HAL Id: hal-02952228
https://hal.inrae.fr/hal-02952228
Submitted on 29 Sep 2020
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
Distributed under a Creative Commons Attribution - NonCommercial| 4.0 International
Reevaluation of Blapimorpha and Opatrinae : addressing
a major phylogeny-classification gap in darkling beetles (
Coleoptera: Tenebrionidae: Blaptinae )
Marcin Kamiński, Ryan Lumen, Kojun Kanda, Dariusz Iwan, M. Andrew
Johnston, Gael Kergoat, Patrice Bouchard, Xing Long Bai, Xiu Min Li, Guo
Dong Ren, et al.
To cite this version:
Marcin Kamiński, Ryan Lumen, Kojun Kanda, Dariusz Iwan, M. Andrew Johnston, et al..
Reeval-uation of Blapimorpha and Opatrinae : addressing a major phylogeny-classification gap in darkling
beetles ( Coleoptera: Tenebrionidae: Blaptinae ). Systematic Entomology, Wiley-Blackwell, In press,
�10.1111/syen.12453�. �hal-02952228�
Reevaluation of Blapimorpha and Opatrinae: addressing
a major phylogeny-classification gap in darkling beetles
(Coleoptera: Tenebrionidae: Blaptinae)
M A R C I N J . K A M I ´
N S K I
1,2, R Y A N L U M E N
1, K O J U N K A N D A
3,
D A R I U S Z I W A N
2, M . A N D R E W J O H N S T O N
4, G A E L J .
K E R G O A T
5, P A T R I C E B O U C H A R D
6, X I N G L O N G B A I
7, X I U
M I N L I
7, G U O D O N G R E N
7and A A R O N D . S M I T H
11Department of Entomology, Purdue University, West Lafayette, Indiana, USA,2Zoological Museum, Museum and Institute of Zoology, Polish Academy of Sciences, Warszawa, Poland,3USDA Systematic Entomology Laboratory, c/o Smithsonian Institution, National Museum of Natural History, Washington, District of Columbia, USA,4Biodiversity Knowledge Integration Center, Arizona State University, Tempe, Arizona, USA,5CBGP, INRAE, CIRAD, IRD, Montpellier SupAgro, University Montpellier, Montpellier, France,6Canadian National Collection of Insects, Arachnids and Nematodes, Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada and7The Key Laboratory of Zoological Systematics and Application, College of Life Sciences, Institute of Life Science and Green Development, Hebei University, Baoding, PR China
Abstract. The taxonomic concepts of Blapimorpha and Opatrinae (informal and
tra-ditional, morphology-based groupings among darkling beetles) are tested using molec-ular phylogenetics and a reassessment of larval and adult morphology to address a major phylogeny-classification gap in Tenebrionidae. Instead of a holistic approach (family-level phylogeny), this study uses a bottom-up strategy (tribal grouping) in order to define larger, monophyletic lineages within Tenebrioninae. Sampling included rep-resentatives of 27 tenebrionid tribes: Alleculini, Amarygmini, Amphidorini, Blaptini, Bolitophagini, Branchini, Cerenopini, Coniontini, Caenocrypticini, Dendarini, Eulabini, Helopini, Lagriini, Melanimini, Opatrini, Pedinini, Phaleriini, Physogasterini, Platyno-tini, Platyscelidini, Praociini, Scaurini, Scotobiini, Tenebrionini, Trachyscelini, Triboli-ini and UlomTriboli-ini. Molecular analyses were based on DNA sequence data from four non-overlapping gene regions: carbamoyl-phosphate synthetase domain of
rudimen-tary (CAD) (723 bp), wingless (wg) (438 bp) and nuclear ribosomal 28S (1101 bp) and
mitochondrial ribosomal 12S (363 bp). Additionally, 15 larval and imaginal charac-ters were scored and subjected to an ancestral state reconstruction analysis. Results revealed that Amphidorini, Blaptini, Dendarini, Pedinini, Platynotini, Platyscelidini and Opatrini form a clade which can be defined by the following morphological features: adults – antennae lacking compound/stellate sensoria; procoxal cavities externally and internally closed, intersternal membrane of abdominal ventrites 3–5 visible; paired abdominal defensive glands present, elongate, not annulated; larvae – prolegs enlarged (adapted for digging); ninth tergite lacking urogomphi. To accommodate this mono-phyletic grouping (281 genera and ∼4000 species), the subfamily Blaptinae sens. nov. is resurrected. Prior to these results, all of the tribes within Blaptinae were classified within the polyphyletic subfamily Tenebrioninae. The non-monophyletic nature of Terebrion-inae has already been postulated by previous authors, yet no taxonomic decisions were
Correspondence: Marcin J. Kami´nski, Purdue University, Department of Entomology, 901 W. State Street, West Lafayette, IN 47907, U.S.A.; and Zoological Museum, Museum and Institute of Zoology, Polish Academy of Sciences, Wilcza 64, 00-679 Warszawa, Poland. E-mail:
made to fix its status. The reinstatement of Blaptinae, which groups ∼50% of the for-mer Tenebrioninae, helps to clarify phylogenetic relations among the whole family and is the first step towards a complete higher-level revision of Tenebrionidae. The Central Asian tribe Dissonomini (two genera, ∼30 species) was not included in Blaptinae due to a lack of representatives in the performed phylogenetic analyses; however, based on morphological features, the tribe is listed as a potential addition to the subfamily.
Introduction
Darkling beetles (Tenebrionidae Latreille) represent one of the most diverse insect families, with over 20 000 described species (Matthews et al., 2010). As a result of their worldwide distri-bution and common xerophily, darkling beetles have been used in a variety of biogeographic studies (Fattorini, 2002a,b; Fat-torini & Fowles, 2005; Condamine et al., 2013; Kami´nski, 2015; Kami´nski et al., 2018; Johnston, 2019). Currently, the fam-ily is subdivided into 10 subfamilies: the nine proposed in Matthews et al. (2010) and Bouchard et al. (2011): Alleculinae Laporte, Diaperinae Latreille, Lagriinae Latreille, Nilioninae Oken, Phrenapatinae Solier, Pimeliinae Latreille, Stenochiinae Kirby, Tenebrioninae Latreille and Zolodininae Watt plus Kuhi-tangiinae Medvedev considered previously as tribe of Pimeli-inae but erected as subfamily by Nabozhenko & Sadeghi (2017). However, this classification system is far from stable, and it is not supported by available phylogenetic data (Doyen & Tschinkel, 1982; Kergoat et al., 2014; Kanda, 2017).
Both molecular and morphological data point to Tenebrion-inae as one of the most problematic subfamilies among dark-ling beetles. The diversity of this grouping is estimated to be about 8000 species, which are distributed in 30 morphologically diverse tribes (Bouchard et al., 2011; Kami´nski et al., 2019a). According to Kergoat et al. (2014), Tenebrioninae is para-phyletic with regard to Alleculinae, Diaperinae, Phrenapatinae and Stenochiinae; a similar scenario was also presented by Kanda (2017). This reflects the fact that, for the majority of its history, Tenebrioninae has been treated as a ‘dumping-ground’ for the whole family and is largely defined by the absence of apomorphies that characterize the other subfamilies (Watt, 1974; Doyen, 1989). A number of informal groupings have been pro-posed within the heterogeneous assemblage of tribes currently in Tenebrioninae. In this study, two of these concepts, ‘Blapi-morpha’ (sensu Skopin, 1964) and the ‘opatrine lineage’ (sensu Doyen & Tschinkel, 1982) are investigated.
The concept of Blapimorpha was first proposed by Skopin (1962, 1964), to group tribes characterized by the following larval features: absence of urogomphi; enlarged forelegs with spines (on ventral side) and asymmetrical chaeto-taxy, presumably modified for digging; bipartite tarsungulus. Skopin (1962, 1964) mentioned that this group includes the majority of tribes listed in Gebien’s (1937, 1939, 1940, 1941
, 1942a,b) system, from Scaurini Billberg up to Cryp-ticini Brullé, except Pimeliini Latreille. Therefore, his con-cept of Blapimorpha includes the following currently recog-nized tribes (Table 1): Amphidorini LeConte, Blaptini Leach,
Cerenopini Horn, Dissonomini Medvedev, Eulabini Horn, Opa-trini, Pedinini, Platyscelidini Lacordaire, Scaurini, Scotobi-ini Solier (representing Tenebrioninae), BranchScotobi-ini LeConte, Coniontini Waterhouse, Caenocrypticini Koch, Lachnogyini Seidlitz, Physogasterini Lacordaire, Praociini Eschscholtz (rep-resenting Pimeliinae), Phaleriini Blanchard, Trachyscelini Blan-chard (representing Diaperinae).
Watt (1974) largely retained the concept of Blapimorpha in his seminal revision of the subfamilial classification of Tenebrion-idae (excluding the pimeliine tribes Coniontini, Caenocrypticini, Physogasterini, Praocini and Branchini). He noted that the larval characters were likely sufficient to support the phylogenetic distinctiveness of this assemblage; only because of the lack of reliable adult characters did he not give it subfamily status. Other authors have criticized the concept of Blapimorpha, stating that the group is defined by evolutionarily convergent features related to soil-dwelling larvae (Doyen, 1972; Schulze, 1969). Iwan & Beˇcváˇr (2000) were the last to discuss the concept of Blapimor-pha. Based on newly available larval material and a comprehen-sive study of the literature, they refuted some of the diagnostic features proposed by Skopin (1962) and Watt (1974). For instance, they showed that the bipartite tarsungulus occurs only in some Opatrini Brullé and Pedinini Eschscholtz, and there-fore it cannot be used to unambiguously define Blapimorpha. They concluded that, although they recognized Blapimorpha as monophyletic, no reliable larval characters were ever proposed to define the group. Only the most general definition was sus-tained, i.e., the absence of urogomphi and enlarged, prominent, digging forelegs with tubercles and asymmetrical chaetotaxy.
The concept of Opatrinae (opatrinoid beetles) is much more commonly recognized among tenebrionid workers ver-sus Blapimorpha (Koch, 1956; Medvedev, 1968; Doyen & Lawrence, 1979; Iwan, 2001, 2004, 2006; Aalbu et al., 2002; Johnston, 2019). In the current classification of Tenebrionidae, species of Opatrinae are included in Tenebrioninae, but as noted by Iwan (2001), this group is abiit, non obiit (dead, but not forgotten). Nineteen years later, the situation has not changed. Although it is not listed as a separate subfamily in the currently accepted classification of darkling beetles (Bouchard
et al., 2011; Bousquet et al., 2018), Opatrinae informally exists
in the collections and minds of many tenebrionid workers (Kami´nski pers. obsrv.). Opatrinoid beetles are distinguished from other Tenebrioninae by the deeply emarginate clypeus (Reitter, 1917). Although the exact composition varies with different researchers, Opatrinae usually includes a restricted list of tribes from Blapimorpha. The most commonly listed tribes are (Iwan & Beˇcváˇr, 2000; Iwan, 2006): Dendarini,
Table 1. List of the genera included in the phylogenetic analysis, with their tribal and subfamilial affiliation
Subfamily Tribe Sampled genera
In-group:Blapimorpha sensu Skopin (1964), except for Dissonomini and Lachnogyini
Blaptinae stat nov.(formerly Tenebrioninae) Amphidorini Eleodes (10), Embaphion, Lariversius, Neobaphion, Trogloderus
Blaptini Agnaptoria (2), Blaps (3), Blaptogonia, Gnaptor, Gnaptorina (4), Prosodes (2)
Dendarini Dendarus (3), Heliopates (3), Hoplarion (2), Litoborus (3), Meglyphus
(2), Melambius (2), Melansis (2), Minorus (3), Oreomelasma,
Phylan (2), Zadenos (5)
Opatrini Adoryacus, Ammobius, Blapstinus (2), Caedius, Gonocephalum (2), Opatrum, Parastizopus, Planostibes, Sulpius, Ulus
Pedinini Anaxius, Drosochrus (2), Leichenum (4), Micrantereus (2), Pedinus (4)
Platynotini Alaetrinus (2), Atrocrates (2), Crypticanus, Eurynotus (3), Gonopus, Melanopterus, Monodius, Oncotus (3), Schelodontes, Sebastianus, Styphacus, Trigonopus, Zidalus
Platyscelidini Bioramix (2), Myatis, Oodescelis (3), Platyscelis (2), Somocoelia
Diaperinae Phaleriini Phaleromella
Trachyscelini Trachyscelis
Pimeliinae Branchini Branchus
Caenocrypticini Caenocrypticus
Coniontini Eusattus
Physogasterini Physogaster
Praocini Praocis
Tenebrioninae Cerenopini Argoporis, Cerenopus
Eulabini Apsena, Eulabis
Melanimini Cheirodes
Scaurini Scaurus
Scotobiini Scotobius
Out-group:Tribes never affiliated with Blapimorpha or Opatrinae
Alleculinae Alleculini Hymenorus
Lagriinae Lagrinii Statira
Tenebrioninae Amarygmini Pimelionotus
Bolitophagini Bolitotherus
Helopini Helops
Tenebrionini Rhinandrus, Tenebrio, Zophobas
Triboliini Tribolium
Ulomini Uloma
Note: Numbers in parentheses indicate the number of included OTUs representing a given genus.
Melanimini Seidlitz, Pedinini, Platynotini, Platyscelidini and Opatrini.
The concepts of Blapimorpha and Opatrinae have not been formally evaluated since their original examination(s) provided above, and have largely been based upon morphology. Addition-ally, while the more recent molecular phylogenies of Kergoat
et al. (2014) and Kanda (2017) tested some of the higher level
(i.e., subfamily) problems within Tenebrionidae, their scope and taxon sampling did not provide enough data to test the con-cepts of Blapimorpha and Opatrinae by themselves. Recent phy-logenetic efforts based on molecular data from independent research groups from China, France, Poland and the United States have provided some insights into the relationships within some of the most commonly included tribes within Blapimorpha and Opatrinae: Amphidorini (Johnston, 2019), Blaptini (Con-damine et al., 2011, 2013; Soldati et al., 2017), Platyscelidini (Bai et al., 2019), Dendarini, Pedinini, Platynotini and Opatrini (Kami´nski et al., 2018, 2019a; Lumen et al., 2020). This has
resulted in a diverse dataset of DNA sequences which when combined, provide a unique opportunity to test the historically morphologically based concepts of Blapimorpha and Opatrinae with molecular data. The aim of this paper is to integrate these data within a phylogenetic framework.
Materials and methods
Taxon sampling
In order to test the monophyly and composition of the ‘Blapi-morpha/Opatrinae’ clade, ingroup taxa representing as many as possible tribes prescribed initially by Skopin (1964) in Blapimorpha were included. In particular, 14 representatives of Amphidorini, 13 of Blaptini, 28 of Dendarini, 13 of Opa-trini, 13 of Pedinini, 19 of Platynotini and nine of Platyscelidini (Table 1). The following tribes were represented by at least one
representative: Branchini, Cerenopini (two species), Conion-tini, Caenocrypticini, Eulabini (two species), Melanimini, Pha-leriini, Physogasterini, Praociini, Scaurini, Scotobiini and Tra-chyscelini (Table 1, Supplementary Material S1). Representa-tives of the following tribes were not included in the analyses due to the lack of well-preserved specimens: Dissonomini and Lachnogyini. However, their potential affiliation to the group of interest based on morphological traits is discussed below. Outgroups from other tribes and/or subfamilies previously not affiliated with Blapimorpha were also included to test the bound-aries and affinities of historically included groups that have since been assigned to other subfamilies (Supplementary Mate-rial S1): Pimelionotus lugens (Fåhraeus) (Amarygmini Gis-tel), Tribolium castaneum (Herbst) (Triboliini GisGis-tel), Tenebrio
molitor L., Rhinandrus sp., and Zophobas atratus (Fabricius)
(Tenebrionini Latreille), Helops sp. (Helopini Latreille), Uloma
tenebrionoides (White) (Ulomini Blanchard), Bolitophagus sp.
(Bolitophagini Kirby), Hymenorus sp. (Alleculini Laporte) and
Statira pluripunctata Horn (Lagriini Latreille).
All sequences of Dendarini, Pedinini, Platynotini and Opa-trini were generated during previous phylogenetic projects (Kami´nski et al., 2018, 2019a,b; Lumen et al., 2020), as were selected Amphidorini (Johnston, 2019). Other sequences were newly generated for this study or were sequenced as part of Kanda’s (2017) unpublished disser-tation. Previously unpublished sequence data are available on GenBank (MT661944-MT661969; MT663963-MT664040; MT647841-MT6478880; Supplementary Material S1). Voucher specimens are deposited in the institutional collections of the authors.
DNA extraction and sequencing
Since the concatenated matrix analysed here is the result of a collective international effort, the extraction and sequenc-ing methods vary between the two included taxonomic sub-sets, i.e., Blaptini + Platyscelidini (first subset) and remain-ing taxa (second subset). Laboratory procedures used for par-ticular subsets are described below. However, regardless of used extraction and sequencing methodology four gene regions were amplified for all included taxa using PCR protocols and primers given in Kanda et al. (2015): nuclear protein-coding genes carbamoyl-phosphate synthetase domain of rudimentary (CAD) (723 bp), wingless (wg) (438 bp), and nuclear riboso-mal 28S (1101 bp) and mitochondrial ribosoriboso-mal 12S (363 bp). Details were also presented in Supplementary Material S2. These regions were chosen because when concatenated, they recovered a strongly supported phylogeny for relationships for tribes traditionally included within the concept of Blapimorpha (i.e., Dendarini, Pedinini, Platynotini and Opatrini (Kami´nski
et al., 2018, 2019a; Lumen et al. 2020)).
First taxonomic subset (Blaptini + Platyscelidini). DNA was
extracted from leg muscles using EZNA® Insect DNA Kits (Omega Bio-tek, USA). Polymerase chain reactions (PCR) were performed with standard settings for primer sequences and thermocycler procedures from Kanda et al. (2015). The
PCR products were subsequently checked by 1% agarose gel electrophoresis and sequencing was performed at GENEWIZ Biotech Co., Ltd. (Suzhou, China) using the same primers as in the PCR.
Second taxonomic subset (remaining taxa). DNA was
extracted from specimens preserved in 95% ethanol and recently pinned specimens using DNeasy Blood & Tissue Kits (Qiagen, Germantown, MD, USA) following the manufacturer’s protocols. Specimens were disarticulated into three parts (head, thorax and abdomen) and inserted into a buffer for proteinase K digestion following protocols in Kanda et al. (2015). Polymerase chain reactions were performed using ExTaq (Takara, Mountain View, CA, USA). Clean-up, quantification and sequencing were performed by the University of Arizona’s Genetics Core Facility (UAGC). Cleaned PCR products were sequenced on a 3730XL DNA Analyzer (Applied Biosystems, Foster City, CA, USA).
DNA analyses
Sequences were aligned in Mesquite 3.61 (Maddison & Mad-dison, 2019) with MAFFTv. 7.130b (Katoh & Standley, 2013) using the L-INS-i method. All alignments were concatenated into a single matrix (2625 bp) for phylogenetic analyses (Supple-mentary Material S3). Data partitions and models of sequence evolution for Bayesian phylogenetic analyses (BI) were assessed in Partitionfinder v. 2.1.1 (Lanfear et al., 2017) implemented on the CIPRES Science Gateway 3.3 (Miller et al., 2010), with the concatenated dataset initially partitioned by gene and codon position (for protein coding genes). Models were com-pared using the greedy algorithm, the unlinked option for branch lengths, and the Bayesian information criteria (BIC). Bayesian analyses were run through CIPRES using MrBayes (v. 3.2.7a) (Ronquist et al., 2012). Two independent runs were performed, each with four chains. Analyses were run for 20 million gener-ations, and parameters were sampled every 1000 generations. A burnin fraction of 25% was used, and convergence was checked by visualising parameters in Tracer v. 1.7.1 (Rambaut
et al., 2018). Maximum likelihood (ML) analysis was conducted
in IQ-TREE v. 1.6.10 (Nguyen et al., 2015) on the CIPRES Sci-ence Gateway. The run was performed with edge-proportional partition models (-spp). Branch support was estimated with 1000 ultrafast bootstrap replicates (Minh et al., 2013), using the ‘bnni’ approach to reduce the risk of overestimating support values (Hoang et al., 2018), and an increased value of maximum num-ber of iterations to stop (−nm 10 000). Models of sequence evo-lution for ML analysis were assessed in IQ-TREE prior to phy-logenetic analysis (Supplementary Material S4). In discussing support for obtained relationships, the following abbreviations are used: UFB ultrafast bootstrap; PP Bayesian posterior prob-ability. Node support is defined as low/weak (UFB = 70–80, PP = 0.90–0.94), moderate (UFB = 81–95, PP = 0.95–0.97), or strong/high (values above those previously mentioned).
Supporting morphological analysis
A comparative study of selected morphological features was performed within Skopin’s (1964) Blapimorpha in order to
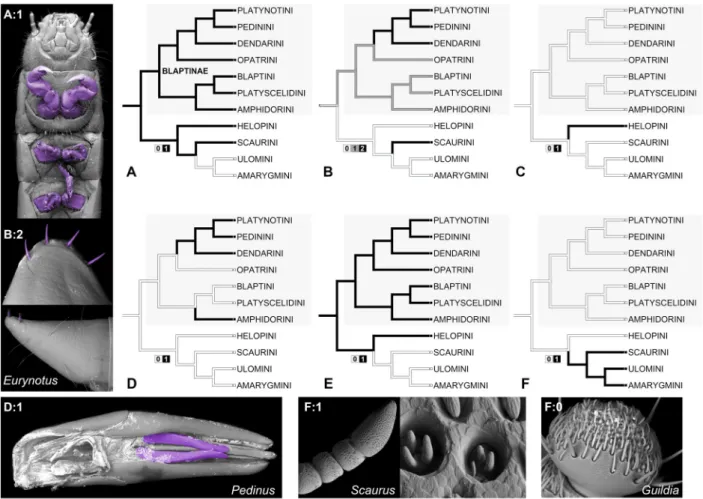
assess the obtained phylogenetic hypotheses (Supplementary Material S5). Analysed characters were selected based on a liter-ature search, which included the following publications: (Koch, 1956; Skopin, 1962, 1964; Medvedev, 1968; Doyen, 1972; Watt, 1974; Doyen & Tschinkel, 1982; Iwan & Beˇcváˇr, 2000; Aalbu et al., 2002; Johnston, 2019; Kami´nski et al., 2019a, b). The distribution of characters within groups was verified by referring to specimens preserved in the following collections: Ditsong National Museum of Natural History (Pretoria, South Africa), Museum and Institute of Zoology, Polish Academy of Sciences (Warsaw, Poland), Field Museum (Chicago, USA), Purdue Entomological Research Collection, Purdue Univer-sity (West Lafayette, USA), Hebei UniverUniver-sity Museum (Hebei, China). In order to trace evolutionary patterns of morphological features, a maximum parsimony ancestral state reconstruction was conducted in Mesquite 3.61 using a simplified phylogenetic topology obtained in MrBayes (Fig. 3; Supplementary Material S5). Photographs were taken using a Canon 1000D body and Canon EF 100 mm f/2.8 Macro USM lens. SEM images were acquired with a Hitachi S-3400 N at the Museum and Institute of Zoology, Polish Academy of Sciences.
Results
Morphological analysis
The analysis performed here enabled a morphologically con-scious interpretation of the phylogenetic results based on molec-ular data. Fifteen larval and imaginal characters were scored and subjected to an ancestral state reconstruction analysis (Supple-mentary Material S4). This enabled delimitation of the newly reinstated subfamily Blaptinae (see below), and rejection of some previously used synapomorphies as diagnostic for opatri-noid beetles (e.g., deeply emarginate clypeus) (Reitter, 1917). The character analysis revealed that Amphidorini, Blaptini, Dendarini, Opatrini, Pedinini, Platynotini and Platyscelidini (referred here as Blaptinae or Blapimorpha sensu novo) can all be clearly delimited from the other Tenebrionidae by the following combination of adult (a) and larval (b) features: (a) antennae lacking compound/stellate sensoria (Fig. 3F); procoxal cavities closed externally and internally, abdomen with inter-sternal membrane of abdominal ventrites 3–5 (see Aalbu et al. 2002); paired abdominal defensive glands present, elongate, not annulated; (b) prolegs enlarged (adapted for digging) (Fig. 3A); ninth tergite lacking urogomphi (Fig. 3C). On the other hand, no synapomorphies were recovered for this diverse taxonomic grouping. Analyses also implied a close relation between Blapti-nae and Helopini. However, both lineages are clearly distin-guishable in both adult and larval stages (Fig. 3). Details are presented in the discussion.
Molecular phylogeny
The monophyly of Blapimorpha as defined by Skopin (1962, 1964) was not supported by any of the used inference methods
(Figs 1, 2). However, the following tribes were recovered together regardless of the used inference method: Amphi-dorini, Blaptini, Dendarini, Opatrini, Pedinini, Platynotini and Platyscelidini (Fig. 1). Support for this clade varied with the used inference method from weak in the ML analysis (UFB: 68) to high in the BI (PP: 1.0). In the ML analyses, represen-tatives of Helopini were recovered sister to Blaptinae; how-ever, the support for this topology was weak (UFB: 56). Fur-thermore, on the ML consensus tree the Blaptinae+Helopini clade is sister to Scaurini; although the support for this group-ing is negligible (UFB: 33). On the other hand, in the Bayesian topology Blaptinae is sister to a clade containing representa-tives of Alleculini, Amarygmini, Helopini, Lagriini, Phaleriini, Scaurini, Trachyscelini and Ulomini (Fig. 2). The branch sup-port for the clade containing Blaptinae and all those tribes is weak (PP: 0.8).
Within Blaptinae both inference methods recovered con-flicting topologies. The differences concerned relations within the opatrinoid clade (Fig. 1). Namely, Opatrini was recov-ered sister to Pedinini+Platynotini in the Bayesian analysis (PP: 1.0 for the Pedinini+Platynotini+Opatrini clade). While in the maximum likelihood analysis it was rendered sister to all other members of the ‘opatrinoid’ clade (UFB: 70 for Dendarini+Pedinini+Platynotini). Relations within the ‘blap-toid’ clade remained unchanged regardless of the used infer-ence method. Branch support for all major phylogenetic lineages within this clade was high in both analyses (Fig. 1).
Within the majority of tribes representing Blaptinae, the recovered topologies did not vary across inference methods (Fig. 2; Supplementary Material S4). The main difference concerned the phylogenetic status of Pedinus Latreille, which was rendered paraphyletic in the ML analysis, and monophyletic with high support (PP: 1.0) in the Bayesian analysis. Some slight topology deviations were also found within Amphidorini. The subfamily Tenebrioninae was recovered as non-monophyletic by both used inference methods (Fig. 2).
Discussion
Concept of Blaptinae
Although some molecular-based phylogenetic studies have been conducted on Tenebrionidae (Kergoat et al., 2014; Kanda, 2017), this is one of the first to address a particular hypothesis formulated by previous authors based on mor-phological data. The polyphyletic nature of Tenebrioninae was already postulated by previous analyses of higher-level classification problems within darkling beetles (e.g., Doyen & Tschinkel, 1982; Kergoat et al., 2014). However, no offi-cial changes to classification have been made to address the issue. The main reason for the lack of classification is likely insufficient data in both morphological (shallow coverage of intertribal diversities) and molecular (poor branch sup-port for deeper nodes) studies. Instead of a holistic approach (family-level phylogeny), this study uses a bottom-up strategy (tribal grouping) in order to define large monophyletic lineages within Tenebrioninae.
Fig 1. Cladograms illustrating phylogenetic relationships with the subfamily Blaptinae. Simplified consensus topologies recovered in Bayesian (left) and ML analyses (right). Triangle height corresponds to sampling effort (wider/larger triangle = more taxa sampled). Branch support values: Bayesian posterior probability (PP: 0.0–1.0) and maximum likelihood ultrafast bootstrap percentage (BP: 0–100). For clarity Opatrini is highlighted with asterisks. [Colour figure can be viewed at wileyonlinelibrary.com].
The recent designation of the ‘opatrinoid’ clade (Dendarini+Pedinini+Platynotini+Opatrini) by Kami´nski
et al. (2019a) has provided an interesting starting point for the
present investigation, as that phylogenetic grouping contains >30% of the species diversity within Tenebrioninae. However, the set of outgroups employed by Kami´nski et al. (2019a) was insufficient to decide if the ‘opatrinoid’ clade warranted subfam-ily status. As such, the concept of Blapimorpha (Skopin, 1962; Watt, 1974; Iwan & Beˇcváˇr, 2000) seemed to be a valuable lead. Especially when considering the phylogenetic analyses conducted by Kanda (2017) suggested a sister relation between the ‘opatrinoid’ clade and Amphidorini+Blaptini.
It is not surprising that Skopin’s (1964) concept (original tribal composition) of Blapimorpha was not supported by the presently conducted phylogenetic analyses, as the group initially consisted of all tenebrionid tribes with soil-dwelling larvae. As hypothe-sized by Doyen (1972), in the majority of those tribes certain features (enlarged prolegs) evolved convergently and should not be used as synapomorphies (see also Schulze, 1969). However,
Doyen’s radical concept did not allow for partial scenarios. As revealed here, the similar morphology of Amphidorini, Blaptini, Dendarini, Pedinini, Platynotini, Platyscelidini and Opatrini lar-vae was likely inherited from a common ancestor (Fig. 3A). This set of tribes is largely convergent with the concept of Opatrinae (Iwan & Beˇcváˇr, 2000). Taking into consideration the taxonomic diversity of this tribal grouping (281 genera, ∼4000 species; ∼50% of Tenebrioninae species), the recovery of this evolu-tionary lineage significantly clarifies phylogenetic relationships within the family Tenebrionidae. In order to fix the concepts presented here around a discrete, monophyletic grouping, res-urrection of the subfamily Blaptinae Leach (type genus Blaps Fabricius; = Opatrinae junior synonym) is proposed (Fig. 2). This decision also highlights the need for extensive revisionary changes in darkling beetle classification, as the current subfamil-ial classification does not accurately reflect phylogenetic rela-tionships within the family (Doyen & Tschinkel, 1982; Kergoat
et al., 2014; Kanda, 2017). In other words, the growing
Fig 2. Detailed phylogeny of Blaptinae. Presented topology is the majority rule consensus of post-burn-in trees obtained in Bayesian analysis of the concatenated CAD, wg, 12S and 28S matrix. Posterior probabilities are displayed above branches in red. Taxa marked with light grey represent subfamilies outside Tenebrioninae. Morphological diversity of the subfamily (A–T): Platynotini (A–D): Platynotus (A), Notocorax (B), Alaetrinus (C),
Anomalipus (D); Pedinini (E–H): Diestecopus (E), Pedinus (F), Emyon (G), Leichenum (H); Opatrini (I–L): Blapstinus (I), Heterotarsus (J), Planostibes
(K), Psammogaster (L); Dendarini (M–N): Litoborus (M), Heliopates (N); Blaptini (O–P): Blaps (O), Prosodes (P); Platyscelidini (Q–R): Bioramix (Q), Oodescelis (R); Amphidorini (S–T): Embaphion (S), Eleodes (T). [Colour figure can be viewed at wileyonlinelibrary.com].
a more natural classification system (Franz, 2005). Future stud-ies should challenge the status of Blaptinae by investigating its relations with other larger evolutionary lineages among Tenebri-onidae.
Morphological delimitation of Blaptinae
The limits of Blaptinae have been comprehensively tested here (except for Dissonomini and Lachnogyini) by contrasting traditional morphology-based hypotheses with molecular data (Figs 1, 2; Supplementary Material S4). Additionally, some of the tribes representing Blapimorpha sensu Skopin (1962, 1964) have already been classified within different subfam-ilies based on discrete characters by previous workers or, outside of convergent traits, are otherwise extremely divergent from Blaptinae morphologically (e.g., Lachnogyini and Tra-chyscelini) (Ferrer, 2003; Masumoto et al., 2012; Nabozhenko & Purchart, 2017). However, in order to assess the reliability of the acquired phylogenetic topologies (Figs 1, 2), a discussion of morphological characters of some presently and historically closely recovered tribes within Tenebrioninae (Amarygmini, Helopini, Scaurini and Ulomini) is conducted here. It should be underlined that the relationships between and among these groups are outside the scope of this work.
A close affiliation between representatives of Blaptinae and Helopini has been postulated by many authors based on different datasets. Tschinkel & Doyen (1980) noted that Amphidorini, Helopini and Opatrini share a trend toward bilobed reservoirs of the abdominal defensive glands (with no common volume between the reservoirs). In the phylogenetic analyses conducted by Doyen & Tschinkel (1982) Helopini and Ulomini were frequently closely recovered as sister to ‘Opatrini’ – an OTU largely corresponding to Blaptinae as defined here. Further-more, Purchart & Nabozhenko (2012) indicated that the larvae of Helopini possess slightly enlarged and elongate prolegs, which might indicate a close affiliation between it and Blapti-nae. However, the delimitation selected here for the subfamily separates it from Helopini mainly by larval morphology. As revealed by Purchart & Nabozhenko (2012), helopine larvae possess an extremely short ninth tergite bearing long urogomphi overlapping segment VIII, and segment VIII bearing spines on the dorsal side (see also Nabozhenko & Gurgenidze, 2006; Matthews & Lawrence, 2015). These features are not present in Blaptinae (Medvedev, 1968, 2001; Yu et al., 2000; Smith
et al., 2014; Kami´nski et al., 2019b). Additionally, Tschinkel
and Doyen (1982) clustered Helopini, Ulomini and ‘Opa-trini’ using characters that are widespread and plesiomorphic within Tenebrionidae (e.g., hindwing flecks, mandibular molae structure and ‘short’ ovipositor coxite 1), which they also noted. The analyses conducted here, regardless of inference method, recovered Helopini outside the ‘urogomphiless’ clade (Fig. 3C) of Blaptinae. Additionally, features of the ovipositor separating Helopini and Blaptini were listed by Tschinkel & Doyen (1980). While this does not rule out a sister relationship with Blaptinae, there is enough evidence here to support Helopini’s exclusion from Blaptinae. Clarifying its position within Tenebrioninae and
relationship with other tribes sampled in this study (Scaurini, Ulomini, Amarygmini) would require more sampling from these groups, and is outside the purview of this study.
Ulomini can be separated from all the tribes of Blaptinae by the following adult features: dorsoventrally flattened antennae bear-ing stellate/placoid sensillae on terminal antennomeres and an exposed pygidium (tergite VII) (Matthews & Bouchard, 2008). Available larval descriptions for Ulomini are scarce; how-ever, those available emphasize differing morphology com-pared to Blaptinae (e.g., shortened prothoracic legs comcom-pared to meso/meta thoracic legs) (Hayashi, 1964, 1966, 1968). Inves-tigating the relation between Helopini, Ulomini and Blaptinae is one of the most promising avenues to further clarify phyloge-netic relations within Tenebrionidae (Tschinkel & Doyen, 1980; Doyen & Tschinkel, 1982; Purchart & Nabozhenko, 2012; Fig. 3).
Although some of the known larval stages of Scaurini (namely Herpiscius sommeri Solier) show strong morpho-logical resemblance to Blaptinae larvae, this phenomenon should be interpreted as a result of evolutionary convergence (Schulze, 1969). Adult representatives of Scaurini can be sep-arated from those of Blaptinae by a number of morpholog-ical characters (see Berry, 1973), of which the most promi-nent is the elongation of the head behind the eyes. Blapti-nae differs from Amarygmini by having symmetric aedeagal tegmina (Bremer & Lillig, 2014). Both Amarygmini and Scau-rini additionally differ from Blaptinae in the presence of stel-late sensoria on the apical antennomeres, which Blaptinae lacks (Medvedev, 1977).
Phylogenetic relationships within Blaptinae
The ‘opatrinoid’ clade of Blaptinae was recovered differently than in previous studies (Kami´nski et al., 2019a). In the results presented here, Platynotini was rendered sister to Pedinini, and the relationship between Dendarini, Playtnotini+Pedinini and Opatrini changed depending on the inference method used (Fig. 1). However, similar to Kami´nski et al. (2019a), relation-ships below the tribal level were relatively stable. The topol-ogy in the ML analyses, despite having lower support than the Bayesian analysis, better converges upon morphological trends within the ‘opatrinoid’ clade. While there are some outliers within specialized Namibian genera (e.g., Periloma Gebien,
Psammogaster Koch) (Schulze, 1963), there is a trend within
opatrine larvae to have many spines on the terminal abdominal segments (Kami´nski et al., 2019b). Similar abdominal struc-tures are also observed in the ‘blaptoid’ clade (Fig. 3B). Con-versely, the rest of the ‘opatrinoid’ clade (Pedinini, Dendarini and Platynotini) show a trend towards a reduction of these spines (Kami´nski et al., 2019b). Furthermore, representatives of Den-darini, Pedinini and Platynotini (except Eurynotina Mulsant and Rey) all possess aedeagal clavae (Fig. 3D). These morphologi-cal trends lend further support to the ML topology. At this point, it should be noted that the phylogenetic relationships within the ‘opatrinoid’ clade have not been ultimately fixed. Future studies employing NGS datasets will likely resolve the current
Fig 3. Evolution of selected morphological features in Blaptinae and selected related tenebrionid tribes: (A) structure of larval prolegs, 0: not enlarged, 1: enlarged; (B) spine arrangement on larval ninth tergite, 0: arrangement different than following, 1: >8 short spines present (most cases), 2: 4–6 elongate spines present (most cases) (see Kami´nski et al., 2019b for exceptions); (C) larval ninth tergite, 0: elongate, without urogomphi; 1: short and equipped with urogomphi (Purchart & Nabozhenko, 2012); (D) aedeagal clavae, 0: absent; 1: present (see Kami´nski et al., 2019a for exceptions); (E) defensive glands, 0: with common volume, 1: without common volume (after Tschinkel & Doyen, 1980); (F) antennal stellate sensoria, 0: absent; 1: present (see Medvedev, 1977). Character optimizations analysed using the maximum parsimony method in Mesquite. SEM images illustrate chosen states used in morphological analysis. Tribal assignment: Guilda (Dendarini), Eurynotus (Platynotini), Pedinus (Pedinini), Scaurus (Scaurini). [Colour figure can be viewed at wileyonlinelibrary.com].
ambiguity in these relationships. On the other hand, the ‘blap-toid’ clade was consistently recovered as monophyletic with a stable topology regardless of the analyses employed (Fig. 1). These results present an interesting biogeographic scenario in respect to the distributions of the Nearctic/Neotropical (Amphi-dorini) and Palearctic (Blaptini and Platyscelidini) ‘blaptoid’ beetles. This exact topology was predicted by Medvedev (2001) based on an examination of morphological data. From a tax-onomic perspective, the delimitation of Amphidorini is unam-biguous based on the presence of aedeagal clavae in the males and undivided ovipositor coxites in the females of all Amphi-dorini genera (Peña, 1971; Doyen & Lawrence, 1979; John-ston, 2016; Lumen et al., 2020), while distinction between Blap-tini and Platyscelidini is more complicated, as the two tribes are much more closely related (Fig. 2). The most reliable features for separating Blaptini and Platyscedilini concern their tarsal struc-ture (see identification key).
Other potential Blaptinae
The Central Asian tribe Dissonomini (two genera, ∼30 species) bears a striking superficial resemblance to Blaptini and Platyscelidini as adults (Fig. 4A); however, larval charac-ters align with the ‘opatrinoid’ clade (i.e., Pedinini). Specif-ically, adults have a reduced scutellum and ‘blaptoid’ dorsal habitus reflecting a closer affiliation with Blaptini/Platyscelidini (Medvedev, 1968). Furthermore, the dilated pro- and mesotar-someres indicates a close relationship between Dissonomini and Platyscelidini (Medvedev, 1968). At the same time, Dissono-mini larvae possess similar morphology of the last abdomi-nal segment to some Pedinini – the presence of four enlarged apical spines (Medvedev, 1968). Platyscelidini can be distin-guished from Dissonomini by the anterior margin of epis-toma lacking a notch in the middle; visible membrane between labrum and epistome in dorsal view; eyes not narrowed by
Fig 4. Morphology of Dissonomus sp. (Tenebrioninae: Dissonomini): dorsal habitus (A); ventral side of head (B); protrochanter (C); aedeagal tegmen (D).. [Colour figure can be viewed at wileyonlinelibrary.com].
expanding temples/genae; middle part of the mentum lacking longitudinal keel (Fig. 4B); outer margins of epipleura reaching sutural angle, or interrupted at middle or before apex of ely-tra (Medvedev, 1968; Abdurakhmanov & Nabozhenko, 2011). Additionally, Dissonomini differs from Pedinini by lacking aedeagal clavae (Fig. 4D), and having an elongate basal por-tion of the aedeagal tegmen (short in Pedinini) (Kami´nski & Iwan 2017). Superficially, representatives of Dissonomini resemble Opatrini, from which they can be differentiated by hav-ing non-opatrinoid protrochanters (see Iwan & Kami´nski 2016) (Fig. 4C), and the already mentioned structure of larval ninth ter-gite, as the majority of Opatrini possess several shorter spines on the dorsal margins (Kami´nski et al., 2019b). To conclude, at this point the exact phylogenetic placement of Dissonomini cannot be assessed based on morphology alone. Due to a lack of ethanol-preserved specimens in this study, representatives of this tribe were not included in the phylogenetic analysis performed here. For this reason, Dissonomini is not officially incorporated within Blaptinae.
Future studies should also aim to resolve the status of the genus Stenolamus Gebien, which in some of the previous clas-sification concepts constituted a separate subtribe Stenolam-ina Koch within Opatini (Koch 1956; Bouchard et al., 2011). However, based on the structure of male terminalia and pro-tochanter Stenolamus was excluded from the ‘opatrinoid’ clade (Dendarini, Pedinini, Platynotini and Opatrini) by Iwan (2004), who did not propose an exact taxonomic placement for it. Furthermore, based on morphological evidence, Stenolamus
cannot be assigned to any of the currently recognized tribes of the ‘blaptoid’ clade (Amphidorini, Blaptini, Platyscelidini). As larvae of Stenolamus are currently unknown, at this time it is impossible to decide if this genus should be classified within Blaptinae.
Taxonomy
Subfamily Blaptinae Leach
=Opatrinae Brullé
Diagnosis : Adults: antennae lacking compound/stellate sen-soria (Fig. 3F); procoxal cavities externally and internally closed, intersternal membrane of abdominal ventrites 3–5 visible; paired abdominal defensive glands present, elongate, not annulated. Larvae: prolegs enlarged (adapted for digging) (Fig. 3A); ninth tergite lacking urogomphi (Fig. 3C).
Tribal composition : Amphidorini, Blaptini, Dendarini, Opa-trini, Pedinini, Platynotini and Platyscelidini. The subtribal and generic classification is presented in Table 2.
Key to the tribes of subfamily Blaptinae based on adults (compiled from Kaszab, 1940; Johnston et al., 2015; Kami´nski et al., 2019a):
1. Gula with stridulatory surface (Fig. 5A) . . . . . . . Platynotini.
- Gula smooth or covered with irregular rugosities . . . . . . . 2.
Table 2. Subtribal and generic classification of the newly formulated darkling beetle subfamily Blaptinae
Tribe Subtribal/generic composition
Amphidorini LeConte
(7 genera)
Eleodes Eschscholtz, Eleodimorpha Blaisdell, Embaphion Say, Lariversius Blaisdell, Neobaphion Blaisdell, Nycterinus Eschscholtz, Trogloderus LeConte
Blaptini Leach
(27 genera)
Blaptina Leach
Ablapsis Reitter, Blaps Fabricius, Coelocnemodes Bates, Dila Fischer von Waldheim, Dilablaps Bogatchev, Hoplitoblaps Fairmaire, Medvedevia Chigray, Nalepa Reitter, Protoblaps Medvedev, Thaumatoblaps Kaszab & Medvedev
Gnaptorina Medvedev
Gnaptor Brullé
Gnaptorinina Medvedev
Agnaptoria Reitter, Asidoblaps Fairmaire, Belousovia Medvedev, Blaptogonia Medvedev, Colasia Koch, Gnaptorina Reitter, Itagonia Reitter, Montagona Medvedev, Nepalindia Medvedev, Pseudognaptorina Kaszab, Sintagona Medvedev, Tagonoides Fairmaire, Viettagona Medvedev & Merkl
Prosodina Skopin
Prosodes Eschscholtz, Tagona Fischer von Waldheim
Remipedellina Semenov
Remipedella Semenov
Dendarini Mulsant & Rey
(38 genera)
Dendarina Mulsant & Rey
Bioplanes Mulsant, Dendarophylan Español, Dendarus Dejean, Heliopates Dejean, Litoboriolus Español, Litororus Reitter, Meglyphus Motschulsky, Microphylacinus Iwan, Kami´nski & Aalbu, Micrositus Mulsant & Rey, Neoisocerus Bouchard, Lawrence, Davies & Newton, Phylacinus Fairmaire, Phylan Dejean, Phylanmania Ferrer, Pythiopus Koch
Melambiina Mulsant & Rey
Allophylax Bedel, Bermejoina Español, Gridelliopus Koch, Guildia Antoine, Hadroderus Koch, Haemodus Gebien, Hanstroemium Koch, Hoplarion Mulsant & Rey, Lasioderus Mulsant & Rey, Litoborus Mulsant & Rey, Melambius Mulsant & Rey, Melansis Wollaston, Melasmana Strand, Minorus Mulsant & Rey, Orarabion Leo & Liberto, Oreomelasma Español, Otinia Antoine, Peyerimhoffius Koch, Psammoardoinellus Leo, Pseudemmallus Koch, Silvestriellum Koch, Tragardhus Koch, Zadenos Laporte de Castelnau, Zoutpansbergia Koch
Opatrini Brullé(117 genera + 6 incertae sedis)
Ammobiina Desbrochers des Loges
Adavius Mulsant & Rey, Ammidium Erichson, Ammobius Guérin-Méneville, Amphithrixoides Bouchard & Löbl, Asiocaedius Medvedev & Nepesova, Brachyidium Fairmaire, Caediexis Lebedev, Caedius Mulsant & Rey, Clitobius Mulsant & Rey, Coeloecetes Blair, Corinta Koch, Cornopterus Koch, Cyptus Gerstaecker, Diaderma Koch, Dilamus Jacquelin du Val, Emmalus Erichson, Falsammidium Koch, Falsocaedius Español, Freyula Koch, Hadrodes Wollaston, Helenomelas Ardoin, Mateuina Español, Messoricolum Koch, Moragacinella Español, Nesocaedius Kolbe, Perithrix Fairmaire, Platyprocnemis Español & Lindberg, Plesioderes Mulsant & Rey, Prodilamus Ardoin, Proscheimus Desbrochers des Loges, Psammestus Reichardt, Pseudoleichenum Ardoin, Raynalius Chatanay, Tarphiophasis Wollaston, Trigonopoda Gebien, Weisea Semenov
Blapstinina Mulsant & Rey
Aconobius Casey, Ammodonus Mulsant & Rey, Austrocaribius Marcuzzi, Blapstinus Sturm, Cenophorus Mulsant & Rey, Conibiosoma Casey, Conibius LeConte, Cybotus Casey, Diastolinus Mulsant & Rey, Goajiria Ivie & Hart, Hummelinckia Marcuzzi, Nevisia Marcuzzi, Nocibiotes Casey, Notibius LeConte, Platylus Mulsant & Rey, Tonibiastes Dejean, Tonibius Casey, Trichoton Hope, Ulus Horn, Xerolinus Ivie & Hart
Heterotarsina Blanchard
Diphyrrhynchus Fairmaire, Heterocheira Lacordaire, Heterotarsus Latreille, Scymena Pascoe
Neopachypterina Bouchard, Löbl & Merkl
Amblysphagus Fairmaire, Eupachypterus Kiirejtshuk, Nabozhenko & Nel, Neopachypterus Bouchard, Löbl & Merkl, Pseudolamus Fairmaire
Opatrina Brullé
Anatrum Reichardt, Brachyesthes Fairmaire, Caediomorpha Blackburn, Ephalus LeConte, Eumylada Reitter, Falsolobodera Kaszab, Gonocephalum Solier, Hadrophasis Ferrer, Jintainum Ren, Melanesthes Dejean, Melanocoma Wollaston, Mesomorphus Miedel, Myladina Reitter, Opatroides Brullé, Opatrum Fabricius, Penthicinus Reitter, Penthicus Faldermann, Phelopatrum Marseul, Polycoelogastridion Reichardt, Reichardtiellina Kaszab, Scleropatroides Löbl & Merkl, Scleropatrum Reitter, Sinorus Mulsant & Revelière, Sobas Pascoe, Socotropatrum Koch, Tidiguinia Español, Trichosternum Wollaston, Wolladrus Iwan & Kami´nski
Sclerina Lacordaire
Eurycaulus Fairmaire, Palaeosclerum Nabozhenko & Kirejtshuk, Platynosum Mulsant & Rey, Sclerum Dejan
Stizopina Lacordaire
Adoryacus Koch, Amathobius Gebien, Blacodatus Koch, Blenosia Laporte de Castelnau, Calaharena Koch, Crististibes Koch, Eichleria Kami´nski, Eremostibes Koch, Helibatus Mulsant & Rey, Luebbertia Koch, Microstizopus Koch, Namazopus Koch, Nemanes Fairmaire, Parastizopus Gebien, Periloma Gebien, Planostibes Gemminger & Harold, Psammogaster Koch, Sphaerostibes Koch, Stizopus Erichson, Sulpius Fairmaire, Syntyphlus Koch
Incertae sedis: Hovarygmus Fairmaire, Pachymastus Fairmaire, Penichrus Champion, Pocadiopsis Fairmaire, Scleroides Fairmaire,
Trigonopilus Fairmaire
Pedinini Eschscholtz(19 genera) Helopinina Lacordaire
Ametrocera Fåhraeus, Anaxius Fåhraeus, Aptila Fåhraeus, Asidodema Koch, Blastarnodes Koch, Diestecopus Solier, Drosochrus Erichson, Micrantereus Solier, Nicandra Fairmaire, Oncopteryx Fairmaire, Oncosoma Westwood, Piscicula Robiche, Psectes Hesse
Leichenina Mulsant
Apsheronellus Bogatchev, Leichenum Dejean
Pedinina Eschscholtz
Table 2. Continued
Tribe Subtribal/generic composition
Platynotini Mulsant & Rey(72 genera)
Eurynotina Mulsant & Rey
Byrrhoncus Koch, Capidium Koch, Colophonesthes Koch, Eurynotus Kirby, Heteropsectropus Kaszab, Hirtograbies Koch, Isoncophallus Koch, Menederes Solier, Menederopsis Koch, Ograbies Péringuey, Oncotus Solier, Phaleriderma Koch, Phylacastus Fairmaire, Psectropus Solier, Schyzoschelus Koch, Stridigula Koch
Platynotina Mulsant & Rey
Adamus Iwan, Alaetrinus Iwan, Amblychirus Koch, Anchophthalmops Koch, Anchophthalmus Gerstaecker, Angolositus Koch, Anomalipus Latrielle, Atrocrates Koch, Atrocrypticanus Iwan, Bantodemus Koch, Clastopus Fairmaire, Claudegirardius Iwan, Colpotinoides Kaszab, Crypticanus Fairmaire, Doyenus Iwan, Ectateus Koch, Eleoselinus Kami´nski, Eucolus Mulsant & Rey, Eviropodus Koch, Glyptopteryx Gebien, Gonopus Latrielle, Hovademus Ardoin, Lechius Iwan, Madobalus Fairmaire, Melanocratus Fairmaire, Melanopterus Mulsant & Rey, Menearchus Carter, Monodius Koch, Nesopatrum Gebien, Notocorax Dejean, Opatrinus Dejean, Parabantodemus Iwan, Paraselinus Kami´nski, Penthicoides Fairmaire, Phallocentrion Koch, Phymatoplata Koch, Platyburak Iwan, Platyburmanicus Iwan, Platycolpotus Iwan, Platynotoides Kaszab, Platynotus Fabricius, Pokryszkiella Iwan, Pseudoblaps Guérin, Pseudonotocorax Iwan, Pteroselinus Kami´nski, Rugoplatynotus Kaszab, Schelodontes Koch, Sebastianus Iwan, Selinopodus Koch, Selinus Mulsant & Rey, Stenogonopus Gebien, Styphacus Fairmaire, Trigonopus Mulsant & Rey, Upembarus Koch, Zidalus Mulsant & Rey, Zophodes Fåhraeus
Platyscelidini Lacordaire(8 genera)
Bioramix Bates, Microplatyscelis Kaszab, Myatis Bates, Oodescelis Motschulsky, Platyscelis Latreille, Somocoelia Kraatz, Somocoeloplatys Skopin, Trichomyatis Schuster
Fig 5. Diagnostic characters for different tribes of the subfamily Blaptinae: Platynotini, stridulatory gula (A); Opatrini, ‘opatrinoid’ trochanter (B); Platynotini, ‘pedinoid’ trochanter (C); Dendarini, subdivided coxites of ovipositor (D); Amphidorini, undivided coxites of ovipositor (E); Pedinini, laterally situated palpifer (F); Dendarini, apically situated palpifer (G). [Colour figure can be viewed at wileyonlinelibrary.com].
2. Protrochanter with elongate base (Fig. 5B) . . . . . . . Opatrini.
- Protrochanter without elongate base (Fig. 5C) . . . 3.
3. Aedeagal tegmen with clavae (Fig. 3D) . . . 4.
- Aedeagal tegmen without clavae . . . 6.
4. Coxites of ovipositor not subdivided into lobes (Fig. 5E) . . . Amphidorini
- Coxites of ovipositor subdivided into lobes (usually 4 plates) (Fig. 5D) . . . 5.
5. Middle part of mentum extending laterally, completely covering lateral wings; palpifer well developed, situated apically on the basistipes (Fig. 5G) . . . Dendarini.
- Mentum with lateral wings well visible; palpifer smaller than basistipes, situated laterally (Fig. 5F) . . . . . . . Pedinini.
6. Male pro- and mesotarsi more-or-less strongly expanded; tomentose ventraly (yellow setae). Projection between tarsal claws with bristles . . . Platyscelidini.
- Tarsi not expanded in males; rarely tomentose ventrally. Projection between tarsal claws without bristles . . . . . . . Blaptini.
Conclusions
• The concept of Blaptinae (=Opatrinae; Blaphimorpha sensu
nov.) has traditionally been recognized by many previous researchers; however, the formal designation of this group was previously suppressed due to a lack of concrete evidence. • Amphidorini, Blaptini, Dendarini, Pedinini, Platynotini, Platyscelidini and Opatrini form a clade supported by new molecular data and morphological features discussed here. To accommodate this monophyletic grouping (281 genera and ∼ 4000 species) the subfamily Blaptinae is resurrected. • Two main clades were recovered within Blaptinae, the
‘blap-toid’ clade (Amphidorini, Blaptini, Platyscelidini) and ‘opa-trinoid’ clade (Dendarini, Pedinini, Platynotini and Opatrini). Topology of the ‘blaptoid’ clade was stable across different inference methods, while the relations with the ‘opatrinoid’ clade were not ultimately fixed.
• The placement of Dissomini in reference to Blaptini is uncertain, although probable based on morphological traits. • The subfamily Tenebrioninae is not monophyletic.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Supplementary Material S1: Specimens used in this study with coding data, corresponding GenBank accession num-bers.
Supplementary Material S2: Thermocycler profiles and PCR primers used in this study.
Supplementary Material S3: Nexus-format matrix of molecular data spanning four non-overlapping gene regions (carbamoyl-phosphate synthetase domain of rudimentary (CAD) (723 bp), wingless (wg) (438 bp), and nuclear ribo-somal 28S (1101 bp) and mitochondrial riboribo-somal 12S (363 bp).
Supplementary Material S4: Maximum likelihood topol-ogy obtained in IQ-TREE analysis.
Supplementary Material S5: Morphological characters used in the ancestral state reconstruction.
Acknowledgements
Funding was provided by the Polish National Science Centre (Sonata 7 Project 2014/13/D/NZ8/02428) and the NSF ARTS Program (DEB #1523605 and DEB #2009247). We are grateful to Ruth Müller for her hospitality during our visits to Transvaal Museum, Pretoria. Rolf Aalbu for assistance in estimating species richness of some Tenebrioninae tribes. Luna Grey for SEM photographs of stellate sensoria in Scaurini. Przemysław Szymroszczyk for photograph of Dissonomus. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. The USDA is an equal opportunity provider and employer. The authors declare there are no conflicts of interest. Moreover, there are no disputes over the ownership of the data presented in the paper and all contributions have been attributed appropriately, via coauthorship or acknowledgement, as appropriate to the situa tion.
Data availability statement
The data that supports the findings of this study are available in the supplementary material of this article.
References
Aalbu, R.L., Triplehorn, A., Campbell, J.M., Brown, K.W., Somerby, R.E. & Thomas, D.B. (2002) 106. Tenebrionidae Latreille 1802.
American Beetles. Volume 2. Polyphaga: Scarabaeoidea through Curculionoidea (ed. by R.H. Arnett, M.C. Thomas, P.E. Skelley and
J.H. Frank), pp. 463–509. CRC Press, Boca Raton.
Abdurakhmanov, G.M. & Nabozhenko, M.V. (2011) Keys and catalogue to darkling beetles (Coleoptera: Tenebrionidae s. str.) of the Caucasus and south of European part of Russia, Moscow, KMK Scientific Press. 361pp.
Bai, X.L., Li, X.M. & Ren, G.D. (2019) A review of the genus
Oode-scelis Motschulsky, 1845 (Coleoptera: Tenebrionidae: Platyscelidini)
from China. Zootaxa, 4656, 401–430.
Berry, R.L. (1973) The Cerenopini and Eulabini, two tribes previously included in the Scaurini (Coleoptera: Tenebrionidae). Annals of the
Entomological Society of America, 66, 70–77.
Bouchard, P., Bousquet, Y., Davies, A.E. et al. (2011) Family-group names in Coleoptera (Insecta). ZooKeys, 88, 1–972.
Bousquet, Y., Thomas, D.B., Bouchard, P., Smith, A.D., Aalbu, R.L., Johnston, M.A. & Steiner, W.E. Jr (2018) Catalogue of Tenebrionidae (Coleoptera) of North America. ZooKeys, 728, 1–455.
Bremer, H.-J. & Lillig, M. (2014) World catalogue of Amaryg-mini, Rhysopaussini and Falsocossyphini (Coleoptera; Tenebrion-idae). Mitteilungen der Münchner Entomologischen Gesellschaft, Jg., 114, 3–176.
Condamine, F.L., Soldati, L., Rasplus, J.Y. & Kergoat, G.J. (2011) New insights on systematics and phylogenetics of Mediterranean
Blaps species (Coleoptera: Tenebrionidae: Blaptini), assessed through
morphology and dense taxon sampling. Systematic Entomology, 36, 340–361.
Condamine, F.L., Soldati, L., Clamens, A.-L., Rasplus, J.-Y. & Kergoat, G.J. (2013) Diversification patterns and process of wingless endemic insects in the Mediterranean Basin: historical biogeography of the genus Blaps (Coleoptera: Tenebrionidae). Journal of Biogeography, 40, 1899–1913.
Doyen, J.T. (1972) Familial and subfamilial classification of the Tene-brionoidea (Coleoptera) and a revised generic classification of the Coniontini (Tentyriidae). Questiones Entomologicae, 8, 357–376. Doyen, J.T. (1989) Reconstitution of Coelometopini, Tenebrionini and
related tribes of America north of Colombia (Coleoptera: Tenebrion-idae). Journal of the New York Entomological Society, 97, 277–304. Doyen, J.T. & Lawrence, J.F. (1979) Relationships and higher classifica-tion of some Tenebrionidae and Zopheridae (Coleoptera). Systematic
Entomology, 4, 333–377.
Doyen, J.T. & Tschinkel, W.R. (1982) Phenetic and cladistic relation-ships among tenebrionid beetles (Coleoptera). Systematic
Entomol-ogy, 7, 127–183.
Fattorini, S. (2002a) Biogeography of the tenebrionid beetles (Coleoptera, Tenebrionidae) on the Aegean Islands (Greece).
Journal of Biogeography, 29, 49–67.
Fattorini, S. (2002b) Relict versus dynamic models for tenebrionid beetles of Aegean Islands (Greece) (Coleoptera: Tenebrionidae).
Belgian Journal of Zoology, 132, 55–64.
Fattorini, S. & Fowles, A. (2005) A biogeographical analysis of the tenebrionid beetles (Coleoptera, Tenebrionidae) of the Island of Thasos in the context of the Aegean Islands (Greece). Journal of
Natural History, 39, 3919–3949.
Ferrer, J. (2003) The systematic position of the ignote tribe Lachnogyini Reitter, 1904, with comments on the evolution of the aedeagus in the subfamilies Pimeliinae and Opatrinae (Insecta, Coleoptera, Tenebrionidae). Spixiana, 26, 51–53.
Franz, M. (2005) On the lack of good scientific reasons for the growing phylogeny/classification gap. Cladistics, 21, 495–500.
Gebien, H. (1937) Katalog der Tenebrioniden (Col.Heteromera). Teil 1. Pubblicazzioni del Museo Entomologico “Pietro Rossi” Duino, 2, 1–883 Udine.
Gebien, H. (1939) Katalog der Tenebrioniden (Col. Heteromera). Teil 2. Mitteilungen der Münchener Entomologischen Gesellschaft, 29, 443–474 (466–497), 739–770 (498–529).
Gebien, H. (1940) Katalog der Tenebrioniden (Col. Heteromera). Teil 2. Mitteilungen der Münchener Entomologischen Gesellschaft, 30, 405–436 (530–561), 755–786 (562–593), 1061–1092 (594–625). Gebien, H. (1941) Katalog der Tenebrioniden (Col. Heteromera). Teil
2. Mitteilungen der Münchener Entomologischen Gesellschaft, 31, 803–834 (658–689), 1131–1146 (690–705).
Gebien, H. (1942a) Katalog der Tenebrioniden (Col. Heteromera). Teil 2. Mitteilungen der Münchener Entomologischen Gesellschaft, 32, 308–346 (706–744).
Gebien, H. (1942b) Katalog der Tenebrioniden (Col. Heteromera). Teil 3. Mitteilungen der Münchener Entomologischen Gesellschaft, 32, 729–750 746–777.
Hayashi, N. (1964) Contributions to the knowledge of the larvae of Cucujoidea II occurring in Japan (Coleoptera: Cucujoidea). Insecta
Matsumurana, 27, 24–30.
Hayashi, N. (1966) A contribution to the knowledge of the larvae of Tenebrionidae occurring in Japan (Coleoptera: Cucujoidea). Insecta
Matsumurana, 1, 141.
Hayashi, N. (1968) Additional notes on the larvae of Lagriidae and Tenebrionidae occurring in Japan (Coleoptera: Cucujoidea). Insecta
Matsumurana, Suppl., 3, 1–12.
Hoang, D.T., Chernomor, O., von Haeseler, A., Minh, B.Q. & Vinh, L.S. (2018) UFBoot2: improving the ultrafast bootstrap approximation.
Molecular Biology and Evolution, 35, 518–522.
Iwan, D. (2001) Comparative study of male genitalia in Opatri-nae sensu Medvedev (1968) (Coleoptera: Tenebrionidae), with notes on the tribal classification. Part I. Annales Zoologici, 51, 351–390.
Iwan, D. (2004) A comparative study of male genitalia in Opatrinae sensu Medvedev (1968) (Coleoptera: Tenebrionidae), with notes on the reinterpreted tribal classification. Part II. Annales Zoologici, 54, 735–765.
Iwan, D. (2006) Interpretation of the tribes Opatrini and Pedinini (sensu Iwan 2004) versus subfamily Opatrinae (sensu Koch 1956 and Medvedev 1968) and “opatrine lineage” (sensu Doyen and Tschinkel 1982). Cahiers Scientifiques, 10, 71–74.
Iwan, D. & Beˇcváˇr, S. (2000) Description of the early stages of
Anomalipus plebejus plebejulus (Coleoptera: Tenebrionidae) from
Zimbabwe with notes on the classification of the Opatrinae. European
Journal of Entomology, 97, 403–412.
Iwan, D. & Kami´nski, M.J. (2016) Toward a natural classification of opatrine darkling beetles: comparative study of female terminalia.
Zoomorphology, 135, 453–485.
Johnston, M.A. (2016) Redefinition of the Eleodes Eschscholtz sub-genera Tricheleodes Blaisdell and Pseudeleodes Blaisdell, with the description of a new species (Coleoptera: Tenebrionidae). Annales
Zoologici, 66, 665–679.
Johnston, M.A. (2019) Phylogenetic revision of the psammophilic
Trogloderus LeConte (Coleoptera: Tenebrionidae), with
biogeo-graphic implications for the intermountain region. PeerJ, 7, e8039. https://doi.org/10.7717/peerj.8039.
Johnston, M.A., Fleming, D., Franz, N.M. & Smith, A.D. (2015) Amphidorini LeConte (Coleoptera: Tenebrionidae) of Arizona: keys and species accounts. The Coleopterists Bulletin, 14, 27–54. Kami´nski, M.J. (2015) Phylogenetic reassessment and biogeography of
the Ectateus generic group (Coleoptera: Tenebrionidae: Platynotina).
Zoological Journal of the Linnean Society, 175, 73–106.
Kami´nski, M. & Iwan, D. (2017) Revision of the subtribe Pedinina (Tenebrionidae: Pedinini). Annales Zoologici, 67, 585–607. Kami´nski, M.J., Kanda, K., Ra´s, M. & Smith, A.D. (2018) Pythiopina,
an enigmatic subtribe of darkling beetles (Coleoptera: Tenebrion-idae: Pedinini): taxonomic revision, microtomography, ecological niche models and phylogenetic position. Systematic Entomology, 43, 147–165.
Kami´nski, M.J., Kanda, K., Lumen, R., Smith, A.D. & Iwan, D. (2019a) Molecular phylogeny of Pedinini (Coleoptera, Tenebrionidae) and its implications for higher-level classification. Zoological Journal of the
Linnean Society, 185, 77–97.
Kami´nski, M.J., Lumen, R., Kubicz, M., Steiner, W., Kanda, K. & Iwan, D. (2019b) Immature stages of beetles representing the ‘Opatrinoid’ clade (Coleoptera: Tenebrionidae): an overview of current knowledge of the larval morphology and some resulting taxonomic notes on Blap-stinina. Zoomorphology, 138, 1–22. https://doi.org/10.1007/s00435-019-00443-7.
Kanda, K. (2017) Phylogenetic Studies in Tenebrionidae (Coleoptera) and Related Families. Unpublished Ph.D. Dissertation Thesis, Oregon State University.
Kanda, K., Pflug, J.M., Sproul, J.S., Dasenko, M.A. & Maddison, D.R. (2015) Successful recovery of nuclear protein-coding genes from small insects in museums using Illumina sequencing. PLoS One, 10, e0143929.
Kaszab, Z. (1940) Revision der Tenebrioniden-tribus Platyscelini (Col. Teneb.). Münchner Entomologische Gesellschaft, 30, 119–235.
Katoh, K. & Standley, D.M. (2013) MAFFT multiple sequence align-ment software version 7: improvealign-ments in performance and usability.
Molecular Biology and Evolution, 30, 772–780.
Kergoat, G.J., Soldati, L., Clamens, A.L. et al. (2014) Higher level molecular phylogeny of darkling beetles (Coleoptera: Tenebrionidae).
Systematic Entomology, 39, 486–499.
Koch C. (1956) Exploration du Parc National de l’Upemba. II. Tene-brionidae (Coleoptera, Polyphaga), Opatrinae, first part: Platynotini, Litoborini and Loensini. Bruxelles: Institut des Parcs nationaux du Congo Belge.
Lanfear, R., Frandsen, P.B., Wright, A.M., Senfeld, T. & Calcott, B. (2017) PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution, 34, 772–773.
Lumen, R., Kanda, K., Iwan, D., Smith, A.D. & Kami´nski, M.J. (2020) Molecular insights into the phylogeny of Blapstinina (Coleoptera: Tenebrionidae: Opatrini). Systematic Entomology, 45, 337–348.
Maddison, W. P. & Maddison, D.R. (2019) Mesquite: a modular system for evolutionary analysis. Version 3.61 http://www.mesquiteproject .org.
Masumoto, K., Katsumi, A. & Lee, C.F. (2012) New Tenebrionid beetles (Coleoptera) from Taiwan (5) descriptions of a species belonging to a new genus and three new species of three different tribe,s and records of six species in new occurrence. Elytra, 15, 25–37.
Matthews, E.G. & Bouchard, P. (2008) Tenebrionid Beetles of Aus-tralia: Descriptions of Tribes, Keys to Genera, Catalogue of Species. Australian Biological Resources Study (ABRS), Department of the Environment and Energy, Canberra, Australia.
Matthews, E.G. & Lawrence, J.F. (2015) Trachelostenini sensu novo: Redescriptions of Trachelostenus Solier, Myrmecodema Gebien and
Leaus Matthews & Lawrence, based on adults and larvae, and
descrip-tions of three new species of Leaus (Coleoptera: Tenebrionidae).
Zootaxa, 4020, 289–312.
Matthews, E.G., Lawrence, J.F., Bouchard, P., Steiner, W.E. & ´Slipi´nski, S.A. (2010) 11.14. Tenebrionidae Latreille, 1802. Handbook of
Zoology. A Natural History of the Phyla of the Animal Kingdom, Vol. IV – Arthropoda: Insecta. Part 38. Coleoptera, Beetles, Vol. 2: Systematics (Part 2) (ed. by R.A.B. Leschen, R.G. Beutel and J.F.
Lawrence), pp. 574–659. Walter de Gruyter, Berlin.
Medvedev, G.S. (1968) Coleoptera. Darkling-beetles (Tenebrionidae), subfamily Opatrinae, tribes Platynotini, Dendarini, Pedinini, Dis-sonomini, Pachypterini, Opatrini (part) and Heterotarsini. Leningrad: Fauna of USSR, Zhestkokrylye, 19.
Medvedev, G.S. (1977) The taxonomic significance of the antennal sensillae of the darkling beetles (Coleoptera: Tenebionidae). Trudi
vsesoyuz-novo entomologicheskovo obshehestva, 58, 61–86 (In
Rus-sian).
Medvedev G.S. (2001) Evolution and system of darkling beetles of the tribe Blaptini (Coleoptera, Tenebrionidae). Meetings in memory of N. A. Cholodkovsky. Iss. 53. St. Petersburg., 332pp.
Miller, M.A., Pfeiffer, W., & Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceed-ings of the Gateway Computing Environments Workshop (GCE). 14 November 2010, New Orleans, LA, pp. 1–8.
Minh, B.Q., Nguyen, M.A. & von Haeseler, A. (2013) Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and
Evolution, 30, 1188–1195. https://doi.org/10.1093/molbev/mst024.
Nabozhenko, M. & Gurgenidze, L.N. (2006) Description of the larva of Cylindrinotus gibbicollis Faldermann, 1837 and notes on the clas-sification of the subtribe Cylindrinotina Español, 1956 (Coleoptera: Tenebrionidae: Helopini). Caucasian Entomological Bulletin, 2, 79–82. https://doi.org/10.23885/1814-3326-2006-2-1-79-82. Nabozhenko, M. & Purchart, L. (2017) Western Palaearctic Trachyscelis
Latreille, 1809 (Coleoptera: Tenebrionidae: Trachyscelini). Annales
Zoologici, 67, 561–575.
Nabozhenko, M. & Sadeghi, S. (2017) Foranotum perforatum gen. et sp. nov. - a new troglobitic darkling beetle (Coleoptera: Tenebrionidae: Kuhitangiinae: Foranotini trib. nov.) from a cave in southern Zagros, Iran. Zootaxa, 4338, 163–172.
Nguyen, L.-T., Schmidt, H.A., von Haeseler, A. & Minh, B.Q. (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution, 32, 268–274. https://doi.org/10.1093/molbev/msu300.
Peña, L.E.G. (1971) Revisión del genero Nycterinus Eschscholtz 1829 (Coleoptera-Tenebrionidae). Boletín del Museo Nacional de Historia
Natural, 32, 129–158.
Purchart, L. & Nabozhenko, M. (2012) Description of larva and pupa of the genus Deretus (Coleoptera: Tenebrionidae) with key to the larvae of the tribe Helopini. Acta Entomologica Musei Nationalis Pragae, 52, 295–302.
Rambaut, A., Drummond, A.J., Xie, D., Baele, G. & Suchard, M.A. (2018) Posterior summarisation in Bayesian phylogenetics using tracer 1.7. Systematic Biology, 67, 901–904.
Reitter, E. (1917) Bestimmungs-Tabelle der palaearktischer Coleopteren. 82. Heft: Tenebrionidae, unterfamilie Asidini. Bande
der Verhandlungen des naturforschenden Vereines in Brunn, 55,
1–74.
Ronquist, F., Teslenko, M., van der Mark, P. et al. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542.
Schulze, L. (1963) The Tenebrionidae of Southern Africa. XXXVIII. On the morphology of the larvae of some Stizopina (Coleoptera: Opatrini). Scientific Papers of the Namib Desert Research Station, 19, 1–23.
Schulze, L. (1969) The Tenebrionidae of Southern Africa. XLII. Description of the early stages of Carchares macer Pascoe and
Herpiscus sommeri Solier with a discussion of some phylogenetic
aspects arising from the incongruities of adult and larval system-atics. Scientific Papers of the Namib Desert Research Station, 53, 139–149.
Skopin, N.G. (1962) [larvae of the subfamily Pimeliinae (Coleoptera, Tenebrionidae)]. Lichinki podsemeystva Pimeliinae (Coleoptera, Tenebrionidae). Trudy Nauchno-Issledovatelskogo Instituta
Zash-chity Rastenii Kazakhstanskoy Akademii Selskokhozyastvennykh Nauk, 7, 191–298.
Skopin, N.G. (1964) Die Larven der Tenebrioniden des Tribus Pyc-nocerini (Coleoptera, Heteromera). Annales de Museé Royal de
l’Afrique Centrale, Tervuren, Serie in 8. Zoological Science, 127,
1–35.
Smith, A.D., Dornburg, R. & Wheeler, Q.D. (2014) Larvae of the genus Eleodes (Coleoptera, Tenebrionidae): matrix-based descrip-tions, cladistic analysis, and key to late instars. Zookeys, 415, 217–268.
Soldati, L., Condamine, F., Clamens, A. & Kergoat, G.J. (2017) Documenting tenebrionid diversity: progress on Blaps Fabricius (Coleoptera, Tenebrionidae, Tenebrioninae, Blaptini) systematics, with the description of five new species. European Journal of
Taxonomy, 282, 1–29. https://doi.org/10.5852/ejt.2017.282.
Tschinkel, W.R. & Doyen, J.T. (1980) Comparative anatomy of the defensive glands, ovipositors and female genital tubes of tenebrionid beetles (Coleoptera). International Journal of Insect Morphology and
Embryology, 9, 321–368.
Watt, J. (1974) A revised subfamily classification of Tenebrionidae (Coleoptera). New Zealand Journal of Zoology, 11, 381–452. Yu, Y.Z., Zhang, D.Z. & Ren, G.D. (2000) Identification of the larvae of
common tenebrionids of the Platyscelini-tribe (Coleoptera) in North China. Entomological knowledge, 37, 160–163.



![Fig 2. Continued. [Colour figure can be viewed at wileyonlinelibrary.com].](https://thumb-eu.123doks.com/thumbv2/123doknet/13869027.446130/9.892.108.808.156.936/fig-continued-colour-figure-viewed-wileyonlinelibrary-com.webp)