Does growth hormone-releasing factor assist follicular development in poor responder patients undergoing ovarian stimulation for in-vitro fertilization?
Texte intégral
(2) C.M.Howles et al.. obtained in a large population of patients (.500) treated at Bourn Hall Clinic, Cambridge, UK, a poor-response cycle was defined as one in which no more than three follicles had a diameter ù16 mm on the day of human chorionic gonadotrophin (HCG) administration or cancellation, or in which more than 41 ampoules of gonadotrophin were needed to achieve complete follicular maturation in a short GnRHa protocol (or 47 ampoules in a long GnRHa protocol). The patients’ partners had satisfactory sperm quality for IVF. Patients who had previously undergone GH replacement therapy or who had diabetes mellitus were excluded from the study. Patients were also excluded if they had medical or surgical conditions judged likely to affect study medication. Written informed consent was obtained from all patients before entry to the study, which was approved by local ethics committees and performed according to the Declaration of Helsinki and the principles of Good Clinical Practice. Study design and drug treatment The study was a randomized, double-blind, placebo-controlled trial conducted at 21 centres in Europe, North America, Australia and Singapore over a 2 year period (1991–1993). Patients were randomly assigned to receive either GRF (Geref®; Ares-Serono) or placebo during IVF. Pituitary down-regulation was then performed with either buserelin (Suprefact®; Hoechst, Frankfurt, Germany) or triptorelin acetate (Decapeptyl®; Ipsen, Paris, France). Buserelin was given either by s.c. injection (500 µg/day for 10 days, followed by 200 µg/day until HCG was administered) or by nasal spray (100 µg five times daily); triptorelin was administered by s.c. injection (100 µg/day). Down-regulation was confirmed by ultrasound scanning and measurement of plasma luteinizing hormone (LH) concentrations at least 10 days after starting GnRHa treatment. Concomitant administration of urinary FSH (Metrodin®; AresSerono) and GRF or placebo was begun when down-regulation had been achieved. FSH was given by i.m. injection at a dose of 300 IU/day for the first 5 days and at doses adjusted according to the ovarian response thereafter; the total dose was not to exceed 70 ampoules (75 IU/ampoule). GRF (500 µg twice daily) was administered by s.c. injection. HCG (5000 IU, i.m.) was administered when at least one follicle had a diameter of ù16 mm and plasma oestradiol concentrations were within acceptable limits for the number of follicles present. GRF treatment was discontinued on the day of HCG administration, or after a maximum of 14 days. Clonidine challenge test All patients underwent a clonidine challenge test before starting the treatment cycle to assess pituitary GH reserve. The test was performed during the first 4 days of the menstrual cycle. Patients received a single oral dose of clonidine (Catapressan®; Boehringer Ingelheim, Paris, France), 0.3 mg, and blood samples (2 ml) were taken 30 and 15 min before dosing, at the time of dosing, and 30, 60, 90 and 120 min after dosing. Blood was allowed to clot for 1 h at room temperature and serum separated by centrifugation at 100 g for 10 min and stored at –20°C before measurement of GH concentrations. Blood pressure was measured 15 min before and 120 min after dosing; the patient remained recumbent throughout the test. Assessments The primary efficacy endpoints were the number of follicles with a diameter ù16 mm on the day of HCG administration and the number of FSH ampoules used. Secondary efficacy endpoints were: duration of FSH treatment; number of follicles recruited, oocytes retrieved and embryos produced; serum GH and oestradiol concentrations at baseline, on days 6, 8 and 10, and on the day of HCG administration;. 1940. response to gonadotrophins and GRF or placebo in relation to the result of the clonidine challenge test; pregnancy rate; plasma IGF-1 concentrations at baseline, on day 6 and on the day of HCG administration. Serum oestradiol and plasma IGF-1 were measured by radioimmunoassay [DPC Coat-a-Count® (Diagnostic Products Corp., Los Angeles, CA, USA) and Nichols SMC® kits (Nichols Institute, Saffron Walden, Essex, UK) respectively]. FSH, LH and GH were measured retrospectively in a central laboratory (SC Bioscience Services Ltd, Cambridge, UK) by immunoradiometric assay with magnetic solid phase separation [FSH/LH: Serono (Geneva, Switzerland) MAIACLONE; GH: Pharmacia and Upjohn (Bridgewater, NJ, USA)]. Safety and tolerability were assessed by collecting information on adverse events throughout the study, measurement of anti-GRF antibodies, and standard haematology and clinical chemistry tests. Anti-GRF antibodies were measured by immunoprecipitation assay developed and validated by the sponsor. Statistical analyses A sample size of 100 patients per group was needed to allow detection of a decrease in the proportion of GRF-treated patients continuing to fail to conceive after two previously unsuccessful IVF attempts following stimulation with gonadotrophins in a pituitary downregulated cycle using a GnRH agonist from 85% to 65% with a power of 90%. Data were analysed on an intention-to-treat basis. Primary efficacy variables were analysed by parametric analysis of variance (ANOVA), controlled for centre. Parametric ANOVA was also used to compare the duration of FSH treatment and the number of oocytes, follicles and embryos in the two treatment groups. Non-parametric ANOVA was used to compare serum GH and oestradiol concentrations, and plasma IGF-1 concentrations. Fisher’s exact test was used to compare pregnancy rates between the two groups. All statistical tests were two-sided and were conducted at the 0.05 significance level. Despite the stringent exclusion criteria, two patients, one treated with GRF and one given placebo, were identified as having violated the study protocol; the partner of the GRF-treated patient was azoospermic and the placebo-treated patient had not had two previous IVF attempts. The violations were not identified until after the statistical analyses had been performed and, therefore, were included in the ITT patient results.. Results A total of 196 patients were randomized to treatment, of whom 96 received GRF and 100 received placebo. The mean age of women receiving FSH alone was 35.4 years, compared with 35.2 years in women receiving FSH plus GRF. The mean weights of the women were similar in the two groups (61.0 versus 59.4 kg respectively). The two groups were also well matched with respect to their gynaecological and obstetric history (Table I). The proportion of patients with primary infertility was slightly higher in the group receiving FSH plus GRF (55 versus 42%), while the proportion of patients with a history of extrauterine pregnancies was higher in the group receiving FSH plus placebo. The latter group also contained a higher proportion of patients showing a positive response to clonidine, defined as an absolute GH peak concentration of at least 10 MIU/ml after clonidine challenge (46 versus 39%). These differences, however, were not significant..
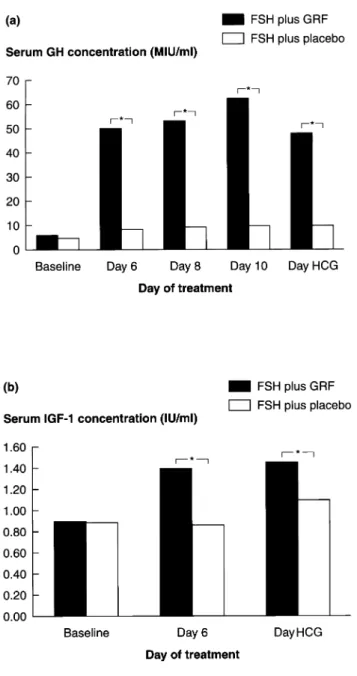
(3) GRF to assist follicular development in poor responders. Table I. Gynaecological and obstetric history and response to clonidine challenge test FSH plus GRF (n 5 96). FSH plus placebo (n 5 100). Baseline serum FSH Duration of infertility (years). 10.6 6 5.3 (2.9–27.0) 7.0 6 3.6 (1–18). 10.4 6 5.0 (2.5–31.0) 6.7 6 3.4 (2–18). Cause of infertility (%) tubal endometriosis unexplained azoospermia. 55 0 40 1. 63 2 35 0. (63.0%) (2.0%) (35.0%) (0%). 1 40 33 14 7 2 3. (1.0) (40.0) (33.0) (14.0) (7.0) (2.0) (3.0). (MIU/ml)a. (57.3%) (0%) (41.7%) (1.0%). Number of previous IVF/embryo transfer attempts (%) 0 0 (0) 2 38 (39.6) 3 21 (21.9) 4 17 (17.7) 5 5 (5.2) 6 4 (4.2) .6 10 (10.5). aMean 6 SD, with range. FSH 5 follicle stimulating hormone; GRF 5 growth hormone-releasing factor; IVF 5 in-vitro fertilization.. Table II. Treatment outcome Number of patients (%). Patients receiving at least one injection of FSH Patients meeting criterion to receive HCGa Oocyte recovery performed At least one oocyte retrieved At least one oocyte fertilized At least one embryo transferred Positive pregnancy testc Clinical pregnancy Live birth. FSH plus GRF. FSH plus placebo. 96 (100%) 83 (86.5%) 84 (87.5%)b 79 (82.3) 75 (78.1%) 62 (64.6%) 18 (18.8%) 8 (8.3%) 5 (5.2%). 100 (100%) 83 (83.0%) 84 (84.0%)b 82 (82.0) 70 (70.0%) 61 (61.0%) 13 (13.0) 8 (8.0%) 4 (4.0). No statistically significant differences were found between the GRF and placebo-treated groups. aCriterion of at least one follicle ù16 mm in diameter. bOne patient in the growth hormone-releasing factor group and two in the placebo group received human chorionic gonadotrophin, but had no follicles ù16 mm in diameter on the day of administration. cPlasma human chorionic gonadotrophin concentration .10 mIU/ml. FSH 5 follicle stimulating hormone; GRF 5 growth hormone-releasing factor; HCG 5 human chorionic gonadotrophin.. Efficacy The median number of follicles with a diameter of ù16 mm was three in both treatment groups (ranges: GRF: 0–13; placebo: 0–11), and the median number of FSH ampoules used was 52 in both groups (ranges: GRF: 20–96; placebo: 28–122). Similarly, there were no significant differences between the groups in the median numbers of follicles above 16 mm in diameter, or in the number of patients with at least one oocyte recovered, one oocyte fertilized, and one embryo transferred (Table II). There were, however, significant differences in serum GH and plasma IGF-1 concentrations between the groups (Figure 1). Treatment with GRF increased serum GH concentrations approximately five- to six-fold, whereas no significant change occurred in placebo-treated patients. Similarly, plasma IGF-1 concentrations increased from ~0.9 IU/ml to ~1.5 IU/ml in patients treated with GRF, but were unchanged in placebo-treated patients.. In addition to the intention-to-treat analysis, which included all randomized patients, a series of subgroup analyses were performed in the following groups of patients: patients receiving at least one injection of FSH and with no major protocol violations; patients who in previous IVF attempts had had three or fewer follicles with a diameter of 16 mm or greater; patients with more than three follicles with a diameter of ù16 mm in previous IVF attempts; patients with basal FSH concentrations of ø10 MIU/ml, and patients with basal concentrations above this figure; patients with peak GH concentrations during the clonidine challenge of ø10 MIU/ml and patients with peak values above this figure. None of these analyses showed any significant differences between the groups. Tolerability Adverse events occurred in 12 (12.5%) patients receiving GRF and 15 (15.0%) patients in the placebo group. The most common adverse events were local reactions at the injection site, which occurred in seven (7.3%) patients receiving GRF and four (4.0%) patients receiving placebo. Two serious adverse events (moderate salpingitis and severe ovarian hyperstimulation syndrome) occurred in the GRF group and four (salpingitis, metrorrhagia, haemoperitoneum and accidental injury) in the placebo group; no patient withdrew from the study because of adverse events. No anti-GRF antibodies were detected in any patient, and there were no significant differences in haematology and clinical chemistry between the groups. Discussion This study was carried out to assess the efficacy of GRF treatment in improving the ovarian response to FSH in women who had previously responded poorly to gonadotrophin treatment. The rationale for such an approach is based on the evidence that GH and IGF-1 play an important role in in-vitro ovarian follicular development and steroidogenesis, and that GRF appears to act locally on granulosa tissue (Jia et al., 1941.
(4) C.M.Howles et al.. the ranges for numbers of follicles achieved and ampoules used were large in both treatment groups and suggestive of possible heterogeneity amongst types of patients included in the study, analyses in various patient subgroups showed no significant differences between GRF and placebo treatment in any subgroup. This would suggest that there was no confounding of a possible treatment effect by inclusion of particular patient subgroups; it has been reported, for example, that the presence of high baseline concentrations of FSH precludes an effect of adjuvant GH (Volpe et al., 1989; Homburg et al., 1991). With almost 200 evaluable patients, this appears to have been the largest study of the effects of GH on follicular development to date. The results of this study are supported by those of two recent, prospective, randomized, controlled studies in smaller numbers of patients, which found that treatment with GH did not increase the number of ovulatory follicles or retrieved oocytes, the dose and duration of gonadotrophin treatment, or the pregnancy rate (Bergh et al., 1994; Hughes et al., 1994). In conclusion, the results of this study suggest that, although GRF treatment is well tolerated and produces a significant increase in GH, which in turn leads to elevated IGF-1 concentrations, GRF does not improve the ovarian responses to gonadotrophins in poorly responsive women undergoing IVF. Acknowledgements. Figure 1. Serum growth hormone (GH) (a) and plasma insulin-like growth factor-1 (IGF-1) (b) concentrations. *P , 0.0001, growth hormone-releasing factor (GRF) versus placebo.. 1986; Mason et al., 1990; Moretti et al., 1990; Bagnato et al., 1991; Adashi and Rohan, 1992; Apa et al., 1995; Doldi et al., 1996; Hugues et al., 1996). Although previous studies with exogenous GH obtained conflicting results (Homburg et al., 1990; Bergh et al., 1994; Hughes et al., 1994), studies with GRF suggested that stimulation of GH production by this hormone was associated with slight improvements in the number of recruited follicles and retrieved oocytes (Hugues et al., 1991; Duffy et al., 1995; Busacca et al., 1996a, b). In the present study, treatment with GRF at a dose of 500 µg twice daily produced significant increases in circulating concentrations of both GH and IGF-1, compared with the concentrations seen in placebo-treated patients. However, GRF did not improve the ovarian response to FSH in terms of the number of follicles above 16 mm in diameter, and had no effect on the amount of FSH needed for ovarian stimulation or the outcome in terms of pregnancy and birth rates. While 1942. The authors would like to thank the other investigators who took part in this study: Canada: I.Tummon (University Hospital, London, Ontario); A.Leader (University of Ottawa, Ottawa, Ontario); France: J.SalatBaroux (Hoˆpital Tenon, Paris); R.Frydman (Hoˆpital Antoine Be´cle`re, Clamart); J.N.Hugues (Hoˆpital Jean Verdier, Bondy Cedex); J.Fenichel (Hoˆpital de l’Archet, Nice); Germany: R.Wiedemann (private practice, Wuerzburg); Italy: C.Flamigni (Universita` degli studi di Bologna, Bologna); E.Cittadini (Universita` degli studi di Palermo, Palermo); U.Montemagno (Universita` degli studi di Napoli, Naples); The Netherlands: J.Schoemaker (Academic Free Hospital, Amsterdam); Singapore: Ng Soon Chye (National University Hospital, Singapore); South Korea: Shin Yong Moon (Seoul National University Hospital, Seoul); Spain: P.Barri (Instituto Dexeus, Barcelona); UK: R.Yates (University Department of Obstetrics and Gynaecology, Royal Infirmary, Glasgow); USA: D.Olive (University of Texas Health Science Center, San Antonio, Texas. In addition, the authors would like to thank the clinical research team and clinical research associates of Ares-Serono Corporate Medical Affairs.. References Adashi, E.Y. and Rohan, R.M. (1992) Intraovarian regulation. Peptidergic signaling systems. Trends Endocrinol. Metab., 3, 243–248. Apa, R., Lanzone, A., Miceli, F. et al. (1995) Growth hormone-releasing factor stimulates meiotic maturation in follicle and cumulus-enclosed rat oocyte. Mol. Cell. Endocrinol., 112, 195–201. Artini, P.G., de Micheroux, A.A. and D’Ambrogio, G. (1996) Growth hormone cotreatment with gonadotrophins in ovulation induction. J. Endocrinol. Invest., 19, 763–779. Bagnato, A., Moretti, C., Frajese, G. et al. (1991) Gonadotrophin-induced expression of receptors for growth hormone-releasing factor in cultured granulosa cells. Endocrinology, 128, 2889–2894. Bergh, C., Hillensjo¨, T., Wikland, M. et al. (1994) Adjuvant growth hormone treatment during in-vitro fertilization: a randomized, placebo-controlled study. Fertil. Steril., 62, 113-120..
(5) GRF to assist follicular development in poor responders Busacca, M., Fusi, F.M., Brigante, C. et al. (1996a) Success in inducing ovulation in a case of premature ovarian failure using growth hormonereleasing hormone. Gynecol. Endocrinol., 10, 277–279. Busacca, M., Fusi, F.M., Brigante, C. et al. (1996b) Use of growth hormonereleasing factor in ovulation induction in poor responders. J. Reprod. Med., 41, 699–703. Doldi, N., Bassan, M., Bonzi, V. et al. (1996) Effects of growth hormone and growth-hormone-releasing hormone on steroid synthesis in cultured human luteinizing granulosa cells. Gynecol. Endocrinol., 10, 101–108. Duffy, D.M., Lindheim, S.R., Vijod, M.A. et al. (1995) Low-dose growth hormone-releasing factor may enhance folliculogenesis in regularly menstruating women: a preliminary study. Fertil. Steril., 63, 756–760. Homburg, R., West, C., Torresani, T. et al. (1990) Cotreatment with human growth hormone and gonadotrophins for induction of ovulation: a controlled clinical trial. Fertil. Steril., 53, 254–260. Homburg, R., West, C., Ostergaard, H. et al. (1991) Combined growth hormone and gonadotropin treatment for ovulation in patients with nonresponsive ovaries. Gynecol. Endocrinol., 5, 33–36. Hughes, S.M., Huang, Z.H., Morris, I.D. et al. (1994) A double-blind crossover controlled study to evaluate the effect of human biosynthetic growth hormone on ovarian stimulation in previous poor responders to in-vitro fertilization. Hum. Reprod., 9, 13–18. Hugues, J-N., Martin-Pont, B., Torresani, T. et al. (1991) Interest of growth hormone-releasing hormone administration for improvement of ovarian responsiveness to gonadotrophins in poor responder women. Fertil. Steril., 55, 945–951. Hugues, J-N., Miro, F., Smyth, C.D. et al. (1996) Effects of growth hormonereleasing hormone on rat ovarian steroidogenesis. Hum. Reprod., 11, 50–54. Jia, N., Kalmijn, J. and Hseuh, A.J.W. (1986) Growth hormone enhances follicle-stimulating hormone-induced differentiation of cultured rat granulosa cells. Endocrinology, 118, 1401–1409. Mason, H.D., Martikainen, H., Beard, R.W. et al. (1990) Direct gonadotrophic effect of growth hormone on oestradiol production by human granulosa cells in vitro. J. Endocrinol., 126, R1–R4. Moretti, C., Fabbri, A., Gnessi, L. et al. (1990) Immunohistochemical localization of growth hormone-releasing hormone in human gonads. J. Endocrinol. Invest., 13, 301–305. Volpe, A., Coukos, G., Barreca, A. et al. (1989) Ovarian response to combined growth hormone-gonadotropin treatment in patients resistant to induction of superovulation. Gynecol. Endocrinol., 3, 125–133. Received on November 5, 1998; accepted on April 22, 1999. 1943.
(6)
Figure
Documents relatifs
In Situ Hybridization Localization of riGnRHR mRNAs in the Brain A–E, Brain sections were hybridized with 35 S-labeled antisense riboprobes for riGnRHR-1 (R-1), riGnRHR-2 (R-2),
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des
Synthetic human growth hormone-releasing factor (h-GRF-1-44-NH2) dose response effect on growth hormone and prolactin secretion in healthy adult men. Growth
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des
Que diraient Samuel et Olivier si on leur demandait de dire la même chose mais en parlant de leur maman et Amélie.. 6 Il s’agit toujours du
The levels of gene expression in all the cell lines were lower that these detected in the breast cancer biopsies, but no direct comparisons can be done, due to the great
Mit der umfassenden Dar- stellung der Politik für die Kriegsbeschädigten im Ersten Weltkrieg und der Zwi- schenkriegszeit von Verena Pawlowsky und Harald Wendelin aber liegt nun eine
Herbort (&) Centre for Ophthalmic Specialized Care (COS), 6, rue Charles-Monnard, 1003 Lausanne, Switzerland e-mail: