chronic bronchitis, the first question is obvious: to whom should an antibiotic be given? The answer, ideally, is that an antibiotic should be given to patients truly suffering from ABECB, rather than to those with exacerbations of non-bacterial aetiology. However, differentiating between these two categories of precipitating factor at the bedside is often difficult. Microbiological investigations of sputum specimens are of limited value because the presence of potentially pathogenic bacteria in the sputa of such patients does not prove that these bacteria caused the exacerbations. The distinction between simple bacterial colonization and infection is very often blurred, even when sampling and microbiological diagnostic techniques are optimal. The most appropriate criteria for identifying patients who will benefit from antibiotics are, in fact, clinical and include an acute and marked increase in cough and sputum production, sputum purulence and dyspnoea. The principal objective in prescribing antimicrobials for bacterial exacerbations in patients with chronic bronchitis of mild or moderate severity is to eradicate the pathogen and to shorten the duration of symptoms. In more severe cases, where patients’ pulmonary reserves are limited, antibiotic therapy is also given with the aim of preventing further disability and hospitalization, while in patients with the most advanced forms of disease, antibiotics may increase the likelihood of survival. Where antibiotics are indicated, oral administration is appropriate in most cases. Several agents have been reported as being effective when given by this route, including co-trimoxazole, tetracyclines, amoxycillin (with or without clavulanate), first- and second-generation cephalosporins, novel macrolides, such as clarithromycin and azithromycin, and novel fluoroquinolones. Selection is usually made on an empirical basis and takes account of the likely pathogens and local susceptibility patterns. Nontypeable strains of Haemophilus influenzae are the most frequently implicated pathogens1 and are associated with the production of TEM-type -lactamases at a frequency which depends on the geographical area.10 Moraxella catarrhalis, most isolates of which produce -lactamases, was once considered to be a commensal organism, but is now recognized as a pathogen in this clinical setting.11 Although Streptococcus pneumoniae is probably a less common cause of ABECB than was previously thought, it remains an important aetiological agent none the less.1 Reduced susceptibility to penicillins and cephalosporins among pneumococci is a growing concern worldwide and a multidrug resistance phenotype which includes resistance to co-trimoxazole, tetracycline, chloramphenicol and macrolides, but not the fluoroquinolones, is a both worrying and realistic prospect.10 In patients with severe and/or chronic diseases, aerobic Gram-negative bacilli, including Pseudomonas aeruginosa,12 are occasionally implicated; 20.
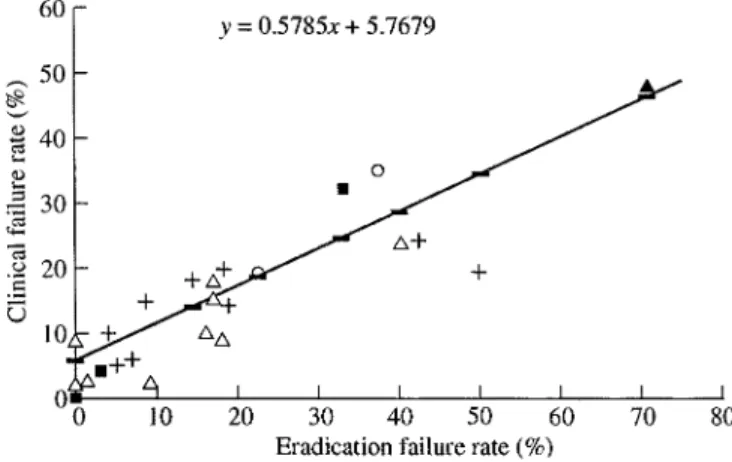
(3) Economic outcomes in ABECB Staphylococcus aureus is also identified, albeit even less frequently.. The direct costs associated with ABECB A report from the USA showed that the total cost of treating patients with AECB was $1.2 billion per annum for those aged 65 years and $419 million for those aged 65 years (1994 charges inflated to 1995 prices).3 Analysis of the disaggregated totals for both age groups revealed that hospital costs accounted for $1.1 billion and $408 million, respectively, while outpatient visits (including all related costs, such as medical care, laboratory tests and diagnostic radiology) contributed $24.9 million and $15.1 million, respectively. The authors concluded that, because a large proportion of the total cost of managing patients with AECB is accounted for by the costs arising from hospitalization, regimens that allow patients to be treated successfully as outpatients are likely to be associated with considerable financial benefits.. Structure of the healthcare costs of ABECB The overall costs of treating patients with any disease have both direct and indirect components. ‘Direct’ healthcare costs apply principally to those resources expended within the healthcare sector.13 They are relatively easy to evaluate and quantify14,15 and include resources used in the diagnosis and treatment of patients with the disease in question, i.e. medication, diagnostic procedures, additional physician visits and, when required, hospitalization. Other direct costs arise from adverse events, drug interactions and treatment failure.13 ‘Indirect’ costs generally include those incurred by sectors of the community other than the healthcare sector and are much more difficult to estimate as they reflect losses in productivity, including more than just paid employment. Other consequences of the disease that are virtually impossible to quantify in financial terms are pain, suffering and a reduction in quality of life. From an economic perspective, a contemporary assessment of antibiotic therapy should include its potential impact on the environment, particularly in relation to the selection of resistant bacteria. Recently, Austin et al. attempted to define the association between the level of antibiotic resistance and the level of antibiotic consumption.16 These investigators drew attention to the importance of both the community setting and commensal organisms and the relevance of these variables to patients with ABECB. However, many questions remain to be addressed before the dynamics of antibiotic resistance can be fully understood, only limited data being available to support the implementation of this theoretical approach. Despite the complexity, it is clear that antibiotics differ in terms of their capacities to promote resistance.17,18 In this regard, there are no data enabling comparisons of the selective pressures exerted by the various antibiotics used as treatment of patients with ABECB. The newer fluoroquinolones have been shown to be less likely than older compounds to select for resistance in S. pneumoniae19 and P. aeruginosa20 and this advantage may assume greater importance in relation to good management and cost-effectiveness when developing antibiotic guidelines in the future, on the grounds that resistance has cost implications, as discussed below. Although the economic impact of treating patients with ABECB is substantial, only a few studies have addressed this aspect of the disease. The results of two approaches to the problem will be considered: the overall cost to the healthcare sector of managing acute exacerbations in patients with chronic bronchitis and the cost-effectiveness of different treatment options.. The impact of antibiotic efficacy on costs Increasing incidences of antibiotic resistance among respiratory pathogens lead to reduced bacteriological and clinical cure rates. Several diseases can serve as models for demonstrating that the MIC can be used to predict clinical outcome. For example, an inverse relationship between the MIC and antibiotic efficacy has been shown in gonorrhoea21 which can be modelled relatively simply and has a single aetiological agent. High MICs have also been associated with poor clinical results in patients with community-acquired pneumonias21 and with lower rates of eradication of pathogens from the middle ear22,23 which, in turn, correlated with higher rates of clinical failure in patients with acute otitis media. A review of 12 studies of patients with ABECB demonstrated a very strong correlation (r 0.91) between eradication failure rates and clinical failure rates (Figure 1).21 These data support the theory that microbiological eradi-. Figure 1. Relationship between eradication failure rates and clinical failure rates in patients with ABECB treated with macrolides ( ), fluoroquinolones ( ), penicillins ( ), cephalosporins ( ) or co-trimoxazole ( ).. 21.
(4) J.-C. Pechère and L. Lacey cation is a major determinant of clinical outcome in this setting and suggest that high MICs are associated with increased rates of clinical failure. It is important that initial therapy is successful as treatment failure can result in marked increases in costs, especially if it leads to hospitalization. The least cost-effective antibiotic, then, is one that is not only clinically ineffective, but that is also associated with a poorer prognosis and an increased likelihood of hospitalization, complications and the need for further treatment, including additional antibiotics. Backhouse et al.24 evaluated the efficacies of four treatment options in the management of patients with ABECB by calculating the total direct cost for each successfully treated patient. They showed that the cost of treatment failure was typically much higher than the acquisition costs of first-line antibiotics. This study confirmed that the cost of treatment failure is high and is driven by the efficacy of the antibiotic regimen used as first-line therapy. In a recent study carried out in Germany, van Barlingen et al.25 investigated the primary factors determining the cost-effectiveness of different antibiotic classes in the management of patients with ABECB. As in the studies described above, these investigators showed that antibiotic acquisition costs account for only a small proportion of total healthcare expenditure (Figures 2 and 3). Not unexpectedly, the costs for patients experiencing more severe. ABECB were greater than those for patients with mild-tomoderate ABECB, because of the higher incidences of hospitalization. Compliance is another issue with a potential economic impact, poor compliance predisposing patients to therapeutic failure. An experimental model demonstrated that either reducing the dosage of an antibiotic or increasing the dosing interval beyond a certain limit promotes the emergence of resistant strains during courses of therapy.26 Similarly, poor compliance, for example when a patient forgets to take a dose or reduces the dosage of an antibiotic without medical advice, leads to under-dosing. Once-daily dosing, treatment regimens of short duration and good tolerability are likely to improve compliance; antibiotics which can be given only once daily are associated with higher rates of compliance (95.2%) than those which are administered on a twice-daily basis (76.2%).27 There may be fewer side effects with short-duration treatment courses than with longer courses. A recent survey of patients’ attitudes to antibiotics demonstrated that short-course therapy, once-daily dosing, good efficacy and few sideeffects are important features of a therapeutic regimen.28 Several novel fluoroquinolones and some macrolides fulfil these criteria. Courses of therapy of short duration reduce overall treatment costs. A recent clinical trial showed that grepafloxacin 400 mg od for 5 days was equivalent to clarithromycin 250 mg bd for 10 days in the treatment of patients with ABECB.29 However, grepafloxacin eradicated pathogens from sputum more rapidly than clarithromycin30,31 and was associated with a higher end-of-treatment bacteriological cure rate (Figure 4).30 A fixed drug budget approach was used to calculate the cost-effectiveness of short-course therapy from these data. This exercise demonstrated that 94% more patients could be successfully treated with grepafloxacin 400 mg for 5 days than with clarithromycin 250 mg bd for 10 days (Figure 5).32 If it is feasible to do so, extending the infection-free interval would diminish both the direct and indirect costs attributed to ABECB. Unfortunately, however, most therapeutic trials involving patients with this disease have. Figure 2. Disaggregated total direct costs (in Deutschmarks) for patients with mild-to-moderate ABECB. Components of total costs: first-line antibiotics ( ), consultations ( ), medication ( ), investigations and interventions ( ), co-medication ( ) and hospitalization ( ).. Figure 4. Bacteriological cure rates in patients with ABECB following treatment with grepafloxacin 400 mg od for 5 days or clarithromycin 250 mg bd for 10 days. (The difference between the regimens is statistically significant.). Figure 3. Disaggregated total direct costs (in Deutschmarks) for patients with severe ABECB. Key: as for Figure 2.. 22.
(5) Economic outcomes in ABECB patients with ABECB. Use of inexpensive drugs is false economy if they are ineffective, as further investigations, interventions and consultations, more expensive antibiotics and, in some cases, costly periods of hospitalization may then be required. Antibiotics that maximize the success of first-line therapy are likely, therefore, to minimize the overall expenditure on healthcare by reducing the high costs associated with treatment failure. Patients prefer antibiotics that are effective and are associated with low incidences of adverse events and which can be given once daily and in courses of short duration, thereby increasing the likelihood of compliance. Enabling patients to return to normality more quickly should also reduce the burden of the disease on them, as well as on their caregivers. Thus, cost-effective antibiotics are those which produce high clinical success rates when used as first-line therapy. In conclusion, optimal economic management of patients with ABECB should be achieved with antibiotics which are active in vitro against the predominant respiratory pathogens, are associated with low rates of resistance, have proven clinical efficacies, are well-tolerated and can be administered once daily in short courses.. Figure 5. Number of patients with ABECB who could be treated successfully with grepafloxacin 400 mg od for 5 days or clarithromycin 250 mg bd for 10 days based on a fixed drug budget approach.. not considered the duration of the infection-free interval between two exacerbations. In a pioneering study of patients with ABECB,33 it was shown that the infectionfree interval was longer in patients treated with a fluoroquinolone than in those treated with a macrolide (142 and 51 days, respectively), although the difference was not statistically significant. If this observation is confirmed, the possibility of extending the infection-free interval with certain antibiotics would warrant further evaluation. Use of the acquisition cost as the principal economic consideration in choosing an antibiotic as therapy is unlikely to result in cost-effective treatment of patients with ABECB. First-line therapy with a well-tolerated antibiotic which is active in vitro against the predominant pathogens, combined with low resistance rates and a convenient once-daily dosing regimen, may reduce overall costs, even if the drugs are more expensive than other suitable agents that do not share these attributes.. References 1. Brown, R. B. (1989). Acute and chronic bronchitis. A practical management strategy. Postgraduate Medicine 85, 249–54. 2. Wilson, R. & Rayner, C. (1995). Bronchitis. Current Opinion in Pulmonary Medicine 1, 177–82. 3. Niederman, M. S., McCombs, J. S., Unger, A. N., Kumar, A. & Popovian, R. (1999). Treatment cost of acute exacerbations of chronic bronchitis. Clinical Therapeutics 21, 576–91. 4. Burrows, B. & Earle, R. H. (1969). Course and prognosis of chronic obstructive lung disease. A prospective study of 200 patients. New England Journal of Medicine 280, 397–404.. Conclusion. 5. Cole, P. J. & Wilson, R. (1989). Host–microbial interrelationships in respiratory infection. Chest 95, 217S–21S.. The first step in reducing the cost of antibiotic treatment of patients with ABECB is to identify those individuals who are most likely to benefit from receiving antibiotics. This will require a stringent clinical analysis of each case. It is likely that antibiotics are currently being overused in this setting and administering them when it is not appropriate to do so has a negative ecological and economic impact. In patients in whom antibiotics are indicated, the primary aims of treatment are to eradicate the pathogens and to relieve symptoms as quickly as possible, thereby helping patients to resume normal daily living. Where empirical therapy is indicated, an antibiotic must cover most, if not all, of the potential pathogens, including those with acquired mechanisms of resistance, in order to maximize the likelihood of a successful outcome with first-line treatment. The acquisition costs of antibiotics account for only small proportions of the total healthcare costs of treating. 6. Cole, P. J. (1995). Bronchiectasis. In Textbook of Respiratory Medicine, 2nd edn, (Brewis, R. A. L., Gibson, G. J., Geddes, D. M. & Corrin, B., Eds), pp. 1286–316. Baillière Tindall, London. 7. Murphy, T. F. & Sethi, S. (1992). Bacterial infection in chronic obstructive pulmonary disease. American Review of Respiratory Disease 146, 1067–83. 8. Saint, S., Bent, S., Vittinghoff, E. & Grady, D. (1995). Antibiotics in chronic obstructive pulmonary disease exacerbations: a metaanalysis. Journal of the American Medical Association 273, 957–60. 9. Anthonisen, N. R., Manfreda, J., Warren, C. P., Hershfield, E. S., Harding, G. & Nelson, N. (1987). Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Annals of Internal Medicine 106, 196–204. 10. Baquero, F. (1998). Increasing role of resistance in LRTIs. Infections in Medicine 15, Suppl. D, 16–27. 11. Verghese, A. & Myers, J. (1990). Bronchitis: emergence of B. catarrhalisas a significant pathogen. Modern Medicine58, 81–97.. 23.
(6) J.-C. Pechère and L. Lacey 12. Cole, P. J. (1993). Evaluating the clinical outcomes of respiratory infection. International Journal of Antimicrobial Agents 3, S15–19.. 24. Backhouse, R., Shakespeare, A. & Hutton, J. (1995). Economic evaluation of alternative antibiotic regimens in the management of acute exacerbations of chronic bronchitis. British Journal of Medical Economics 8, 11–25.. 13. Drummond, M. F., O’Brien, B., Stoddart, G. L. & Torrance, G. W. (1997). Methods for the Economic Evaluation of Health Care Programmes, 2nd edn. Oxford University Press. 14. Ballow, C. H. (1995). Cost considerations in oral antibiotic therapy. Advances in Therapy 12, 199–206.. 25. van Barlingen, H. J. J., Nuijten, M., Volmer, T., Sanderson, D. J. & Lacey, L. (1998). Model to evaluate the cost-effectiveness of different classes of antibiotics in the management of acute bacterial exacerbations of chronic bronchitis in Germany. Journal of Medical Economics 1, 201–18.. 15. Wool, C., Cerutti, R., Garbagna, N. & Grossi, E. (1996). A costeffective study of four different antibiotics in the treatment of acute exacerbations of chronic obstructive pulmonary disease. British Journal of Medical Economics 10, 159–68.. 26. Michéa-Hamzehpour, M., Auckenthaler, R., Regamey, P. & Pechère, J. C. (1987). Resistance occurring after fluoroquinolone therapy of experimental Pseudomonas aeruginosa peritonitis. Antimicrobial Agents and Chemotherapy 31, 1803–8.. 16. Austin, D. J., Kristinsson, K. G. & Anderson, R. M. (1999). The relationship between the volume of antimicrobial consumption in human communities and the frequency of resistance. Proceedings of the National Academy of Sciences, USA 96, 1152–6.. 27. Cockburn, J., Gibberd, R. W., Reid, A. L. & Sanson-Fisher, R. W. (1987). Determinants of non-compliance with short term antibiotic regimens. British Medical Journal 295, 814–8. 28. Branthwaite, A. & Pechère, J. C. (1996). Pan-European survey of patients’ attitudes to antibiotics and antibiotic use. Journal of International Medical Research 24, 229–38.. 17. Milatovic, D. & Braveny, I. (1987). Development of resistance during antibiotic therapy. European Journal of Clinical Microbiology 6, 234–44.. 29. Langan, C., Marr, C., Staley, H. & the Raxar ABECB Study Group. (1999). A multicenter study of short-course grepafloxacin therapy versus clarithromycin in the treatment of patients with acute bacterial exacerbation of chronic bronchitis (ABECB). Clinical Microbiology and Infection 5, Suppl. 3, 275 (Abstract P712).. 18. Carmeli, Y., Troillet, N., Eliopoulos, G. M. & Samore, M. H. (1999). Emergence of antibiotic resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrobial Agents and Chemotherapy 43, 1379–82.. 30. Langan, C., McKaig, G., Thakker, B., Marr, C. & Staley, H. (1999). Comparison of time to pathogen eradication in patients with acute bacterial exacerbation of chronic bronchitis (ABECB) with either grepafloxacin or clarithromycin. Journal of Antimicrobial Chemotherapy 44, Suppl. A, 96 (Abstract 256).. 19. Köhler, T., Michéa-Hamzehpour, M., Plésiat, P., Kahr, A. L. & Pechère, J. C. (1997). Differential selection of multidrug efflux systems by quinolones in Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy 41, 2540–3. 20. Davies, T. A., Pankuch, G. A., Dewasse, B. E., Jacobs, M. R. & Appelbaum, P. C. (1999). In vitro development of resistance to five quinolones and amoxicillin clavulanate in Streptococcus pneumo niae. Antimicrobial Agents and Chemotherapy 43, 1177–82.. 31. Tran, J. Q., Ballow, C. H., Forrest, A., Hyatt, J. M., Sands, M. F., Peloquin, C. A. et al. (2000). Comparison of the abilities of grepafloxacin and clarithromycin to eradicate potential bacterial pathogens from the sputa of patients with chronic bronchitis: influence of pharmacokinetic and pharmacodynamic variables. Journal of Antimicrobial Chemotherapy 45, Topic T1, 9–17.. 21. Pechère, J. C. (1998) Modelling and predicting clinical outcomes of antibiotic therapy. Infections in Medicine 15, Suppl. E, 46–54.. 32. Vogel, F., Volmer, T., Marr, C., Barnett, G. & Lacey, L.F. (1999). Economic evaluation of grepafloxacin compared with clarithromycin in the treatment of acute bacterial exacerbations of chronic bronchitis (ABECB) in Germany. Clinical Microbiology and Infection 5, Suppl. 3, 146 (Abstract P229).. 22. Dagan, R., Abramson, O., Leibovitz, E., Greenberg, D., Lang, R., Goshen, S. et al. (1997). Bacteriologic response to oral cephalosporins: are established susceptibility breakpoints appropriate in the case of acute otitis media? Journal of Infectious Diseases 176, 1253–9.. 33. Chodosh, S., Schreurs, A., Siamai, G., Barkman, H. W., Anzueto, A., Shan, M. et al. (1998). Efficacy of oral ciprofloxacin vs. clarithromycin for treatment of acute bacterial exacerbations of chronic bronchitis. Clinical Infectious Diseases 27, 730–8.. 23. Gehanno, P., Lenoir, G. & Berche, P. (1995) In vivo correlates for Streptococcus pneumoniae penicillin resistance in acute otitis media. Antimicrobial Agents and Chemotherapy 39, 271–2.. 24.
(7)
Figure


Documents relatifs
Le bonhomme de neige un bonnet un bonnet une carotte une carotte un balai un balai il construit il construit un jardin un jardin il accroche il accroche Le bonhomme de
Dans les entreprises de 10 salariés ou plus de l’ensemble de l’économie hors agriculture et hors emplois publics, 84,2 % des salariés travaillent à temps complet au
sont de plus en plus partie prenante dans le suivi des maladies chroniques des patients, tant en milieu hospitalier que communautaire.. L’accès aux valeurs de laboratoire
Vous est-il arrivé de vous demander quel intérêt pour- rait-il y avoir à échanger davantage avec l’étranger?. L’ac- te d’échanger n’a-t-il pas pour fondement l’idée
Les résultats ont montré que le modèle était capable de reproduire les résultats expérimentaux concernant l’effet de la pression de saturation, de la vitesse de
Prioritising outcomes for evaluating eosinophil- guided corticosteroid therapy among patients with acute COPD exacerbations requiring hospitalisation: a Delphi consensus
Implications for the Classroom The Interlanguage theory, that assumes that an active and independent learning mind makes its own generalizations upon grappling with
The scope of the study was to analyze the timing of diagnosis of persistent LSVC, the timing of diagnosis of associated anomalies of the coronary sinus, and the global impact