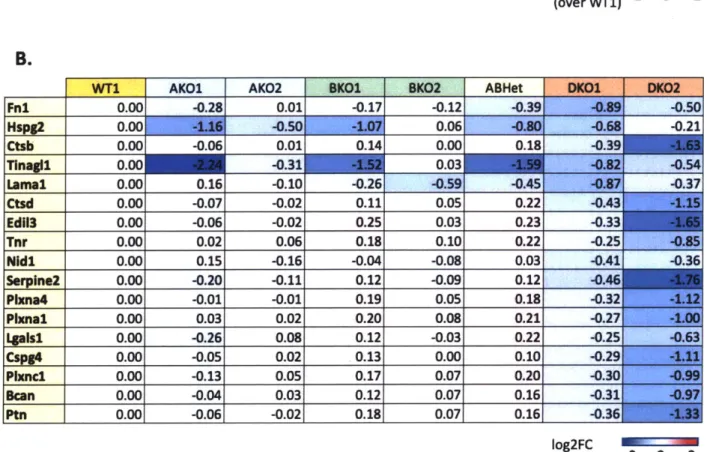
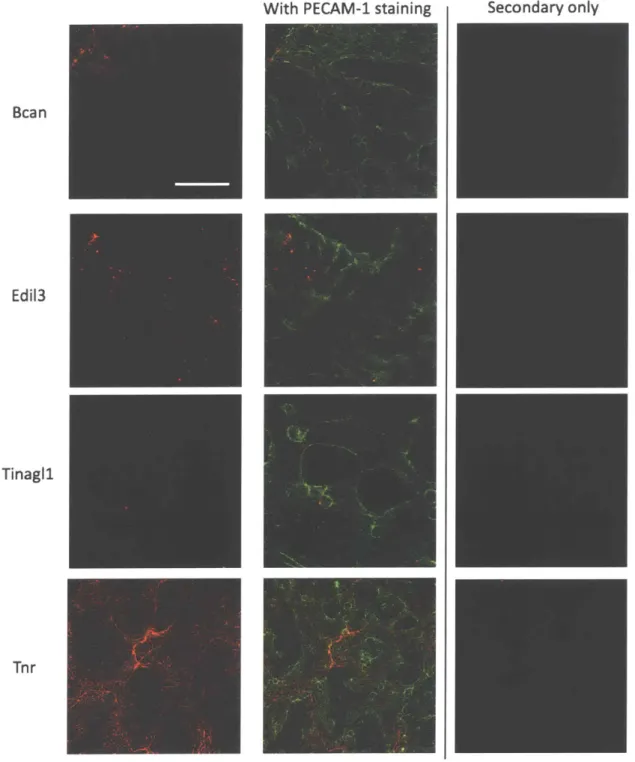
Alternatively spliced isoforms of Fibronectin, Tenascin-R
and other potential players in early vasculogenesis
By
Thao Huong Nguyen
B.S., Biochemistry Bates College, 2011
SUBMITTED TO THE DEPARTMENT OF BIOLOGY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF
DOCTOR OF PHILOSOPHY AT THE
MASSACHUSETTS INSTITUTE OF TECHNOLOGY SEPTEMBER 2019
© 2019 Massachusetts Institute of Technology. All rights reserved.
Signature ofAuthor:... Certified By:...
S ig
A ccepted By:... MASSACHUS INSTITUTE OFTECHNOLOGYSEP 10
2019
Signature redacted
...
...
Department of Biology August 30, 2019nature redacted
...
Richard 0. Hynes Daniel K. Ludwig Professor for Cancer Research Thesis SupervisorSicjnature redacted
t, ... Amy E. Keating Professor of Biology Co-Director, Biology Graduate CommitteeAlternatively spliced isoforms of Fibronectin, Tenascin-R and other potential players in early vasculogenesis
By
Thao Huong Nguyen
Submitted to the department on August 30, 2019, in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biology
Abstract
The absence of both the EIIIA and EIIIB domains of fibronectin (FN) has been shown to negatively affect blood vessel formation and maintenance. Vascular defects have been observed in the yolk sacs of EIIIA/B double-null embryos by as early as embryonic day E9.5, and these defects are likely due to alterations in the extracellular matrix (ECM). Therefore, I have
conducted this study to investigate the differences in the ECM composition of the yolk sac in the presence and absence of EIIIA and EIIIB. I first collected yolk sacs at E9.5 from wild type, EIIIA-null, EIIIB-null, EIIIA/B heterozygous and EIIIA/B double-null mouse embryos, enriched for ECM content, and used quantitative proteomics to analyze their ECM composition. From these data, we identified a set of matrisome proteins that had decreased abundance in EIIIA/B double-null yolk sacs but were relatively unchanged in single-null and heterozygous yolk sacs compared to wild type. Some of these proteins could play a role in ECM remodeling or directly affect angiogenesis, and their reduced level in double-null yolk sacs might contribute to the vascular defects seen in the double-null tissue. Subsequently, I carried out further studies with tenascin-R (TN-R), one of proteins that was downregulated in the ECM of EIIIA/B double-null yolk sac. TN-R has been previously described to be restricted to the central nervous system, and our finding of TN-R in the yolk sac is novel. TN-R is localized to the mesoderm layer of yolk sacs. TN-R fibers partially overlap with FN, and TN-R area coverage in EIIIA/B double-null yolk sacs are decreased compared to wild type, suggesting that the presence of EIIIA/B promotes TN-R assembly in the yolk sac ECM. In addition, TN-R colocalizes with blood vessels in both the yolk sac and the retina, suggesting that TN-R might participate in vasculogenesis and
angiogenesis at these locations. Together, this study extends our understanding of yolk sac ECM, provides insight into the role of EIIIA and EIIIB domains, identifies novel expression patterns of ECM proteins, and opens up the possibility of a novel function for TN-R.
Thesis Supervisor: Richard 0. Hynes
Acknowledgements
"Baa tiec nao ri cing phii tin" is the Vietnamese proverb's equivalence of "All good things must come to an end." As my time at MIT is approaching the end, I am filled with mixed
feelings, but throughout this journey I am grateful for the opportunity to grow and for the support that I received along the way to reach the finish line.
First, I would like to thank my advisor, Richard Hynes, who has been very patient in rewiring me over the years to become more self-confident and more definitive in my opinions. I learned a lot from his careful judgement and thorough scientific investigation, and shall carry these lessons into my future endeavors.
I would like to thank my thesis committee members, Frank Gertler and Monty Krieger, for their experimental advice and encouragement particularly at the down moments of different research projects. I would also like to thank Jack Lawler for serving as the external member for my thesis defense.
The companionship and scientific expertise of the Hynes lab members have been crucial to my experience in the lab. I would like to thank Genevieve Abbruzzese, Steffen Rickelt, Noor Jailkhani and Chenxi Tian for their input on my thesis project, advice on experiments and encouragement. I am grateful to Jess Hebert for always lending me his ears, editing many of my writings, and suggesting good music to play during long experiments. I would like to thank David Benjamin for putting a humorous and positive spin on everything. In addition, I want to thank Patrick Murphy, Alexandra Naba, John Lamar, Bigyan Bista and Jeffrey Schindler for their feedback during my early years in the lab. Finally, I want to thank Amanda Del Rosario for her expertise in mass spectrometry and Carles Boix for his help with data analysis.
Music has always been an important part of my life, and I am glad I could continue to develop my performing and artistic skills at MIT. Playing the character Christmas Eve in the musical
"Avenue
Q"
for the second time with the MIT Musical Theater Guild signaled that I had stayed at MIT long enough. My best memories included singing with the MIT Chamber Chorus under the conducting of Bill Cutter, learning to stretch my voice beyond its limit and getting a free therapy session through the magic of Kerry Deal, and forming great friendships through the love of music with Srinivasan Raghuraman, Carles Boix, Liang Yu, Piotr Suwara, Oron Propp,Alexey Bailitskiy and many others. Singing as high as the stratosphere, literally speaking, helped me disconnect from worrying thoughts and become something different from myself for a short while, and I am thankful that MIT has so many musical activities to offer.
Part of me has been forever changed through the decisions I had to make to help build the Association of Vietnamese Students and Professionals in the U.S during its early years. I've also gained many brothers and sisters, including 'anh' Phu Nguyen, by organizing a series of summer events for the young Vietnamese community in different cities for several years.
Being very far away from family for a long time is not easy, and I was fortunate to have kind and loving roommates that made home feel like home. I want to acknowledge Yasmin Pei-Chau for
being a caring friend whose energy always cheers me up, and Phammela Abarzua for being there for me through the toughest moments of my late twenties.
Finally, I would like to thank my family for always believing in me. My father once told me I could be whatever I wish to be, my mother often made sure she commented the opposite of how I felt to keep me level-headed in all situations, and my grandfather taught all of his grandchildren "to live and not merely exist." I would like to thank my sister and brother-in-law for always cheering me on and inviting me to spend time at their home just to sleep and eat. Everyone's resolution this year was for me to graduate, and it seems their wish is finally fulfilled.
Table of Contents
T itle P ag e . ... 1
A b stract ... 3
A cknow ledgem ents ... 5
C hapter 1: Introduction ... 9
The extracellular matrix and the matrisome ... 10
Vasculogenesis and angiogenesis ... 12
The extracellular matrix in angiogenesis and vasculogenesis ... 14
F ibronectin ... . 18
Fibronectin and splice variants in vessel formation and maintenance... 22
Chapter 2: Quantitative mass spectrometric analysis of ECM in normal and EIIIA/B knockout yolk sac ... . 34
Chapter 3: Tenascin-R expression associated with angiogenesis in yolk sac and retina.... 66
Chapter 1.
Introduction
The extracellular matrix and the matrisome
In multicellular organisms, the non-cellular components termed extracellular matrix (ECM) provide structural support and biochemical and mechanical cues that are important for many biological processes (Hynes, 2009). A list of ECM proteins and associated factors termed
the matrisome has been constructed bioinformatically. The core ECM components of the
matrisome were defined as proteins that have characteristic ECM domains such as epidermal growth factor (EGF)-like and fibronectin type III-like domains, and excludes those that have non-characteristic ECM domains such as kinase and phosphatase domains (Naba et al., 2012a). Adding ECM-associated factors and with some manual annotation, this resulted in a list of over a thousand matrisome proteins in humans and mice. The Matrisome was divided into two main groups: 'core matrisome' and 'matrisome associated' proteins (Hynes and Naba, 2012 for the full list consult http://matrisomeproject.mit.edu).
'Core matrisome' proteins (~300 proteins) are ECM proteins that are often large and frequently crosslinked into mostly insoluble structures (Hynes, 2012). This category is further subdivided into collagens, glycoproteins and proteoglycans. Collagens are the most abundant proteins in mammals (-30% of total protein mass) (Ricard-Blum, 2011) and also the most abundant among matrisome proteins. The 28 members of the collagen family share a common triple-helix structure and the presence of the triplet Gly-X-Y repeats, where X is frequently proline and Y is frequently 4-hydroxyproline. Collagens can be further subdivided based on their supramolecular assemblies: fibrillar collagens (such as Col I), fibril-associated with interrupted triple-helices (such as Col IX) and network-forming collagens (such as Col IV) (Ricard-Blum, 2011; Yurchenco, 2011). The elastic properties of collagens are dependent on different kinds of crosslinking, which are tissue and collagen-type specific (Eyre and Wu, 2005).
Glycoproteins, proteins that have covalently attached oligosaccharides, make up the second core matrisome category. Well studied proteins in this group typically have multiple repeating domain structures and are multimeric. This includes fibronectin (FN) (Schwarzbauer and DeSimone, 2011), core basement membranes laminins and nidogens (Yurchenco, 2011), thrombospondins (Adams and Lawler, 2011) and tenascins (Chiquet-Ehrismann and Tucker, 2011). The ECM glycoproteins perform a wide range of roles, including assembling the ECM, promoting cell adhesion, and binding growth factors (Hynes and Naba, 2012).
The last category of 'core matrisome' proteins comprises proteoglycans, a subgroup of glycoproteins that have attached glycosaminoglycan (GAG) chains. The GAG chains can be chondroitin sulfate, dermatan sulfate, keratan sulfate and heparan sulfate (Iozzo and Schaefer, 2015). The addition of GAGs gives proteoglycans a high negative charge, thus they can
sequester both water and divalent cations and function as space fillers or lubricants (Sarrazin et al., 2011). Proteoglycans can be present in pericellular space such as perlecan and agrin, while some can regulate activity of growth factors and cytokines by protecting them from proteolysis (Iozzo and Schaefer, 2015; Sarrazin et al., 2011).
The first category of 'matrisome-associated' proteins are ECM regulators. This group encompasses crosslinking enzymes such as tissue transglutaminase and factor XIII, which
crosslinks fibrin and fibronectin (Carr et al., 1987), and lysyl oxidase, which is necessary to form crosslinks in collagens (Eyre and Wu, 2005). Another major group of ECM regulators are
proteases, whose functions include processing proproteins (such as pro-collagens) into their final forms (Canty and Kadler, 2005) and remodeling the ECM by cleaving ECM proteins (Lu et al., 2011). Key families of extracellular proteases in this category are: Matrix Metalloproteinases
(MMPs), A Disintegrin and Metalloproteinases (ADAMs) and cathepsins. The category of ECM regulators also includes protease inhibitors, such as the Serpine family (Law et al., 2006).
Secreted factors are the next group of matrisome-associated proteins. This group includes growth factors such as Epidermal Growth Factor (EGF), vascular endothelial growth factor (VEGF) family, the transforming growth factor (TGF) family, platelet-derived growth factor (PDGF) family etc., which are known to bind specifically to ECM glycoproteins such as
fibronectin and proteoglycans (Hynes, 2009). Other secreted factors in this group are cytokines and chemokines, which can be bound and sequestered by the ECM, including to GAGs much like growth factors (Vaday and Lider, 2000). The list of secreted factors also includes secreted proteins that might bind to the ECM, even if they have not previously been shown to do so. This was done to allow this list to reveal novel ECM interactions (Naba et al., 2012b).
The final matrisome protein category is ECM-affiliated proteins, which are miscellaneous proteins that share some architectural or biochemical similarities with ECM proteins (mucins, ficolins) or are experimentally observed to be associated with ECM proteins (annexins, galectins etc.) The ECM-affiliated protein list is purposefully broad and includes an entire protein family if any members of that family were identified experimentally to bind the ECM (Naba et al., 2012a). The list includes families of heavily glycosylated mucins, carbohydrate-binding lectins, semaphorins and their receptors the plexins, transmembrane receptors syndecans, annexins and galectins.
Vasculogenesis and angiogenesis
One process where the ECM plays a key role is in the formation and maintenance of the circulatory system. Blood vessel formation is classically divided into 2 categories:
vasculogenesis, the de novo creation of vessels from precursor cells, and angiogenesis, the formation of new vessels from preexisting vessels (Patan, 2004). Vasculogenesis occurs early in development due to the need to distribute oxygen and metabolites throughout the growing organism. In this process, progenitor cells in the mesoderm (angioblasts) differentiate into endothelial cells, form lumens and construct a primitive vascular plexus (Goldie et al., 2008). The first wave of vasculogenesis happens in the yolk sac and allantois, a structure responsible for placental development and formation of the umbilical vessels. The second wave takes place later in development in the embryo proper to develop the heart, aorta, veins and coronary vasculature (Ferguson III et al., 2005). After these initiating events, the extension of the vascular network in the organism during pre- and postnatal development and throughout life occurs through
angiogenesis. Angiogenesis consists of two distinct processes, sprouting angiogenesis and intussusceptive angiogenesis. Sprouting angiogenesis, is characterized by sprouts of endothelial cells from an existing vessel growing towards an angiogenic stimulus. Intussusceptive
angiogenesis, discovered much more recently, happens through the invasion of the interstitial tissues into existing vessels, which splits an existing vessel into two (Patan, 2004).
Growth factors and cytokines are key regulators of vascular development. Some examples are VEGF, platelet-derived growth factor (PDGF), fibroblast growth factors (FGF), TGF-f, and the angiopoietins (Ang). Sources of these growth factors include endothelial cells, fibroblasts, smooth muscle cells, platelets, inflammatory cells, and cancer cells (Kubis and Levy, 2003; Ucuzian et al., 2010). Genetic deletion studies have provided an understanding of the roles and timing of many factors in vessel development (Heinke et al., 2012). VEGF and its receptors are perhaps the most well studied, and they play a pivotal role in vessel formation (Shibuya, 2011). Haploinsufficiency of VEGF-A is enough to cause embryonic lethality (Carmeliet et al.,
1996), and knocking-out its receptors VEGFR-l and VEGFR-2 also causes lethality by E8.5-9.5 (Fong et al., 1995; Shalaby et al., 1997). The angiopoietins and their receptors, Tiel and Tie-2, seem to play a role later in the maturation and expansion of the vasculature. Tie2-null and AngI-null mice manage to form early blood vessels but die between E9.5 and E12.5 because of multiple cardiovascular defects (Dumont et al., 1994; Suri et al., 1996). The PDGFs are important for recruitment of pericytes to stabilize blood vessels. Deficiency of PDGF-B chain leads to lack of pericytes, overproliferation of endothelial cells and an increase in vessel leakiness (Leveen et al., 1994; Lindahl et al., 1997). TGF- can stimulate progenitor cell differentiation into pericytes and smooth muscle cells as well as the synthesis of ECM (Roberts et al., 1992; Armulik et al., 2005), and, therefore, is thought to play a role in vessel stabilization (Heinke et al., 2012). However, the absence of a primitive vessel network in TGF-$ null mice suggests that it is also important for differentiation of endothelial cells (Dickson et al., 1995). In summary, a number of soluble signaling factors act concertedly in collaboration with ECM to control the initiation and development of blood vessel networks.
The extracellular matrix in angiogenesis and vasculogenesis
In quiescent vessels, directly underneath the monolayer of endothelial cells is the
basement membrane. The primary constituents of this basement membrane are Col IV, laminins, nidogens and perlecan (Yurchenco, 2011). Endothelial basement membrane also contains von Willebrand factor (Knittel et al., 1995), and low levels of fibronectin are seen in angiogenic vessels (Astrof and Hynes, 2009). Beneath the intimal endothelial layer are smooth muscle cells (in large vessels) or pericytes (in small vessels). Pericytes share the same basement membrane with endothelial cells, but smooth muscle cells in larger vessels form their own ECM including
-1
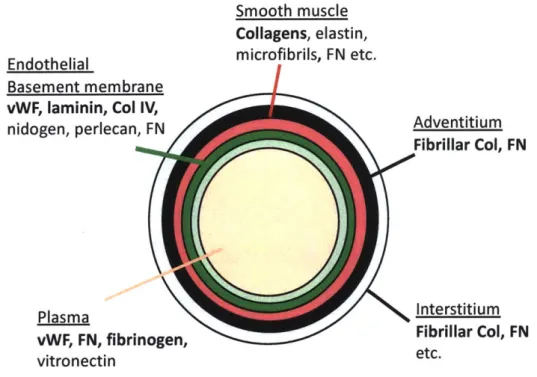
elastin, collagens and fibrillin microfibrils (Wagenseil and Mecham, 2009). Outside the smooth muscle cell layer is an adventitial layer containing fibroblasts, fibrillar collagens, FN, among other ECM proteins (Fig. 1, Bergmeier and Hynes, 2012).
Smooth muscle Collagens, elastin,
Endothelial microfibrils, FN etc.
Basement membrane vWF, laminin, Col IV,
nidogen, perlecan, FN Adventitium
Fibrillar Col, FN
Plasma Interstitium
vWF, FN, fibrinogen, Fibrillar Col, FN
vitronectin etc.
Figure 1: Extracellular matrix proteins abundant in different layers of blood vessels
(Bergmeier and Hynes, 2012). The monolayer of endothelial cells (pale green) resides above a basement membrane (green) consisting primarily of laminins, Col IV, von Willebrand factor (vWF), nidogens and perlecans, and some FN. The layer beneath varies depending on vessel types. In small vessels, pericytes surround the endothelial layer and contribute ECM to the basement membrane. In large vessels, surrounding the endothelial and basement membrane layer are smooth muscle cells and a matrix (red) rich in collagens, elastin and fibrillins, and FN; the surrounding connective tissue adventitium (black) contains fibrillar collagens, FN and other ECM proteins. The interstitium (grey) typically consists of fibrillar collagens, FN and diverse other ECM proteins. Plasma, another source of matrix proteins, has abundant amounts of vWF, FN and fibrinogen. When vessels become leaky (stimulated by growth factors or injury), plasma proteins can leak into the vessel wall and become part of the vessel's provisional matrix.
In addition to soluble cues, the ECM surrounding blood vessels is another important modulator of vascular development. The role of the ECM is best understood in sprouting angiogenesis in adult animals. Angiogenesis is generally believed to begin with stimulatory signals from angiogenic factors to activate endothelial cells and degrade the basement membrane (Chang et al., 2009). Subsequently, vessels become leaky and proteins from blood flush into the interstitial layer and form a provisional, proangiogenic matrix including interstitial collagen, fibrin, fibronectin and vitronectin (Senger, 1996). Endothelial cells need attachment to the ECM through integrin receptors to proliferate (Giancotti and Ruoslahti, 1999; Meredith and Schwartz, 1997). Endothelial cells also use ECM proteins such as fibrin and Col I to migrate in response to cytokine signals (van Hinsbergh et al., 2001; Senger et al., 2002). Furthermore, the ECM
provides a scaffold for endothelial cells to undergo morphogenesis, cluster together and form cord-like structures (Davis and Camarillo, 1995). These cords can mature to form tubes with hollow lumens in an integrin-dependent manner within Col I gel and fibrin matrix (which are major components of the provisional matrix) (Bayless et al., 2000; Davis and Camarillo, 1996). To prevent regression of the endothelial cell tubes and stabilize the new vessels, pericytes are recruited to the new vessels and begin making a new basement membrane with the endothelial cells (Saunders et al., 2006; Stratman et al., 2009).
ECM proteins can bind soluble factors and affect their availability. For example, the matrix protein thrombospondin-1, which itself is anti-angiogenic (Armstrong and Bornstein, 2003), binds various angiogenic cytokines including VEGF, HGF and FGF-2 and might interfere with their activities. Another example is the sequestration of the TGF-$/latency-associated
peptide (TGF-
p/LAP)
complex by latent TGF-P binding proteins (LTBPs). Inactive TGF-p is stored in the ECM through binding of LTBPs with fibrillins or FN. (Dallas et al., 2005; Isogai etal., 2003). Mutation in the fibrillin-1 leads to excessive activity of TGF-, leading to many vascular abnormalities (Ramirez and Dietz, 2009). Therefore, the ECM not only acts to provide proliferation signals and physical scaffolds for the morphogenesis of endothelial cells but also can regulate activity of cytokines and enzymes.
We have gained much of our understandings of angiogenesis in the adult thanks to a plethora of powerful in vitro and in vivo assays (Tahergorabi and Khazaei, 2012). However, the role of the ECM in vasculogenesis is not well understood due to the lack of in vitro models that mimic the embryonic ECM (Vailh6 et al., 2001). As a result, knockout mice have been the primary tool to study vasculogenesis. Knockouts of basement membrane proteins nidogens, perlecan, vitronectin and von Willebrand factor show no obvious defects in angiogenesis (Hynes, 2007). Knockout of basement membrane laminins and Col IV leads to lethality due to
mechanical forces and stress exerted on the vasculature after a nascent vasculature is formed (Senger and Davis, 2011). Knockout of FN leads to early and clear cardiovascular defects
(George et al., 1993; Astrof and Hynes, 2009), suggesting that it is an essential gene for initiation and development of early vessels. I will discuss more about the FN in the next section. One reason that it has been difficult to establish in vitro models of the embryonic ECM is that its composition is markedly different from the adult ECM. In contrast to the adult ECM, the embryonic ECM is known to have less fibrillar collagen but more tenascins, hyaluronan,
proteoglycans and fibronectin (Senger and Davis, 2011). We currently do not have a full account of embryonic ECM. Given that vasculogenesis occurs exclusively in the context of the
embryonic ECM, a better understanding of the composition of the embryonic ECM will facilitate future studies of vasculogenesis and developmental angiogenesis.
Fibronectin
Fibronectin (FN) is a ubiquitous glycoprotein that is found in blood plasma and in the ECM of most tissues. A single FN monomer has a molecular weight ranging from 230 kDa to 270 kDa with this size difference mostly due to the inclusion or exclusion of alternatively spliced domains (Hynes 1990). FN is secreted as a dimer with the two monomers linked by disulfide bonds at the C-terminal end in an antiparallel fashion (An et al., 1992). Plasma FN exists as soluble dimers and is made by hepatocytes in the liver (Owens and Cimino, 1982; Tamkun and Hynes, 1983), while tissue FN (also called cellular FN) is found primarily in complexes
containing multiple FN and other matrix proteins that are bound together by both covalent and non-covalent interactions (Barry and Mosher, 1988; Schwarzbauer and DeSimone, 2011).
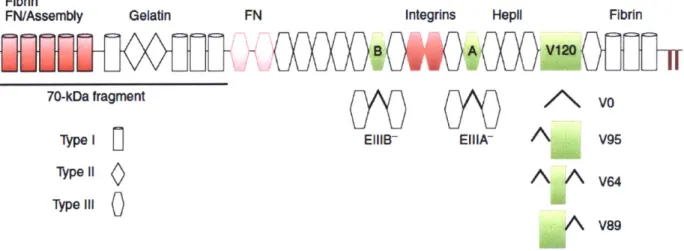
FN consists of three different types of modules with distinctive structures called
fibronectin type I, type II and type III domains (Fig. 2). Type I and II repeats have intra-domain disulfide bonds to maintain their conformations. Meanwhile, type III repeats lack disulfide bonds and therefore are flexible and can undergo conformational changes. Besides 12 type I repeats, 2 type II repeats and 15 type III repeats that are constitutively expressed, FN has three alternatively spliced domains: EIIIA, EIIIB, which are both type III, and a variable region called the V
domain (or IIICS) that can be excluded (VO) or included completely (V120) or partially (V95, V64 and V89) (Schwarzbauer, 1991a). At least one FN monomer must have the V domain for the dimer to be secreted, which is likely why the V domain is widely expressed in plasma FN
(Schwarzbauer et al., 1989) and essentially all tissues (Ffrench-Constant and Hynes, 1989; Oyama et al., 1989). On the other hand, the EIIIA and EIIIB domains are only rarely found in plasma FN. They are widely expressed in early embryogenesis but their inclusion becomes
restricted to specific locations in adult animals (Ffrench-Constant and Hynes, 1989; Oyama et al., 1989; Peters and Hynes, 1996; Peters et al., 1996).
Fibrin
FN/Assembly Gelatin FN Integrins Hepil Fibrin
70-kDa fragment V
Type I EIIIB- EIII
A
V95ypel1
A A
v 64
Type11
0A
AV89
Figure 2: Overview of domain organization of fibronectin (Schwarzbauer and DeSimone, 2011). FN is made up of 12 type I (cylinders), 2 type II (diamonds), 15 type III (hexagons) domains and 3 alternatively spliced domains EIIIA, EIIIB and V (green). The V region can be completely included (V120), or partially included (V95, V64 and V89). Two disulfide bonds at the C-terminus link FN together as dimers. RGD in IIIio and the synergistic 1119 domain interact with integrins. Fibrin, gelatin/collagen I, FN and heparin-binding domains are depicted. In red are domains important for FN fibrillogenesis.
FN interacts with several cell surface receptors and matrix molecules and some of these interaction sites have been identified (Pankov and Yamada, 2002). There are two well-defined integrin binding sites in FN: the RGD sequence in the IIIio domain (Pierschbacher and Ruoslahti, 1984) or the LDV motif in CS1 segment of the V region (Guan and Hynes, 1990; Humphries et al., 1987). Some integrins require the 1119 repeat for their maximal interaction with FN (Aota et al., 1991). There are two heparin-binding sites on FN, one at the N-terminal and one in theIII2.
sulfate glycosaminoglycans (Barkalow and Schwarzbauer, 1994; Hynes, 1990). Collagen/gelatin binding occurs at 16-9
and111,2(Owens
and Baralle, 1986), and binding to fibrin happens at thefirst type I repeats and the C-terminal region (Hynes, 1990).
Many proteins, including FN, are crosslinked and form fibrillar structures in the ECM. The current model for the assembly of FN into fibrils indicates that the FN dimer is assembled into a stable matrix via a multi-step process that depends on cell binding, FN-FN intermolecular interactions and crosslinking (Mao and Schwarzbauer, 2005). The secreted FN dimer is soluble and compact partly due to binding between type III modules III12-14 of one monomer to type III modules 1112-3 of the other monomer (Johnson et al., 1999). When integrin receptors bind to soluble FN at the RGD and synergy sites in modules 1119-10 (Aota et al., 1994; Pierschbacher and Ruoslahti, 1984), the receptors cluster, firstly bringing bound FN molecules closer together and, due to cytoskeletal forces acting through the integrins extending the FN into an open
conformation to expose cryptic binding sites. Deletion mutations and antibody blockade experiments have shown that the 70-kDa N-terminal fragment (McDonald et al., 1987;
Schwarzbauer, 1991b) and the type III1-2 domains (Chernousov et al., 1987; Sechler et al., 2001) are critical for the assembly of FN. It is also evident that heparan sulfate, which binds to FN at the HeplI domain within type 11112-14, also assists in bringing multiple FNs together and promote-ing assembly (Raitman et al., 2018).
In vitro, the assembly of a number of other matrix proteins is dependent on the assembly
of FN. For example, collagen I fibers align with FN fibers early in their assembly (Li et al., 2003). Collagen I fibrillogenesis is also abrogated when its binding to FN is disrupted by blocking antibodies (McDonald et al., 1982) or when a FN matrix fails to form in cultures of FN-deficient embryonic fibroblasts (Velling et al., 2002). Fibrinogen (Pereira et al., 2002),
thrombospondin-1 (Sottile and Hocking, 2002), fibrillin-1 (Kinsey et al., 2008), fibulin-1
(Godyna et al., 1995), and latent TGF-p binding protein (LTBP)-1 (Dallas et al., 2005) are other proteins that need a pre-formed FN matrix to support their assembly. From these data, it has become apparent that FN is a matrix organizer required for the deposition of many ECM proteins.
FN has multiple important biological functions. In adults, FN participates in multiple steps during wound healing (Clark, 1990; Grinnell, 1984). When injuries occur, the first response is forming blood clots consisting of platelets and fibrin to prevent hemorrhage. When the level of plasma FN is reduced in heterozygous FN+/- mice or by conditionally knocking out FN in the liver, the initiation and growth of blood clots induced by arterial injury is delayed (Matuskova Jana et al., 2006; Ni et al., 2003). A detailed survey of the coagulation process showed that when blood vessels are damaged and the subendothelial matrix is exposed, plasma FN (pFN) is among the first molecules to adhere to the site of injury and is then followed by the accumulation of platelets (Wang et al., 2014). In addition, pFN can also be crosslinked to fibrin by factor XIIIa (Mosher, 1975) and the incorporation of FN increases the mechanical strength of the fibrin clot (Wang et al., 2014). After the wound is sealed by a blood clot, additional FN is deposited by platelets (Sakai et al., 2001), macrophages, fibroblasts (Brown et al., 1993), and endothelial cells (Takamiya et al., 2006) to support tissue repair. Plasma FN is neuroprotective following transient cerebral ischemia and traumatic insult but is not required for skin wound healing (Sakai et al., 2001; Tate et al., 2007), suggesting that plasma FN likely plays an important role in diminishing acute damage, while FN deposited by cells is responsible for reconstructing the injured tissue.
Fibronectin and splice variants in vessel formation and maintenance
Current in vivo data implicate FN in vascular morphogenesis. During postnatal
development, FN regulates angiogenesis, as shown by the fact that inducible knockout of FN in the retina significantly hinders growth, branching and coverage of the vessel network (Turner et al., 2017). Embryos with FN gene inactivation rapidly develop abnormalities in the mesoderm, neural tube and vasculature from embryonic day E8 and die in gestation. By E8.5, the dorsal aortae either are not present, or form but are abnormal and bloated (George et al., 1993). These defects are more severe in the 129S4 background than in the C57BL/6J strain (Astrof et al., 2007a). The yolk sac vasculature is also abnormal in knockout animals; the two layers of the yolk sac (endoderm and mesoderm) appear to split apart, and blood floats in between the two layers instead of being confined to blood islands or vessels (George et al., 1993). In addition, knockout of FN decreased capillary plexus formation in embryoid bodies and this phenomenon is rescued by addition of FN to the culture system (Francis et al., 2002). These observations suggest that FN is essential to and plays a direct role in vascular formation and development.
Fibronectin has two type III domains that are alternatively spliced, EIIIA and EIIIB (Fig. 1). The EIIIA and EIIIB domains of FN are expressed during embryonic development (Peters and Hynes, 1996; Peters et al., 1996), in injury (Brown et al., 1993; Ffrench-Constant et al.,
1989; Kilian et al., 2008), and in tumors (D'Ovidio et al., 1998; Kaczmarek et al., 1994; Pujuguet et al., 1996) but mostly absent in healthy adult tissue. EIIIA is proinflammatory, prothrombotic and has been associated with several pathological conditions. Significant upregulation of EIIIA is observed in fibrotic lungs, and EIIIA-null mice were protected from lung fibrosis after exposure to bleomycin (Muro et al., 2008). In the ApoE-null mouse model of atherosclerosis, mice with EIIIA depletion have fewer atherosclerotic lesions (Tan et al., 2004)
and atherosclerosis is exacerbated in mice constitutively expressing EIIIA (Doddapattar et al., 2015). EIIIA is a ligand for Toll-like-receptor (TLR-) 4 (Okamura et al., 2001) and integrins a9p land a4p1 (Liao et al., 2002), and TLR-4 has been shown to partake in several EIIIA-related pathological conditions (Bhattacharyya et al., 2014; Doddapattar et al., 2015). Much less is known about how EIIIB participates in biological processes. EIIIB-null mice develop normally, and embryonic fibroblasts from these mice have a small reduction in in cellular growth and FN assembly (Fukuda et al., 2002).
The absence of one of the extra domains does not have major impacts on mouse development (Fukuda et al., 2002; Tan et al., 2004), but deficiency in both EIIIA and EIIIB domains has significant consequences for cardiovascular development and maintenance that resemble the phenotype of FN-null mice. Mice lacking both EIIIA and EIIIB exons exhibit embryonic lethality with incomplete penetrance by E10.5 with defects including vascular hemorrhage, a failure to remodel embryonic and yolk sac vasculature, and heart malformation (Astrof et al., 2007b). The few surviving double-null mice in the C56/BL6 background lose protection against arterial aneurysm and have increased hemorrhage and hypertrophy in the carotid artery under disturbed flow condition (Murphy and Hynes, 2014). A reduction in vessel coverage and tip cell number in the developing retinal vascular plexus in postnatal pups has also been observed in the double-null mice (Turner et al., 2017). The presence of either EIIIA and EIIIB are therefore important for vascular formation and maintenance. In vitro, culturing FN-null embryonic fibroblasts with cellular FN (purified from the conditioned media of fibroblasts, which contains both EIIIA and EIIIB) produced a better FN, fibrillin-1, fibulin-4 and LTBP-4 matrix than culturing with just plasma FN (Kumra et al., 2018). This suggests that EIIIA and EIIIB's presence promotes matrix assembly for FN and other matrix proteins subsequently.
This thesis seeks to investigate the ECM environment in yolk sac and the impact of the absence of EIIIA and EIIIB on the composition of the yolk sac ECM and angiogenesis.
• In chapter 2, I will describe a quantitative analysis of the yolk sac ECM in EIIIA and EIIIB knockout backgrounds. I will then explore what the data imply about the role of EIIIA and EIIIB in the matrix and in vascular development in the yolk sac.
• In chapter 3, I will describe my follow-up study of one of the candidate genes we found in our proteomics data, tenascin-R. I will validate expression of tenascin-R and show that its expression is associated with angiogenesis.
• In chapter 4, I will summarize the findings in this thesis, propose further studies and discuss outstanding questions and implications from this work.
References
Adams, J.C., and Lawler, J. (2011). The Thrombospondins. Cold Spring Harb. Perspect. Biol. 3, a009712.
An, S.S.A., Jimenez-Barbero, J., Petersen, T.E., and Llinas, M. (1992). The two polypeptide chains in fibronectin are joined in antiparallel fashion: NMR structural characterization. Biochemistry 31, 9927-9933.
Aota, S., Nagai, T., and Yamada, K.M. (1991). Characterization of regions of fibronectin besides the arginine-glycine-aspartic acid sequence required for adhesive function of the cell-binding domain using site-directed mutagenesis. J. Biol. Chem. 266,15938-15943.
Aota, S., Nomizu, M., and Yamada, K.M. (1994). The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J. Biol. Chem. 269, 24756-24761.
Armstrong, L.C., and Bornstein, P. (2003). Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 22, 63-71.
Armulik, A., Abramsson, A., and Betsholtz, C. (2005). Endothelial/Pericyte Interactions. Circ. Res. 97, 512-523.
Astrof, S., and Hynes, R.O. (2009). Fibronectins in Vascular Morphogenesis. Angiogenesis 12, 165-175.
Astrof, S., Kirby, A., Lindblad-Toh, K., Daly, M., and Hynes, R.O. (2007a). Heart development in fibronectin-null mice is governed by a genetic modifier on chromosome four. Mech. Dev. 124, 551-558.
Astrof, S., Crowley, D., and Hynes, R.O. (2007b). Multiple cardiovascular defects caused by the absence of alternatively spliced segments of fibronectin. Dev. Biol. 311, 11-24.
Barkalow, F.J., and Schwarzbauer, J.E. (1994). Interactions between fibronectin and chondroitin sulfate are modulated by molecular context. J. Biol. Chem. 269, 3957-3962.
Barry, E.L., and Mosher, D.F. (1988). Factor XIII cross-linking of fibronectin at cellular matrix assembly sites. J. Biol. Chem. 263, 10464-10469.
Bayless, K.J., Salazar, R., and Davis, G.E. (2000). RGD-Dependent Vacuolation and Lumen Formation Observed during Endothelial Cell Morphogenesis in Three-Dimensional Fibrin Matrices Involves the av3 and a501 Integrins. Am. J. Pathol. 156, 1673-1683.
Bergmeier, W., and Hynes, R.O. (2012). Extracellular Matrix Proteins in Hemostasis and Thrombosis. Cold Spring Harb. Perspect. Biol. 4.
Bhattacharyya, S., Tamaki, Z., Wang, W., Hinchcliff, M., Hoover, P., Getsios, S., White, E.S., and Varga, J. (2014). Fibronectin EDA Promotes Chronic Cutaneous Fibrosis Through Toll-like Receptor Signaling. Sci. Transl. Med. 6, 232ra50.
Brown, L.F., Dubin, D., Lavigne, L., Logan, B., Dvorak, H.F., and Van de Water, L. (1993). Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am. J. Pathol. 142, 793-801.
Canty, E.G., and Kadler, K.E. (2005). Procollagen trafficking, processing and fibrillogenesis. J. Cell Sci. 118, 1341-1353.
Carmeliet, P., Ferreira, V., Breier, G., Pollefeyt, S., Kieckens, L., Gertsenstein, M., Fahrig, M., Vandenhoeck, A., Harpal, K., Eberhardt, C., et al. (1996). Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435.
Carr, M.E., Gabriel, D.A., and McDonagh, J. (1987). Influence of factor XIII and fibronectin on fiber size and density in thrombin-induced fibrin gels. J. Lab. Clin. Med. 110, 747-752.
Chang, S.-H., Kanasaki, K., Gocheva, V., Blum, G., Harper, J., Moses, M.A., Shih, S.-C., Nagy, J.A., Joyce, J., Bogyo, M., et al. (2009). VEGF-A induces angiogenesis by perturbing the cathepsin-cysteine protease inhibitor balance in venules, causing basement membrane degradation & mother vessel formation. Cancer Res. 69, 4537-4544.
Chernousov, M.A., Faerman, A.I., Frid, M.G., Printseva, O.Y., and Koteliansky, V.E. (1987). Monoclonal antibody to fibronectin which inhibits extracellular matrix assembly. FEBS Lett. 217,124-128.
Chiquet-Ehrismann, R., and Tucker, R.P. (2011). Tenascins and the Importance of Adhesion Modulation. Cold Spring Harb. Perspect. Biol. 3, a004960.
Clark, R.A.F. (1990). Fibronectin Matrix Deposition and Fibronectin Receptor Expression in Healing and Normal Skin. J. Invest. Dermatol. 94, s128-s134.
Dallas, S.L., Sivakumar, P., Jones, C.J.P., Chen,
Q.,
Peters, D.M., Mosher, D.F., Humphries, M.J., and Kielty, C.M. (2005). Fibronectin Regulates Latent Transforming Growth Factor-Q (TGF$) by Controlling Matrix Assembly of Latent TGFf-binding Protein-1. J. Biol. Chem. 280,18871-18880.
Davis, G.E., and Camarillo, C.W. (1995). Regulation of Endothelial Cell Morphogenesis by Integrins, Mechanical Forces, and Matrix Guidance Pathways. Exp. Cell Res. 216, 113-123. Davis, G.E., and Camarillo, C.W. (1996). An a2pl Integrin-Dependent Pinocytic Mechanism Involving Intracellular Vacuole Formation and Coalescence Regulates Capillary Lumen and Tube Formation in Three-Dimensional Collagen Matrix. Exp. Cell Res. 224, 39-51.
Dickson, M.C., Martin, J.S., Cousins, F.M., Kulkarni, A.B., Karlsson, S., and Akhurst, R.J. (1995). Defective haematopoiesis and vasculogenesis in transforming growth factor-beta 1 knock out mice. Development 121, 1845-1854.
Doddapattar, P., Gandhi, C., Prakash, P., Dhanesha, N., Grumbach, I.M., Dailey, M.E., Lentz, S.R., and Chauhan, A.K. (2015). Fibronectin splicing variants containing extra domain A promote atherosclerosis in mice through Toll-like receptor 4. Arterioscler. Thromb. Vasc. Biol. 35,2391-2400.
D'Ovidio, M.C., Mastracchio, A., Marzullo, A., Ciabatta, M., Pini, B., Uccini, S., Zardi, L., and Ruco, L.P. (1998). Intratumoral microvessel density and expression of ED-A/ED-B sequences of fibronectin in breast carcinoma. Eur. J. Cancer 34, 1081-1085.
Dumont, D.J., Gradwohl, G., Fong, G.H., Puri, M.C., Gertsenstein, M., Auerbach, A., and Breitman, M.L. (1994). Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 8,1897-1909.
Eyre, D.R., and Wu, J.-J. (2005). Collagen Cross-Links. In Collagen: Primer in Structure, Processing and Assembly, J. Brinckmann, H. Notbohm, and P.K. Muller, eds. (Berlin, Heidelberg: Springer Berlin Heidelberg), pp. 207-229.
Ferguson III, J.E., Kelley, R.W., and Patterson, C. (2005). Mechanisms of Endothelial
Differentiation in Embryonic Vasculogenesis. Arterioscler. Thromb. Vasc. Biol. 25, 2246-2254. Ffrench-Constant, C., and Hynes, R.O. (1989). Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development 106, 375-388.
Ffrench-Constant, C., Van de Water, L., Dvorak, H.F., and Hynes, R.O. (1989). Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J. Cell Biol. 109,903-914.
Fong, G.-H., Rossant, J., Gertsenstein, M., and Breitman, M.L. (1995). Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376, 66.
Francis, S.E., Goh, K.L., Hodivala-Dilke, K., Bader, B.L., Stark, M., Duncan, D., and Hynes, R.O. (2002). Central Roles of a5pl Integrin and Fibronectin in Vascular Development in Mouse Embryos and Embryoid Bodies. Arterioscler. Thromb. Vasc. Biol. 22, 927-933.
Fukuda, T., Yoshida, N., Kataoka, Y., Manabe, R., Mizuno-Horikawa, Y., Sato, M., Kuriyama, K., Yasui, N., and Sekiguchi, K. (2002). Mice Lacking the EDB Segment of Fibronectin Develop Normally but Exhibit Reduced Cell Growth and Fibronectin Matrix Assembly in Vitro. Cancer Res. 62, 5603-5610.
George, E.L., Georges-Labouesse, E.N., Patel-King, R.S., Rayburn, H., and Hynes, R.O. (1993). Defects in mesoderm, neural tube and vascular development in mouse embryos lacking
fibronectin. Development 119, 1079-1091.
Godyna, S., Mann, D.M., and Argraves, W.S. (1995). A quantitative analysis of the incorporation of fibulin-1 into extracellular matrix indicates that fibronectin assembly is required. Matrix Biol. J. Int. Soc. Matrix Biol. 14, 467-477.
Goldie, L.C., Nix, M.K., and Hirschi, K.K. (2008). Embryonic vasculogenesis and hematopoietic specification. Organogenesis 4, 257-263.
Grinnell, F. (1984). Fibronectin and wound healing. J. Cell. Biochem. 26, 107-116.
Guan, J.-L., and Hynes, R.O. (1990). Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor a4pl. Cell 60, 53-61.
Heinke, J., Patterson, C., and Moser, M. (2012). Life is a pattern: vascular assembly within the embryo. Front. Biosci. Elite Ed. 4, 2269-2288.
van Hinsbergh, V.W., Collen, A., and Koolwijk, P. (2001). Role of fibrin matrix in angiogenesis. Ann. N. Y. Acad. Sci. 936, 426-437.
Humphries, M.J., Komoriya, A., Akiyama, S.K., Olden, K., and Yamada, K.M. (1987). Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J. Biol. Chem. 262, 6886-6892. Hynes, R.O. (1990). Fibronectins (New York: Springer-Verlag).
Hynes, R.O. (2007). Cell-matrix adhesion in vascular development. J. Thromb. Haemost. 5, 32-40.
Hynes, R.O. (2009). The Extracellular Matrix: Not Just Pretty Fibrils. Science 326, 1216-1219. Hynes, R.O. (2012). The evolution of metazoan extracellular matrix. J. Cell Biol. 196, 671-679. Hynes, R.O., and Naba, A. (2012). Overview of the Matrisome-An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb. Perspect. Biol. 4.
Iozzo, R.V., and Schaefer, L. (2015). Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 42, 11-55.
Isogai, Z., Ono, R.N., Ushiro, S., Keene, D.R., Chen, Y., Mazzieri, R., Charbonneau, N.L., Reinhardt, D.P., Rifkin, D.B., and Sakai, L.Y. (2003). Latent Transforming Growth Factor-binding Protein 1 Interacts with Fibrillin and Is a Microfibril-associated Protein. J. Biol. Chem. 278,2750-2757.
Johnson, K.J., Sage, H., Briscoe, G., and Erickson, H.P. (1999). The compact conformation of fibronectin is determined by intramolecular ionic interactions. J. Biol. Chem. 274, 15473-15479. Kaczmarek, J., Castellani, P., Nicolo, G., Spina, B., Allemanni, G., and Zardi, L. (1994).
Distribution of oncofetal fibronectin isoforms in normal, hyperplastic and neoplastic human breast tissues. Int. J. Cancer 59, 11-16.
Kilian,0 ., Dahse, R., Alt, V., Zardi, L., Hentschel, J., Schnettler, R., and Kosmehl, H. (2008). mRNA Expression and Protein Distribution of Fibronectin Splice Variants and High-Molecular
Weight Tenascin-C in Different Phases of Human Fracture Healing. Calcif. Tissue Int. 83, 101-111.
Kinsey, R., Williamson, M.R., Chaudhry, S., Mellody, K.T., McGovern, A., Takahashi, S., Shuttleworth, C.A., and Kielty, C.M. (2008). Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J. Cell Sci. 121, 2696-2704.
Knittel, T., Neubauer, K., Armbrust, T., and Ramadori, G. (1995). Expression of Von Willebrand factor in normal and diseased rat livers and in cultivated liver cells. Hepatology 21, 470-476. Kubis, N., and Levy, B.I. (2003). Vasculogenesis and Angiogenesis: Molecular and Cellular Controls. Interv. Neuroradiol. 9, 239-248.
Kumra, H., Sabatier, L., Hassan, A., Sakai, T., Mosher, D.F., Brinckmann, J., and Reinhardt, D.P. (2018). Roles of fibronectin isoforms in neonatal vascular development and matrix integrity. PLOS Biol. 16, e2004812.
Law, R.H., Zhang,
Q.,
McGowan, S., Buckle, A.M., Silverman, G.A., Wong, W., Rosado, C.J., Langendorf, C.G., Pike, R.N., Bird, P.I., et al. (2006). An overview of the serpin superfamily. Genome Biol. 7, 216.Leveen, P., Pekny, M., Gebre-Medhin, S., Swolin, B., Larsson, E., and Betsholtz, C. (1994). Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev.8,1875-1887.
Li, S., Van Den Diepstraten, C., D'Souza, S.J., Chan, B.M.C., and Pickering, J.G. (2003). Vascular smooth muscle cells orchestrate the assembly of type I collagen via alpha2betal integrin, RhoA, and fibronectin polymerization. Am. J. Pathol. 163, 1045-1056.
Liao, Y.-F., Gotwals, P.J., Koteliansky, V.E., Sheppard, D., and Water, L.V.D. (2002). The EIIIA Segment of Fibronectin Is a Ligand for Integrins a901 and a401 Providing a Novel Mechanism for Regulating Cell Adhesion by Alternative Splicing. J. Biol. Chem. 277, 14467-14474.
Lindahl, P., Johansson, B.R., Leveen, P., and Betsholtz, C. (1997). Pericyte Loss and Microaneurysm Formation in PDGF-B-Deficient Mice. Science 277, 242-245.
Lu, P., Takai, K., Weaver, V.M., and Werb, Z. (2011). Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 3.
Mao, Y., and Schwarzbauer, J.E. (2005). Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 24, 389-399.
Matuskova Jana, Chauhan Anil K., Cambien Beatrice, Astrof Sophie, Dole Vandana S., Piffath Crystal L., Hynes Richard 0., and Wagner Denisa D. (2006). Decreased plasma fibronectin leads
to delayed thrombus growth in injured arterioles. Arterioscler. Thromb. Vasc. Biol. 26, 1391-1396.
McDonald, J.A., Kelley, D.G., and Broekelmann, T.J. (1982). Role of fibronectin in collagen deposition: Fab' to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J. Cell Biol. 92, 485-492.
McDonald, J.A., Quade, B.J., Broekelmann, T.J., LaChance, R., Forsman, K., Hasegawa, E., and Akiyama, S. (1987). Fibronectin's cell-adhesive domain and an amino-terminal matrix assembly domain participate in its assembly into fibroblast pericellular matrix. J. Biol. Chem. 262, 2957-2967.
Meredith, J.E., and Schwartz, M.A. (1997). Integrins, adhesion and apoptosis. Trends Cell Biol.
7, 146-150.
Mosher, D.F. (1975). Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J. Biol. Chem. 250, 6614-6621.
Muro, A.F., Moretti, F.A., Moore, B.B., Yan, M., Atrasz, R.G., Wilke, C.A., Flaherty, K.R., Martinez, F.J., Tsui, J.L., Sheppard, D., et al. (2008). An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 177, 638-645.
Murphy, P.A., and Hynes, R.O. (2014). Alternative splicing of endothelial fibronectin is induced by disturbed hemodynamics and protects against hemorrhage of the vessel wall. Arterioscler. Thromb. Vasc. Biol. 34, 2042-2050.
Naba, A., Clauser, K.R., Hoersch, S., Liu, H., Carr, S.A., and Hynes, R.O. (2012a). The Matrisome: In Silico Definition and In Vivo Characterization by Proteomics of Normal and Tumor Extracellular Matrices. Mol. Cell. Proteomics MCP 1].
Naba, A., Hoersch, S., and Hynes, R.O. (2012b). Towards definition of an ECM parts list: An advance on GO categories. Matrix Biol. J. Int. Soc. Matrix Biol. 31, 371-372.
Ni, H., Yuen, P.S.T., Papalia, J.M., Trevithick, J.E., Sakai, T., Fassler, R., Hynes, R.O., and Wagner, D.D. (2003). Plasma fibronectin promotes thrombus growth and stability in injured arterioles. Proc. Natl. Acad. Sci. 100, 2415-2419.
Okamura, Y., Watari, M., Jerud, E.S., Young, D.W., Ishizaka, S.T., Rose, J., Chow, J.C., and Strauss, J.F. (2001). The Extra Domain A of Fibronectin Activates Toll-like Receptor 4. J. Biol. Chem. 276, 10229-10233.
Owens, M.R., and Cimino, C.D. (1982). Synthesis of fibronectin by the isolated perfused rat liver.Blood 59, 1305-1309.
Owens, R.J., and Baralle, F.E. (1986). Mapping the collagen-binding site of human fibronectin by expression in Escherichia coli. EMBO J. 5, 2825-2830.
Oyama, F., Murata, Y., Suganuma, N., Kimura, T., Titani, K., and Sekiguchi, K. (1989). Patterns of alternative splicing of fibronectin pre-mRNA in human adult and fetal tissues. Biochemistry 28,1428-1434.
Pankov, R., and Yamada, K.M. (2002). Fibronectin at a glance. J. Cell Sci. 115, 3861-3863. Patan, S. (2004). Vasculogenesis and Angiogenesis. In Angiogenesis in Brain Tumors, M. Kirsch, and P.McL. Black, eds. (Boston, MA: Springer US), pp. 3-32.
Pereira, M., Rybarczyk, B.J., Odrljin, T.M., Hocking, D.C., Sottile, J., and Simpson-Haidaris, P.J. (2002). The incorporation of fibrinogen into extracellular matrix is dependent on active assembly of a fibronectin matrix. J. Cell Sci. 115, 609-617.
Peters, J.H., and Hynes, R.O. (1996). Fibronectin isoform distribution in the mouse. I. The alternatively spliced EIIIB, EIIIA, and V segments show widespread codistribution in the developing mouse embryo. Cell Adhes. Commun. 4, 103-125.
Peters, J.H., Chen, G.E., and Hynes, R.O. (1996). Fibronectin isoform distribution in the mouse. II. Differential distribution of the alternatively spliced EIIIB, EIIIA, and V segments in the adult mouse. Cell Adhes. Commun. 4, 127-148.
Pierschbacher, M.D., and Ruoslahti, E. (1984). Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30.
Pujuguet, P., Hammann, A., Moutet, M., Samuel, J.L., Martin, F., and Martin, M. (1996). Expression of fibronectin ED-A+ and ED-B+ isoforms by human and experimental colorectal cancer. Contribution of cancer cells and tumor-associated myofibroblasts. Am. J. Pathol. 148, 579-592.
Raitman, I., Huang, M.L., Williams, S.A., Friedman, B., Godula, K., and Schwarzbauer, J.E. (2018). Heparin-fibronectin interactions in the development of extracellular matrix insolubility. Matrix Biol. 67, 107-122.
Ramirez, F., and Dietz, H.C. (2009). Extracellular Microfibrils in Vertebrate Development and Disease Processes. J. Biol. Chem. 284, 14677-14681.
Ricard-Blum, S. (2011). The Collagen Family. Cold Spring Harb. Perspect. Biol. 3.
Roberts, A.B., McCune, B.K., and Sporn, M.B. (1992). TGF-p: Regulation of extracellular matrix. Kidney Int. 41, 557-559.
Sakai, T., Johnson, K.J., Murozono, M., Sakai, K., Magnuson, M.A., Wieloch, T., Cronberg, T., Isshiki, A., Erickson, H.P., and Fassler, R. (2001). Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat. Med. 7, 324-330.
Sarrazin, S., Lamanna, W.C., and Esko, J.D. (2011). Heparan Sulfate Proteoglycans. Cold Spring Harb. Perspect. Biol. 3.
Saunders, W.B., Bohnsack, B.L., Faske, J.B., Anthis, N.J., Bayless, K.J., Hirschi, K.K., and Davis, G.E. (2006). Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J. Cell Biol. 175, 179-191.
Schwarzbauer, J.E. (1991a). Fibronectin: from gene to protein. Curr. Opin. Cell Biol. 3, 786-791.
Schwarzbauer, J.E. (1991b). Identification of the fibronectin sequences required for assembly of a fibrillar matrix. J. Cell Biol. 113, 1463-1473.
Schwarzbauer, J.E., and DeSimone, D.W. (2011). Fibronectins, their fibrillogenesis, and in vivo functions. Cold Spring Harb. Perspect. Biol. 3, a005041.
Schwarzbauer, J.E., Spencer, C.S., and Wilson, C.L. (1989). Selective secretion of alternatively spliced fibronectin variants. J. Cell Biol. 109, 3445-3453.
Sechler, J.L., Rao, H., Cumiskey, A.M., Vega-Col6n, I., Smith, M.S., Murata, T., and
Schwarzbauer, J.E. (2001). A novel fibronectin binding site required for fibronectin fibril growth during matrix assembly. J. Cell Biol. 154, 1081-1088.
Senger, D.R. (1996). Molecular framework for angiogenesis: a complex web of interactions between extravasated plasma proteins and endothelial cell proteins induced by angiogenic cytokines. Am. J. Pathol. 149, 1-7.
Senger, D.R., and Davis, G.E. (2011). Angiogenesis. Cold Spring Harb. Perspect. Biol. 3, a005090.
Senger, D.R., Perruzzi, C.A., Streit, M., Koteliansky, V.E., de Fougerolles, A.R., and Detmar, M. (2002). The al
P
Iand a2 1 Integrins Provide Critical Support for Vascular Endothelial Growth Factor Signaling, Endothelial Cell Migration, and Tumor Angiogenesis. Am. J. Pathol. 160, 195-204.Shalaby, F., Ho, J., Stanford, W.L., Fischer, K.-D., Schuh, A.C., Schwartz, L., Bernstein, A., and Rossant, J. (1997). A Requirement for Flkl in Primitive and Definitive Hematopoiesis and Vasculogenesis. Cell 89, 981-990.
Shibuya, M. (2011). Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis. Genes Cancer 2, 1097-1105.
Sottile, J., and Hocking, D.C. (2002). Fibronectin Polymerization Regulates the Composition and Stability of Extracellular Matrix Fibrils and Cell-Matrix Adhesions. Mol. Biol. Cell 13, 3546-3559.
Stratman, A.N., Malotte, K.M., Mahan, R.D., Davis, M.J., and Davis, G.E. (2009). Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 114, 5091-5101.
Suri, C., Jones, P.F., Patan, S., Bartunkova, S., Maisonpierre, P.C., Davis, S., Sato, T.N., and Yancopoulos, G.D. (1996). Requisite Role of Angiopoietin-1, a Ligand for the TIE2 Receptor,
during Embryonic Angiogenesis. Cell 87,1171-1180.
Tahergorabi, Z., and Khazaei, M. (2012). A Review on Angiogenesis and Its Assays. Iran. J. Basic Med. Sci. 15, 1110-1126.
Takamiya, M., Kumagai, R., Nakayashiki, N., and Aoki, Y. (2006). A study on mRNA
expressions of fibronectin in dermal and cerebral wound healing for wound age estimation. Leg. Med. 8, 214-219.
Tamkun, J.W., and Hynes, R.O. (1983). Plasma fibronectin is synthesized and secreted by hepatocytes. J. Biol. Chem. 258, 4641-4647.
Tan, M.H., Sun, Z., Opitz, S.L., Schmidt, T.E., Peters, J.H., and George, E.L. (2004). Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood 104,
11-18.
Tate, C.C., Garcia, A.J., and LaPlaca, M.C. (2007). Plasma fibronectin is neuroprotective following traumatic brain injury. Exp. Neurol. 207, 13-22.
Turner, C.J., Badu-Nkansah, K., and Hynes, R.O. (2017). Endothelium-derived fibronectin regulates neonatal vascular morphogenesis in an autocrine fashion. Angiogenesis 20, 519-531. Ucuzian, A.A., Gassman, A.A., East, A.T., and Greisler, H.P. (2010). Molecular Mediators of Angiogenesis. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 31, 158.
Vaday, G.G., and Lider, 0. (2000). Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J. Leukoc. Biol. 67, 149-159. Vailh6, B., Vittet, D., and Feige, J.-J. (2001). In Vitro Models of Vasculogenesis and Angiogenesis. Lab. Invest. 81, 439.
Velling, T., Risteli, J., Wennerberg, K., Mosher, D.F., and Johansson, S. (2002). Polymerization of Type I and III Collagens Is Dependent On Fibronectin and Enhanced By Integrins al1$and a2pl. J. Biol. Chem. 277,37377-37381.
Wagenseil, J.E., and Mecham, R.P. (2009). Vascular Extracellular Matrix and Arterial Mechanics. Physiol. Rev. 89, 957-989.
Wang, Y., Reheman, A., Spring, C.M., Kalantari, J., Marshall, A.H., Wolberg, A.S., Gross, P.L., Weitz, J.I., Rand, M.L., Mosher, D.F., et al. (2014). Plasma fibronectin supports hemostasis and regulates thrombosis. J. Clin. Invest. 124, 4281-4293.
Yurchenco, P.D. (2011). Basement Membranes: Cell Scaffoldings and Signaling Platforms. Cold Spring Harb. Perspect. Biol. 3, a004911.
Chapter 2.
Quantitative mass spectrometric analysis of ECM in normal
and EIIIA/B knockout yolk sacs
This chapter was written by Thao Nguyen, with editing by Richard Hynes and Jess Hebert. All experiments in this chapter were performed by Thao Nguyen, except for mass spectrometry, which was performed by Amanda Del Rosario. Violin plot of RNA-seq read distribution and k-means clustering analysis were performed by Carles Boix.
Abstract
The absence of both the EIIA and EIIIB domains of fibronectin (FN) has been shown to negatively affect blood vessel formation and maintenance. Vascular defects have been observed in the yolk sacs of EIIIA/B double-null embryos as early as E9.5 and these defects are likely due to alterations in the extracellular matrix in the absence of the domains. Using an established method to profile the ECM by proteomics, we identified 108 ECM proteins in the yolk sac including known components of the endothelial basement membrane and ECM regulators as well as proteins known to be involved in angiogenesis. We also surveyed the matrix compositions of yolk sacs with different combinations of EIIIA and EIIIB domains and identified a number of proteins with decreased abundance in the double-null yolk sacs. Several of these proteins could be important for yolk sac angiogenesis, and therefore their depletion in the ECM might lead to vascular defects in EIIIA/B double-null yolk sacs.
Introduction
The yolk sac, the membrane that encloses the embryo, is a major site of vasculogenesis, the de novo formation of blood vessels. Blood and endothelial cell precursors (angioblasts) migrate from the mesoderm and aggregate to form blood islands by E7.5 in the yolk sac. Subsequently, the angioblasts on the outer edges of blood islands differentiate into endothelial cells and migrate to form tubes, and blood islands fuse to form a primitive capillary network that extends across the yolk sac by E8.5. After that, to accommodate the expanding yolk sac, this network of small vessels undergoes angiogenesis and rapidly reorganizes to form larger vessels, create branch points, sprout new capillaries and prune unnecessary vessels from existing ones (Goldie et al., 2008; Garcia and Larina, 2014). Numerous soluble effectors and transcription