Editorial
Closure devices: the solution for repair of direct access apertures
after valved stent implantation?
Sutures have been part of surgery from the very beginning. Despite major improvements made since the use of hemp yarn and catgut, the fact remains that most modern suture material creates holes in the tissue, which are larger than the suture that follows the needle. This in turn induces quite often some degree of unwelcome bleeding. For the application mentioned here, where an apical aperture of the heart has to be closed after the implantation of a valved stent[1,2], the risk of potential or eventual suture tearing is of major concern. There are a number of tricks to control apical bleeding including the preventive and therapeutic use of buttressed sutures with felt or pericardium, gluing of an apical Chinese hat made from pericardium or Teflon felt[3], and even the application of a net for external support or a core cap[4].
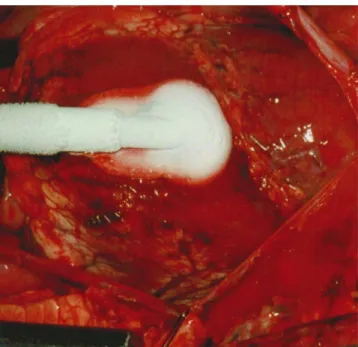
For solving a challenging problem, it is sometimes helpful to get inspiration from other disciplines. Construction of tunnels through mud, like for under crossing a river, may serve here as en example. The technical solution, which is applied under such circumstances, is to freeze temporarily the entire zone to be crossed. Tunnel construction with standard equipment for rock mining becomes then possible. Well, we have tried this approach some years ago. The protocol included the laceration of the left ventricle, freezing the left ventricular wall and the blood within the laceration by the means of a cryoprobe which was driven by liquid nitrogen (Fig. 1). Once the bleeding was ‘frozen,’ a patch was attached to the epicardium with a running suture, and during thawing the entire ‘repair’ was consolidated by injection of fibrin glue. Although this approach had some success in the animal lab, and larger ventricular orifices could be managed with more freezing power, care has to be taken to avoid major coronary arteries and other vital structures. However, for closure of the apical orifice after direct access implantation of a valved stent, it may not be necessary to use the rather heavy equipment derived from mining or arrhythmia surgery. As a matter of fact, Pawelec-Wojtalik et al.[5—7]have previously suggested to use for this purpose, muscular closure devices designed for percutaneous VSD closure. Interestingly enough, the device selected expands on both sides as well as within the access orifice and does not rely on sutures, clips, staples, or other tension members.
Of course, there is already a large clinical experience with closure devices within the heart for treatment of septal defects[8]. However, not much is known about the healing properties of such a devices used as seal towards the outside of the circulatory system where secondary bleeding results in cardiac tamponade with all its consequences. At the time of writing, a PubMed search with the term ‘closure device’ brings up 231 items. Most of them deal with the permanent closure of femoral access sites, ASDs, and VSDs, or their respective complications. There are only 15 animal studies and none deals with the problem of permanent repair of left ventricular wall perforations. For more about the future of direct access site device closure, see Ref.[9].
www.elsevier.com/locate/ejcts European Journal of Cardio-thoracic Surgery 30 (2006) 2—3
Fig. 1. Control of major bleeding (up to 60 cm jets) by the means of a cryoprobe driven with liquid nitrogen after left ventricular laceration. The ventricular wall and the blood within the laceration are frozen together prior to suturing of a pericardial patch and consolidation with glue.
1010-7940/$ — see front matter # 2006 Elsevier B.V. All rights reserved. doi:10.1016/j.ejcts.2006.04.021
References
[1] Zhou JQ, Corno AF, Huber CH, von Segesser LK. Self-expandable valved stent of large size: off-bypass implantation in pulmonary position. Eur J Cardiothorac Surg 2003;24:212—6.
[2] Huber CH, Tozzi P, Corno AF, Marty B, Ruchat P, Gersbach P, Nasratulla M, von Segesser LK. Do valved stents compromise coronary flow? Eur J Cardiothorac Surg 2004;25:754—9.
[3] Panagiotou M, Koletsis E, Markakis K, Mourtzis N. Chinese-hat patch glue repair of incomplete apical ventricular rupture. Inter Cardiovasc Thorac Surg 2006;5:197—9.
[4] Lembcke A, Dushe S, Enzweiler CNH, Kloeters C, Wiese TH, Hermann KA, Hamm B, Konertz WE. Passive external cardiac constraint improves seg-mental left ventricular wall motion and reduces akinetic area in patients with non-ischemic dilated cardiomyopathy. Eur J Cardiothorac Surg 2004;25:84—90.
[5] von Segesser LK. Direct percutaneous valve replacement: the next step? Eur J Cardiothorac Surg 2004;26:873—4.
[6] Pawelec-Wojtalik M, Antosik P, Wasiatycz G, Wojtalik M. Use of muscular VSD Amplatzer occluder for closing right ventricular free wall perforation after hybrid procedure. Eur J Cardiothorac Surg 2004;26:1044—6.
[7] Pawelec-Wojtalik M, von Segesser LK, Ma L, Bukowska D. Closure of left ventricle perforation with the use of muscular VSD occluder. Eur J Cardi-othorac Surg 2005;27:714—6.
[8] Pawelec-Wojtalik M, Wojtalik M, Mrowczynski W, Surmacz R, Quereshi SA. Comparison of cardiac function in children after surgical and Amplatzer occluder closure of secundum atrial septal defects. Eur J Cardiothorac Surg 2006;29:89—92.
[9] Pavelec-Wojtalik M, Nozynski J, Wojtalik MA, Piaszcynski M, Surmacz R, Bukowska D, Mrowczynski W. Is device closure for direct access valved stent implantation safe? Eur J Cardiothorac Surg 2006;30:4—9.
Ludwig K. von Segesser*
Department of Cardio-Vascular Surgery, Centre Hospitalier Universitaire Vaudois, CHUV, CCV, BH 10-275, CH-1011 Lausanne, Switzerland
*
Corresponding author. Tel.: +41 21 314 22 79; fax: +41 21 314 22 78 E-mail address:Ludwig.von-segesser@chuv.hospvd.ch