Characterization of the Highly Active Polyhydroxyalkanoate
Synthase of Chromobacterium Sp. Strain Usm2
The MIT Faculty has made this article openly available. Please share
how this access benefits you. Your story matters.
Citation Bhubalan, K. et al. “Characterization of the Highly Active
Polyhydroxyalkanoate Synthase of Chromobacterium sp. Strain USM2.” Applied and Environmental Microbiology 77 (2011): 2926-2933. Web. 4 Nov. 2011. © 2011 American Society for Microbiology
As Published http://dx.doi.org/10.1128/AEM.01997-10 Publisher American Society for Microbiology
Version Author's final manuscript
Citable link http://hdl.handle.net/1721.1/66933
Terms of Use Creative Commons Attribution-Noncommercial-Share Alike 3.0 Detailed Terms http://creativecommons.org/licenses/by-nc-sa/3.0/
Characterization of the highly active PHA synthase of Chromobacterium sp. USM2 1
2
Kesaven Bhubalan1, Jo-Ann Chuah1, Fumi Shozui2, Christopher J. Brigham3, Seiichi 3
Taguchi2, Anthony J. Sinskey3,4,5, Chokyun Rha6, Kumar Sudesh1* 4
5 6
1
School of Biological Sciences, Universiti Sains Malaysia, 11800 Penang, Malaysia. 7
2
Division of Biotechnology and Macromolecular Chemistry, Graduate School of 8
Engineering, Hokkaido University, N13-W8, Kita-ku, Sapporo 060-8628, Japan. 9
3
Department of Biology, 4Engineering Systems Division, 5Division of Health Sciences 10
Technology, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, 11
Massachusetts 02139 12
6
Biomaterial Science and Engineering Laboratory, Massachusetts Institute of Technology, 77 13
Massachusetts Avenue, Cambridge, Massachusetts 02139 14 15 * Corresponding author 16 Mailing address: 17 Kumar Sudesh 18
Ecobiomaterial Research Laboratory, 19
School of Biological Sciences, 20
Universiti Sains Malaysia, 21 11800 Penang. 22 MALAYSIA 23 Tel: +60-4-6534367 24 Fax: +60-4-6565125 25
ABSTRACT 1
Synthesis of bacterial polyhydroxyalkanoate (PHA) is very much dependent on the 2
expression and activity of the key enzyme PHA synthase (PhaC). Enhanced gene expression 3
and enzyme evolution techniques have brought forth numerous improved and active 4
synthases. Nevertheless, the search for a natural synthase with such properties is still 5
widespread. In a recent study, the PhaC of a locally isolated Chromobacterium sp. USM2 6
(PhaCCs) exhibited high level of PHA accumulation when expressed in mutant Cupriavidus 7
necator PHB−4. It showed the ability to use the 3-hydroxybutyrate (3HB), 3-hydroxyvalerate 8
(3HV) and 3-hydroxyhexanoate (3HHx) monomers in PHA biosynthesis. In vitro assay of 9
recombinant PhaCCs expressed in Escherichia coli showed that its activity towards the 10
polymerization of 3-hydroxybutyryl-CoA was nearly 8-folds higher (2132 ± 70 U/g) 11
compared to that of the model strain C. necator (253 ± 21 U/g). Specific activity using a 12
Strep2-tagged, purified PhaCCs was 238 ± 98 U/mg, almost 5-folds higher than previous 13
studies using purified PhaC from C. necator. High poly(3-hydroxybutyrate) [P(3HB)] 14
accumulation in E. coli of up to 76 ± 2 wt% was observed within 24 h of cultivation. PhaCCs 15
has proven to be the first naturally occurring highly active PHA synthase with superior 16
polymerizing ability. 17
18
Keywords: Polyhydroxyalkanoate (PHA); Chromobacterium sp.; PHA synthase; High 19
synthase activity; Poly(3-hydroxybutyrate) 20
21 22 23 24
INTRODUCTION 1
Unlike petrochemical polymers, biosynthesis of bacterial polymer is very much 2
dependent on the catalytic activities of various enzymes involved. Polymerization rates and 3
yields vary based on the biosynthesis pathway of the organism and the monomer supply. One 4
such biopolymer that has attracted much interest is polyhydroxyalkanoate (PHA). Owing to 5
its desirable properties, PHA makes an excellent candidate as biodegradable replacements for 6
conventional plastics (7). PHA had gained much interest for applications in various 7
industries such as medicine, pharmacology, agriculture, packaging and cosmetics (2, 26, 44). 8
PHA has been produced using wild-type as well as recombinant microorganisms (16-18). The 9
biosynthesis of this bacterial polymer is controlled by both monomer supplying enzymes and 10
PHA synthase (PhaC) which is the key enzyme involved in polymerization (28, 35, 37). 11
The dominant role played by PhaC in determining polymer composition provided an 12
impetus to extensive investigations of PHA synthases. So far, the PhaC of Cupriavidus 13
necator (PhaCCn) (Class 1) has been studied in some mechanistic detail and is the benchmark 14
commonly used to evaluate the performance of other synthases (11, 13, 33). Some studies 15
have also been carried out on the synthase of Allochromatium vinosum (Class III) (12, 22). 16
Nevertheless, PhaC is a complex enzyme and its complete structure and properties are yet to 17
be fully understood. It is known that the activity and affinity towards the polymerization of 18
different hydroxyalkanoate-CoA substrates vary based on the different classes of PHA 19
synthases. Efforts have been taken to alter and improve the properties of natural synthases 20
towards attaining higher activity and wider substrate specificity via enzymatic evolution (37). 21
Several successful studies reported mutant synthases with up to 1 – 4-fold increased activity 22
(1, 25, 41). Nevertheless, the search for a natural synthase with comparable properties is still 23
widespread. 24
The property of PHA is dependent on its monomeric composition which is determined 1
by PhaC (28, 35). Recently, Bhubalan et al cloned the PHA synthase gene (phaCCs) from a 2
locally isolated Chromobacterium sp. USM2 and heterologously expressed the gene in a 3
PHA negative mutant of C. necator PHB–4 (5). Poly(3-hydroxybutyrate-co-3-4
hydroxyvalerate) [P(3HB-co-3HV)] copolymer with high 3-hydroxyvalerate (3HV) fraction 5
was synthesized from mixtures of fructose and sodium valerate. Besides, 3-hydroxyhexanoate 6
(3HHx) monomer was successfully incorporated when crude palm kernel oil (CPKO) was fed 7
as the sole carbon source, resulting in the production of poly(3-hydroxybutyrate-co-3-8
hydroxyhexanoate) [P(3HB-co-3HHx)] copolymer. P(3HB-co-3HV) and P(3HB-co-3HHx) 9
copolymers are known to possess improved mechanical and thermal properties compared to 10
P(3HB) homopolymer. When a combination of sodium valerate or sodium propionate with 11
CPKO was fed to a C. necator PHB–4 strain containing heterologously-expressed phaCCs, 12
high contents of polymer comprising 3HB, 3HV and 3HHx monomers were produced (4). 13
Some of the P(3HB-co-3HV-co-3HHx) terpolymer produced was found to possess 14
elastomeric properties. However, not many microorganisms have the suitable PhaC with the 15
ability to incorporate monomers of both short-chain-length (scl-) and medium-chain-length 16
(mcl-) PHA. The ability of PhaCCs to produce scl-mcl-PHA highlighted the potential of this 17
synthase to be further exploited. 18
In this study, the PHA synthase of Chromobacterium sp. USM2 was further 19
characterized via in vitro and in vivo assays using Escherichia coli JM109 to fully understand 20
its PHA synthesizing ability. We also purified a recombinantly-expressed Strep2-tagged 21
version of PhaCCs, to examine the unique abilities of this enzyme. The results obtained 22
showed that PhaCCs is a highly active enzyme in its natural form and is expressed in high 23
concentrations in E. coli. The ability to obtain high concentrations of synthase in vivo might 24
significant amount of pure protein. Once this is possible, then attempts can be made to 1
determine the three-dimensional structure of this complex enzyme which to date, still remains 2
an impenetrable barrier. 3
4
MATERIALS AND METHODS 5
6
Bacterial strains, plasmids and culture conditions 7
E. coli JM109 was used for all standard genetic engineering and its transformants 8
were used for PHA biosynthesis. The plasmids used in this study are listed in Table 1. E. coli 9
JM109 was grown at 37 oC in LB broth consisting of the following components: per liter; 10 10
g casein enzymic hydrolysate, 5 g yeast extract and 10 g NaCl at pH 7.0. In order to 11
determine the functional expression of the cloned gene in vivo, PHA biosynthesis was carried 12
out by transferring 1.5 mL [3 % (v/v)] of inoculum from a preculture grown for 12 h in LB 13
into 50 mL of fresh LB in 250 mL Erlenmeyer flasks supplemented with 2% (w/v) of 14
glucose. The cultures were incubated at two different temperatures of 30 °C and 37 °C for 72 15
h on a reciprocal shaker at 180 rpm. Ampicillin at a final concentration of 100 µg/mL was 16
added to maintain plasmid stability. For maintenance purposes, bacterial cultures from the 17
exponential growth phase were stored at −20 °C in 20% (v/v) glycerol. 18
For extraction of crude protein, E. coli JM109 transformants were grown at 30 °C in 2 19
mL of LB broth for 14 h. An aliquot of 17.5 µL [1% (v/v)] was inoculated to 1.75 mL of 20
fresh LB broth and was further grown for 9 h at 30 °C. Ampicillin at a final concentration of 21
100 µg/mL was added for plasmid maintenance. For Strep2-PhaCCs expression and 22
purification, E. coli BL21(DE3) was used as a host strain. Expression of Strep2-PhaCCs was 23
performed as follows. Cells with Strep2-PhaCCs expression plasmid were grown in 1 L LB 24
broth supplemented with 100 µg/mL ampicillin until an OD600 = 0.6. Enzyme synthesis was 25
induced by addition of 0.1 mM (final concentration) of IPTG and allowed to incubate for 2 h 1
at 30°C on a reciprocal shaker at 180 rpm. Cells were then pelleted and protein was purified 2
as described below. 3
4
DNA manipulation and plasmid construction 5
Plasmid isolation and DNA manipulation was carried out according to standard 6
procedures (29). All the restriction enzymes were used according to the manufacturers’ 7
protocols such as TaKaRa, Toyobo and Roche respectively. All the other chemicals used 8
were of analytical grade. The plasmid pGEM"AB(phaCCs) in Figure 1 was constructed to 9
determine the functional expression of PhaCCs synthase enzyme in E. coli JM109 via in vitro 10
and in vivo experiments. First, the plasmid vector pGEM"C1AB was digested with XbaI and 11
PstI to segregate phaC1 and then ligated with a synthetic linker XbaI-EcoRI-EcoRV-Asp718-12
HindIII-PstI which was derived by annealing a set of complementary primers (FXbaIPstILK 13
and RXbaIPstIL) (nucleotide sequences are shown in Table 2). The resultant vector was 14
named pGEM"AB(L). Next the phaCCs gene was cloned using the forward primer FEcoRICs 15
and the reverse primer RAsp718Cs (Table 2) from the plasmid vector pBBR1MCS-C2. The 16
resulting 1.7 kb EcoRI−Asp718 phaCCs fragment was purified and then digested with the 17
corresponding enzymes and ligated with pGEM"AB(L) which was also digested with the 18
same enzymes. The resultant vector was named pGEM"AB(phaCCs). DNA sequencing for 19
confirmation of new plasmid constructs was carried out by the dideoxy chain termination 20
method with the Prism 310 Genetic Analyzer DNA sequencer (Applied Biosystems) and the 21
CEQ2000XL DNA Analysis System (Beckman Coulter) using the BigDye terminator cycle 22
sequencing ready reaction kit (Applied Biosystems) and Dye Terminator Cycle Sequencing 23
with Quick Start Kit (Beckman Coulter), respectively. 24
Plasmid pET-phaCCs was constructed using pET51b (Novagen) as the parent 1
plasmid. For construction of a strep2-phaCCs, the phaCCs on the plasmid pBBR1MCS-C2 2
was amplified by PCR using the forward primer Strep2phaCCsFW and the reverse primer 3
Strep2phaCCsRV (Table 2) to introduce the unique restriction site BamHI 5’ to the phaCCs 4
open reading frame, and the unique restriction site HindIII, 3’ to the phaCCs open reading
5
frame. The amplified gene was subcloned into the pET51b digested with BamHI and HindIII, 6
followed by ligation to produce the plasmid pET-phaCCs. The portion of pET-phaCCs 7
containing the tagged phaCCs gene was sequenced by MIT Biopolymers Laboratory. 8
9
Preparation of crude protein samples 10
E. coli JM109 harboring either pGEM"AB(phaCCs), pGEM'CAB or pGEM"AB(L) 11
was cultured as discussed above. Cells were harvested by centrifugation and whole-cell 12
extract of the transformant was prepared by resuspending the cells in 2 mL of ice cold 40 mM 13
potassium phosphate buffer (pH 7.5) and subsequent disrupting by sonication (5 s, 3 times) 14
on ice using UD-200, TOMY sonicator. A soluble fraction was obtained as resulting 15
supernatant when the disrupted cells were centrifuged at 13,700 × g for 10 min at 4 °C and 16
the insoluble fraction was obtained from the precipitate. Protein concentrations were 17
determined using Bradford assay (6). 18
19
Expression and purification of Strep2-Tagged PhaCCs 20
E. coli BL21(DE3)/pET-phaCCs was cultured as discussed above. Cells (6.5 − 7.8 g 21
wet weight) were pelleted by centrifugation at 2988 × g at 4 °C. The cell pellet was 22
resuspended in 25 mL Buffer A (100 mM Tris-HCl, pH 8.0) and lysed using a French 23
pressure cell (2 passes at 12,000 psi). The resulting cell lysate was centrifuged at 100,000 × 24
g to remove cell debris. The clarified lysate was loaded onto a Strep-tactin column (IBA, 25
GmbH, Göttingen, Germany; 10 mL column volume) preequilibrated with 80 mL buffer B 1
(100 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA). The lysate and column were 2
incubated at 4 °C for 15 − 20 min. The column was eluted and washed with 5 × 10 mL 3
buffer B. Strep2-PhaCCs was eluted from the column with 6 × 5 mL fractions of buffer C 4
(Buffer B + 2.5 mM desthiobiotin). The Strep-tactin column was regenerated according to the 5
manufacturer’s instructions. Protein concentration of each fraction was determined by 6
Bradford assay. The pooled fractions were then concentrated using a Vivaspin 15R 7
concentrator (Sartorius AG, Göttingen, Germany) to 5.5 – 25.5 mg protein/mL, and dialyzed 8
twice versus 100 mM Tris-HCl (pH 8.0), containing 0.5 mM EDTA and 0.5 mM 9
dithiothreitol, for 12 − 16 h using a Slide-a-Lyzer dialysis cassette (Thermo Scientific). 10
Aliquots of 100 µL of the protein preparation were stored at −80°C. Protein concentrations 11
of pooled, concentrated fractions were determined by Bradford assay and confirmed 12
spectrometrically at A280, using the molar absorption coefficient 110,810 M-1 cm-1. Strep2-13
PhaCCs purification was performed three separate times. 14
15
In vitro enzymatic assay of crude PhaCCs 16
The activity of PHA synthase from crude extract was determined by measuring the 17
amount of CoA released from 3HB-CoA during the polymerization of 3HB-CoA (40). The 18
assay mixture contained 2 mM 3HB-CoA, 40 mM potassium phosphate buffer (pH 7.5, 30 19
°C), 10 mM 5,5'-dithio-bis(2-nitrobenzoic acid) (DTNB) and 1 mg/mL BSA. The reaction 20
was initiated by adding 35 − 40 µg of protein obtained from the soluble fraction of disrupted 21
cells into the above reaction mixture and the absorbance at 412 nm was measured at 30 °C. 22
The concentration of CoA was determined spectrometrically (11) using a molar absorption 23
coefficient of 15,600 M-1 cm-1 at 412 nm using Hitachi U-3900H Spectrophotometer. One 24
unit of enzyme activity (U) is defined as the amount of enzyme that catalyzed the release of 1
1.0 µmol CoA/min. Enzymatic assays were performed in triplicate. 2
3
In vitro enzymatic assay of Strep2-PhaCCs 4
Assays were carried out as previously described (42). Final enzyme concentrations of 5
7.5 − 30 nM of Strep2-PhaCCs and 600 µM of 3HB-CoA (final concentrations) were used. 6
The concentration of CoA was determined spectrometrically(11) using a molar absorption 7
coefficient of 13,600 M-1 cm-1 at 412 nm using an Agilent 8453 Spectrophotometer. 8
Preparations of Strep2-PhaCCn were used as a control and purified as described elsewhere (9). 9
One unit of enzyme activity is defined as described above. Enzymatic assays were performed 10
in triplicate. 11
12
Western blot analysis 13
A total of 10 µg of proteins prepared from both soluble and insoluble fractions of the 14
disrupted E. coli JM109 transformants were separated using 12.5 %-sodium dodecyl sulfate 15
polyacrylamide gel electrophoresis (SDS-PAGE). Separated proteins from the soluble 16
fraction were then transferred onto a polyvinylidene fluoride (PVDF) membrane (Immun-17
BlotTM PVDF Membrane [Bio-Rad]) using a Criterion Blotter (Bio-Rad). An immunoblot 18
analysis of PHA synthase was carried out using specific rabbit anti-serum raised against the 19
C-terminal peptides of PhaCCn as described by Murata et al (23). PhaC protein was detected 20
using goat anti-rabbit IgG conjugated with alkaline phosphatase as a secondary antibody. 21
22
Gas chromatography (GC) and polymer isolation 23
Methanolysis of the lyophilized cells in the presence of 15% (v/v) sulfuric acid and 24
85% (v/v) methanol was carried out prior to determining P(3HB) content through GC 25
analysis (8). P(3HB) was extracted by refluxing lyophilized cells with chloroform for 4 h at 1
60 ºC. The polymer solution was then purified by precipitation with chilled methanol. The 2
purified polymer was then air dried in a fume cupboard. 3
4
Gel permeation chromatography (GPC) 5
Average molecular weight was estimated using a Shimadzu 10A GPC system and a 6
10A refractive index detector with Shodex K-806M and K-802 columns. Chloroform was 7
used as the eluent at a flow rate of 0.8 mL/min and analysis was carried out at 40 ºC. Sample 8
concentration of 1.0 mg/mL was used. The calibration curve was generated using polystyrene 9
standards with a low polydispersity. 10
11
Transmission electron microscopy (TEM) 12
E. coli JM109 harboring pGEM"AB(phaCCs) was cultured for 24 h in LB 13
supplemented with 2% (w/v) glucose, as mentioned above. TEM analysis was carried out to 14
observe the accumulation of PHA granules and the changes in cell morphology under the 15
electron microscope (Philip CM 12/ STEM and JLM-2000FX11). Cells were harvested and 16
fixed in McDowell-Trump fixative at 4 °C for 24 h (21). The cell pellets were then post-fixed 17
with 1% osmium tetroxide (OsO4) at room temperature. Cells were dehydrated in an 18
increasing ethanol series (50, 75, 95 and 100%) and then transferred to 100% acetone. Cells 19
were embedded at 60 °C for 24 − 48 h in Spurr’s low viscosity resin (34). Ultra-thin sections 20
were prepared, mounted on copper grids and stained with uranyl acetate and lead citrate for 21
electron microscope examination at an acceleration voltage of 80 kV (Philip CM 12/ STEM 22
and JLM-2000FX11). 23
RESULTS 1
2
In vitro assay of crude PhaCCs in E. coli 3
The ability of C. necator PHB−4 transformant harboring phaCCs (GenBank accession 4
no. HM989943) to utilize CPKO and 3HV precursors for the biosynthesis of PHA polymers 5
comprising of 3HB, 3HV and 3HHx monomers served as groundwork to further investigate 6
the interesting properties of this synthase. Hence, in this study PhaCCs was characterized via 7
in vivo and in vitro assays to better understand its PHA synthesizing ability. The phaCCs gene 8
was cloned into plasmid pGEM"AB harboring the phaACnand phaBCn genes of C. necator 9
H16 and subsequently expressed in E. coli JM109. E. coli harboring pGEM'CAB plasmid 10
which contains the PHA biosynthetic genes (phaCAB) of C. necator H16 was used as the 11
positive control. The construction of pGEM"AB(phaCCs) is shown in Figure 1. 12
The expression level of PhaCCs was evaluated through a series of in vitro assays. 13
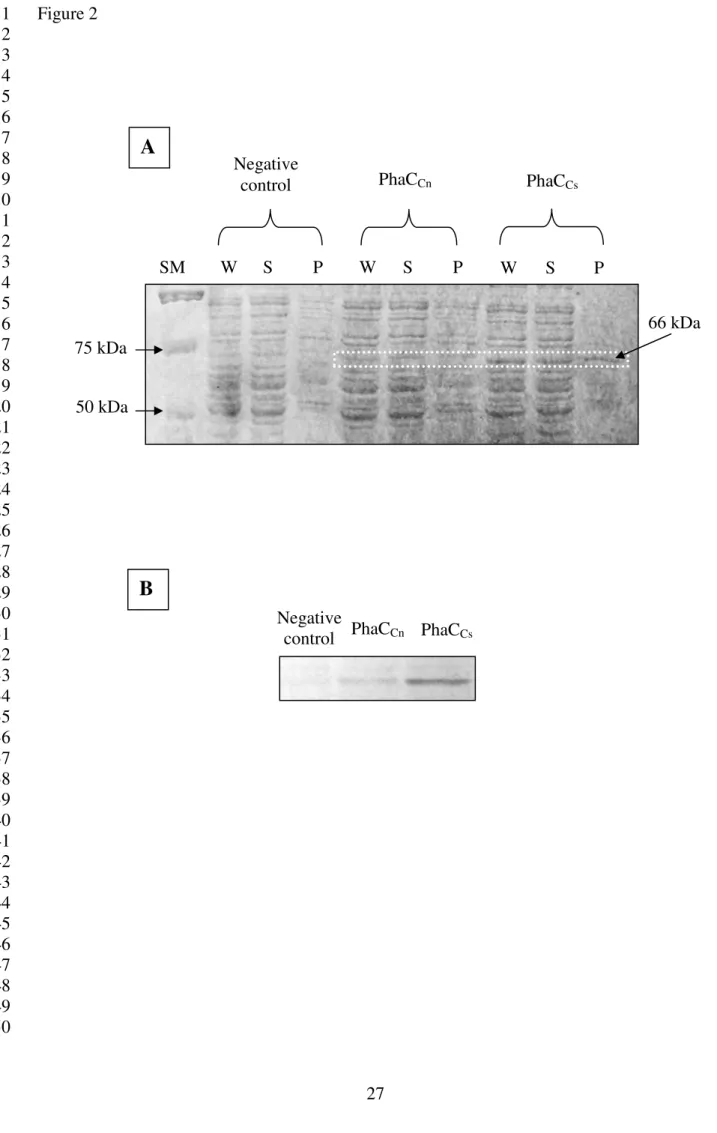
From the SDS-PAGE analysis [Figure 2(A)] of the crude cell lysates, it can be seen that the 14
expression level of PhaCCs appeared to be higher compared to that of PhaCCn using the same 15
background strain. Distinct bands at approximately 66 kDa in size corresponded to the sizes 16
of the synthases. Detection of protein bands in the precipitate (cell pellets) confirmed the 17
presence of some insoluble portions of protein. A more distinctly observed elevation in the 18
concentration of PhaCCs was seen through Western blot analysis. As shown in Figure 2(B), 19
the intensity of PhaCCs band was much greater as compared to that of PhaCCn. This 20
demonstrated the presence of higher concentration of PhaCCs in the bacterial cells. As 21
expected, no protein band was detected with the negative control. 22
In order to investigate the activity of PhaCCs, 3HB-CoA was used as the substrate and 23
the release of CoA during polymerization was measured to determine the total enzyme 24
activity. The total activity of PhaCCs was measured using the soluble fraction of the crude 25
extract. PhaCCs demonstrated superior ability in polymerizing 3HB-CoA as compared to that 1
of PhaCCn. The total activity of PhaCCs (2132 ± 70 U/g) was nearly 8-folds higher than that of 2
PhaCCn (253 ± 21 U/g). The high activity of PhaCCs could be associated with its elevated 3
level of expression in E. coli. It was assumed that the availability of higher concentration of 4
PhaCCs in the cells will ensure efficient and faster accumulation of polymer upon the addition 5
of a carbon substrate. Results obtained from in vivo evaluation of PhaCCs confirmed the 6
above hypothesis. 7
8
In vivo evaluation of PhaCCs 9
The E. coli transformant harboring phaCCs was found to accumulate high amounts of 10
P(3HB) [76 ± 2 wt%] within 24 h of cultivation using glucose as the carbon source (Table 3). 11
The total P(3HB) concentration reached a maximum value of 7.2 ± 0.2 g/L at 48 h of 12
cultivation. E. coli strains are known to grow at an optimal temperature of 37 °C. However, 13
the optimal temperature for growth and PHA accumulation by Chromobacterium sp. USM2 14
had been previously identified as 30 °C (5). Therefore, E. coli transformant harboring phaCCs 15
was cultivated at a lower temperature of 30 °C to determine the effect of lowered temperature 16
on overall growth and productivity. A significant difference was noticed in the P(3HB) 17
accumulation by this transformant at 30 °C, compared to 37 °C (Figure 3). P(3HB) content up 18
to 88 ± 1 wt% (48 h) was synthesized at 30 °C compared to a maximum of 76 ± 2 wt% (24 h) 19
at 37 °C. The polymerization of P(3HB) by PhaCCs in E. coli appeared to be better at 30 °C. 20
As shown in Figure 4(A), the E. coli transformant was packed with granules of various sizes. 21
Some cells contained mainly smaller granules as shown in Figure 4(B). The Mw of P(3HB) 22
produced averaged at 5 × 105 Da with a high polydispersity of 6.0. 23
Enzymatic activity of purified Strep2-PhaCCs 1
To further investigate the polymerization ability of PhaCCs, we constructed a Strep2-2
tagged PhaCCs for expression in, and purification from, E. coli. Strep2-PhaCCs was cloned 3
into E. coli BL21(DE3) and purified as described in Materials and Methods. The specific 4
activity of this highly purified, Strep2-PhaCCs, was 238 ± 98 U/mg, which is significantly 5
greater than that of purified synthase from C. necator from previously published results 6
(specific activity = 40 U/mg (42)). The Strep2-PhaCCs purified enzyme also exhibited a lag 7
phase in activity (Figure 5), consistent with previous results using purified Class I PhaC 8
proteins (ex. PhaC from C. necator) isolated from E. coli (11, 42, 43). The lag phase in 9
activity of Strep2-PhaCCs is more prevalent when lower concentrations of enzyme are used. 10
Preliminary enzymatic assay experiments using 3HV-CoA also suggested that the specific 11
activity of Strep2-PhaCCs is roughly twice as great as that of PhaCCn, using this substrate 12
(data not shown). 13
14
DISCUSSION 15
In vitro and in vivo characterization of PhaCCs in E. coli, carried out by heterologous 16
expression of phaCCs along with phaACn and phaBCn under the control of C. necator promoter 17
in a pGEM"AB(phaCCs) expression plasmid, showed increased level of synthase expression 18
and activity. As seen in Figure 2(A) of the crude cell lysates, a distinct band of approximately 19
66 kDa in size corresponded to the class I PHA synthases (27, 28). A more distinct band 20
exhibited by PhaCCs compared to that of PhaCCn suggested that PhaCCs was expressed at a 21
higher level. This finding was further confirmed by Western blot analysis [Figure 2(B)]. The 22
total activity of PhaCCs towards the polymerization of 3HB-CoA was nearly 8-folds higher 23
compared to that of PhaCCn. This suggested that the total enzymatic activity of PhaCCs can be 24
partially correlated with its elevated level of expression in vivo. 25
The ability to polymerize 3HB-CoA varies according to the classes of PHA synthases. 1
PHA synthases belonging to class I, III and IV show higher preference towards the 2
polymerization of 3HB-CoA (35, 37). The expression and activity of these genes in E. coli 3
are commonly used as benchmarks to compare the performance of other heterologous PHA 4
biosynthesis genes. In this study, the activity of the heterologous PhaCCn expressed in E. coli 5
was comparable to that observed in the wild-type C. necator whereby its activity in cell 6
extracts is known to range from 180 to 330 U/g during PHA accumulation stages (10, 14, 30). 7
The activity of several PHA synthases in E. coli had been documented. Alterations in 8
their specific activity and expression level were achieved through enzyme evolution studies. 9
The PhaCCn harboring a F420S mutation is known to exhibit 2.4-fold higher specific activity 10
towards the polymerization of 3HB-CoA compared to the wild-type PhaCCn (38). Meanwhile, 11
PhaCCn harboring a double mutation at G4D and F420S exhibited increased synthase 12
concentration in vivo and enhanced polymer accumulation (25). In a similar study, the 13
synthase activity in extracts of A. punctata cells and its mutants were found to be in the range 14
of 118 – 768 U/g (1). The mutant synthases exhibited 1 – 5-fold increased activity compared 15
to the wild-type synthase. Wild-type and mutant synthases of Pseudomonas sp. 61-3 16
exhibited activity of less than 50 U/g towards the polymerization of 3HB-CoA (41). In a 17
recent study, the enzymatic activities of the PHA synthase of Aeromonas caviae (PhaCAc) and 18
some of its mutants when expressed in C. necator PHB−4grown on fructose were reported to 19
be in the range of 18 – 249 U/g (40). When compared with the activity levels of these well-20
known wild-type and mutated PHA synthases, PhaCCs exhibited a clearly much higher 3HB-21
CoA polymerizing activity. 22
It is interesting to note that PhaCCs revealed a homology of 46% with PhaCCn but only 23
34% with PhaCAc even though PhaCCs is also known to incorporate 3HHx. As mentioned 24
and polymer accumulation. The mutant synthases which harbors mutations at F420S, G4D or 1
G4D/F420S showed improved activities and higher concentration in vivo (24, 25, 38). It was 2
found that the amino acids sequences at the corresponding positions of 4 and 420 in PhaCCs 3
did not show any alterations such as G4D or F420S. The amino acids at these positions were 4
identified as phenylalanine. This indicated that the PHA synthase of Chromobacterium sp. 5
USM2 was highly active in its natural form. Characterization of PhaCCs in vitro showed that 6
this enzyme was produced at high concentrations in the E. coli cells. Such a high level of 7
expression exhibited by PhaCCs in E. coli might be correlated with a high degree of 8
translation capability due to efficient codon usage (32). 9
Besides the high level of expression, the PhaCCs also showed a very high level of 10
activity, approximately 8-folds higher than that of PhaCCn. Furthermore, the activity of 11
purified PhaCCs was shown to be at least 3 to 5 times greater than the activity of pure PhaCCn. 12
It was reported previously that mutant synthase of A. caviae had an increased specific activity 13
towards 3HB-CoA of approximately 1.6-folds compared to the wild-type (0.016 U/mg) (15). 14
PhaCCs exhibits much higher preference towards 3HB-CoA compared to the other Class I 15
PHA synthases such as PhaCCn and PhaCAc. The characteristics of PhaCCs in its wild-type 16
strain Chromobacterium sp. USM2 or in the C. necator PHB−4 transformant are yet to be 17
investigated in order to determine if these elevated levels of protein expression and enzymatic 18
activity are strain-dependent. Nevertheless, the findings from this study have given us 19
invaluable insights on the interesting properties of this synthase. 20
Results of in vivo evaluation on PhaCCs correlated with results obtained from the in 21
vitro experiments. The synthase could efficiently polymerize P(3HB) when glucose was fed 22
as the carbon source. Rapid accumulation was noticed as the cells were able to achieve 76 ± 2 23
wt% of P(3HB) within 24 h of cultivation. Highest P(3HB) content of 88 ± 1 wt% was 24
accumulated at 48 h. This accounted for a P(3HB) concentration of 7.2 ± 0.2 g/L. Previously, 25
E. coli transformants harboring phaCCn are known to accumulate P(3HB) in the range of 60 – 1
70 wt% (36). By observing the residual cell biomass (Table 3), it can be clearly seen that the 2
increase in total cell biomass was caused by increasing amounts of polymer accumulation. 3
The average Mw of P(3HB) synthesized by PhaCCs (5 × 105 Da) was found to be lower than 4
that produced by some E. coli transformants harboring different PHA synthases such as 5
PhaCCn (9.7 × 105 Da) and mutant PHA synthase of Pseudomonas sp. 61-3 (7.2 × 105 Da) (3, 6
39). The lower molecular weight of the resulting polymer could be attributed to the high in 7
vivo concentration of the synthase (31). As observed in Figure 4B, many small granules were 8
present in some of the transformants. It is possible that higher concentrations of synthase in 9
vivo could have resulted in the formation of many small granules which leads to the 10
formation of short P(3HB) chains, thus increasing the polydispersity. 11
The Chromobacterium sp. USM2 synthase produced higher amounts of P(3HB) at 30 12
°C compared to at 37 °C. Significant difference was noticed in the polymer accumulation 13
whereby a reduction in P(3HB) content of approximately 18% was noticed at the end of 14
cultivation at 37 °C (Figure 3). Nevertheless, residual cell biomass values (data not shown) 15
indicated that cell growth was not affected by the different cultivation temperature. Higher 16
accumulation of P(3HB) at a lower temperature could be correlated with the temperature 17
optimum of PhaCCs. Since the wild-type Chromobacterium sp. USM2 is known to grow and 18
accumulate PHA at an optimum temperature of 30 °C, the synthase is probably more active at 19
this temperature. The performance of PHA synthase is known to be affected by varying 20 temperatures (25). 21 22 CONCLUSION 23
The PHA synthase of a locally isolated Chromobacterium sp. USM2 has been 24
very high levels of expression and specific activity towards the polymerization of 3HB-CoA 1
compared to the PHA synthase of model strain C. necator. The activity of this natural 2
synthase was found to be higher than some of the engineered mutant synthases. This finding 3
questioned the possible existence of other such natural synthases which are yet to be 4
discovered. Rapid accumulation of P(3HB) in vivo within a short period of time further 5
confirmed the in vitro findings. The naturally active PhaCCs can potentially be developed as a 6
model synthase to compare the activity of other synthases. 7
8
ACKNOWLEDGEMENTS 9
This study was supported by Techno Fund provided by The Ministry of Science, 10
Technology and Innovation, Malaysia (MOSTI). K.B and J.C acknowledge National Science 11
Fellowship awarded by MOSTI and USM’s Fellowship Scheme respectively for financial 12
support. We are grateful for the guidance and suggestions provided by Dr. Ken’ichiro 13
Matsumoto and Dr. Miwa Yamada. We also thank Prof. JoAnne Stubbe and Dr. Ping Li for 14
the generous gift of some of the 3HB-CoA used in this study and Ms. Jingnan Lu for helpful 15
assistance with protein purification. 16
17
REFERENCES 18
19
1. Amara, A. A., A. Steinbüchel, and B. H. A. Rehm. 2002. In vivo evolution of the 20
Aeromonas punctata polyhydroxyalkanoate (PHA) synthase: isolation and 21
characterization of modified PHA synthases with enhanced activity. Appl. Microbiol. 22
Biotechnol. 59:477−482. 23
2. Anderson, A. J., G. W. Haywood, and E. A. Dawes. 1990. Biosynthesis and 24
composition of bacterial poly(hydroxyalkanoates). Int. J. Biol. Macromol. 12:102− 25
105. 26
3. Antonio, R. V., A. Steinbüchel, and B. H. A. Rehm. 2000. Analysis of in vivo 27
substrate specificity of the PHA synthase from Ralstonia eutropha: formation of 28
novel copolyesters in recombinant Escherichia coli. FEMS Microbiol. Lett. 182:111− 29
117. 30
4. Bhubalan, K., D. N. Rathi, H. Abe, T. Iwata, and K. Sudesh. 2010. Improved 1
synthesis of P(3HB-co-3HV-co-3HHx) terpolymers by mutant Cupriavidus necator 2
using the PHA synthase gene of Chromobacterium sp. USM2 with high affinity 3
towards 3HV. Polym. Degrad. Stab. 95:1436−1442. 4
5. Bhubalan, K., K. H. Yong, Y. C. Kam, and K. Sudesh. 2010. Cloning and 5
expression of the PHA synthase gene from a locally isolated Chromobacterium sp. 6
USM2. Mal. J. Microbiol. 6:81−90. 7
6. Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of 8
microgram quantities of protein utilizing the principle of protein-dye binding. Anal. 9
Biochem. 72:248−254. 10
7. Braunegg, G., G. Lefebvre, and K. F. Genser. 1998. Polyhydroxyalkanoates, 11
biopolyesters from renewable resources: physiological and engineering aspects. J. 12
Biotechnol. 65:127−161. 13
8. Braunegg, G., B. Sonnleitner, and R. M. Lafferty. 1978. A rapid gas 14
chromatographic method for the determination of poly-β-hydroxybutyric acid in 15
microbial biomass. Eur. J. Appl. Microbiol. 6:29−37. 16
9. Cho, M., C. Brigham, A. Sinskey, and J. Stubbe. 2010. Purification and 17
characterization of a polyhydroxyalkanoate synthase from its native organism, 18
Ralstonia eutropha. Manuscript in preparation. 19
10. Fukui, T., and Y. Doi. 1997. Cloning and analysis of the poly(3-hydroxybutyrate-co-20
3- hydroxyhexanoate) biosynthesis genes of Aeromonas caviae. J. Bacteriol. 21
179:4821−4830. 22
11. Gerngross, T. U., K. D. Snell, O. P. Peoples, A. J. Sinskey, E. Csuhai, S. 23
Masamune, and J. Stubbe. 1994. Overexpression and purification of the soluble 24
polyhydroxyalkanoate synthase from Alcaligenes eutrophus: evidence for a required 25
posttranslational modification for catalytic activity. Biochemistry 33:9311−9320. 26
12. Jia, Y., T. J. Kappock, T. Frick, A. J. Sinskey, and J. Stubbe. 2000. Lipases 27
provide a new mechanistic model for polyhydroxybutyrate (PHB) synthases: 28
characterization of the functional residues in Chromatium vinosum PHB synthase. 29
Biochemistry 39:3927−3936. 30
13. Jia, Y., W. Yuan, J. Wodzinska, C. Park, A. J. Sinskey, and J. Stubbe. 2001. 31
Mechanistic studies on class I polyhydroxybutyrate (PHB) synthase from Ralstonia 32
eutropha: Class I and III synthases share a similar catalytic mechanism. Biochemistry 33
40:1011−1019. 34
14. Kichise, T., T. Fukui, Y. Yoshida, and Y. Doi. 1999. Biosynthesis of 35
polyhydroxyalkanoates (PHA) by recombinant Ralstonia eutropha and effects of 36
PHA synthase activity on in vivo PHA biosynthesis. Int. J. Biol. Macromol. 25:69−77. 37
15. Kichise, T., S. Taguchi, and Y. Doi. 2002. Enhanced accumulation and changed 38
monomer composition in polyhydroxyalkanoate (PHA) copolyester by in vitro 39
evolution of Aeromonas caviae PHA synthase. Appl. Environ. Microbiol. 68:2411− 40
2419. 41
16. Lee, S. Y. 1996. Bacterial polyhydroxyalkanoates. Biotechnol. Bioeng. 49:1−14. 42
17. Lee, S. Y. 1996. Plastic bacteria? progress and prospects for polyhydroxyalkanoate 43
production in bacteria. Trends Biotechnol. 14:431−438. 44
18. Li, R., H. Zhang, and Q. Qi. 2007. The production of polyhydroxyalkanoates in 45
recombinant Escherichia coli. Bioresource Technol. 98:2313−2320. 46
19. Matsumoto, K., K. Takase, E. Aoki, Y. Doi, and S. Taguchi. 2005. Synergistic 47
synthase: enhancement of PHA production and alteration of polymer molecular 1
weight. Biomacromolecules 6:99−104. 2
20. Matsusaki, H., H. Abe, K. Taguchi, T. Fukui, and Y. Doi. 2000. Biosynthesis of 3
poly(3-hydroxybutyrate-co-3-hydroxyalkanoates) by recombinant bacteria expressing 4
the PHA synthase gene phaC1 from Pseudomonas sp. 61-3. Appl. Microbiol. 5
Biotechnol. 53:401−409. 6
21. McDowell, E. M., and B. F. Trump. 1976. Histologic fixatives suitable for 7
diagnostic light and electron-microscopy. Arch. Pathol. Lab. Med. 100:405−414. 8
22. Müh, U., A. J. Sinskey, D. P. Kirby, W. S. Lane, and J. Stubbe. 1999. PHA 9
synthase from Chromatium vinosum: cysteine 149 is involved in covalent catalysis. 10
Biochemistry 38:826−837. 11
23. Murata, T., K. Takase, I. Yamato, K. Igarashi, and Y. Kakinuma. 1997. 12
Purification and reconstitution of Na+-translocating vacuolar ATPase from 13
Enterococcus hirae. J. Biol. Chem. 272:24885−24890. 14
24. Normi, Y. M., T. Hiraishi, S. Taguchi, H. Abe, K. Sudesh, N. Najimudin, and Y. 15
Doi. 2005. Characterization and properties of G4X mutants of Ralstonia eutropha 16
PHA synthase for poly(3-hydroxybutyrate) biosynthesis in Escherichia coli. 17
Macromol. Biosci. 5:197−206. 18
25. Normi, Y. M., T. Hiraishi, S. Taguchi, K. Sudesh, N. Najimudin, and Y. Doi. 19
2005. Site-directed saturation mutagenesis at residue F420 and recombination with 20
another beneficial mutation of Ralstonia eutropha polyhydroxyalkanoate synthase. 21
Biotechnol. Lett. 27:705−712. 22
26. Park, S. J., J. I. Choi, and S. P. Lee. 2005. Short-chain-length 23
polyhydroxyalkanoates: synthesis in metabolically engineered Escherichia coli and 24
medical applications. J. Microbiol. Biotechnol. 15:206−215. 25
27. Potter, M., and A. Steinbüchel. 2005. Poly(3-hydroxybutyrate) granule-associated 26
proteins: impacts on poly(3-hydroxybutyrate) synthesis and degradation. 27
Biomacromolecules 6:552−560. 28
28. Rehm, B. H. A. 2003. Polyester synthases: natural catalysts for plastics. Biochem. J. 29
376:15−33. 30
29. Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning laboratory 31
manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New 32
York. 33
30. Schubert, P., A. Steinbüchel, and H. G. Schlegel. 1988. Cloning of the Alcaligenes 34
eutrophus genes for synthesis of poly-β-hydroxybutyric acid (PHB) and synthesis of 35
PHB in Escherichia coli. J. Bacteriol. 170:5837−5847. 36
31. Sim, S. J., K. D. Snell, S. A. Hogan, J. Stubbe, C. Rha, and A. J. Sinskey. 1997. 37
PHA synthase activity controls the molecular weight and polydispersity of 38
polyhydroxybutyrate in vivo. Nat. Biotechnol. 15:63−67. 39
32. Slater, S. C., W. H. Voige, and D. E. Dennis. 1988. Cloning and expression in 40
Escherichia coli of the Alcaligenes eutrophus H16 poly-β-hydroxybutyrate 41
biosynthetic pathway. J. Bacteriol. 170:4431−4436. 42
33. Song, J. J., S. Zhang, R. W. Lenz, and S. Goodwin. 2000. In vitro polymerization 43
and copolymerization of 3-hydroxypropionyl-CoA with the PHB synthase from 44
Ralstonia eutropha. Biomacromolecules 1:433−439. 45
34. Spurr, A. R. 1969. A low-viscosity epoxy resin embedding medium for electron 46
microscopy. J. Ultrasruc. Res. 26:31−43. 47
35. Steinbüchel, A., and T. Lütke-Eversloh. 2003. Metabolic engineering and pathway 1
construction for biotechnological production of relevant polyhydroxyalkanoates in 2
microorganisms. Biochem. Eng. J. 16:81−96. 3
36. Taguchi, K., S. Taguchi, K. Sudesh, A. Maehara, T. Tsuge, and Y. Doi. 2002. 4
Metabolic pathways and engineering of PHA biosynthesis, p. 217−247. In Y. Doi and 5
A. Steinbüchel (ed.), Biopolymer Handbook. Wiley-VCH, Germany. 6
37. Taguchi, S., and Y. Doi. 2004. Evolution of polyhydroxyalkanoate (PHA) 7
production system by "enzyme evolution": successful case studies of directed 8
evolution. Macromol. Biosci. 4:145−156. 9
38. Taguchi, S., H. Nakamura, T. Hiraishi, I. Yamato, and Y. Doi. 2002. In vitro 10
evolution of a polyhydroxybutyrate synthase by intragenic suppression-type 11
mutagenesis. J. Biochem. 131:801−806. 12
39. Taguchi, S., M. Yamada, K. Matsumoto, K. Tajima, Y. Satoh, M. Munekata, K. 13
Ohno, K. Kohda, T. Shimamura, H. Kambe, and S. Obata. 2008. A microbial 14
factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc. Natl. 15
Acad. Sci. USA 105:17323−17327. 16
40. Takase, K., K. Matsumoto, S. Taguchi, and Y. Doi. 2004. Alteration of substrate 17
chain-length specificity of type II synthase for polyhydroxyalkanoate biosynthesis by 18
in vitro evolution: in vivo and in vitro enzyme assays. Biomacromolecules 5:480−485. 19
41. Tsuge, T., S. Watanabe, S. Sato, T. Hiraishi, H. Abe, Y. Doi, and S. Taguchi. 20
2007. Variation in copolymer composition and molecular weight of 21
polyhydroxyalkanoate generated by saturation mutagenesis of Aeromonas caviae 22
PHA synthase. Macromol. Biosci. 7:846−854. 23
42. Yuan, W., Y. Jia, J. Tian, K. D. Snell, U. Müh, A. J. Sinskey, R. H. Lambalot, C. 24
T. Walsh, and J. Stubbe. 2001. Class I and III polyhydroxyalkanoate synthases from 25
Ralstonia eutropha and Allochromatium vinosum: characterization and substrate 26
specificity studies. Arch. Biochem. Biophys. 394:87−98. 27
43. Zhang, S., T. Yasuo, R. W. Lenz, and S. Goodwin. 2000. Kinetic and mechanistic 28
characterization of the polyhydroxybutyrate synthase from Ralstonia eutropha. 29
Biomacromolecules 1:244−251. 30
44. Zinn, M., B. Witholt, and T. Egli. 2001. Occurrence, synthesis and medical 31
application of bacterial polyhydroxyalkanoate. Adv. Drug Deliver. Rev. 53:5−21. 32 33 34 35 36 37 38 39 40
FIGURE CAPTIONS 1
2
Figure 1: Construction of the pGEM"AB(phaCCs) expression plasmid harboring the PHA 3
synthase gene of Chromobacterium sp. USM2 with promoter (PCn), terminator (TCn) and 4
monomer supplying genes phaACn and phaBCnof C. necator. pGEM"C1AB plasmid harbors 5
the PHA synthase gene of Pseudomonas sp. 61-3 with promoter (PCn), terminator (TCn) and 6
monomer supplying genes phaACn and phaBCnof C. necator. pGEM"AB(L) harbors the 7
synthetic XbaI-EcoRI-EcoRV-Asp718-HindIII-PstI linker with promoter (PCn), terminator 8
(TCn) and monomer supplying genes phaACn and phaBCnof C. necator. 9
10
Figure 2: (A) SDS-PAGE analysis of crude extracts of PhaCCn and PhaCCs. E. coli cells 11
harboring plasmid pGEM"AB(L) was used as negative control. A total of 10 µg of sample 12
was loaded into each well. SM = Size marker, W = Whole-cell extract, S = Supernatant 13
(soluble fraction), P = Precipitate (cell pellets). (B) Comparison of the expression levels of 14
PhaCCn and PhaCCs in E. coli transformants using Western blot analysis. E. coli cells 15
harboring plasmid pGEM"AB(L) was used as negative control. A total of 10 µg of protein 16
from the supernatant was used for the analysis. 17
18
Figure 3: Comparison of P(3HB) production at 30 °C and 37 °C by E. coli JM109 19
transformant harboring phaCCs. Cells were incubated for 72 h at 180 rpm in LB medium 20
supplemented with 2 % (w/v) of glucose and 100 µg/mL of ampicillin. Data shown are means 21
of triplicates. Means with different letters are significantly different (Tukey’s HSD, p<0.05). 22
23
Figure 4: TEM showing P(3HB) granules in E. coli JM109 transformant harboring 24
pGEM”AB(phaCCs). Cells were cultured for 24 h at 30 °C, 180 rpm in LB supplemented with 25
2% (w/v) glucose and 100 µg/mL ampicillin. It could be observed that some cells contained 26
granules of various sizes (A), while others contained a high number of smaller granules (B). 27
28
Figure 5: Time course of CoA release from 3HB-CoA catalyzed by purified Strep2-PhaCCs 29
(black boxes) and purified Strep2-PhaCCn (black triangles). In the experiment represented by 30
this figure, 30 nM of Strep2-PhaCCs and 30 nM of Strep2-PhaCCn were used. The dashed line 31
indicates when the 3HB-CoA substrate was completely used up. 32 33 34 35 36 37 38 39 40 41 42 43 44
Table 1: Strains and plasmids used in this study. 1
Bacterial strains and plasmids
Relevant phenotype Source or reference Bacterial strains:
Escherichia coli
JM109
E14–(mcrA), recA1, gryA96, thi-1,
hsdR17(rk-, mk+), supE44, relA1, D(lac-proAB), [F’ traD36, proAB,
lacIqZ∆M15]
Stratagene
BL21(DE3) F–, ompT, gal, dcm, lon, hsdSB(rB -mB-), λ(DE3 [lacI lacUV5-T7 gene 1
ind1 sam7 nin5])
Novagen
Plasmids:
pBBR1MCS-C2 pBBR1MCS-2 derivative harboring approximately 2.0 kb fragment of
phaCCs from Chromobacterium sp. USM2 with putative promoter
(5)
pGEM'CAB pGEM-T derivative; with C. necator promoter and terminator harboring
phaCCn, phaACn, phaBCn
(20)
pGEM"C1AB pGEM-T derivative; with C. necator promoter and terminator harboring
phaC1Ps, phaACn, phaBCn
(19)
pGEM"AB pGEM-T derivative; with C. necator promoter and terminator harboring
phaACn, phaBCn
This study
pGEM"AB(L) pGEM-T derivative; with C. necator promoter and terminator harboring
phaACn, phaBCn, synthetic
XbaI-EcoRI-EcoRV-Asp718-HindIII-PstI linker
This study
pGEM"AB(phaCCs) pGEM-T derivative; with C. necator promoter and terminator harboring
phaCCs, phaACn, phaBCn
This study
pET51b Protein expression vector for N-terminally Strep2-tagged proteins.
pET-phaCCs pET51b derivative; with phaCCs open reading frame inserted into
BamHI/HindIII restriction site.
This study 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23
Table 2: Primer sequences used in this study. Restriction sites are underlined. 1
Primer name Sequence (5’−3’)
FEcoRICs GCGGCCAACCAGGAATTCATGC RAsp718Cs GGGACGGTACCTTCGGTTCAG FXbaIPstILK CTAGATAAGAAGGAGATGAATTCGATATCGGTACCA AGCTTCTGCA RXbaIPstIL GAAGCTTGGTACCGATATCGAATTCATCTCCTTCTTAT Strep2phaCCsFW CAAGGATCCGATGCAGCAGTTTGTCAATTCCCT Strep2phaCCsRV CTTAAGCTTTCAGTTCAAGGCGGCGA 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16
Table 3: Biosynthesis of P(3HB) by E. coli JM109 transformant harboring phaCCsa. 1 2 P(3HB), poly(3-hydroxybutyrate) 3 a
Incubated for 72 h at 30 oC at 180 rpm in LB medium supplemented with 2 % (wt/v) of 4
glucose and 100 µg/mL of ampicillin. 5
b
P(3HB) content in freeze-dried cells were determined via gas chromatography (GC). 6
c
P(3HB) concentration = P(3HB) content x CDW 7
d
Residual biomass = CDW − P(3HB) concentration 8
Data shown are means of triplicates. 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 Cultivation time (h) CDW (g/L) P(3HB) content (wt%)b P(3HB) concentration (g/L)c Residual biomass (g/L)d 12 2.1 ± 0.1 52 ± 3 1.1 ± 0.1 1.0 ± 0.1 24 5.9 ± 0.1 76 ± 2 4.5 ± 0.2 1.4 ± 0.1 36 7.5 ± 0.3 86 ± 1 6.4 ± 0.3 1.1 ± 0.0 48 8.3 ± 0.1 88 ± 1 7.2 ± 0.2 1.0 ± 0.0 60 8.2 ± 0.2 85 ± 3 6.9 ± 0.2 1.3 ± 0.3 72 7.9 ± 0.2 83 ± 1 6.5 ± 0.2 1.3 ± 0.1
Figure 1 1 2 3 4 5 6 7
Figure 2 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 PhaCCn PhaCCs Negative control W S P W S P SM W S P Negative
control PhaCCn PhaCCs
75 kDa
50 kDa
66 kDa
A
Figure 3 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28
Figure 4 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 B A
Figure 5 1 2 3 4 5 6