British Journal of Anaesthesia 1994; 73: 826-832
Effects of halothane and isoflurane anaesthesia on microcirculatory
blood flow in musculocutaneous flaps
G. H . SIGURDSSON, A. BANIC, A. M. WHEATLEY AND D . METTLER
Summary
Hypoperfusion and necrosis in musculocutaneous flaps used for reconstruction of tissue defects is still a significant clinical problem. Although the causes of hypoperfusion are frequently surgical in nature, little is known about the effects of anaesthetic management on blood flow in flaps or the outcome of flap surgery. We compared in minipigs the effects of halothane and isoflurane anaesthesia in equi-potent doses on microcirculatory blood flow (MBF) in the skin and muscle part of musculocutaneous flaps and also in intact (control) skin and muscle. Measurements were made during stable normo-volaemic conditions and during mild to moderate hypovolaemia (withdrawal of 5%, 10% and 15% of total blood volume). Multi-channel laser Doppler flowmetry (LDF) was used to measure MBF and electromagnetic flowmetry (EMF) for total flap blood flow. During normovolaemic conditions there was no significant difference between the two groups in central haemodynamic or respiratory data. After 15% blood loss, however, there was a significant decrease in mean arterial pressure and cardiac output in the halothane group while there was no significant change in the isoflurane group
(P < 0.05). MBF in control skin, control muscle
and flap muscle remained approximately 10-15% higher in the isoflurane than in the halothane group throughout the study. In the isoflurane group, MBF in flap skin was unchanged during normovolaemia and there was less than 10% decrease during hypovolaemia. In the halothane group hypo-volaemia caused a significant decrease in MBF in flap skin: 27% decrease after 5% blood loss, 45% decrease after 10% blood loss and 49% decrease after 15% blood loss compared with 5%, 20% and 21 %, respectively, in intact skin. We conclude that during normovolaemic conditions MBF was well maintained in musculocutaneous flaps in minipigs both with halothane and isoflurane anaesthesia; however, during mild to moderate hypovolemia MBF decreased markedly in flap skin with halothane anaesthesia while it remained unchanged with isoflurane. (Br. J. Anaesth. 1994; 73: 826-832)
Key w o r d s
Anaesthetics volatile, halothane. Anaesthetics volatile, isoflurane. Measurement techniques, flowmetry. Blood, flow. Skin, blood flow. Pig.
Musculocutaneous flaps are used frequently in plastic surgery to reconstruct muscle and skin defects after trauma or radical surgery of malignant tumours [1,2]. With improved understanding of the anatomy and physiology of flaps and improved microsurgical techniques, the outcome of arterial (only attached by the intact feeding artery and vein) and free musculo-cutaneous flap transfers have become more pre-dictable. However, there is still a failure rate (flap necrosis) which can cause severe morbidity [3—6]. It has been shown that flap ischaemia is frequently caused by obstruction of the feeding artery or other surgical problems [3, 5, 7, 8].
Flap operations are frequently extensive and prolonged procedures (8-12 h is not uncommon) which may result in hypothermia [9, 10], progressive fluid and blood loss leading to vasoconstriction and decreased blood flow [11, 12] and possibly flap ischaemia. Although the effects of general anaes-thesia on arterial pressure, cardiac output and regional blood flow are well documented [13-17], little is known about the effects of anaesthetics, such as halothane and isoflurane, on blood flow in free or arterial flaps. Free and arterial flaps are unique tissues in that, although they have an arterial blood supply and venous drainage, they lack lymphatic drainage, which frequently causes flap oedema. In addition, while the flap is completely denervated, the proximal part of the supplying artery is innervated and the vessels in the flap respond to mechanical and humoral stimuli [18]. Thus arterial and free flaps may not respond to anaesthetics in the same way as tissues in situ.
With the recent availability of multichannel laser Doppler flowmeters, continuous monitoring of micro-circulatory blood flow (MBF) simultaneously in multiple organs has become possible [19-21]. Using this technique we have compared the effects of halothane and isoflurane on blood flow in both flap skin and muscle and in contralateral muscle and skin
(in situ) in normovolaemic minipigs and during mild
to moderate hypovolaemia. To mimic conditions during free flap surgery, the flap feeding artery was
G. H. SIGURDSSON, MD, PHD, (Department of Anaesthesia and Intensive Care); A. BANIC, MD, PHD (Department of Plastic and Reconstructive Surgery); A. M. WHEATLEY, PHD (Department of Visceral and Transplantation Surgery); D. METTLER, DVM (Sur-gical Research Unit); University of Berne, Inselspital, CH-3010 Berne, Switzerland. Accepted for publication: June 16, 1994.
clamped for 90 min (approximates the ischaemia encountered during the performance of two microvascular anastomoses) and both the flap artery and vein were denervated pharmacologically.
Materials and methods
This study was performed according to the NIH guidelines for the use of experimental animals and was approved by the Animal Ethics Committee of Canton Berne. Fourteen fasted minipigs (23-28 kg) were given ketamine 10 mg/kg body weight i.m. followed 10 min later by metomidate 5 mg/kg body weight and azaperone 2 mg/kg body weight i.v. for tracheal intubation. Anaesthesia was maintained with either 0.50 + 0.05% halothane (end-tidal con-centration; group H, n = 7) or 1.00 + 0.09% isoflurane (end-tidal concentration; group I, n = 7) and 70% nitrous oxide in oxygen [22, 23]. Inhaled and exhaled concentrations of nitrous oxide, halothane and isoflurane were monitored continu-ously with a multi-gas analyser (Hellige SMU 611, Hellige, Freiburg, Germany). Continuous i.v. infusions of fentanyl 4 ug/kg body weight/h and pancuronium 0.5 mg/kg body weight/h were ad-ministered to both groups. The lungs were ventilated with a volume-controlled ventilator with a positive end-expiratory pressure (PEEP 3-4 cm H2O) (Tiberius 19, Dragerwerk, Liibeck, Germany). Tidal volume was maintained at 10 ml/kg body weight and ventilatory frequency adjusted (13-18 b.p.m.) to maintain PaCo2 at 4.5-5.5 kPa. Abdominal aortic, pulmonary artery (Arrow, Reading, PA, USA) and central venous catheters were inserted via the femoral artery and veins.
The pectoralis ascendens muscle with overlying skin was chosen as a musculocutaneous flap. The skin incision was made in the midline over the sternum and extended slightly laterally over the jugular vein to the neck. A skin island, measuring 15 x 10 cm with its long axis parallel to the sternum and the distal tip at the xiphoid, was designed and skin incision completed. The skin was dissected free from the pectoralis transversus and the descendens muscles and from the jugular vein. After transection of both muscles, the pectoralis ascendens [24] (pectoralis profundus [25]) muscle was exposed. The pectoralis ascendens muscle was detached from its origin on the humerus in order to expose the brachial vessels and the brachial nerve plexus. The feeding artery of the pectoralis ascendens muscle is the external thoracic artery that derives typically as the first branch of the axillary artery at the level of the first rib. The length of the external thoracic artery measures about 2 cm and the diameter about 1-1.5 mm. The brachial artery measures about 1 cm in length between the appearance from the chest and the origin of the external thoracic artery and has a diameter of about 3 mm. The rest of the muscle with the skin island was dissected free and all perforators ligated.
A laser Doppler flow (LDF) probe was sutured centrally on the muscle surface of the flap. Through a small skin incision over the pectoralis muscle on the contralateral side another LDF probe was
sutured on the surface of the intact muscle. Two LDF probes were also sutured centrally on the flap skin and on the contralateral intact skin. An electromagnetic flow (EMF) probe of the appropriate size was placed around the feeding artery to the flap. The probe size was determined during surgery such that the diameter of the artery was approximately 30 % larger than the measurement window.
After completion of surgery, the flap feeding artery was clamped for 90 min to simulate the ischaemia time during the performance of two microvascular anastomoses in free flap surgery. Thereafter, the flap was transposed from the body and placed on a thermostatically controlled warming device to keep the flap temperature constant at 36.5-37.5 °C (Micro-Temp-Pump SMS-2000, Seabrook Medical Systems, Medizintechnik, Giimligen, Switzerland) which was monitored con-tinuously with a needle probe thermometer (Ther-mometer TTX-181, Pro vet, Naumburg, Germany) placed centrally in the flap muscle. Before declamping and until the end of the experiment, the feeding artery and the draining vein were irrigated externally every 20 min with 1 % lignocaine to ensure complete denervation.
After a 20-min stabilization period measurements of LDF, EMF, respiratory and central haemo-dynamic variables were performed for 60 min during stable anaesthetic and haemodynamic conditions. The animals were then exposed to graded hypo-volaemia. Five percent of the total blood volume (TBV was estimated to be 7 % of body weight) was taken every 15 min until 15 % of the TBV had been removed. Blood was collected in sterile blood bags containing anticoagulant solution. Thirty minutes after the last bleed the shed blood was reinfused over 30 min and continuous monitoring of cardiovascular and haemodynamic variables continued for an ad-ditional 60 min. At the end of the experiment, the animals were killed with an overdose of anaesthetic. During surgery the animals received Ringer's lactate 6 ml kg"1 h"' and 5 % glucose 2 ml kg"1 h"1, which kept central venous and pulmonary capillary wedge pressures constant. After surgery the rate of infusion of Ringer's lactate was reduced to 3 ml kg"1 h"1. The body temperature of the animals was maintained at 37.5 + 0.5 °C using two heating blankets.
HAEMODYNAMIC MONITORING
Mean arterial pressure (MAP), central venous press-ure (CVP), mean pulmonary artery presspress-ure (PAP) and pulmonary capillary wedge pressure (PCWP) were recorded with quartz pressure transducers (129A, Hewlett-Packard, Andover, MA, USA) and displayed continuously on a multi-modular monitor (Hellige SMU 611, Hellige AG, Freiburg, Germany) and recorder (Hellige SMR 821, Hellige AG, Freiburg, Germany). Heart rate (HR) was measured from the ECG which was also monitored con-tinuously. Cardiac output (CO) was measured by a thermodilution technique (mean value of three measurements, cardiac output module, Hellige SMU 611, Hellige AG, Freiburg, Germany). Central
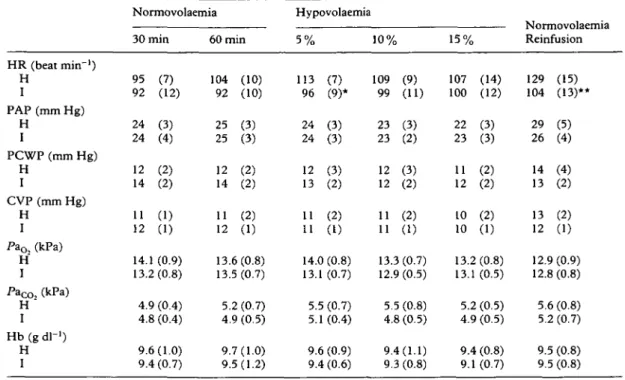
Table I Mean (SD) haemodynamic, respiratory and laboratory data in the two groups (H = halothane, I = isoflurane) during normovolaemia (30 and 60 min after end of surgery), during 5%, 10% and 15% blood loss, and 15 min after reinfusion of shed blood. Heart rate (HR), mean pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP), central venous pressure (CVP), haemoglobin concentration (Hb), arterial oxygen tension (Pa^) and arterial carbon dioxide tension (Pacc,2) were measured. *P < 0.05, **P < 0.01 between groups HR (beat min"1) H I PAP (mm Hg) H I PCWP (mm Hg) H I CVP (mm Hg) H I PaOz (kPa) H I PaCO2 (kPa) H I Hb (g dl"1) H I Normovolaemia 30 min 95 (7) 92 (12) 24 (3) 24 (4) 12 (2) 14 (2) 11 (1) 12 (1) 14.1 (0.9) 13.2(0.8) 4.9 (0.4) 4.8 (0.4) 9.6(1.0) 9.4 (0.7) 60 min 104 92 25 25 12 14 11 12 (10) (10) (3) (3) (2) (2) (2) (1) 13.6 (0.8) 13. 5. 4 9. 9. .5 (0.7) .2 (0.7) .9 (0.5) 7(1.0) .5(1.2) Hypovolaemia 5 % 113 96 24 24 12 13 11 11 (7) (9)* (3) (3) (3) (2) (2) (1) 14.0(0.8) 13.1 5.5 5.1 9.6 (0.7) (0.7) (0.4) (0.9) 9.4 (0.6) 10% 109 (9) 99 (11) 23 (3) 23 (2) 12 (3) 12 (2) 11 (2) 11 (1) 13.3(0.7) 12.9 (0.5) 5.5 (0.8) 4.8 (0.5) 9.4(1.1) 9.3 (0.8) 15% 107 (14) 100 (12) 22 (3) 23 (3) 11 (2) 12 (2) 10 (2) 10 (1) 13.2 (0.8) 13.1 (0.5) 5.2(0.5) 4.9 (0.5) 9.4 (0.8) 9.1 (0.7) Reinfusion 129 (15) 104 (13)** 29 (5) 26 (4) 14 (4) 13 (2) 13 (2) 12 (1) 12.9 (0.9) 12.8 (0.8) 5.6 (0.8) 5.2 (0.7) 9.5 (0.8) 9.5 (0.8)
venous blood temperature was recorded from the thermistor in the pulmonary artery catheter. Blood samples for haemoglobin (Hb) and packed cell volume analysis were obtained from the aortic artery catheter.
RESPIRATORY MONITORING
Expired minute volume ventilation, tidal volume, ventilatory frequency, PEEP, peak and end-inspira-tory pressures, inspired and end-tidal carbon dioxide concentration and inspired and expired oxygen concentrations were monitored continuously throughout the study. Respiratory compliance (chest wall and lung) was calculated as expiratory tidal volume ( F T ) divided by end-inspiratory airway pressure minus PEEP (Paw). Both values were recorded simultaneously from the ventilator. Blood samples for arterial blood-gas analysis were obtained from the aortic artery catheter and analysed im-mediately (temperature corrected) in a blood-gas analyser (Ciba Corning 278 Blood Gas System, Diagnostics Corp., Medfield, MA, USA).
ELECTROMAGNETIC FLOWMETRY
Blood flow in the feeding artery of the flap was monitored continuously with a square-wave electro-magnetic flowmeter (EMF) using a blood flow transducer (Flo-Probe SP 7515-020 or -030, Spectramed Inc., Oxnard, CA, USA) connected to a blood flowmeter (Gould SP2202, Spectramed Inc., Oxnard, CA, USA).
LASER DOPPLER FLOWMETRY
MBF was monitored continuously with two double-channel laser Doppler systems (Periflux 4001 Mas-ter, Perimed AB, Jarfalla, Sweden) allowing four simultaneous LDF measurements. Standard probes (PF 408) were used for monitoring skin, and angled probes (PF 404) for monitoring muscle blood flow. The flow probes measured both flap skin and muscle, and also contralateral muscle and skin (control). The time constant of the flowmeter output amplifier was set at 3 s. LDF data were acquired online via a multichannel interface (Mac Paq MP 100, Biopac Systems Inc., Goleta, CA, USA) with acquisition-analysis software (Acqknowledge 881 3.0, Biopac Systems Inc.) to a portable computer (Macintosh Powerbook 180C, Apple Computer Inc., Cupertino, CA, USA).
A detailed description of the theory of LDF operation and practical details of LDF measure-ments have been described previously [20, 26, 27]. Briefly, low energy laser light from a solid state diode laser operating at 780 nm (Perimed PF4001) is guided to the measurement site via an optical fibre. Two identical adjacent fibres receive back scattered light from the tissue which is then transmitted to independent photodetectors. This back scattered portion consists of light scattered from the static tissue matrix which has not been Doppler shifted and a spectrally broadened component resulting from interactions with moving blood cells. Optical mixing of these components at the photodetector surface produces an electrical signal containing all the Doppler frequency shift information. Further processing within the frequency range 20 Hz-25 kHz
Anaesthesia and blood flow in myocutaneous flaps
produces an output voltage which varies linearly with the product of mean blood cell speed and concentration [28, 29]. The product of mean blood cell speed and concentration is referred to correctly as blood cell flux, but as flow rate may also be defined as volume flux, then as long as the number of red cells within a volume of blood remains constant, blood cell flux is proportional to volume flux or flow of blood. T h e sensitivity of the laser Doppler system was checked using a calibration standard consisting of a suspension of 2-mm latex spheres (Perimed, Jarfalla, Sweden). This produced a standard deflection of 2.5 V or 250 PU on the recorder.
STATISTICS
The Wilcoxon rank sum test (non-parametric) was used to describe differences between the two an-aesthetic groups, between flap skin and control skin or flap muscle and control muscle. All data are presented as mean (SEM). P < 0.05 was considered
statistically significant.
Results
Results for systemic haemodynamic, respiratory and laboratory data are summarized in table 1 and figures
1 and 2. Total flap blood flow, measured by E M F , is presented in figure 3. Microcirculatory blood flow, measured by L D F in the muscle and skin of the flaps, and also in control skin and muscle, is presented in figures 4 and 5.
During normovolaemic conditions there was no statistically significant difference between groups H and I in any of the variables monitored. During hypovolaemia (15',, blood loss) however, there was a significant decrease in MAP and CO in group H (P
< 0 . 0 5 ; fig 1 and 2) but no significant change in group I. MAP decreased by 7 ",, in group H and 5 ",, in group I after 5 ",, blood loss, 17 ",, in group H and 9",, in group I after 10",, blood loss and 30",, in group H and 15",, in group I after 15",, blood loss (fig.lj. CO decreased in a similar fashion to MAP in both groups during blood loss frig. 2;. Total blood flow (EMF) to the musculocutaneous flap decreased by 35",, in group H and 8",, in group I after 5",, blood loss, 50",, in group H and 26",, in group I after 10",, blood loss, and 60",, in group H and 4 0 % in group I after 15",, blood loss (fig. 3).
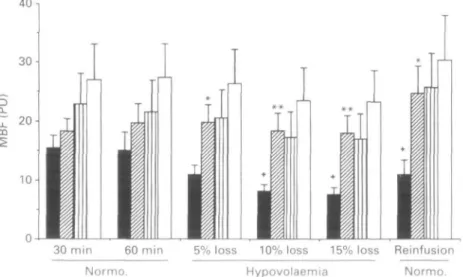
M B F (LDF) in control skin and muscle, and in flap muscle was approximately 10-15 ",, higher in the isoflurane than in the halothane group throughout the study ffigs 4, 5). In flap skin, M B F was unchanged during hypovolaemia in group I 'no change after 5",, blood loss, 7",, decrease after 10",, blood loss and 9",, decrease after 15",, blood loss compared with 4",,, 14",, and 15",,, respectively, in intact skin;. In group H, however, there was a significant decrease in blood flow in flap skin '21",, decrease after 5",, blood loss, 45",, decrease after 10",, blood loss and 49",, decrease after 15",, blood loss compared with 5",,, 20",, and 21",,, respect-ively, in intact skin;.
ively, in intact skin).
40
Normo Normo.
Figure 1 Mean arterial pressure MAP in the halothane • >
and isoflurane Q groups during normovolaemia 'Normo.) (30 and 60 min after end of surgery during 5".,, 10",,and 15",, blood loss, and 15 min after reinfusion of shed blood (mean, SEMi. *P < 0.05 between groups.
1.0
Normo. Normo.
Figure 2 Cardiac output (Co) in the halothane • and
isoflurane e g ) groups during normovolaemia (Normo.) (30 and 60 min after end of surgery;, during 5 ",,, 10",, and 15 ",, blood loss, and 15 min after reinfusion of shed blood mean, SEM,-. *P < 0.05 between groups.
Normo. Hypovolaemia Normo.
Figure ? Total blood flow T B F j to the musculocutaneous
flaps, measured by electromagnetic flowmetry in the halothane • and isoflurane ' 0 groups during normovolaemia Normo. '30 and 60 min after end of surgery,, during 5 ',,, 10",, and 15",, blood loss, and 15 min after reinfusion of shed blood
mean, SEM;.
Discussion
The pig model was chosen for this study for two main reasons. First, anatomically the vascularization of pig skin is quite similar to humans [30-32] and pathophysiologically it also appears more relevant ui pig sum lb quite similar to numans
830 o_ 80 -I 70 60 50 -4 0 • 30 -20
1
T
l
1
1
I
30 min 60 min 5% loss 10% loss 15% loss Reinfusion
Normo. Hypovolaemia Normo.
Figure 4 Microcirculatory blood flow (MBF) in flap muscle ( • = halothane, §| = isoflurane) and control muscle (M — halothane, D = isoflurane), measured by laser Doppler flowmetry (perfusion units, PU) during
normovolaemia (Normo.) (30 and 60 min after end of surgery), during 5 %, 10% and 15 % blood loss, and 15 min after reinfusion of shed blood (mean, SEM). *P < 0.05 between flap and control muscle in the halothane group.
CO
30 min 60 min 5% loss 10% loss 15% loss Reinfusion
Normo. Hypovolaer Normo
Figure 5 Microcirculatory blood flow (MBF) in flap skin ( • = halothane, H = isoflurane) and control skin (fll halothane, • = isoflurane), measured by laser Doppler flowmetry (perfusion units, PU) during normovolaemia (Normo.) (30 and 60 min after end of surgery), during 5%, 10% and 15% blood floss, and 15 min after reinfusion of shed blood (mean, SEM). *P < 0.05, **P < 0.01 between groups in flap skin blood flow; fP < 0.05 between flap and control skin in the halothane group.
for human comparison than rodents [32]. The latter have higher concentrations of the enzyme xanthine oxidase in skin and therefore produce higher con-centrations of oxygen radicals during ischaemia and reperfusion than appears to be the case in pigs and humans [33]. Second, the central and coronary circulations of the pig are remarkably similar to those of humans [34]. The anaesthetics used were administered in equipotent doses. One MAC for halothane in pigs is 0.7% and for isoflurane 1.4% [35]. As our intension was to simulate clinical conditions during free flap surgery we chose to use a combination of volatile anaesthetics with nitrous oxide [35] and an opioid, which is a more common practice than using volatile agents alone.
During normovolaemic conditions, there was no significant difference between the two anaesthetic groups in any of the central haemodynamic variables
(table 1, and figs 1 and 2). This may appear to contrast with other studies which indicated that halothane depresses myocardial contractility and CO more than isoflurane [35—38]. However, as pointed out above, relatively low doses of the volatile anaesthetics (0.70 MAC) were used in our study. Merin, Verdouw and de Jong have shown that when halothane was given in a low concentration (0.65 MAC) in 60 % nitrous oxide, CO decreased by only 10% compared with awake controls, while a high concentration (1.5 MAC) decreased CO by 32% [34]. In the present study, haemorrhagic hypo-volaemia produced a lower MAP and CO in group H than in group I (figs 1, 2) perhaps because of less pronounced effects of isoflurane on myocardial contractility and coronary blood flow in pigs com-pared with halothane [22, 23, 34, 35, 37, 39]. These results are also in agreement with those of Seyde and
Longnecker for hypovolaemic rats [17], but in contrast with those of Weiskopf and colleagues for hypovolaemic dogs [40].
The cause of the marked difference in effects of the two volatile agents on blood flow in the skin of a denervated flap during hypovolaemia may not appear obvious. Severe blood loss may cause up to 10-fold increase in noradrenaline and up to 50-fold increase in adrenaline concentrations in circulating blood [41], and both catecholamines are potent vaso-constrictors in skin [42, 43]. Although the flaps were denervated, circulating catecholamines could still have reached the flap and caused vasoconstriction [44, 45]. Bond and colleagues studied the effects of hypovolaemia on blood flow (EMF) in denervated skin in dogs [43, 46]. They found that increased cutaneous vascular resistance in haemorrhage was caused by elevated plasma catecholamines and was unaffected by sympathetic nerves or circulating angiotensin [43]. Thus it is possible that high concentrations of circulating catecholamines con-tributed to the decrease in blood flow in flap skin. However, this does not explain the difference between the two groups.
Most general anaesthetics depress the cardio-vascular response to stress caused by blood loss or surgical stimuli [17,40], but the literature on the effects of halothane and isoflurane on plasma catecholamines during anaesthesia and surgery is conflicting. During oral surgery in children, halothane—nitrous oxide caused significantly higher plasma concentrations of catecholamines than isoflurane-nitrous oxide anaesthesia [47] and during hypotensive anaesthesia, lower catecholamine con-centrations were found in patients given isoflurane than in patients given halothane and sodium nitro-prusside [48]. Others have not found any difference in plasma concentrations of catecholamines between the two volatile agents, either during undisturbed anaesthesia or during surgery [49,50]. Several mechanisms by which anaesthetics can influence circulating concentrations of catecholamines have been studied. First, they can influence noradrenaline release (spillover rate) from nerve endings and the clearance rate from the circulation. In high doses (1.0—2.0 MAC) both anaesthetics decrease nor-adrenaline spillover rate and clearance in dogs [44,45]. The effects of lower doses of volatile anaesthetics combined with nitrous oxide are, how-ever, not known. Secondly, volatile anaesthetics can influence stimulus-secretion coupling in the adrenal medulla. Both halothane and isoflurane inhibit carbachol-induced secretion of adrenaline and nor-adrenaline in bovine chromaffin cells [51].
During normovolaemia, systemic blood flow (CO) and pressure (MAP), and skin flow were comparable in the two groups, while during hypovolaemia all decreased significantly more in group H than in the group I. Thus it may not be reasonable to assume that reduced systemic flow and pressure resulted in decreased blood flow and perfusion pressure in flap skin. Additionally, systemic hypotension may have resulted in more pronounced systemic catecholamine release in group H than in group I [41, 48], causing cutaneous vasoconstriction and further reduction in
MBF [43] in group H. While this may explain the difference in MBF between the two groups, it does not clarify why MBF decreased more in denervated flap skin than in intact control skin in group H. One explanation might be that the 90 min of flap ischaemia with subsequent reperfusion and later hypotension caused some microcirculatory dysfunc-tion (e.g. endothelial injury), which was not de-tectable with the LDF technique as long as systemic flow and pressure remained normal. However, when systemic pressure decreased the perfusion pressure of the flap may have decreased below a critical level, resulting in a further decrease in flap skin MBF. Whether halothane and isoflurane have any specific effects on endothelial cells or on the compliance (flexibility) of erythrocytes and leucocytes during ischaemic and reperfusion injury in skin is not known.
After restoring circulating blood volume by re-infusion of shed blood, CO and MAP returned to baseline levels in both groups (figs 1 and 2). In addition, flap muscle blood flow in both groups and flap skin blood flow in group I increased to levels even slightly higher than baseline (figs 4 and 5). In group H, however, flap skin flow remained below baseline levels, even after restoration of blood volume, indicating that some, not immediately reversible, microcirculatory disturbance had occurred.
Acknowledgements
This study was supported by the Swiss National Fund for Scientific Research (grants Nos 32-40761.94 and 32-32192.91) and a grant from the Stanley Thomas Johnson Foundation. We thank the staff of the Surgical Research Unit, Inselspital, Berne; and O. Beslac, H. Gisiger and G. Vucic for technical assistance.
References
1. Banic A, Wulff K. Latissimus dorsi free flap for total repair of extensive lower leg injuries in children. Plastic and Reconstructive Surgery 1987; 79: 769-773.
2. Khouri RK, Shaw WW. Reconstruction of the lower extremity with microvascular free flaps. Journal of Trauma 1989; 29: 1086-1090.
3. Furnas H, Rosen JM. Monitoring in microvascular surgery. Annals of Plastic Surgery 1991; 26: 265-272.
4. Hallock GG, Altobelli JA. Assessment of TRAM flap perfusion using laser Doppler flowmetry: An adjunct to microvascular augmentation. Annals of Plastic Surgery 1992; 29: 122-127.
5. Hidalgo DA, Jones CS. The role of emergent exploration in free tissue transfer. A review of 150 consecutive cases. Plastic and Reconstructive Surgery 1990; 86: 492^99.
6. Hallock GG. Intimal staining for visibility enhancement during microanastomoses. Annals of Plastic Surgery 1992; 26: 122-124.
7. Hjortdal VE, Hansen ES, Hauge E. Myocutaneous flap ischemia: flow dynamics following venous and arterial obstruction. Plastic and Reconstructive Surgery 1992; 89: 1083-1091.
8. Daniel RK, Kerrigan CL. Skin flaps: An anatomical and hemodynamic approach. Clinics in Plastic Surgery 1979; 6: 181-200.
9. Sessler DI, Rubinstein EH, Moayeri A. Physiologic responses to mild perianesthetic hypothermia in humans. Anesthesiology 1991; 75: 594-610.
10. Hynson JM, Sessler DI, Glosten B, McGuire J. Thermal balance and tremor patterns during epidural anesthesia. Anesthesiology 1991; 74: 680-690.
11. Svanes K. Effects of temperature on blood flow. In: Kaley G, Altwa JA, eds. Microcirculation. Baltimore: University Park Press, 1980; 21-39.
12. Banic A, Kouris K, Lewis DH, Christenson JT. The effect of temperature on skin perfusion in a cutaneous flap model. Surgical Research Communication 1990; 8: 203-214. 13. Colanruoni A, Bertuglia S, Intaglietta M. Effects of anesthesia
on the spontaneous activity of the microvasculature. In-ternational Journal of Microcirculation: Clinical and Ex-perimental 1984; 2: 13-28.
14. Longnecker DE. The microcirculation. In: Prys-Roberts C, ed. The Circulation in Anaesthesia: Applied Physiology and Pharmacology. Oxford: Blackwell Scientific Publications,
1990; 167-178.
15. MacDonald DJF. Anaesthesia for microvascular surgery: A physiological approach. British Journal of Anaesthesia 1985; 57: 904-912.
16. Roberts C. Regulation of the circulation. In: Prys-Roberts C, ed. The Circulation in Anaesthesia: Applied Physiology and Pharmacology. Oxford: Blackwell Scientific Publications, 1990; 179-207.
17. Seyde WC, Longnecker DE. Anesthetic influences on regional hemodynamics in normal and hemorrhaged rats. Anesthesiology 1984; 61: 686-698.
18. Cochrane DF. Anaesthesia for microvascular surgery. Baillieres Clinical Anaesthesiology 1987; 1: 747-759. 19. Banic A, Kouris K, Lewis DH. Evaluation of the
microcirculation in a sheep island pedicle flap model with a laser Doppler flow meter (LDF) and 99m-Tc labelled red blood cells (RBCs). Journal of Reconstructive Microsurgery 1990;6: 345-351.
20. Wheatley AM, Zhao D. Intraoperative assessment by laser Doppler flowmetry of hepatic perfusion during orthotopic liver transplantation in the rat. Transplantation 1993; 56: 1315-1318.
21. Oberg PA. Laser Doppler flowmetry. Critical Reviews of Biomedical Engineering 1990; 18: 125-163.
22. Manohar M, Parks C. Regional distribution of brain and myocardial perfusion in swine while awake and during 1.0 and 1.5 MAC isoflurane anaesthesia produced without or with 50% nitrous oxide. Cardiovascular Research 1984; 18: 44-353.
23. Manohar M, Parks C. Porcine regional brain and myocardial blood flows during halothane-O2 and halothane-nitrous oxide anesthesia: comparisons with equipotent isoflurane anesthesia. American Journal of Veterinary Research 1984; 45: 465-473.
24. Berg R. Angewandte und topographische Anatomic der Haustiere. Jena: G. Fischer Verlag, 1988; 180.
25. Nickel R, Schummer A, Seiferle E. Atlas der Anatomie der Haustiere. Berlin, Hamburg: Paul Parey Verlag, 1984;
102-103.
26. Almond NE, Wheatley AM. Measurement of hepatic per-fusion in the rat by laser Doppler flowmetry. American Journal of Physiology 1992; 262: G203-G209.
27. Wheatley AM, Almond NE, Stuart ET, Zhao D. Interpret-ation of the laser Doppler signal from the liver of the rat. Microvascular Research 1993; 45: 290-301.
28. Nilsson GE, Tenland T, Oberg PA. Evaluation of a laser Doppler flowmeter for measurement of tissue blood flow. IEEE Transactions on Biomedical Engineering 1980; 27: 597-604.
29. Bonner RF, Nossal R. Model for laser Doppler measurements of blood flow in tissue. Applied Optics 1982; 20: 1097-2107. 30. Thomson JG, Kerrigan CL. Hydrogen clearance: assessment of technique for measurement of skin-flap blood flow in pigs. Plastic and Reconstructive Sugery 1991; 88: 657-663. 31. Pang CY. Assessment of microsphere technique for
measure-ment of capillary blood flow in random skin flaps in pigs. Plastic and Reconstructive Surgery 1984; 74: 513-520. 32. Pang CY, Forrest CR, Morris SF. Pharmacological
aug-mentation of skin flap viability: A hypothesis to mimic the surgical delay phenomenon or a wishful thought. Annals of Plastic Surgery 1989; 22: 293-306.
33. Picard-Ami LA, MacKay A, Kerrigan CL. Effect of allopurinol on the survival of experimental pig flaps. Plastic and Reconstructive Surgery 1992; 89: 1098-1103.
34. Merin RG, Verdouw PD, de Jong JW. Dose-dependent depression of cardiac function and metabolism by halothane in swine. Anesthesiology 1977; 46: 417^123.
35. Lundeen G, Manohar M, Parks C. Systemic distribution of blood flow in swine while awake and during 1.0 and 1.5 MAC isoflurane anesthesia with or without 50% nitrous oxide. Anesthesia and Analgesia 1983; 62: 499-512.
36. Brower RW, Merin RG. Left ventricular function and compliance in swine during halothane anesthesia. Anesthesiology 1979; 50: 409^15.
37. Gilbert M, Mori M, Myhre ES. Hemodynamic dose-responses to halothane and isoflurane are different in swine with and without critical coronary artery stenosis. Anesthesia and Analgesia 1989; 68: 752-758.
38. Manohar M, Parks C. Porcine brain and myocardial blood flows during halothane-02 and halothane-nitrous oxide anesthesia: comparisons with equipotent isoflurane anesthesia. American Journal of Veterinary Research 1984; 45: 465^173.
39. Kalman S, Eintrei C. Central circulation during halothane—diethyl-ether azeotrope and isoflurane. Ada Anaesthesiologica Scandinavica 1991; 35: 736—740. 40. Weiskopf RB, Townsley MI, Riordan KK, Chadwick K,
Baysinger M, Mahoney E. Comparison of cardiopulmonary responses to graded hemorrhage during enflurane, halothane, isoflurane and ketamine anesthesia. Anesthesia and Analgesia 1981; 60: 481-491.
41. Watts DT. Adrenergic mechanisms in hypovolemic shock. In: Mills LC, Moyer JH, ed. Shock and Hypotension. New York: Grune & Stratton, 1965; 385-391.
42. Green HD, MacLeod JA, Anderson DA, Denison DAJ. Comparison of the blockade produced by dibenzyline, ilidar, tolazoline and phentolamine on the vasomotor responses in the skin induced by sympathetic nerve stimulation with the blockade of its responses to 1-norepinephrine and 1-epi-nephrine. Journal of Pharmacology and Experimental Therapeutics 1954; 112: 218-230.
43. Bond RF, Lackey GF, Taxis JA, Green HD. Factors governing cutaneous vasoconstriction during hemorrhage. American Journal of Physiology 1970; 219: 1210-1215. 44. Deegan R, He HB, Wood AJJ, Wood M. Effect of enflurane
and isoflurane on norepinephrine kinetics: A new approach to assessment of sympathetic function during anesthesia. Anesthesia and Analgesia 1993; 77: 49-54.
45. Deegan R, He HB, Wood AJJ, Wood M. Effect of anesthesia on norepinephrine kinetics: comparison of propofol and halothane anesthesia in dogs. Anesthesiology 1991; 75: 481^88.
46. Bond RF, Manley ESJ, Green HD. Cutaneous and skeletal muscle vascular responses to hemorrhage and irreversible shock. American Journal of Physiology 1967; 212: 488-497. 47. Johannesson GP, Lindahl SGE, Sigurdsson GH, Norden N.
Halothane, enflurane and isoflurane anesthesia for adenoidectomy in children, using two different pre-medications. Acta Anaesthesiologica Scandinavica 1987; 31: 233-238.
48. Macnab MS, Manninen PH, Lam AM, Gelb AW. The stress response to induced hypotension for cerebral aneurysm surgery: a comparison of two hypotensive techniques. Canadian Journal of Anaesthesia 1988; 35: 111-115. 49. Crozier TA, Morawietz A, Drobnik L, Rieke H, Sydow M,
Radke J. The influence of isoflurane on peri-operative endocrine and metabolic stress responses. European Journal of Anaesthesiology 1992; 9: 55-62.
50. Adams HA, Russ W, Leisin M, Borner U, Gips H, Hempelmann G. Untersuchungen zur endokrinen Stress-Antwort bei Halothan-, Enfluran- und Isofluran-Narkosen fur unfallschirurgische Eingriffe. Der Anaesthesist 1987; 36: 159-165.
51. Pocock G, Rickards CD. The action of volatile anaesthetics on stimulus-secretion coupling in bovine adrenal chromafnn cells. British Journal of Pharmacology 1988; 95: 209-217.