Publisher’s version / Version de l'éditeur:
Journal of Microscopy, 227, September 3, pp. 191-202, 2007-09-01
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE. https://nrc-publications.canada.ca/eng/copyright
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la
première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
NRC Publications Archive
Archives des publications du CNRC
This publication could be one of several versions: author’s original, accepted manuscript or the publisher’s version. / La version de cette publication peut être l’une des suivantes : la version prépublication de l’auteur, la version acceptée du manuscrit ou la version de l’éditeur.
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Low temperature bitumen stiffness and viscous paraffinic nano and micro size domains by cryogenic AFM and PDM
Masson, J-F.; Leblond, V.; Margeson, J. C.; Bundalo-Perc, S.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
NRC Publications Record / Notice d'Archives des publications de CNRC:
https://nrc-publications.canada.ca/eng/view/object/?id=4d4efaab-4bd6-4137-a414-bba4e3d70919 https://publications-cnrc.canada.ca/fra/voir/objet/?id=4d4efaab-4bd6-4137-a414-bba4e3d70919
http://irc.nrc-cnrc.gc.ca
L o w - t e m p e r a t u r e b i t u m e n s t i f f n e s s a n d
v i s c o u s p a r a f f i n i c n a n o - a n d m i c r o - d o m a i n s
b y c r y o g e n i c A F M a n d P D M
N R C C - 4 9 7 1 0
M a s s o n , J - F . ; L e b l o n d , V . ; M a r g e s o n , J . ;
B u n d a l o - P e r c , S .
A version of this document is published in / Une version de ce document se trouve dans: Journal of Microscopy, v. 227, no. 3, Sept. 2007, pp. 191-202
The material in this document is covered by the provisions of the Copyright Act, by Canadian laws, policies, regulations and international agreements. Such provisions serve to identify the information source and, in specific instances, to prohibit reproduction of materials without written permission. For more information visit http://laws.justice.gc.ca/en/showtdm/cs/C-42
Les renseignements dans ce document sont protégés par la Loi sur le droit d'auteur, par les lois, les politiques et les règlements du Canada et des accords internationaux. Ces dispositions permettent d'identifier la source de l'information et, dans certains cas, d'interdire la copie de documents sans permission écrite. Pour obtenir de plus amples renseignements : http://lois.justice.gc.ca/fr/showtdm/cs/C-42
Low temperature bitumen stiffness and
viscous paraffinic nano and micro domains by cryogenic AFM and PDM
J-F. Masson,* V. Leblond, J. Margeson, S. Bundalo-Perc
Institute for Research in Construction, National Research Council Canada, Ottawa, Ontario, Canada, K1A 0R6
*To whom correspondence should be addressed. Phone: (613) 993-2144. Fax: (613) 952-8102.
e-mail: jean-francois.masson@nrc.gc.ca
Summary
In an effort to better understand the structure and behaviour of bitumen in low temperature, we describe the first use of cryogenic atomic force microscopy (AFM) and phase detection microscopy (PDM) to characterize bitumen nano- and micro-structures.
The results were interpreted in light of glass transition temperatures (Tgs) for bitumen
fractions. The domains visible by microscopy, the catana, peri, and para phases, were attributed to domains rich in asphaltenes, naphthene and polar aromatics, and saturates, respectively. Between –10 °C and –30 °C, AFM images revealed topographic features
not visible in AFM images acquired at room temperature. Based on PDM and Tgs, the
features were assigned to viscous unfrozen saturates. Upon cooling to –72°C, unfrozen domains of 20 nm to 400 nm were observed. These domains were found in the paraphase rich in saturates and in the periphase rich in naphthene aromatics and polar aromatics. The findings indicate that new viscous domains form upon cooling to low temperatures due to phase segregation, and that some bitumens are never entirely rigid in low temperatures.
Keywords: asphalt, AFM, atomic force microscopy, bitumen, composition, microscopy,
Introduction
More than four thousand years ago, in the Bronze Age, natural bitumen was used to waterproof the buried end of wood pylons (Jones, 1963). Centuries later, filled bitumen, or asphalt, was used by Sumerians and Assyrians to waterproof walls and join materials (Lambert, 2005; Speight, 1999). With the advent of the modern petroleum industry in the
second half of the 19th century, man-made bitumen was produced as a residual of the
distillation of petroleum crude oil. As a consequence of its greater availability, the use of bitumen expanded. Close to two hundred applications are now known (Asphalt Institute 1989), the most important ones being in roofing and paving.
Our understanding of bitumen composition, its properties, and the relationship between the two has evolved tremendously in the last century (Brown et al., 1957; Traxler, 1961; Hoidberg, 1965; Petersen, 1984; Brûlé et al. 1986; Goodrich et al., 1986;
Speight, 1999). Bitumen contains heavy hydrocarbons, typically in the range C24-C150.
With the number of possible isomers exceeding 1015, the complexity of the mixture
precludes any precise molecular identification. As a result, physico-chemical characterization of bitumen often involves its fractionation into chemical families (see Masson et al., 2001 and references therein). Composition is most often reported in weight percent of saturates, naphthene aromatics, polar aromatics, and asphaltenes (S-NA-PA-As), or in terms of saturates, aromatics, resins and asphaltenes (S-A-R-(S-NA-PA-As), or simply in terms of asphaltenes and maltenes, the latter being the mixture of the first three fractions. The balance of these four families of compounds is thought to govern bitumen characteristics.
properties, bitumen performance sometimes fails to meet expectations. This is especially true of low temperature applications. To address this issue, we have studied the structure of bitumen by cryo-microscopy and we have related the results to material stiffness. To achieve a resolution of the bitumen structure down to a few nanometers, we used atomic force microscopy (AFM), which provided a topographic profile of the surface, and phase detection microscopy (PDM), which provided an indication of material stiffness and adhesion. The use of these microscopy methods for bitumen analysis was described before (Loeber et al., 1996; Pauli et al., 2001; Jäger et al., 2004; Masson et al., 2006a)
We report here on the first application of cryo-AFM/PDM for bitumen analysis. To assess bitumen stiffness, we used modulated differential scanning calorimetry (MDSC) and measured glass transition temperatures. It was found that various bitumen domains can be attributed to specific bitumen fractions, and that upon cooling to low temperatures viscous nano and micro domains form in the maltenes due to segregation. The scope of the work is limited to bitumens that show a catana phase (bee-like structures) at room temperature (Masson et al., 2006a).
Materials and methods
Materials
Three bitumens from the Materials Reference Library (MRL) of the Strategic Highway Research Program (SHRP) in the U.S.A were investigated: AAK, AAN, and AAS. Numerous publications are available on the chemistry and properties of the MRL bitumens (Jones, 1993; Michon et al., 1997a, 1997b; Usmani, 1997). The three bitumens were selected because they contain fused aromatic rings of different average sizes
(Michon et al., 1997a, 1997b) and they all show multiple phases in PDM at room temperature (Masson et al., 2006a). The composition of the bitumens is shown in Table 1. Sample preparation and AFM analysis
Bitumen films were heat cast to maintain the bitumen solid-state structure as described before (Masson et al., 2006a). The microscopy work was performed on a JEOL JSPM-5200 (JEOL Ltd.,Peabody, MA) with MikroMasch (MikroMasch, Portland, OR) silicon tips of nominal tip radius, spring constants and free resonance frequencies of 10 nm, 40
Nm-1 and 170 kHz, respectively. With this instrument, the sample stage is located under
a bell jar that can be evacuated and cooled or heated. In this work, the bell-jar was first evacuated down to about 1 mPa, after which, the sample was cooled at 1°C/min to a fixed temperature where it was equilibrated 10 min before images were acquired. AFM and PDM images were acquired simultaneously. After the acquisition of images, which took about 1h, the sample was further cooled to the next observation temperatures. Typical imaging temperatures were 22°C, –10°C, –27°C, –55°C and –72°C. These temperatures were selected based on glass transition temperatures obtained by MDSC. The acquisition of images was fairly straightforward. At the temperatures of observation, the bitumens did not pollute the tips with sticky residues. In the reported AFM images, the higher a topographic feature (expressed in nm), the lighter is its shade. In the PDM images, the greater the sample-tip interaction (or phase-lag signal, expressed in degree), the darker is the shade.
Glass transition temperatures
Glass transition temperatures of the bitumens were obtained by plotting the derivative of the heat capacity against time. The heat capacity was measured by modulated differential
scanning calorimetry (MDSC) as detailed before (Masson and Polomark, 2001; Masson et al., 2002). Here the bitumen sample was cooled slowly at 1°C/min from 22°C down to –100°C. This rate was the same as the microscopy cooling rate. MDSC was used to monitor the effect of this cooling on bitumen by heating the bitumen back up at 5°C/min from –100°C to 150°C with a modulation period of 60s and amplitude of ±0.8°C.
Results and Discussion
Basic morphology
The basic morphology of the bitumens investigated was obtained at 22°C under vacuum. Fig. 1 shows the typical characteristics of this morphology as obtained by AFM and PDM (Masson et al., 2006a). Bitumens AAK, AAN and AAS all showed the same characteristics at room temperature. The AFM image at the top of Fig. 1 showed a flat background in which a catana phase is dispersed. This phase is defined by its succession of pale and dark lines (Masson et al., 2006a). For this reason, short isolated domains are often referred to as ‘bees’ or ‘bee structures’ (Loeber et al., 1996; Pauli et al., 2001; Jäger et al., 2004). The pale and dark lines respectively indicate a rise and a drop of the topographic profile against the featureless (flat) backround. The rise is bitumen dependant and varies between about 20 to 300 nm (Jäger et al.; 2004: Masson et al., 2006a). In contrast to AFM, PDM reveals three phases, the catana, the peri, and the para phases, the composition of which will be detailed shortly. As the names imply (Masson et al. 2006a), the periphase immediately surrounds the catanaphase, whereas the paraphase, in a lighter shade, is neighbor to the periphase. The shades in the PDM images indicate the relative stiffness of the phases as obtained from sample-tip
interactions. The softer the phase, the greater the sample-tip interaction, and the darker the shade in the PDM image.
Glass transition temperatures
The basic morphology in Fig. 1 is obtained at a temperature between the glass transition
temperatures (Tg) of the asphaltenes and the maltenes. The Tg is the temperature at
which, upon cooling, an amorphous material goes from a viscous fluid behaviour to a hard, stiff, glassy state, or vice versa upon heating (Eisenberg, 1993). An amorphous
material of constant composition shows a single Tg, whereas an inhomogeneous blend
shows more than one Tg (Olabisi, 1979).
Bitumen is a mixture, and this mixture is not totally homogeneous because it
shows more than one Tg. With MDSC, Tgs are shown by maxima in Fig 2. Previous
MDSC work showed that the asphaltenes have a glass transition above room temperature, between 40°C and 60°C (Masson and Polomark, 2001; Masson et al., 2002). The
maltenes have a large Tg below room temperature (Masson and Polomark, 2001), which
is most often taken as the Tg of bitumen measured by standard DSC (Turner and
Branthaver, 1997). The separation of bitumen in two gross phases with Tgs above room
temperature and Tgs below room temperature is consistent with the topography obtained
by AFM at room temperature, which shows a biphasic structure (top of Fig. 1). Accordingly, early AFM studies of bitumen have led to the conclusion that the glassy catanaphase is borne of asphaltenes (Loeber et al., 1996; Pauli et al., 2001). Models for bitumen rheology also consider bitumen a biphasic system where asphaltenes are dispersed in maltenes (Lesueur et al., 1996).
particular, more details become visible and a multi-phase bitumen system emerges (Masson and Polomark, 2001; Masson et al., 2002; Jäger et al., 2004; Masson et al., 2006a). This is evident in the PDM image in Fig. 1, and it is visible in the MDSC graph
in Fig. 2 as sub-zero Tg peaks with shoulders, and as secondary Tg peaks above room
temperature, the latter being the clearest with bitumen AAS. Broad and uneven Tg peaks
are typical of partly segregated blends (Olabisi, 1979). The results in Fig. 1 and 2 thus demonstrate that a biphasic bitumen representation is too simplistic and that bitumen
phases with Tgs above and below room temperature contain secondary phases.
Both MDSC and PDM results are consistent with the complex composition of maltenes, the fractions of which increase in molecular mass, aromaticity and polarity as S < NA < PA, or S < A < R (Speight, 1999). The terminology for the fractions can be confusing, however, because the A or NA fraction often contain little conjugated structures like alkylated-benzenes or other structures with 4n+2 pi electrons (n is an integer), which defines molecular aromaticity (Turney, 1979). Table 2 provides the
generic composition of the fractions in more classical terms, along with typical Tg.
The temperature of the glass transition is affected by composition. It increases when the molecular structure becomes more rigid due to the increase in molecular weight, the presence of rings, or polar groups (Shen and Eisenberg, 1966). Given the
molecular weight, aromaticity and heteroatom content of the bitumen fractions, the Tg of
these fractions increases as S < NA < PA < As, or S < A < R < As (Table 2).
PDM images obtained from bitumen are based on a variation in Tg, or
composition. Indeed, PDM is sensitive to sample-tip interactions that arise from differences in material stiffness (Anczykowski et al., 1996) and surface adhesion
(Winkler et al.,1996) and for organic materials with a glass transition, stiffness and
adhesion are not independent. The glassy state below Tg provides for low adhesion,
whereas the viscous and rubbery state above Tg provides higher adhesion (Dunning,
1977).
Given the relationship between composition, Tg, stiffness and adhesion, the
different domains in the PDM images of bitumen (Fig. 1) can be assigned to specific
compositions based on Tgs. The catanaphase can be assigned to the most rigid and most
polar bitumen fraction, the asphaltenes (Loeber et al., 1996; Pauli et al., 2001), although not all asphaltenes form such a phase (Masson et al. 2006a). The periphase that surrounds the catana phase can be attributed to a blend of less polar material, the resins and the aromatics (or the naphthene and polar aromatics). The paraphase, which is the farthest from the most polar phase, can be assigned to the non-polar saturates. Thus in the microscopy discussion below, the paraphase becomes synonymous with domains rich in saturates or alkanes, and the periphase becomes interchangeable with domains rich in alkylated cyclic structures, i.e., NA, PA, A or R. (Table 2).
AFM at the glass transition temperature of the maltenes
Bitumen AAK. The effect of cooling on the AFM and PDM images of bitumen was investigated by means of an atomic force microscope with a cooling stage. Amongst the
bitumens investigated, bitumen AAK showed the warmest Tg from the maltenes, with a
Tg centered at –10 °C (Fig. 2). The AFM image of this bitumen at –10 °C showed much
more topography than at 22 °C, as seen from a comparison of Figs 1 and 3. In addition to the catanaphase rich in asphaltenes, two features became visible in the AFM image of the bitumen matrix at –10°C: faint and fairly large domains, and less numerous bright
looking ones (label V, for viscous). The small and bright domains were about 45 nm above the background, whereas the larger and paler domains were about 18 nm in height (Fig. 4). This either indicated the growth of crystalline material rising over the background, or an uneven volume contraction of matter upon cooling. We will see later how these two possibilities are distinguished.
The PDM image of bitumen AAK at –10 °C showed fewer features than at 22°C
(bottom of Fig. 3). With a temperature in the middle of the Tg region, much of the
maltenes were in the rigid glassy state, as were the asphaltenes. For this reason, the stiffness sensitive PDM signal showed little contrast between the catanaphase and its surrounding periphase.
It is noteworthy that the PDM image in Fig. 3 also revealed dispersed domains of 100-200 nm that showed in the darkest shade (label V). This phase correlated with the bright looking maxima in the upper AFM image. The nature of these features is better
appreciated by cooling the bitumen further through the Tg of its maltenes. Hence, Fig. 5
shows the AFM and PDM images at –27°C. The AFM image showed more visible and more numerous domains rising over the background than at –10°C (Fig. 3). The height of the domains was about 85 nm. In the phase image, these domains provided a very clear contrast to the background, where they appeared as black domains with an average diameter of about 360 nm, about twice the size at –10 °C. It is noteworthy that each domain left streaks on the image from the rastering of the surface by the AFM probe. This was a clear and important indication that these domains were viscous in nature and
thus, well above the Tg of its components, and not stiff crystalline material as hinted to
Bitumen AAS. This bitumen also revealed new structural features upon cooling. In the
AFM image of AAS at the Tg of –15°C (Fig. 6), the light shaded paraphase protruded
from the surface by about 45 nm, giving an overall perspective on the various domains much like that obtained with the PDM image at 22°C. This comparison confirmed that the paraphase was rich in saturates.
The reason for the protruding of the saturates-rich paraphase in the AFM image of
Fig. 6 is related to the relationship between Tg and volume. There is an important volume
contraction upon cooling through the Tg (Eisenberg, 1993). At –15°C the saturates of
AAS had not been cooled through their Tg and they had not contracted like the
surrounding more polar material. Consequently, in the topographic (AFM) image, the paraphase protruded from the surface. The PDM image in Fig. 6 showed no contrast between the glassy catanaphase and periphase, and only a slight contrast between the paraphase and the glassy phases.
Bitumen AAN. Upon cooling to the Tg of the maltenes in bitumen AAN (–19 °C), the
saturates-rich paraphase once again became clearly visible against the glassy maltenes-rich periphase (Fig. 7). This phase was not visible at 22°C in the AFM image. In contrast to the other bitumens in this study, the domains of saturates in AAN showed inclusions. The 5 µm x 5 µm images in Fig. 7 showed these inclusions as white dots in the AFM image and as dark dots in the PDM image, their size being about 70 nm in diameter and 22 nm in height. Because the white dots are reminiscent of grains of salt, we have referred to the collection of inclusions as the salphase since it was observed at 22°C in some bitumens (Masson et al., 2006a). Fig. 7 is thus far the clearest expression of this phase.
AFM below the glass transition of the maltenes
In Fig 2, the Tg of the maltenes extends down to about –45°C. The change in slope and
the curve shape below this temperature suggested that more bituminous matter still
remained unfrozen, in accordance with the Tg of the saturates (Table 2). The bitumens
under investigation were thus cooled further down to –55°C.
Bitumen AAK. Fig. 8 shows the images of this bitumen below its main Tg. The AFM
image showed the asphaltenes-rich catanaphase, along with light shaded domains that protruded from the surface by about 80 nm. The PDM image showed these domains as dark quasi-circular domains of 300-400 nm in diameter. It is noteworthy that there was a clear increase in the number and the average size of these domains as the temperature decreased from –10°C (Fig. 3) to –27°C (Fig. 5) and to –55°C (Fig.8).
Upon cooling, the progressive transformation of the phases in the PDM images followed that in the AFM images except that the catana phase was absent. At –55 °C,
within the Tg region of the saturates, the PDM image showed a bi-phasic structure, in
which isolated and quasi-circular viscous domains were quite distinct from a background that seemed inhomogeneous (bottom of Fig. 8). In an attempt to find a reason for the inhomogeneity, smaller scans of 5 µm x 5 µm were performed on bitumen AAK (Fig. 9). The PDM image revealed that the apparently isolated quasi-circular domains in the PDM image of Fig. 8 were not truly isolated. Fig. 9 shows that they were within the initial paraphase that had become glassy, and which had become almost invisible against the
glassy background of high-Tg material. The AFM image in Fig. 9 showed that the glassy
periphase was still slightly above the surface by 10 nm and that the viscous paraphasic material was protruding further from the surface, at 40-55 nm.
Bitumen AAS. At –55°C, this bitumen showed domains of viscous alkanes of about 100 nm on average (Fig. 10), which was smaller than for bitumen AAK (Fig. 8). A comparison of the AFM and the PDM images in Fig. 10 showed that many of these domains were aligned outside the resins and aromatics rich periphase, as indicated by the dotted oval Fig. 10. The largest viscous domains in bitumen AAS were found in the paraphase, as indicated by the largest oval in the figure. Smaller scans revealed that the viscous domains could be as small as 10 nm as indicated by the dotted arrow in the PDM image of Fig. 11. This size was consistent with the embedded salphase observed at –19 °C in the paraphase of bitumen AAN (Fig. 7), and at +22°C in other bitumens (Masson et al., 2006a). This implies that the salphase can be observed at many temperatures, that it
contains unfrozen material with a Tg below the observation temperature, and that this Tg
is in the lowest measured for bitumen fractions (Table 2). Given the relationship between
Tg, polarity and molecular weight (Eisenberg, 1993; Shen and Eisenberg, 1966), the
salphase must contain the lowest molecular weight amorphous alkanes of the paraphase. The enlarged topographic images of bitumen AAS at the top of Fig. 11 clearly showed a salphase within the periphase. This was unexpected. The earlier results at room temperature (Masson et al., 2006a) and those at –19°C in Fig. 7 suggested that the salphase would be found exclusively in the saturates-rich paraphase. Fig. 11 clearly demonstrated that it could also form in the periphase. Given the earlier conclusion that the periphase contains A and R, or NA and PA (Table 2), the salphase in the periphase of Fig 11 clearly established that micro and nano viscous domains also formed in the
periphase. And again, based on the relationship between Tg, polarity and molecular
attributed to domains of amorphous alkyl-rich segments.
Bitumen AAN. Upon cooling to –55 °C, the salphase was also found in the periphase of bitumen AAN, where it was about 20 nm to 200 nm (Fig. 12). The small domains of the salphase showed most clearly on the PDM image.
To end the sequence of experiments, all three bitumens were cooled to –72°C to determine if the viscous bitumen phases would freeze. However, the AFM and PDM images were not significantly different from those obtained at –55°C, which
demonstrated that the alkane- or alkyl- rich salphase was still above its Tg at –72°C.
Volume loss upon cooling
The effect of temperature on volume was noteworthy. With every stepwise decrease in temperature from 22 °C to –70 °C, the AFM probe had to travel a greater distance to make contact with the bitumen surface due to its constant loss of volume (the exact distance was not noted, however). The continuous tapping of the AFM probe at a single location on the bitumen surface and the monitoring of the probe deflection as the temperature is continuously lowered would allow for a precise measure of volume loss with temperature, and thus allow for an accurate measurement of the glass transition associated to a specific phase in the bituminous mixture, in contrast to that obtained after the isolation of bitumen fractions (Claudy et al., 1991; Masson et al., 2002). As indicated before, the glass transition temperature can be assessed from a change in volume with temperature (Eisenberg, 1993). One difficulty of this experiment, however, resides in being able to place the AFM probe on a predetermined phase without the benefit of a surface scan to identify the phase being probed.
Origin of the salphase
Bitumen is a mixture. Like many mixtures, its components can segregate upon cooling. For instance, the steric hardening of bitumen is caused from the segregation of asphaltenes from the maltenes (Masson et al., 2005). The AFM and PDM observations of new domains that become visible upon cooling are also consistent with segregation. In this case, the segregation is visible in the paraphase and in the periphase, and according to microscopy and MDSC results, it involves material of low glass transition temperature, in other words, alkanes and alkyl segments. A progressive segregation is most clearly shown by bitumen AAK cooled from 22°C (Fig. 1), to –10°C (Fig. 3), to –27°C (Fig. 5) and to –55°C (Fig. 8).
The temperature at which segregation occurs in segregating blends is shown on a phase diagram (Olabisi, 1979; Paul and Bucknall, 2000; Ott and Boerio-Goates, 2000). Segregation upon cooling indicates the existence of an upper critical solution temperature (UCST) as illustrated in Fig. 13. In accordance with this diagram, upon cooling a binary homogeneous mixture below the UCST and under the spinodal curve, a mixture
segregates into two phases. The intersection of the tie-line (shown at TR in the figure)
with the spinodal curve provides the composition of two new phases after equilibrium is reached. With the material at hand, the binary mixture may be considered a mixture of immiscible alkyl segments in the paraphase, or a mixture of immiscible alkyl and cyclic segments in the periphase. In either case, the position of the UCST or the spinodal remain unknown for bitumens.
Segregation as illustrated in Fig. 13 can be a very slow process, especially at low temperatures. However, its occurrence in bitumen is supported by simple calculations of
diffusion rates and diffusion lengths.
The diffusion rate can be expressed as (Einstein, 1956)
D = kT/6πηr (Eq. 1)
where D is the diffusion rate, kT is the kinetic energy, η is the viscosity of the continuous phase, and r is the radius of the diffusing particle. The average molecular mass of saturates is 400 Da, with the lowest molecular weight being about 100 Da (Peramanu et al., 1999). If we assume that diffusing low molecular mass alkanes form a sphere of radius r and that they have a molecular mass between 100 and 350 Da (below the average) and a density of 0.9 g/mL (to calculate the volume and the radius of the diffusing sphere), then for temperatures of –10°C, –30°C and –50°C, the diffusion rates
vary from 0.20 to 0.01 µm2/h (Table 3). To calculate these rates, the viscosity of the
continuous phase was taken as the complex viscosity of a naphthenic oil at –10°C, –30°C and –50°C (Masson et al., 2006c).
The diffusion rate is related to the diffusion length L and time t by (Einstein, 1956)
L = (2Dt)1/2 (Eq. 2)
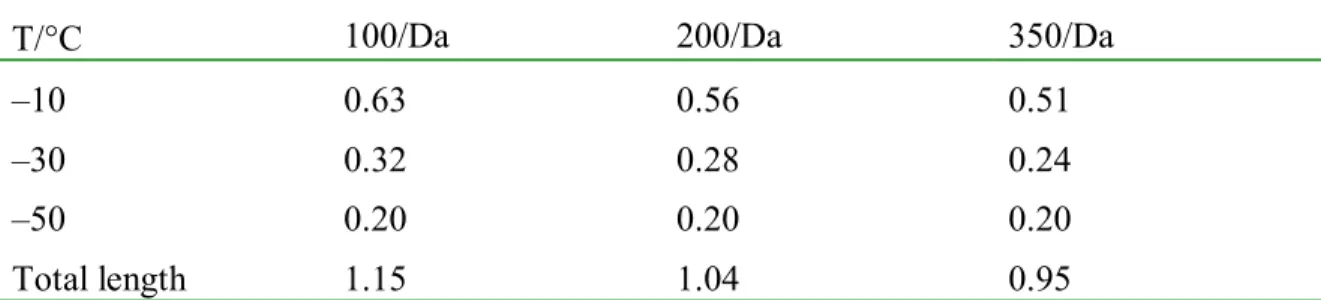
The diffusion rates in Table 3 thus lead to hourly diffusion lengths of 0.63µm to 0.20µmm (Table 4). Given that samples were slowly cooled from room temperature to about –10°C, –30°C and –50°C, where they were maintained isothermally for 1h, the total diffusion time exceeds 3h and the total diffusion length approaches 1µm (Table 4).
scale of the paraphase (Figs 7 and 9). Consequently, the diffusion of low molecular weight material within the paraphase and the periphase is rapid enough that an embedded salphase can form upon cooling, which supports the earlier contention that the salphase is the result of phase segregation, as illustrated in the phase diagram of Fig. 13. The absence of a salphase at 22°C (Fig. 1) indicated that for the bitumens studied, the UCST was below room temperature, but its observation at 22°C in other bitumens (Masson et al., 2006a) showed that this was not always the case.
Finally, the existence of an unfrozen salphase in the bitumens cooled down to – 72°C indicates that bitumens with multiple phases at room temperature would never be entirely rigid in various low temperature applications. It may thus be important to establish a relationship between the salphase and the low temperature physical characteristics of bitumen.
Summary and conclusion
We have described the use of cryogenic AFM and PDM to characterize nano- and macro-structures in bitumens that show catana, peri, and para phases at room temperature. These phases were related to the existence of domains with different glass transition
temperatures (Tgs). Given that Tg is governed by composition, the catana, peri, and para
phases were assigned to asphaltenes, naphthene and polar aromatics (or aromatics and resins), and saturates, respectively.
The microscopy work at –10 to –30 °C, within the Tg region of the maltenes,
showed that bitumen contracted, but not all bitumen phases contracted equally. Topographic features of about 85 nm in height, not visible in AFM images acquired at
room temperature, were revealed. These features were associated to the unfrozen paraphase rich in saturates. The PDM images concurred with the viscous nature of this phase.
Upon cooling down to –55°C, below the Tg of the maltenes, unfrozen alkanes in
the saturates-rich paraphase and in the alkyl-aromatics-rich periphase became visible. The unfrozen material was found in collections of quasi-circular domains of 20 nm to 400 nm, which was called the salphase. Based on a simple diffusion model, the salphase was considered to result from phase segregation within the paraphase and the periphase. Upon
cooling, amorphous and low molecular weight alkanes or alkyl segments of low Tg
formed domains of their own within larger domains rich in saturates, naphthene aromatics and polar aromatics. It would thus seem that bitumens with multiple phases at room temperature are never entirely rigid in low temperatures. This may have important implications for their use in low temperature applications.
References
Anczykowski, A., Krüger, D. & Fuchs, H. (1996) Cantilever dynamics in quasinoncontact force microscopy: Spectroscopic aspects. Phys. Rev. B, 53, 15485– 15488.
Asphalt Institute (1989) The Asphalt Handbook. Manual Series no. 4 (MS-4). The Asphalt Institute, Lexington, Kentucky.
Brown, A.B., Sparks, J.W. & Smith, F.M. (1957) Steric hardening of asphalts. Assoc. Asphalt Paving Technol. Tech. Sess. 26, 486-494.
Brûlé, B., Ramond, G., & Such, C. (1986) Relationships between composition, structure, and properties of road asphalts: state of the research at the French Public Works Central laboratory. Transp. Res. Rec. 1096, 22-34.
Claudy P., Létoffé J.-M., King G.N., Brûlé, B. & Planche J.-P. (1991) Characterization of paving asphalts by differential scanning calorimetry. Fuel Sci. Techn. Int. 9, 71-92. Dunning, H.R. (1977) Pressure sensitive adhesives: formulations and technology Noyes Data Corp., Park Ridge, N.J.
Einstein, A. (1956) Investigations on the theory of Brownian movement. Dover Publications, New York.
Eisenberg A. (1993) The glassy state and the glass transition, in: Physical Properties of Polymers, 2nd ed., American Chemical Society, Washington, DC.
Goodrich, J. L., Goodrich, J. E., & Kari, W. J. (1986) Asphalt composition tests: their application and relation to field performance. Transp. Res. Rec. 1096, 146-167.
Jäger, A., Lackner, R., Eisenmenger-Sittner, Ch., & Blab, R. (2004) Identification of four material phases in bitumen by atomic force microscopy. Road Mat. Pav. Design. 5, 9-24.
Jones, P.M. (1963) Bituminous materials. Canadian Building Digest CBD-38, Institute for Research in Construction, National Research Council Canada, Ottawa, Ontario. Jones, D.R. (1993) SHRP Materials Reference Library for Asphalt Cements: A Concise Data Compilation. Report SHRP-A-645, Strategic Highway Research Program, National Research Council, Washington, DC.
Hoidberg, A.J. (1965) Bituminous Materials: Asphalts, Tars, and Pitches. Interscience: New York.
Lambert, J.B. (2005) The deep history of chemistry. Bull. Hist. Chem. (ACS Div. Hist. Chem.) 30 (1), 1-9.
Lesueur, D., Gérard, J.-F., Claudy, P., Létoffé, J.-M., Planche, J.-P. & Martin, D. (1996) A structure-related model to describe asphalt linear viscoelasticity. J. Rheol. 40, 813–836. Loeber, L., Sutton, O., Morel, J., Valleton, J.-M. & Muller, G. (1996) New direct observations of asphalts and asphalt binders by scanning electron microscopy and atomic force microscopy. J. Microsc. 182, 32–39.
Masson, J-F. & Polomark, G.M. (2001) Bitumen microstructure by modulated differential scanning calorimetry. Thermochim. Acta 374 105–114, and Erratum in 413 (2004) 273.
Masson, J-F., Price, T. & Collins, P. (2001) Dynamics of bitumen fractions by thin-layer chromatography/flame ionization detection. Energy Fuels, 15, 955–960.
Masson, J-F., Polomark, G.M. & Collins, P. (2002) Time-Dependent Microstructure of Bitumen and Its Fractions by Modulated Differential Scanning Calorimetry. Energy Fuels 16, 470-476.
Masson, J-F., Collins, P. & Polomark, G. (2005) Steric hardening and the ordering of asphaltenes in bitumen. Energy Fuels 19, 120-122.
Masson, J-F., Leblond, V. & Margeson, J. (2006a) Bitumen morphologies by phase-detection atomic force microscopy. J. Microsc. 221. 17–29.
Masson J-F., Polomark, G.M., Bundalo-Perc, S. & Collins P. (2006b) Melting and glass transitions in paraffinic and naphthenic oils. Thermochim. Acta 440, 132–140.
Michon, L., Martin, D., Planche, J.-P. & Hanquet, B. (1997a) Estimation of average structural parameters of bitumens by 13C nuclear magnetic resonance spectroscopy. Fuel
76, 9–15.
Michon, L., Hanquet, B., Diawara, B., Martin, D. & Planche, J.-P. (1997b) Asphalt study by neuronal networks: correlation between chemical and rheological properties. Energy Fuels 11, 1188–1193.
Olabisi, O, Robeson, L.M. & Shaw, M.T. (1979) Polymer-Polymer Miscibility. Academic Press, New York.
Ott J.B. & Boerio-Goates, J. (2000) Chemical Thermodynamics. Academic Press, San Diego.
Paul, D.R. & Bucknall, C.B. (2000) Polymer Blends. Wiley-Interscience, New York. Pauli, A.T., Branthaver, J.F., Robertson, R.E. & Grimes, W. (2001) Atomic force microscopy investigation of SHRP asphalts. Symposium on Heavy Oil and Resid Compatibility and Stability, pp. 110–114. Petroleum Chemistry Division, American Chemical Society, San Diego, California.
Peramanu, S., Pruden, B.B. & Rahimi, P. (1999) Molecular weight and specific gravity distributions for Athatbasca and Cold Lake bitumens and their saturates, aromatic, resin and asphaltene fractions. Ind. Eng. Chem. Res. 38, 3121-3130.
Petersen, J.C. (1984) Chemical composition of asphalt as related to asphalt durability: state of the art. Transp. Res. Rec. 999, 13-30.
Shen, M.C. & Eisenberg, A. (1966) Glass transitions in polymers. Prog. Solid State Chem. 43, 407-481.
Speight, J.G. (1999) The Chemistry and Technology of Petroleum, 3rd edn., Marcel Dekker, New York.
Ternay, A. (1979) Contemporary Organic Chemistry, W.B. Saunders Company, Philadelphia.
Traxler, R. N. (1961) Asphalt : its composition, properties and uses, New York, Reinhold.
Turner, T. F. & Branthaver, J. F. (1997) DSC studies of asphalts and asphalt components, in Asphalt Science and Technology, A.M. Usmani (Ed.), Marcel Dekker, New York. Usmani, A. M. (1997) Asphalt Science and Technology, Marcel Dekker, New York.
Winkler, R.G., Spatz, J.P., Sheiko, S., Möller, M., Reineker, P. & Marti, O. (1996). Imaging material properties by resonant tapping-force microscopy: a model investigation. Phys. Rev. B, 54, 8908–8912.
Table 1. Bitumen characteristics.
Table 2. Typical composition of isolated bitumen fractions and their Tg.
Table 3. Diffusion rates in µm2/h for alkanes of low molecular weight*.
Table 4. Hourly diffusion length in µm for alkanes of low molecular weight*.
Fig. 1. AFM topographic (top) and PDM images of bitumen AAS at 22°C under vacuum (15 µm x 15 µm). Respective differentials are 165 nm and 175°.
Fig. 2. Tgs for bitumens as obtained by the derivative of the heat capacity. The dotted
lines highlight the breadth of the sub-zero Tg region and the temperature range for
AFM-PDM observations in this work.
Fig. 3. AFM topographic (top) and PDM images of bitumen AAK at –10°C (15 µm x 15 µm). Respective differentials are 95 nm and 175°. V refers to viscous domains. See text for details.
Fig. 4. Topographic profile along the arrow of the AFM image for bitumen AAK in Fig.
3.
Fig 5. AFM topographic (top) and PDM images of bitumen AAK at –27°C (15 µm x 15 µm). Respective differentials are 180 nm and 170°.
Fig. 6. AFM topographic (top) and PDM images of bitumen AAS at –15°C (15 µm x 15 µm). Respective differentials are 125 nm and 50°.
Fig. 7. AFM topographic (top) and PDM images of bitumen AAN at –19°C (15 µm x 15 µm). Respective differentials are 30 nm and 110°.
Fig. 8. AFM topographic (top) and PDM images of bitumen AAK at –55°C (15 µm x 15 µm). Respective differentials are 125 nm and 95°.
Fig. 9. AFM topographic (top) and PDM images of bitumen AAK at –55°C (5 µm x 5 µm). Respective differentials are 80 nm and 150°.
Fig. 10. AFM topographic (top) and PDM images of bitumen AAS at –55°C (15 µm x 15 µm). Respective differentials are 70 nm and 130°.
Fig. 11. AFM topographic (top) and PDM images of bitumen AAS at –55°C (5 µm x 5 µm). Respective differentials are 75 nm and 50°.
Fig. 12. Topographic image (top) and friction image of bitumen AAN at –55°C (15 µm x 15 µm). Respective differentials are 170 nm and 75°.
Fig. 13. Typical phase diagram for a binary mixture, with the temperature on the ordinate
and the volume fraction on the abscissa. An upper critical solution temperature (UCST)
and spinodal curve delimits one- and two-phase regions. TR is the temperature of
reference, or temperature of observation, φ is the initial volume composition, and φ’ and φ” are the compositions of the segregated phases at equilibrium.
Table 1. Bitumen characteristics.
Bitumen Compositiona Fused Ringsb
S NA PA As
AAK 5 30 42 20 3
AAN 10 40 34 16 4
AAS 4 40 38 17 5
a
From Jones (1993). bfrom Michon et al. (1997a, 1997b)
Table 2. Typical composition of isolated bitumen fractions and their Tg
Fractiona Compositionb Tg/°C References
c
S n- and iso-alkanes –88 to
–60
Claudy et al. 1991 ; Masson et al. 2002, Masson et al., 2006b A or NA Alkylated cyclopentanes and
cyclohexanes
–15 to –34
Claudy et al. 1991 ; Masson et al. 2002
R or PA Alkylated and cycloalkylated aromatic rings
10 to 20 Masson and Polomark, 2001; Masson et al. 2002
As Alkylated condensed
aromatic rings
40 to 60 Masson and Polomark, 2001
a
S, saturates; A, aromatics; NA, naphthene aromatics; R, resins; PA, polar aromatics; As, asphaltenes
b
Speight, 1999; cfor the Tgs, measured by DSC
Table 3. Diffusion rates in µm2/h for alkanes of low molecular weight*.
T/°C 100/Da 200/Da 350/Da
–10 0.20 0.16 0.13 –30 0.05 0.04 0.03 –50 0.02 0.01 0.01 ∗η (–10°C) = 10 kPa.s; η (–30°C) = 40 kPa.s; η (–50°C) = 100 kPa.s.
Table 4. Hourly diffusion length in µm for alkanes of low molecular weight.
T/°C 100/Da 200/Da 350/Da
–10 0.63 0.56 0.51 –30 0.32 0.28 0.24 –50 0.20 0.20 0.20