Publisher’s version / Version de l'éditeur:
Journal of Coatings Technology, 49, 629, pp. 50-58, 1977-06
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Effect of free film preparation method on physical properties of organic
coatings
Yaseen, M.; Ashton, H. E.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=be119249-13ed-48e7-8ead-910a883a0c86 https://publications-cnrc.canada.ca/fra/voir/objet/?id=be119249-13ed-48e7-8ead-910a883a0c86S e r
TH1
N21d no. 730 c. 2
BLDG
:ional Research Council of Canada
- -- -
,iseil national de recherches du Canada
? .y,$+~;t$
< 7EFFECT OF FREE FILM PREPARATION METHOD
ON PHYSICAL PROPERTIES OF ORGANIC
COATINGS
by M. Yaseen and H.E. Ashton
Reprinted from
Journal of Coatings Technology Vol. 49, No. 629, June 1977, p. 50-58
DBR Paper No. 730
Division of Building Research
RESUME
Des feuils de vernis phe'noliques et de re'sines alkydes non pigmente'es de'gage's de leur subjectile ont e'te' pre'pare's en utilisant des proce'de's d'arnalgarnation au rnercure et de papier photographique. L'absorp- tion d'eau, l'impre'gnation de vapeur d'eau e t les proprie'te's me'caniques des feuils ont Cte Ctudie'es en utilisant des ressorts ou quartz, des re'cipients Payne et une installation de mise l'essai de la traction.
I1 est de'montre' que des re'sultats plus fiables pour l'absorption d'eau et l'impre'gnation sont obtenus avec des feuils.tir6.s de papier d'e'tain. La me'thode de preparation n'influence pas la re'sistance 2 la traction ou 6 l'klongation des revctements.
Effect of Free Film Preparation Methoa
On Physical Properties
Of Organic Coatings
M. Yaseen and H. E. Ashton
National Research Council of Canada*
Free films of phenolic varnishes and unpigmented alkyd In the first category, Elm2 applied clear and pig- resins were prepared by mercury amalgamation and pho- mented coatings to silvered glass panels and obtained tographic Paper techniques. The water absorption, water the free films by amalgamation of the silver layer. vapor permeation, and mechanical properties of the films Browne3 compared all three types-tinplate, gummed were studied using quartz springs, Payne cups, and a paper sealed with linseed oil, and silicone parting agent tensile testing machine.
It is shown that more reliable results for water absorp- applied on glass and other substrates. He selected tin- tion and permeation are obtained with films from tinfoil. plate for t' be weathered and gummed paper The method of preparation does not affect the tensile for those to be immersed in water. In weathering tests,
strength or elongation of the coatings. the free films were obtained after exposure had been
comuleted. KEY WORDS: Free film; Mercury; Tinplate; Tinfoil; Per-
meability; Relative humidity; Accelerated weathering; Tung oil.
INTRODUCTION
Within the past two decades there has been a trend towards measurement of the engineering or basic prop- erties of organic coatings in place of the empirical tests so long in use. In some cases the property of interest can only be measured using a free film; in others it is considered desirable to determine the property as sepa- rate from the influence of the substrate. In the former category are mechanical properties, when measured by tensile testers, and vapor permeability properties. In the latter case, water absorption of coatings has been shown to be greater when they are attached to a sub- strate than in the form of a free film.' Regardless of purpose, before free films can be used they must be prepared; the method of preparation should affect their properties as little as possible.
Various methods of obtaining free films have been developed by investigators interested in studying some property of organic coatings. The procedures can basi- cally be divided into three types: (1) those that use mercury or a metallic surface that can be amalgamated
I with mercury; (2) those for which some form of water-
! soluble or water-sensitive material is used as a sub- strate; and (3) those that use substrates possessing low surface tension, e.g., silicone release agents or poly- tetrafluoroethylene.
* Div. of Building Research, Ottawa, Canada KIA OR6,
~ o s c h , et aL4 encountered difficulties with amalga- mation procedures using tin-plated steel and silver- plated glass. They were successful with glass plates coated with a thin layer of methyl cellulose. Soaking in water for 30 min, however, was required to remove the methyl cellulose from the free films. Evans5 used hiding power charts coated with partially hydrolyzed poly- vinyl acetate, but a soaking period of two hours was required to obtain the free films.
Schurr, et a1.6 concluded that for exposure tests, tin- foil backed by a metal support is better than tinplate, which may contain pinholes. This method has subse- quently been adopted by the American Society for Test- ing and material^.^ Free films of high polymers are often obtained by pouring their solutions onto pools of mercury and allowing the solvents to e v a p ~ r a t e . ~ This procedure is not usual for coatings, possibly because the normal coating application techniques of brush and drawdown blade cannot be used.
A simple procedure for preparing free films was de- veloped in the DBRINRC laboratory by Harris. The coating is drawn down on matte or semi-matte photo- graphic paper, which may be outdated. On drying, the coated paper is placed on moistened blotting paper to wet the gelatin layer without soaking the coating film, which can then be readily stripped off. This method has been used for several years in DBR's laboratory and by other workers.1° .
In spite of long use of the photographic paper method, however, occasional doubts have arisen at DBRINRC regarding its validity for preparing free films to be tested for water absorption and permeability. One reason is that the films have already been exposed to
Table 1-Composition of Clear Finishes Para-Phenylphenolic Varnishes NRP Formula NO. Alkyds
Oil Content % Volatile Content Varnlsh Properties Approx. Percentage Aromatic Mineral Per Cent G - H Type Lengtha of sollds Solvent Spirits Solids Viscosity
Tung 20 66.7 33.3 66.7 50 C Tu ng 40 80.0
-
100.0 50 B-C Linseed 20 66.7 30.6 69.4 5 1 B-C Linseed 40 80.0 10 90 50 C-D Soya 20 66.7 28.6 71.4 50 B D.H. castor 20 66.7 33.3 66.7 50 C Oil Content Percentage TY pe of solids % Phthalic Content Percentage Iaomw of Solids Per cent G-H Solids Viscosity Soya Soya Soya Linseed Soya Safflower Safflower Soya Soya Soya Ortho Ortho Ortho Ortho Ortho Ortho Is0 Is0 Is0 Is0 50 A-B 50 A-B 40 G-H 50 A-B 50 A-B 60 A I 60 A 1 50 C-D 50 C-D 50 C-D(a) Imp gal per 100 Ib resin.
(b) Per cent oil content of these alkyds calculated from reported fatty acid content. The oil content of the other commercial alkyds is as reported by the manufacturer,. (c) Alkyds prepared in the laboratory.
water, although not to the extent they would be in soaking. Results might, therefore, not be comparable with those obtained with films prepared by the two other basic procedures. A second objection is that traces of photographic gelatin might be left on the film. Such a hydrophilic material could easily have some effect on properties involving water. A third objection, which also applies to other procedures where the film is stripped, is that the force required to remove the film from the substrate might be sufficient to alter the mechanical properties of the materials, especially those with low tensile strength and high elongation.
Free films obtained by mercury amalgamation are not subjected to mechanical stresses, contamination with water-soluble material, or soaking in water. The disadvantages associated with the method include: high cost of mercury and good quality tinfoil; toxicity- because a large surface area of mercury is exposed and mercury is easily spilled; and occasional contamination with traces of amalgam. A problem that usually applies only to low surface tension substrates is crawling of the wet coating after application, resulting in a non-uniform film. This frequently occurs with unpigmented finishes that have a low viscosity.
Because of the questions regarding the validity of the photographic paper method and the disadvantages of the amalgamation procedure, it was decided to deter- mine whether preparation methods do, in fact, affect properties of free films. A first attempt was made sev- eral years ago, using water absorption as the criterion and tinfoil, tinplate, and photographic paper as sub-
strates." It was found that films from foil had a statisti- cally lower water absorption, but the immersion tech- nique used then was not sensitive enough to measure water absorption of clear fjnishes precisely.12 As far as could be determined, films prepared on the other two substrates showed equal absorption.
In the work presented here, the more sensitive quartz-spring balance was used to compare water ab- sorption properties of films prepared by the tinfoil and photographic paper methods. Permeability to water vapor and changes in tensile properties during acceler- ated weathering were also studied. Permeability was determined with Payne cups at several temperatures and humidities, and mechanical properties were meas- ured on a tensile machine both before and after acceler- ated weathering. Because low surface tension sub- strates have not been generally or successfully used in this laboratory, the procedure was not included in the test program.
EXPERIMENTAL
Materials
Two types of clear finish were examined: phenolic varnishes and alkyd resins. The varnishes have been described p r e v i ~ u s l y . ' ~ Some of the alkyd resins have also been used in previous absorption tests" and in natural exposure studies.14 In order to have alkyds with oil contents similar to those of the varnishes, four extra-long oil alkyds (also commercial products) were
ldded to the test series. Finally, two alkyds prepared in Lhe DBR laboratory were included in some of the tests to determine how resins of known composition com- pare with commercial products. The composition of the materials is summarized in Table 1.
Coating Application
A Gardner Mechanical Drive equipped with a perfo- rated plate was used in the application of coatings to both substrates. To prepare films on tinfoil, a sheet of white bond paper was placed on the metal plate while vacuum was applied. The sheet was smoothed, taped on three sides, and a second sheet placed on the first. Two sheets of bond paper were required to prevent formation of dimples at the perforations in the plate. With the vacuum still running, the tinfoil was rolled onto the paper, with firm pressure on the roll to reduce wrinkle formation. Finally, the foil was made com- pletely smooth by wiping it with a tissue. Coating was applied on the foil to a wet film thickness of 2 mils (50 pm), with the doctor blade mechanically driven at a uniform speed. When the blade had cleared the end of the foil, the vacuum was released and the coated foil (together with the second bond sheet) transferred to a piece of hardboard kept level to prevent flow of the wet coating. When three or four drawdowns had been com- pleted the board was placed in a conditioned drying room maintained at 2322°C and 5025% rh.
Because of the thickness of photographic paper, only one sheet of bond paper was needed under it to prevent dimples. The two were taped together and the combina- tion placed on the perforated plate and made even under vacuum. Following application of the coating, the two sheets were transferred to hardboard and taped to it to prevent curling of the photographic paper. Dry- ing proceeded as before.
Film Preparation
Free films were obtained after the coatings had dried for three weeks. They were stripped from photographic paperg but not washed or soaked to remove traces of gelatin. Washing ,would be difficult to carry out with long-oil materials, which are very soft and tacky. After stripping, the films were placed on separate sheets of waxed paper, with the original upper surface facing down, and allowed to condition for at least an additional three weeks.
Except for samples to be tested for permeability, coatings on tinfoil were first cut into 1-in. wide tensile strips and taped to provide gauge lengths of 3 or 4 in. (76 or 101 mm). They were placed on a mercury bath and, when the coating was floating freely after amalgamation of the foil, were picked up at a taped end with tweezers. The film was placed on waxed paper and the original underside carefully brushed with a camel's hair brush to remove traces of mercury and amalgam. To obtain a relatively clean surface, it seemed to be necessary to use a fairly large amount of mercury in the amalgama- tion bath. Free films from tinfoil were kept under the same conditions as those obtained from photographic paper.
Water Absorption
Basically, the apparatus13 consists of quartz spnngs, each mounted in a separate glass tube. Four tubes are connected to one manifold and the parts of the tubes where the samples are located are immersed in a water bath, the temperature of which is closely controlled. Identical conditions can thus be maintained for all four samples. The water vapor source is immersed in an- other water bath in which the temperature can be varied to change the water vapor pressure in the sample tubes. Vapor pressure is determined by a micromanometer and spring extension is measured by a cathetometer.
For the water absorption determination, free films were cut into pieces about 1.5
x
3 in. (38x
76 mm). Four pieces of each sample were spaced on a platinum loop to allow free access of vapor to all surfaces. The films were evacuated to constant weight and then sub- jected to several cycles of absorption near 99% rh and desorption at 0% rh. This procedure is necessary to remove trapped solvent and obtain constant absorption values. l 2Equilibrium absorption of water in the known weight of free film was determined at various vapor pressures and constant temperature. The ratio between the theo- retical vapor pressure of water at the temperature of determination and the pressure of vapor in the system was calculated and expressed in terms of per cent rela- tive humidity. The amount of water absorbed in the coating at a particular vapor pressure under the condi- tion of equilibrium is reported here as per cent water absorption at a given relative humidity.
Water Vapor Permeability
The rate of permeation under steady-state conditions was determined with the Payne cup in accordance with Procedure A of ASTM Method E96, using magnesium perchlorate as desiccant. In all cases, the side of the film originally in contact with the substrate faced the desiccant. After the films were sealed in place, the cups were put in a specially designed cabinet in which both temperature and relative humidity could be varied. Either the temperature was kept constant and relative humidity increased in steps, or the converse. Permea- tion through each type of film was determined in tripli- cate and separate sets were used for the variation of temperature and relative humidity. The cups were weighed inside the cabinet to determine the amount of water vapor transmitted through the films.
Rate of permeation of water vapor through the free film at different relative humidities and constant tem- perature, or at different temperatures and constant rela- tive humidity, was calculated in terms of the amount of water vapor (g) permeating in one hour through unit area (m2) and unit thickness of 1 mil (25 pm).
Tensile Tests
Films were pulled on a Tinius-Olsen U-CeltronicB tester equipped with a 12,000 g cell. This tester permits the load to be decreased in steps down to 120 g for full-scale deflection. It was usually operated in the 600,
1
1200, or 2400 g ranges depending upon the strength of 2.4
the films. Tensile specimens were of 3-in. (76-mm)gauge
length and were taped to this size with waterproof 2.2 plastic-coated pressure-sensitive cloth tape, which
acted as a grip for the machine jaws. Rate of strain was " O - 0.12 in. (3 mm) per min, which is 4% per min. Because 1.8 of the variability encountered in tensile testing thin
organic coating films, 10 samples were pulled for each 1 . 6 -
unexposed material. For materials exposed to acceler- B=
ated weathering, this was later reduced to seven and, c5
-
1.4with free films from photographic paper, was even + a
0:
lower in some cases because of breakage.
=:
1.2-The load, in grams, applied to the 1-in.-wide strip of Z coating, either at break or at deformation (yield point), = y 1.0-
+
was determined directly from the recording chart on the 4 s
machine. Tensile strength was calculated (in pounds 0 . 8 -
per square inch) from the maximum load either at the
point of break or at deformation, ignoring any possible 0. 6
decrease in cross-sectional area because of breaking.
The degree of extension of the coating at break or at 0 . 4
deformation (whichever occurred first) was taken as the
measure of flexibility and is reported here in terms of 0. 2
per cent elongation. Per cent elongation was calculated
from the extension rate and time for breaking to occur. OO
I I I I I I I
-
FREE F I L M S P R E F A R E D F R O M T I N F O l l P H O T O G R A P H I C P A P E R 2 0 - G A L L I N S E E D 9 0 2 7 0 V I I Z P * 4 0 - G A L L I N S E E D 0 D 9 0 5 P 8-
4 0 - G A L T U N G 9011 b 9 0 1 P b-
-
-
-
d-
-
1'0 :O 3 b 4 b 5 b 6 b7b
sb 9 b l o oWhen results from an individual specimen were low R E L A T I V E H U M I D I T Y . 70
for and tensi1e strength in com~arison Figure 1-Absorption of water vapor in phenolic varnish films with other films of the same material, they were re-
jected as probably due to edge defects. This differs from
the procedure followed in ASTM D2370 where the low- days before pulling. The tinfoil strips were first amal- est five readings are automatically discarded without gamated, the free films retaped to 3 in. and pulled as regard for whether both values are low. above. This retaping procedure was used because in previous work failures always occurred at the edge of
Accelerated Weathering the tape if exposed strips were pulled directly. With retaped strips, some failures occurred at other locations To determine the effect of weathering on tensile in the test specimens.
properties, films were exposed in a twin carbon-arc Weather-Ometera. The cycle was 12 hr light without
water and 12 hr high relative humidity without light. RESULTS AND DISCUSSION The latter was substituted for the 12 hr of water spray in
the cycle customarily used in the DBR laboratory to Discussion of the results will be limited mainly to the test clear finishes on wood.~5 hi^ substitution was effect of the two substrates on free film properties. The necessary because the force of the spray frequently effect of coating composition on these properties will be breaks free films that have been exposed for any length considered in in subsequent papers.
of time. In subsequent tests of clear finishes applied to
wood panels it was found that it took twice as long to Appearance
obtain the same results with the light-humidity cycle as Free films obtained from tin foil were uniformly with the light-water spray cycle. This time might be glossy and smooth. After any mercury or amalgamation reduced for free films that absorb water more rapidly product had been carefully removed, the underside ap- than does a three-coat system applied to wood. peared in most cases to be identical to the original top Free films obtained from photographic paper were surface. Free films from photographic paper had a tex- cut into 1 in. x 4 in. (26 x 101 mm) strips and mounted tured appearance on the side in contact with the paper. on exposure racks. A Teflon@ backing was placed be- In general, these films were smooth and uniform but not hind the film to provide a support to which the free films so bright and glossy as those obtained from tinfoil. would not stick. Coatings applied to tinfoil were cut into
the same size strips, with the foil still attached. They Water Absorption
were taped on the racks also with a Teflon backing. At The effect of substrate on water absorption is illus- intervals, exposed films were taken from the weather- trated in Figure for phenolics and in Figure for ing machine. Free from photographic paper were alkyds. In most cases the per cent water absorption in
taped to a gauge length of 3 in. (76 mm) and free films obtained from photographic paper is higher
at 23 + 20C and 50 -+ rh for One seven
than that of the same type of film obtained from tinfoil.
Weather-Ometer 1s a reg~stered trademark of Atlas Electr~c D e v ~ c e s , Inc. Teflon IS a When determinations are made at 50% the difference reg~stered trademark OF E. I . du Pont de Nemours & CO., Inc. between the free films prepared by the two different
6 2 . 5 % S O Y A - 0 , P H T H A L l C 5 6 . 5 % S O Y A - 0 , P H T H A L I C 7 0 % S A F F L O W E R - 0 , P H T H A L l C F R E E T I N F O I L 9 1 3 1 0 1 0 5 6 1 E 1 T A LMS P R E P A R E D FROM P H O T O G R A P H I C P A P E R 9 1 3 P
11
1 0 5 6 P E l ? A A 0 R E L A T I V E H U M I D I T Y , %Figure 4--Permeation of water vapor through alkyd films
humidities. Only with tung oil varnishes (not shown) did films from foil transmit less water vapor.
At first glance, it is puzzling that films from foil should absorb less but transmit more water than those from photographic paper. It may be explained on the basis of the equation for permeation through attached layers, as proposed by Barrer:16
where P and 1 are the permeability coefficient and thickness of the composite film, and PI, P,, P,
.
.
.
, andl,, 1,,1,.
. .
correspond to the individual layers. Accord- ingly, a layer attached to the underside of a coating should reduce the rate of permeation through it. Con- sequently, the resistance to water vapor transmission of films detached from photographic paper depends not only upon the inherent permeability of the coating itself but also upon its reactivity to the photographic film.Tung oil, with a higher content of conjugated unsatu- ration than other oils, reacted either with itself or the phenolic resin in the course of varnish preparation. Because of this and the rapid-drying properties of tung varnishes, the interfacial interaction between the coat- ing (if applied to photographic paper) and the photo- graphic matter should be at a minimum, especially those with lower oil contents. Where there is no interac- tion or adsorption at the interface of coating and photo- graphic paper, the rates of permeation through free films prepared by both methods should be the same for each coating, within experimental limits. This is shown to be the case here.
With the other varnishes, it is more probable that a thin layer of gelatinous product from the substrate will
Table %Repeatability of Water Vapor Permeation Results for Phenolics
Range as Percentage of Mean Per Cent RH 893 902 1022 1024 901 905 50 57.5 70 80 87 97 Mean Range 50 57.5 70 80 87 90 Mean Range Tlnfoll Fllrns Photographlc Paper Fllrns 3.98 6.12 15.36 9.04 4.34 7.32 12.92 12.28 4.44 7.98 14.90 12.26 5.86 7.60 14.08 11.22 4.66 5.96 10.84 8.00 5.50 8.40 12.56 11.02
be adsorbed on the underside of the films. The lower rates of permeation through the other varnish films obtained from photographic paper show that either chemical reaction or adsorption has taken place be- tween active groups present in the film former and the chemicals in the photographic material. Such a chemi- cally adsorbed layer on the underside of the coating should slow down permeation through it. It can be seen that the greatest difference is with 40 gal linseed var- nish. Soya and dehydrated castor varnishes containing
80% oil should show even greater differences. Alkyds, as showninFigure 4, also exhibit lower rates of permeation of water vapor through films obtained from photographic paper. Here again, the largest dif- ference between the two substrates occurs with 70% safflower alkyd, which should be the slowest drying in the group.
Another explanation of the low rate of permeation through films obtained from photographic paper may be that the material adhering to the film was in the very low relative humidity environment inside the cup. Owing to the response of gelatinous materials to changes in rela- tive humidity the layer would probably be in a shrunk- en, compact state at low relative humidity and offer greater resistance to water vapor transmission. A third alternative is that, with a polar substrate, the coating molecules at the interface might be more highly oriented than in films cast on tinfoil. If sufficient orien- tation were to occur, the permeability might be differ- ent.
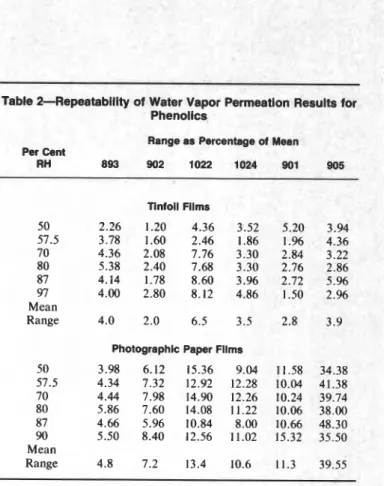
The repeatability of the permeation measurements on both phenolics and alkyds was estimated by calculat- ing the range of each trio of measurements as a per cent of their mean. It is evident, from the results summa- rized in Tables 2 and 3, that in almost all cases the precision with tinfoil films is superior to that with
Table 3--Repeatability of Water Vapor Permeation Results for Alkyds
Range as Percentage of Mean Per Cent RH 912 913 91 6 1055 1058 El G Tlnfoil Films 50 14.00 2.00 13.88 13.03 5.06 9.20 7.14 9.92 65 12.48 1.90 11.48 12.57 5.40 9.48 5.60 10.00 80 13.22 2.10 1 1.34 12.55 5.18 8.88 6.00 8.68 90 12.18 3.04 11.27 11.15 5.36 8.02 6.26 6.92 97 12.88 2.76 10.95 10.50 4.28 7.66 6.86 6.64 Mean Range 12.95 2.4 11.8 12.0 5.1 8.65 6.4 8.4
Photographic Paper Flims
50 17.48 13.06 14.50 15.53 43.20 3 1.34 29.40 18.78 65 20.86 13.04 13.37 15.14 39.40 33.22 31.64 19.14 80 22.18 13.34 12.61 13.77 46.18 32.74 36.00 19.66 90 24.44 13.82 11.65 13.00 41.06 35.38 37.30 19.24 97 20.00 12.36 11.36 11.47 35.24 36.68 39.58 19.86 Mean Range 21.0 31.1 12.7 13.8 41 .O 33.9 34.8 19.3 FREE F I L M S P R E P A R E D F R O M PO-GAL T U N G 8 9 3 T 0 T E M P E R A T U R E . "F 150% R H I
Figure %Temperature effect on permeation of water vapor- Phenolic 5 2 0 0 4 8 0 0
.-
VI CL-
4 0 0 0 ar + v, 3 2 0 0 F R E E F I L M S P R E P A R E D F R O M L I N S E E D 9 0 2 T 0 9 0 2 P 8 0 0 ~ I 2 0 - G A L D H C 1 0 2 4 T 1 0 2 4 P I 4 0 0-
4 0 - G A L-
L I N S E E D 9 0 5 T A 9 0 5 P A 0 I 1 I I I 1 I I I D l 2 3 4 5 6 7 8 9 1 0 D A Y S O F E X P O S U R E , W - 0 - M E T E R Figure &Tensile strength of phenolic varnish filmsbehavio: . -.
photographic paper films, probably owning to var- different coatings vary greatly in
iability in the amount of gelatinous material adhering to apparent that the values for the same coatlng trc
the latter. the two substrates are the same, within the expe
mental error of the method.
Water Vapor Permeation and Temperature Films of phenolic varnishes attain maximi
Figure 5 illustrates that higher temperatures have a strength after four to six days' exposure to accel
greater effect on rate of pmneation of water vapor ated weathering, and maximum strength decrea5
through phenolic varnish films obtained from photo- with increasing oil content. Alkyds have much 1 0 ~
graphic paper than on films obtained from tin. The tensile strengths (note scale difference) and do not
greatest change'in permeation rates occurs with 40 gal attain their maximum until after 30 to 40 days of ex-
linseed; 20 gal tung films from foil always transmit less posure. Again, the materials containing more oil
water vapor. These findings are consistent with the have lower tensile strength.
hypothesis that a thin layer of photographic material Similar results were obtained for changes in elon-
adheres to the under surface of the film and that it gation during accelerated weathering (not illus-
b a ~ m e s n~ore activated at higher temperatures, pro- trated); i.e., the two classes of material differed
moting the process of permeation. markedly in flexibility retention, but the substrate
had no effect. It appears that changes in mechanical
Accelerated Weathering Tests- properties of phenolic varnishes and alkyds during
Tensile Properties accelerated weathering are not affected by the
methods of free film preparation studied here. Changes in the tensile strengths of phenolic var-
nishes and aklyds, after various periods of exposure
to artificial weathering, are illustrated in Figures 6 CONCLUSIONS
and 7. It is apparent that the tensile strengths of the On the basis of this study, it is concluded that films
obtained from tinfoil provide more reliable informa- tion on the water absorption and water vapor per-
1700
-
meation properties of clear finishes. Consequently, itis recommended that for such studies free films of
1600
-
coatings obtained from photographic paper shouldnot be used. It is also suggested that, in cases where
1500
-
-
water forms a part of the process, it would be betternot to use a substrate that might cause physical or
1400
-
-
chemi-sorption of hydrophilic material on free films1300
-
-
obtain from it.The mechanical properties of tensile strength and
1200
-
-
elongation of a coating are not affected by themethod of free film preparation. It can therefore be
.-
2 1100-
-
concluded that coating films prepared by either thephotographic paper or tinfoil method, whichever is
-
more convenient, can be used for determiningY mechanical properties.
-
F R E E F I L M S PREPARED F R O M
-
ACKNOWLEDGMENTTIN F O I L P H O T O G R A P H I C P A P E R The authors acknowledge with thanks, the prep-
aration of the varnishes and films by G.A. O'Doh- erty, L.R. Dubois, and R.C. Seeley. Mr. Seeley also measured the mechanical properties of the film and
H. Schultz made the adsorption and permeation
measurements.
This paper is a contribution from the Div. of Building Research, National Research Council of Canada, and is published with the approval of the Director of the Division.
References
(I) Funke, W . , J. Oil& Colour Chemists' Assoc.. 46, p. 975
0 6 12 18 3 0 4 0 (1963).
(2) Elm, A . C . , Official DIGEST,^^, No. 322, 701 (1951).
DAYS - O F E X P O S U R E , W - 0 - M E T E R (3) Browne, F . L . , J. For. Prod. Research S o c . , 111, 5 , p. 391
Figure 7-Tensile strength of alkyd films (1953).
er- ;es ier
(4) Bosch, W., F.J. Macekonis, and P.H. Martin, Official DI-
GEST, 26, NO. 359, 1219 (1954).
(5) Evans, R.M., Official DIGEST, 33, NO. 439, 970 (1961).
(6) Schurr, G.G., T.K. Hay, and M. VanLoo, JOURNAL OF PAINT
T E C H N O L O G Y . ~ ~ , NO. 501, 591 (I%@.
(7) ASTM D2370: Method of Test for Elongation and Tensile Strength of Free Films of Paint, Varnish, Lacquer and Re- lated Products, With a Tensile Testing Apparatus. ASTM Book of Standards, Part 27, Philadelphia, Pa.
(8) Bigelow, S.L. and A. Gemberling, J. Am. Chem. Soc., 29,
11, 1576 (1907).
(9) Harris, J., Official DIGEST,^^, No. 372, 30 (1956).
(10) Long, J.S., S. Thames, and 0. Smith., Official DIGEST, 37, No. 488, 1050 (1965).
(11) Ashton, H.E., Official DIGEST.^^, No. 470, 232 (1964). (12) Yaseen, M. and H.E. Ashton, J. Oil & Colour Chemists' As-
sac., 53, 12, 1015 (1970).
(13) Yaseen, M. and H.E. Ashton, ibid. 53, 11, 977 (1970). (14) Ashton, H.E., JOURNAL OF PAINT TECHNOLOGY, 39, NO. 507,
212 (1967).
(15) Ashton, H.E., Paint Varnish Prod., 59, 9, 34 (1969).
(16) Barrer, R.M., "Diffusion in and through Solids," London: Cambridge University Press, (1941).
Reprinted from the June, 1977 issue of the JOURNAL OF COATINGS TECHNOLOGY Volume 49; Number 629; Pages 50-58
Copyright 1977 by the Federation of So~~tietles for Coatings Technology, Phliadelphla, Pennsylvania, U.S.A.