Elucidation of the Structure of Organic Compouncls
by Therrnal Fragmentation
by W. Simon, P. Kriemler, J. A. Voelimin, and H. Steiner, Department for Organic Chemistry, Swiss Federal lnstitute of Technology, Zurich, Switzerland
Abstract
The thermal fragmentation of organic compounds is described using a tech-nique in which the sample is coated on a ferromagnetic conductor. This con-ductor can be reproducibly heated to an accurately defined temperature (Curie point) in 20 to 30 milliseconds. Tech-niques are described to study fragmen-tation mechanisms.
A great variety of fragmentation techniques are in widespread use in organic chemistry for structural analysis. Such a reaction has been used, for instance, in 1927 which led to the first correct skeleton for steroids (Figure 1) (1). Pyrolysis as well as reaction gas chromatogra-phy have similar potentialities in the elucidation of the structure of organic compounds. In pyrolysis gas chromatography the samples are thermally fragmented in a stream of an inert carrier gas, and the re-action products are passed directly into a gas chromatography column where they are separated (2). It has been shown on different occa-sions that the pyrolysis gas chro-matogram may be used as a
finger-print the material studied (3, 4 ) . Under certain conditions the structure of the fragments obtained correlates closely with the structure of the starting material (5). The successful use of pyrolysis gas chro-matography in the structural eluci-dation of organic compounds and in the qualitative and quantitative analysis of mixtures is possible un-der the following ideal conditions
(5-8).
a) The fragmentation of the sam-ple must be carried out at such a high dilution and the fragments so quickly stabilized that no undesir-able recombination reactions can occur.
b) The sample must be heated to a constant pyrolysis temperature as quickly and as reproducibly as possible.
c) The pyrolysis temperature must not be too high, since unchar-acteristic fragments (methane, car-bon, hydrogen) may otherwise b2 formed preferentially.
If conditions (a) to (c) are satisfied, the fragments should be closely related to the structure of the original compound, and the
py-rolysis gas chromatogram of a mix-ture can be deduced by adding to-gether the signals of the pyrolysis chromatograms of the individual components.
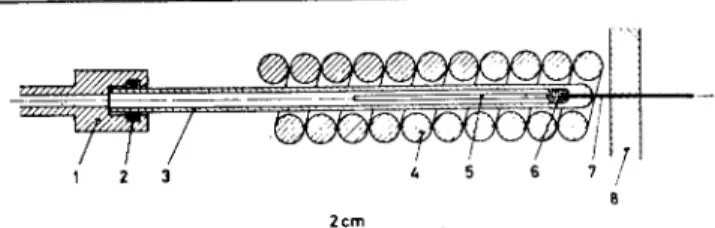
A pyrolysis unit that operates close to the ideal conditions has been described earlier (5,7) (see Figure 2). A thin layer of the sam-ple under investigation is coated on a ferromagnetic conductor (5 in Figure 2) which is warmed up in 20-30 milliseconds to a constant temperature using high frequency induction heating. The arrangement can be attached to inlet systems of standard gas chromatographs. The po-7er consumption of a conductor in a high frequency induction coil depends on the magnetic field in-side the coil, the radius, specific re-sistance, and magnetic permeabil-ity of the conductor, as well as the frequency applied (5). Owing to the dras'ic change in the magnetic permeability of the ferromagnetic conductor at the Curie point, the energy input drops at that tempera-ture. If the ene,rgy loss of the con-ductor above the Curie point is equal or larger than the energy
up-"
2 3
2cm
Figure 2. Pyrolysis Unit (10). 1—Carrier gas connector; 2--0-ring; 3—Glass capillary; 4—High frequency induction coil (copper) connected to an oscillator working at 0.45 MHz; 5— Ferromagnetic conductor (wire: 0.5 x 20 mm); 6—Sintered glass beads; 7—Platinum iridium capillary (0.3 mm inner
diameter); 8—Serum cap of inlet system of a gas
chromato-graph. 350° Se METHYLCYCLOPENTENO -PHENANTHRENE (DIELS HYDROCARBON) Figure 1. Selenium Dehydrogenation of Steroids
CHOLESTEROL
/Fe "Fe (Zn),CoNi (33167%) Ni Fe (60/40%) NiCr Fe (51/1/48%) Ni Fe (45/55%) Ni ,Co Ni (60140%) \ CH2/ (.1)
0-0
300 -(x500) (.20) 100 -300° 400° 500°. 600° . 7.00° •Nu Nu Fe Nitr Fe Ni Fe Co Ni Fe (45155) (51/1/48) (60/40) (33/67)
800° 900° °C
• e
Co Ni Co Ni (45/55) (60/40) Figure 5. Yield of Fragments in the Pyrolysis of the
Sodium Salt of Benzoic Acid
NH2 HOOC- C' H - CH2- CH2- 5- CH3 GLC. PERKIN-ELMER 226 GOLAY K 1540 (150 001") 1.2 ML N2 /MIN 3 MIN 40°;PROGR.31.2./MIN;145° 514 MET. (27.1N 1-N Ne0H ) 10 10 X
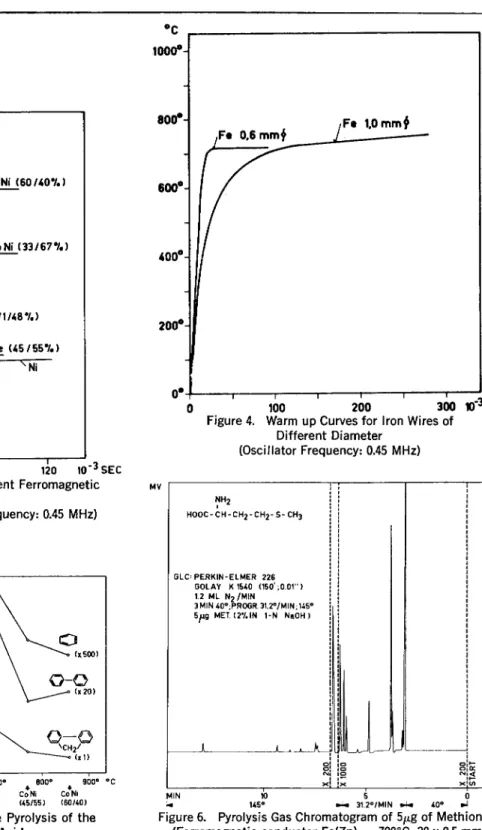
take from the field, the conductor will warm up only to that tempera-ture. When an oscillator with a cer-tain range of frequencies and a con-ductor of a limited range of diame-ters is used, the final temperature (Curie temperature) depends only on the ferromagnetic material and is independent of any fluctuations in experimental conclitions (5). In Figure 3 warm up curves are given for different commercially available ferromagnetic conductors (5) . By
increasing the radius of the conduc-tor, the energy uptake above the Curie point may become larger than the energy losses. In this case the warm up will not stop at the Curie point and will be slower for a given oscillator due to the higher mass of the conductor of larger diameter (Figure 4) . So far no catalytic ef-fects have been observed when us-ing ferromagnetic conductors of dif-ferent composition (8) . The behav-iour of the conductors in the thermal
fragmentation was not changed even by coating them with gold or plati-num. Usually the yield in the char-acteristic fragments shows an opti-mum at about 700°C in agreement with results obtained by the fila-ment technique (9) (Figure 5) . Since the dead volume of the py-rolysis zone is small ( 5 ,u1.) and the pyrolysis time short, GO sys-tems of high resolution may be used. A pyrolysis gas chromatogram of 5 itg of methionine is given in Figure
MV
0° 1 I
0 30 60 90 120 10-3 SEC
Figure 3. Warm up Curves for Different Ferromagnetic Conductors
(Diameter: 0.5-0.6 mm; Oscillator Frequency: 0.45 MHz)
REL. INT.
100 200 300 10-3SEC
Figure 4. Warm up Curves for !ron Wires of Different Diameter
(Oscillator Frequency: 0.45 MHz)
MIN 10 5 0
145° 31 2°/MIN .4. 40° .1 Egure 6. Pyrolysis Gas Chromatogram of 5iug of Methionine
(Ferromagnetic conductor Fe(Zn), ,-..,700°C; 20 x 0.5 mm) (ic 1000°
eoce
400° 200° e 1000° 600° 600° 400° 200°7-717- •
g
TOTAL ION CURRENT
....r . . ELIdElt 800.
GLASS. C-APILLARY (50,t 039'rnm f)EMULPHOR
HL lis /MIN , g :
. 8 MIN 40°, PROGR. 31.2.1141N, 3O ,6 PRO U0 N. IN 1_44, Nia0H,2 NS HITACHI PERKIN-ELMER R1MU- 6 D
16 10011
12
....31211/MIN
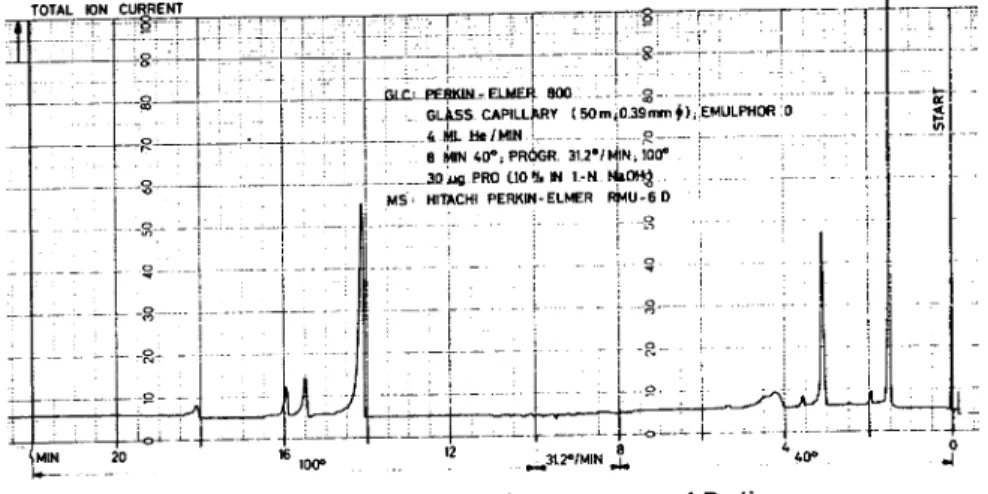
Figure 7. Pyrolysis Gas Chromatogram of Proline
(Ferromagnetic conductor: Fe(Zn); 20 x 0.5 mm; end temperature: ,--700°C)
0 1 MIN 0°
Ow0
MIN REL. INT. 60 40 30 20 -x 1000)r-6-0
H2 0
COOH
Fe (Zn)
D D
700°C
Figure 10. Thermal Fragmentation of Benzoic Acid,c(s
•5 'k 06
151. 05
5.90
6. It was obtained using a tubular column without a sample splitter.
Since the thermal fragmentation is preferably performed on samples in the pg range [see (a) above], mass spectrometry is the method of choice for the determination of the structure of the pyrolysis products. A detailed description of a direct instrumental combination of ther-mal fragmentation (TF) /gas chro-matography (GC) /mass
spectrome-try (MS) in which fragments as small as 0.01 to 0.001 gg may be identified has been given elsewhere (10, 11). A typical pyrolysis gas chromatogram obtained with such an arrangement is given in Figure 7. The mass spectrometer is used as the GC detector, the total ion current being recorded.
To obtain information about the mechanism of the pyrolysis de-scribed, a program to study simple
molecules is in progress in our labo-ratory. A schematic pyrolysis gas chromatogram for the sodium salt of benzoic acid (200 pg) as ob-tained in the TF/GC/MS combina-tion, including the structure of the fragments, is given in Figure 8. It is obvious that undesirable recom-bination reactions have occurred. This is not surprising since even if the distribution of the sample on the surface of the wire were uniform, the thickness of the film would be of the order of 10,000 molecules (5). The probability for recombination reactions decreases with decreas-ing sample size. Thus, the rela-tive yield of biphenyl decreases as the quantity of sodium benzo-ate on the ferromagnetic wire is reduced (Figure 9) . A reduction of the amount of benzoic acid from 20 to 2 gg gives a reduction in the relative amount of biphenyl formed of about two. Using about 0.2 ,ug benzoic acid, biphenyl can no longer be detected; the amount formed must have been reduced at least 1,000 times. It has been assumed earlier (5) that the decrease in the yield of benzene with increasing sample size is probably due to the
TOTAL ION CURRENT
- C5 H6 MWt 134 [--
0 0
C12 H12 0—cH2 154 toFigure 8. Thermal Fragmentation of 200 pg of Benzoic Acid (Sodium Salt)
(Ferromagnetic conductor: Fe(Zn); 20 x 0.5 mm; end temperature: --700°C)
GLC: Perkin-Elmer 800
Open tubular column 50 ft.; 0.02 in.; Carbowax K 1540 10 ml He/Min.
3.7 min. 50°C; Prog. 16.7°C/min.; 180°C. MS: Hitachi Perkin-Elmer RMU-6D
10
-0— CHO
rc)_cH,
6 'h C6H5COON IN 1-N NaGI
Figure 9. Relative Yield in the Formation of Biphenyl and Benzene (5)
(Pyrolysis of Sodium Benzoate; Ferromagnetic conductor: Fe(Zn); 20 x 0.5 mm, ~700°C)
Fe /In/Ce/NI/e 600 C PROPORT1ONAL COUNTER RECORDER , CH,
Figure 11. Instrumental Combination of Pyrolysis and Radio Gas Chromatography
ACTIVITY [9.] { CO2 , 82 , CH4 0-CH3 1-C. 2-C. 3-C C. 985 23.1 0.4 0.9 1.9 055 853 58.5 93 Na OH Fe(2n),700°C cH =CH2 0-cH2c.
Pyrolysis of Labelled Phenylalanine
0_
3 2 1CH2 -CH -COOH
983
Figure 12.
fact that the hydrogen atom used to form benzene from the benzoate presumably comes from the water retained by excess NaOH on the surface of the conductor after evapo-ration of the solvent. The relative amount of water present decreases with increasing sample size. The thermal fragmentation of benzoic acid—d, clearly shows (Figure 10) that the hydrogen atom in question originates from other benzoic acid molecules (12) . The decreasing ben-zene formation at high benzoic acid concentration (Figure 9) is, there-fore, due to competing recombina-tion reacrecombina-tions.
With amino acids the structure of the pyrolysis fragments in gen-eral correlates closely with the structure of the starting material (5, 10). The thermal fragmentation of mixtures gives signals as expected from the simple addition of the
sig-nals (10) from the chromatograms of the individual acids. Similar re-sults are obtained in the fragmen-tation of peptides (10) . At present this seems to be in contrast to the findings of Merritt (13). With a somewhat different technique he observes a fragmentation depending on the peptide linkage (13).
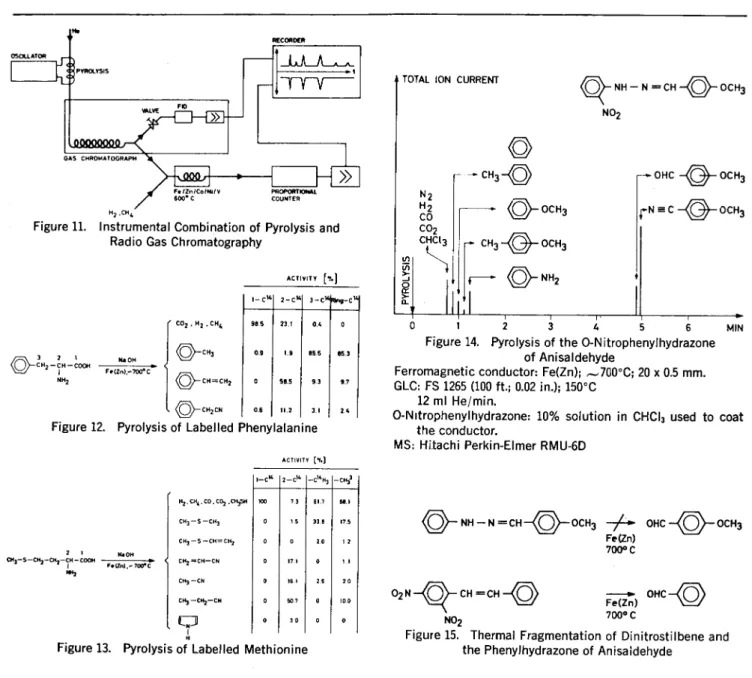
More detailed information about the mechanism of the fragmentation may be obtained using tracer tech-niques. The arrangement given in Figure 11 shows the possibility of recording simultaneously the pyrol-ysis gas chromatograms as well as the pyrolysis radio gas chromato-gram (14) of C14- and H3-labelled compounds. The results obtained for labelled phenylalanine are pre-sented in Figure 12. Several con-clusions are possible using this in-formation: The aromatic nucleus is fragmented to only a negligible
ex-tent. The bond between the carbon atom 3 and the aromatic nucleus remains almost intact (12), in con-trast to the bond between C2 and C3. A considerably more compli-cated situation is shown in Figure 13 for the fragmentation of meth-ionine (12).
In certain instances there is a parallel between the thermal frag-mentation and the fragfrag-mentation induced by electron impact. Figure 14 shows schematically the pyroly-sis gas chromatogram obtained in the fragmentation of o-nitrophenyl-hydrazone of anisaldehyde (12). The most interesting fragment formed is anisaldehyde. No alde-hyde is observed in the pyrolysis of the corresponding phenylhy-drazone; but it is, however, obtained in the thermal fragmentation of 2,4-dinitrostilbene (Figure 15). The results can be rationalized
assum-TOTAL ION CURRENT
0
— CH3—0
10—
OCH3 cH3 oc4-13 NH2(R_
NH — N CH OCH3 NO2 N2 H2 CO CO2 CHCI3 3 4 5 MINFigure 14. Pyrolysis of the 0-N itrophenylhydrazone of Anisaldehyde
Ferromagnetic conductor: Fe(Zn); ,-700°C; 20 x 0.5 mm. GLC: FS 1265 (100 ft.; 0.02 in.); 150°C
12 ml He/min.
0-Nitrophenylhydrazone: 10% solution in CHCI3 used to coat the conductor.
MS: Hitachi Perkin-Elmer RMU-6D
ACTIVIT [e.] 1-C38 2-C38 -C38H3 -CH? H. , C0 .0O2 ,071358 100 73 617 65.1 CH3-5 -CH3 0 1 5 33.1 175 6/13 -5 -CH=CH, 0 0 20 1.2 90 08 CH2=CH-CN 0 17.1 0 Fear9,- 7007C CH3 -CN 0 WI 25 20 CH3 -C112-CN 0 507 0 100
LJ
0 30 0Figure 13. Pyrolysis of Labelled Methionine
4(1)— NH — N CH OCH3 OHC OCH3
Fe (Zn) 700°C 02N CH CH Fe(In) OHC NO2 700°C
Figure 15. Thermal Fragmentation of Dinitrostilbene and the Phenylhydrazone of Anisaidehyde
2 1 013-5-043-04,111-COON
NO2 m/e =316 [CH30 CO kei'12 CH Fe - 700° C C20 H42 • CH3 CH3 CH3 1 I / CO0—CH2—CH.---C—CH2CH2—CH2— 2 CH—CH2 CH2—CH2—CH \ CH3 NO2 m /e =135 C8 Hg NO 135.06841 C8 H7 02 135.04460 C6 H3 N2 02 135.01945 C6 H5 N3 0 135.04326
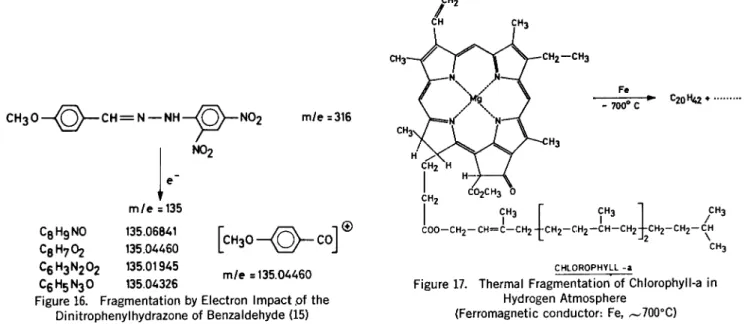
Figure 16. Fragmentation by Electron Impact .of the Dinitrophenylhydrazone of Benzaldehyde (15)
CHLOROPHYLL -a
Figure 17. Thermal Fragmentation" of Chlorophyll-a in Hydrogen Atmosphere
(Ferromagnetic conductor: Fe, ,-700°C)
CH3 0 CH=N —NH
e-m/e =135.04460
ing that a transfer of an oxygen atom of the nitro group has taken place. A similar rearrangement is observed in the corresponding mass spectra (Figure 16) (15) . High resolution mass spectroscopy has shown that an electron impact frag-ment of mass number 135.04460 corresponding to C8H702 is ob-tained (15) .
Since in mass spectrometry re-arrangement reactions such as the McLafferty rearrangement (16) are quite common and since even ion molecule reactions are observed very frequently (17, 18) , it is expected that thermal fragmentation reac-tions will be much more complex. This is mainly due to the fact that component concentrations are us-ually much higher than in mass spectrometry.
The application of the technique described is of course especially at-tractive for the study of compounds of high complexity which are diffi-cult to study by other techniques
(Figure 17) (19, 20) .
The examples given show that the pyrolysis technique described has potentialities for the elucidation of the structure of organic compounds (20-24) . A more detailed under-standing of the mechanism of the fragmentation is needed for the suc-cessful application of this method. Because of the complexity of the fragmentation process, pyrolysis techniques may give information not obtained in electron impact frag-mentation (21, 22) . At present the method seems to be particularly at-tractive for compounds not
acces-sible to mass spectrometry.
Acknowledgment
The present work has been sup-ported by the Schweizerischer Nationalfonds zur Foerderung der wissenschaftlichen Forschung (Re-search Project No. 3602) and the Eidg. Volkswirtschafts-Stiftung.
Literature Cited
1. Diets, 0., Gaedke, W., and Koerding, P., Ann. 459, 1(1927).
2. Kokes, R. J., Tobin Jr., H., and Em-• mett, P. H., J. Amer. Chem. Soc. 77,
5860 (1955).
3. Reiner, E., Symposium on Pyrolysis and Reaction Gas Chromatography, Ecole Polytechnique, September 15-16, 1966, Paris.
4. Oyama, V. I., Symposium on Pyroly-sis and Reaction Gas Chromatogra-phy, Ecole Polytechnique, Septem-ber 15-16, 1966, Paris.
5. Simon, W., and Giacobbo, H., Chem.-Ing.-Techn. 37, 709 (1965); Simon, W., and Giacobbo, H., Angew. Chem. int. Ed. 4, 938 (1965).
6. Levy, R. L., Symposium on Pyrolysis and Reaction Gas Chromatography, Ecole Polytechnique, September 15-16, 1966, Paris.
7. Giacobbo, H., and Simon, W., Pharm. Acta Helv. 39, 162 (1964); Giacobbo, H., and Simon, W., in Festschrift zum 60. Geburtstag von Jakbo Buechi (X. Perlia, Ed.), Verlag Schweizer Apothekerverein, Zurich, 1963, S. 199. 8. Jones, C. E. R., Moyles, A. F., Nature
191, 663 (1961); 189, 222 (1961). 9. Lehmann, F. A., and Brauer, G. M.,
Anal. Chem. 33, 673 (1961).
10. Voellmin, J. A., Kriemler, P., Omura, I., Seibl, J., and Simon, W., Micro-chem. J. 11, 73 (1966).
11. Voellmin, J. A., Omura, I., Seibl, J.,
Grob, K., and Simon, W., Helv. 49,
1768 (1966).
12. Kriemler, P., Voellmin, J. A., and Simon, W., Helv., in preparation. 13. Merritt Jr., C., and Robertson, D. H.,
Symposium on Pyrolysis and Reac-tion Gas Chromatography, Ecole Polytechnique, September 15-16, 1966, Paris.
14. Simon, H., Muellhofer, G., and Me-dina, R., "Radioisotope Sample Measurennent Techniques in Medi-cie and Biology," International Atomic Energy Agency, Vienna, 1965. 15. Seibl, J., private communication. 16. McLafferty, F. W., Appl.
Spectro-scopy 11, 148 .(1957); Anal. Chem. 31,
82 (1959).
17. Biemann, K., Mass Spectrometry
Organic Chemical Applications, Mc-Graw-Hill Book Company, Inc., New York, San Francisco, Toronto, Lon-don, 1962.
18. Brown, P., Djerassi, C., Schroll, G., Jakobsen, H. J., and Lawesson, S.
0., J. Amer. Chem. Soc. 87, 4559
(1965).
19. Whitten, D. G., Bentley, K. E., and
Kuwada, D., J. Org. Chem. 31, 322
(1966).
20. Levy, R. L., Gesser, H., Halevi, E. A., and Saidman, S., J. Gas Chromatog.
2, 254 (1964).
21. Brauer, G. M., Journal of Polymer Science 8, 3 (1965).
22. Keulemans, A. I. M., Symposium on Pyrolysis and Reaction Gas Chro-matography, Ecole Polytechnique, September 15-16, 1966, Paris. 23. Janak, J., Nature 185, 684 (1960). 24. Dhont, J. H., Nature 200, 882 (1963);
Analyst 89, 71 (1964).
Manuscript received November 18, 1966