Selective versus non-selective antiarrhythmic
approach for prevention of atrial fibrillation after
coronary surgery: is there a need for pre-operative
risk stratification?
A prospective placebo-controlled study using low-dose sotalol
U. Klo
¨ ter Weber, S. Osswald, M. Huber, P. Buser, K. Skarvan*, P. Stulz†,
C. Schmidhauser‡ and M. Pfisterer
*Division of Cardiology, Department of Internal Medicine, Department of Anesthesiology and †Clinic for Cardiothoracic Surgery, University Hospital, Basel, Switzerland; ‡Brunner and Hess, Statistics and Software
Solutions Inc., Zurich, Switzerland
AimThis study evaluated the advantages of ‘selective’ over ‘non-selective’ antiarrhythmic prevention of atrial fibril-lation after coronary surgery based on a new risk prediction algorithm.
Methods and Results In a retrospective analysis of a
prospective randomized trial, a model for risk prediction was determined based on clinical data of the control group (A; n=107) and tested in a test group (B; n=107, treated with low dose sotalol). Using this algorithm, the effect of a ‘selective’ antiarrhythmic approach in high-risk patients was compared to a ‘non-selective’ approach, where all patients were treated. In total, 75 (35%) patients developed atrial fibrillation and 14 (7%) side-effects led to discontinu-ation of study medicdiscontinu-ation. Based on the risk prediction algorithm, 36% of group A patients were classified as high-risk patients with an incidence of atrial fibrillation of 76% compared to 26% in low-risk patients (P<0·0001). The selective approach, i.e. treatment of high-risk patients only
reduced the incidence of atrial fibrillation from 76% to 50% (P=0·0295) compared to a reduction from 44% to 26% (P=0·0065) when all patients were treated. More impor-tantly, with the non-selective approach 100% of patients were exposed to the possible side-effects of sotalol and costs compared to 24% only with the selective approach (P<0·0001).
Conclusions: Thus, a selective approach based on a
clinical risk prediction algorithm should improve the cost-effectiveness and safety of low-dose sotalol in the prevention of atrial fibrillation after coronary bypass surgery.
(Eur Heart J 1998; 19: 794–800)
Key Words:Antiarrhythmic prevention, atrial fibrillation, heart surgery, risk stratification.
See page 684 for the Editorial comment on this article
Introduction
Atrial fibrillation, although not life threatening, is one of the commonest complications after coronary surgery[1–3]
and often prolongs hospital stay due to inter-mittent haemodynamic instability or thromboembolic complications[3–4]. On the other hand, prophylactic use
of antiarrhythmic drugs for prevention of atrial fibril-lation exposes 60–70% of patients without postoperative atrial fibrillation to the costs and possible side-effects of this therapy (e.g. proarrhythmia, bradycardia, hypo-tension etc.)[5–14]. This emphasizes the importance of
pre-operative risk stratification which ideally would allow for accurate identification of high-risk patients in whom preventive antiarrhythmic therapy may be justified[15–17].
Prior studies have shown an incidence of atrial fibrillation up to 50% in patients after coronary bypass grafting, with a peak incidence on the 2nd to 3rd postoperative day[1–3,5]. Some of these studies
Revision submitted 20 October 1997, and accepted 30 October 1997.
Correspondence: Matthias Pfisterer, MD, FESC, FACC, Professor of Cardiology, Head, Division of Cardiology, Dept. of Internal Medicine, University Hospital, CH-4031 Basel, Switzerland.
rated clinical, anaesthetic, surgical or peri-operative parameters as the most important risk factors for the development of postoperative atrial fibrillation[4,16].
The purpose of this prospective study was to develop an appropriate risk prediction algorithm based on clinical, electrocardiographic and haemodynamic variables, and to test this algorithm in a second indepen-dent population receiving routine antiarrhythmic drug therapy to prevent postoperative atrial fibrillation after coronary surgery.
Patients and methods
The protocol was approved by the local Human Ethical Committee. Between June 1994 and March 1995, all patients undergoing elective coronary artery bypass grafting at the University Hospital of Basel were screened for the study. Patients were eligible if they were in sinus rhythm before surgery, were not on antiarrhyth-mic drugs (except beta-blockers, which had to be stopped at least 24 h before surgery) and had no additional surgical procedures during the same opera-tion (e.g. aneurysmectomy, valve replacement, carotid surgery etc.). Thus, 220 consecutive patients were included and gave written informed consent to partici-pate in this study. They were randomized to a baseline population (group A; n=110, receiving placebo) or to a test population (group B; n=110, receiving low-dose sotalol 80 mg twice daily) for 3 months starting on the day of surgery. Study drug was administered in a prospective double-blind fashion. Six patients were excluded for this analysis for intra-operative death (n=1) or incomplete data (n=5) leaving a study popu-lation of 214 patients (A; n=107, B: n=107). Based on the findings in group A (baseline group), a risk predic-tion algorithm separating high from low-risk patients was retrospectively developed and tested in group B (test group).
This method allowed the comparison of a ‘selective’ antiarrhythmic approach (efficacy of sotalol in high-risk patients only) with a ‘non-selective’ antiarrhythmic approach (efficacy of sotalol in all patients) and estimated the value of risk prediction in the setting of preventive antiarrhythmic therapy for avoidance of atrial fibrillation after coronary surgery.
Clinical, haemodynamic and surgical parameters were collected and a standard 12-lead electrocardiogram was recorded in each patient 24–48 h before surgery. P-wave duration was measured using a magnifying glass and a scale in milliseconds in leads II and aVR by two independent investigators, who were not aware of the clinical outcome (variability 5·6&14 ms). P-wave dis-persion was defined as the difference between the short-est and longshort-est P-wave duration measured in any of the 12 leads (variability 0·7&15 ms). Additional 12-lead electrocardiograms were obtained on days 0, 1, 2, 4, 8 after surgery and before discharge. During the first 2 postoperative days, patients were followed by
continu-ous electrocardiogram monitoring in the intensive care unit. Afterwards, central and peripheral heart rate was controlled by specially trained nurses at 6 h intervals up to hospital discharge and, if heart rate exceeded 120 beats . min"1 or an irregular pulse suggested atrial fibrillation, additional 12-lead electrocardiograms were obtained to confirm the diagnosis. Clinically relevant episodes of atrial fibrillation were defined as either symptomatic atrial fibrillation episodes (hypotension, dizziness, shortness of breath, angina) or symptomatic episodes at a rate §120 beats . min"1. For statistical analysis, more organized atrial arrhythmias such as atrial flutter were also counted as atrial fibrillation, if they fulfilled the above criteria except for sinus tachycardia.
After discharge, patients were followed by their referring physicians and electrocardiograms were obtained, when the clinical situation suggested atrial fibrillation. At the end of the 30-month follow-up period, all patients were clinically examined and a 12-lead electrocardiogram was obtained. If more than one episode of atrial fibrillation occurred, the first one was used to calculate the time between surgery and the onset of atrial fibrillation.
Statistical analysis
All values are given as mean&one standard deviation. For univariate analysis, continuous variables were com-pared by the Student’s t-test, and where appropriate by analysis of variance. Categorical data were analysed by contingency table analysis. Logistic regression was used to identify predictive factors in a multivariate way and to estimate the probability of an event based on the formula: P=1/(1+e"Z), where Z is the linear combina-tion of all predictive co-variates: Z=B0+B1X1+B2X2+
. . . BpXp(statistic software SPSS 6.1.3). For estimation
of the significance of a variable on the predictive accu-racy of the algorithm, a ‘backward stepwise’ method was used, where all variables were eliminated until the log probability changed <0·01% with further elimination of a variable (2Log test). In order to develop an algorithm with high predictive accuracy, five obvious outliers (standardized residual >2·0 with a significant leverage regarding the calculation of the coefficients) in group A were eliminated from the multivariate analysis based on stringent statistical criteria; to test this algorithm in the ‘real’ study situation, the data of these patients were included such that the results were based on all patients again. Various risk stratification algorithms were devel-oped based upon the findings from univariate and multivariate regression analysis in group A (baseline group). The algorithm with the highest predictive accu-racy was then chosen for risk prediction in group B (test population). The value of risk prediction was estimated by comparing the efficacy of prevention (incidence of atrial fibrillation in group A (baseline group) and group B (test group)) with the ‘non-selective’ (all patients
treated) and the ‘selective’ approach (only high-risk patients treated). A P-value of <0·05 was considered to indicate statistical significance.
Results
A total of 214 consecutive patients (female 23, male 191) undergoing elective coronary bypass surgery were enrolled in the study. The mean age was 60&9 years (Table 1). Atrial fibrillation occurred in 75/214 patients (35%) 111&30 (median 43, range 4–1944) h after
opera-tion. In total, there were 62 (83%) episodes of true atrial fibrillation and 13 (17%) episodes of atrial flutter. There were five side-effects leading to discontinuation of study medication in group A and nine in group B (P=0·35). By univariate analysis, of all the clinical, angiographic and surgical variables tested in group A (Table 1), only age (P=0·0143), history of previous atrial fibrillation (P=0·0097), left ventricular ejection fraction (P=0·0086), P-wave duration on the 12-lead electrocardiogram (P=0·0001) and P-wave dispersion (P=0·0009) were predictive for postoperative atrial fib-rillation (Table 2). By logistic regression, a history of
Table 1 Baseline characteristics. The comparison (P value) was not significant Baseline Group A (placebo) n=107 Test Group B (sotalol) n=107 Clinical parameters
Age (years) 60&10 61&9
Male gender (%) 87 92
Hypertension (%) 60 50
Diabetes mellitus (%) 15 20
Hypercholesterolaemia (%) 60 64
History of smoking (%) 58 54
Previous atrial fibrillation (%) 5 4
Previous myocardial infarction (%) 59 58
Chronic obstructive lung disease (%) 8 5
Beta-blocking drugs (%) 72 83
Angiographic findings
1-vessel disease (%) 4 2
2-vessel disease (%) 21 23
3-vessel disease (%) 75 75
LV end-diastolic pressure (mmHg) 16&7 16&7
LV ejection fraction (%) 60&13 61&10
Mitral regurgitation (%) 13 9
LV hypertrophy (%) 7 8
Intra-operative parameters
Vein grafts (n/patient) 2&1 2&1
Internal mammary grafts (n/patient) 0·7&0·5 0·7&0·5 Distal anastomoses (n/patient) 3·0&1·0 3·0&1·0 Extracorporal bypass time (min) 93&28 98&27 Aortic cross clamp time (min) 57&18 58&20
Intubation time (h) 20&5 21&5
Pericardial closure (%) 41 33
Endarterectomy (%) 10 8
LV=left ventricular.
Table 2 Predictors of atrial fibrillation in baseline group A (placebo)
Parameters 60 (56%)no AF 47 (44%)AF
P-values
Uni-variate Multi-variate
Age (years) 58&10 63&9 0·0143 0·0731
Previous atrial fibrillation 0 (0%) 5 (11%) 0·0097 ns No pre-operative betablocker 13 (43%) 17 (57%) 0·097 0·0145 LV ejection fraction 63&13 57&12 0·0086 0·0069 P-wave duration (ms) 105&13 117&15 0·0001 0·0001 P-wave dispersion (ms) 41&12 49&12 0·0009 ns LV=left ventricular.
atrial fibrillation and P-wave dispersion were no longer significant, whereas no beta-blockers before oper-ation became significant (P=0·0145). Age (P=0·0731) remained borderline significant. Multiple models for prediction were then calculated (Methods). Best predic-tion was achieved by keeping age, pre-operative beta-blockers, left ventricular-ejection fraction and P-wave duration in the algorithm which read: Z=0·7877 (1=no pre-operative beta-blocker)+0·0548 (age in years)+["0·0666 (left ventricular-ejection fraction in %)]+0·1095 (P-wave duration in ms)"11·7267.
This algorithm estimated the statistical prob-ability for postoperative atrial fibrillation in every patient with a factor ranging from 0 to 1 (0=lowest risk, 1=highest risk). In order to find the optimal breakpoint which separated best high-risk from low-risk patients, positive and negative predictive accuracy were plotted for each breakpoint (Fig. 1). It appeared that a break-point between 0·5–0·6 predicted high-risk patients with the highest predictive accuracy (Fig. 1). Therefore, the breakpoint with a positive predictive value of 76% and a negative predictive value of 74% was set to a value of 0·55 for definition of high and low-risk patients (values <0·55=low-risk, values§0·55=high-risk). Fig. 2 shows the effect of risk prediction by comparing the incidence of atrial fibrillation between all group A patients and sub-groups classified as low and high risk. Figure 3 shows the validation of this risk prediction of group A in group B.
Non-selective approach
In group A (baseline), the incidence of atrial fibrillation was 44% compared to 26% (P=0·0065) in group B (test population) which suggested a 40% reduction by sotalol in non-selected patients. Figure 4 depicts the rate of atrial fibrillation according to this ‘non-selective’
approach in relation to its occurrence over time in group A as compared to group B patients. However, by this ‘non-selective’ antiarrhythmic approach chosen for this double-blind placebo-controlled analysis 56% of patients who did not develop atrial fibrillation were unnecessarily treated and exposed to the possible side-effects and costs of sotalol.
1.0 100
0
Breakpoint for probability
P ercentage (%) 0.5 80 60 40 20 0.1 0.2 0.3 0.4 0.6 0.7 0.8 0.9
Figure 1 The plot shows receiver-operator curves for positive/negative predictive values ( , , respectively) calculated for each breakpoint (=value for probability of atrial fibrillation) derived by the computer model (see Methods). Based on the findings in group A (baseline group), the breakpoint for risk prediction in group B (test group) was set at the value of highest predictive accuracy at 0·55.
High-risk 100
0
Incidence of atrial fibrillation (%
) 80 60 40 20 29/38 p = 0.0001 26% All patients 44% 18/69 47/107 76% Stratified patients Low-risk
Figure 2 The overall incidence of atrial fibrillation in group A (baseline group) is shown on the left. On the right, the incidence of atrial fibrillation in the same patients stratified according to risk group (algorithm) is compared.
Group A baseline population n = 107 64% 36% Group B test population n = 107 24% 76% NS
Figure 3 Validation of risk prediction of group A (base-line group) in group B (test group). =high risk;
=low risk.
90 100
Time after surgery (days)
Cumulative incidence (%) 50 80 60 40 20 10 20 30 40 60 70 80 0 0
Figure 4 The graph depicts the cumulative incidence of atrial fibrillation after coronary bypass surgery in group A (baseline group ( )) and group B (test group ( )).
Selective approach
With the ‘selective’ approach, only 26/107 (24%) high-risk patients would have been treated, and only about one fourth of treated high-risk patients having no benefit from therapy (Fig. 2) would have been exposed to the potential side-effects of prophylactic sotalol. Based on estimations derived from the negative predictive accuracy (74%) of the algorithm (Fig. 1), 15/81 (19%) patients identified as ‘low-risk’ patients would have been misclassified, and about 30–40% (=estimated efficacy of sotalol; Figs 1 and 2) of these patients might actually have had a benefit from prophylactic sotalol therapy but would not have received this treatment with the ‘selec-tive’ approach. This, however, would have resulted in 5–6 (5%) patients only, who would have been lost for therapy compared to 66 (81%) patients, in whom therapy would have been withheld correctly.
Figure 5 compares the efficacy of low-dose sota-lol prevention between a ‘non-selective’ approach, where all patients were treated, and a ‘selective’ approach, where only high-risk patients were treated. The overall efficacy of sotalol prevention did not change with either approach.
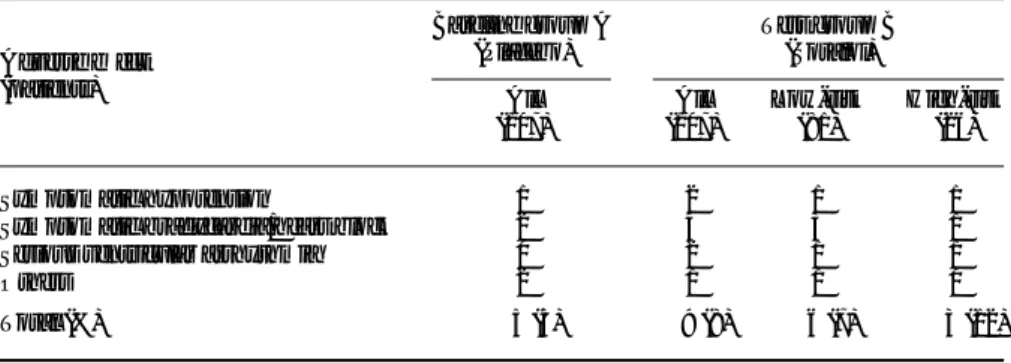
Table 3 summarizes the observed side-effects of study medication in all placebo and sotalol treated patients. Side-effects in the sotalol group are stratified according to the predicted risk groups, which allows an estimation of how many side-effects would have been avoided by the ‘selective’ antiarrhythmic approach. Note that two thirds of all sotalol-related side-effects occurred in low-risk patients.
Discussion
In addition to previously published predictors of post-operative atrial fibrillation such as age, left ventricular-ejection fraction, no beta-blocker and history of atrial fibrillation[3,4,17,18] prolonged P-wave duration in the normal 12-lead electrocardiogram has been identified by the present investigation as by far the strongest predic-tor. This parameter has not yet been used for risk prediction, although this parameter is easily available in every patient. The incorporation of these independent parameters into a new risk prediction algorithm proved to be a powerful tool for prospective identification of high-risk patients with a 2·9-fold increased incidence of postoperative atrial fibrillation. The high accuracy of this algorithm (sensitivity 62%, specificity 85%) allowed the comparison of selective (treatment of high-risk patients only) vs a non-selective (all patients treated) antiarrhythmic approach to prevent postoperative atrial fibrillation. The overall efficacy of sotalol prevention did not change with either approach, but with the selective approach only 24% of patients were treated compared to 100% with the non-selective approach. The calculated loss of patients who were misclassified by the algorithm, but sustained atrial fibrillation that would have been suppressed by sotalol, was 5% only.
Many other studies have addressed the issue of postoperative atrial fibrillation, its prevention by peri-operative antiarrhythmic treatment[5–14] and
pre-operative risk assessment[15–17]. However, thus far, no
risk prediction algorithm has been tested in a second independent patient population to distinguish between a non-selective and a selective therapy approach. Since only the non-selective approach could actually be studied in one study population, the analysis of the
'Selective' approach 100
0
High-risk patients
Incidence of atrial fibrillation (%)
80 60 40 20 29/38 p = 0.0295 –26% 'Non-selective' approach All patients 47/107 p = 0.0065 –18% 13/26 28/107
Figure 5 Drug efficacy (sotalol vs placebo) with the ‘non-selective’ (all patients treated ( )) and the ‘selective’ antiarrhythmic approach (high-risk patients treated only ( )) is compared.
Table 3 Incidence of study drug-related side-effects
Adverse effect (patients) Baseline group A (Placebo) Test group B (Sotalol) All (107) All (107) Low-risk (81) High-risk (26) Symptomatic hypotension 1 2 1 1
Symptomatic bradycardia/heart block 2 4 3 1
Serious ventricular arrhythmia 0 2 1 1
Others 2 1 1 0
selective approach had to be based on calculations. However, the results of this analysis make the selective approach most attractive yielding similar efficacy with reduced side-effects and probably costs.
Signal averaged P-wave duration has recently been found to be useful in predicting the risk of post-operative atrial fibrillation[19,20], P-wave duration on the
signal-averaged electrocardiogram was 156&20 ms in patients who developed atrial fibrillation compared to 145&19 ms in those who did not (P<0·01), whereas P-wave duration on the standard 12-lead electrocardio-gram (102&26 ms vs 96&27 ms) did not differ signifi-cantly. Compared to our study, P-wave duration on the standard 12-lead electrocardiogram was shorter in that study, which points to a need for standards for P-wave measurement in order to make results comparable. Biologically, P-wave duration correlates with the degree of intra-atrial conduction delay, which is not only a marker of local conduction disturbances, but also the pathophysiological basis for electric heterogeneity pre-disposing to reentry and the occurrence of atrial fibril-lation[21]. These intra-atrial conduction disturbances
may be corrected by re-synchronization of both atria by permanent bi-atrial pacing which may be effective in preventing atrial fibrillation and may offer an alternative to drugs in the future[22].
Peri-operative beta-blockade has been shown to reduce the incidence of postoperative atrial fibril-lation[5,9,10]. Elevated norepinephrine levels after heart surgery, peaking with the highest incidence of atrial fibrillation, suggest that the sympathetic system has an important role in the pathogenesis of postoperative atrial fibrillation[23]. Despite these findings, in our study, an increased risk for atrial fibrillation was found if patients were not on beta-blockers before therapy. How-ever, this was not the case if beta-blockers were with-drawn. On the other hand, similar to previous findings[7]
low-dose sotalol significantly reduced the rate of atrial fibrillation. It was not the scope of this study to discern beta-adrenergic blocking from the class III antiarrhyth-mic effects of sotalol. The marked proarrhythmic side-effects of d-sotalol observed in the Survival with oral D-sotalol trial (SWORD[24]) were not confirmed in the
present study with d,l-sotalol, although one possible proarrhythmic event was noted. This led to the proposal that in patients after coronary bypass surgery low-dose sotalol should be restricted to the first 9 days after surgery[25], the period of the highest rate of
postopera-tive atrial fibrillation. Overall, however, low-dose sotalol side-effects were mild and relatively infrequent com-pared to those of other drugs used for the same indication[8,11–14].
Limitations
Although this study was a prospective double-blind placebo-controlled trial, the risk prediction algorithm was developed in a retrospective way, based on findings
in the baseline, placebo group (logistic regression). This limits the strength of the algorithm, so that its true predictive accuracy has to be tested in further prospec-tive studies. Applied to the second independent patient group, however, it resulted in similar rates of high- and low-risk patients. Furthermore P-wave measurement on the 12-lead electrocardiogram depended to some degree of the subjective opinion of two investigators. However, inter-observer comparisons and double-blind assessment of the electrocardiograms ensured high reproducibility of the test results and elimination of any possible bias with regard to outcome or treatment assignment.
Conclusions
Clearly identified risk factors such as age, left ventricular ejection fraction and prolonged P-wave duration sup-port the hypothesis, that patients who develop atrial fibrillation after surgery have a preexisting arrhyth-mogenic substrate that can be recognized before opera-tion. In our study, the development of a simple risk prediction algorithm including these parameters proved to be a powerful tool to identify patients who are at highest risk for postoperative atrial fibrillation and who will benefit most from antiarrhythmic prevention. More importantly, the high negative predictive accuracy of our algorithm allows correct identification of patients who do not develop atrial fibrillation and should not receive antiarrhythmic therapy. Thus, pre-operative risk assess-ment is very helpful, not necessarily to increase the efficacy of antiarrhythmic prevention, but to avoid over-treatment of low-risk patients with potentially harmful drugs. In that respect, pre-operative risk assessment combined with a ‘selective’ antiarrhythmic approach with low-dose sotalol increases the cost-effectiveness and safety of antiarrhythmic prevention.
This study was supported by a research grant from Bristol-Meyers-Squibb, Inc., CH-6340 Baar, Switzerland. In part, this study was presented at the Annual Meeting of the American College of Cardiology 1996 in Orlando, FL.
References
[1] Lauer MS, Eagle KA, Buckley MJ, DeSanctis RW. Atrial fibrillation following coronary artery bypass surgery. Prog Cardiovasc Dis 1989; 31: 367–78.
[2] Groves PH, Hall RJ. Atrial tachyarrhythmias after cardiac surgery. Eur Heart J 1991; 12: 458–63.
[3] Hashimoto K, Ilstrup DM, Schaff HV. Influence of clinical and hemodynamic variables on risk of supraventricular tachy-cardia after coronary artery bypass. J Thorac Cardiovasc Surg 1991; 101: 56–65.
[4] Creswell LL, Schuessler RB, Rosenbloom M, Cox JL. Hazards of postoperative atrial arrhythmias. Am Thorac Surg 1993; 56: 539–49.
[5] Andrews TC, Reimold SC, Berlin JA, Antman AM. Preven-tion of supraventricular arrhythmias after coronary artery bypass surgery. A meta-analysis of randomized control trials. Circulation 1991; 84: II 236–44.
[6] Nystrom UJ, Edvardsson N, Berggren H, Pizzarelli GP, Radegran K. Oral sotalol reduces the incidence of atrial
fibrillation after coronary artery bypass surgery. Thorac Cardiovasc Surg 1993; 41: 34–7.
[7] Suttorp MJ, Kingma JH, Peels HO et al. Effectiveness of sotalol in preventing supraventricular tachyarrhythmias shortly after coronary artery bypass grafting. Am J Cardiol 1991; 68: 1163–9.
[8] Butler J, Harriss DR, Sinclair M, Westaby S. Amiodarone prophylaxis for tachycardias after coronary artery surgery: a randomised, double blind, placebo controlled trial. Br Heart J 1993; 70: 56–60.
[9] Daudon P, Corcos T, Gandjbakhch I, Levasseur JP, Cabrol A, Cabrol C. Prevention of atrial fibrillation or flutter by acebutolol after coronary bypass grafting. Am J Cardiol 1986; 58: 933–6.
[10] Suttorp MJ, Kingma JH, Tjon-Joe-Gin RM et al. Efficacy and safety of low- and high-dose sotalol versus propranolol in the prevention of supraventricular tachyarrhythmias early after coronary artery bypass operations. J Thorac Cardiovasc Surg 1990; 100: 921–6.
[11] Wafa SS, Ward DE, Parker DJ, Camm AJ. Efficacy of flecainide acetate for atrial arrhythmias following coronary artery bypass grafting. Am J Cardiol 1989; 63: 1058–64. [12] McAlister HF, Luke RA, Whitlock RM, Smith WM.
Intra-venous amiodarone bolus versus oral quinidine for atrial flutter and fibrillation after cardiac operations. J Thorac Cardiovasc Surg 1990; 99: 911–8.
[13] Gentili C, Giordano F, Alois A, Massa E, Bianconi L. Efficacy of intravenous propafenone in acute atrial fibrillation complicating open-heart surgery. Am Heart J 1992; 123: 1225–8.
[14] Conolly SJ, Mulji AS, Hoffert DL, Davis C, Shragee BW. Randomized placebo-controlled trial of propafenone for treatment of atrial tachyarrhythmias after cardiac surgery. J Am Coll Cardiol 1987; 10: 1145–8.
[15] Leitch JW, Thomson D, Baird DK, Harris PJ. The impor-tance of age as a predictor of atrial fibrillation and flutter after coronary artery bypass grafting. J Thorac Cardiovasc Surg 1990; 100: 338–42.
[16] Frost L, Molgaard H, Christiansen EH, Hjortholm K, Paulsen PK, Thomsen PE. Atrial fibrillation and flutter after coronary artery bypass surgery: epidemiology, risk factors and preventive trials. Int J Cardiol 1992; 36: 253–61.
[17] Fuller JA, Adams GG, Buxton B. Atrial fibrillation after coronary artery bypass grafting. Is it a disorder of the elderly? J Thorac Cardiovasc Surg 1989; 97: 821–5.
[18] Mendes LA, Connelly GP, McKenney PA et al. Right cor-onary artery stenosis: an independent predictor of atrial fibrillation after coronary artery bypass surgery. J Am Coll Cardiol 1995; 25: 198–202.
[19] Steinberg JS, Zelenkofske S, Wong SC, Gelernt M, Sciacca R, Menchavez E. Value of the P-wave signal-averaged ECG for predicting atrial fibrillation after cardiac surgery. Circulation 1993; 88: 2618–22.
[20] Hutchinson LA, Steinberg JS. A prospective study of atrial fibrillation after cardiac surgery: multivariate risk analysis using p wave signal-averaged ECG and clinical variables. Ann Noninvas Cardiol 1996; 1: 133–40.
[21] Allessie MA, Lammers WJ, Bonke FI, Hollen J. Experimental evaluation of Moe’s multiple wavelet hypothesis of atrial fibrillation. In: Zipes D, ed. Cardiac Electrophysiology and Arrhythmias. New York: Grune and Stratton, 1985: 265–75. [22] Daubert JC, Gras D, Leclerq C, Baisset JM, Victor F, Mabo P. Biatrial synchronous pacing: a new therapeutic approach to prevent refractory atrial arrhythmias. J Am Coll Cardiol 1995; 25: 230A.
[23] Kalman JM, Munawar N, Howes LG et al. Atrial fibrillation after coronary bypass grafting is associated with sympathetic activation. Ann Thorac Surg 1995; 60: 1709–15.
[24] Waldo AL, Camm AJ, deRuyter H et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. Lancet 1996; 348: 7–12.
[25] Klo¨ter Weber U, Pfisterer M, Osswald S, Huber M, Buser P, Stulz P. Low dose Sotalol to prevent supraventricular arrhyth-mias after CABG surgery and its effects on hospital stay. J Am Coll Cardiol 1996; 27 II: 309A.