HAL Id: inserm-00382734
https://www.hal.inserm.fr/inserm-00382734
Submitted on 28 Dec 2010
HAL is a multi-disciplinary open access
archive for the deposit and dissemination of
sci-entific research documents, whether they are
pub-lished or not. The documents may come from
teaching and research institutions in France or
abroad, or from public or private research centers.
L’archive ouverte pluridisciplinaire HAL, est
destinée au dépôt et à la diffusion de documents
scientifiques de niveau recherche, publiés ou non,
émanant des établissements d’enseignement et de
recherche français ou étrangers, des laboratoires
publics ou privés.
JAK2V617F detection and dosage of serum
erythropoietin: first steps of the diagnostic work-up for
patients consulting for elevated hematocrit.
François Girodon, Eric Lippert, Pascal Mossuz, Irène Dobo, Nathalie
Boiret-Dupré, Jean-François Lesesve, Sylvie Hermouet, Vincent Praloran
To cite this version:
François Girodon, Eric Lippert, Pascal Mossuz, Irène Dobo, Nathalie Boiret-Dupré, et al..
JAK2V617F detection and dosage of serum erythropoietin: first steps of the diagnostic work-up for
patients consulting for elevated hematocrit.. Haematologica, Ferrata Storti Foundation, 2007, 92 (3),
pp.431-2. �inserm-00382734�
Letters to the Editor
haematologica/the hematology journal | 2007; 92(03) | 431|
JAK2V
617Fdetection and dosage of serum
erythropoietin: first steps of the diagnostic
work-up for patients consulting for elevated
hematocrit
The predictive values of common biological crite-ria for the diagnosis of polycythemia vera were studied in a cohort of patients with high
hemat-ocrit. We found JAK2V617Fand erythropoietin assays
were the most relevant first tests. Classification of
patients according to their JAK2V617Fstatus and
ery-thropoietin levels facilitated the choice of further diagnostic investigations.
Haematologica 2007;92:431-432
The discovery that more than 90% of people with polycythemia vera (PV) have the V617F mutation of JAK2 ( JAK2V617F )1-5calls for a re-assessment of the usefulness of
Polycythemia Vera Study Group (PVSG) or World Health Organization (WHO) criteria for diagnosing PV. New guidelines have recently been proposed; however, they were not based on biological and clinical data from large
series of untreated patients.6-8 In the present study, in
which the clinical and biological data of 419 untreated patients (168 with known JAK2 status) consulting for an elevated hematocrit were collected, we compared the PVSG, WHO and new PV criteria and tried to determine the most efficient diagnostic strategy. Males with a hema-tocrit (Hct) >50% and females with a Hct >48% were included after informed consent, following the guidelines of the ethical committee of "Région Bourgogne". The patients were evaluated before treatment, except for a small number who needed an emergency phlebotomy. The 57 patients with a Hct >60% (males) or >56% (females) were considered as having true erythrocytosis and red cell mass (RCM) was not measured. Standardized endogenous erythroid colony (EEC) assays, dosage of
serum erythropoietin and detection of JAK2V617Fby
quan-titative polymerase chain reaction in blood granulocytes
were performed as described elsewhere.5,9
The PVSG criteria were more stringent than the WHO criteria since 39 patients diagnosed as having PV by the WHO criteria were considered to have idiopathic
ery-throcytosis (IE) according to the PVSG. JAK2V617F was
present in 13/13 WHO-positive/PVSG-negative PV patients who were tested. The WHO criteria were then used as a reference to evaluate the value of other PV markers in 168 patients for whom JAK2 status could be assessed. The positive and negative predictive values
(PPV, NPV) of JAK2V617Fwith the WHO criteria as the
ref-erence were 92% and 97%, respectively. We used an alternate classification to evaluate the PPV and NPV of EEC, serum erythropoietin level and bone marrow histol-ogy (BMH). This classification was based on the WHO criteria minus the studied parameter which was replaced
by JAK2V617F, considered as a major criterion. This
modi-fied classification allowed us to determine the PPV and NPV of EEC (95% and 83%, respectively), low (<3.3 IU/L) serum erythropoietin (86% and 89%, respectively) and bone marrow histology (91% and 77%, respective-ly). Although JAK2 status had excellent PPV and NPV, used singly none of the studied parameters was sufficient to establish or refute the diagnosis of PV. Among the 71 patients with the JAK2 mutation, six did not fulfil the WHO criteria (Table 1). Four had low erythropoietin lev-els: patients A86 and A98, initially classified as having
apparent erythrocytosis eventually developed a complete
phenotype of PV with a RCM >1.25 and EEC formation. For patients N89 and B164 (who had only low serum ery-thropoietin and were thus classified as having idiopathic
Table 1. Characteristics of JAK2V617F-positive patients who did not have PV according to the WHO-PV criteria.
Patient Sex Diagnosis Hct RCM Platelets WBC Epo BMH Splenomegaly JAK2V617F EEC Revised
(WHO) (%) (×109/L) (×109/L) (IU/L) (%) diagnosis
A86 F AE 55.7 1.14 389 9.2 2.2 + no 79 − PV A98 F AE 53.2 1.20 408 8.8 1.8 + no 65 − PV N89 F IE 68.3 2.02 168 9.25 0.6 nd no 32 − PV B164 M IE 62.0 2.06 327 9.1 0.6 nd nd 43 − PV B153 M IE 52.9 1.33 127 6.2 9.7 − no 19 − IE D109 M IE 59.1 1.26 327 10.2 6.4 nd no 14 − IE
Hct: hematocrit; RCM: red cell mass; WBC: white blood cell count; Epo: erythropoietin; EEC: endogenous erythroid colony assay; BMH +: bone marrow histology in favor of PV; BMH - : bone marrow histology; IE : idiopathic erythrocytosis; AE: apparent erythrocytosis; nd: no data; not in favor of PV; splenomegaly (palpable and/or confirmed with ultrasound); revised diagnosis was obtained according to our strategy as described in the text.
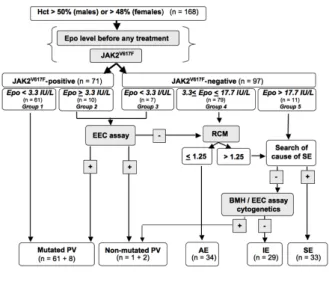
Figure 1. Diagnostic work-up of erythrocytoses with JAK2V617F detection and serum erythropoietin (Epo) dosage as the first steps. Patients were classified in groups 1 (JAK2V617F
-positive, low serum Epo), 2 (JAK2V617F
-positive, normal/high Epo), 3 ( JAK2V617F -negative, low serum Epo), 4 (JAK2V617F
-negative, normal serum Epo) and 5 (JAK2V617F
-negative, high serum Epo). For each group, the most efficient exploration strategy is indicated. Hct: hemat-ocrit; EEC: endogenous erythroid colony; RCM: red cell mass; PV: polycythemia vera; AE: apparent erythrocytosis; IE: idiopathic ery-throcytosis; SE: secondary erythrocytosis.
Letters to the Editor
| 432| haematologica/the hematology journal | 2007; 92(03)
erythrocytosis, a very high hematocrit, the presence of JAK2V617F and the absence of a cause of secondary ery-throcytosis led to the diagnosis of PV. Two other
JAK2V617F -positive patients had normal erythropoietin levels. They do not have any WHO criteria of PV and show no signs of evolution in the absence of treatment 2 years after diagnosis. For these reasons, despite their
JAK2V617F positivity, these patients were considered to have idiopathic erythrocytosis. Ninety-seven patients did not have the JAK2 mutation. Three of them fulfilled the WHO criteria of PV, with EEC. One had low erythropo-etin levels and two had normal levels, but the assays were done after phlebotomy.
In line with recent reports,6-8we show, in a large cohort
of patients, that the presence of JAK2V617F has the best
PPV and NPV for the diagnosis of PV. However, one can-not rely on a single test to make or reject the diagnosis of PV. Indeed, our study confirms that the absence of
JAK2V617Fdoes not exclude the diagnosis of PV and that,
as previously reported,10 JAK2V617F can be found in
patients not fulfilling PV criteria who are, therefore, clas-sified as having idiopathic erythrocytosis.
We then asked whether a combination of tests could reach a PPV and/or NPV of 100%. This was possible only by combining JAK2 status, erythropoietin levels and EEC status and retaining the diagnosis when two of these three criteria were in favor of PV. Since EEC is not rou-tinely available in every hospital, we evaluated the inter-est of first subgrouping patients according to their JAK2 status and erythropoietin levels to guide further explo-rations (Figure 1). This strategy allowed a diagnosis of PV to be affirmed in 61/72 patients finally classified as hav-ing PV (group 1) and definitively excluded PV for 11
patients with erythropoietin >17.7 IU/L (group 5).9 For
patients in other groups, a confirmatory test (mainly EEC)
should be performed if JAK2V617Fis detected or if serum
erythropoietin concentration is low (groups 2 and 3). In groups 4 and 5 (normal/high erythropoietin), RCM or a careful search for a cause of secondary erythrocytosis should be the first steps. This strategy prioritizes the most relevant investigations, thus saving time and money.
François Girodon,* Eric Lippert,° Pascal Mossuz,#Irène Dobo,@ Nathalie Boiret-Dupré,ˆ Jean-François Lesesve,§ Sylvie Hermouet,** Vincent Praloran° FG and EL contributed equally to this work *Laboratoire d’Hématologie, CHU de Dijon, Dijon, France; °Laboratoire d’Hématologie, CHU de Bordeaux, Bordeaux, France; #Laboratoire d’Hématologie, CHU de Grenoble, Grenoble, France; @Laboratoire d’Hématologie, CHU d’Angers, Angers, France; ˆLaboratoire d’Hématologie, CHU de Clermont-Ferrand, Clermont-Ferrand, France; § Laboratoire d’Hématologie, CHU de Nancy, Nancy, France; **Laboratoire d’Hématologie, CHU de Nantes, Nantes, France
Key words: erythrocytosis, polycythemia vera, JAK2V617F, erythropoietin, myeloproliferative disorders.
Funding: this work was supported by grants from the Programme Hospitalier de Recherche Clinique of the Région Bourgogne and the Grand Ouest and Aquitaine committees of the Ligue Nationale contre le Cancer.
Acknowledgments: we thank Dr. Céline Schaeffer (CHU Dijon) and Dr. Magali Donnard (CHU Limoges) for collecting patients’ data, Dr. Malik Fardeheb and Dr. Christine Binquet (Centre d’Investigation Clinique - Epidémiologie Clinique/Essais Cliniques, Univ de Bourgogne, Dijon, France) for their help in analyzing the data. We are indebted to colleagues from the Departments of Clinical Haematology of the Centres Hospitaliers of Angers, Bayonne, Bordeaux, Châlon-sur-Saône, Clermont-Ferrand, Dax, Dijon, Laval, Libourne, Limoges, Lorient, Nancy, Nantes, La Roche-sur-Yon, and Saumur for providing samples.
Correspondence: François Girodon, Laboratoire d’Hématologie, Hôpital du Bocage, CHU de Dijon, Dijon, France. Phone: interna-tional +33.380293047. Fax: internainterna-tional +33.380293660. E-mail: francois.girodon@chu-dijon.fr
References
1. James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144-8.
2. Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Cancer Genome Project. Acquired muta-tion of the tyrosine kinase JAK2 in human myeloprolifera-tive disorders. Lancet 2005;365:1054-61.
3. Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005;352: 1779-90.
4. Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387-97.
5. Lippert E, Boissinot M, Kralovics R, Girodon F, Dobo I, Praloran V, et al. The JAK2-V617F mutation is frequently present at diagnosis in patients with essential thrombo-cythemia and polythrombo-cythemia vera. Blood 2006;108:1865-7. 6. James C, Delhommeau F, Marzac C, Teyssandier I,
Couedic JP, Giraudier S, et al. Detection of JAK2 V617F as a first intention diagnostic test for erythrocytosis. Leukemia 2006;20:350-3.
7. Tefferi A, Spivak JL. Polycythemia vera: scientific advances and current practice. Semin Hematol 2005; 42: 206-20. 8. Tefferi A, Pardanani A. Mutation screening for JAK2V617F:
when to order the test and how to interpret the results. Leuk Res 2006;30:739-44.
9. Mossuz P, Girodon F, Donnard M, Latger-Cannard V, Dobo I, Boiret N, et al. Diagnostic value of serum erythropoietin level in patients with absolute erythrocytosis. Haemato-logica 2004;89:1194-8.
10. Percy MJ, Jones FG, Green AR, Reilly JT, McMullin MF. The incidence of the JAK2 V617F mutation in patients with idiopathic erythrocytosis. Haematologica 2006;91: 413-4.