Bioinorganic Chemistry of the Human Host-Defense Protein
Calprotectin
by
Megan Brunjes Brophy B. A. Chemistry Reed College, 2010
Submitted to the Department of Chemistry in Partial Fulfillment of the Requirements of the Degree of
DOCTOR OF PHILOSOPHY IN BIOLOGICAL CHEMISTRY at the
Massachusetts Institute of Technology June 2015
Massachusetts Institute of Technology, 2015 All Rights Reserved
Signature of Author:
Signature redacted
/
V
/ '/ Department of Chemistry May 8, 2015 Certified by: Accepted by:Signature redacted
6/7
Elizabeth M. Nolan Associate Professor Thesis SupervisorSignature redacted
Robert W. Field Haslam and Dewey Professor of Chemistry Chairman, Departmental Committee for Graduate StudiesMASSACHUSETTS INSTITUTE
OF TEcHNOLOLGY
JUN 2 4 2015
This doctoral thesis has been examined by a committee of the Department of Chemistry as follows:
Signature
redacted--Catherine L. Drennan HHMI Professor and Investigator and Professor of Chemistry and Biology Committee Chairsperson
Signature redacted
Elizabeth M. Nolan Associate Professor of Chemistry Thesis Supervisor
Signature redacted
JoAnne Stubbe Novartis Professor of Chemistry and Professor of Biology Committee Member
Bioinorganic
Chemistry of the Human Host-Defense Protein
Calprotectin
by
Megan Brunjes Brophy
Submitted to the Department of Chemistry on May 8, 2015 in partial fulfillment of the requirements for the Degree of Doctor of Philosophy in Biological Chemistry.
Abstract
The human innate immune system responds to bacterial and fungal pathogens by
releasing the metal-chelating protein calprotectin (CP) at sites of infection and in the upper
layers of the epidermis. CP is a Mn(II)- and Zn(ll)-binding protein. The work described in this thesis elucidates the metal-binding properties of CP, and correlates these properties with in
vitro growth inhibition of bacteria and fungi. We report that the metal-binding properties of CP are modulated by Ca(ll), and we propose a working model in which CP responds to physiological Ca(Il)-ion gradients to become a potent Zn(ll)- and Mn(Il)-chelating agent in the extracellular space. Individual chapter summaries follow.
Chapter 1: Bioinorganic Chemistry of the Host Pathogen Interaction
Transition metal ions are required for all forms of life. During the course of infection, pathogenic microorganisms must acquire transition metals from the host. Three metals of interest from this standpoint are iron, zinc, and manganese. This chapter describes bacterial metal-ion homeostasis machineries, and metal-requiring processes with a focus on Zn(II) and Mn(II). This chapter then highlights the S100 family of Ca(ll)-binding proteins and discuses the Zn(Il)-, Cu(ll)-, and Mn(Il)-binding properties of S100B, S100A12, S100A7,
S10OA15, and S100A8/S100A9. Finally, an overview of the scope of this thesis is
Chapter 2: Calcium Ion Gradients Modulate the Zinc(Il) Affinity and Antibacterial Activity of Human Calprotectin
Calprotectin (CP) is a human neutrophil protein that is produced and released by neutrophils at sites of infection, where it prevents the growth of microorganisms by sequestering bioavailable zinc(II) and manganese(II). In this chapter, we present metal-binding studies to elucidate the Zn(ll)-metal-binding properties of CP. We report unique optical absorption and EPR spectroscopic signatures for the interfacial His3Asp and His4 sites of
human CP by using Co(II) as a spectroscopic probe. Zinc competition titrations employing colorimetric and fluorimetric Zn(II) sensors establish that CP coordinates two Zn(II) ions / CP
heterodimer. The Ca(ll)-insensitive Zn(ll) sensor ZP4 is used to determine the Kd of CP for Zn(II) in Ca(Il)-deplete and Ca(Il)-replete conditions. These competition titrations afford apparent Kdsitel = 133 58 pM and Kdsite2 = 185 219 nM in the absence of Ca(II). In the presence of excess Ca(Il) these values decrease to Kd,sitel 5 10 pM and Kd,site2 : 240 pM. In
vitro antibacterial assays indicate that the metal-binding sites and Ca(ll)-replete conditions
are required to inhibit the growth of Gram-negative and Gram-positive bacteria. We propose a model in which Ca(II) ion gradients modulate the antibacterial activity and Zn(Il)-binding properties of human CP.
Chapter 3: High-Affinity Manganese Coordination by Human Calprotectin Is Calcium-Dependent and Requires the Histidine-Rich Site at the Dimer Interface
In this chapter, we report that the His4 motif at the S10OA8/S100A9 dimer interface of
CP is required for high-affinity Mn(II) coordination. We identify a low-temperature EPR spectroscopic signal for this site that is consistent with high-spin Mn(II) in an octahedral coordination sphere. This site could be simulated with zero-field splitting parameters D = 270 MHz and EID = 0.30 (E = 81 MHz). This analysis, combined with studies of mutant
proteins, suggests that (A8)Hisl7, (A8)His27, (A9)His9l, (A9)His95 and two as-yet unidentified ligands coordinate Mn(ll) at site 2. These studies support a model in which CP
responds to Ca(ll) ion gradients to become a potent metal-ion chelator in the extracellular space.
Chapter 4: Contributions of the C-terminal Tail of S100A9 to High-Affinity Manganese Binding by Human Calprotectin
This chapter examines the role of the S100A9 C-terminal tail to high-affinity Mn(ll) coordination by human CP. We present a 16-member mutant family with mutations in the S100A9 C-terminal tail (residues 96-114), which houses three histidine and four acidic residues, to evaluate its contribution to Mn(ll) sequestration. These studies confirm that two His residues at positions 103 and 105 complete the octahedral coordination sphere of CP in solution.
Appendix 1: Sequence Alignments of Transition-Metal Binding S100 Proteins
Sequence alignments of S100A7, S100A8, S100A9, S100A12, S100A15, and
S100B proteins from multiple organisms are presented.
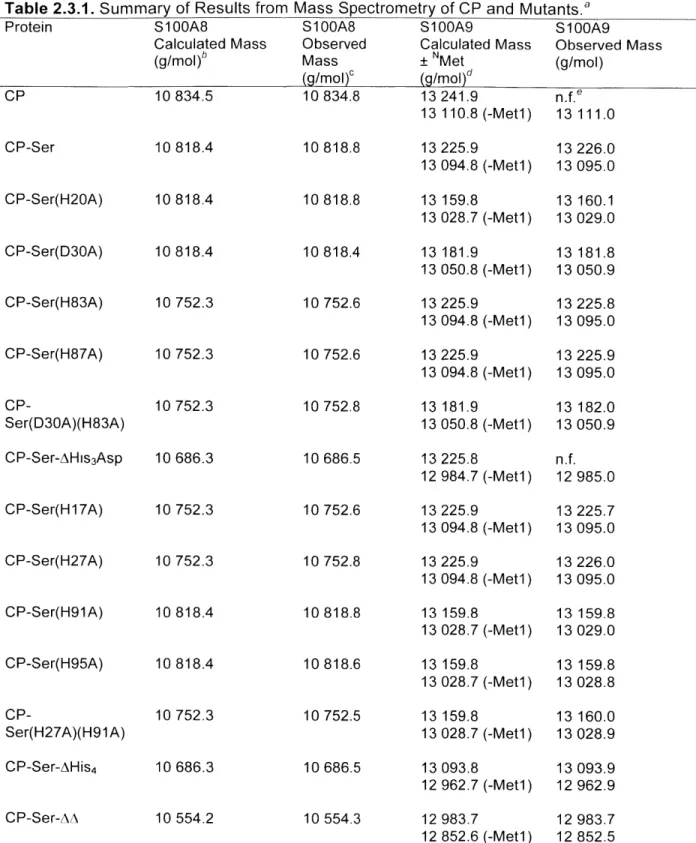
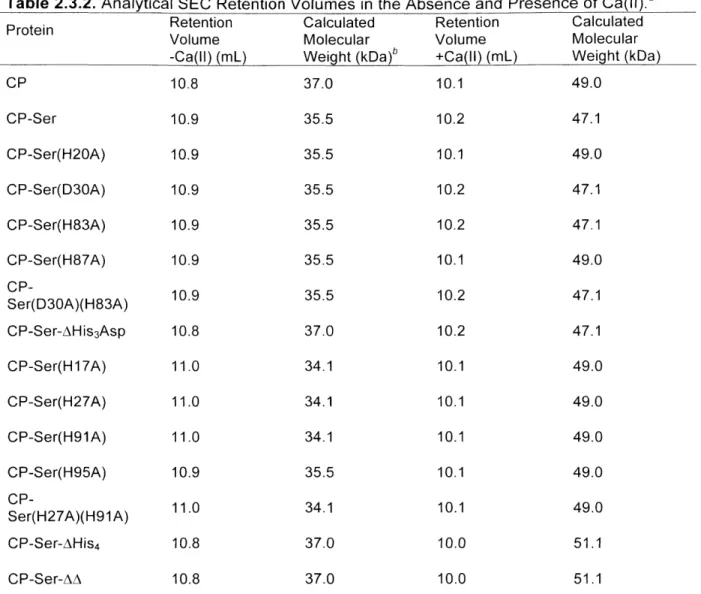
Appendix 2: Characterization of CP Mutant Proteins by Circular Dichroism and Analytical Size Exclusion Chromatography
Additional characterization of CP and mutant proteins employed in Chapters 2-4 is presented.
Appendix 3: Structures of Sensors Used In this Work
Appendix 4: Manganese Binding Properties of Human Calprotectin under Conditions of High and Low Calcium
This appendix represents a collaborative work with the Drennan Lab (MIT) and Britt Lab (UC Davis) to study the Mn(Il)-CP complex in low- and high-Ca(II) conditions. We report a crystal structure of Mn(Il)-, Ca(Il)-, and Na(l)-bound CP with Mn(II) exclusively coordinated to the His6 motif. Electron spin-echo envelope modulation and electron-nuclear double resonance experiments demonstrate that the six coordinating histidine residues are spectroscopically equivalent. The observed 15N ( = %/) hyperfine couplings (A) arise from
two distinct classes of nitrogen atoms: the coordinating E-nitrogen of the imidazole ring of each histidine (A = [3.45, 3.71, 5.91] MHz) and the distal 6-nitrogen (A = [0.11, 0.18, 0.42]
MHz). In the absence of Ca(II), the affinity of CP for Mn(II) drops by two to three orders of magnitude, and Mn(II) coordinates to the His6 site as well as other sites on the protein.
Acknowledgements
The first person I need to acknowledge is my graduate advisor, Elizabeth Nolan. I first met Liz during my MIT visit weekend in spring 2010. For the Friday night dinner all of the biological prospective students, as well as several faculty members, walked from the Marriott hotel in Kendall Square to Simmons. Somehow, probably because she was walking the fastest, Liz ended up in front. I wanted to talk to her about her research program and interests, so I propelled myself to the front of the group. I don't actually remember what I asked her, but I do remember that Liz was walking so quickly I had to jog every few strides to keep up. After I came to MIT and Liz became my research adviser, we worked together to get the calprotectin project off the ground. Things move quickly in a new lab, and there were several times during the first few years that I still felt like I was running to keep up with the pace. Fortunately, Liz doesn't leave people behind. Thank you, Liz, for being a mentor to me over the last five years. I have grown and changed so much as a scientist and as a person,
and I am truly grateful for the opportunity to work with you.
The work presented in this thesis details studies with at least 34 proteins (including mutants), many of which have been prepared multiple times over the years. This work represents the efforts of many people, including Dr. Joshua Hayden, Lisa Cunden, Aleth Gaillard, Toshiki Nakashige, and Hope Flaxman. Joshua and I worked together on the initial phases of the calprotectin project, and he performed the Zn(II) competition assays, low-temperature Mn(II) EPR, and determined the affinities of CP for Zn(II) and Mn(II). Joshua also taught me almost everything I know about EPR, and he provided valuable assistance in interpreting the low-temperature Co(II) EPR data. Lisa Cunden purified and characterized the CP-Ser(H27D) mutant. Aleth, Toshiki, and I worked on the C-terminal tail aspect of the
titrations with CP-Ser-AAA and CP-Ser-A101 mutants, and Toshiki assisted with the collection of EPR data.
One of the best things about graduate school has been that daily I come into lab and I get to work with some of the smartest, kindest, and most talented people that I have ever met. Tengfei, Haritha, Piotr, Yoshi, Joshua, Andy, Lisa, Aleth, Yunfei, Jill, Toshiki, Simone, Fabien, Julie, Sumin, Hope, 1-Ling, Shion, Phoom, Jules, Rose, Anmol, Fangting, Tim, Justin and Claire (in no particular order) helped make even the rough days fun and enjoyable. Joshua was the first post-doc to work on the calprotectin project with me, and we worked closely on the first two projects. Joshua's good nature and sense of humor made all of those long days collecting EPR data, trouble shooting the EPR, trying to get the cryostat to cool down (and stay cool!) actually fun and full of much-needed laughter. Lisa was the first undergraduate who worked with me on my 'baby' protein, and I'm so glad that I was able to con her into coming back to keep working on S100 proteins. Justin always had a smile on his face, and was always ready to listen Disney music and Taylor Swift. Andy, Jill, and Tengfei became my friends and confidants, and the week they all moved on to the next stage of their careers was one of the saddest. The best thing about science is the people
that you get to work with, and the worst is eventually saying goodbye to them. Phoom.
Phoom. Phoooooom. No, Phoom. Haritha, it has been so wonderful to get to know you over the years. Thanks for the Indian food, and for trying to make it *slightly* less spicy for my poor English tastebuds. I'm glad that my attempts to eat spicy food gave you something to laugh about. Fabien, Simone, and Julie have been great hiking buddies for trips up to the White Mountains. Jules knows an Archer quote for every situation and we shared many conversations during the Serial craze of 2014. Hope is an undergraduate who has worked with me on S100A7 and S100A15, and it has been ajoyto have her in lab.
MIT would not have been the same without my fellow biological students: Kanchana, Amy, Haritha, Austin, and Ali. Austin was always excited to talk about biochemistry, but was
equally comfortable in a conversation about DC superheroes or Disney movies. Kanchana, Amy, and Ali, thanks for all of the coffee, talks, dinners, drinks, and friendship. If I'm still sane, it's because of you. I also want to thank Crystal for hosting innumerable movie nights and book club meetings over the years, and for always being ready with a bottle of wine.
Even MIT chemistry graduate students can't spend all of their time in lab, and I've been lucky to have so many amazing friends in Cambridge. Joni, Ilya, Kathryn, Katie, David, Flourish, Nick, Karen, and Verity. Thank you for feeding my cat when I was out of town, feeding me when I was too busy, for all of the movie nights, debates about which translation of the Iliad best reflected the original Greek, and arguments about The Fellowship of the Ring. (The Great Tom Babombadil Schism.) Thank you to Katie and David for making sure that I ate real food whenever I was sick or overwhelmed, and thank you for Joni and Ilya for always being ready for brunch food. Joni and Verity, I do not thank you for hiding unicorns in my apartment every time I went out of town. Now I have a plethora of unicorn paraphernalia, including a ceramic unicorn head, that I have to haul across the country. What am I supposed to do with a ceramic unicorn head?
I also have to acknowledge two of my professors and mentors from Reed, Arthur Glasfeld and Maggie Geselbracht. Arthur taught my introductory chemistry class and is a huge part in why I majored in chemistry at all as an undergraduate. Maggie taught the sophomore-level inorganic chemistry class, and is a huge part of why I remained a chemistry major after a love/hate relationship with the first semester of organic chemistry. Maggie's enthusiasm for all things inorganic chemistry was infectious, and her ability to make complex topics accessible was unparalleled. She loved colors, coordination chemistry, and symmetry. Maggie passed away in September after a lengthy battle with lymphoma. She kept a blog during her treatments, and used it as an opportunity to teach her friends and family about Rituxan, and platinum anti-cancer drugs. I had my first undergraduate
research experience in Maggie's lab, and I wish I could have shared more of my graduate
research with her.
Arthur was the professor who introduced me to bioinorganic chemistry, and he introduced me to the biological importance of manganese. When I first told Arthur that I wanted to learn more about metals in biology, he recommended that I read Lippard and Berg. His continued advice has also proved invaluable, and his enthusiasm for all things manganese has been much appreciated over the years. When I wrote to Arthur during my first year to tell him about my project with Liz, I received the following response, "As for calprotectin, you make me very happy. I'd thought that protein would make an interesting target, but also figured that some hotshot out there would be all over it. And so it is - good luck!"
I also want to take this opportunity to acknowledge my collaborators over the years: Derek Gagnon, Troy Stich, and Dave Britt performed advanced EPR spectroscopic measurement on Mn(Il)-CP, and Sarah Bowman and Cathy Drennan have worked on the crystal structure of Mn(Il)-, Ca(II)- and Na(l)-bound CP. Cathy, in addition to being a wonderful collaborator, is also my thesis chairsperson and has given me valuable advice
over the years. Mickie Killian and Thomas Brunold have performed MCD experiments on
Co(Il)-bound CPs and helped to provide valuable insight into the coordination sphere at the His6 site.
I had the opportunity to TA for JoAnne Stubbe and John Essigman in the first-semester Biological Chemistry class (5.08) two years in a row. I learned a lot about teaching and organization from both of them. JoAnne is also on my thesis committee, and I'd like to thank her for coming to all of my oral exams and for many helpful discussions over the years.
Finally, I need to thank my mother for literally everything. Thank you for coming to visit during the Boston winters. Thank you for supporting me every step of the way, and
thank you for reading "The Immortal Life of Henrietta Lacks" and "The Poisoner's Handbook" with me. Thank you for visiting in Boston in January even though it was so cold, and thank you for coming in the spring and fall when we could go outside.
"A man would make but a very sorry chemist if he attended to that department of human knowledge alone." Mary Shelley, Frankenstein, 1818
"Be of good cheer. If science teaches us anything, it teaches us to accept our failures, as well as our successes, with quiet dignity and grace." Mel Brooks and Gene
Table of Contents
A bstract...3
Acknow ledgem ents
...
9
Table of Contents...
15
List of Tables
...
21
List of Figures
...
23
Chapter 1: Bioinorganic Chemistry of the Host Pathogen Interaction...29
1.1 Introduction ... 30
1.2 Iron and Nutritional Im m unity ... 30
1.3 Acquisition and Utilization of Zn(II) by Microorganism s ... 32
1.3.1 Zn(II) Uptake by Bacteria... 32
1.3.2 Additional Zn(Il) Transport Systems ... 38
1.3.3 Microbial Utilization of Zn(ll) ... 38
1.4. Manganese Uptake and Utilization by Bacteria... 39
1.4.1 Manganese Uptake by Bacteria... 39
1.4.2 Microbial Utilization of Mn(ll) ... 43
1.5 Zinc and Manganese Sequestration by the Human Immune System... 48
1.5.1 S100 Proteins: A Family of Metal-binding Proteins ... 48
1.5.2 S100B and S100A12 ... 51
1.5.3 S100A7 and S10OA15 ... 53
1 .5 .4 C a lp ro te c tin ... 5 5 1.6 Thesis Overview ... 57
Chapter 2: Calcium Ion Gradients Modulate the Zinc(II) Affinity and
Antibacterial Activity of Human Calprotectin
...
75
2.1 Intro d uctio n ...
76
2.2 E xperim ental...
79
2.2.1 Materials and General Methods
...
79
2.2.2 General Methods for Optical Absorption and Fluorescence Spectroscopic
M e a s u re
m e n ts ...
8 0
2.2.3 Cloning and Mutagenesis of S100A8 and
S100A9
Genes... 81
2.2.4 Overexpression and Purification of
CP
and Mutants... 86
2.2.5 Protein Mass Spectrometry
...
92
2.2.6 Analytical Size Exclusion Chromatography
...
93
2.2.7 Circular Dichroism Spectroscopy
...
94
2.2.8 Electron Paramagnetic Resonance Spectroscopy
...
94
2.2.9 Zinc Stoichiometry Determination...
95
2.2.10 Zinc Affinity Determination with FZ3... 95
2.2.11 Zinc Affinity Determination with ZP4...
96
2.2.12 Calcium-Dependent Zn(II) Sequestration by
CP ...
96
2.2.13 Antimicrobial Activity Assays
...
97
2.3 Results and Discussion... 99
2.3.1 Design, Preparation, and Characterization of a Calprotectin Mutant Family... 99
2.3.2 Calprotectin Binds Co(II) at the Interfactial His3Asp and His4 Motifs ... 106
2.3.3 Calprotectin Coordinates Zinc(ll) at the Interfacial His
3Asp and His
4Sites
...
111
2.3.4 Calprotectin Coordinates Zinc(Il) with Picomolar Affinity in a Ca(lI)-Dependent
2.3.5 Antibacterial Activity of CP Is Ca(ll)-Dependent and Requires at Least One Metal-B in d in g S ite ... 1 1 8
2.4 Summary and Perspectives ... 123
Acknowledgements... 123
References ... 124
Chapter 3: High-affinity Manganese Coordination by Human Calprotectin Is
Calcium-dependent and Requires the Histidine-rich Site at the Dimer
Interface
...
127
3.1 Introduction ... 128
3.2 Experimental... 130
3.2.1 Materials and General Methods... 130
3.2.2 Preparation of Mutant CP and Design and Purification of CP-Ser(H27D)... 131
3.2.3 Analytical Size Exclusion Chromatography ... 132
3.2.4 Manganese Quantification by Atomic Absorption Spectroscopy ... 133
3.2.5 Optical Absorption and Fluorescence Spectroscopy ... 133
3.2.6 Circular Dichroism Spectroscopy... 134
3.2.7 General Methods for Electron Paramagnetic Resonance Spectroscopy... 135
3.2.8 Room-Temperature EPR Spectroscopy ... 135
3.2.9 Low-Temperature EPR Spectroscopy ... 136
3.2.10 Mn(ll) Competition Experiments with ZP1 ... 137
3.3. Results and Discussion... 139
3.3.1 Analytical SEC Reveals that the His4 Site Formed at the CP (uI ) Dimer Interface is Required for Mn(II) Coordination... 139 3.3.2 Room-Temperature Mn(II) EPR Titrations Confirm the Requirement of the His4
3.3.3 The Mn(ll) Affinity of the His4 Site is Modulated by Calcium Ions... 145
3.3.4 Low-Temperature Mn(II) EPR Spectroscopy Supports Octahedral Coordination at th e H is4 S ite ... 1 4 9 3.3.5 CP Prefers to Coordinate Zn(ll) at the His4 Site ... 153
3.4 Summary and Perspectives ... 154
Acknowledgements ... 155
R eferen ces ... 156
Chapter 4: Contributions of the C-terminal Tail of S10OA9 to High-Affinity
Manganese(ll) Binding by Human Calprotectin... 159
4.1 Intro d uctio n ... 160
4.2 E xperim ental... 163
4.2.1 Materials and General Methods ... 163
4.2.2 Electrospray Ionization Mass Spectrometry ... 164
4.2.3 Analytical Size Exclusion Chromatography ... 164
4.2.4 Circular Dichroism Spectroscopy ... 164
4.2.5 Mn(ll) Competition Experiments with ZP1 ... 165
4.2.6 Electron Paramagnetic Resonance Spectroscopy ... 166
4.2.7 Room-Temperature EPR Spectroscopy ... 167
4 .2 .8 S ite-D irected M utagenesis ... 167
4 .2 .9 P reparatio n of M utant C P ... 169
4.3. Results and Discussion... 175
4.3.1. Design and Preparation of the Human S100A9 Mutant Family... 175
4.3.2 The C-Terminal Tail Contributes to Ca(Il)-Dependent Oligomerization... 178
4.3.3 Analytical SEC and Thermal Denaturation Studies Support Contribution of the C-Terminal Tail and HHH Motif to Mn(II) Coordination in Solution... 180
4.3.4. Mn(li)-Binding Titrations by ZP1 Competition and RT-EPR Confirm a Role for the C -T e rm in a l T a ill ... 1 8 3
4.3.5. Low-Temperature EPR Spectroscopy Supports the Contribution of the HXH M o tif ... 1 8 7
4.3.6. The S100A9 HXH Motif is Conserved ... 189
4.4 Summary and Perspectives ... 192
Acknowledgements... 194
R efe re n c es ... 19 5
Appendix 1: Sequence Alignments of Transition Metal Binding S100
P ro te in s
...
199
Appendix 2: Additional Characterization of CP and Mutant Proteins...207
Calprotectin Protein Nomenclature ... 208
Circular Dichroism Spectroscopy ... 209
Analytical Size Exclusion in the Absence and Presence of Ca(ll)... 225
Manganese(II) Analytical Size Exclusion Chromatography ... 257
ZP1 Competition Curves for S100A9 C-terminal Tail Mutants ... 260
Low-Temperature EPR of S100A9 C-terminal Tail Mutants... 266
Appendix 3: Structures of Colorimetric and Fluorescent Sensors Employed in
th is W o rk
...
269
Appendix 4: Manganese Binding Properties of Human Calprotectin under
Conditions of High and Low Calcium
...
273
A b s tra ct... 2 7 5 A 4 .1 Intro d uctio n ... 276
A4.3 Results and Discussion ... 288
Biographical Note...
341
List of Tables
Chapter 1 Table 1.4.1 Table 1.5.1 Chapter 2 Table 2.2.1 Table 2.2.2 Table 2.2.3 Table 2.2.4 Table 2.2.5 Table Table Table 2.2.6 2.3.1 2.3.2 Chapter 3 Table 3.3.1 Table 3.3.2 Table 3.3.3 Chapter 4 Table 4.1.1 Table 4.2.1 Table 4.2.2Metal-ligand bond distances in selected Mn(Il) solute-binding proteins H u m a n S 10 0 p rote ins ...
Primers employed for sub-cloning and site-directed mutagenesis of
S 1 0 0 A 8 ...
Primers employed for sub-cloning and site-directed mutagenesis of S 1 0 0 A 9 ...
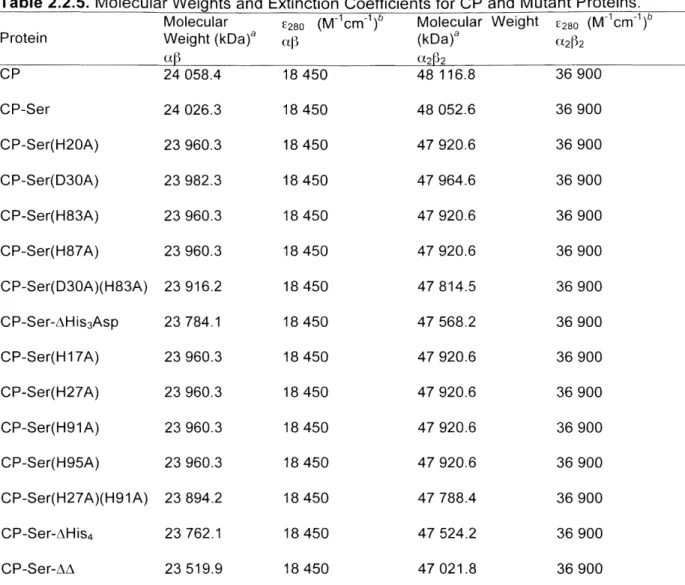
Templates and primer pairings employed in site-directed mutagenesis Calprotectin protein nomenclature... Molecular weights and extinction coefficients for CP and mutant p ro te in s ...
Molecular weights and extinction coefficients for CP subunits... Summary of results from mass spectrometry of CP and mutants... Analytical SEC retention volumes in the absence and presence of C a (ll)... ... ... ... ... ... ...
Mn(ll) quantification of protein-containing SEC samples... Slope analysis for room temperature EPR titrations... Reported zero-field splitting parameters for mononuclear Mn(ll)...
Mononuclear Mn(ll) coordination motifs... Primers employed for site-directed mutagenesis of the S100A9 C-te rm in a l ta il... Template plasmids and primer pairings...
43 49 84 85 86 90 91 92 102 103 140 145 152 162 168 169
Table 4.2.3 Table 4.2.4 Table 4.3.1 Table 4.3.2 Table 4.3.3 Table 4.3.4 Table 4.3.5 Appendix 2 Table A2.1 Appendix 4 Table 1 Table 2 Table 3 Table S1 Table S2 Table S3 Table S4 Table S5 Table S6
Calprotectin protein nomenclature...
.
...
Molecular weights and extinction coefficints for CP subunits... Amino acid sequences of the C-terminal tail of CP-Ser and mutants... Mass spectrometric analysis of human CP and mutants... Analytical SEC retention volumes and calculated molecular weights...Melting temperatures of CP-Ser and mutants... Slope analysis of room temperature EPR titrations...
Calprotectin protein nomenclature...
Ca(ll)-coordination by S100A8 and S100A9...
Zero-field splitting parameters... Hyperfine coupling in CP-Ser...
Calprotectin protein nomenclature... Mass spectrometry of 1 5N-labeled CP...
Crystallographic data and refinement statistics... Average Mn(ll)-ligand distances... A ve ra g e bo nd a ng les ...
Representative Ca(Il)- and Na(l)-ligand bond distances...
173 174 176 177 178 182 186 208 277 292 297 322 322 323 324 324 325
List of Figures
Chapter 1 Figure 1.2.1 Figure 1.3.1 Figure 1.3.2 Figure 1.3.3 Figure 1.4.1 Figure 1.4.2 Figure 1.4.3 Figure 1.5.1 Figure 1.5.2 Figure 1.5.3 Figure 1.5.4 Figure 1.5.5 Figure 1.5.6 Chapter 2 Figure 2.1.1 Figure Figure Figure Figure Figure Figure Figure Figure 2.3.1 2.3.2 2.3.3 2.3.4 2.3.5 2.3.6 2.3.7 2.3.8Structures of siderocalin and lactoferrin... Zn(Il)-homeostasis machinery in Escherichia coi...
Sequence alignment of Zn(II)-coordinating lipoproteins... Crystal structure of Zur bound to DNA... Mn(ll)-homeostasis machinery in Staphylococcus aureus... Structures of MntC and PsaA... Examples of Mn(il)-dependent enzymes... Sequence alignment of human S100 proteins... S tru ctu re s of S 10G B ...
S tru ctu re of S 100 A 12 ...
S tructu re of S 100 A 7 ...
Structures of the S100A8 and S100A9 homodimers... Structure of human calprotectin (S100A8/S1lG A9)...
Proposed antimicrobial mechanism and structural features of human
C P ... ... ... ... ... ... ... ... ... ... ... ... ... ... .. . ... .. .... ... ... ... ... ... ... ... ... ... ... ...
SDS-PAGE of samples from a representative preparation of CP-Ser... SDS-PAGE of purified proteins employed in this study... CD spectra of CP in the absence and presence of calcium... A n a lytica l S E C of C P ...
Calcium-binding to CP and CP-Ser monitored by SEC... Thermal denaturation of CP-Ser... Cobalt binding to CP monitored by optical absorption spectroscopy... Titration of CP-Ser-AHis3Asp with Co(ll)...
31
33
35
37
39
41
44
50
52
53
55
56
57
78
99
101
104
104
105
106
107
107
Figure 2.3.9 Figure 2.3.10 Figure 2.3.11 Figure 2.3.12 Figure 2.3.13 Figure 2.3.14 Figure 2.3.15 Figure 2.3.16 Figure 2.3.17 Figure Figure 2.3.18 2.3.19 Figure 2.3.20 Figure 2.3.21 Figure 2.3.22 Figure 2.3.23 Figure 2.3.24 Chapter 3 Figure 3.1.1 Figure 3.3.1 Figure 3.3.2 Figure 3.3.3 Figure 3.3.4 Figure 3.3.5 Figure 3.3.6
Titration of CP-Ser with Co(1) in the presence of Ca(lI)... Low-temperature EPR spectroscopic signals of Co(II)-bound CP...
EPR power saturation experiments... CD spectra of CP-Ser with Co(ll)... Displacement of Co(II) from CP-Ser by Zn(ll)... Titration of CP-Ser with a 1:1 mixture of Co(ll):Zn(II)...
Zinc response of 25 pM Zincon in the presence of 10 pM CP...
Fluorescence response of FZ3 to Zn(II) in the presence of CP...
Fluorescence response of ZP4 to Zn(II) in the presence of CP
m u ta n ts ...
Fluorescence response of ZP4 to Zn(ll) in the presence of CP...
Fluorescence response of ZP4 to Zn(ll) in the presence of CP, D30A, a n d H 2 7 A /H 9 1A ...
CD spectra of CP with Zn(ll)... Antibacterial activity of CP and CP-Ser at t = 24 h...
Growth of S. aureus 25923 in the presence of CP...
Antibacterial activity of CP mutants at t = 8 h and t = 24 h... Effect of BME on bacterial growth inhibition...
Structure and amino acid sequences of calprotectin... Analytical SEC of CP and mutant proteins... Circular dichroism spectra of CP-Ser with Mn(II)... Thermal denaturation of CP-Ser and mutants... Room temperature EPR titration for CP-Ser...
Room temperature EPR titration for CP-Ser and mutants... Dissociation constant plots obtained from room temperature EPR titra tio n s ... 108 110 110 111 112 112 113 114 115 116 117
118
119 120 121 122 129 140 141 142 143 144 146Figure 3.3.7 Figure 3.3.8 Figure 3.3.9 Figure 3.3.10 Chapter 4 Figure 4.1.1 Figure 4.3.1 Figure 4.3.2 Figure 4.3.3 Figure 4.3.4 Figure 4.3.5 Figure 4.3.6 Figure 4.3.7 Figure 4.3.8 Figure 4.3.9 Figure 4.4.1 Appendix 1 Figures Al.1-A1.6 Appendix 2 Figures A2.1-A2.16 Figures A2.17-A2.48 Figures A2.49-A2.51
ZP1 competition assays for CP-Ser and metal-binding site mutants... Low-temperature Mn(II) EPR spectroscopy... Low-temperature EPR titrations of CP-Ser with Mn(II)... Time course displacement of Mn(II) from the His4 site...
Structural features of human CP-Ser... Ca(ll)-binding titrations monitored by analytical SEC... Analytical SEC of CP-Ser and selected mutant proteins... Thermal denaturation of A101 and CP-Ser-AAA...
ZP1 and C-terminal tail mutants compete for Mn(II)...
Room temperature EPR titrations of A101 and CP-Ser-AAA... Low-temperature EPR spectra of CP-Ser and C-terminal tail mutants. The Mn(ll) coordination sphere of CP site 2... Sequence alignment of S100A9s highlights the conserved HxH motif.. Low-temperature EPR of CP-Ser-mT with Mn(II)... Mononuclear Mn(II) coordination motifs...
Sequence alignments of S100 proteins from different organisms...
Circular dichroism spectra of CP and mutants...
Analytical SEC of CP and mutants...
Analytical SEC of CP mutants with Mn(II)...
148 150 152 153 161 179 181 182 185 186 188 188 189 191 193 200-205 209-224 225-256 257-259
Figures A2.52-A2.57 Figures A2.58-A2.60 Appendix 3 Figure A3.1 Figure A3.2 Figure A3.3 Figure A3.4 Appendix 4 Figure 1 Figure 2 Figure 3 Figure 4 Figure 5 Figure 6 Figure 7 Figure 8 Figure 9 Figure 10 Figure 11 Figure 12 Figure 13 Figure S1 Figure S2 Figure S3 Figure S4
ZP1 competitions for C-term inal tail m utants...
Low-temperature EPR spectra of C-terminal tail mutants with Mn(ll)...
Structure
Structure
Strucutre
Structure
o f a p o Z in c o n
...
o f a p o M F 2 ... o f a p o Z P 1 ... o f a p o Z P 4 ...Structure of Ca(ll)-, Na(I)-, and Mn(ll)-bound CP-Ser...
Hydrogen-bonds at the Mn(II)-His6 site of CP-Ser... X-band CW EPR spectra... Mims ENDOR of 15N-CP-Ser... 3-pulse and 4-pulse ESEEM... Frequency domain 4-pulse ESEEM...
2H
20/H20 3-pulse ESEEM...
Exchangeable proton counting... X-band CW EPR Ca(ll)... 4-pulse E S E E M C a (l )... M im s E N D O R C a (l )... P roto n co unting C a (l )...
2H
20/H20 3-pulse ESEEM Ca(l)...
S D S -P A G E o f C P ...
Overlay of 4XJK and 1XK4... Overlay of 4XJK and 4GGF... Electron density of Mn(ll), Ca(ll), and Na(l) ions...
260-265
266-268
270
270
271
271
289
291
294
297
298
299
301
302
305
307
308
309
311
326
327
328
329
Figure S5 CW EPR of CP-Ser and tail mutants... 330 Figure S6 Q-band Mims ENDOR... 331 Figure S7 Q-band HYSCORE of AHis3Asp... 332 Figure S8 Q-band HYSCORE of 15N-AHis3Asp... 333 Figure S9 Q-band HYSCORE of 15N(A8)-AHis
3Asp... 334 Figure S10 Q-band HYSCORE of 15N(A9)-AHis3Asp... 335 Figure S1 1 Q-band proton Davies ENDOR... 336 Figure S12 Room-temperature EPR... 337 Figure S13 14N Pulse ESEEM... 338
Chapter 1:
Interaction
Bioinorganic Chemistry of the Host Pathogen
1.1 Introduction
During the course of infection, pathogenic microorganisms must acquire essential nutrients, including transition metal ions, from the host.1-4 This chapter summarizes
metal-ion uptake systems that are employed by microorganisms, and the utilizatmetal-ion of these metals with a focus on zinc(II) and manganese(II). This chapter will also describe the mammalian systems that are employed to sequester these essential nutrients from pathogenic bacteria and fungi.
1.2 Iron and Nutritional Immunity
Nearly all microorganisms require iron for growth, and iron is employed in a variety of enzyme cofactors including hemes, non-heme iron centers, and iron-sulfur clusters. Microbes may acquire iron by employing membrane-embedded transporters and by using small-molecule chelators to scavenge iron from the host.5~9 Moreover, Neisseria meningitidis is able to pirate Fe(lll) from the human iron-binding protein transferrin,' and Staphylococcus
aureus and Mycobacterium tuberculosis import heme from the host as an iron source.1 12
The human host proteins transferrin, lactoferrin, and siderocalin (also called lipocalin-2 or neutrophil gelatinase-associated lipocalin (NGAL)) deplete bioavailable iron in blood serum, secretory fluids, and sites of infection to limit the pool of iron available to microorganisms (Figure 1.2.1).131 5 Transferrin and lactoferrin coordinate Fe(Ill) with high affinity (Kd 1020 M),161'7 and siderocalin captures and hydrolyzes ferric-bound enterobactin.18
Iron provides a historical paradigm for the importance of metals at the host pathogen interface. The sequestration of iron by the innate immune system and iron homeostasis mechanisms, both microbial and mammalian, are relatively well understood and provide a basis for subsequent studies with manganese and zinc.
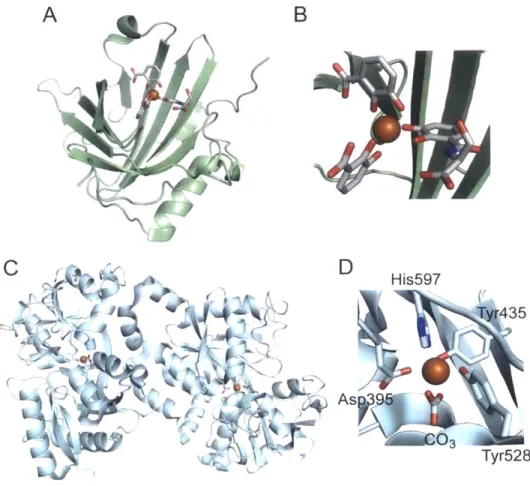
A
Co
~r4(~
( )4B
D
His597 Ty435 As9 Tyr528Figure 1.2.1. Structures of siderocalin and lactoferrin. (A) and (B) show human siderocalin with hydrolyzed ferric enterobactin. (PDB ID: 1L6M).14 (C) and (D) show diferric human lactoferrin, and an expansion of Fe(Ill) coordinated to the C-terminal lobe is shown in D.
1.3
Acquisition and Utilization of Zn(II) by Microorganisms
1.3.1 Zn(II) Uptake by Bacteria
Bacteria employ a large arsenal of proteins to maintain an appropriate level of cytoplasmic zinc, and zinc acquisition is a virulence factor of many bacteria including
Salmonella enterica serovar Typhimurium,20-22 Escherichia coli,23
-26 Campylobacter
jejuni,
2728 Pseudomonas aeruginosa,29 Yersinia pestis,3 Acinetobacter baumanii, 32
Moraxella catarrhalis 33 Vibrio parahaemolyticus, 34
Listeria monocytogenes,35 Streptococcus
pneumoniae,3 6 Brucella abortus, 37 38 Haemophilus influenzae,39 Proteus mirabilis,4 0 and
Haemophilus decreyi.41 A summary of the Zn(ll) homeostasis machinery that has been
identified in E. coli is shown in Figure 1.3.1. In general, the Zn(Il)-uptake systems are regulated at the transcriptional level by the zinc-uptake regulator Zur, and the Zn(II) exporters are regulated by the zinc transport regulator ZntR (vide infra).
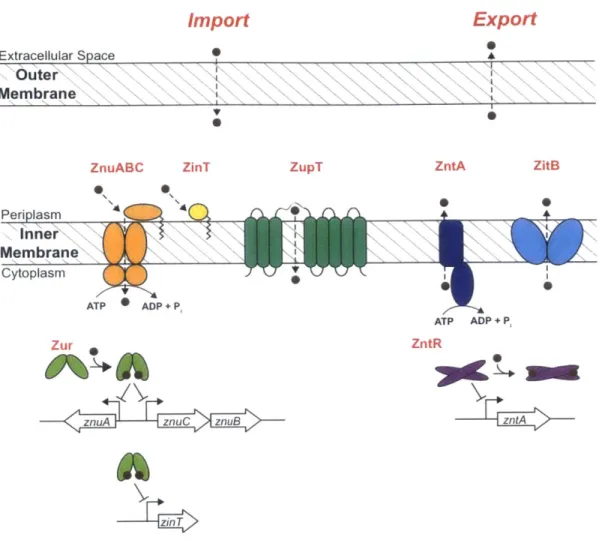
The primary high-affinity Zn(II) importer of many bacteria is the three-component ATP-binding cassette ZnuABC.2 1 22 26 This transporter is typically embedded in the inner membrane of Gram-negative bacteria and consists of a periplasmic Zn(Il)-binding lipoprotein (ZnuA), a dimeric transmembrane permease (ZnuB), and a cytoplasmic ATP-hydrolase (ZnuC). ZnuABC facilitates the active import of Zn(ll) by coupling ion transport to ATP hydrolysis; however, the molecular details of the metal-ion transfer are not well understood and there is no available structure of the intact transporter. Moreover, Gram-negative bacteria such as E. coli have an outer membrane that acts a permeability barrier. To the best of our knowledge, no outer-membrane Zn(II) transporters have been identified for E.
Import
Export
a Extracellular SpaceOuter
Membrane V 0 S*ZnuABC ZinT ZupT ZntA ZitB
Periplasm n n er
Membrane
Cytoplasm ATP ADP + P, ATP ADP + P, Zur ZntRznuA znuC znuB zt
-6
Figure 1.3.1. Zn(Il)-homeostasis machinery in E. coli. Zinc(ll) diffuses across the outer
membrane into the periplasm. Periplasmic Zn(II) is transported across the inner membrane
into the cytoplasm by ZnuABC, ZinT, and ZupT. The Zn(Il)-binding transcriptional regulator
proteins Zur and ZntR coordinate Zn(II) and regulate the transcription of znuA, znuCznuB,
and zinTor zntA, respectively.
The periplasmic solute-binding protein ZnuA is soluble, and there are several
available structures of ZnuA from different organisms including E. coli,424 3 Salmonella,44 andSynechocystis 6803.45 ZnuA is a Cluster 9 solute-binding protein that is characterized by two
(a/P)
4lobes that are linked by a backbone helix. The E. coli and Salmonella ZnuA proteins
contain a conserved disulfide bond in the C-terminal lobe domain. The role of this disulfide is
unknown, and it may contribute to the stability of ZnuA or regulation of Zn(ll) binding.
42The
two structures of
E.
coli ZnuA reveal a primary Zn(ll)-binding site in the cleft between the two
addition to the primary Zn(II)-binding site, ZnuA has a His-rich loop that is disordered in the
available crystal structures (Figure 1.3.2); however, this region likely contributes to Zn(II)
binding by ZnuA. Indeed, E. coli ZnuA outcompeted MagFura-2 (Kd,Zn(II) = 20 nM) for 2 equivalents of Zn(II) / ZnuA, confirming the presence of a second high-affinity Zn(lI) site. The flexible nature of the His-rich loop has made it difficult to resolve crystallographically, and its role in Zn(II) binding is not known. This loop may facilitate Zn(II) transfer to the ZnuB subunit, sense high levels of periplasmic Zn(II), or assist in recruitment of Zn(II) from the periplasm .42,4,47
Gram-negative organisms including E. coli and Salmonella express an additional
lipoprotein called ZinT that is involved in Zn(II) uptake.4 3 ZinT may contain one or two Zn(Il)-binding sites depending on the bacterium. The Salmonella ZinT is regulated by Zur and contributes to growth in Zn(Il)-deplete media; however, mutation of zinT did not attenuate the virulence of Salmonella in mice.48 Regardless, ZnuA and ZinT form a 1:1 complex in the presence of Zn(II). Later studies determined that ZinT coordinates Zn(II) with Kd = 22 2 nM, and that the Zn(Il)-bound form of ZinT is sufficient for complex formation with ZnuA. On the basis of these studies, it has been proposed that ZinT acts as a Zn(II)
chaperone for ZnuA in the periplasm.48 Gram-negative bacteria also employ the ZIP-family
E. coli ZnuA S. pneumoniae AdcAII S. pneumoniae AdcA S. aureus USA3002351 E. coli ZinT E. coli ZnuA S. pneumoniae AdcAII S. pneumoniae AdcA S. aureus USA300_2351 E. coli ZinT E. coli ZnuA S. pneumoniae AdcAII S. pneumoniae AdcA S. aureus USA30023S1 E. coli ZinT E. coli ZnuA S. pneumoniae AdcAII S. pneumoniae AdcA S. aureus USA300_23S1 E. coli ZinT E. coli ZnuA S. pneumoniae AdcAII S. pneumoniae AdcA S. aureus USA300_2351 E. coli ZinT E. coli ZnuA S. pneumoniae AdcAII S. pneumoniae AdcA S. aureus USA3002351 E. coli ZinT E. coli ZnuA S. pneumoniae AdcAII S. pneumoniae AdcA S. aureus USA300-2351 E. coli ZinT E. coli ZnuA S. S. S. E. pneumoniae AdcAII pneumoniae AdcA aureus USA300_2351 coli ZinT E. coli ZnuA S. S. S. E. pneumoniae AdcAII pneumoniae AdcA aureus USA3002351 coli ZinT MLHKK-TLLFAA--- LSAALWGG---ATQAADAAWASLKPVGFIASAIADGVTETEVLL 53 --- GQKESQTGKGXKIVTSFYPIYAXVKEVSGDLNDVRXI- 37 -MKKI-SLLLASLCALFLVACSN--QKQADGKLNIVTTFYPVYEFTKQVAGDTANVELLI 56 MKKKLGMLLLVPAVTLSLAACCNDDCKDKDGKVTIKTTVYPLQSFAEQIGCKHVKVSSIY 60 PDGASEOYSLRPSDVKRLQNADLVVWVGPEMEAFMQKPVSKLPGAKQVTIAQLEDVKPL QSSSGINSFEPSANDIAAIYDADVFVYHSHTLESWAGSLDPNLKKSKVKVLEASEGXTLE GAGTEPHEYEPSAKAVAKIQDADTFVYENENMETWVPKLLDTLDKKKVKTI-KA- TGDML PAGTDLNSYEPTQKDILSASKSDLFMYTCDNLDPVAKKVASTIKDKDKKLSLEDKLDKAK ---His-rich loop LMK- -SIHGDDD--- DHDHAEKSDEDHHHGDFNMHLWLSPEIARATAVAIHGKLVELMPQ RVP---GLE--- DV-EAGDGVDEKTLYDPHTWLDPEKAGEEAQIIADKLSEVDSE LLP ---- GGEEEEGDHDH-GEE---GHHHEFDPVWLSPVRAIKLVEHIRDSLSADYPD LLTDQHEHGEEHEHECHDH-EKEEHHHHHGGYDPKVWLDPKINQTFAKEIKDELVKKDPK ---SRAKLDANLKDFEAQLASTETQVGNELAPLKGKGYFVFHDAYGYFEKQFGLTPLGHFTVN HKETYQKNAQAFIKKAQELTKKFQPKFEKATQKTFVTQNTAFSYLAKRFGLNQLGIAGIS KKETFEKNAAAYIEKLQSLDKAYAEGLSQAKQKSFVTQOAAFNYLALDYCLKQVAISGLS HKDDYEKNYKKLNDDLKKIDNDMKQVTKDKQGNAVFISIIESIGYLADCYGFVQKGIQNMN ---PEIQPGAQRLHEIRTQLVEQKATCVFAEPQFRPAVVESVARGTSVRMGTLDPLGTNIKLG PEQEPSPRQLTEIQEFVKTYKVKTIFTESNASSKVAETLVKSTGVGLKTLNPLESDPQND PDAEPSAARLAELTEYVKKNKIAYIYFEENASQALANTLSKEAGVKTDVLNPLESLTEED AE-DPSQKELTKIVKEIRDSNAKYILYEDNVANKVTETIRKETDAKPLKFYNMESLNKEQ 113 97 114 120 168 145 165 179 228 205 225 239 288 265 285 298 --- 9 KTSYSEFLSQLANQYASCLKGD--- KTYLENLEENXSILAEELK---TKAGENYISVMEKNLKALKQTTDQEGPAIEP---EKAEDTKTVQNGYFEDAAVKDRTLSD QKKDNITYQSLMKSNIENIGKALDSG-VKVKDDKAESKHDKAISDGYFKDEQVKDRELSD --- MAIRLYKLAVALGVFIVSAPAFSHGHHSHGKPLTEVEQKAANCVFDDANVQNRTLSD 310 284 342 357 57 YAGNWQSVYPFLEDGTFDQVFDYKAKLTGKMTQAEYKAYYTKGYQTDVTKINITDNTMEF 402 YAGEWQSVYPYLKDGTLDEVMEHKAENDPKKSAKDLKAYYDKGYKTDITNIDIKGNEITF 417 WDGVWQSVYPLLQSGKLDPVFQKKADADKTKTFAEIKDYYHKGYATDIEMIGIEDGIVEF 117 ---VQGGQSKKYTYKYVGKKILTYKKGNRGVRFLFEATDAD-AGQFKYVQFSDHNIAPVKAEH TKDGKKHTGKYEYNGKKTLKYPKNRGVRFMFKLVDGNDKDLPKFIQFSDINNIAPKKAEH HRNNETTSCKYDYDGYKILTYKSGKKCVRYLFECKDPE-SKAPKYIQFSDHIIAPRKSS ---FHIFFGGTSQETLFEEMDNWPTYYPDNLSCQEIAQEMLAH 501 FIIFMGNDN-DALLKEMDNWPTYYPSKLNKDQIKEEMLAN 516 FHIFMGNDSQQSLLNEMENWPTYYPYQLSSEEWEEMMSH 216 461 477 176
Figure 1.3.2. Sequence alignment of Zn(Il)-coordinating lipoproteins from E. coli, S.
pneumoniae, and S. aureus. Zn(Il)-coordinating residues are shown in bold. The ZnuA-like
proteins/domains are highlighted in orange, and the ZinT-like proteins/domains are
highlighted in blue. A histidine-rich loop that is characteristic of Zn(lI)-solute binding proteins
is indicated by a bold line.
The Zn(Il)-uptake machinery of positive bacteria differs from that of
Gram-negative bacteria and is, in general, not well understood. S. pneumoniae is one human
pathogen that has been the focus of recent work on Zn(II) transport.36 56
-5 9 S. pneumoniae
acquires Zn(II) through an ABC transporter called AdcABC. By analogy to E. coli ZnuABC, AdcA is an extracellular solute-binding protein, AdcB is a transmembrane permease, and AdcC is an ATP hydrolase. The amino acid sequence of AdcA reveals two Zn(Il)-binding domains: an N-terminal ZnuA-like domain, and a C-terminal ZinT-like domain (Figure 1.3.2).
S. pneumoniae also expresses a second Zn(Il)-binding lipoprotein, AdcAll, that is
homologous to ZnuA and lacks the ZinT-like domain. Both AdcA and AdcAll are employed for Zn(ll) uptake via AdcBC.36 58 Additional polyhistidine triad (PhT) proteins appear to deliver Zn(II) to AdcAll.5 9
Zinc(II) uptake by other Gram-positive pathogens is not as well understood. For example, S. aureus appears to utilize a zinc uptake regulator protein; however, it is not known how S. aureus acquires Zn(II) from the environment.60 Putative AdcB / AdcC homologues have been identified as mreA and mreB, but they do not appear to be transcribed in Zn(Il)-deficient conditions.60 A BLAST search revealed that the S. aureus
genome encodes an AdcA homolog (Figure 1.3.2), but this gene does not appear to be part
of a zinc-transport operon, and it is not clear how its transcription is regulated. In a mouse model of S. aureus infection, Zn(II) is depleted from abscesses;61
however, it is currently unclear how S. aureus may compete with the host for Zn(II). S. aureus has been employed as a model organism to study metal-ion sequestration by the host, and it will be important to elucidate staphylococcal Zn(ll) homeostasis mechanisms and requirements.
Bacteria concentrate Zn(II) from in vitro growth media;56
,
62
however, excess cytoplasmic Zn(ll) is toxic to bacterial cells. Zn(ll) efflux proteins include E. coli ZntA, a P-type ATPase that actively transports Zn(II), and ZitB, a cation diffusion facilitator protein (Figure 1.3.1). ZntA confers resistance to the toxic effects of the metals Zn(ll), Pb(II), and
Cd(ll).63 Each ZntA protein binds two equivalents of Zn(II) with Ka = 108 M-1.64 Recent
structural studies of apo ZntA from Shigella sonnei supports the presence of a high-affinity site in the transmembrane domain, and a second high-affinity site is likely located in the N-terminal cytoplasmic domain.64'65 The cation diffusion facilitator proteins ZitB and Yiip are H+
/ Zn 2+ antiporters that contribute to Zn(II) resistance of E. coli.
66-69
Zn(II) homeostasis is tightly regulated by E. coli cells. The transcription of
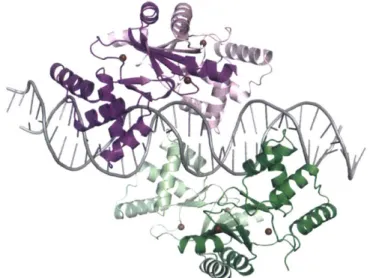
Zn(ll)-uptake proteins, including ZnuABC and ZinT, is regulated by the metalloregulatory protein Zur (Figures 1.3.1 and 1.3.3). A crystal structure of E. coli Zur bound to its cognate DNA
sequence was recently reported, and reveals that two Zur dimers bind to this DNA fragment.7 0 Zur has a femtomolar affinity for Zn(II), and the Zn(ll)-bound form of Zur binds to
DNA and represses the transcription of Zn(Il)-uptake proteins. 2 Zn(ll) efflux is upregulated by the Zn(ll)-bound form of ZntR, which also chelates Zn(II) with femtomolar affinity.71 Recent studies indicate that ZntR responds to nanomolar concentrations of chelatable Zn(I1).72 The high affinity of these proteins for Zn(II) supports the notion that the concentrations of "free" Zn(II) within the bacterial cell are tightly regulated.
Figure 1.3.3. Crystal structure of Zur bound to cognate DNA (PDB ID: 4MTD).70 The two dimers are shown in purple/pink and green/sea foam. The duplex DNA is colored grey, and Zn(ll) ions are shown as chocolate spheres.
1.3.2 Additional Zn(ll) Transport Systems
The Zn(li)-homeostasis proteins above are found in a variety of bacteria and are relatively well studied. E. coli and S. Typhimurium also express a ZIP-family member named ZupT that contributes to Zn(II) acquisition and virulence.23
,
54
,
55 N. meningitidis expresses a
TonB-dependent membrane protein, ZnuD, in Zn(l[)-deficient conditions that may be utilized for zinc or heme import. 375 N. meningitidis may also acquire Zn(ll) by pirating zinc from the human zinc-binding protein calprotectin.6 The fungal pathogen Aspergillus fumigatus reguires the Zn(ll) transporter ZrfC for virulence,7 and Candida albicans scavenges Zn(II)
with the extracellular protein Pral.78 The Y. pestis siderophore yersiniabactin also contributes to Zn(ll) acquisition.30 The Zn(Il)-homeostasis mechanisms of many pathogens
remain unexplored, and it is likely that additional Zn(Il)-uptake and -efflux systems will be discovered in the future.
1.3.3 Microbial Utilization of Zn(ll)
Zinc is essential cofactor for many proteins and may be important for either structural integrity or reactivity. The E. coli 70S ribosome coordinates up to 8 equivalents of Zn(II).79,80
It is unclear why these ribosomes concentrate Zn(II); however, it has been proposed that these ribosomes may be used as a rainy day Zn(ll) store.81
There are several bacterial virulence factors that require Zn(II) as a cofactor. Metallo-p-lactamases require Zn(II) as a cofactor,82 -8 5 and metalloproteinases such as S. aureus aureolysin require Zn(l1).86'87
Moreover, the Zn(Il)-enzyme LasB is required for Pseudomonas swarming,8 8 and Zn(II)
modulates envelope stress in enteropathogenic E. coli.89 Zn(ll) may also influence the formation of S. aureus and E. coli biofilms;5
2 '90 however, the molecular basis for this
phenomenon is not well understood. In summary, bacteria utilize Zn(II) as an essential cofactor in many enzymes; however, it is not always clear how Zn(II) contributes to pathogen survival and virulence in the mammalian host. It is likely that Zn(II) plays a
multifaceted role in bacterial pathogenesis, and additional work will need to be done to clarify the molecular role of Zn(ll) in many organisms.
1.4. Manganese Uptake and Utilization by Bacteria
1.4.1 Manganese Uptake by Bacteria
Animal models of infection suggest that Mn(ll) uptake systems are required for 57 ,92
virulence in a variety of bacterial pathogens including S. aureus,91 S. pneumoniae,
Borrelia burgdorferi,3 S. Typhimurium, and Y. pestis.95 The mechanisms of Mn(ll)
acquisition by microbes as well as the microbial processes and virulence factors that require Mn(ll) are an active area of current research initiatives (Figure 1.4.1).2
Out
4
In
LMW Mn(ll)-complexes Mn-SOD 0+Classlb
RNR FosB Other enzymes WC -tR ntA ntB mntc MnBA MntH MnR mntHFigure 1.4.1 Model of Mn(ll) homeostasis in S. aureus and examples of some Mn(ll)-containing enzymes.91
Bacteria primarily rely on two classes of Mn(Il)-importers, Nramp-type transporters
and ATP-binding cassette (ABC) importers, to shuttle divalent manganese into the
cytoplasm.96 The Nramp-type transporters (e.g. MntH from S. aureus, Figure 1.4.1) are comprised of multiple membrane-embedded helices. A paucity of structural or biochemical information about these transporters is available,97 making studies of Nramp-type machinery a rich area for exploration. One recent study reports the crystal structure of ScaDMT from
Staphylococcus capitis, and represents the first structural information for this family of
transporters.98 The ABC-type importers are three- or four-component systems comprised of
(i) a soluble extracellular or periplasmic binding protein, (ii) a transmembrane permease, and (iii) a cytoplasmic ATP hydrolase. Noteworthy examples of ABC-type importers that are expressed by pathogens for Mn(II) acquisition include PsaABC of S. pneumoniae and MntABC of S. aureus. In recent years, structural and biochemical studies of the solute-binding proteins from S. pneumoniae (PsaA) and S. aureus (MntC) have informed the mechanism of bacterial Mn(II) capture.99-102 Structural studies revealed that various Mn(II) solute-binding proteins display nearly identical secondary and tertiary structures.99,100 103-105 These solute-binding proteins are characterized by two (U/)4 lobes that are linked by a
backbone (a-helix (Figure 1.4.2). In order to concentrate metals from the environment into
the cytoplasm, solute-binding proteins must coordinate a cognate metal with high affinity and subsequently deliver the metal to the transmembrane protein. Because no Mn(II) solute-binding protein has been crystallized with its transmembrane partner, the mechanism of
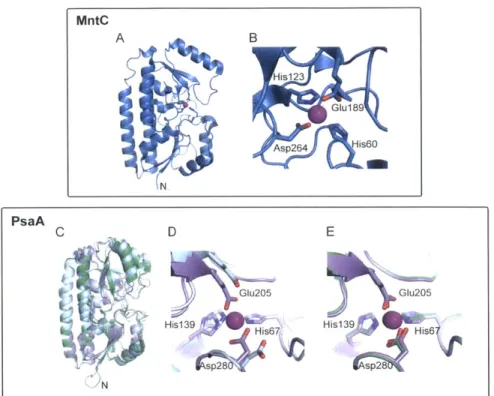
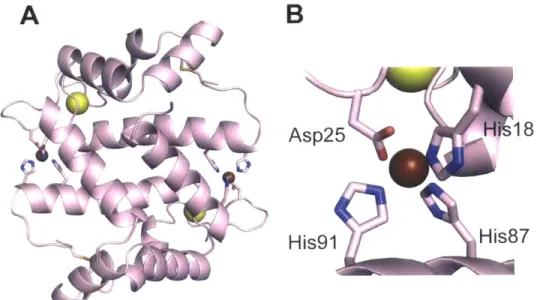
MntC A B His123 Glu189 Asp264 His60 N, PsaA C D E His139u 65 H Is3205 Hisl 39 6 His139p 6 )NI
Figure 1.4.2 Structures MntC (A) and (B) and PsaA (C)-(E). The Mn(ll)-bound monomer of
S. aureus MntC and an expansion of the Mn(Il)-binding site are shown in (A) and (B). PDB:
4K3V 99. (C) Overlay of apo PsaA (light blue), Mn(ll)-bound PsaA (lavender), and Zn(Il)-bound PsaA (green). (D) and (E) Overlays of the Mn(ll)-binding site of PsaA with apo PsaA (D) and the Zn(ll)-binding site of Zn(ll)-bound PsaA (green). Apo PsaA, PDB: 3ZK7;102 Mn(Il)-bound PsaA, PDB: 3ZTT; 100 Zn(Il)-bound PsaA, PDB: 1PSZ.1 06
S. pneumoniae PsaA is a pneumococcal virulence factor, and small-molecule
Mn(ll)-uptake inhibitors are a promising target for drug design.10' Pneumococcal PsaA (34.6 kDa) has been crystallized in the apo, Mn(ll)-bound, and Zn(ll)-bound forms (Figure 1.4.2 panels C-E).100,102
,106 The structure of Zn(Il)-PsaA is of interest because Zn(II) is toxic to S.
pneumoniae at high concentrations. Zn(II) inhibits Mn(II) uptake by the PsaABC system,
which is attributed to Zn(II) coordination at the Mn(II) site of PsaA.61 08 PsaA also coordinates Cd(II), and Cd(ll) competes with Mn(II) for PsaA.1 09 The overall fold of PsaA is very similar in the apo, Zn(Il)- and Mn(Il)-bound structures, but the length of the backbone helix varies between the apo and metal-bound forms. In apo PsaA, the backbone helix extends an additional turn, indicating that Zn(II) and Mn(II) coordination induces unwinding of this helix as the lobe domains change conformation.102 Zn(II) and Mn(II) are both
coordinated by His67, His139, Glu205, and Asp280 (Table 1.4.1, Figure 1.4.2 panels D and
E).1 001106 Zn(II) and Mn(II) coordination result in similar changes to the secondary structure of
PsaA and the protein adopts a "closed" conformation with both metals. PsaA coordinates Zn(II) in a tetrahedral geometry with Glu205 and Asp280 each coordinating Zn(II) in a monodentate manner (Table 1.4.1). An overlay of Mn(Il)- and Zn(Il)-bound PsaA suggests that there is a small rearrangement of the metal-binding residues between the two forms. The Mn(II) coordination sphere of Mn(II)-PsaA has been described as four-coordinate;100 however, Glu205 and/or Asp280 may afford bidentate interactions (Table 1.4.1). Additional biophysical studies are needed to address this ambiguity and to fully elucidate the molecular basis for how PsaA facilitates Mn(ll) delivery to its transmembrane partner PsaC.
The staphylococcal solute-binding protein MntC (35 kDa) is expressed during infection, and is a current target for the development of a multivalent vaccine against S.
aureus.91'110 MntC was recently crystallized with a putative Mn(ll) ion in the metal-binding
pocket (Figure 1.4.2 panels A and B).99 The Mn(ll)-binding site is similar to that of PsaA and formed by His50, His123, Glu189, and Asp264 (Figure 1.4.2, Table 1.4.1); however, the Mn(II) center is five-coordinate. Asp264 provides a bidentate ligand whereas a monodentate
interaction is observed for Glu189. Isothermal titration calorimetry (ITC) experiments
performed in the presence of the Mn(II) competitor citrate (Kd,Mn(II) ~ 10-4 M) revealed that MntC binds Mn(ll) with nanomolar affinity (Kd = 4.4 0.9 nM at 25 0C and pH 6.0)99 MntC also coordinates Zn(ll) and Cd(ll). In contrast to Mn(ll), binding of these d10 metal ions appears to be irreversible and may render the transporter inactive.99 The irreversibility of Zn(ll) binding is similar to the behavior reported for PsaA,102 and indicates that elevated
Table 1.4.1. Metal-ligand bond distances in selected Mn(II) solute-binding proteins. Protein Residue / Coordinating atom Metal-Ligand Bond Distance
(A)
Mn(ll)-MntCa b His50 / NE2 2.1
His123/ NE2 2.1
Glu189 /Oc 1 2.3
Glu189 I Oc2 2.8
Asp264/ 061 2.2
Asp264/ 062 2.3
Mn(Il)-PsaA d His67 / NE2 2.1
His139 Nv2 2.1 Glu205 0E1 2.1 Glu205 I OE2 2.4 Asp280 061 2.1 Asp280 / 062 2.4 Zn(il)-PsaAce His67 / Nc2 2.0 His139/ NE2 2.0 Glu205 / 01 2.0 Glu205 / Oc2 2.6 Asp280/061 2.0 Asp280 / 062 2.8
a Numbering corresponds to the soluble, cysteine-less construct of MntC. b PDB: 4K3V
Numbering corresponds to the full-length PsaA d PDB: 3ZTT e PDB: 1 PSZ
1.4.2 Microbial Utilization of Mn(II)
Following cellular uptake, manganese becomes incorporated into biomolecules. Microbial enzymes that employ manganese as a cofactor contribute to defense against oxidative stress, nucleotide biosynthesis, primary metabolism, and antibiotic resistance (Figure 1.4.3). Recent investigations have focused on how host-mediated Mn(II) sequestration modulates oxidative killing as a result of Mn(II) chelation,11 1 13 - and additional
Mn(li)-dependent microbial processes warrant consideration in future work. The rest of this section discusses the role of Mn(II) superoxide dismutase and ribonucleotide reductase in pathogenesis, and considers the consequences of Mn(ll)-sequestration by mammalian calprotectin (discussed in more detail in section 1.5.4) on bacterial Mn homeostasis.
2 02~ + 2 H+ Mn-SOD 02+H202 O 0 0 0 1 1 B , 1 B HO-P-O-P-O HO-P-O-P-O OH OH 0 0 RNR OH OH H H
~H
H OH OH OH H 00 S OH 1L-\, 0 SH + HO-P CH3 FosB I HO_CH3 OH OH OH 0 OH HO-I H O OH 0 OH 0 lo aG HOO OH H' --~
H H' - O HO OH HO OHFigure 1.4.3 Examples of Mn(l[)-dependent eznymes employed by bacteria.
Pathogens must survive the harsh chemical conditions of the immune response, including a host-mediated oxidative burst. Microorganisms therefore produce a number of detoxifying enzymes, including superoxide dismutases (SODs), to overcome oxidative stress. SODs catalyze the disproportionation of superoxide into oxygen and hydrogen peroxide, and require one of four metal cofactors: Fe, Mn, Ni, or Cu/Zn. 14 The Fe, Mn, and Cu/Zn forms have been identified in human pathogens. Recent investigations addressing how the Mn(li)-sequestering protein calprotectin (CP, vide infra) influences bacterial susceptibility to oxidative stress have focused on S. aureus and a mouse model of S. aureus infection. 1,111113 S. aureus strains produce two SODs, SodA and SodM.1 5'11 6 SodA and
SodM both exhibit a His3Asp primary coordination sphere and appear to require Mn as the redox-active cofactor; however, the identity of the cognate metal ion has not been rigorously determined for either enzyme, and some SODs are active with Mn or Fe.117-119 Given the
expression of these two SODs, S. aureus may be particularly susceptible to oxidative stress under Mn(ll)-limiting conditions, providing an appropriate model organism to examine the effect of Mn(II) sequestration on SOD activity in vitro and in an animal model of S. aureus infection. Although not rigorously established to occur in S. aureus, small-molecule Mn