Publisher’s version / Version de l'éditeur:
Journal of The Electrochemical Society, 129, 1, pp. 85-89, 1982-03-06
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1149/1.2123797
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Transport properties of Nafion® membranes in concentrated solution
environments
Yeager, H. L.; O'Dell, B.; Twardowski, Z.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=9ca50f7e-5776-4103-bdb1-b6cbe37b62dd
https://publications-cnrc.canada.ca/fra/voir/objet/?id=9ca50f7e-5776-4103-bdb1-b6cbe37b62dd
125, 1981 (1978).
6. R. N. Fleck, D. N. Hanson, and C. W. Tobias, L a w - f e n c e R a d i a t i o n Laboratory., B e r k e l e y , CA, UCRL-11612 ( S e p t e m b e r 1964).
7. L. L a p i d u s , " D i g i t a l C o m p u t a t i o n for C h e m i c a l E n - gineers," p. 153, M c G r a w - H i l l , New Y o r k (1962). 8. A. J. A h l b e r g , E. N. Nilson, a n d J. L. Walsh, "The
C H A N G I N G E L E C T R O D E P R O F I L E S 85 T h e o r y of Splines a n d T h e i r Applications," A c a - demic Press, New Y o r k (1967).
9. G. A. P r e n t i c e and C. W. Tobias, AIChE J., In press. 1O. O. Kardos, Plating, 61, 129 (1974).
11. G. A. Prentice, Ph.D. Dissertation, U n i v e r s i t y o f
California, Berkeley, L a w r e n c e B e r k e l e y L a b o r a - tory, LBL-11694 ( D e c e m b e r 1980).
Transport Properties of Nafion R Membranes
in Concentrated Solution Environments
H. I..Yeager,* B. O'Dell, and Z. Twardowski
Department oi Chemistry, University oJ Calgary, Calgary, Alberta, Canada T2N 1N4
A B S T R A C T
The t r a n s p o r t p r o p e r t i e s of p e r f l u o r i n a t e d cation e x c h a n g e m e m b r a n e s in c h l o r - a l k a l i cell solution e n v i r o n m e n t s h a v e been studied using a n e w l y d e - signed m e m b r a n e test cell. A r a d i o t r a c e r - w e i g h t m e t h o d was used to m e a s u r e s o d i u m ion a n d w a t e r t r a n s p o r t n u m b e r s for two Nation| perfluorosulfonate m e m b r a n e s . A homogeneous film shows r a p i d l y decreasing sodium ion t r a n s - p o r t n u m b e r s w i t h i n c r e a s i n g NaOH c a t h o l y t e concentration, r e a c h i n g a v a l u e of 0.56 mol F -1 w i t h 12.5M solution at 80~ A f a b r i c - b a c k e d m a t e r i a l w i t h a s u l f o n a m i d e e x c h a n g e site l a y e r yields c o n s i d e r a b l y h i g h e r values w i t h a m o r e c o m p l i c a t e d c o n c e n t r a t i o n dependence. W a t e r t r a n s p o r t n u m b e r s for the l a t t e r m e m b r a n e a r e also higher; a v a l u e of a b o u t 3 mol F -1 is found for t y p i c a l e x - p e r i m e n t s in w h i c h the a n o l y t e solution is 5M NaC1. Results are discussed in t e r m s of the m e c h a n i s m of ionic m e m b r a n e t r a n s p o r t in r e l a t i o n to p o l y m e r
w a t e r a n d e l e c t r o l y t e sorption. R e c e n t l y we h a v e r e p o r t e d self-diffusion coefficients for s o d i u m ion in s e v e r a l NafionR perfluorosulfonate ion e x c h a n g e m e m b r a n e s in c o n c e n t r a t e d s o d i u m h y - d r o x i d e solutions at e l e v a t e d t e m p e r a t u r e s (1). The d e t e r m i n a t i o n of m e m b r a n e diffusion coefficients is p a r t of an e x p e r i m e n t a l p r o g r a m to p r o v i d e p a r a m - eters for a m u l t i c o m p o n e n t t r a n s p o r t m o d e l of m e m - b r a n e c h l o r - a l k a l i cells (2-4). In a d d i t i o n to m e a s u r e - m e n t s of ionic diffusion and m e m b r a n e w a t e r and e l e c t r o l y t e contents (1), it is also n e c e s s a r y to d e - t e r m i n e the d y n a m i c p r o p e r t i e s of the m e m b r a n e u n d e r conditions t y p i c a l of a n o p e r a t i n g cell. These conditions include c o n c e n t r a t e d solution environments, e l e v a t e d t e m p e r a t u r e s , and high c u r r e n t densities. H e r e we r e p o r t a n e w cell design for m e a s u r e m e n t of the s o d i u m ion a n d w a t e r t r a n s p o r t n u m b e r s in ion e x c h a n g e m e m b r a n e s u n d e r these conditions.
A r a t h e r e x t e n s i v e l i t e r a t u r e exists on the d e t e r - m i n a t i o n of ionic and w a t e r t r a n s p o r t n u m b e r s in ion e x c h a n g e m e m b r a n e s . The m a j o r i t y of this w o r k has b e e n r e v i e w e d b y L a k s h m i n a r a y a n a i a h (5, 6). Two m e t h o d s for ionic t r a n s p o r t m e a s u r e m e n t s are H i t t o r f - t y p e electrolysis e x p e r i m e n t s and i n d i r e c t emf m e t h - ods. In s i m i l a r fashion, m e m b r a n e w a t e r t r a n s p o r t n u m b e r s can b e m e a s u r e d b y electrolysis techniques or b y s t r e a m i n g p o t e n t i a l m e a s u r e m e n t s . Aside f r o m the s y s t e m a t i c discrepancies w h i c h h a v e b e e n o b - s e r v e d b e t w e e n emf and the t r u e electrolysis results (5), the f o r m e r techniques a r e i n a p p r o p r i a t e for this w o r k because w e wish to m e a s u r e m e m b r a n e p r o p - erties u n d e r conditions of high c u r r e n t density. Elec- trolysis m e t h o d s b a s e d on m e a s u r i n g changes in e i t h e r s o l u t i o n v o l u m e o r w e i g h t can be employed. Volume m e t h o d s a r e g e n e r a l l y m o r e convenient, b u t a r e sus- c e p t i b l e to e r r o r s due to m e m b r a n e m o v e m e n t and
a r e difficult to use at e l e v a t e d t e m u e r a t u r e s . S i n h a a n d Bennion (7) h a v e d e t e r m i n e d p o t a s s i u m ion t r a n s p o r t n u m b e r s for a cation e x c h a n g e m e m b r a n e
* Electrochemical Society Active Member.
Key words: current efficiency, ion exchange, electrolysis.
in 2-10M p o t a s s i u m h y d r o x i d e solutions at r o o m t e m - p e r a t u r e . Low c u r r e n t densities, 12 A m - 2 o r l e s s ,
w e r e u s e d a n d results w e r e d e t e r m i n e d b y m e a s u r e - m e n t of solution v o l u m e changes a n d t i t r i m e t r i c analysis. The a v e r a g e r e l a t i v e s t a n d a r d d e v i a t i o n o b - t a i n e d for the p o t a s s i u m ion t r a n s p o r t n u m b e r w a s
6% even for these c a r e f u l l y p e r f o r m e d m e a s u r e m e n t s . The need to create a m e a s u r a b l e c o n c e n t r a t i o n change d u r i n g electrolysis w i t h this a p p r o a c h p r e s e n t s a p a r t i c u l a r p r o b l e m for ionic t r a n s p o r t n u m b e r m e a - s u r e m e n t s in c o n c e n t r a t e d solution e n v i r o n m e n t s . If c o n c e n t r a t i o n changes a r e k e p t s m a l l it is difficult t o o b t a i n sufficient a c c u r a c y in solution a n a l y s i s to ob- t a i n a r e l i a b l e result. If l a r g e r c o n c e n t r a t i o n changes are produced, such m e m b r a n e p r o p e r t i e s as w a t e r a n d e l e c t r o l y t e content are altered. I n the l a t t e r case i n t e r p r e t a t i o n of results becomes difficult. M e a r e s and S u t t o n (8) h a v e d e s c r i b e d an e l a b o r a t e m e t h o d for m e a s u r i n g m e m b r a n e ionic t r a n s p o r t n u m b e r s at con- s t a n t c h e m i c a l composition using r a d i o t r a c e r s . A l - t h o u g h this a p p a r a t u s is not r e a d i l y a d a p t a b l e to the e x p e r i m e n t a l conditions of i n t e r e s t here, it d e m o n - strates t h a t the use of r a d i o t r a c e r s can be effective in l a r g e l y r e m o v i n g the p r o b l e m of c o n c e n t r a t i o n changes in the m e a s u r e m e n t of m e m b r a n e t r a n s p o r t p a r a m - eters. Thus t h e e x p e r i m e n t s which a r e d e s c r i b e d h e r e a r e b a s e d on the r a d i o t r a c e r d e t e r m i n a t i o n of sodium ion t r a n s p o r t n u m b e r s , c o m b i n e d w i t h a w e i g h t m e t h o d for the m e a s u r e m e n t of w a t e r t r a n s - p o r t numbers. Results a r e discussed in t e r m s of the factors w h i c h influence the o v e r a l l t r a n s p o r t c h a r a c - teristics of an ion e x c h a n g e m e m b r a n e in a c h l o r - a l k a l i cell.
Experimental
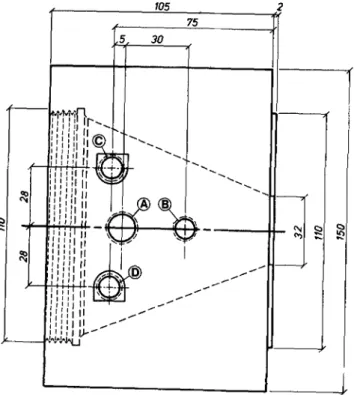
A~paratus.--A d i a g r a m of the m e m b r a n e t r a n s p o r t test cell is shown in Fig. 1 and 2. The c y l i n d r i c a l cell is constructed e n t i r e l y of Teflon, w i t h an o u t s i d e d i a m e t e r of 15 cm. The Teflon s t i r r i n g a s s e m b l y in each h a l f - c e l l is h e l d in p l a c e using a p r e s s - f t b e a r - i n g t h e t h r e a d e d e n d cap. Teflon-coated m a g n e t i c
86 J. Electrochem. Soc.: E L E C T R O C H E M I C A L S C I E N C E A N D T E C H N O L O G Y January 1982
77'
- - t - - i - +
-'-/T
/
CAP MAGNET BEARING STIRRER MEMBRANE STIRRER BEARING MAGNET CAP
Fig. 1, Ion exchange membrane transport cell
s t i r r i n g b a r s p r o v i d e coupling to e x t e r n a l l y p l a c e d m a g n e t s w h i c h are a t t a c h e d to constant speed motors. Solutions a r e s t i r r e d at speeds of 600 r p m to avoid concentration p o l a r i z a t i o n at m e m b r a n e surfaces a n d to hasten t e m p e r a t u r e equilibration. The ion e x c h a n g e m e m b r a n e is p l a c e d b e t w e e n the h a l f - c e l l s using sili- cone r u b b e r gaskets, and the cell a s s e m b l y is t h e n c l a m p e d t o g e t h e r w i t h brass end p l a t e s a n d t h r e a d e d bolts (not s h o w n ) . The i n t e r i o r c o m p a r t m e n t of each h a l f - c e l l is conical in shape; this design allows
105 2
7 ~
Fig. 2. Top view of a half-cell, with dimensions in mm
for e v e n d i s t r i b u t i o n of c u r r e n t lines of flux t h r o u g h the m e m b r a n e , o p t i m u m d r a i n a g e for solution r e - covery, and gas b u b b l e d i s e n g a g e m e n t space above the solution level.
C i r c u l a r p l a t i n u m m e s h electrodes of 6 cm d i a m are used in the cell; p l a t i n u m w i r e leads are fitted t h r o u g h t h r e a d e d Teflon fittings in openings B. The electrodes h a v e c e n t e r holes to a c c o m m o d a t e t h e s t i r r e r shafts. Openings A are used for solution d e - l i v e r y and s a m p l e w i t h d r a w a l . W e i g h e d d r y i n g tubes filled w i t h m o l e c u l a r sieves a r e fitted h e r e d u r i n g the e x p e r i m e n t so t h a t w a t e r lost b y e v a p o r a t i o n can be d e t e r m i n e d . Solutions are h e a t e d w i t h 80W c a r t r i d g e h e a t e r s ( W a t l o w Electric M a n u f a c t u r i n g C o m p a n y ) placed in each h a l f - c e l l ; t e m p e r a t u r e control ( • 1 7 6 is achieved using a p l a t i n u m t e m p e r a t u r e sensor and p r o p o r t i o n a l t e m p e r a t u r e c o n t r o l l e r ( C o l e - P a r m e r I n s t r u m e n t C o m p a n y ) . The h e a t e r s and t e m p e r a t u r e sensor a r e i n s e r t e d into t i t a n i u m t u b u l a r h o l d e r s w h i c h are fitted into openings C a n d D (Fig. 2).
Procedure.--The m e m b r a n e is e q u i l i b r a t e d w i t h a p - p r o p r i a t e solutions at e l e v a t e d t e m p e r a t u r e in t h e cell for up to 24 hr. These solutions are t h e n c a r e f u l l y r e m o v e d b y syphoning and r e p l a c e d w i t h w e i g h e d portions of i d e n t i c a l solutions, a b o u t 330 ml for each half-cell. A f t e r t e m p e r a t u r e e q u i l i b r a t i o n is achieved, the anode c o m p a r t m e n t is doped w i t h sodium-22 r a d i o t r a c e r , an initial w e i g h e d s a m p l e of anode solu- tion is removed, and constant c u r r e n t ( • is initiated. The exposed m e m b r a n e a r e a is 8 cm2; a c u r r e n t of 1.6A is used in all e x p e r i m e n t s to y i e l d a c u r r e n t d e n s i t y of 2 k A m -2. Electrolysis is continued for 1-2 h r u n t i l solution concentrations h a v e changed b y a b o u t 1%. F i n a l l y , anode and cathode solutions are s y p h o n e d and weighed, and samples of cathode s o l u t i o n a r e a n a l y z e d for r a d i o a c t i v i t y and c o m p o s i - tion b y titration. T y p i c a l l y 0.5% of the o r i g i n a l r a d i o - active sodium-22 is t r a n s f e r r e d d u r i n g an e x p e r i m e n t . E x p e r i m e n t s w e r e conducted in w h i c h solutions w e r e i n i t i a l l y w e i g h e d into a d r y cell, and the cell
was disassembled to d e t e r m i n e last traces ( < l g ) of solution left after syphoning. Both procedures yield i d e n t i c a l results, confirming the accuracy of the simpler approach.
The s o d i u m ion t r a n s p o r t n u m b e r , tNa+, is calcu- lated f r o m the weight c o n c e n t r a t i o n a n d radioactiv- ity of the anode solution a n d the total a m o u n t of t r a n s f e r r e d radioactivity. The t r u e sodium ion t r a n s - port n u m b e r describes the n e t a m o u n t of sodium ion t r a n s p o r t e d f r o m the anode to the cathode com- p a r t m e n t ; back-diffusion of sodium ion generates a n e r r o r w h e n u s i n g radiochemical methods, p a r - t i c u l a r l y at low c u r r e n t densities (8). E x p e r i m e n t s w e r e therefore p e r f o r m e d where the cathode solu- tion was doped w i t h r a d i o t r a c e r a n d its appearance i n the anode solution was monitored. Corrections to
tNa+ were less t h a n 0.5%, as w o u l d be expected at a c u r r e n t density of 2 kA m -2.
To calculate the t r a n s p o r t n u m b e r of water, tH~o (mol F - l ) , the weight of w a t e r i n the cathode solu- tion before a n d after electrolysis is d e t e r m i n e d from solution weight concentrations a n d weights. The t r a n s - port n u m b e r is t h e n given by
(AWtH20) F
tH20 -- -~- 1 [1]
18.01 (i,A) (t,sec)
The second t e r m i n Eq. [1] corrects for the c o n s u m p - tion of w a t e r in the cathode reaction. Estimated s t a n - d a r d d e v i a t i o n for tNa+ a n d tH20 are 0.015 a n d 0.5 mol F - l , respectively.
Materials.--Two Nation m e m b r a n e s were studied: 1150 e q u i v a l e n t weight u n b a c k e d film a n d Nation 295. The l a t t e r m a t e r i a l is a n 1150 EW m e m b r a n e which is backed w i t h a n open w e a v e Teflon fabric (70% open a r e a ) , a n d the cathode surface of the m e m b r a n e has b e e n treated w i t h e t h y l e n e d i a m i n e to produce w e a k l y acidic s u l f o n a m i d e exchange sites. Various properties of these m e m b r a n e s in concentrated sodium h y d r o x i d e solution h a v e b e e n reported p r e v i o u s l y (1).
Results and Discussion
S o d i u m ion a n d water t r a n s p o r t n u m b e r s for 1150 EW are listed in Tables I and II. The values i n Table I are those of e x p e r i m e n t s in which identical NaOH solutions were used in anode and cathode c o m p a r t - ments. I n Table II all e x p e r i m e n t s utilized 5M NaC1 as the anode s o l u t i o n while cathode solutions w e r e either
Table I. Sodium ion and water transport numbers for 1150 EW Nation, sodium hydroxide solution
Solution concen-
T e m p e r - ~ration t~a + tH~o
a t u r e (~ (tool 1-D (tool F-D (tool F-D
80 5,0" 0.78 2.4 9.8 0.64 0.5 10.9 0.62 1.2 11.2 0.61 0.6 12.8 0,56 0.5 90 8.0 0.76 3.0 9,8 0.64 0.4 13.0 0.60 0.9
9 Molar concentrations w o r e determined at r o o m temperature.
Table II. Sodium ion and water transport numbers for 1150 EW Nation, sodium chloride and sodium hydroxide solutions
Anode C a t h o d e T e m p e r - s o l u t i o n s o l u t i o n tN~+ t~2o a t u r e (~ (real 1-1) ( t o o l 1 -z) ( m o l F -1) ( m o l F -z ) 70 N a t 1 , 5.0 N a C l , 5.0 0.99 3.6 80 5.0 5.0 0.99 2.8 90 5.0 5.0 1.00 2.3 80 N a t 1 , 5.0 N a O H , 5.0 0.80 2.1 80 5.0 9.5 0.69 1.9 80 5.0 11.0 0.62 1.9 80 ,5.0 12.4 0.58 1.7
5M NaC1 or various concentrations of NaOH. As seen in the tables a n d Fig. 3, tNa+ decreases smoothly with i n - creasing NaOH c o n c e n t r a t i o n for 1150 EW. This would be expected due to i n c r e a s i n g electrolyte sorption into the m e m b r a n e phase. However, it has b e e n observed that a l t h o u g h electrolyte i n v a s i o n does occur for Nation m e m b r a n e s i n concentrated NaOH solutions, the a m o u n t of sorbed electrolyte r e m a i n s r e l a t i v e l y con- stant over the c o n c e n t r a t i o n ranges used in this w o r k (1, 9). M e m b r a n e w a t e r contents for 1100 EW (9) a n d Nation 295 (1) are seen to be g r e a t l y affected though, a n d so the o n l y m a j o r c h a n g e in m e m b r a n e composi- tion is the reduction in w a t e r content w i t h i n c r e a s i n g electrolyte concentration. (This is reflected i n Table I by the g e n e r a l l y lower tH20 values for 1150 EW as NaOH c o n c e n t r a t i o n increases.) Mauritz a n d c o - w o r k - ers suggest t h a t a t u n n e l i n g process of the form
H
N a + O - - H O H - ~ N a + O H - H20 5 - 8 +
would enhance h y d r o x i d e ion t r a n s p o r t i n e n v i r o n - m e n t s of decreasing w a t e r content due to increased polarization of the O - - H b o n d (9). This e x p l a n a t i o n appears reasonable in light of the m e a s u r e d t r a n s p o r t n u m b e r s a n d the r a t h e r u n u s u a l electrolyte sorption characteristics of these m e m b r a n e s .
A n i n t e r e s t i n g aspect of the tSa+ values for 1150 EW in concentrated solution is their r e l a t i v e l y large m a g - nitude. I n general the low c o n c e n t r a t i o n of exchange sites (about 1.0 X 10 -3 m o l / g swollen polymer) in 1150 EW would not be expected to cause sufficient D o n n a n exclusion of electrolyte to allow tNa+ to rise m u c h above 0.5. I n comparison, K r e s s m a n a n d co- workers m e a s u r e d tNa+ for a heterogeneous sulfonate ion exchange m e m b r a n e w i t h m u c h l a r g e r ion e x - change capacity and f o u n d that in concentrated NaOH solution at 25~ tNa+ falls below 0.5 for solution con- c e n t r a t i o n s l a r g e r t h a n 4M (10). We would a t t r i b u t e the difference to the ion clustered morphology of Nation. The ion clusters generate centers of high e x - change site c o n c e n t r a t i o n r a t h e r t h a n a m o r e e v e n d i s t r i b u t i o n t h r o u g h o u t the m e m b r a n e phase as for c o n v e n t i o n a l materials (11). The ion c l u s t e r i n g p h e - n o m e n o n has b e e n shown to produce a v a r i e t y of u n -
m o E i.O ~- 0 . ( ~ - 0 . 8 - - - 0 . 7 - - 7 -F- 0.(5-- 0 . 5 - 0 I I I f I _ i 2 4 6 8 10 12 14 N a O H C o n c e n t r a t i o n , m o l L -T Fig. 3. Sodium ion transport number vs. NaOH catholyte concen- tration. O , 0 : 1 1 5 0 EW membrane; [~, I1: Hafion 295. Anolyte solution is NaOH far light symbols and 5M NaC| for dark symbols.
88 J. Electrochem. Soc.: E L E C T R O C H E M I C A L S C I E N C E A N D T E C H N O L O G Y January 1982
u s u a l t h e r m o d y n a m i c a n d t r a n s p o r t properties for Nation m e m b r a n e s i n dilute solution e n v i r o n m e n t s (12- 15). The tNa+ values of n e a r u n i t y in NaC1/NaC1 systems i n T a b l e II are p a r t i c u l a r l y noteworthy. The m e m b r a n e concentrations of sodium ion and chloride ion i n 1150 EW e q u i l i b r a t e d w i t h 5M NaC1 solution at 80~ are 1.9 • 10 -8 a n d 0.4 X 10-3 mol cm -~, respectively (16), i n d i c a t i n g t h a t while there is b r e a k - d o w n of D o n n a n exclusion a n d s u b s e q u e n t electrolyte sorption, chloride does n o t p a r t i c i p a t e i n conduction of c u r r e n t at a high c u r r e n t density.
The t r a n s p o r t n u m b e r s i n Table I for N a O H / N a O H systems are seen to have little t e m p e r a t u r e d e p e n - dence. A similar r e s u l t was o b t a i n e d for the t e m p e r a - t u r e dependence of self-diffusion of s o d i u m ion i n 1150 EW for 9.5M NaOH solution, where the activation e n e r g y of diffusion f r o m 60 ~ to 90~ was o n l y 2.5 kcal mo1-1 (1).
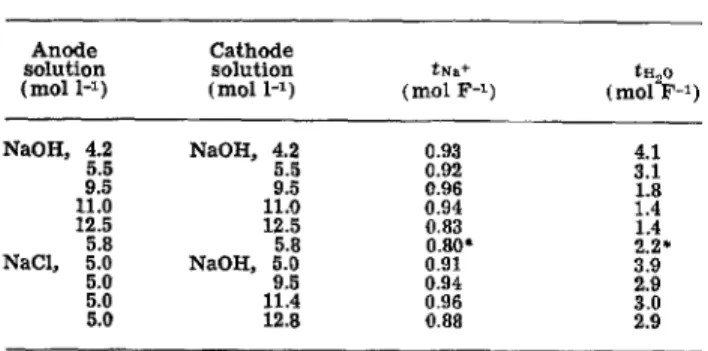
T r a n s p o r t n u m b e r s for Nation 295 are p r e s e n t e d i n Table III for e x p e r i m e n t s using identical NaOH solu- tions a n d for those in which the anode solution was fixed at 5M NaC1. These results m a y be compared to other m e a s u r e m e n t s of the t r a n s p o r t properties of Nation 295. Hora a n d Maloney report a n average caustic c u r r e n t efficiency of 88% for this m e m b r a n e w h e n used i n a chlor-alkali cell (17). Maloney also reports a c u r r e n t efficiency of 93% at 5M NaC1, 10M NaOH concentrations a n d 2 k A m - a c u r r e n t density (18) Berzins m e a s u r e d a w a t e r t r a n s p o r t n u m b e r of 3.2 tool F -1 for 9M NaOH caustic s t r e n g t h w i t h a m e m - b r a n e of the 295 composition b u t without fabric b a c k - ing (19). I n a r e c e n t report, Dotson a n d co-workers (20, 21) m e a s u r e d tNa+ and tH20 for Nation 295 at 85 ~ a n d 1-3 kA m -2 c u r r e n t density. For e x p e r i m e n t s w h e r e identical NaOH c o n c e n t r a t i o n s are used as a n o - lyre a n d catholyte, t h e y report t~2o values f r o m 4.0 to 1.5 over the c o n c e n t r a t i o n range studied in Table III, i n excellent a g r e e m e n t w i t h o u r results. However, their tNa+ values are all about 0.1 mol F - 1 lower t h a n ours, although t h e y observe a peak in tNa+ at about l l M NaOH as seen in Fig. 3. No e x p l a n a t i o n can be offered here for this discrepancy. These workers h a v e observed that the t r a n s p o r t properties of Nation 295 are i n d e - p e n d e n t of c u r r e n t density i n the 1-3 kA m -2 r a n g e (18, 20, 21). Thus we have not varied c u r r e n t density here b u t have focused instead on the influence of other parameters.
As seen in Table III and Fig. 3, the dependence of tNa+ on NaOH c o n c e n t r a t i o n has change d r a m a t i c a l l y w i t h the i n t r o d u c t i o n of the sulfonamide layer on the cathode surface of the m e m b r a n e . At i n t e r m e d i a t e concentrations tNa+ a c t u a l l y increases w i t h increasing c o n c e n t r a t i o n followed b y a rapid decrease as the NaOH cathode solution concentration is raised about 12M. The s u l f o n a m i d e l a y e r is characterized by both reduced w a t e r c o n t e n t a n d sorbed electrolyte a n d thus serves as a b a r r i e r to h y d r o x i d e t r a n s p o r t (I, I7). As discussed b y iVfauritz et al., i n e n v i r o n m e n t s of e x - t r e m e l y low w a t e r Ievels, as would be p r e s e n t i n the s u l f o n a m i d e layer, ion p a i r i n g would reduce the effect
Table III. Sodium ion and water transport numbers, Haflon 295, 80~ Anode Cathode s o l u t i o n s o l u t i o n tNa+ $H20 (tool 1 -z) ( m o l 1 -z ) ( m o l F -1 ) (tool F -1 ) NaOH, 4.2 N a O H , 4.2 0.93 4.1 5.5 5.5 0.92 3.1 9.5 9.5 0.96 1.8 11.0 11.0 0.94 1.4 12.5 12.5 0.83 1.4 5.8 5.8 0.80" 2.2* NaCI, 5.0 N a O H , 5.0 0.91 3,9 5.0 9.5 0.94 2.9 5.0 11.4 0.96 3.0 5.0 12.8 0.88 2.9 * T r e a t e d m e m b r a n e s u r f a c e f a c i n g a n o d e solution. of a p r o t o n t u n n e l i n g m e c h a n i s m of hydroxide t r a n s - port (9). Similar b u t less p r o n o u n c e d i m p r o v e m e n t s in c u r r e n t emciency are seen even w i t h ~ a f i o n com- posite m e m b r a n e s w h e r e the cathode surface of the m e m b r a n e is simply a higher e q u i v a l e n t weight s u l - fonate form, yielding fewer exchange sites (19).
The presence of a m i n i m u m i n the tNa+ plots for Nation 295 c a n be related to the effects of electro- osmosis, in general t~zo is a function of tNa+ and other m e m b r a n e parameters, b u t also s t r o n g l y depends on anolyte c o n c e n t r a t i o n for cation exchange m e m b r a n e s (5, 21). S o d i u m ion, as the m a j o r c u r r e n t carrier, t r a n s - ports net positive a m o u n t s of water across the m e m - brane. The frictional i n t e r a c t i o n b e t w e e n this w a t e r and h y d r o x i d e serves to increase tNa+. K r e s s m a n and Tye provide a general discussion of the effects of elec- troosmosis on cation t r a n s p o r t n u m b e r for cation ex- change m e m b r a n e s i n concentrated solution e n v i r o n - m e n t s (22). T h e y predict a m i n i m u m can be o b t a i n e d in the c o n c e n t r a t i o n dependence of tNa+ for a suffi- ciently large electroosmotic effect, e v e n if anode a n d cathode solutions are of the same concentration. With fixed NaC1 c o n c e n t r a t i o n a n d increasing NaOH con- centrations in the cathode compartment, a n osmotic c o m p o n e n t to water t r a n s p o r t might also be expected. This would serve to increase w a t e r - h y d r o x i d e fric- tional interaction. As seen in Fig. 3, t h e result for Nation 295 is to shift the tNa+ curve to h i g h e r con- centrations of NaOH for the mixed electrolyte e x p e r i - ments. For the 1150 EW m e m b r a n e , where $H20 values are smaller, m u c h smaller differences in tNa+ are seen b e t w e e n the two types of experiment.
A l t h o u g h w a t e r t r a n s p o r t provides a positive con- t r i b u t i o n to t~a+, the presence of large a m o u n t s of w a t e r in a m e m b r a n e serves to favor h y d r o x i d e m i - gration relative to t h a t of sodium ion. These opposing effects lead to c u r r e n t efficiencies g e n e r a l l y lower t h a n 90% for homogeneous Nation materials in h i g h l y con- c e n t r a t e d NaOH solution e n v i r o n m e n t s . Berzins m e a - sured c u r r e n t efficiencies a n d w a t e r t r a n s p o r t n u m b e r s for three Nation m e m b r a n e s in NaC1/NaOH systems at 80~ a n d 8 kA m -2 c u r r e n t density: 0.1 m m thick 1100 EW, 0.05 m m 1500 EW, a n d a composite m e m b r a n e composed of the two homogeneous films (19). The 1100 EW m e m b r a n e shows c u r r e n t efficiencies of 55%, 40%, and 70% at NaOH concentrations of 6M, 10M, a n d 14M, respectively. For the 1500 EW m e m b r a n e , c u r r e n t efficiency steadily declines from 85% to 55% over this c o n c e n t r a t i o n range. The w a t e r t r a n s p o r t n u m b e r i n - creases for 1100 EW from 2 to 3.5 mol F -1 while for 1500 EW it decreases from 3 to 2 mol F -1 with increas- ing NaOH concentration. The performance of the com- posite film is v e r y s i m i l a r to t h a t of the 1500 EW m e m - brane, w i t h the exception of slightly increased w a t e r transport. As predicted b y K r e s s m a n a n d Tye, a m i n i - m u m i n c u r r e n t efficiency is seen for the m e m b r a n e w i t h l a r g e r w a t e r content and, p r o b a b l y due to a n os- motic contribution, the 1100 EW m e m b r a n e a c t u a l l y has a superior c u r r e n t efficiency at highest N a O H c o n - centrations. Thus the overall p e r f o r m a n c e of a homo- geneous m e m b r a n e is the n e t result of complicated interactions a m o n g cation transport, eleetroosmosis, osmosis, a n d the effect of m e m b r a n e w a t e r content on the hydroxide t r a n s p o r t mechanism. We would a t t r i b - ute the higher c u r r e n t efficiency of Nation 295 over the 1100 EW/1500 EW composite m e m b r a n e to the lower acidity of sulfonamide exchange sites, which would r e - duce w a t e r content, e n h a n c e ion pairing, and thus discourage h y d r o x i d e ion transport.
The w a t e r t r a n s p o r t n u m b e r for Nation 295 in NaC1/ NaOH e x p e r i m e n t s is seen to r e m a i n at about 3, i n - d e p e n d e n t of NaOH concentration. This value is similar to t h a t of 1150 EW in 5M NaCI solution. It is i n t e r e s t i n g to note that the sulfonamide layer does not appear to restrict water transport. A n increase in w a t e r concen- tration in the 1150 EW portion of the 295 m e m b r a n e due to electroosmosis m i g h t be responsible for this
T R A N S P O R T P R O P E R T I E S behavior, perhaps resulting in intramembrane pres-
sure gradients (2).
Krishtalik discusses the performance of a hypotheti- cal two layer cation exchange membrane in chior-alkali electrolysis (23). One layer would be highly conduct- ing, but have low selectivity for transport of cations over anions, and the second layer (such as the sulfon- amide layer" in Nation 295) would have opposite prop- ,erties. His treatment yields the result that when the selective layer faces the hydroxide solution, the cur- rent efficiency of the composite membrane would be the same or slightly higher than that of the layer when used alone as a homogeneous film. If the placement of the composite membrane is reversed in the cell, the current efficiency should be somewhat better than us- ing the nonselective film alone, but much lower than that of the composite membrane in the opposite place- ment. One experiment was performed here for Nation 295 in NaOH solutions, in which the sulfonamide layer faced the anode compartment. As predicted, the tNa+ value of 0.80 is marginally higher than the 0.78 re- sult for 1150 EW, but much smaller than the value of 0.92 when the membrane is used in the opposite con- figuration.
In conclusion, the current efficiency of a chlor- alkali membrane cell is seen to be a complicated func- tion of several membrane and solution parameters. These interactions become more complex in composite membranes. General features which lead to high cur- rent efficiencies are large ion exchange capacity to en- hance cation diffusion and reject electrolyte sorption, low water content to prevent hydroxide migration by a tunneling effect, and osmotic or electroosmotic water transport to increase the frictional interaction between water and hydroxide ion. The optimization of mem- brane performance therefore depends on the difficult task of simultaneously improving each of these prop- erties.
Acknowledgments
Dr. R. L. Dotson of Olin Chemicals provided valu- able design assistance and Mr. Fred Jarvis supervised construction of the membrane transport cell. N. Tyrer and B. Kipling performed some experimental portions of this work. The authors thank E. I. du Pont de Nemours and Company for providing membrane samples. Financial support for this research was pro- vided by the Olin Corporation, Chemicals Division, Southeast Process Technology, Electrochemical Tech- nology Group.
Manuscript submitted March 6, 1981; revised manu- script received ca. July 1, 1981. This was Paper 394 pre- sented at the St. Louis, Missouri, Meeting of the So- ciety, May 11-16, 1980.
Any discussion of this paper will appear in a Discus- sion Section to be published in the December 1982 JOURNAL. All discussions for the December 1982 Discus- sion Section should be submitted by Aug. 1, 1982.
Publication costs o] this article were assisted by The University o] Calgary.
REFERENCES
1. H. L. Yeager, B. Kipling, and R. L. Dotson, This Journal, 1~7, 303 (1~80).
2. D. N. Bennion, R. L. Dotson, and J. M. Ford, Paper 394 presented at The Electrochemical Society Meeting, St. Louis, MO, May 11-16, 1980.
3. R. L. Dotson, H. L. Yeager, and J. M. Ford, Paper 395 presented at The Electrochemical Society Meeting, St. Louis, MO, May 11-16, 1980.
4. J. M. Forct and R. L. Dotson, Paper 396 presented at The Electrochemical Society Meeting, St. Louis, MO, May 11-16, 1980.
5. N. Lakshminarayanaiah, "Transport Phenomena in Membranes," Chap. 5, Academic Press, New York
(1969).
6. N. Lakshminarayanaiah, This Journal, 116, 338 (1969).
7. M. Sinha and D. N. Bennion, ibid., 125, 556 (1978). 8. P. Meares and A. H. Sutton, J. Colloid Interlace
Sci., 28, 118 (1968).
9. K. A. Mauritz. K. J. Branchick, C. L. Gray, and S. R. Lowry, Polym. Prep. Am. Chem. Soc., Div. Polym. Chem., 20, 122 (1980).
10. T. R. E. Kressman, P. A. Stanbridge, and F. L. Tye,
Trans. Faraday Soc,. 59, 2139 (1963).
11. S. C. Yeo and A. Eisenberg, J. Appl. Polym. Sci., 21, 875 (1977).
12. H. L. Yeager and B. Kipling, J. Phys. Chem., 83, 1836 (1979).
13. H. L. Yeager and A. Steck, Anal. Chem., 51, 862 (1979).
14. A. Steck and H. L. Yeager, ibid., 52, 1215 (1980). 15. H. L. Yeager and A. Steck, in "Proceedings of the
Symposium on Ion Exchange," R. S. Yeo, Editor, The Electrochemical Society Softbound Proceed- ings Series, Pennington, NJ (1981).
16. Unpublished results.
17, C. J. Hora and D. E. Maloney, Paper 441 presented at The Electrochemical Society Meeting, Atlanta, GA, Oct. 9-14, 1977.
18. D. E. Maloney, Paper presented at the Symposium on Advances in Chlor-Alkali Technology, Society of Chemical Industry, London, England, June 13- 15, 1979.
19. T. Berzins, Paper presented at the 71st Annual Meeting. American Institute of Chemical Engi- neers, Miami Beach, FL, Nov. 12-16, 1978. 20. R. L. Dotson, R. W. Lynch, and G. E. Hilliard, Paper
611 presented at The Electrochemical Society Meeting, Hollywood, FL, Oct. 5-10, 1980.
21. R. L. Do,tson, R. W. Lynch, and G. E. Hilliard, in "Proceedings of the Symposium on Ion Ex- change," R. S. Yeo, Editor, The Electrochemical Society Softbound P~oceedings Series, Penning- ton, NJ (1981).
22. T. R. E. Kressman and F. L. Tye, Trans. Faraday Soc., 55, 1441 (1959).