9 Springer-Verlag 2000 Printed in Austria
Atomic-force microscopy imaging of plasma membranes purified
from spinach leaves
M. Cr~vecoenr 1'*, E. Lesniewska 2, V. Vi~ 2, J. P. Goudonnet:, H. Greppin 1, and C. Le Grimellec 3
1 Physiologie et Biochimie V6g6tales, Universit6 de Gen~ve, Geneva, 2 Laboratoire de Physique, CNRS 5027, Universit6 de Bourgogne, Dijon, and 3 Centre de Biochimie Structurale, INSERM U414, Universit6 de Montpellier, Montpellier
Received August 18, 1999 Accepted December 6, 1999
Summary.
Plasma membranes purified from spinach leaves by aqueous two-phase partitioning were examined by atomic-force microscopy (AFM) in phosphate buffer, and details on their struc- ture were reported at nanometric scale. Examination of the fresh membrane preparation deposited on mica revealed a complex orga- nization of the surface. It appeared composed of a first layer of mate- rial, about 8 nm in thickness, that practically covered all the mica surface and on which stand structures highly heterogeneous in shape and size. High-resolution imaging showed that the surface of the first layer appeared relatively smooth in some regions, whereas different characteristic features were observed in other regions. They con- sisted of globular-to-elliptical protruding particles of various sizes, from 4-5 nm x-y size for the smallest to 40-70 nm for the largest, and of channel-like structures 25-30 nm in diameter with a central hole. Macromolecular assemblies of protruding particles of various shapes were imaged. Addition of the proteolytic enzyme pronase led to a net roughness decrease in regions covered with particles, indi- cating their proteinaceous nature. The results open fascinating per- spectives in the investigation of membrane surfaces in plant cells with the possibility to get structural information at the nanometric range.Keywords:
Atomic-force microscopy; Plasmalemma; Phase parti- tion; Leaf cells; Spinach.Abbreviations:
AFM atomic-force microscopy; EM electron microscopy; TMAFM tapping-mode atomic-force microscopy.Introduction
I n plants the p l a s m a m e m b r a n e , the o u t e r p e r m e a b i l - ity b a r r i e r o f the cell, has a n u m b e r o f essential phys- iological functions. T h e s e include t r a n s p o r t of ions a n d o t h e r solutes o u t a n d into the cells, h o r m o n e b i n d i n g
* Correspondence and reprints: Physiologie et Biochimie Vdgdtales, Universit6 de Gen6ve, 3 Place de l'Universit6, CH-1211 Gen6ve 4, Switzerland.
a n d response, synthesis a n d a s s e m b l y of cell wall m a t e - rials. T h e p l a s m a m e m b r a n e plays an i m p o r t a n t role in interactions with p a t h o g e n s a n d in p e r c e p t i o n of external e n v i r o n m e n t a l signals with their s u b s e q u e n t transmission into the cells, w h e r e a c a s c a d e of events is t r i g g e r e d ( L e s h e m 1992, L e b r u n - G a r c i a et al. 1999). A k e y role has also b e e n p r o p o s e d for this m e m b r a n e in s o m e g r o w t h a n d d e v e l o p m e n t p r o c e s s e s ( G r e p p i n et al. 1991, L e s h e m 1992, M a s s o n et al. 1994). K n o w l - edge of the structural o r g a n i z a t i o n of the p l a s m a m e m b r a n e is a f u n d a m e n t a l r e c o g n i z e d step in u n d e r - standing its different cellular functions in relation with such processes. P a r t o f this k n o w l e d g e has b e e n a c q u i r e d b y c u r r e n t e l e c t r o n m i c r o s c o p y ( E M ) which, unfortunately, does n o t allow the o b s e r v a t i o n of native m e m b r a n e s . Biophysical m e t h o d s h a v e p r o v e d to be also useful f o r a clearer u n d e r s t a n d i n g of o r g a n i z a t i o n and d y n a m i c s of t h e p l a s m a m e m b r a n e ( Q u i n n a n d Williams 1990, L e s h e m 1992).
T h e a t o m i c - f o r c e m i c r o s c o p e ( A F M ) , i n v e n t e d b y Binnig et al. (1986), allows t h e surface o f biological samples to be i m a g e d at high r e s o l u t i o n u n d e r physi- ological c o n d i t i o n s a n d has b e e n used to i m a g e a wide v a r i e t y o f biological samples, a m o n g t h e m h a v e b e e n m e m b r a n e s , b o t h native a n d reconstituted. It is actu- ally c o n s i d e r e d as a p o w e r f u l tool f o r the study of m e m b r a n e surfaces with r e p r o d u c i b i l i t y a n d with a lateral r e s o l u t i o n o f a b o u t 0.5 n m a n d an extraordi- n a r y vertical r e s o l u t i o n o f a b o u t 0.1 n m ( D a n k e r et al. 1997, L e s n i e w s k a et al. 1998, MiJller et al. 1999). This is m u c h higher t h a n that of light m i c r o s c o p e s a n d c o m p a r a b l e with e l e c t r o n microscopes, with the great
a d v a n t a g e to o b s e r v e samples in b u f f e r w i t h o u t par- ticular p r e p a r a t i o n (Lal a n d J o h n 1994, S h a o et al. 1996). M e m b r a n e surfaces of different intact cells h a v e b e e n directly e x a m i n e d b y A F M in b u f f e r or in their g r o w t h m e d i u m ( B u t t et al. 1990a, 1991; H e n d e r s o n et al. 1992; Hfiberle et al. 1991; H 6 r b e r et al. 1992; L e G r i m e l l e c et al. 1994; O b e r l e i t h n e r et al. 1997). T h e A F M has b e e n u s e d to i m a g e t h e surface t o p o g r a p h y of n a t u r a l a n d r e c o n s t i t u t e d t w o - d i m e n s i o n a l crystals of m e m b r a n e p r o t e i n s such as gap junctions, b a c t e - r i o r h o d o p s i n , c h o l e r a toxin, a n d Escherichia coli p o r i n in a q u e o u s c o n d i t i o n s w i t h o u t fixation ( B u t t et al. 1990b, 1991; H o h et al. 1991, 1993; S c h a b e r t et al. 1995; H e y m a n n et al. 1997). I m a g e s o f isolated biological m e m b r a n e s h a v e b e e n r e p o r t e d for p l a s m a m e m b r a n e ( L e G r i m e l l e c et al. 1995, Lfirmer et al. 1997) a n d n u c l e a r e n v e l o p e ( D a n k e r et al. 1997, R a k o w s k a et al. 1998). So far, h o w e v e r , in spite o f t h e g r e a t qualities a n d potentialities o f f e r e d b y A F M to investigate native m e m b r a n e surfaces, it does n o t a p p e a r to h a v e b e e n u s e d f o r h i g h - r e s o l u t i o n i m a g i n g o f t h e p l a s m a m e m b r a n e in p l a n t cells. I n plants, this m e m b r a n e is accessible with difficulty d u e to t h e p r e s e n c e o f the cell wall. P a r t o f o u r k n o w l e d g e o f fine s t r u c t u r e o f this m e m b r a n e c o m e s f r o m studies with p r o t o p l a s t s t h a t are r o u t i n e l y o b t a i n e d f r o m a g r e a t v a r i e t y of plant tissues ( F o w k e 1986, 1988; F o w k e et al. 1986; L e s h e m 1992). A n o t h e r a p p r o a c h consists in p r e p a r i n g a highly purified fraction o f this m e m b r a n e , with p a r t i t i o n in a q u e o u s d e x t r a n - p o l y e t h y l e n e glycol ( P E G ) two- p h a s e systems as t h e m o s t c o m m o n m e t h o d used. This p r o c e d u r e s e p a r a t e s t h e m e m b r a n e vesicles a c c o r d i n g to their surface properties. T h e p l a s m a m e m b r a n e - d e r i v e d vesicles are p r e f e r e n t i a l l y f o u n d in t h e P E G - rich u p p e r p h a s e a n d are essentially c y t o p l a s m i c side-in ( K 6 r n e r et al. 1985, L a r s s o n et al. 1987, Sand- s t r o m et al. 1987, Sandelius a n d M o r r 6 1990). Two- p h a s e p a r t i t i o n i n g is r o u t i n e l y u s e d to p u r i f y the p l a s m a m e m b r a n e of spinach leaf cells in view of its b i o c h e m i c a l , structural, a n d f u n c t i o n a l c h a r a c t e r i z a - tion in t h e c o u r s e o f g r o w t h a n d flowering ( P e n e l et al. 1988, Crespi et al. 1989, G r e p p i n et al. 1991). P a r t of o u r structural i n f o r m a t i o n was a c q u i r e d b y classical E M , with o b s e r v a t i o n s of u l t r a t h i n sections p e r f o r m e d either in p l a s m a m e m b r a n e p r e p a r a t i o n s o r in leaves. O u r objective in this p a p e r is to exploit the t r e m e n - d o u s potentialities of t h e A F M to go f u r t h e r in o u r k n o w l e d g e o f this m e m b r a n e n a m e l y its t o p o g r a p h i c a l o r g a n i z a t i o n , u n d e r physiological conditions, w i t h o u t p a r t i c u l a r p r e p a r a t i o n so crucial in E M . We focus o u r
a t t e n t i o n o n the d e t e r m i n a t i o n o f t h e c o n d i t i o n s for e x p l o r i n g in b u f f e r the surface o f purified p l a s m a m e m b r a n e . O u r results d e m o n s t r a t e t h a t f e a t u r e s w e r e o b s e r v e d on the p l a s m a m e m b r a n e surface that w e r e c o m p a r a b l e to those d e s c r i b e d b y A F M o n t h e surface o f p l a s m a m e m b r a n e s f r o m o t h e r biological samples.
Material and methods
Plant material
Spinach plants (Spinacia oleracea L. cv. Nobel; Samen Mauser, Winterthur, Switzerland) were grown four weeks in a growth chamber with short-day illumination (8 h daily). The temperature was set at 23 + 0.5 ~ and the relative humidity was maintained at 80% + 10% during the day and 60% + 10% during the night. Light (400 gmol/m 2. s) was provided by white fluorescent tubes (22432-0; Sylvania, Danvers, Mass., U.S.A.).
Plasma membrane isolation
Isolation of pure plasma membrane from spinach leaves was per- formed by phase partitioning in an aqueous polymer two-phase system (Kjellbom and Larsson 1984), from a crude extract prepared as follows. Leaves (20 g) were homogenized in 80 ml of medium containing 50 mM HEPES (pH 7.5), 500 mM sucrose, 10 mM KC1, i mM MgCI2, and 10 mM ascorbic acid. After a centrifugation at low speed (6,000 g, 10 rain), membranes were collected by high-speed centrifugation (30,000 g, 30 rain). The crude membrane pellet was used for phase partitioning and the upper fraction obtained at the end of the partition was centrifuged at 30,000 g for 30 rain. The pellet was suspended in 5 mM phosphate buffer (pH 7.5) to which vitamin C (10 raM) was added. The purity of the fraction was routinely checked by EM analyses, on the basis of a morphological and a cytochemical marker to verify its enrichment in plasma membrane vesicles. Membrane thickness was determined on film negatives of vesicles photographed at x25,000, with the soft Imagenia of a Biocom image analyzer system (Les Ulis, France). Plasma mem- branes were also identified on the basis of phosphotungstic acid (PTA) staining of sections at low pH as previously reported (Perroud et al. 1997).
AFM imaging
Protein content of the plasma membrane fraction was appreciated by the Bio-Rad micro-assay (Bio-Rad Laboratories, Munich, Federal Republic of Germany) based on the Bradford method (Bradford 1976). Aliquots of 25 gl corresponding to various protein concentrations were applied for 10 rain to the surface of freshly cleaved muscovite green mica (New York Mica Co., New York, N.Y., U.S.A.). The samples were then washed three times with buffer to remove membranes that were not firmly attached to the substrate and they were imaged with a Nanoscope III AFM (Digital Instru- ments Inc., Santa Barbara, Calif., U.S.A.) equipped with a D type scanner (12 gm). Few preparations were observed in air, most of them being imaged in buffer solution. Various V-shaped silicon nitride cantilevers (Park Scientific Instruments, Sunnyvale, Calif., U.S.A.) with spring constant k equal to 0.01 to 0.06 N/m, are mounted on a bimorph which could be modulated normally to the sample surface. The cantilever spring constant k and its resonant fre- quency v are related by k = 2. (7~vL) 3
W(D3/E) 1/2,
where E and W arethe length and the width of the cantilevers, E is the elastic modulus in the vertical direction, and p the density of the materiai of the can- tilever. In order to remove the contaminants, the tips were exposed to UV ozone for 10 min. The UV-ozone cleaning permits the removal of the hydrocarbons. In contact mode the force was previ- ously adjusted for each scan image at the lowest possible value (i.e., about 20 pN). The measured forces after imaging never exceeded 100pN. In oscillating-contact mode (i.e., tapping-mode AFM, TMAFM), the resonance fl'equency for cantilevers of 10 mN/m spring constant was locked at 29 kHz. According to the experiment, the driven amplitude varied between 1 and 5 nm and corresponded to an energy of 5 to 125 pJ. Such low amplitude corresponds to the amplitude used in MAC mode AFM which senses piconewton forces (Molecular Imaging, Phoenix, Ariz., U.S.A.). The setpoint was adjusted such that the damping represented less than 20% of the driven amplitude. All reported images were made with 512 by 512 pixets definition with typical scan rates of 1-5 Hz. No striking dif- ference between height images in contact and oscillating-contact modes were recorded. Images of the local viscoelastic properties of the membranes can be obtained in the contact mode from force versus distance curves (Weisenhorm et al. 1993) or by force modu- lation (Radmacher et al. 1992). In force modulation mode, we have recorded the amplitude and the phase of the oscillation signal cor- responding in first approximation to the local elasticity and local vis- cosity. In this case, the resonance frequency was locked at 8 kHz. The setpoint was adjusted, from force plots, such that the variation in amplitude for imaging represented less than 5 nm. Amplitude and phase images have been obtained simultaneously with the topo- graphical images. These images can provide information on the lateral heterogeneity in membranes by investigating the local vis- coelastic properties,
Results
Electron microscopy results (data not shown) indicated that most m e m b r a n e s in our m e m b r a n e preparations were 8 nm thick with a typical clear dark- light-dark pattern. In addition the bulk of the vesicles were stained with phosphotungstic acid at low pH. The morphological and cytochemical results indicated that our preparation was c o m p o s e d by 90 to 95% of vesi- cles derived from plasma m e m b r a n e with minor con- taminating membranes, as in previous experiments (Crespi et al. 1989, P e r r o u d et al. 1997).
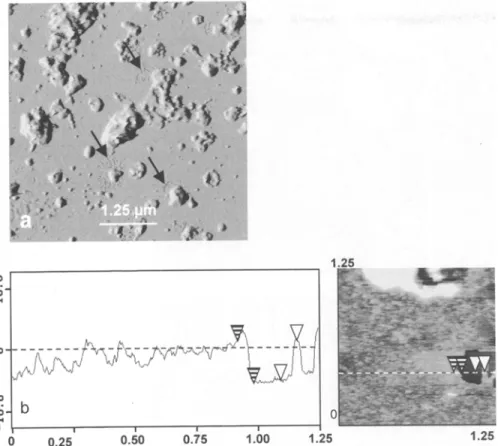
In our first A F M assay, plasma m e m b r a n e vesicles at a protein concentration of 25 gg/ml were adsorbed to freshly cleaved mica and observed in buffer. In these conditions it was very difficult to find a region of the mica surface uncovered by sample. In view of this surprising covering of the mica surface, m e m b r a n e s were imaged either in air at the same protein concen- tration or in buffer at lower protein concentrations ranging from 20 to 5 gg/ml. A typical overview of a plasma m e m b r a n e (25 ~tg of protein per ml) imaged in air is shown in Fig. I a. Scanning of large zones (10 by 10 ~tm) revealed a complex organization of the surface of the m e m b r a n e sample with a first layer of material that practically covered all the mica surface. However,
zones were found in which the underlying mica was imaged (Fig. i a). The same observation was m a d e for m e m b r a n e s observed in buffer at protein concentra- tions of 5 to 10 ~tg/ml (not shown). Estimates of the apparent thickness of the first layer from the analysis of different sections through such regions where the mica was observed gave values of 6.1 + 0.5 nm for m e m b r a n e s imaged in air (Fig. 1 b) and of 8.2 + 0.5 n m for membranes imaged in buffer independently of the protein concentration. As in air, the organization of the sample surface was heterogeneous at low resolu- tion when the m e m b r a n e preparation was imaged at a protein concentration of 25 gg/ml under phosphate buffer. A first layer covering most of the mica surface was observed, on which stand structures highly het- erogeneous in shape and size, corresponding likely to m e m b r a n e sheets and vesicles, with the presence of both flat and smooth areas.
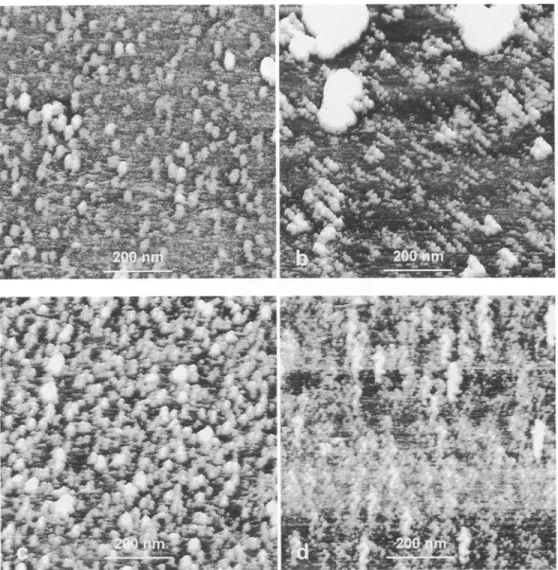
Using smaller scans, the surface of the first layer a p p e a r e d relatively smooth in some regions and in other regions covered by different structures as shown in Fig. 2. Some areas were covered exclusively by globular-to-elliptical structures which were heteroge- neous in size and emerged from the sample surface (Fig. 2 a). The x-y size of these protrusions ranged from 5 n m for the smallest to 70 nm for the largest. Some of the largest protruding particles appeared associated (Fig. 2b). Aggregates of smaller protrusions were also imaged in some areas as well as a distribution of pro- truding particles along lines (Fig. 2c). Some of these arrangements, which were repeatedly observed on dif- ferent samples and with different A F M tips, looked like hippocampus (Fig. 2 d). It is n o t e w o r t h y that the direction of these alignments was different from that of the sCanning direction and independent of the A F M probe.
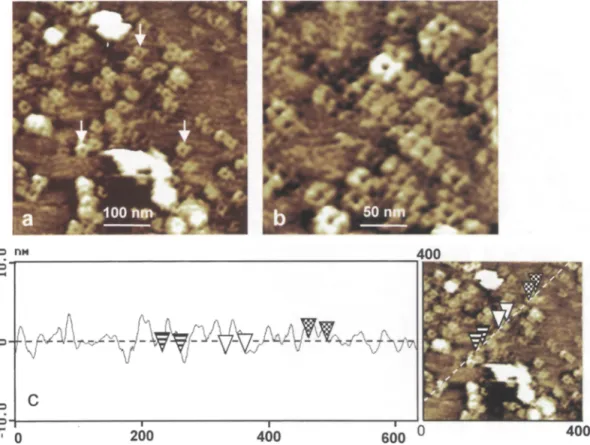
In order to prove that the A F M tip does not induce important modification on the observed structures, we have scanned some area s continuously. In this case, the force applied was adjusted before the first recorded image. It was observed that repetitive scanning of the same area of the preparation every 5 min for 30 min did not create new structures and did not cause notice- able distortion or damage to the sample (Fig. 3 a-c). However, "flip-flop" of some proteins could occur under the applied pressure. In order to get some infor- mation as to the chemical nature of the protruding particles, m e m b r a n e s at a concentration of 25 gg/ml were incubated, at r o o m temperature, in the presence of the proteolytic enzyme pronase and then adsorbed on mica. Such a treatment however resulted in the
Fig. 1. a Imaging in air at low magnification (10 by 10 gm) of plasma membranes from spinach leaves by oscillating-contact mode AFM (TMAFM; deflection mode; scan size, 10 gm; scan rate, 2 Hz; Z range, 0.05 nm). b Section analysis showing the thickness of plasma mem- branes imaged in air. Vertical distances between the pairs of striped and open arrowheads were respectively 6.1 and 6.01 nm
d e t a c h m e n t of the sample f r o m the mica surface during scanning. T h e r e f o r e the e n z y m e was added in situ to the sample already imaged. Scanning of the s a m e m e m b r a n e areas b e f o r e and 30 rain after p r o n a s e t r e a t m e n t resulted in a net decrease of rough- ness of the m e m b r a n e surface in regions c o v e r e d with particles (Fig. 3 d).
In addition to protruding particles as those in Figs. 2 and 3, the p r e s e n c e of channel-like structures was o b s e r v e d in s o m e locations of the samples (Fig. 4). T h e y w e r e frequently found in close proximity to large protruding structures either isolated or aggregated (Fig. 4a). 250 by 250 n m scans indicated that they a p p e a r e d in m o s t cases c o m p o s e d of 4 subnnits dis- p o s e d a r o u n d a central hole or depression (Fig. 4b). T h e d i a m e t e r of channel structures r a n g e d f r o m 25 to 30 n m (Fig. 4 c) and that of their central hole was a b o u t 5 nm, estimated f r o m section analysis.
It has to be p o i n t e d out that the surface of mica on which buffer alone or P E G in buffer were a d s o r b e d a p p e a r e d s m o o t h (roughness less than 1 nm) for similar scan sizes as those used for scanning of m e m - branes. M o r e o v e r , the different features i m a g e d w e r e
r e p e a t e d l y found u p o n scanning of p l a s m a m e m b r a n e s purified f r o m different batches of leaves and with two different A F M equipments.
M e m b r a n e samples were in s o m e cases k e p t at 4 ~ in buffer to which vitamin C was added, for o n e or two days b e f o r e their adsorption on mica. For such samples, the f o r m a t i o n of two distinct zones, a s m o o t h and a rough surface on which stand globular structures and channel-like structures, was o b s e r v e d (Fig. 5). For such regions the force m o d u l a t i o n m o d e was used and b o t h p h a s e and topographical signals are given in Fig. 5. The image of the local viscosity (Fig. 5 b ) shows the p h a s e separation on the m e m b r a n e , and the t o p o g r a p h i c image (Fig. 5 a) shows the existence of m a c r o d o m a i n s in the m i c r o m e t e r range which are e m b e d d e d in a d a r k e r matrix. The step height m e a - sured b e t w e e n light domains and the matrix is 0.8 + 0.1 nm. The s a m e o b s e r v a t i o n was m a d e for p l a s m a m e m - b r a n e s scanned next day after their adsorption on mica on which they w e r e k e p t at r o o m t e m p e r a t u r e in buffer. A F M images of m e m b r a n e s k e p t at - 8 0 ~ until their e x a m i n a t i o n in buffer w e r e of c o m p a r a - tively lower quality than those of fresh m e m b r a n e s .
Fig. 2. a Submicron scans of the surface of fresh plasma membranes from spinach leaves in phosphate buffer. Globular structures of various sizes were observed, b-d AFM images illustrating different arrangements of protruding particles on the plasma membrane surface: align- ments and aggregates (b and e) and hippocampus-like structures (d) observed independently of the AFM probe and the scanning direc- tion. (TMAFM; scan size, 800 rim; scan rate, 2 Hz; Z range, 15 nm)
D i s c u s s i o n
Since its a p p e a r a n c e in 1986 (Binnig et al. 1986), the A F M has p r o v e n to be an excellent tool to investi- gate structures of m a n y different biological samples ( R a t n e s h w a r and Scott 1994, Shao et al. 1996). It has d e m o n s t r a t e d its ability to image o v e r a wide range of lateral magnification down to the molecular level, in aqueous e n v i r o n m e n t and without sample p r e p a r a t i o n technique so crucial in EM. Cell biologists have exploited these r e m a r k a b l e properties of the A F M to study m e m b r a n e proteins and m e m b r a n e structure. High-resolution imaging of different m e m b r a n e pro- teins such as gap junctions and b a c t e r i o r h o d o p s i n has b e e n r e p o r t e d which c o m p l e m e n t s other structural
results o b t a i n e d by negative staining and X-ray crys- tallography (Butt et al. 1990b; H o h et al. 1991; Lal et al. 1993; Mtfller et al. 1995, 1999). I n f o r m a t i o n on the p l a s m a m e m b r a n e has also b e e n o b t a i n e d by direct imaging of the surface of intact cells in buffer or in their growth m e d i u m , with some features in good a g r e e m e n t again with those o b t a i n e d by alternative techniques (Lal and John 1994, Lesniewska et al. 1998). So far h o w e v e r there are only a few reports on the use of A F M to study plant cells. I m a g e s have b e e n r e p o r t e d for cell wall materials isolated f r o m the p a r e n c h y m a of different tissues (Kirby et al. 1996) and for pollen grains and cellulose microfibrills (van der Wel et al. 1996). The lower side of leaves f r o m a small Indian tree and f r o m the w a t e r lily
Nymphaea odorata
Fig.3. a-c Three successive images of
the same area of the plasma membrane imaged in buffer and recorded after 5, 15, and 30rain. No drastical topographical modification between scans is noticed (TMAFM; scan size, 500 nm; scan rate, 2 Hz; Z range, 20 nm). d Image of the same region of the plasma membrane after pronase (0.25% in phosphate buffer) treat- ment. The mean roughness is decreased by a factor of 2 to 3 (TMAFM; scan size, 500 nm; scan rate, 0.8 Hz; Z range, 20 nm). By using the contact mode AFM we can remove partially the plasma membrane and image the mica surface
has b e e n o b s e r v e d u n d e r w a t e r but no details below 200 n m were resolved (Butt et al. 1990a).
T h e A F M images we described h e r e r e p r e s e n t the first ones that are c o n c e r n e d with the p l a s m a m e m - b r a n e f r o m plant tissues. This m e m b r a n e was purified by p h a s e partition, a very current p r o c e d u r e used for its isolation and the fraction o b t a i n e d was of high purity as c h e c k e d by EM.
T h e m e a s u r e d thickness of o u r m e m b r a n e on mica is a b o u t 6 n m for dry samples and 8 n m for samples e x a m i n e d in buffer. These values were in the s a m e range as those r e p o r t e d for s o m e other biological m e m b r a n e s visualized by A F M and c o r r e s p o n d to a single m e m b r a n e layer. T h e minimal a p p a r e n t thick- ness of p l a s m a m e m b r a n e s f r o m M a d i n - D a r b y canine kidney ( M D C K ) cells grown on glass coverslips gave values close to 6 n m for dried samples and close to 8 n m for living samples in buffer, b o t h values o b t a i n e d in the flattest zones (Le G r i m e l l e c et al. 1995). A similar value of 8 n m was r e p o r t e d for the a v e r a g e thickness of basal cell m e m b r a n e s of M D C K cells pre- p a r e d by a lysis-squirting p r o t o c o l and imaged in buffer (Ziegler et al. 1998). T h e a p p a r e n t height of the
purple m e m b r a n e e x a m i n e d by A F M in buffer was found to vary f r o m 5.6 n m to 11 nm, depending on the p H of the buffer solution (Butt et al. 1990b, 1991; Miiller et al. 1995). The surfaces imaged in the p r e s e n t study m o s t p r o b a b l y c o r r e s p o n d to the extracellular surface of the m e m b r a n e as m o s t vesicles collected f r o m the u p p e r p h a s e of a two-phase system are mainly or exclusively in a cytoplasmic-side-in orienta- tion (Sandelius and Morr6 1990). It is likely that u p o n deposition on mica vesicles o p e n e d and m a i n t a i n e d this orientation as r e p o r t e d for phospholipid vesicles containing proteins (Contino et al. 1994, Salafsky et al. 1996).
S u b m i c r o m e t e r scanning of p l a s m a m e m b r a n e p r e p a r a t i o n s in buffer revealed the p r e s e n c e of repro- ducible features, n a m e l y protruding particles and channel-like structures. The particles occupy the m a j o r p a r t of the surface scanned and are h e t e r o g e n e o u s in size. Their minimal size was a p p r o x i m a t e l y 15 to 20 n m in air and 4 to 5 n m in buffer, indicating a g r e a t e r resolution u p o n imaging in liquid m e d i u m .
Protruding particles of different sizes have b e e n described on different m e m b r a n e surfaces visualized
Fig. 4a-e. Imaging at low magnification of plasma m e m b r a n e s from spinach leaves under phosphate buffer, a 500 n m scan of the mem- brane surface to show the presence of channel-like structures (arrows) and a few globular structures (TMAFM; scan size, 500 nm; scan rate, 1 Hz; Z range, 20 nm). b 250 n m scan of the m e m b r a n e surface showing channel-like structures with a central depression (TMAFM; scan size, 250 nm; scan rate, 1 Hz; Z range, 20 nm). c Section analysis profile through a channel-like structure. Horizontal distances b e t w e e n the right and middle pairs of arrowheads were respectively 25.5 and 29.4 n m
Fig. 5a, b. Imaging at low magnification of plasma membranes from spinach leaves under buffer showing the formation of smooth sur- faces (arrow) in the proximity of regions covered with particles (force modulation mode; scan size, 1.6 rim; scan rate, 1 Hz; Z range, 50 nm; phase, 10~ a Topography, b viscosity
by AFM and were observed on both cytoplasmic
(Le Grimellec et al. 1995, Ziegler et al. 1998) and
extracellular faces (Le Grimellec et al. 1995, Lfirmer
et al. 1997) of plasma membrane. Their proteinaceous
nature was established by different approaches,
namely pronase and gold-labeled-concanavalin A
treatments and ethanol dehydration (Hfiberle et al.
1991, HSrber et al. 1992, Le Grimellec et al. 1994,
1995). The marked decrease of roughness in regions of
our samples covered with particles upon the addition
of pronase is also in favor of their proteinaceous
nature. This protease digests proteins at the membrane
surface and reduces the x-y size and the number of
protrusions on different plasma membranes visualized
by A F M (Le Grimellec et al. 1994, 1995; L~irmer et al.
1997). The fact that particles occupy most of the mem-
brane surface is in agreement with the well-known
high protein-to-lipid ratio of this membrane that
generally exceeds one (Houslay and Stanley 1982).
In spinach a value of 0.98 was reported for plasma
membrane (Penel et al. 1988) and a high density of
intramembrane particles was observed on freeze-
fracture surfaces (Cr6vecoeur unpubl.). Such particles
were described on most plant plasma membranes visu-
alized by this technique and were generally believed
to be integral proteins (Platt-Aloia and Thomson 1989,
Webb and Steponkus 1993).
As for other membranes (H6rber et al. 1992; Le
Grimellec et al. 1994, 1995; Ziegler et al. 1998), high-
magnification AFM reveals that the surface of spinach
plasma membrane is heterogeneous with regard to dis-
tribution of particles and channels. The heterogeneity
was repeatedly found on the major part of the same
sample surface and on membranes prepared from dif-
ferent batches of plants. In addition it was observed
with both AFM equipments used. It could be attrib-
uted to the fact that the plasma membranes came from
the different tissues composing spinach leaves, e.g.,
lower and upper epidermis, palisade and spongy
parenchyma, and vascular bundles. They have various
roles in the functioning of the leaf. Differences in
plasma membrane between different cell types have
been revealed by immunocytochemical and biochem-
ical studies. For instance, the auxin transport inhibi-
tor N-l-naphthylphthalamic acid was predominantly
localized in pea stems in the plasma membrane of
parenchyma cells sheating the vascular bundle (Jacobs
and Gilbert 1983). An auxin-binding protein was
mainly localized at the plasma membrane of the outer
epidermal cells (LONer and Kl~mbt 1985). In stems
and leaves, plasma membrane H+-ATPase is essen-
tially present in tissues specialized in nutrient trans-
port like phloem and in guard cells. This localization
has been subsequently confirmed by studies of gene
expression (DeWitt et al. 1991, Michelet et al. 1994,
Michelet and Boutry 1995).
The heterogeneity in membrane surfaces could also
be related to distinct microdomains. It has become
evident recently that the two membrane faces are
organized in plane into a mosaic of supramolecular
domains, in plant as well as in animal cells (Edidin
et al. 1991, Masson et al. 1992, Edidin 1997).
Distribution of protrusions along lines in a direction
different from that of scanning has been reported for
AFM imaging of other membrane surfaces (H6rber et
al. 1992, Le Grimellec et al. 1995, Ehrenh6fer et al.
1997, Ziegler et al. 1998) and by high-resolution scan-
ning electron microscopy (Walther and Hentschel
1989). In plant cells, arrays of intramembrane particles
have been described on plasma membrane surfaces
examined by freeze-fracture. They have been related
in some cases to ordered membrane-associated syn-
thetic complexes involved in cellulose microfibril
biogenesis (Mueller and Brown 1980, Brown et al.
1996, Blanton and Haigler 1996).
In conclusion, our images show that structural infor-
mation at the nanometer range can be achieved by
AFM examination of purified plasma membranes in
buffer, without any fixation or preparation. As in other
AFM studies of biological membranes, additional
experiments are now required to progress in the iden-
tification of structures imaged by AFM. However, our
first objective in the future will be to compare plasma
membranes from vegetative plants and from plants
induced to flowering, focusing our interest on mechan-
ical properties of the membrane surface. It was shown
that photoperiodic floral induction results in various
biochemical and biophysical modifications at the
plasma membrane level, some of them indicating a
change in membrane fluidity in spinach as well as in
other plants (Borochov et al. 1995; Crespi et al. 1993,
1997). We have also the objective to exploit the rec-
ognized AFM potential not only as an imaging tool but
also as a system for analyzing viscoelastic and mechan-
ical properties of our membrane samples as reported
for different living biological cells (Hoh and Schoe-
nenberger 1994, Radmacher et al. 1996) and for artifi-
cial membranes (Vi6 et al. 1998).
Acknowledgments
The authors gratefully acknowledge Mrs. Evelyne Vazquez for her technical participation in preparing plasma membranes. This work was supported by grants from the R6gion Bourgogne, the R6gion Languedoc-Roussillon, and the Minist6re de la Recherche (France).
References
Binnig G, Quate CF, Gerber CH (1986) Atomic force microscope. Phys Rev Lett 56:930-933
Blanton RL, Haigler CH (1996) Cellulose biogenesis. In: Smallwood M, Knox R Bowles D (eds) Membranes: specialized functions in plants. Bios Scientific Publishers, pp 57-75
Borochov A, Spiegelstein H, Halevy A H (1995) Involvement of signal transduction pathway components in photoperiodic flower induction in Pharbitis nil. Physiol Plant 95:393-398
Bradford MM (1976) A rapid and sensitive method for the quanti- tation of microgram quantities of protein utilizing the principle of protein binding. Anal Biochem 72:248-254
Brown RM, Saxena IM, Kudlicka K (1996) Cellulose biosynthesis in higher plants. Trends Plant Sci 1:149-156
Butt HJ, Wolff EK, Gould SAC, Northern BD, Peterson CM, Hansma PK (1990a) Imaging cells with the atomic force micro- scope. J Struct Biol 105:54-61
- Downing KH, Hansma PK (1990b) Imaging the membrane
protein bacteriorhodopsin with an atomic force microscope. Biophys J 58:1473-1480
- Prater CB, Hansma PK (1991) Imaging purple membranes dry
and in water with the atomic force microscope. J Vac Sci Technol B 9:1193-1196
Contino PB, Hasselbacher CA, Ross JB, Nemerson Y (1994) Use of an oriented trans-membrane protein to probe the assembly of a supported phospholipid bilayer. Biophys J 67:1113-1116 Crespi R Cr6vecoeur M, Penel C, Greppin H (1989) Changes in
spinach plasmalemma after gibberellic acid treatment. Plant Sci 62:63-71
. . . . (1993) Plasma membrane sterols and flowering induction. Plant Sci 89:153-160
- Perroud PF, Martinec J, Greppin H (1997) Flowering and mem- brane functions. In: Greppin H, Penel C, Simon P (eds) Travelling shot on plant development. University of Geneva, Geneva, Switzerland, pp 201-215
Danker T, Mazzanti M, Tonini R, Rakowska A, Oberleithner H (1997) Using atomic force microscopy to investigate patch- clamped nuclear membrane. Cell Biol Int 21:747-757
DeWitt ND, Harper JF, Sussman MR (1991) Evidence for a plasma membrane proton pump in phloem cells of higher plants. Plant J 1:121-128
Edidin M (1997) Lipid microdomains in cell surface membranes. Curt Opin Struct Biol 7:528-532
- Kuo SC, Sheetz MP (1991) Lateral movements of membrane
glycoproteins restricted by dynamic cytoplasmic barriers. Science 254:1379-1382
Ehrenh6fer U, Rakowska A, Schneider SW, Schwab A, Oberleith- ner H (1997) The atomic force microscope detects ATP-sensitive protein clusters in the plasma membrane of transformed MDCK cells. Cell Biol Int 21:737-746
Fowke LC (1986) The plasma membrane of higher plant protoplasts. In: Chadwick CM, Garrod DR (eds) Hormones, receptors and cellular interactions in plants. Cambridge University Press, Cambridge, pp 217-239
- (1988) Structure and physiology of the protoplast plasma mem-
brane. Plant Cell Tissue Organ Cult 12:151-157
- Griffing BG, Mersey BG, Tanchak MA (1986) Protoplasts for studies of cell organelles. In: Fowke CC, Constabel F (eds) Plant protoplasts. CRC Press, Boca Raton, pp 39-52
Greppin H, Bonzon M, Crespi R Crbvecoeur M, Degli Agosti R, Penel C, Tacchini P (1991) Communication in plants. In: Penel C, Greppin H (eds) Plant signalling, plasma membrane and change of state. Universit6 de Gen~ve, Geneva, Switzerland, pp 139-177 H~berle W, H6rber JKH, Binnig G (199t) Atomic force microscopy
on living cells. J Vac Sci Technol B 9:1210-1213
Henderson E, Haydon PG, Sakaguchi DS (1992) Actin filament dynamics in living glial cells imaged by atomic force microscopy. Science 257:1944-1946
Heymann JB, Mtiller D J, Mitsuoka K, Engel A (1997) Electron and atomic force microscopy of membrane proteins. Curr Opin Struct Biol 7:543-549
Hob JH, Schoenenberger CA (1994) Surface morphology and mechanical properties of MDCK monolayers by atomic force microscopy. J Cell Sci 107:1105-1114
- Lal R, John SA, Revel JR Arnsdorf MF (1991) Atomic force microscopy and dissection of gap junctions. Science 253: 1405- 1408
- Sosinsky GE, Revel JR Hansma PK (1993) Structure of the extra-
cellular surface of the gap junction by atomic force microscopy. Biophys J 65:149-163
H0rber JK, H~berle W, Ohnesorge F, Binnig G, Liebich HG, Czerny CR Mahnel M, Mayr A (1992) Investigation of living cells in the nanometer regime with the scanning force microscope. Scanning Microsc 6:919-930
Houslay MD, Stanley KK (1982) Dynamics of biological mem- branes. Wiley, Chichester
Jacobs M, Gilbert SF (1983) Basal localization of the presumptive auxin transport carrier in pea stem cells. Science 220:1297-1300 Kirby AR, Gunning AE Waldron KW, Morris V J, Ng A (1996) Visu-
alization of plant cell walls by atomic force microscopy. Biophys J 70:1138-1143
Kjellbom P, Larsson C (1984) Preparation and polypeptide compo- sition of chlorophyll-free plasma membranes from leaves of light- grown spinach and barley. Plant Physiol 62:501-509
K6rner LE, Kjellbom R Larsson C, Moiler IM (1985) Surface prop- erties of right-side-out plasma membrane vesicles isolated from barley roots and leaves. Plant Physiol 79:72-79
Lal R, John SA (1994) Biological applications of atomic force microscopy. Am J Physiol 266:C1-C21
- Kim H, Garavito RM, Arnsdorf MF (1993) Molecular resolution
imaging of reconstituted biological channels using atomic force microscopy. Am J Physiol 265:C851-C856
L~irmer J, Schneider SW, Danker T, Schwab A, Oberleithner H (1997) Imaging excised apical plasma membrane patches of MDCK cells in physiological conditions with atomic force microscopy. Pflugers Arch 434:254-260
Larsson C, Widell S, Kjellbom P (1987) Preparation of high-purity plasma membranes. Methods Enzymol 148:558-568
Lebrun-Garcia A, Bourque S, Binet MN, Ouaked F, Wendehenne D, Chiltz A, Schaffner A, Pugin A (1999) Involvement of plasma membrane proteins in plant defense responses: analysis of the cryptogein signal transduction in tobacco. Biochimie 81: 663- 668
Le Grimellec C, Lesniewska E, Cachia C, Schreiber JR de Fornel F, Goudonnet JP (1994) Imaging of the membrane surface of MDCK cells by atomic force microscopy. Biophys J 67:36-41 - - Giocondi MC, Cachia C, Schreiber JR Goudonnet JP (1995)
Imaging of the cytoplasmic leaflet of the plasma membrane by atomic force microscopy. Scanning Microsc 9:401-411
Leshem Y (1992) Plant membranes biophysics development and senescence. In: Leshem Y (ed) Plant membranes: a biophysical
approach to structure, development and senescence. Kluwer, Dordrecht, pp 113-154
Lesniewska E, Giocondi MC, Vi6 V, Finot E, Goudonnet JR Le Grimellec C (1998) Atomic force microscopy of renal cells: limits and prospects. Kidney Int Suppl 65:$42-$48
L6bler M, Klfimbt D (1985) Auxin-binding protein from coleoptiles membrane of corn (Zea mays L.). J Biol Chem 260:9854-9859 Masson F, Rakotomavo M, Rossignol M (1992) Characterization in
tobacco leaves of structurally and functionally different mem- brane fractions enriched in vanadate sensitive H+-ATPase. Plant Sci 92:129-142
- Santoni V, Rossignol M (1994) Functional and structural changes at the plasma membrane during the induction of flowering in tobacco leaves. Flowering Newsl 17:39-43
Michelet B, Boutry M (1995). The plasma membrane H+-ATPase: a highly regulated enzyme with multiple physiological functions. Plant Physiol 108:1-6
- Lukaszewicz M, Dupriez V, Boutry M (1994) A plant plasma membrane proton-ATPase gene is regulated by development and environment and shows signs of translational regulation. Plant Cell 6:1375-1389
Mueller SC, Brown RM (1980) Evidence for an intramembrane component associated with a cellulose microfibril synthesizing complex in higher plants. J Cell Biol 84:315-326
Mt~ller DJ, Schabert FA, Bttldt G, Engel A (1995) Imaging purple membranes in aqueous solutions at subnanometer resolution by atomic force microscopy. Biophys J 68:1681-1686
- Sass HJ, Mfiller SA, B~ildt G, Engel A (1999) Surface structures of native bacteriorhodopsin depend on the molecular packing arrangement in the membrane. J Mol Biol 285:1903-1909 Oberleithner H, Geibel W, Guggino W, Henderson RM, Hunter M,
Schneider SW, Schwab A, Wang W (1997) Life on biomembranes viewed with the atomic force microscope. Wien Klin Wochenschr 109:419-423
Penel C, Auderset G, Bernardini N, Castillo F, Greppin H, Morr6 DJ (1988) Compositional changes associated with plasma membrane thickening during floral induction of spinach. Physiol Plant 73: 134-146
Perroud PF, Crespi E Cr6vecoeur M, Fink A, Tacchini R Greppin H (1997) Detection and characterization of GTP-binding proteins on tonoplast of Spinacia oleracea. Plant Sci 122:23-33
Platt-Aloia KA, Thomson WW (1989) Advantages of the use of intact plant tissues in freeze-fracture electron microscopy. J Elec- tron Microsc 13:289-299
Quinn PJ, Williams WP (1990) Structure and dynamics of plant membranes. In: Marwood JL, Bowyer JR (eds) Methods in plant biochemistry, vol 4. Academic Press, pp 297-340
Radmacher M, Tillmann RW, Fritz M, Gaub HE (1992) From mol- ecules to cells: imaging soft samples with the atomic force micro- scope. Science 257:1900-1905
- Fritz M, Kacher CM, Cleveland JP, Hansma PK (1996) Measur- ing the viscoelastic properties of human platelets with the atomic force microscope. Biophys J 70:556-567
Rakowska A, Danker T, Schneider SW, Oberleithner H (1998) ATP- induced shape change of nuclear pores visualized with the atomic force microscope. J Membr Biol 163:129-136
Ratneshwar L, Scott AJ (1994) Biological applications of atomic force microscopy. Am J Physiol 266:C1-C21
Salafsky J, Groves JT, Boxer SG (1996) Architecture and func- tion of membrane proteins in planar supported bilayers: a study with photosynthetic reaction centers. Biochemistry 35: 14773- 14781
Sandelius AS, Morr6 DJ (1990) Plasma membrane isolation. In: Larsson C, Moller JM (eds) The plant plasma membrane. Springer, Berlin Heidelberg New York Tokyo, pp 44-75 Sandstrom RR de Boer AH, Lomax TL, Cleland RH (1987) Latency
of plasma membrane H+-ATPase in vesicles isolated by aqueous phase partitioning. Plant Physiol 85:693-698
Schabert F, Henn C, Engel A (1995) Native Escherichia coli OmpF porin surfaces probed by atomic force microscopy. Science 268: 92-94
Shao Z, Mou J, Czajkowski DM, Yang J, Yuan JY (1996) Biological atomic force microscopy: what is achieved and what is needed. Adv Phys 45:1-86
van der Wel NN, Putman CAJ, van Noort SJT, de Grooth BG, Emons AMC (1996) Atomic force microscopy of pollen grains, cellulose microfibrils and protoplasts. Protoplasma 194:29-39
Vi6 V, Van Mau N, Lesniewska E, Goudonnet JP, Heitz H, Le Grimellec C (1998) Distribution of ganglioside GMI between two-component, two-phase phosphatidylcholine monolayers. Langmuir 14:4574-4583
Walther R Hentschel J (1989) Improved representation of cell surface structure by freeze substitution and backscattered elec- tron imaging. Scanning Microsc Suppl 105:201-211
Webb MS, Steponkus PL (1993) Freeze-induced membrane ultra- structural alterations in rye (Secale cereaIe) leaves. Plant Physiol 101:955-963
Weisenhorm AL, Khorsandi M, Kasas S, Gotzos V, Butt HJ (1993) Deformation and height anomaly of soft surfaces studied with an AFM. Nanotechnology 4:106-113
Ziegler U, Vinckier A, Kernen R Zeisel D, Biber J, Semenza G, Murer H, Groscurth P (1998) Preparation of basal cell mem- branes for scanning probe microscopy. FEBS Lett 436: 179- 174