Publisher’s version / Version de l'éditeur:
Vous avez des questions? Nous pouvons vous aider. Pour communiquer directement avec un auteur, consultez la première page de la revue dans laquelle son article a été publié afin de trouver ses coordonnées. Si vous n’arrivez pas à les repérer, communiquez avec nous à PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca.
Questions? Contact the NRC Publications Archive team at
PublicationsArchive-ArchivesPublications@nrc-cnrc.gc.ca. If you wish to email the authors directly, please see the first page of the publication for their contact information.
https://publications-cnrc.canada.ca/fra/droits
L’accès à ce site Web et l’utilisation de son contenu sont assujettis aux conditions présentées dans le site LISEZ CES CONDITIONS ATTENTIVEMENT AVANT D’UTILISER CE SITE WEB.
Technical Translation (National Research Council of Canada), 1970
READ THESE TERMS AND CONDITIONS CAREFULLY BEFORE USING THIS WEBSITE.
https://nrc-publications.canada.ca/eng/copyright
NRC Publications Archive Record / Notice des Archives des publications du CNRC :
https://nrc-publications.canada.ca/eng/view/object/?id=16c7538a-0572-4e81-90ac-1ee26028127e https://publications-cnrc.canada.ca/fra/voir/objet/?id=16c7538a-0572-4e81-90ac-1ee26028127e
NRC Publications Archive
Archives des publications du CNRC
For the publisher’s version, please access the DOI link below./ Pour consulter la version de l’éditeur, utilisez le lien DOI ci-dessous.
https://doi.org/10.4224/20375481
Access and use of this website and the material on it are subject to the Terms and Conditions set forth at
Adsorption of sodium lignosulfonates by sodium montmorillonite from aqeous solutions.
Koyama, M.; National Research Council of Canada. Division of Building Research
PREFACE
L i g n o s u l f o n a t e i s a by-product o f s u l f i t e l i q u o r from the) paper-making p r o c e s s a n d f i n d s d i v e r s e ap?' l c a t i o n s s u c h a s i n c o n c r e t e , a g r i c u l t u r e , a d h e s i v e s , c e r a m i c s , gypsum p l a s t e r , d y e s t u f f s and t a n n i n g .
L i g n o s u l f o n i c a c i d and i t s s a l t s a r e t h e most w i d e l y used w a t e r - r e d u c i n g and s e t - r e t a r d i n g a d m i x t u r e s i n c o n c r e t e p r a c t i c e . A d d i t i o n s of l i g n o s u l f o n a t e promote g r i n d i n g e f f i c i e n c y i n t h e cement c l i n k e r and a l s o a c t a s a l u b r i c a n t f o r cement-based g r o u t , r e d u c e the f r o a t s u s c e p t i b i l i t y and i n c r e a s e t h e c o m p r e s s i v e s t r e n g t h o f c o n c r e t e .
The l i g n o s u l f o n a t e m o l e c u l e i s complex a n d a n u n d e r s t a n d - i n g o f i t s a d s o r p t i v e b e h a v i o u r i n cement and c l a y s y s t e m s s h o u l d e n a b l e i t s more e f f i c i e n t u s e and s h o u l d a l s o l e a d t o new
a p p l i c a t i o n s . Very febr p u b l i c a t i o n s , however, a r e a v a i l a b l e on t h i s a s p e c t . I n t h i s p a p e r a n e x a m i n a t i o n h a s b e e n made o f t h e e f f e c t s o f pH and a d d i t i o n s of sodium c h l o r i d e on t h e a d s o r p t i v e b e h a v i o u r o f sodium l i g n o s u l f o n a t e on a montmori l l o n i t e c l a y . The r e s u l t s a r e o f g r e a t p e r t i n e n c e t o c e m e n t - w a t e r - l i g n o s u l - f o n a t e s y s t e m s . The D i v i s i o n wishea t o t h a n k NRC T r a n s l a t i o n s S e c t i o n f o r a r r a n g i n e t o h a v e t h i s p a p e r t r a n s l a t e d and t o D r . V.S.
Ramachandran of t h i s D i v i s i o n who checked t h e t r a n s l a t i o n . Ottawa
December 1970
N. 3. Hutcheon D i r e c t o r
NATIONAL RESEARCH COUNCIL OF CANADA
T e c h n i c a l T r a n s l a t i o n 1433
T i t l e : Adsorption of sodium l i g n o s u l f o n a t e s by sodium m o n t r n o r i l l o n i t e from aqueous s o l u t i o n s
Author : Makoto Koyama
Reference: Kogyo Kagaku Z a s s h i ( J o u r n a l of t h e Chemical S o c i e t y of Japan, I n d u s t r i a l Chemistry S e c t i o n ) ,
73
( 5 ) :
1033-1038, 1970A d s o r p t i o n o f Sodium L i g n o s u l f o n a t e s by
Sodium l l o n t m o r i l l o n i t e from Aqueous S o l u t i o n s tl ( ~ e c e i v e d S e p t . 1, 1 9 6 9 )
Nakoto KOYAMA '2
( S y n o p s i s )
The samples of sodium l i g n i n o s u l f o n a t e s u s e d f o r t h e e x p e r i m e n t s had b e e n f r a c t i o n a t e d by t h e r i o l e c u l a r w e i b h t s , and t h e i r a v e r a g e m o l e c u l n r meiyht w a s 36,000. The a d s o r p t i o n
e x p e r i m e n t s were c o ~ d u c t e d u n d e r wide r a n g e s of piI m d of he c o n c e n t r a t i o n of sodium c h l o r i d e .
The e x p e r i m e n t a l r e s u l t s showed :,hat t h e a d s o r p t i o n i s o t h e r m was a Langmuir :,ype. F i r s t , t h e amount o f a d s o r p t i o n was d e t e m - i n e d by e x p e r i m e n t s u n d e r v a r i o u s c o n d i t i o n s . Lihen t h e a d s o r p t i o n was made from s o l u t i o n s which d i d n o t c o n t a i n sodium c h l o r i d e , t h e amount of s a t u r a t i o n a d s o r p t i o n i n c r e a s e d
a s t h e pH s o l u t i o n d e c r e a s e d . For e x m p l e , t h e amount o f a d s o r p t i o n w a s 1 . 0 mg p e r 1 g of c l a y a t pH 7 , 8.7 mg s t pH 4
and 60.6 rng a t p H 1.0. On t h e o t h e r hand, t h e a d s o r p t i o n from 0.4 N s o d i u v c h l o r i d e s o l u t i o n s remained n e a r l y t h e same w i t h i n
a pH r a n g e between 1 . 5 and 8 ( a b o u t 45 mpIg c l a y ) . Nhen p~ was r e t a i n e d a t a b o u t 6-7, and o n l y t h e ~ o n c e n r ~ r a t i o n s o f sodium c h l o r i d e were changed, t h e a a o i m t s of a d s o r p ; , i o n i n c r e : + s e d a s t h e
1: T h i s p a p e r i s P a r t I of A d s o r p t i o n o f L i g n i n S u l f o n a t e s by Clay-Minerals.
% 2: Maitoto KOYAMA Tokyo Kogyo S h i k e n j o
(Tokyo T e c h n o l o g i c a l T e s t i n g I n s t i t u t e ) : Honmachi
,
Shibuyaku, Tokyo-to.
c o n c e n t r a t i o n of sodium c h l o r i d e i n c r e e s e d , b u t t h e maximal ad- s o r p t i o n due t o t h e chani;e of c o n d i t i o n s was a t t r i b t ~ t e d m a i n l j t o t h e change of t h e s i z e of s o d i u ~ l i g n i n o s u l f o n a t e s i n t h e s o l u t i o n s .
Next, u s i n g 0.4 N sodium chlo12ide s o l u t i o n s , t h e a r e a of a d s o r p t i o n s ~ n - f a c e a t t h e s a t u r a t e d a d s o r p t i o n was determined. It was found t h a t t h e s u r f a c e arlea W A S n e w l y c o n s t a n t w i t h i n a
3
wide r a n g e of pH between 1.8 and 7.8 ( 5 0 - 6 0 m ' / g c l a y ) , ~ n d ~ b o v e pH 7.8, i t d e c r e a s e d d r a s t i c a l l y . Namely, t h e a r e a of t h e s ~ ~ r f a c e on which l i g n i n s u l f o n h t e s c o u l d be adsorbed w a s n e a r l y c o n s t a n t a t n e u t r a l and a c i d i c pH, r e g a r d l e s s of t h e
pH s o l u t i o n v a l u e s .
One of t h e many u s a g e s of sodium l i g n i n o s u l f o n ~ t e s
a3
( h e r e a f t e r a b b r e v i a t e d a s LsA) i s t h e a d d i t i o n t o mud w a t e r f o r boring1). I n t h i s c a s e , t h e LSA was u s e d mainly a s a d i s p e r s i n g a g e n t of r n o n t m o r i l l o n i t e , and t h e niechanism of t h e d i s p e r s i n g a c t i o n was e x p l a i n e d a s t h e a d s o r p t i o n . I n a d d i t i o n t o t h i s a p p l i c a t i o n , t h e LSA had besil usel? i n c e r a r r i c s 2 ) , cement manu-4 ) f a c t u r i n g 3 ) e n d f o r c o n d i t i o n i n g of s o i l
.
t 3: Mtiin component of b i s u l f i t e d i g e s t i n g s o l u t i o n i n p r e p a r a t i o n o f pulp. G l o b u l a r , w a t e r
s o l u b l e macromoleci~le
.
1: Bunkickti OKII\TO "Boring Mud Water" Gihod.0 Pub. Co., Tokyo ( 1 9 6 6 ) p. 119.
2 9 3 : "Lignln" R e s e ~ ~ r c h I n s t i t u t e f o r Chemical Marketing ( 1 9 6 4 ) .
4: S h i g e r u &TISONO, Nogiken-ho ( R e s e a r c h Heport o f t h e I n s t i t u t e of A g r i c u l t u r ~ l Technology)
The major o b j e c t i v e s o f t h i s r e s e a r c h work were t o b b t a i n b a s i c d a t a which might be v a l u a b l e i n d e v e l o p i n g new u s e s f o r LSA
and o t h e r l i g n i n d e r i v a t i v e s . and more e f f i c i e n t c o n d i t i o n s o f u s i n g them.
T h i s r e p o r t i s t h e f i r s t p a r t of t h i s s e r i e s o f b a s i c r e s e a r c h , and t h e a u t h o r s examined t h e e f f e c t s o f pH and sodium
c h l o r i d e on t h o a d s o r p t i o n o f LSA on m o n t m o r i l l o n i t e s a t u r a t e d w i t h sodium i o n s , and found t h a t t h e s ~ ~ r f a c e a r e a o f t h e a d s o r p t i o n
was c o n s t a n t .
I n t h e a f o r e - d e s c r i b e d f i e l d s of a p p l i c a t i o n of LSk, e l l t h e s y s t e m s c o n s i s t of c l a y and w a t e r , and therefore t h e pro- p e r t i e s of t h e systems concerned a r e t h e i r r h e o l o g i c a l ones. However, s i n c e c l a y sllrf a c e s have d i f f e r e n t c h a r a c t e r i s t i c s a t l a y e r s u r f a c e s ( s u r f a c e s p a r a l l e l t o t h e l a y e r s o f s t r a t i f i e d s t r u c t u r e ) and a t t e r ~ i n a l s u r f a c e ( p e r p e n d l < : u l a r t o t h e l a y e r s ) , and, i n a d $ i t i o n t o t h i s d i f f e r e n c e , s i n c e p a r t i c l e s t a k e d i f f e r e n t s t e r i c p o s i t i o n s depending on t h e c o n d i t i o n o f t h e i r m u t u a l 5 combinations, t h e r h e o l o g i c p r o p e r t i e s a r e i n d e e d q u i t e comp- l i c a t e ? .
M e n macromolecular a n i o n s tire u s e d t o improve t h e r h e o l o g i c p r o p e r t i e s o f t h e s e s y s t e m s , t h e p o s i t i o n s a t which t h e v a c r o -
m o l e c u l a r a n i o n s a r e adsorbed pay influexlce t h e r h e o l o g i c p r o - p e r t i e s of t h e system. T h e r e f o r e , when t h e a d s o r p t i o n o f t h e aacromoleculrrr a n i o n s a r e s t u d i e d , n o t o n l y t h e d e t e r ~ q i n k t i o n of
5. H . van Olphen, " A n 1 n t ; r o d u c t i o n t o Clay C o l l o i d
Chemistry" I n t e r s c e i e n c e Pub., N e w York ( 1 9 6 3 )
t h e amount o f a d s o r p t i o n b u t a l s o t h e s i t e s of %he a d s o r p t i o n must be s t u d i e d . However, i t i s e x t r e q e l y d i f f i c u l t t o conduct
t h e ~d s o r p t i o n experiment r e p a r a t e l y on t h e l a y e r s u r f a c e and t h e t e r m i n ~ l s u r f a c e , and i n f a c t t h e r e h&s n o t been any refison- able method d e v e l o p s d t o d e t e r m i n e t h e s i t e s o f a d s o r p t i o n . I n t h i s r e p o r t , t h e a u t h o r s p a i d p a r t i c u l a r s t t e n t 5 o n t o c l a r i f y t h e s i t e s o f a d s o r p t i o n .
The f a c t o r s which govern t h e a d r o r p t i o n a r e a s f o l l o w s . 6
>
The a d s o r p t i o n c o h e r e n c e of macromolecular a n i o n s t o c l a y i s
a t t r i b u t e d t o s ~ v e r & l f a c t o r s , n a r f e l y , s t a t i c e l e c t r i c i t j , chemical bonding, hydrogen bonding and Ven d e r Waals f o r c e . S i n c e LSA c o n t a i n s a l a r g e number of s u l f o n a t e g r o u p s , t h e s t a t i c e l e c t r i c i t y i s p r o b a b l y t h e a o s t c r i t i c e l l y i m p o r t a n t f a c t o r , and t h e r e f o r e LSA i s p r o h a b l y adsorbed on a p o s i t i v e l y charged s i d e o f c l a y p a r t i c l e s .
On t h e o t h e r hand, t h e e l e c t r i c a l l y charged s t a t e of t h e s u r f a c e of c l a y p a r t i c l e s d i s p e r s e d i n w a t e r i s c u r r e n t l y con- s j d e r e d a s f o l l o w s . When t h e cla:, p a r t i c l e h o l d s an i o n which
i s e a u i v a l e n t t o one e l e c t r i c v a l e n c e i n t e r m s of e x c h a n g e a b l e c a t i o n , t h e e l e c t r l c p o t e n t i a l on i t s l a y e r s u r f a c e i r always n e g a t i v e , b u t , h a t on i t s t e r m i n a l s u r f a c e i s d e t e r m i n e d by t h e
pH o t t h e Wueous environment, a s i s t h e c a s e of tllumina s u r f u c e . When t h e e x c h a n g e a b l e c a t i o n s a r e two o r t h r e e v a l e n c e - e q u i v a l e n t , o r when o x i d e s of i r o n o r alumir~um a r e adsorbed on t h e s u r f a c e ,
6 : Daizo K i d a , Macro. o l e c u l a ( K o b u n s h i ) ,
13
306 ( 1 9 6 4 ) .t h e hole o r a p a r t of t h e l a y e r s u r f a c e become p o s i t i v e l y charged.
From t h e s e known f a c t s and r e a s o n i n g s , t h e f a c t o r s which c o n t r o l t h e a d s o r p t i o n of LSA by c l a y a r e assumed t o be p H of s o l u k i o n s , s p e c i e s of exchangeable c a t i o n s , and s p e c i e s and c o n c e n t r a t i o n of p o s i t i v e i o n s e x i s t i n g i n t h e s o l u t i o n s . Accordingly, i f s u f f i c i e n t d a t a f o r t h e a d s o r p t i o n of LSA by
c l a y i s t o be o b t a i n e d , i t becomes n e c e s s a r y t o p r e p a r e a v a r i e t y c l a y samples c o n t a i n i n g v a r i o u s exchangeable c a t i o n s and examine t h e e f f e c t s of pH and d i f f e r e n t s p e c i e s of s a l t s on e a c h c l a y sample.
As w i l l be d e s c r i b e d l a t e r , t h e LSA used f o r t h e e x p e r i - ment h a s a ~ o l e c u l a r diameter (assuming LSA i s g l o b u l a r ) of 100
2
7)
i n 0.4 N sodium c h l o r i d e s o l u t i o n . According t o r e f e r e n c e
,
some m o n t m o r i l l o n i t e p a r t i c l e s have about t h e same diameter. Therefore, i t may be a p p r o p r i a t e t o assume t h a t m o n t m o r i l l o n i t e i s adsorbed on LSA b u t t h e a u t h o r s 1 experiments were c a r r i e d o u t b a ~ e d on t h e concept t h a t LSA was adsorbed on t h e m o n t m o r i l l o n i t eS U F ~ ~ ~ 8 9
2. EXPERIMENTS AND MhTEHIkLS
2.1. P r e p a r a t i o n of Samples.
2.1.1. LSA. A s a a t a r t i n g m a t e r i a l , a calcium-base waste s o l u t i o n of t ~ e a t m e n t of p u l p with a c i d i c s u l f i t e was used.
7: Handbook of Clay (Nendo and book) Japanese
F i r s t , 1,he w a s t e was c o n v e r t e d t n sodium-base by a base
s u b s t i t u t i o n 8 ' , and Ghe wanra .,as condensed 9, t o a b o u t 50% con- c o n t r s t i o n of s o l i d m a t t e r u s i n g a m u l t i - e f f i c i e n c y b o i l e r . LSA was i s o l a t e d and p u r i f i e d a s shown i n f ' i g u r e 1, and t h e b a r i u m
s a l t s o l u t i o n was t r e a t e d vqith e t k r n o l s o l u t i o n s ( o f d i f f e r e n t e t h a n o l c o n c e n t r a t i o n ) t o y i e l d 1 2 d i f f e r e n t p r e c i p i t a t e s by a
f r a c t i o n a l p r e c i p i t a t i o n m ~ t h o d . Kach f r a c t i o n wap2 c o n v e r t e d t o sodium s a l t and s t o r e d a f t e r vacuum d r y i n g . The sample u s e d i n t h i s e x p e r i m e n t was t h e f r a c t i ~ n A - 3 ( f r a c t i o n w i t h t h e t h i r d l a r g e s t m o l e c ~ ~ l a r w e i g h t ) . ?'he y i e l d of t h i s f r a c t i o n was 13.2% o f t h e t o t a l of a l l 1 2 f r s c t i o n s . L S A i n t h i s f r a c t i o n had a n a v e r a g e m o l e c u l a r w e i g h t o f 36,000. The c o n t e n t of e a c h a c i d i c g r o u p i s a s f o l l o w s , The s i l l f o n u t e g r o u p was 194 r n e q / 1 0 0 * L ~ ~ , and weakly a c i d i c g r o u p s , n a v l r l y p ~ a ( 8 , 8-11 and )11 were 38,
4 3 and 62 meq/100 g * L S A , r e s p e c t i v e l y ( a l l b a s e a on h a - s a l t o f LSA)
.
D e t e r ~ i n a t i o n o f mol.ecul.ar w e i g h t was c o n d u c t e d by an o s m o l j t i c v e t h o d u s i n g a 0.5 N sodlum c h l o r i d e s o l u t i o n , and t h e a c i d i c s r o u p was d e t e r m i n e d by 2 ~ n t i t r i r n e t r i c e l e c t r o -c o n d u c t i v i t y method
l e )
.
8. T . S e i g s n j i , 2;. T k n r a i , F i , Ogewa and ot;hers. A b s t r b c t s of t h e 1 7 t h r n n u a l Meeting of Nikka
( J a p a n e s e Chemical S o c i s c y ) ( 1 9 6 4 ) , p. 20.
9. '1'. S e i g e n j i , M, O y m u a r ~ d H. I i d a , t i b s t r a c t s o f t h e 1 7 t h Annual :ectli:g sf N i ~ k a ( 1 9 6 4 ) p. 20. 10. H. n o , : i m a , A. Fie.yhsbi, ~ r l d I . T a c h i . J o u r n a l o f
t h e J ~ p d n e s e S o c ? , i ~ f ~ , - oi"' P h p e r and Pulp Technology,
F i g u r e 1: I s o l k i t i o n and P u r i f i c a t i o n of LSA
Na-base s u l f i t e p u l p waste
c a t i o n exchange
o ow ex
50W)(LSA was c o n v e r t e d t o H-form) n-butariol DCHA&
\
butan01 l a y e r ( LSA-DCHA )1
aqueous l a y e r b u t a n o l l a y e r ( DCHA ) aqueous i a p r (LSA, ~ a - f o r m )1
benzene aq.'
l a y e r benzenei l a y e r ( LSNa ) ( r e s i d u a l b u t a n o lc a t i o n exchange (LSA, H-form) BaC03 t o p H 6.5 o r lovier
c e n t r i f u g a t i o n (BaCOg, BaSU4 removed) LSA-Ba s a l t
D
2.1.2 Sodium M o n t m o r i l l o n i t e ( h e r e a f t e r N a - m o n t m o r i l l o n i t e ) . M o n t m o r i l l o n i t e No. 20, a s t a n d a r d c l a p 4 of A P T , was used a s a
s t a r t i n g m a t e r i a l , and i t wa:? p u r i f i e d by t h e f o l l o w i n g method, a n d was u s e d a s a sa?lple o f t h i s e x p e r i ~ l e n t . The p r o c e d u r e o f p u r i f i c a t i o n i s a s f o l l o w s .
Two hundred and f i f t y grams of c l a y was m o i s t u r e d viith w a t e r , and 500 g of a 35% hydro,;en p e r o x i d e s o l u t i o n w a s added t o t h e wet c l a y t o decompose o r g a n i c s u b s t a n c e s , and t h e n i t was c o n v e r t e d t o N a - m o n t m o r i l l o n i t e , t h r i c e L r e a t i n g w i t h a 2 N sodium c h l o r i d e s o l u t i o n . It was w e l l washed w i t h w a t e r u n t i l no
c h l o r i d e i o n c o u l d be d e t e c t e d . A t t h e l a s t washing, o n l y
p ~ r t i c l e s d i s p e r s e d i n w a t e r were c o l l e c t e d , and t h e s u s p e n s i o n w a s c e n t r i f u g e d ( 1 2 , 0 0 0 rpm, S O m i n u t e s ) . The p r e c i p i t a t e s were c o l l e c t e d and a i r - d r i e d , and f u r t h e r d r i e d i n a vacuum d e s i c c a t o r
B
(40°c, -400 mm Hg ) w i t h c a l c i u m c h l o r i d e , f o r f i v e h o u r s . T h e d r i e d m a t t e r was p l a c e d i n a p o r c e l a i n m o r t a r and c r u s h e d t o p a s s a 6 0 - ~ e s h s i e v e , and t h e powder was s t o r e d i n a d e s i c c a t o r c o n t a i n i n g 50% s u l f u r i c a c i d .
DTA c u r v e s of t h e s t a r t i n g r a t e r i a l &nd t h e p ~ r i f i e d m a t e r i a l a r e shown i n f i s u . r e 2. ?'he m a j o r peaks of X-ray d i f -
f r a c t i o n p a t t e r n of s a r l ~ p l e s were found a t d , 15.28. ( s t r o n g p e a k ) , 4.9 t o 5 . 2 8 (weak p e a k ) and 3.1% (medium p e a k ) . 'r'ne c o n d i t i o n s of X-ray d i f f r a c t i o n were CuKK r a y ( 2 0 kV, 30 r n ~ ) and 2O/rnin.,
9 4 : American P e t r o l e u m I n s t i t u t e Clay M i n e r a l
and t h e samples examined were prepared by a i r - d r y i n g samples of water-suspended c l a y on a g l a s s p l a t e d f o r a microscope.
Fig. 2. DTA Curves of Na-montmorillonite a: p u r i f i e d sample b: c r u d e c l a y s t a n d a r d : A1203 c o n t a i n e r : p l a t i n u m temp. c o n t r o l : 5O~/rnin 2.2. Adsorption Experiment. I n a 50 m l ground g l a s s Erlenmeyer f l a s k , a 1 0 m l s o l u t i o n of LSA prepared by a predetermined method, and 200 mg of a s a r p l e c l a y were p l a c e d and t h e f l a s k w a s s e t a s i d e w i t h o u t d i s t u r b a n c e u n t i l a d s o r p t i o n e p i l i l i b r a t i o n w a s e s t a b l i s h e d . When t h e ad-
s o r p t i o n completed, t h e suspension was c e n t r i f u g e d ( 8 , 0 0 0 rpm, 1 0 min.) and t h e c o n c e n t r a t i o n o f LSA i n the s u p e r n a t a n t w a s
determined, and t h e differenc.e between t h i s v a l u e und t h e con- c e n t r a t i o n b e f o r e t h e a d s o r p t i o n was c a l c u l a t e d , a s t h e m o u n t of a d s o r p t i o n . The experiments were c a r r i e d o u t changing t h e
p H of s o l u t i o n , c o n c e n t r a t i o n of sodium chloraide and c o n c e n t r a t i o n of LSA, The c o n c e n t r a t i o n o f LSA v a r i e d between 0.0025 and ( 1 0 3 5 ) 0.3%, t h e p H v a r i e d between 0.6 and 9 , and t h e conceni;rations
-10-
of sodium chloride were either 0.4
N
or zero. The time needed for eauilibrium adsorption was 15 days in the systems including0.4 N sodiur chloride solution, but it was 2 4 hours in all the others. Determination of LSA was conducted by an optical density method in the ultra-violet region, using a Hitachi EPS self -recording photopeter, Model 2U, and for the pH determination, a Hitachi-
Horiba pH meter, Model rf-4 fitted with a compound electrode. Since the water adsorption of sample clay during the periods of experiments reached as much as 0.5 ml per 200 mg., all the calculations of the amounts of adsorption of LSA were based on the 0.5 ml of adsorbed water per 200 mg of clay. The experiments were conducted at room temperature.
2.3. Determination of LSA by Optical Density Method.
Since LSA has a strong absorption in the ultra-violet region, detection and determination of LSA at as low as 0.0005$ of
concentration can be carried out, if the determinations could be done at certain
pH
range. However, under the afore-described experiaental conditions, theLSA
determination becomes somewhat complicated because some substances eluted from clay or colloidal c l ~ y itself have some ultra-violet absorption. Therefore, aseparate determination of LSA is needed. It was found that the eluiltes from clay had a mild peek at approxisktely 240 m p and, on both sides of this peah, there should be wave lenkths at which the optical dens1 ties are identical. As S L I C ~ , two vuave lenkths,
231
r
y
and 256y4uwere chosen, and the optical densities of mixtures of LSA and clay-eluates at these wdve lengths wered e t e r n d n e d , and t h e d i f f e r e n c e of t h e o p t i c a l d e n s i t i e s a t t h e s e two vravelengths were c a l c l ~ l a t e d . It was l e a r n e d t h a t t h i s
d i f f e r e n c e i s p r o p o r t i o n a l co he c o n c e n t r a t i o n of LSA, a s t h e s e wave l e n g t h s corresponded t o a s n o u l d e r and minimal a d s o r p t i o n of LSA i t s e l f . When t h i s method was a p p l i e d t o s o l u t i o n s w i t h d i f f e r e n t c o n c e n t r a t i o n s of c l a y - e l u a t e s , and a c a l i b r a t i o n c u r v e was o b t a i n e d , a l i n e a r r e l a t i o n s h i p between t h e a c t u a l con-
c e n t r a t i o n of LSA and i t s o p t i c a l d e n s i t y w a s found, and t h e d e v i a t i o n s of t h e d e t e r r i n a t i o n v a l u e s from t h i s ~ t r a i ~ h t l i n e of c a l i b r a t i o n were, i n e v e r y c a s e , l e s s t h a n 2$ of t h e maximal c o n c e n t r a t i o n o f LSA which w a s a c t u a l l y used t o p r e p a r e t h e c a l i b r a t i o n l i n e . The deterrriination o f o p t i c a l d e n s i t i e s w a s conducted i n a 0.01 N h y d r o c h l o r i c a c i d s o l u t i o n .
3 . RESULTS
AND
D I S C I J S S I O N .Adsorption i s o t h e r m s o b t a i n e d by e x p e r i m e n t s a r e shown i n f i g u r e s 3 ~ i n d 4. 'lbe r e s - ~ l t s a g r e e q u i t e v~rell w i t h Lar;gmuirZs
8 5
a d s o r p t i o n i s o t h e r m e q u a t i o n (Fig. 5 ) . Taking t h e a c c e p t e d t h e o r y t h a t LSA i s an a r ~ i o n i c , g l o b u l a r macro-molecule with t h r e e dimensional n e t s t r u c t i ~ r e , b u t w i t h o u t d e f i n i t e s p a t i a l o r i e n t a t i o n i n t o c o n s i d e r a t i o n , t h i s a d s o r p t i o n i s p r o b a b l y a r a t h e r s i m p l e , 8 5 : KbC
*
=m
C : m o u n t o f e q u i l i b r i u m a d s o r p t i o n( w
L S A / ~ C l a y ) b: amount of s a t : l r a t e d a d s o r p t i o n(w/f3)
C: e q u i l i b r i u m conc. ( g L S A / ~ ~ O m l ) K: a.dsorpi;ion ecluilibrium c o n s t a n tu n i - m o l e c u l a r , l a f e r a d s o r p t i o n w i t h r e l t i t i v e l y ab'undant un-
adsorbed s p a c i n g , and the amount o f s 8 t u i 3 a t e d a d s o ~ p t i o n may
p o s : i b l y be o b t a i n e d . The a d s o r p t i o n s d t v a r i o u s pH
a r e
shownIn fi;:ure 6. F u r t h e r , t h e e f f e c t o f c o r ? c e n t r 2 a t i o ~ o f sodium c h l o r i d e ( F i g . 7 ) , t h e e f f e c t o f b o t h pH and s o d i u m c h l o r i d e
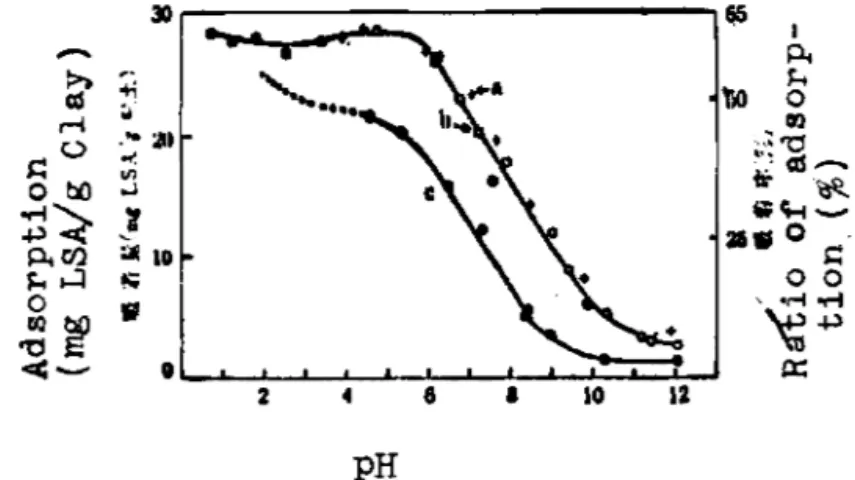
c o n c e n t r a t i o n ( F i g . 8 ) , and t h e r e s u l t s o f d e t e r m i n a t i o n i n b a s i c s i d e ( F i g , 9 ) were also examined, First, t h e r e s u l t s of t h e s e
e x p e r i m e n t s cen be summarized a s f o l l o w s :
-
:
1
.4
I
a
n
at-LSA Conc, ( g/'lOOmi] LSA Con@. (g/100rnl]
Fig,. 3, P d s o r p t i o r . Isot'nerm F i g . 4 . Adso?ption I s o t h e r m
( v v i t h o u t K a C l j (0.4N N a C l s y s t e m )
Fig. 5: Langmuirls S t r a i g h t Fig. 6. S a t u r a t e d Adsorption
Line Black c i r c l e : 0.4N va. pH
NcCl system. V~hite
c i r c l e : Vuithout NaCl a: 0.4N IiaC1 system
Figures show pH b: without NaCl
N a C l Conc.
( N )
Fig. 7. R e l a t i o n s h i p between NaCl cono. a d s o r p t i o n .
a:
LSA i n i t i a l cone. 0.1%.b: LSA i n i t i a l conc. 0.2%. S o l u t i o n p H 6-7.
l-l 4J a 0 l-l 4J cd cc
Fig. 8. R e l a t i o n s h i p between p H , NaCl conc. and adsorpLion.
( 1 n i t i a . l conc. of LSA was 0.05%) a , b , c , d : conc. o f NaCl 0.2W, 0.04N,
0,01N, 0, r e s p e c t i v e l y ) .
The r a t i o o f adsorbed LSA a g a i n s t t h e added LSA.
Fig. 9. Change of a d s o r p t i o n by changing pH from n e u t r a l t o b a s i c . (LSA i n i t i a l conc. 0.1%) a , b , c : sodium c h l o r i d e conc. 0.4, 0.2, 0.04N, r e s p e c t i v e l y . 65 1 PC k *\io 0 m .:a I.
.
h-"s
P k w . + a m 0 F: 0 0 .. d l-lk g
30 3) 10 0 #@ 2 4 8 8 10 I2 P:'-*.*..
‘-
L +(1) Vvithout sodium c h l o r i d e , b o t h e q u i l i b r i u m a d s o r p t i o n and s a t u r a t e d a d s o r p t i o n i n c r e a s e a s t h e p H l o w e r s ( f i g . 3,6). ( 2 ) Ifhen n e u t r a l , t h e e q u i l i b r i u m e d s o r p t i o n i n c r e a s e s as sodium c h l o r i d e c o n c e n t r a t i o n i n c r e a s e s , b u t above 0.2N o f sodium c h l o r i d e c o n c e n t r ~ t i o n t h e a d s o r p t i o n r e m a i n s c o n s t a n t
id.
7 ) .( 3 ) I n 0.4 N sodium c h l o r i d e s o l u t i o n s , t h e amounts of saturbated a d s o r p t i o n a r e n e a r l y c o n s t a n t ( a b o u t 45 mg/g) between pH 1 . 5 and 8 ( F i g . 6 ) .
( 4 ) The a d s o r p t i o n c a n t a k e p l t c e a t a s h i g h pH a s 11 ( F i g . 9 ) .
Thus t h e a u t h o r s c o u l d d e t e r m i n e t h e m o u n t of a d s o r b e d LSA
by \la-montmorillonite a t v a r i o u s sodium c h l o r i d e c o n c e n t r a t i o n and wide r a n g e o f pH.
I t i s u n d o u t e d l y t r u e t h a t t h e a d s o r p i i o n i s : u b j e c t e d t o t h e e f f e c t s of pH, sodium c h l o r i d e c o n c e n t r a t i o n , and LSA ccn- c e n t r a t i o n . I n o r d e r t o a n a l y z e t h e d e g e e o f ~ h e s e e f f e c t s i n - d i v i d u a l l y and a l s o t o c l a r i f y t h e mechanism of f u n c t i o n i n g of t h e s e i n f l u e n t i a l f a c t o r s , t h e a u t h o r s exanlined t h e e x p e r i m e n t a l r e s u l t s i n t h e f o l l o w i n g manner. A s f a c t o r s which d e c r e a s e o r i n c r e a s e t h e m o u n t of a d s o r p t i o n , t h e f o l l o w i n g a r e c o n s i d e r e d : (1) change of s p a t i a l a r r a n g e l l e n t of LSA m o l e c u l e i n t h e s o l u t i o r ~ , ( 2 ) d i i ' f e r e n c e i n t h e mode of a d s o r p t i o n , t h a t i s , e i t h e r on t h e t e r m i n a l s u r f a c e o n l y o r b o t h on t e r m i n a l and l a j e r s u r f a c e , and ( 3 ) change of t h e r a t i o c f t h e a d s o r b a b l e s u r f a c e o f c l a y s u r f a c e , due t o t h e change o f a r e a o f c o v e r a g e by LSA. S i n c e t h e r e l a t i o n s h i p between t h e LSA c o n c e n t r a t i o n and t h e amount o f a d s o r p t i o n can be e x p l a i n e d by a d s o r p t i o n i s o t h e r m , t h i s w i l l
n o t be d i s c u s s e d .
F i r s t , t h e f a c t o r ( 1 ) i s p r o b a b l y t h e most i m p o r t a n t , a s macromolecular e l e c t r o l y t e ? g e n e r e l l y a r e s u b j e c t e d t o t h e e f f e c g s
of c o n c e n t r a t i o n of e l e c t r o l y t e s , and t h e same can be expected f o r LSA n o l e c l ~ l e . For exar?ple l1 ), LSA w i t h m o l e c u l a r r.eight of
0
36,000 h a s i t s m o l e c u l a r - c h h i n t e r m i n a l d i s t a n c e o f a b o u t 400 A
0
i n p u r e w a t e r , b u t o n l y nbout KjO A i n a 0.5 N sodium c h l o r i d e
s o l u t i o n . Also, when a r e d u c t i \ , e v i s c o s i t y o f LSA aqueous s o l u t i o n was determined under d i f f e r e n t c o n c e n t r a t i o n s of sodium c h l o r i d e , i t was found t h a t t h e r e d u c t i v e v i s c o s i t y r a p i d l y d e c r e z s e d a s t h e sodium c h l o r i d e c o n c e n t r a t i o n i n c r e a s e d up t o 0.15 N, but above t h a t c o n c e n t r a t i o n , i t rernained n e a r l y c o n s t a n t . Thus t h e s p a t i a l expanse of t h e LSA ~ o l e c u l e d e c r e a s e s a s t h e sodium c h l o r i d e
c o n c e n t r a t i o n i n c r e a s e s , r e a c h i n g a t t h e minimal expanse a t t h e c o n c e n t r a t i o n o f 0.15bJ. Corresponding t o t h i s d e c r e a s e i n t h e s p a t i a l expanse, tihe m o u n t of LSA which can adsorb on a c e r t a i n a r e a of c l a y most l i k e l y i n c r e a s e s . I t i s assumed t h a t t h e a f f e c t of h y d r o c h l o r i c a c i d vihich was u s e d t o c o n t r o l t h e s o l u t i o n p H had t h e s m e i n c l i n a t i o n , b u t t h e a u t h o r s d i d n o t c o n f i r m t h i s . A s t o che f a c t o r s ( 2 ) and ( S ) , f i b l ~ r e 9 c l e a r l y i l l u s t r a t e s the d e g r e e of t h e i r c o n t r i b u t i o n s . Nhmely, f o r t h e f a c t o r ( 2 ) , s i n c e t h e e l e c t r i c c h a r g e distribution on the l a y e r s u r f a c e i s n o t a l t e r e d by change of t h e s o l u t i o n pH, arid s i n c e t h e r e a p p e a r s t o t o be no s e l e c t i v e a d s o r p t i o n when t h e r e Is no e v i d e n c e of 11) G . K o j i n a , A. Nakai and I. T a c h i J o u r n a l o f t h e J a p a n e s e S o c i e t y cf Pnper and P u l p Technology (Pami-pa-Gikyo-shi) -9 1 4 831 ( 1 9 6 0 ) .
m u l t i p l e - c h a r g e d p o s i t i v e i o n s i n i;he s o l u t i o n , t h e amount of a d s o r p t i o n s h o u l d remain c o n s t a n t r e g a r d l e s s o f t h e s o l u t i o n pH. However, as shown i n t h e f i , l l r e , t h e a d s o r p t i o n becomes c l o s e t o z e r o a t h i g h e r pH. T h e r e f o r e , no a d s o r p t i o n t a k e s p l a c e on t h e l a y e r s u r f a c e . A? f o r t h e f a c t o r ( 3 ) , t h e f o l l o w i n g c o n c l u s i o n i s o b t a i n e d . \%en t h e sodium c h l o r i d e c o n c e n t r a t i o n i n f i g u r e 9 was e i t h e r 0.2 o r 0.4
N,
t h e s i z e o f LSA m o l e c u l e i s minimum,a s d i s c u s s e d i n t h e s e c t i o n of the f b c t o r ( I ) , a n d i f t h e ad- s o r p t i o n t a k e s p l a c e o n l y d t t h e t e r i n i n a l s u r f a c e o f c l a y , t h e
c a u s e of t h e c h ~ n g e o f amount o f a d s o r p ; i o n s h o u l d be o n l y t h e change of a d s o r p t i o n characterlstj c which w a s caused by t h e change of pH, on
t h e
t e r m i n a l surf ace. The t e r m i n a l s u r f ace t e l ~ c l s t o h a v e h i g h e r p o t e n t i a l and l a r g e r d e n s i t y of p o s i t i v e c h a r g e as t h es o l u t i o n pH l o w e r s , j u s t i n t h e same way a s i s known o f alumina s u r f a c e 1 2 ) . T h e r e f o r e , t h e amount o f a d s o r p t i o n i s e x p e c t e d t o i n c r e a s e a s t h e pH l o w e r s , and t h i s was proven by t h e e x p e r i m e n t . A t t h e a c i d i c pH r a n g e , ;he amount of a d s o r , j t i o n becomes n e t ~ r l y c o n s t a n t , and t h i s c a n be e x p l a i n e d by t h e s t e a d y i n c r e a s e of
p o s i t i v e c h a r g e d e n s i t y on the t e r m i n a l s u r f a c e accompanied by t h e i n c r e a s e i n a d s o r p t i o n o f LSA, and by s a t u r a t i o n a d s o r p i i o n , which does n o t a l l o w f u r t h e r a d s o r p t i o n , a t c e r t a i n , l i m i t i n g c h a r g e
d e n s i t y . Although f i g u r e 8 shows t h e same t e n d e n c y , t h e a d s o r p t i o n s t a r t s t o i n c r e a s e a g a i n a t l o w e r pH. T h i s o c c u r s when t h e sodium c h l o r i d e c o n c e n t r a t i o n i s l o w e r t h a n 0.15N, and a t t h i s con- c e n t r a t i o n , t h e s p a t i a l expanse o f t h e LSA m o l e c u l e h a s n o t r e a c h e d 1 2 ) J . A . Yopns and D.W. F u e r s t e n a u , J. C o l l o i d S c i . , 1 9 , 6 1 ( 1 9 6 4 ) .
-
t h e minimum b u t t h e a d s o r p o t i o n becomes almost o o n s t a n t a t n e a r n e u t r a l pH, but l a t e r when h y d r o c h l o r i c a c i d i s added t o lower
t h e s o l u t i o n ' , t h e a d s o r p t i o n s t a r t s t o t a k e p l a c e a g a i n ,
Next, t h e charge d i s t r i b u t i o n on t h e ~ e r m i n a l s u r f a c e may
change a s t h e s a l t c o n c e n t r a t i o n i n t h e s o l u t i o n changes, even when t h e s o l u t i o n pH i s maintained consr,ant. Therefore, t h e t e r m i n a l s u r f a c e may be more s e n s i t i v e t o t h e change of concent- r a t i o n of sodium c h l o r i d e , i f t h e p o s i t i v e charge d e n s i t y i s
s m a l l e r a s i n a b a s i c s o l u t i o n . If t h e c o n c e n t r a t i o n of sodium
d
c h l o r i d e i s lowered, t h e p o s i t i v e charge d e n s i t y i s lowered and, a s a r e s u l t , i t s e f f e c t i s t h e same as r a i s i n g t h e s o l u t i o n pH.
Therefore,
the a c t u a l pHa t
which t h emaximal
a d s o r p t i o n o c c u r sshould be
a t a lower value
t h a n5.5,
ahown in f i g u r e9.
However, t h e experimental r e s u l t s showed t h a t t h e change of t h e concent- r a t i o n of sodium c h l o r i d e does n o t change t h e maximum a d s o r p t i o npH, 2 s f a r a s t h e sodium c h l o r i d e c o n c e n t r a t i o n i s h i g h e r t h a n
0.04 N . I n s:lmrnary, t h e f a c t o r ( 3 ) i n f l u e n c e s r i s e and f a l l of t h e t e r m i n a l p o t e n t i a l s of c l a y , and i t s e f f e c t i s shown a t n e u t r a l and b a s i c pH, but i f t h e sodium c h l o r i d e c o n c e n t r a t e i s low, t h e m o u n t of a d s o r p t i o n i s a l s o v e r y low and t h e f a c t o r ( 1 ) c o n t r i b u t e s
q u i t e s i g n i f i c a n t l y . I n o t h e r words, t h e degree o f he e f f e c t of f 3 ) on t h e amount of a d s o r p t i o n i s d i f f i c u l t t o determine.
The experimental r e s u l t s i n d i c a t e t h a t t h e s i z e of LSA
molecule i n sodium c h l o r i d e s o l u t i o n s of c o n c e n t r a t i o n h i g h e r
8 T r a n s l a t o r ' s Note: The t r a n s l a t o r cannot f o l l o w
t h a n 0.2N i s almost c o n s t b n t , and t h e chan4e o f pH does n o t a l t e r t h e s i z e . T h e r e f o r e , t h e amount% of a d s o r p t i c n from t h e sodium c h l o r i d e s o l u ~ i o n s of c o n c e n t r a t i o n s h i d h e r t h a n 0.2N and t h e s i z e of t h e LSA m o l e c u l e s i n t h e s o l u t i o n a r e s u f f i c i e n t d a t a needed t o c a l c u l a t e t h e a r e a o f sodium n l o n t m o r i l l o n i t e on which
LSA i s adsorbed, and r e l a t i o n s h i p hetween t h e c a l c u l a t e d s r e a a.nd t h e s o l u t i o n pH becomes c l e a r .
L e t u s d i s c u s s t h e a d s o r p t i o n a r e a . The a r e a was c a l - c u l a t e d from t h e amount of s a t u r a t i o n a d s o r p t i o n and t h e s i z e of t h e LSA molecule i n t h e s o l u t i o n s o f d e t e r m i n h t i o n s . Assuming t h e shape of t h e LSA m o l e c u l r t o be g l o b u l a r , t h e v a l u e ,
(y2$
3/2/Mt6 was s k e n of F l o r y t s v i s c o s i t y e o u a t i ~ n ' ~ ) ~ ~ ] r
24
( r )a s a m o l e c u l a r d i a m e t e r . S i n c e t h e LSA molecule i n 0.4N sodium c h l o r i d e s o l u t i o n i s asrumad t o be a r e a s o n a b l y s t i f f b a l l , t h e a p p l i c a t i o n of t h i s e q u a t i o n i s adequate. I n o r d e r t o deterrt i n e t h e a r e a of adsorpGion v e r y a c c u r a t e l y , I t i s n e c e s s a r y t o know t h e occupied a r e a b y t h e LSA molecule, b u t i t i s n e a r l y i m p o s s i b l e .
1 6 : : l i m i t i n g v i s c o s i t y , r : d i s t a n c e between
b o t h t e - m i n a l s of r n o l e c u l t r c h a i n , M: m o l e c u l d r 100 ml/g.
P. J. F l o r y , " P r i n c i p l e s of Polymer ~ h e m i s t r ~ " , C o r n e l l Univ. P r e s s , New "ork ( 1 9 5 3 ) p. 611.
"Macro-molecular Chemistry S e r i e s ( 2 ) Physic a1 P r o p e r t i e s o f Macro-molecules" Ed. 1~1acro-
~ o l e c ~ l l e r Society., Chi j i n Shoin Pub. Co. ( 1 9 6 3 ) p. 40.
T h e r e f o r e , i t was s u b s t i t u t e d ~ 4 t h t h e areti. o f a c i r c l e with t h e - 2
&
d i a m e t e r ( r ).
F i r s t , t h e l i m i t i n g v i s c o s i t y C73
o f LSA i n a 0.4N sodium c h l o r i d e s o l u t i o n w a s d e t e r m i n e d , teachp pH. The r e l a t i o n o f t h e LSA c o n c e n t r a t i o n C and t h e r e 6 u c t i v e v i s c o s i t y a s a s t r a i g h t l i n e a t e v e r y pH v a l u e $ . From t h e d e t e r m l n c i t i o nv a l u e , m e l a t i o n s h i p between the two v a l u e s ,
f73,
c , ~ ) * .
and p Hwas o b t a i n e d and shown i n f i g u r e 1 0 . From t h e s e r e s u l t s , i t was found t h a t t h e LSA m o l e c u l e h a s n e a r l y t h e s a m e m o l e c u l a r s i z e i n 0.4N sodium c h l o r i d e s o l u t i o n s r e g a r d l e s s o f t h e i r s o l u t i o n p H . S i n c e t h e r e was p r a c t i c a l l y no d i f f e r e n c e i n t h e
t
't
v a l u e s a t d i f f e r e n t pH, t h e p o s : . . i b i l i t y of a s s o c i b t i o n of m o l e c u l e s was t h o u g h t t o be v e r y s m a l l . Only when t h e s o l u t i o n p H w a s 0.6, t h e q J v a l u e was l a r g e , p r o b a b l y owing t o r e s t r i c t e d d i s s o c i a s i o n of s u l f o n a t e g r o u p s a t t h i s p H and t h e r e s u l t i n g a s s o c i a t i o n .F i g u r e 11. shows t h e r e l a t i o n s h i p between t h e pH and
a d s o r p t i o n q u a n t i t y ( F i g . 6 ) and t h e m o l e c u l a r s i z e of LSA ( F i g . 1 0 ) . T h i s a d s o r p t i o n a r e a v a l u e i s a t o t a l s u r f a c e a r e a upon which
LSP. molectiles a r e a d s o r b e d . Assitmine; t h a t t h e LSA m o l e c u l e s c a n c o v e r and occupy t h e c l a y s u r f a c e w i t h o u t s p a c e between t h e
m o l e c u l e s , t h e v a l u e i s e q u i v a l e n t w i t h t h e ~ u r f a c e a r e a o f
N a - m o n t m o r i l l o n i t e on which LSA c a n be a d s o r b e d . A s t h e m o l e c u l a r dimension of LSA u s e d f o r t h i s e x p e r i m e n t was a p p r o x i m a t e l y 100% i n d i a m e t e r , which i s n e ~ r l y 5 t i m e s a s l a r g e a s t h e t h i c k n e s s o f a u n i t l a y e r o f N a - m o n t m o r i l l o n i t e , one m o l e c u l e o f LSA c a n combine
Fig. 10 0 1 I . . a , . , ,
.
I 0 2 4 6 8 (>I I Fig. 11 Adsorption area v s pH Since t h e t h i c k n e s s of an e l e c t r i c double l a y e r 1 5 ) of a s u l f o n a t e 0 i o n i n 0.4W sodium c h l o r i d e s o l u t i o n seems t o be l e s s t h a n 10k, a p o r t i o n of t h e m o n t m o r i l l o n i t e s u r f a c e on which no a d s o r p t i o n can t a k e p l a c e because of a mutual r e p u l s i o n of two o r more molecules of LSA may be v e r y small. Thereifore, when a c h a r g ed e n s i t y on t h e c l a y s u r f a c e i s above a c e r t a i n s t a n d a r d , LSA
must h e adsorbed q u i t e densely on t h e c l a y s u r f a c e . Yeking i n t o account t h e f a c t t h a t t h e p o s i t i v e p o t e n t i a l ut the t e r m i n a l
s u r f a c e becomes l a r g e r a s t h e s o l u t i o n pH l o w e r s , . t h e curve of f i g u r e 11 can be i n t e r p r e t e d a s f o l l o w s . Namely, a t t h e p H range h i g h e r t h a n 8 , t h e p o s i t i v e charge d e c r e a s e s r a p i d l y and a t approxi-
15: S. Tojima "Basic Electro-chemistry (Kiso Denki-
a7
m a t e l y pH 9, ZPC a p p e a r s , w h i l e t h e f l a t p o r t i o n between pH 1.8
and 8 shows t h a t t h e a d s o r p t i o n took p l a c e v e r y d e n s e l y . I n
c o n c l u s i o n t h e s u r f a c e a r e a y o n which LSA can be a d s o r b e d , o f Na-montmorillonite i n 0.4N sodiunl c h l o r i d e s o l u t i o n i s
50-60 m2/g, and a t pH lower t h a n 7.8, t h e whole o f t h i n a r e a c a n be u t i l i z e d f o r a d s o r p t i o n , b u t above pH 7.8, o n l y a p o r t i o n cf t h e a r e a c a n a d s o r b LSA. A discus:.ed i n t h e p r e v i o u s s e c t i o n , t h i s a d s o r b a b l e a r e a i s t h e s u r f a c e area o f t h e t e r m i n a l s u r f a c e . The i n c r e a s e of adsorbed s u r f a c e a r e a below pH 1.8 i s p r o b a b l y
due t o t h e a s s o c i a t i o n o f m o l e c u l e s , which was a l r e a d y d i s c u s s e d , and t h e a d s o r p t i o n on l a j e r s u r f n c e and p o s s i b l e a d s o r p t i o n on t h e polymolecular l a y e r . I n o t h e r words, t h i s i n c r e a s e i s p~dobably an a p p a r e n t i n c r e a s e o f a d s o r p t i o n a r e a .