HAL Id: hal-03044749
https://hal.archives-ouvertes.fr/hal-03044749
Submitted on 7 Dec 2020
HAL is a multi-disciplinary open access archive for the deposit and dissemination of sci-entific research documents, whether they are pub-lished or not. The documents may come from teaching and research institutions in France or abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est destinée au dépôt et à la diffusion de documents scientifiques de niveau recherche, publiés ou non, émanant des établissements d’enseignement et de recherche français ou étrangers, des laboratoires publics ou privés.
Optical microscopy to study single nanoparticles
electrochemistry: From reaction to motion
Jean-Marc Noël, Jean-François Lemineur
To cite this version:
Jean-Marc Noël, Jean-François Lemineur. Optical microscopy to study single nanoparticles elec-trochemistry: From reaction to motion. Current Opinion in Electrochemistry, Elsevier, 2021, 25, pp.100647. �10.1016/j.coelec.2020.100647�. �hal-03044749�
Optical microscopy to study single nanoparticles electrochemistry: from
reaction to motion
Jean-Marc Noël and Jean-François Lemineur*
Université de Paris, ITODYS, CNRS, UMR 7086, 15 rue J-A de Baïf, F-75013 Paris, France *Corresponding author: JeanFrancois.Lemineur@u-paris.fr
Abstract:
Nanoparticles (NPs) based electrochemical devices are generating a growing interest and optical microscopy has recently proven to be a powerful tool to apprehend their electrochemical behavior. Through several striking examples, this review demonstrates how label-free optical imaging coupled to an electrochemical actuation can be used to probe
operando the physical and electrochemical properties of single NPs, with high resolution and
sensitivity and without additional emitters. Such an approach can be particularly relevant to establish clear structure-motion/reactivity relationships required to optimize NPs exploited as electrode materials.
Keywords:
Nanoelectrochemistry, high resolution optical microscopy, single entity, super-localization. Introduction:
Nanoparticles (NPs) are increasingly used in the field of electrochemistry because of the outstanding physical and chemical properties they exhibit and the many advantages they can provide as electrode materials. [1,2]
The integration of NPs in electrochemical (EC) devices raised many new challenges that rely on controlling their structure, composition and motion in order to reach constant, optimal and homogeneous reactivity. In addition, the few amounts of materials constituting the NPs make them more vulnerable to undesirable phenomena such as passivation or degradation, which can cause premature aging of the devices. In this context, it is essential to resolve the NPs structure, to evaluate their nanoscale motion and to probe their reactivity, ideally
operando and at the single NPs level, in order to assess clear structure-motion/reactivity
relationships and their evolution over time.
The emergence of nano-electrochemistry [3] and its ability to record current down to <1pA have allowed significant advances in the field of single nano-entities electrochemistry. [4,5] For instance, it became possible to follow the EC activity of isolated atoms and clusters, [6] to decipher and to quantify NPs reaction by studying stochastic EC impacts of single NPs, [7,8,9,10] and to image in situ the reactivity of immobilized individual nano-entities during EC processes, [11,12,13] using nanoscale electrodes/pipettes. Also, high frequency EC measurements recently evidenced the influence of the NP/electrode interaction dynamic on
the NPs EC response. [14,15,16,17] However, EC tools remain often blind to operando changes in NPs structure, to the presence of multiple heterogeneous reactive sites over the NPs surface, and to NPs motion.
Alternatively, the coupling of electrochemistry to high resolution imaging techniques, capable of resolving NPs, ideally operando, could overcome these drawbacks. Electron Microscopy is a typical example as it enables high -in situ or ex situ- spatial resolution observations of dynamic EC phenomena at the single NP level, including nucleation and growth, [18,19] transformation [20] and motion [21]. However, it remains challenging to implement and limited to low temporal resolutions and depth of field. Also, the illumination beam can strongly influence the NPs reactivity, [22] making any quantitative assessment difficult.
Herein, we review some of the recent label free methodologies and operando studies published in the last two years that should help the readers to apprehend the crucial role of OMs in the understanding of NPs structure, motion and reaction and their relationships in the context of single NPs electrochemistry.
Optical microscopy:
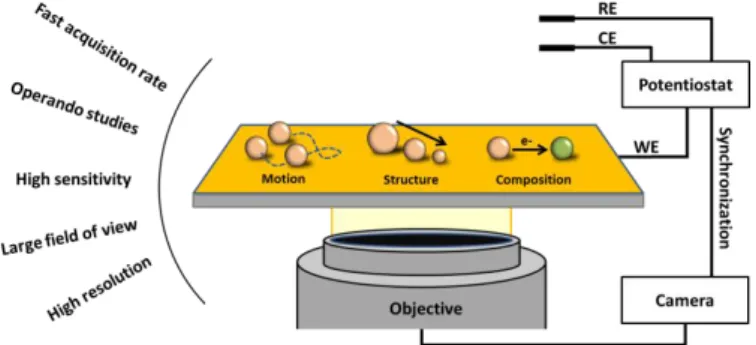
OMs are gaining more and more popularity. [23] They possess many advantages starting with the ability to visualize NPs in-situ, in real time with high temporal (up to 1 kHz) and high spatial (down to <20 nm) resolution, which is perfectly adapted to the accurate operando tracking of NP structural or compositional changes and discrete motion during EC activity, as summarized in Figure 1. In addition, OMs possess a wide field of view ensuring a high throughput screening and the possibility to collect huge amounts of data for post-processing and machine learning. [24]
Figure 1. Representation of a typical opto-electrochemical microscope and some of its key advantages. Very briefly, one could separate OMs into two classes: the label-free techniques such as Dark-Field (DFM), Surface Plasmon Resonance (SPRM) and Interference Reflection (IRM) Microscopies, on which we focus herein, and those relying on additional emitting probes such as fluorescence or Raman microscopies.
OMs are, by definition, limited by the diffraction of light and well separated single NPs (e.g. separated by distances larger than the diffraction limit) appear as Airy spots with radius roughly equal to 1.22 λ N.A. (λ and N.A. standing for the wavelength of light and the objective numerical aperture, respectively). Therefore, both dynamic nanoscale structural and chemical information are not readily accessible but can be inferred from the relative spot intensity, either by calibrating the system (by using nano-gauges or ex-situ measurements) or by correlation with quantitative optical models. One should also mention spectroscopy which often provides additional chemical information about the nano-systems and sometimes increases the monitoring sensitivity. However, it is at the cost of lower temporal resolution and greater instrumental complexity. [25] Because of the brevity of this opinion, we will only consider intensity changes, spectrally resolved or not.
NPs displacement can also be evaluated by tracking the translation of the NP centroid (i.e. the center of mass of the airy spot) and if needed, with a resolution >10 times better than the diffraction limit. The location of an emitter can indeed be determined with nanometer precision by fitting its optical pattern by a Gaussian function, a mathematical procedure called super-localization. In addition, for scattering NPs acting as emitters, super-localizing the centroid position and tracking operando its displacement can potentially reveal the asymmetric modification of the NPs (local chemical structure, physical alteration, surface chemistry, electronic density, etc.). [26]
1. Monitoring NPs reaction through local intensity variation
Since the seminal work of Tao et al. [27] on the imaging of catalytic hydrogen evolution reaction at single Pt NPs based on local refractive index variation probed by SPRM, followed by the demonstration of the use of DFM to quantitatively track individual Ag NPs electrodeposition one year later by Hill and Pan, [28] many recent reports used and extended the methodology that consists in interpreting local intensity changes to other relevant systems and other optical set-ups.
The EC behavior of Ag NPs, a model system, has been extensively studied by DFM or SPRM and their dissolution/transformation mechanism at the vicinity of an electrode has been uncovered in the presence of various electrolytes. [29,30,31,32,33] As an example, Tschulik
et al. [34,35,36] recently followed the Ag NPs compositional change into AgCl NPs and
further into Ag2O3/AgClO2 NPs by exploiting single NPs color intensity fluctuation, while the
latter transformation is electrochemically hidden by water splitting and/or chloride oxidation.
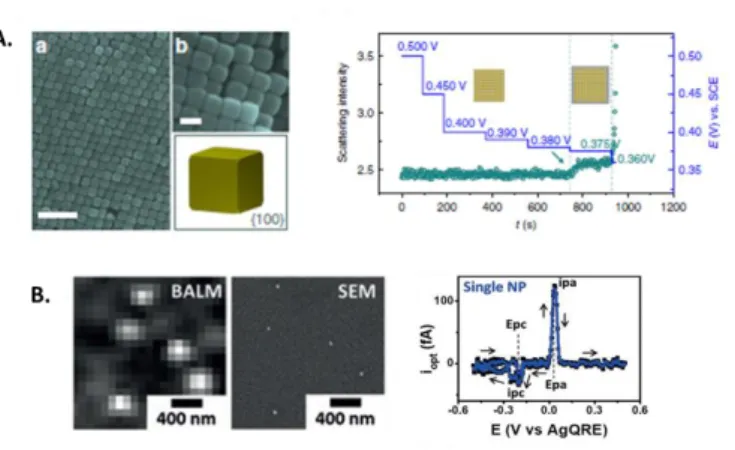
Figure 2. A) Electrodeposition of Ag atomic layer on single Au NPs (imaged ex-situ by SEM) monitored by DFM through the recording of the time dependent scattering intensity fluctuation under different potential. Reprinted from Ref. [37]. Creative Commons CC BY license. B) Correlated SEM-IRM images of Ag NPs and optical current related to the formation and subsequent dissolution of a 21 ± 2 nm Ag NPs. Reprinted from Ref. [41]. Copyright 2018 Wiley-VCH.
As it is often required to reduce NPs sizes and to tune their surfaces with atomic precision, especially in the field of electrocatalysis, one of the current challenges in operando OMs is to enhance the limit of detection. To this end, Ren et al. [37] designed a new DFM optical path that effectively suppresses the refractive index mismatch and increases the excitation and collection efficiency. To illustrate the potential of the method, they followed the under-potential deposition of sub-monolayer of Ag atoms dynamically on single Au NPs with different facets, as exemplified in Figure 2A. Alternatively, one can take advantages of IRM in combination with antireflection coating [38,39,40] for detecting small NPs, including non-plasmonic NPs, and as an example for probing size dependent single NPs electrochemistry. [41] Indeed, recording local intensity variation during EC deposition/dissolution of Ag NPs confirmed that the final NPs size was controlled by the nucleation probability and that the higher surface free energy of smaller NPs was responsible for their easier oxidation.
Estimate of current can also be inferred from the optical response by mathematically post-processing single NPs intensity transients, iopt, as shown in Figure 2B. In addition to be
quantitative, if for example correlated to NPs size variation through correlative ex situ SEM images, optical current already helped to i) separate the role of non-Faradic EC currents, [25] ii) confirm the presence of metal nanoclusters at the early stage of Ag NPs formation, [41] iii) study the overlap of the NP diffusion layer during electrodeposition [42] and iv) decipher a complex electrocatalytic mechanism related to Co NPs. [43]
Finally, optical monitoring has proven to be useful to evaluate the effect of NPs and electrodes chemical functionalization, frequently employed to improve NPs stability and to avoid NPs desorption. For example, OMs have shown that the presence of molecules often decreases reactivity more than other parameters, such as NPs size or shape and reduces electron transfer between NPs and current collector. [44,45,46]
The ability to spatially resolve NPs with high temporal and high spatial resolution offered by OMs gives the opportunity to apprehend NP motion occurring during and often induced by EC processes. Optical tracking is, for example, widely used to evaluate motion speed of micro- and nano- motors, often bipolar particles propelled by redox reactions such as water splitting, [47] galvanic replacement reaction, [48] reduction of metal-halide, [49] etc. [50]
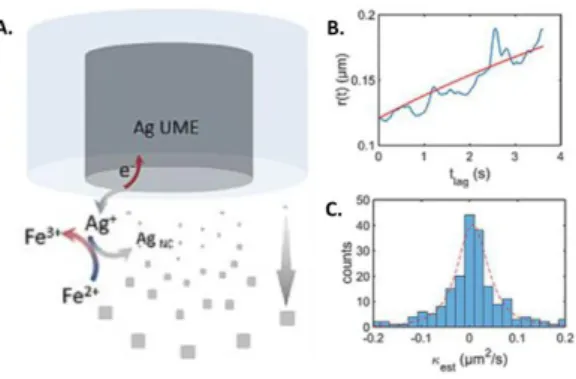
Figure 3. A) Principle of NPs electro-synthesis using Ag sacrificial microelectrode and reversible redox reducer. B) Single NP radius evolution over time calculated from NPs optical tracking and time resolved mean square displacement analysis. C) Single NPs growth rate dispersion graph obtained from DFM monitoring. Reprinted from Ref. [51]. Copyright 2018, Wiley-VCH.
Recently, DFM was used to monitor the growth of NPs electro-synthesized within the electrode diffusion layer using reversible redox reducers and metallic ion precursors, continuously generated anodically from a sacrificial metallic microelectrode, as schematized in Figure 3A. Through “time resolved” mean square displacement analyses, Brownian NPs were sized dynamically, individually and in real time (Figure 3B), allowing to evaluate the growth rate (κest) for every NPs as presented in Figure 3C.[51] This methodology was further
employed to unravel shape-selective mechanisms of NPs electro-synthesis and to highlight the relationship between the NPs growth rate and the standard potential difference between the reactants.[52]
By using IRM to optically track NPs together with the super-localization approach, the mechanism of EC conversion of AgCl/AgBr NPs into Ag was deciphered. [53] In particular, a correlation between intermittent optical intensity changes and motion of the NP centroid suggested a multistep process in which the NP displacement was actuated by the EC reaction and the local release of ions that propelled the NP toward other reactive sites up to the full NP conversion.
Optical approach can also complement EC impact experiments, through “optical impacts” allowing for instance to analyze mass transport in 3D, [30] visualize the effect of the ionic strength [54] and of the electrostatic forces [55] on the NPs-electrode collision dynamic, and detect NPs collisions without faradic reaction. [56]
The centroid finding strategy, routinely used in single molecule imaging is capable of achieving spatial localization with a resolution of <20 nm. [57] Recently, the localization based super-resolution imaging has been used to probe local changes in the NPs during EC actuation.
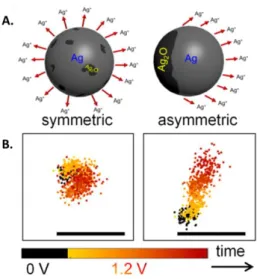
Figure 4. A. Symmetric and asymmetric Ag NP dissolution influenced by the oxide layer homogeneity at the NP surface. B. Trajectories of the centroid of a NP that dissolves in a symmetric or asymmetric manner. Reprinted from refs. [26] and [58]. Copyright 2018 and 2019 American Chemical Society.
By employing DFM together with the super-localization approach, Willets et al. [58] studied the anodic dissolution of Ag NPs with a spatial resolution higher than the object size. The
operando tracking of the NP centroid displacement during oxidation revealed two distinct
and random behaviors schematized in Figure 4A: a homogeneous NP reaction and an asymmetric dissolution traduced by the NP centroid displacement (Figure 4B, right) and attributed to the inhomogeneous repartition of the oxide layer that passivates the NPs surface.
In another DFM study, Wang and co-workers [59] evidenced by imaging the periodic non-faradic charging and discharging of single plasmonic nanorods and by tracking the spatial motion of the NP optical centroid that the electron density distribution can be displaced and higher in specific NPs regions when charges are electrochemically injected.
Kanoufi et al. [60] optically monitored single Co oxide sub-microparticles growing and reacting anodically at the tip end of a nano-electrode. By achieving this, they collected interesting mechanistic information about the NPs formation and electrocatalytic properties. Notably, super-localizing the edges of relatively large particles (i.e. not entirely diffraction limited) evidenced their breathing (reversible volume expansion) during their involvement into water splitting.
Label-free OMs is a promising strategy to unravel the most complex EC processes occurring at single NPs through the analysis of both intensity variation and displacement of optical patterns, especially in combination with a super-localization procedure. Very often, optical models also complement the operando monitoring and allow to interpret quantitatively optical fluctuations. Through the examples shown herein, we evidenced the usefulness of such an optical strategy to study the EC synthesis, deposition, transformation, or catalysis of individual NPs.
As a perspective, we believe that the coupling of the two single entity approaches (i.e. operando imaging and local probe electrochemistry) and, as a result, the recording of the EC current related to the very same single optically monitored NPs should bring significant advances in the understanding of structure/reactivity relationships and their evolution under operating conditions. Few attempts have been recently made in this direction as the Optically Targeted Electrochemical Cell Microscopy (OTECCM) developed by Hill et al. and used to study both optically and electrochemically the electrocatalytic CO2 reduction at
single Cu NPs [61] and hydrazine oxidation at single Au nanorods. [62] Funding sources
This work was financially supported by the Emergence call from Université de Paris within the Investissement d’Avenir program under reference ANR-18-IDEX-0001, the CNRS and by Université de Paris.
References
[1] S. E. F. Kleijn, S. C. S. Lai, M. T. M. Koper, P. R. Unwin, Electrochemistry of Nanoparticles, Angew. Chem. Int. Ed. 53 (2014) 3558-3586.
[2] Y. Sun, N. Liu, Y. Cui, Promises and challenges of nanomaterials for lithium-based rechargeable batteries, Nat. Energy 1 (2016) 16071.
[3] S. Lemay, H. White, Electrochemistry at the Nanoscale: Tackling Old Questions, Posing New Ones, Acc. Chem. Res. 49 (2016) 2371-2371.
[4] F.T. Patrice, K. Qiu, Y.L. Ying, Y.T. Long, Single Nanoparticle Electrochemistry, Annu. Rev. Anal. Chem. 12 (2019) 347-370.
[5] L. A. Baker, Perspective and Prospectus on Single-Entity Electrochemistry, J. Am. Chem. Soc. 140 (2018) 15549-15559.
[6] M. Zhou, J. E. Dick, A. J. Bard, Electrodeposition of Isolated Platinum Atoms and Clusters on Bismuth-Characterization and Electrocatalysis, J. Am. Chem. Soc. 139 (2017) 17677-17682.
[7] P. A. Defnet, T. J. Anderson, B. Zhang, Stochastic Collision Electrochemistry of Single Silver Nanoparticles, Curr. Opin. Electrochem. (2020) DOI: 10.1016/j.coelec.2020.06.004
[8] B. M. Quinn, P. G. Van’t Hof, S.G. Lemay, Time-resolved electrochemical detection of discrete adsorption events, J. Am. Chem. Soc. 126 (2004) 8360-8361.
[9] X. Xiao, A. J. Bard, Observing single nanoparticle collisions at an ultramicroelectrode by electrocatalytic amplification, J. Am. Chem. Soc. 129 (2007) 9610-9612.
[10] Y. Zhou, N. V. Rees, R. G. Compton, The Electrochemical Detection and Characterization of Silver Nanoparticles in Aqueous Solution, Angew. Chem. Int. Ed. 50 (2011) 4219-4221.
[11] B. Tao, L. C. Yule, E. Daviddi, C. L. Bentley, P. R. Unwin, Correlative Electrochemical Microscopy of Li-Ion (De)intercalation at a Series of Individual LiMn2O4 Particles, Angew. Chem. Int. Ed. 58 (2019) 4606-4611.
[12] J. Kim, C. Renault, N. Nioradze, N. Arroyo-Curras, K. C. Leonard, A. J. Bard. Electrocatalytic Activity of Individual Pt Nanoparticles Studied by Nanoscale Scanning Electrochemical Microscopy, J. Am. Chem. Soc. 138 (2016) 8560-8568.
[13] T. Sun, H. Zhang, X. Wang, J. Liu, C. Xiao, S. U. Nanayakkara, J. L. Blackburn, M. V. Mirkin, E. M. Miller, Nanoscale mapping of hydrogen evolution on metallic and semiconducting MoS2 nanosheets, Nanoscale Horiz.
4 (2019) 619-624.
[14] S. M. Oja, D. A. Robinson, N. J. Vitti, M. A. Edwards, Y. Liu, H. S. White, B. Zhang, Observation of Multipeak Collision Behavior during the Electro-oxidation of Single Ag Nanoparticles, J. Am. Chem. Soc. 139 (2017) 708-718.
[15] D. A. Robinson, Y. Liu, M. A. Edwards, N. J. Vitti, S. M. Oja, B. Zhang, H. S. White, J. Am. Chem. Soc. 139 (2017) 16923-16931.
[16] J. Ustarroz, M. Kang, E. Bullions, P. R. Unwin, Impact and oxidation of single silver nanoparticles at electrode surfaces: one shot versus multiple events, Chem. Sci. 8 (2017) 1841-1853.
[17] H. Ma, J.-F. Chen, H.-F. Wang, P.-J. Hu, W. Ma, Y.-T. Long, Exploring dynamic interactions of single nanoparticles at interfaces for surface-confined electrochemical behavior and size measurement, Nat. Commun. 11 (2020) 2370.
[18] I. M. Ornelas, P. R. Unwin, C. L. Bentley, High-Throughput Correlative Electrochemistry-Microscopy at a Transmission Electron Microscopy Grid Electrode, Anal. Chem. 91 (2019) 14854-14859.
[19] H. E. M. Hussein, R. J. Maurer, H. Amari, J. J. P. Peters, L. Meng, R. Beanland, M. E. Newton, J. V. Macpherson, Tracking Metal Electrodeposition Dynamics from Nucleation and Growth of a Single Atom to a Crystalline Nanoparticle, ACS Nano 12 (2018) 7388-7396.
[20] N. Ortiz Peña, D. Ihiawakrim, M. Han, B. Lassalle-Kaiser, S. Carenco, C. Sanchez, C. Laberty-Robert, D. Portehault, O. Ersen, Morphological and Structural Evolution of Co3O4 Nanoparticles Revealed by in situ
Electrochemical Transmission Electron Microscopy during Electrocatalytic Water Oxidation, ACS Nano 13 (2019) 11372-11381.
[21] J. Ustarroz, I. M. Ornelas, G. Zhang, D. Perry, M. Kang, C. L. Bentley, M. Walker, P. R. Unwin, Mobility and Poisoning of Mass-Selected Platinum Nanoclusters during the Oxygen Reduction Reaction, ACS Catal. 8 (2018) 6775-6790.
[22] M. Wang, C. Park, T. J. Woehl, Quantifying the Nucleation and Growth Kinetics of Electron Beam Nanochemistry with Liquid Cell Scanning Transmission Electron Microscopy, Chem. Mater. 30 (2018) 7727-7736.
[23] W. Wang, Imaging the chemical activity of single nanoparticles with optical microscopy, Chem. Soc. Rev. 47 (2018) 2485-2508.
[24] J. Zhou, B. Huang, Z. Yan, J.-C. G. Bünzli, Emerging role of machine learning in light-matter interaction, Light Sci. Appl. 8 (2019) 84.
[25] C. P. Byers, B. S. Hoener, W.-S. Chang, S. Link, C. F. Landes, Single-Particle Plasmon Voltammetry (spPV) for Detecting Anion Adsorption, Nano Lett. 16 (2016) 2314-2321.
[26] K. A. Willets, Supercharging Superlocalization Microscopy: How Electrochemical Charging of Plasmonic Nanostructures Uncovers Hidden Heterogeneity, ACS Nano 13 (2019) 6145-6150.
[27] X. Shan, I. Diez-Pérez, L. Wang, P. Wiktor, Y. Gu, L. Zhang, W. Wang, J. Lu, S. Wang, Q. Gong, J. Li, N. Tao, Imaging the electrocatalytic activity of single nanoparticles, Nat. Nanotech. 7 (2012) 668-672.
[28] C. M. Hill, S. Pan, A Dark-Field Scattering Spectroelectrochemical Technique for Tracking the Electrodeposition of Single Silver Nanoparticles, J. Am. Chem. Soc. 135 (2013) 17250-17253.
[29] C. A. Little, C. Batchelor-McAuley, K. Ngamchuea, C. Lin, N. P. Young, . G. Compton, Coupled Optical and Electrochemical Probing of Silver Nanoparticle Destruction in a Reaction Layer, ChemistryOpen 7 (2018) 370-380.
[30] V. Brasiliense, A. N. Patel, A. Martinez-Marrades, J. Shi, Y. Chen, C. Combellas, G. Tessier, F. Kanoufi, Correlated Electrochemical and Optical Detection Reveals the Chemical Reactivity of Individual Silver Nanoparticles, J. Am. Chem. Soc. 138 (2016) 3478-3483.
[31] V. Sundaresan, J. W. Monaghan, K. A. Willets, Monitoring Simultaneous Electrochemical Reactions with Single Particle Imaging, ChemElectroChem 5 (2018) 3052-3058.
[32] Y. Fang, W. Wang, X. Wo, Y. Luo, S. Yin, Y. Wang, X. Shan, N. Tao, Plasmonic Imaging of Electrochemical Oxidation of Single Nanoparticles, J. Am. Chem. Soc. 136 (2014) 12584-12587.
[33] L. Sun, W. Wang, H.-Y. Chen, Dynamic Nanoparticle-Substrate Contacts Regulate Multi-Peak Behavior of Single Silver Nanoparticle Collisions, ChemElectroChem 5 (2018) 2995-2999.
** [34] K. Wonner, M. V. Evers, K. Tschulik, Simultaneous Opto- and Spectro-Electrochemistry: Reactions of
Individual Nanoparticles Uncovered by Dark-Field Microscopy, J. Am. Chem. Soc. 140 (2018) 12658-12661. Hidden EC processes revealed at the single NPs level by coupling OM to electrochemistry.
[35] K. Wonner, M.V. Evers, K. Tschulik, The electrochemical dissolution of single silver nanoparticles enlightened by hyperspectral dark-field microscopy, Electrochim. Acta 301 (2019) 458-464.
[36] K. Wonner, C. Rurainsky, K. Tschulik, Operando Studies of the Electrochemical Dissolution of Silver Nanoparticles in Nitrate Solutions Observed with Hyperspectral Dark-Field Microscopy, Front. Chem. 7 (2020) 1-12.
** [37] S. Hu, J. Yi, Y.-J. Zhang, K.-Q. Lin, B.-J. Liu, L. Chen, C. Zhan, Z.-C. Lei, J.-J. Sun, C. Zong, J.-F. Li, B. Ren,
Observing atomic layer electrodeposition on single nanocrystals surface by dark field spectroscopy, Nat. Commun. 11 (2020) 2518.
Atomic layer electrodeposition probed on single NPs illustrating that high sensitivity to EC processes can be reached by OM.
[38] S. Campidelli, R. A. Khachfe, K. Jaouen, J. Monteiller, C. Amra, M. Zerrad, R. Cornut, V. Derycke, D. Ausserré, Backside absorbing layer microscopy: Watching graphene chemistry, Sci. Adv. 3 (2017) e1601724. [39] R. A. Khachfe, D. Ausserre, Backside absorbing layer microscopy: a universal relationship between physical thickness and reflectivity, Opt. Express 28 (2020) 4836-4844.
[40] K. Jaouen, R. Cornut, D. Ausserré, S. Campidelli, V. Derycke, Ideal optical contrast for 2D material observation using bi-layer antireflection absorbing substrates, Nanoscale 11 (2019) 6129-6135.
* [41] J.-F. Lemineur, J.-M. Noël, D. Ausserré, C. Combellas, F. Kanoufi, Combining Electrodeposition and Optical
Microscopy for Probing Size-Dependent Single-Nanoparticle Electrochemistry, Angew. Chem. Int. Ed. 57 (2018) 11998-12002.
The use of conductive antireflection substrate and IRM in single NPs electrochemistry exemplified by the monitoring of small NPs electrodeposition and stripping.
[42] J.-F. Lemineur, J.-M. Noël, C. Combellas, F. Kanoufi, Optical monitoring of the electrochemical nucleation and growth of silver nanoparticles on electrode: From single to ensemble nanoparticles inspection, J. Electroanal. Chem. (2020) 114043.
[43] V. Brasiliense, J. Clausmeyer, A. L. Dauphin, J.-M. Noël, P. Berto, G. Tessier, W. Schuhmann, F. Kanoufi, Opto-electrochemical In Situ Monitoring of the Cathodic Formation of Single Cobalt Nanoparticles, Angew. Chem. Int. Ed. 56 (2017) 10598-10601.
[44] J.-F. Lemineur, J.-M. Noël, C. Combellas, D. Ausserré, F. Kanoufi, The promise of antireflective gold electrodes for optically monitoring the electro-deposition of single silver nanoparticles, Faraday Discuss. 210 (2018) 381-395.
[45] J. G. Smith, P. K. Jain, The ligand Shell as an Energy Barrier in Surface Reactions on Transition Metal Nanoparticles, J. Am. Chem. Soc. 138 (2016) 6765-6773.
[46] R. Liu, X. Shan, H. Wang, N. Tao, Plasmonic Measurement of Electron Transfer between a Single Metal Nanoparticle and an Electrode through a Molecular Layer, J. Am. Chem. Soc. 141 (2019) 11694-11699.
[47] G. Loget, A. Kuhn, Electric field-induced chemical locomotion of conducting objects, Nat. Commun. 2 (2011) 535.
[48] J. Bastos-Arrieta, C. Bauer, A. Eychmüller, J. Simmchen, Galvanic replacement induced electromotive force to propel Janus micromotors, J. Chem. Phys. 150 (2019) 144902.
[49] C. Zhou, X. Chen, Z. Han, W. Wang, Photochemically Excited, Pulsating Janus Colloidal Motors of Tunable Dynamics, ACS Nano 13 (2019) 4064-4072.
[50] J. G. S. Moo, C. C. Mayorga-Martinez, H. Wang, B. Khezri, W. Z. Teo, M. Pumera, Nano/Microrobots Meet Electrochemistry, Adv. Funct. Mater. 27 (2017) 1604759.
[51] V. Brasiliense, J.-M. Noël, K. Wonner, K. Tschulik, C. Combellas, F. Kanoufi, Single Nanoparticle Growth from Nanoparticle Tracking Analysis: From Monte Carlo Simulations to Nanoparticle Electrogeneration, ChemElectroChem 5 (2018) 3036-3043.
* [52] J.-M. Noël, M. M. Vieira, V. Brasiliense, J.-F. Lemineur, C. Combellas, F. Kanoufi, Effect of the driving force
Investigation of NPs growth mechanisms during local NPs electrosynthesis by operando optical tracking of single NPs.
** [53] J.-F. Lemineur, J.-M. Noël, A. Courty, D. Ausserré, C. Combellas, F. Kanoufi, In Situ Optical Monitoring of
the Electrochemical Conversion of Dielectric Nanoparticles: From Multistep Charge Injection to Nanoparticle Motion, J. Am. Chem. Soc. 142 (2020) 7937-7946.
Optical intensity variation correlated to nanoscale centroid motion to decipher the EC conversion mechanism of single NPs.
[54] J.-F. Lemineur, T. J. Stockmann, J. Médard, C. Smadja, C. Combellas, F. Kanoufi, Optical Nanoimpacts of Dielectric and Metallic Nanoparticles on Gold Surface by Reflectance Microscopy: Adsorption of Bouncing? J. Anal. Test. 3 (2019) 175-188.
[55] R. Hao, Y. Fan, B. Zhang, Imaging Dynamic Collision and Oxidation of Single Silver Nanoparticles at the Electrode/Solution Interface, J. Am. Chem. Soc. 137 (2017) 12274-12282.
[56] J.-F. Lemineur, J.-M. Noël, C. Combellas, F. Kanoufi, Revealing the sub-50ms electrochemical conversion of silver halide nanocolloids by optical microscopy and stochastic electrochemistry, Nanoscale 12 (2020) 15128-15136.
[57] J. Biteen, K. A. Willets, Introduction: Super-Resolution and Single-Molecule Imaging, Chem. Rev. 117 (2017) 7241-7243.
** [58] V. Sundaresan, J. W. Monaghan, K. A. Willets. Visualizing the Effect of Partial Oxide Formation on Single
Silver Nanoparticle Electrodissolution, J. Phys. Chem. C 122 (2018) 3138-3145.
Super-localization procedure to reach high spatial resolution and to reveal heterogeneities in NPs surface composition and EC alteration.
** [59] T. Liu, S. Liu, W. Jiang, W. Wang, Tracking Sub-Nanometer Shift in the Scattering Centroid of Single Gold
Nanorods during Electrochemical Charging, ACS Nano 13 (2019) 6279-6286.
NPs centroid displacement tracking of motionless NPs by OM to probe electron density inhomogeneities induced by the EC charge injection at the NPs surface.
* [60] V. Brasiliense, J. Clausmeyer, P. Berto, G. Tessier, C. Combellas, W. Schuhmann, F. Kanoufi, Monitoring
Cobalt-Oxide Single Particle Electrochemistry with Subdiffraction Accuracy, Anal. Chem. 90 (2018) 7341-7348. OM monitoring combined to edges super-localization to study the anodic formation of single Co based NPs and to evidence NPs EC breathing.
[61] J. D. Walmsley, J. W. Hill, P. Saha, C. M. Hill, Probing Electrocatalytic CO2 Reduction at Individual Cu
Nanostructures via Optically Targeted Electrochemical Cell Microscopy, J. Anal. Test. 3 (2019) 140-149.
** [62] P. Saha, J. W. Hill, J. D. Walmsley, C. M. Hill, Probing Electrocatalysis at Individual Au Nanorods via
Correlated Optical and Electrochemical Measurements, Anal. Chem. 90 (2018) 12832-12839.
Combination of nanopipette and OM to probe electrochemically and optically single NPs and to further establish structure/reactivity relationships.