Publisher’s version / Version de l'éditeur:
Industrial Biotechnology, 7, 2, pp. 129-135, 2011-04-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.1089/ind.2011.7.129
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Direct fermentation of triticale starch to lactic acid by Rhizopus oryzae
Xiao, Zhizhuang; Wu, Meiqun; Beauchemin, Manon; Groleau, Denis; Lau,
Peter C.K.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=bc39aac0-59d7-48de-8969-d33a0e2021b7
https://publications-cnrc.canada.ca/fra/voir/objet/?id=bc39aac0-59d7-48de-8969-d33a0e2021b7
Zhizhuang Xiao, Meiqun Wu, Manon Beauchemin, Denis Groleau,
and Peter C.K. Lau*
Biotechnology Research Institute, National Research Council Canada, Montreal, Quebec H4P 2R2, Canada
* Author for correspondence
Biotechnology Research Institute, National Research Council Canada, 6100 Royalmount Avenue, Montreal, Quebec H4P 2R2, Canada Phone: +001 (514) 496-6325; Fax: +001 (514) 496-6265 Email: peter.lau@cnrc-nrc.gc.ca
Submitted: 1 December 2010; Revised: 19 February 2011; Accepted: 10 March 2011
KEYWORDS: biorefinery; Rhizopus oryzae; triticale, starch platform, lactic acid, calcium carbonate
ABBREVIATIONS: LA, lactic acid; DASGIP, Drescher Arnold
Schneider — Gesellschaft für Informations — und Prozesstechnologie; PLA, polylactide, or polylactic acid
Abstract
The production of lactic acid from triticale starch was demonstrated for the first time. Direct fermentation by Rhizopus oryzae in batch mode led to a conversion yield of 0.74 g lactic acid/g starch. Kinetics analy-sis of the fermentation suggested a multiphase process which conanaly-sist- consist-ed of quick hydrolysis of starch, rapid glucose accumulation followconsist-ed by fast production of lactic acid. In batch fermentation mode, correct
timing and proper dosage of a neutralizing agent (calcium carbonate) were found to be critical factors that affect the organic acid yield. Addition of CaCO3at the time point when glucose accumulation had reached its peak (24 h) resulted in the highest lactic acid yield. The best ratio (by weight) of triticale starch to CaCO3 for lactic acid production was 1:1. It was important to maintain a pH of about 5 during the whole fermentation process. Fermentation carried out in an optimized, commercially available small-scale parallel bioreac-tor (DASGIP) yielded up to 0.87 g lactic acid/g triticale starch when R. oryzae spores were directly added to the fermentation medium. Our fermentation results indicated that triticale starch from this nonfood crop is a promising renewable feedstock for production of lactic acid.
Introduction
S
tarch, being the most abundant storage carbohydrate on earth, is a major feedstock for biorefineries outside of lig-nocellulosic materials.1 Starchy materials such as wheat,corn, cassava, potato, rice, rye, and barley have been used in lactic acid (LA; 2-hydroxypropionic acid) production, a valuable carboxylic acid widely used in the food, cosmetic, pharmaceutical, and chemical industries.2,3 LA is best known for its utilization in
the production of the biodegradable, compostable, and biocompat-ible polylactic acid (PLA), which provides an environment-friendly alternative to biodegradable plastics derived from petrochemical materials.4–7 In the chemical industry, via decarboxylation,
decar-bonylation, dehydration, or reduction with hydrogen, LA can be converted to commodity chemicals such as acetaldehyde, acrylic acid, and pro panoic acid.8
Fermentative production of LA by microbes, in contrast to chemi-cal synthesis, e.g., by hydrolysis of lactonitrile using strong acids, has several advantages, most importantly, the production of optically pure L(+) or D(–)-lactic acid instead of a racemic mixture.2–4,9,10 The
L(+)-isomer is the biologically important isomer and is a preferred substrate in most industrial applications. Among the numerous LA producers, the Lactobacillus, Bacillus, and Rhizopus genera are considered as commercially viable, and each has its advantages
Direct fermentation of
triticale starch to lactic acid
by Rhizopus oryzae
and disadvantages.4 Unlike the lactobacilli, LA-producing Rhizopus
strains (R. oryzae, R. arrhizus) generate L(+)-LA as a sole isomer of LA.11,12 In addition, the Rhizopus fungi naturally produce the
amylo-lytic enzymes for starch saccharification; they require few complex nutrients and only a small amount of inorganic salts for growth; they are tolerant to low pHs; and fungal biomass is easier to separate from the fermentation broth than bacterial biomass, thus facilitating downstream processes.12,13
As the first human-made hybrid crop of durum wheat (Triticum spp.) and rye (Secale spp.), the century-old triticale (X Triticosecale Wittmack) remains a largely unexplored crop, as its grain and forage are used primarily as animal feed.14–16 Triticale is generally known
for its adaptability to unfavorable soils, drought tolerance, cold hardiness, disease resistance, and low-input requirements. Canadian spring (e.g., Carman, Pronghorn, and AC Ultima) and winter varieties (e.g., Pika and Bobcat) have superior adaptation to stress conditions such as drought, marginal, or acidic soils.14,16 Some 3 million acres of
triticale are projected to be grown in the Canadian Prairies by 2015 (Canadian Triticale Biorefinery Initiative; www.ctbi.ca). Like other cereals, triticale is characterized by high starch and other carbohy-drate content. Total starch levels in triticale are equal to or higher than for wheat.16 Triticale grain dry matter contains 62.4%–70.9%
starch, a content that is dependent upon cultivar and year.17 A recent
scanning electron microscopy study of triticale starch granules dur-ing endosperm and seed development indicated unique and inherent structures, such as channels or pores that may facilitate the flow of hydrolytic enzymes into the granule matrix.18 In addition, triticale
starch has the apparent advantages of low phenolic acids content that otherwise negatively impacts starch hydrolysis, and a lower gelatinization temperature (of 65–68°C) compared to those of barley, wheat, and corn (in the range of 72–75°C; personal communication, T. Vasanthan, University of Alberta).
Triticale starch is envisaged to provide an important carbohydrate platform for a biorefinery value chain.In the present work, the feasi-bility of LA production from triticale starch was assessed via fungal fermentation by R. oryzae sb NRRL 29086 in a “direct,” or single-step mode, otherwise known as simultaneous saccharification and fermentation (SSF; for reviews3,9,12). We examined the effects of spore
inoculum size, temperature, and addition of calcium carbonate on the production of LA in batch fermentations and compared the yields to those obtained in a DASGIP parallel bioreactor system.
Material & methods
TRITICALE STARCH AND ANALYSIS
Starch of 99% purity prepared from Triticale var. AC Ultima, a Canadian winter cultivar, was kindly supplied by Dr. Thavaratnam Vasanthan (University of Alberta) and Dr. Hong Qi (Alberta Agriculture and Rural Development). The high-purity starch (<2.0% w/w protein contamination) was prepared from ground triticale grain flour using a wet-milling procedure that was originally developed for gluten containing wheat grains.1 Briefly, the isolation involved a “dough
and wash” technology — preparing a dough or slurry with deionized
water, followed by dilute alkaline washing and separation of the protein-enriched fiber fraction from starch milk by centrifugation, and then water washing.19 The composition of starch was determined
by acid hydrolysis as follows: 1 g starch was hydrolyzed with 100 mL 0.6 N HCl after heat treatment at 121°C for 20 min. The hydrolysate was used to determine the glucose concentration. Theoretically, 1 g dry starch can be hydrolyzed into 1.1 g glucose using HCl. Glucose and LA were monitored using an HPLC system (Waters; Milford, Massachusetts, USA) consisting of a pump model 600 and an auto-sampler model 717 Plus equipped with a refractive index detector from Waters (model 2414). The separation column, Transgenomic ICSep IC-ION-300 (300 mm × 7.8 mm OD) from Transgenomic (New Haven, Connecticut, USA) was used for both analyses of glucose and LA. The mobile phase was 0.01N H2SO4 at 0.4 mL/min. Analysis was carried out at 35°C. Compounds were quantified using standards including glucose (BDH; Mississauga, Ontario, Canada) and L(+)-LA (Sigma; St. Louis, Missouri, USA).
FUNGAL CULTURE AND SPORE COLLECTION
R. oryzae sb NRRL 2908620,21 was grown on DifcoTM potato dextrose
agar (Becton, Dickinson, and Company; Sparks, Maryland, USA) plates at 30°C for 10 d. Spores were collected using an inoculating loop and suspended in Vogel medium, pH 5.0 (2.5 g sodium citrate, 5 g KH2PO4, 2 g NH4NO3, 0.4 g MgSO4•7H
2O, 0.2 g CaCl2•2H2O, 2.5 mg
FeSO4, 0.98 mg MnSO4•H
2O, 0.83 mg ZnCl2, and 1.0 mg CoCl2 in 1 000
mL). Spores were counted by conventional means under a micro-scope using a hemacytometer22 (Bright-Line Hemacytometer; Hausser
Scientific, Horsham, Pennsylvania, USA).
EFFECT OF TEMPERATURE ON FERMENTATION
Duplicate 500 mL flasks, each containing 100 mL Vogel medium (pH 5.0) supplemented with 3 g triticale starch, were inoculated with 1×106 R. oryzae spores per mL. Fermentations were carried out in a
shaker agitated at 200 rpm and kept at 24, 28, 30, or 32°C. Two-mL aliquots were taken from each flask at various time intervals, centri-fuged, and the supernatant fluids analyzed for the presence of LA by HPLC as described above.
EFFECT OF INNOCULUM SIZE
Duplicate 500 mL flasks, each containing 100 mL of Vogel medium (pH 5.0) supplemented with 3% (w/v) triticale starch, were inoculated with a series of spore concentrations ranging from 400 to 1×106
R. oryzae spores/mL. Fermentations were carried out in a shaker at the agitation rate of 200 rpm and at 28°C unless specified. Two-mL aliquots were taken from each flask (at 16 h and at then at 4–16 h intervals afterwards, through 72 h), centrifuged, and the supernatant fluids analyzed for LA as described above.
EFFECT OF CALCIUM CARBONATE ON THE KINETICS OF
LA FERMENTATION
R. oryzae spores were inoculated into 100 mL of Vogel medium containing 3% (w/v) triticale starch at a final spore concentration at
PEER
REVIEW
130 INDUSTRIALBIOTECHNOLOGY A P R I L 2 0 1 1
1×104 per mL. One gram of sterile calcium carbonate powder was
added to the culture medium at 0, 16, 20, 24, and 40 h during the fermentation at 28°C. To determine the effect of CaCO3 dosage on LA production, different amounts of CaCO3 were added to the medium 24 h after start of the fermentation. Fermentation without addition of CaCO3 was used as control. Each condition was carried out in dupli-cate. Samples taken at different time points were subjected to analysis for glucose and LA by HPLC and pH measurement.
FERMENTATION IN DASGIP PARALLEL BIOREACTOR
For initial scale-up of the fermentation, a 2 L DASGIP parallel bio-reactor system (DASGIP BioTools, Shrewsbury, Massachusetts, USA) was used. R. oryzae spores were inoculated into 1 000 mL of Vogel medium containing 3% (w/v) triticale starch at a final spore concen-tration of 1×104 per mL. Initial pH of the fermentation medium was
adjusted to 5.0 ± 0.1 by addition of 28% NH4OH. The fermentation was performed at 28°C. The dissolved oxygen was maintained by fixing the agitation rate at 250 rpm and the inlet-gas flow rate at 0.1 vvm (L/min). To avoid foaming, 200 µL of antifoam agent Mazu® DF 204 (BASF, Mount Olive, New Jersey, USA) was added to the medium.
Results
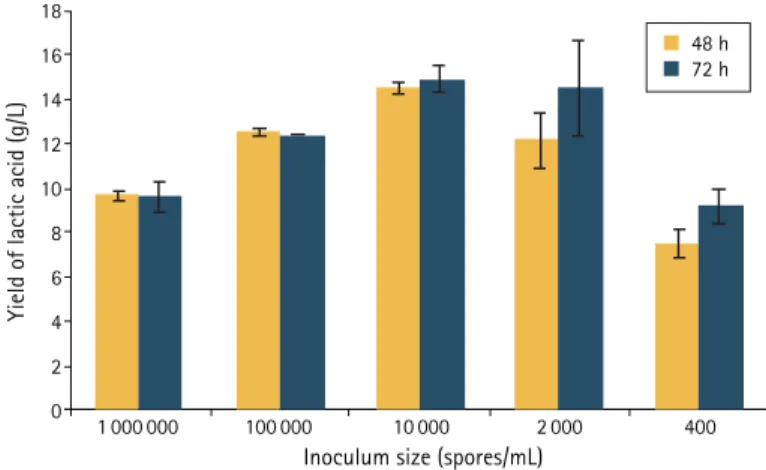
The effect of temperature on the conversion of triticale starch to LA by the R. oryzae strain was first determined over four temperature points; the highest yield of LA at two selected time points (24 h and 48 h) was obtained at 28°C (Figure 1). Inoculum size (spores/mL) also affected the yield of LA, the highest obtained when the number of R. oryzae spores was 1×104/mL after inoculation of flasks
contain-ing 3% (w/v) triticale starch (Figure 2). The product yield decreased by 23% when either a higher inoculum size (1×106 spores/mL) or a
much lower inoculum size (400 spores/mL) was used; however, good yields were also obtained with an inoculum of 2 000 spores/mL and a fermentation duration of 72 h.
Analysis of the kinetics of the fungal fermentation of triticale starch to LA at 28°C with an inoculum size of 1×104 spores/mL
suggested five critical time points or phases during the whole fer-mentation process (Figure 3). In phase 1 (0–16 h), the fungi rapidly consumed starch, and only small amounts of glucose accumulated, with no detectable LA accumulation. In phase 2 (16–20 h), starch degradation coincided with a sharp increase in glucose accumulation; however, LA production was marginally detected during this period. In phase 3 (20–24 h), most of the starch had been degraded, glucose concentration reached its highest level, and production of LA was rapidly observed when pH was dropped rapidly from 5.0 to about 3.0 (Figure 3). In phase 4 (24–40 h), glucose was consumed rapidly, while LA production approached its maximal level. In the last phase
Figure 2. Effect of spore inoculum size on the yield of lactic acid in the fermentation of triticale starch by R. oryzae. Each data point represents mean ± SD (n = 3). The p value of the data point for inoculum size 1×104 spores/mL, compared to others, is <0.001.
18 16 14 12 10 8 6 4 2 0 1 000 000 100 000 10 000 2 000 400
Yield of lactic acid (g/L)
Inoculum size (spores/mL)
■ 48 h ■ 72 h
Figure 1. Effect of temperature on the fermentation of triticale starch into lactic acid by R. oryzae. Each data point represents mean± SD (n = 3). The p value of the data point for 28°C/48 h, compared to others, is <0.001. 10 9 8 7 6 5 4 3 2 1 0 24 28 30 32
Yield of lactic acid (g/L)
Temperature (°C)
■ 24 h ■ 48 h
Figure 3. Kinetics of direct fermentation of triticale starch to lactic acid by R. oryzae and change of pH during fermentation; lactic acid ; glucose, ; triticale starch, ; pH, .
18 16 14 12 10 8 6 4 2 0 6 5 4 3 2 1 0 0 10 20 30 40 50 60 70 Biomass (g/L) pH Fermentation time (h)
(after 40 h), glucose consumption tapered off, while LA accumulation reached a plateau.
When a larger inoculum size of 1×106 spores/mL was used,
observed LA production occurred earlier than in cases when smaller inocula were used (data not shown); both glucose accumulation and LA production were lower (this latter effect shown in Figure 1).
We examined next the influence of a time-course addition of the neutralizing agent CaCO3 on LA production. The results showed that timing greatly influenced LA production (Figure 4A, Table 1), glucose accumulation (Figure 4B), and pH values in the fermentation medium (Figure 4C ). Compared to the control where no CaCO3 was added, CaCO3 supplementation during the 20–40 h period (phases 3, 4, and 5) increased LA production; however, addition of CaCO3 at 0 h and 16 h (phase 1 and 2) raised the pH to about 6.0 (Figure 4C ), causing a delay as well as reduced glucose accumulation (Figure 4B ) and, hence, decreased LA production (Figure 4A, Table 1). CaCO3 supplementation at 24 h yielded 74.0 ± 2.1 g LA from 100 g starch, thus leading to an 80% increase in yield compared to when CaCO3 was added immediately at the beginning of the fermentation.
The effect of CaCO3 dosage (starch-to-CaCO3 ratio) and quantity of CaCO3 on LA production was also examined (Table 2). The results indicated that the best yield of LA was obtained at the starch-to-CaCO3ratio (by weight) of 1:1. Adding more CaCO3provided no addi-tional benefit. However, a low CaCO3 dosage (starch-to-CaCO3ratio of 6:1) was still beneficial since that fermentation produced 41% more LA than without the neutralizing agent.
Instead of shake flasks, the DASGIP parallel bioreactor system was employed to provide a comparison of LA yield. Initial experiments examined the effect of temperature (30°C versus 28°C), rate of agita-tion, inoculum concentration and format (spores versus mycelium), aeration level, and influence of sparger design (Rushton-type impel-ler), as well as the use of chemical antifoam (Mazu DF204). After sev-eral initial runs, examples of promising yields of LA were determined (Table 3). LA production reached 86.7±4.3 g from 100 g starch after 72 h of fermentation performed in a 2 L-scale DASGIP system. With
PEER
REVIEW
132 INDUSTRIALBIOTECHNOLOGY A P R I L 2 0 1 1
Table 1. Time effect of CaCO
3addition on the
production of lactic acid by R. oryzae
LA yield (g/L)
LA yield (g/100 g starch)
Control (no CaCO3) 14.9±1.5 49.7±5.0
+ CaCO3 at 0 hr 12.3±0.1 41.0±0.3*
+ CaCO3 at 16 hr 12.8±0.1 42.7±0.3a
+ CaCO3 at 20 hr 19.1±0.3 63.7±1.0**
+ CaCO3 at 24 hr 22.2±0.7 74.0±2.1**
+ CaCO3 at 40 hr 20.3±0.1 67.7±0.3**
Note: Ratio of CaCO3 to starch = 1:3. Each data point represents mean ± SD (n = 3).
anot significant; *, p value < 0.05; **, p value < 0.01; versus control
Figure 4. (A) Lactic acid yield as a function of CaCO3 addition at
vari-ous times; (B) glucose accumulation profile; (C) pH change during the course of triticale starch fermentation to lactic acid by R. oryzae.
25 20 15 10 5 0 0 10 20 30 40 50 60 70
Yield of lactic acid (g/L)
Fermentation time (h) no addition of CaCO3 1 g of CaCO3 at 0 h 1 g of CaCO3 at 16 h ■ 1 g of CaCO3 at 20 h ▲ 1 g of CaCO3 at 24 h ● 1 g of CaCO3 at 40 h
A
0 10 20 30 40 50 60 70 18 16 14 12 10 8 6 4 2 0 Glucose accumulation (g/L) Fermentation time (h) no addition of CaCO3 1 g of CaCO3 at 0 h 1 g of CaCO3 at 16 h ■ 1 g of CaCO3 at 20 h ▲ 1 g of CaCO3 at 24 h ● 1 g of CaCO3 at 40 hB
7 6 5 4 3 2 1 0 0 10 20 30 40 50 60 70 pH Fermentation time (h) no addition of CaCO3 1 g of CaCO3 at 0 h 1 g of CaCO3 at 16 h ■ 1 g of CaCO3 at 20 h ▲ 1 g of CaCO3 at 24 h ● 1 g of CaCO3 at 40 hC
J.11.PRV72 Xiao 129-135.indd 132 4/21/11 4:17 PMa fixed agitation rate of 250 rpm, aeration rates ranging between 0.03 and 0.1 L/min did not significantly affect the LA yield. However, inoculum size was found to be important, as observed previously in shake flasks experiments.
Discussion
This study is a benchmarking effort to assess the feasibility of LA production from triticale starch via fungal fermentation employing an R. oryzae strain, giving LA yields from starch (0.74–0.87 g/g) that are competitive with other starchy materials. Yu and Hang11
studied the direct fermentation of several starchy agricultural com-modities and found the following order of merit in terms of LA yield: rice > corn > cassava > barley > oats. The yields of LA and mode of production by various starchy materials are compared in Table 4. Rice and corn yielded about 45 g and 35 g of LA from 100 g starch, respectively, when Rhizopus spores were used as inoculum in the flask experiments.11,23 An oat starch conversion yield of 68 g LA/100 g
starch was achieved in 10 L airlift bioreactors when spores were used as inoculum.24 Sweet potato starch produced 72 g LA from 100
g starch in 5 L stirred bioreactors when freshly prepared R. oryzae mycelium from spores was used.25 Up to 85 g LA was produced from
100 g corn starch in 3 L airlift bioreactors when the starch was pre-hydrolyzed by α-amylase, also using freshly prepared mycelium from spores as inoculum.26 In the DASGIP bioreactor system described
herein, our study shows that simultaneous saccharification and fer-mentation of triticale starch by R. oryzae spores produced 86.7±4.3 g LA from 100 g starting starch material — a yield that was about 10% higher than those obtained in shake flasks.
LA fermentations by Rhizopus strains have been carried out at temperatures in the range of 27°C to 35°C.12 The optimal temperature
for LA production by R. oryzae strain NRRL 29086 was 28°C. Yu and Hang11 reported that increasing the inoculum size of R. oryzae strain
NRRL 395 from an initial level of 1×105 spores/mL to 3×105 spores/
mL or more did not improve the LA yield obtained with a number of agricultural commodities including barley, oat, cassava, corn, and rice. However, our results indicated that R. oryzae inoculum size significantly influenced LA production, 1×104 spores/mL being the
optimal concentration.
Table 2. Effect of CaCO
3dosage on the production of lactic acid by R. oryzae
CaCO3 dosage (g/ 100 mL) 0 0.5 1 2 3 6
Ratio of starch to CaCO3 - 6 3 1.5 1 0.5
Yield of lactic acid (g/L) 12.8±2.7 18.1±0.5 19.8±0.2 20.2±0.1 21.0±0.5 20.8±1.8
Yield of lactic acid (g/100 g starch) 42.7±9 60.3±1.7* 66.0±0.7* 67.3±0.3** 70.0±1.7** 69.3±6*
Note: Each data point represents mean ± SD (n = 3). The significance is calculated relative to the production of LA without CaCO3 (*, p value < 0.05; **, p value < 0.01).
Table 3. Lactic acid fermentation of triticale starch by R. oryzae using a 1L DASGIP parallel bioreactor
system
Inoculum size (spores/mL)
Agitation speed (rpm)
Aeration rate (L/min)
Lactic acid yield (g from 100 g starch)
2.4 × 103 250 0.1 86.7 ± 4.3
1.0 × 104 250 0.1 82.3 ± 4.1
1.0 × 104 250 0.03 81.0 ± 4.0
Note: Each data point represents mean ± SD (n = 4).
Table 4. Comparison of results of the present work to previous reports
Carbon source/
feedstock
Inoculum type
Fermentation vessel
Yield of lactic acid
(g/L)
Yield of lactic acid
(g/100 g starch)
Reference
triticale starch spore flask 22 74 this study
triticale starch spore DASGIP bioreactor 26.1 87 this study
rice spore flask 45 45 11
corn spore flask unknown 35 23
oat starch spore airlift bioreactor 51.7 68 24
sweet potato starch mycelium stirred bioreactor 43.3 72 25
Little information is known about the kinetics of the directed fermentation of starch to LA by R. oryzae. In our study, the profiles obtained for starch, glucose accumulation, LA production, and pH during the entire fermentation process suggested that LA production proceeded according to the following: First, rapid hydrolysis of the starch provided nutrients and energy for fungal growth and for the amylolytic enzyme production (phase 1 and 2). Second, fast accumu-lation of glucose was observed due to amylolytic enzymes secreted by the fungus (phase 2 and 3). Third, rapid production occurred of LA that was subsequently secreted into the fermentation medium (phase 4). The kinetics of LA production appeared similar when a much higher inoculum size (1×106 spores/mL) was used compared to the
optimal inoculum size (1×104 spores/mL). However, the use of larger
inoculum sizes led to earlier production of LA, although yields were lower. It is assumed here that cultures that were started with a larger inoculum size grew faster and required more energy for growth than those started with a smaller inoculum size. Hence, determination of the appropriate inoculum size appears essential for optimal production of LA by R. oryzae. As expected, pH dropped during the fermenta-tion as LA was being produced (Figure 3B ). Among several neutral-izing agents, including sodium hydroxide, ammoniacal solution, and sodium bicarbonate, CaCO3 was found to be most effective for LA production from potato starch via R. oryzae.25 Several other studies
involved the use of CaCO3, as well as experimenting on the time of addition, dosage, and ratio of fermentation substrate-to-CaCO3.11,23,26–29
Our results indicated that time of addition together with dosage of CaCO3 was an important factor for LA production. Addition of CaCO3 immediately at the beginning of fermentation was not a good strategy, as it actually reduced LA yields compared to the control conditions. Most of the LA yield occurred between 24 and 40 h (phase 4 and 5). Addition of CaCO3 at the 24 h time point, at which time glucose accu-mulation had reached its peak, resulted in the highest LA production (Table 1). The best ratio (by weight) of triticale starch-to-CaCO3 for LA production was 1:1. With the addition of the right amount of CaCO3 at the right time, the pH of the fermentation broth could be maintained around 5 during the whole fermentation process.
The DASGIP parallel bioreactor offers various features for automated control while permitting accelerated process development.30 In this
study, LA production at the 2 L scale under a set of optimized conditions exceeded that observed in shake flasks. However, further challenges still need to be met, such as higher LA yields (g/L) and a shorter fermentation cycle. In this context, further optimization of key parameters, such as the use, or not, of fresh mycelium needs to be considered. Also, to meet the cost issues associated with starch processing, triticale flour and whole grains should be considered in future experiments.
In conclusion, triticale offers an interesting prospect of a nonfood crop for an important carbohydrate platform in a biorefinery value chain.
A C K N O W L E D G M E N T S
Funding of this work was provided by the Canadian Triticale Biorefinery Initiative (CTBI) of the Agricultural Bioproducts
Innovation Program of Agriculture and Agri-Food Canada. We thank members of the CTBI for their continuous support, and John Lu and Thava Vasanthan for discussion. We are grateful to S. Deschamp for his expert help in chemical analysis. This manuscript is issued as NRCC publication number 53354.
R E F E R E N C E S
1. Grull DR, Jetzinger F, Kozich M, Wastyn MM, and Wittenberger R. Industrial starch platform — Status quo of production, modification and application. In:
Biorefineries — Industrial processes and products. Status quo and future direc-tions. Kamm B, Gruber PR, and Kamm M (eds), vol 2, 61-95, Wiley-VCH Verlag
GmbH & Co. KGaA, Weinheim (2006).
2. Wee YJ, Kim JN, and Ryu HW. Biotechnological production of lactic acid and its recent applications. Food Tech Biotechnol 44, 163-172 (2006).
3. John RP, Anisha GS, Nampoothiri KM, and Pandey A. Direct lactic acid fer-mentation: Focus on simultaneous saccharification and lactic acid production.
Biotechnol Adv 27, 145-152 (2009).
4. Gruber P, Henton DE, and Starr J. Polylactic acid from renewable resources. In: Biorefineries — Industrial processes and products. Status quo and future
directions. Kamm B, Gruber PR, and Kamm M (eds), vol 2, 381-407, Wiley-VCH
Verlag GmbH & Co. KGaA, Weinheim (2006).
5. Datta R, and Henry M. Lactic acid: Fecent advances in products, processes, and technologies — A review. J Chem Technol Biotechnol 81, 1119-1129 (2006). 6. Nampoothiri KM, Nair, NR, and John RP. An overview of the recent
develop-ments in polylactide (PLA) research. Bioresource Technol 101, 8493-8501 (2010).
7. Pang X, Zhuang X, Tang Z, and Chen X. Polylactic acid (PLA): Research, development and industrialization. Biotechnol J 5, 1125-1136 (2010). 8. Katryniok B, Paul S, and Dumeignil F. Highly efficient catalyst for the
decarbox-ylation of lactic acid to acetaldehyde. Green Chem 12, 1910-1913 (2010). 9. Reddy G, Altaf Md, Naveena BJ, Ventkateshwar M, and Kumar EV. Amylolytic
bacterial lactic acid fermentation — A review. Biotechnol Adv 26, 22-34 (2008). 10. John RP, Nampoothiri KM, and Pandey A. Fermentative production of lactic
acid from biomass: An overview on process developments and future perspec-tives. Appl Microbiol Biotechnol 74, 524-534 (2007).
11. Yu RC and Hang YD. Kinetics of direct fermentation of agricultural commodi-ties to L(+) lactic acid by Rhizopus oryzae. Biotechnol Lett 11, 597-600 (1989). 12. Zhang ZY, Jin B, and Kelly JM. Production of lactic acid from renewable
materials by Rhizopus fungi. Biochem Eng J 35, 251-263 (2007). 13. Liu Y, Liao W, and Chen S. Co-production of lactic acid and chitin using a
pelletized filamentous fungus Rhizopus oryzae cultured on cull potatoes and glucose. J Appl Microbiol 105, 1521-1528 (2008).
14. Salmon DF, Mergoum M, and Gomez-Macpherson H. Triticale production and management. In: Triticale improvement and production. Mergoum M and Gomez-Macpherson H. (eds), 27-37; Plant production and protection paper 179, Food and Agriculture Organization of the United Nations, Rome, Italy (2004).
15. Oettler G. The fortune of a botanical curiosity — Triticale, past, present, and future. J Agric Sci 143, 329-346 (2005).
16. Triticale Production and Utilization Manual. Alberta Agriculture, Food and Rural Development (2005).
17. Buresova I, Sedlaclova I, Famera O, and Lipavsky J. Effect of growing conditions on starch and protein content in triticale grain and amylose content in starch.
Plant Soil Environ 56, 99-104 (2010).
18. Li C-Y, Li W-H, Lee B, Laroche A, Cao L-P, and Lu Z-X. Morphological charac-terization of triticale starch granules during endosperm development and seed germination. Can J Plant Sci 91, 57-67 (2011).
PEER
REVIEW
134 INDUSTRIALBIOTECHNOLOGY A P R I L 2 0 1 1
19. Kandil A, Li J, Vasanthan V, Bressler, DC, and Tyler RT. Compositional changes in whole grain flours as a result of solvent washing and their effect on starch amylolysis. Food Res Int 44, 167-173 (2011).
20. Henriksson G, Akin DE, Hanlin RT, Rodriguez C, Archibald DD, Rigsby LL, and Eriksson KL. Identification and retting efficiencies of fungi isolated from dew-retted flax in the United States and Europe. Appl Environ Microbiol 63, 3950-3956 (1997).
21. Xiao Z, Wang S, Bergeron H, Zhang J, and Lau PCK. A flax-reting endopoly-galacturonase-encoding gene from Rhizopus oryzae. Antonie van Leeuwenhoek 94, 563-571 (2008) .
22. Smith CS, Salde SJ, Nordheim EV, Cascino JJ, Harris RF, and Andrews JH. Sources of variability in the measurement of fungal spore yields. Appl Environ
Microbiol 54, 1430-1435 (1988) .
23. Hang YD. Direct fermentation of corn to L (+)-lactic acid by Rhizopus oryzae.
Biotechnol Lett 11, 299-300 (1989).
24. Koutinas AA, Malbranque F, Wang R, Campbell GM, and Webb C. Development of an oat-based biorefinery for the production of L (+)-lactic acid by Rhizopus
oryzae and various value-added coproducts. J Agric Food Chem 55, 1755-1761
(2007)
.
25. Yen HW, Chen TJ, Pan WC, and Wu HJ. Effects of neutralizing agents on lac-tic acid production by Rhizopus oryzae using sweet potato starch. World J
Microbiol Biotechnol 26, 437-441 (2010).
26. Yin P, Nishina N, Kosakai Y, Yahiro K, Park Y, and Okabe M. Enhanced produc-tion of L(+)-lactic acid from corn starch in a culture of Rhizopus oryzae using an air-lift bioreactor. J Ferment Bioeng 84, 249-253 (1997).
27. Zhou Y, Domínguez JM, Cao N, Du J, and Tsao GT. Optimization of L-lactic
acid production from glucose by Rhizopus oryzae ATCC 52311. Appl Biochem
Biotechnol 77-79, 401-407 (1999).
28. Liu Y, Liao W, Liu C, and Chen S. Optimization of L (+)-lactic acid produc-tion using pelletized filamentous Rhizopus oryzae NRRL 395 Appl Biochem
Biotechnol 131, 844-853 (2006).
29. Huang LP, Jin B, and Lant P. Direct fermentation of potato starch wastewater to lactic acid by Rhizopus oryzae and Rhizopus arrhizus. Bioproc Biosys Eng 27, 229-238 (2005).
30. Bareither R and Pollard D. A review of advanced small-scale parallel bioreac-tor technology for accelerated process development: Current state and future needs. Biotechnol Prog 27, 2-14 (2010)